Abstract
Brain-derived neurotrophic factor (BDNF) affects synaptic plasticity and neural structure and plays key roles in learning and memory processes. Recent evidence also points to important, yet complex, roles for BDNF in rodent models of cocaine abuse and addiction. Here we examine the role of prefrontal cortical (PFC) BDNF in reward-related decision making and behavioral sensitivity to, and responding for, cocaine. We focus on BDNF within the medial and orbital PFC, its regulation by cocaine during early postnatal development and in adulthood, and how BDNF in turn influences responding for drug reinforcement, including in reinstatement models. When relevant, we draw comparisons and contrasts with experiments using natural (food) reinforcers. We also summarize findings supporting, or refuting, the possibility that BDNF in the medial and orbital PFC regulate the development and maintenance of stimulus-response habits. Further investigation could assist in the development of novel treatment approaches for cocaine use disorders.
Keywords: instrumental, prelimbic, operant, orbitofrontal, addiction, review, goal-directed
Introduction
Substance use disorders are profound public health concerns, with significant costs for affected individuals and the economy as a whole (Miller & Hendrie, 2008). In 2013, 1.5 million Americans aged 12 and over were current cocaine users (SAMHSA, 2014) and in 2010, cocaine was the leading cause of emergency room visits involving illicit drug usage (SAMHSA, 2012). Despite this, there is still currently no FDA-approved pharmaceutical treatment for cocaine dependence.
Substance use disorders are characterized by drug use despite negative consequences. Altered plasticity and aberrant changes in so-called “reward” and learning and memory circuits are thought to underlie, in part, maladaptive reward-related decision making in addiction (Everitt & Robbins, 2005; Hyman et al., 2006; Robbins et al., 2008; Dong & Nestler, 2014; Everitt, 2014). Understanding the mechanisms mediating drug-induced neurobiological changes that drive behaviors interpreted as drug-seeking in rodents could provide avenues to novel therapeutics that could break compulsive drug use in humans.
Brain-derived neurotrophic factor (BDNF) is involved in neural organization and synaptic plasticity during development and in adulthood (Huang & Reichardt, 2003; Binder & Scharfman, 2004; Park & Poo, 2013). Through activation of its high affinity receptor, tropomyosin receptor kinase B (trkB), BDNF activates signaling cascades that affect gene transcription and synaptic structure and plasticity (Atwal et al., 2000; Binder & Scharfman, 2004; Park & Poo, 2013). Genetic polymorphisms associated with reduced BDNF signaling (Egan et al., 2003) appear to increase the risk for the development of stimulant addiction (Cheng et al., 2005; Su et al., 2014). Additionally, blood serum BDNF levels rise during early periods of cocaine withdrawal (Von Diemen et al., 2014; Viola et al., 2014; Corominas-Roso et al., 2013,2015). Individuals with higher serum BDNF have been shown to relapse later than those with lower levels (Corominas-Roso et al., 2015); however, higher BDNF levels can also correlate with greater craving and loss of behavioral control (Corominas-Roso et al., 2013).
Abundant pre-clinical research has aimed at better understanding how brain BDNF is affected by cocaine exposure and how BDNF affects drug-seeking and decision-making behaviors. This review focuses on BDNF in the prefrontal cortex (PFC). The PFC plays an important role in learning and memory, decision-making processes, and in both the expression and inhibition of cocaine-reinforced behaviors (Robbins et al., 2008; Torregrossa et al., 2008; Peters et al., 2009; Lucantonio et al., 2012; Moorman et al., 2014). We first briefly summarize the neuroanatomy of the rodent PFC, as well as tasks commonly used to model aspects of drug abuse and addiction in rodents. We then review evidence that acute and repeated cocaine exposure affects BDNF and Bdnf expression in the PFC. Next, we consider whether drug-induced changes in BDNF levels in the PFC play a role in the development of behaviors interpreted as drug-seeking or is instead “protective.” We summarize the effects of direct manipulation of PFC BDNF expression on food- and drug-reinforced responding and on PFC-dependent decision making and discuss evidence for the “therapeutic-like” potential of manipulating trkB systems.
We focus this review in particular on PFC neurocircuits – including both medial and orbital regions of the PFC – and we refer readers to Russo et al. (2009), McGinty et al. (2010), Ghitza et al. (2010), Schmidt et al. (2013), Barker et al. (2014), and Li & Wolf (2015) for additional discussions regarding drug-mediated regulation of BDNF expression and activity throughout the multiple corticolimbic regions implicated in substance use disorders.
The rodent PFC: A brief overview
Broadly speaking, the rodent PFC can be divided into a lateral region, the orbitofrontal cortex (oPFC), and a medial region, referred to as the medial prefrontal cortex (mPFC). The mPFC can be further subdivided into the anterior cingulate cortex, prelimbic cortex (PL), infralimbic cortex (IL), and the medial oPFC (Ongur & Price, 2000) (fig. 1). The mPFC as a whole receives projections from multiple areas of the limbic systems involved in encoding reward salience and value, including the hippocampus and amygdala (e.g., Jay & Witter, 1991; McDonald, 1991; Gabbott et al., 2006; Mátyás et al., 2014; Zingg et al., 2014). This allows it to integrate information from multiple sources into decision-making processes, and to coordinate motor output via downstream structures. For instance, the PL innervates the nucleus accumbens (NAC) core and the basolateral and lateral nuclei of the amygdala, while the IL innervates the NAC shell and the basal, central, and medial amygdala (Sesack et al., 1989, McDonald et al., 1996; Heidbreder & Groenewegen, 2003). These two highly-studied structures (the PL and IL) regulate reward-related decision making, often with opposing influences (reviewed Moorman et al., 2014).
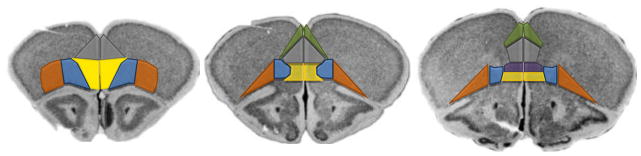
Figure 1. Regions of the rodent prefrontal cortex.
The mouse PFC can be divided into the anterior cingulate cortex (green), PL (gray), IL (purple), medial oPFC (yellow), ventrolateral oPFC (blue), and lateral oPFC (orange). The agranular insula, often studied in concert with the lateral oPFC, is lateral to the lateral oPFC. Regions outlined on coronal images from the Mouse Brain Library (Rosen et al., 2000).
The medial oPFC is positioned ventrally to the PL and IL, at the base of the medial wall (fig. 1). It innervates a thin strip of the dorsomedial striatum immediately adjacent to the ventricles, with projections extending ventrally to the NAC (e.g., see Schilman et al., 2008; Rodriguez-Romaguera et al., 2015). The medial oPFC has received less attention than the more dorsal regions of the mPFC, but despite this, recent reports indicate that the medial oPFC regulates the expression of conditioned fear and repetitive stereotyped behavior (Ahmari et al., 2013; Rodriguez-Romaguera et al., 2015). Further, inactivation of the medial oPFC induces perseverative-like responding for food reinforcers (Gourley et al., 2010), and this may be due to an inability to retrieve outcome-related information to guide response strategies (Bradfield et al., 2015). Medial oPFC inactivation also attenuates cocaine-primed reinstatement, an animal model of relapse (Fuchs et al., 2004). Together, these findings suggest that the medial oPFC regulates aspects of reward-related decision making in both food- and drug-related contexts.
The more lateral regions of the oPFC are essential for stimulus-dependent reward-related decision making and for integrating reward salience and expectancies to allow for “on-the-fly” response selection (Lucantonio et al., 2012; Stalnaker et al., 2015). The oPFC receives projections from the basal amygdala, and it innervates the amygdala and centrolateral and ventral striatum (Ongur & Price, 2000; Schilman et al., 2008; Hoover & Vertes, 2011; Gremel & Costa, 2013; Zingg et al., 2014).
There is much debate regarding what the oPFC does and does not do (cf., Stalnaker et al., 2015). This may be complicated by assuming homogeneity of oPFC projections. The ventrolateral subregion of the oPFC, situated between the lateral oPFC and the medial oPFC, has overlapping, as well as distinct, projection properties, relative to the other oPFC subregions. For example, the ventrolateral oPFC innervates the dorsal striatum but largely spares the NAC; it also innervates the basolateral amygdala but to a lesser degree than the lateral oPFC (Schilman et al., 2008; Rodriguez-Romaguera et al., 2015; Zimmermann et al., 2015). These distinctions likely position the lateral and ventrolateral oPFC to differentially regulate reward-related behaviors.
Animal models of drug seeking and habit formation
In order to study the molecular- and circuit-level changes associated with cocaine exposure and addiction, researchers must model drug seeking and related behaviors using tractable experimental conditions. We will briefly summarize behavioral tasks relevant to this review. First, conditioned place preference (CPP) can be used to examine the development and extinction of a Pavlovian association between a previously neutral context and cocaine administration. Preference for a cocaine-paired context, and how long it lasts when cocaine is withheld, is thought to reflect a subject’s sensitivity to the drug and its ability to acquire and extinguish the context-drug association.
Cocaine self-administration studies allow subjects to control drug intake and can be used to quantify the acquisition and maintenance of a drug-reinforced response, binge-like behavior, and the reinstatement of drug seeking following extinction. In this case, mice or rats perform an operant response (e.g., lever press or nose poke) to receive a cocaine reinforcer. The reinforcer is most commonly a direct intravenous infusion, although cocaine can also be self-administered orally (e.g., Macenski et al., 1998; Miles et al., 2003; Gabriele et al., 2009). Cocaine delivery is often paired with a stimulus, such as a light or a tone. Thus, self-administration studies typically involve components of action-outcome conditioning — associating an operant response with reinforcer delivery — and stimulus-outcome conditioning — associating a cue with the drug.
To assess the reinstatement of cocaine seeking, an experimenter-administered cocaine injection, presentation of the drug-paired stimulus, or acute stressor (termed drug, cue, and stress-induced reinstatement, respectively) is used to reinstate operant responding. The amount of responding in extinction (no reinforcer is delivered during reinstatement) is considered a marker of drug seeking (for further discussion, see Marchant et al., 2013).
Another pair of tasks can be used to assess whether rodents use action-outcome (goal-directed) or stimulus-response (habitual) response strategies. Although these tasks typically use food as the reinforcers, they are relevant to issues of drug abuse because stimulus-elicited habits are considered etiological factors in the development and maintenance of addiction (Jentsch & Taylor, 1999; Everitt & Robbins, 2005; Schwabe et al., 2011; Torregrossa et al., 2011). In these tasks, the value of a reinforcer is reduced via prefeeding or transient LiCl-induced gastric malaise (outcome devaluation). Alternatively, the contingency between a trained response and the reinforcer is violated (action-outcome contingency degradation). Animals using goal-directed behavioral response strategies will adjust (decrease) their responding. Animals that have developed habits, however, will continue responding, as previously (for further review of these tasks, see Yin et al., 2008; Balleine & O’Doherty, 2010).
Cocaine rapidly regulates mPFC BDNF and Bdnf, and acute BDNF infusion can decrease cocaine-related responding
BDNF is a member of the neurotrophin family that, in mammals, includes nerve growth factor, neurotrophin-3, and neurotrophin 4/5. BDNF is initially synthesized as a 32-kD pro-peptide (referred to as “pro-BDNF”) and is then cleaved into a 14-kD mature form. pro-BDNF preferentially binds and activates the p75 pro-apoptotic receptor. Meanwhile, mature BDNF preferentially stimulates the trkB receptor. Ligand binding and trkB receptor autophosphorylation initiate multiple intracellular signaling cascades through the MAP kinase, PI3-kinase, and the PLCγ pathways. Through these, BDNF regulates neuronal activity and synaptic and structural plasticity during both pre- and postnatal development, and in the mature brain (Reichardt, 2006; Lu et al., 2014).
Cocaine dynamically regulates trkB, BDNF protein, and Bdnf mRNA expression in the mPFC (summarized in table 1). In mature rodents, acute experimenter-administered cocaine can increase Bdnf mRNA within ~2 hours of exposure, and this is associated with an increase in expression of the mature form of BDNF 24 hours after injection (Le Foll et al., 2005; Fumagalli et al., 2007,2009). In contrast, 22–72 hours after repeated exposure to cocaine, either experimenter- or self-administered, Bdnf levels drop (McGinty et al., 2010; Fumagalli et al., 2007). Accordingly, BDNF replacement in the PL suppresses cocaine-related responding in extinction and in reinstatement tests using cocaine-associated cues or a cocaine prime (Berglind et al., 2007,2009; Whitfield et al., 2011; McGinty et al., 2010) (table 1). Blockade of mPFC trkB activity occludes these effects, indicating that local BDNF-trkB binding can, at least in part, account for suppressive effects on cocaine seeking (see McGinty et al., 2010; Whitfield et al., 2011). Within the ventromedial PFC, local infusions of BDNF into the IL enhance the extinction of cocaine-CPP (Otis et al., 2014). IL BDNF infusions also rescue cocaine-induced deficiencies in fear extinction recall (Kabir et al., 2013).
Table 1. Postnatal cocaine exposure regulates mPFC BDNF systems, and PL BDNF regulates appetitive conditioning: A summary.
Report synopses are provided at left, with the corresponding references at right. These studies highlight the temporally dynamic regulation of BDNF or Bdnf following acute (gray cells) vs. repeated (white cells) cocaine. Epigenetic factors (dark green cells) and effects of early-life cocaine exposure (light green cells) are also reported. The bottom half of the table addresses the effects of direct manipulations of PL BDNF on reinstatement (beige cells); cocaine- vs. food-reinforced responding (blue cells); and cocaine-CPP and habits (orange cells).
| Cocaine regulates Bdnf and BDNF (Tissue samples collected from the mPFC except those marked “*,” connoting samples collected from the frontal cortex) | |
|---|---|
| Brief synopsis | Reference |
| Acute cocaine (20 mg/kg) increases Bdnf 2–3 hours following exposure, and expression is typical by 5 hours. Methamphetamine has similar effects. | Le Foll et al., 2005 |
| Acute cocaine (40 mg/kg) increases Bdnf exon I and IV 4 hours following exposure. | Liu et al., 2006* |
| Acute cocaine (5 mg/kg) increases Bdnf mRNA 2–24 hours after exposure; expression of mature BDNF protein is increased at the 24 hour time point. | Fumagalli et al., 2007 |
| Acute cocaine (10 mg/kg) increases Bdnf , TrkB (full-length), synaptic trkB, and ERK1/2 phosphorylation within 2 hours of injection. Chronic stressor exposure blocks these effects. | Fumagalli et al., 2009 |
| Repeated cocaine self-administration (1 hr/day; 10 days) and experimenter- administered cocaine (20 mg/kg/day; 10 days) does not impact Bdnf expression as measured 1, 30, or 90 days (self-administration) or 4 hours (experimenter-administered) after cocaine. | Liu et al., 2006* |
| Repeated cocaine exposure (non-contingent; 5 mg/kg/day; 5 days) increases Bdnf and CREB expression and phosphorylation 2 hours after the last exposure. However, both pro-BDNF and mature BDNF protein levels are reduced 2 and 72 hours after repeated cocaine exposure. | Fumagalli et al., 2007 |
| Repeated cocaine self-administration (2 hr/day; 10 days) increases Bdnf expression when assessed 22 hours following the last infusion, but only if a cocaine-associated cue is present. Following 15 days of abstinence Bdnf is upregulated regardless of cue presence. | Hearing et al., 2008 |
| Repeated cocaine self-administration reduces Bdnf expression within 22 hours of a final infusion, and then BDNF expression levels increase above control within 21 days. | McGinty et al., 2010 |
| Repeated cocaine self-administration (2 hr/day; 14 days) increases Bdnf (exon IV) and BDNF levels when measured 1 week after the last exposure. Cocaine increases the association of phosphorylated CREB with Bdnf exon IV. | Sadri-Vakili et al., 2010 |
| Repeated cocaine self-administration or yoked exposure (14 days) increases mature BDNF and Bdnf exon I within 24 hours of the last session, but Bdnf exon IV is reduced and Bdnf exon VI is unchanged. One week later, BDNF protein levels are unchanged. | Fumagalli et al., 2013 |
| Repeated cocaine self-administration (24 hr/day; 4 trials/hr; 10 days) increases Bdnf exon IV when tested 14 days following the last session. | Peterson et al., 2014 |
| Repeated cocaine self-administration (6 hr/day; 10 days) does not modify Bdnf or BDNF when tested 45 days after exposure. | Li et al., 2013 |
| Repeated cocaine exposure (non-contingent; 25 mg/kg/day; 5 days) increases BDNF and trkB expression 25 days after administration. Protein levels were assessed following a cocaine prime (7.5 mg/kg) given one day prior to euthanasia. | Zhang et al., 2015 |
| The male offspring of cocaine self-administering rats are cocaine-resilient and have increased mPFC Bdnf exon IV, and BDNF. Resilience can be blocked with a trkB antagonist, which augments cocaine self-administration. | Vassoler et al., 2013 |
| Sign-tracking rats, known to have higher rates of cocaine-seeking behavior in reinstatement, have lower levels of BDNF. | Morrow et al., 2015* |
| Early-life cocaine exposure (10 mg/kg/day; postnatal days 28–42) increases Bdnf exon IV, pro-BDNF, mature BDNF, and synaptic trkB. This is detectable 48, but not 3, days following exposure. Concurrently, levels of tPA, the enzyme responsible for the cleavage of pro-BDNF into mature BDNF, are upregulated. Phosphorylation of Akt, mTOR, and S6K also increases. | Giannotti et al., 2014 |
| Early-life cocaine exposure (15 mg/kg/day; postnatal days 18–24) increases BDNF expression at 8 and 14 days following exposure (but not 1 or 3 days). No changes to trkB. | Lu et al., 2010 |
| Bdnf and BDNF in the PL regulate appetitive decision making | |
| Brief synopsis | Reference |
| Acute BDNF infusion suppresses cue- and cocaine-induced reinstatement of cocaine seeking and normalizes ERK phosphorylation in the downstream NAC, but not dorsal striatum. No effects on the reinstatement of food seeking. | Berglind et al., 2007 |
| Acute BDNF infusion suppresses the reinstatement of cocaine seeking and normalizes extracellular glutamate levels in the NAC. | Berglind et al., 2009 |
| Acute BDNF infusion suppresses the reinstatement of cocaine seeking, and effects are associated with local trkB-ERK1/2 activation. | Whitfield et al., 2011 |
| Acute BDNF infusion immediately following repeated cocaine self-administration can enhance the extinction of a cocaine-reinforced response. Effects are most robust during initial training. | Berglind et al., 2007 |
| Viral-mediated Bdnf knockdown enhances the extinction of a food-reinforced operant response; effects are most robust during initial training. BDNF infusion has no effects at a concentration that decreases adrenal gland weight. | Gourley et al., 2009a |
| Viral-mediated Bdnf knockdown increases cocaine-reinforced responding on a progressive ratio schedule of reinforcement. No effects on response acquisition. | Sadri-Vakili et al., 2010 |
| Viral-mediated Bdnf knockdown decreases food-reinforced responding on a progressive ratio schedule of reinforcement. | Gourley et al., 2012a; see fig. 2 |
| Viral-mediated Bdnf knockdown interferes with cocaine-CPP. | Choi et al., 2012 |
| Acute BDNF infusion induces habit-like behavior in typical mice. | Gourley et al., 2012a |
| Viral-mediated Bdnf knockdown is unable to protect against habits induced by adolescent cocaine exposure. | Hinton et al., 2014 |
The PL preferentially innervates the NAC core, and as with PL BDNF, trkB activity in the NAC core appears to oppose cocaine-seeking behaviors. Specifically, siRNA-mediated knockdown of the trkB receptor increases cue-induced responding when rats are tested immediately following a period of cocaine self-administration (Li et al., 2013). This is significant because cortical projections provide a primary source of BDNF in the striatum, which contains little Bdnf mRNA (Altar et al., 1997). Accordingly, PL-selective knockdown of Bdnf decreases BDNF protein expression in the striatum (Gourley et al., 2009a,2012a). Conversely, BDNF infusion increases BDNF expression in the NAC, and levels of phosphorylated (active) ERK1/2 also increase (Berglind et al., 2007; McGinty et al., 2010). Infusions of BDNF into the mPFC also normalize levels of extracellular glutamate and activity of the vesicular trafficking protein synapsin in the NAC following cocaine exposure (Berglind et al., 2009; Sun et al., 2014). These findings are particularly provocative given that the reinstatement of drug seeking after extinction is thought to reflect, at least in part, disturbances in glutamatergic neurotransmission in a mPFC-NAC pathway, specifically, depleted levels following repeated cocaine exposure, followed by robust up-regulation after re-exposure to cocaine or cocaine-related cues (Kalivas, 2009).
A history of cocaine exposure increases mPFC BDNF and Bdnf
In the aforementioned studies of McGinty and colleagues, in which BDNF was infused into the PL of cocaine self-administering rats, BDNF was largely infused immediately following a period of cocaine self-administration, coinciding with low BDNF levels. In other experiments, infusions later in the drug abstinence period were ineffective (Berglind et al., 2007), suggesting the possibility that enhancing mPFC BDNF signaling (i.e., by replacing BDNF tone) has protective benefits during a quite narrow time window. This may be because endogenous BDNF protein and Bdnf mRNA appear to increase in the days and weeks following cocaine exposure, eventually exceeding typical levels (Hearing et al., 2008; McGinty et al. 2010; Sadri-Vakili et al., 2010; Zhang et al., 2015) (summarized table 1).
A history of early-life experimenter-administered cocaine exposure also progressively increases mPFC BDNF, leading to increased mPFC BDNF expression in adulthood (Lu et al., 2010; Giannotti et al., 2014). Notably, however, a recent study by Simchon-Tenenbaum et al., 2015 did not replicate this finding. This discrepancy may be due to differences in tissue dissection strategies, since Simchon-Tenenbaum et al. appeared to extract the whole PFC for analysis, which would include lateral (oPFC), in addition to medial, subregions. As will be discussed below, the oPFC may respond differently to cocaine exposure, which could preclude the detection of elevated mPFC BDNF in tissue samples containing both the mPFC and oPFC.
Chronic deviations in typical mPFC BDNF-trkB tone influence locomotor sensitization and cocaine- and food-reinforced behaviors
The prolonged augmentation of mPFC BDNF expression following cocaine exposure (see above) could conceivably be associated with increased behavioral sensitivity to the drug. Consistent with this notion, TrkB knockdown broadly throughout the mPFC modestly blunts the motoric response to cocaine in sensitized mice (Lu et al., 2010). Additionally, the development of cocaine-induced locomotor sensitization is delayed in Bdnf+/− mice (Horger et al., 1999). Locomotor sensitization can also be blocked by a combination of dopamine D1/D2 receptor and 5-HT3 receptor antagonists, which interferes with cocaine-induced increases in mPFC BDNF (Zhang et al., 2015). These studies suggest that mPFC BDNF-trkB could support the development and expression of cocaine-induced locomotor sensitization.
Drug-induced mPFC BDNF over-expression could also conceivably increase cocaine-seeking behaviors. In one study, cocaine increased mPFC levels of Bdnf exon IV, and aerobic exercise normalized Bdnf exon IV levels and reduced cocaine self-administration in tandem, suggesting that mitigating drug-related increases in mPFC Bdnf could be associated with cocaine resilience (Peterson et al., 2014). In a similar vein, PL-targeted Bdnf knockdown can blunt cocaine-CPP (Choi et al., 2012).
Despite these findings, blocking drug-related increases in mPFC BDNF does not necessarily protect against cocaine vulnerabilities in all contexts. For example, mPFC-targeted Bdnf knockdown increases, rather than decreases, cocaine self-administration on a progressive ratio schedule of reinforcement (Sadri-Vakili et al., 2010), even while decreasing responding for food reinforcement (Gourley et al., 2012a; fig. 2). Also, inhibiting Bdnf enhances the cytotoxic properties of cocaine in cultured cells (Yan et al., 2007), and PL-targeted Bdnf knockdown failed in one report to block biases towards habit-based decision making induced by adolescent cocaine exposure (Hinton et al., 2014).
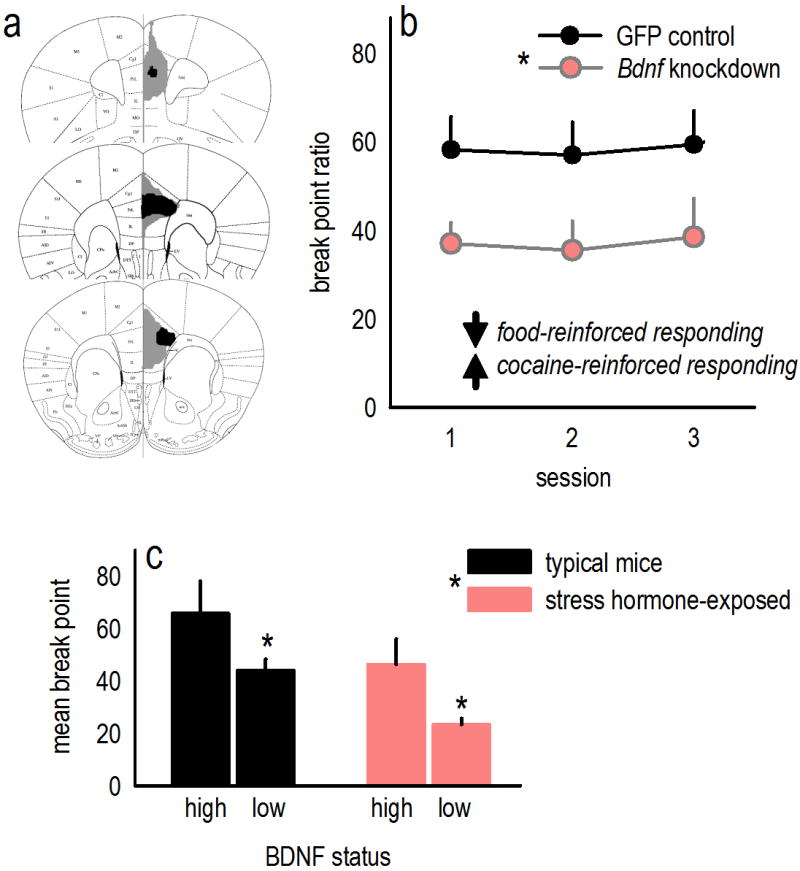
Figure 2. PL BDNF regulates responding for “natural reward”.
(a) Viral vectors expressing Cre Recombinase were delivered to the PL of ‘floxed’ Bdnf mice, generating a site-selective knockdown. Control mice received a viral vector expressing Green Fluorescent Protein (GFP). The largest viral vector spread is represented in gray, the smallest in black. (b) Bdnf knockdown caused a persistent drop in responding for food reinforcers on a progressive ratio schedule of reinforcement; meanwhile, knockdown increases cocaine-reinforced responding on a progressive ratio schedule (Sadri-Vakili et al., 2010). (c) We re-analyzed data from typical mice or mice chronically exposed to the stress hormone corticosterone in Gourley et al. (2012a), generating groups based on a median split of endogenous PL BDNF levels. Corticosterone reduced BDNF overall. Additionally, “high” BDNF was associated with high break point ratios, while “low” BDNF was associated with low break point ratios, again pointing to differential roles for mPFC BDNF in regulating food- vs. cocaine-reinforced responding on a progressive ratio schedule (cf., Sadri-Vakili et al., 2010). Figure components are compiled or reprinted from Gourley et al., 2012a. Bars and symbols represent group means+SEMs, *p<0.05.
As discussed, BDNF is subject to anterograde transport, such that selective BDNF overexpression in the dorsomedial PFC induces BDNF over-expression in the downstream amygdala (McGinty et al., 2010), and selective Bdnf knockdown in the oPFC reduces BDNF levels in the amygdala (Gourley et al., 2013a; Zimmermann et al., 2015). Interestingly, inhibition of BDNF-trkB signaling in the amygdala [another structure subject to cocaine-induced increases in BDNF expression (Grimm et al., 2003)] interferes with the extinction of cocaine-CPP (Heldt et al., 2014). Together, these findings further suggest that BDNF in certain PFC circuits supports key behavioral inhibitory functions.
These and other findings have led to the perspective that cocaine-induced increases in mPFC BDNF may have some protective properties. Indeed, the male offspring of cocaine self-administering rats are cocaine-resilient and also have higher levels of Bdnf and BDNF in the mPFC than control counterparts (Vassoler et al., 2013). Cocaine resilience in these rats is entirely blocked by administration of a trkB antagonist, providing evidence that mPFC BDNF-trkB systems can have protective properties. Additionally, typical rats that learn to approach reward-related stimuli (sign-trackers), instead of the location of food reinforcer delivery (goal-trackers), in a Pavlovian conditioned approach task have lower levels of PFC BDNF (Morrow et al., 2015). Sign-trackers also exhibit greater drug-seeking behavior in reinstatement tests (Saunders & Robinson, 2010,2011; Yager & Robinson, 2013), again suggesting that PFC BDNF may be “protective” against certain drug-reinforced behaviors.
Increases in BDNF following cocaine abstinence are linked to cocaine-induced long-term potentiation in the mPFC, and mechanistically, this may occur via the reduction of cell-surface ionotropic GABAA receptors (Lu et al., 2010). This discovery led to subsequent investigations utilizing viral-mediated gene silencing of the predominant GABAA subunit, GABAAα1, in the mPFC. Particularly when knockdown was initiated early in life, GABAAα1 silencing induced a deferral to habit-based responding in food-reinforced operant conditioning tasks, mimicking the effects of cocaine (Butkovich et al., 2015). GABAAα1-deficient mice were also delayed in acquiring a cocaine-reinforced response, but even when cocaine exposure was controlled, GABAAα1 silencing had no effects on the reinstatement of cocaine seeking following extinction (Butkovich et al., 2015). These findings suggest that chronic changes in dorsomedial PFC GABAAα1 systems (linked to changes in BDNF or other factors) do not obviously account for relapse in cocaine addiction.
Does mPFC BDNF influence habit-based behavior?
Several independent groups have reported using reinforcer devaluation and instrumental contingency degradation tasks that a history of repeated cocaine or amphetamine exposure can induce outcome-insensitive habits (Schoenbaum & Setlow, 2005; Nelson & Killcross, 2006,2013; Nordquist et al., 2007; LeBlanc et al., 2013; Corbit et al., 2014; Hinton et al., 2014). Further, instrumental responding for cocaine can quickly become dominated by habit-like strategies (Miles et al., 2003; Zapata et al., 2010). Additionally, acute cocaine exposure can disrupt the consolidation of new action-outcome associative learning and memory, resulting in a deferral to habit-based response strategies (Gourley et al., 2013b), and pairing cocaine with a food-reinforced response also results in behavioral insensitivity to the devaluation of the food reinforcer (Schmitzer-Torbert et al., 2015). Thus, cocaine exposure biases response strategies towards habits.
The relationship between cocaine-induced augmentation of mPFC BDNF and the development and maintenance of cocaine-related habits is, in our view, opaque. Firstly, PL-directed BDNF infusion in mice can induce habit or habit-like behaviors, similar to the effects of cocaine (Graybeal et al., 2011; Gourley et al., 2012a). This may be because mPFC Bdnf increases during the initial acquisition of a food-reinforced instrumental response, but then decreases with proficiency (Rapanelli et al., 2010). Aberrant drug-induced elevations in mPFC BDNF that persist after task proficiency has been achieved could conceivably disrupt typical intracellular signaling essential for goal-directed action selection, causing mice to defer to habit-based decision making.
One caveat to this model is that experiments using BDNF infusions may rely on BDNF concentrations that exceed physiological levels (Li & Wolf, 2015). Additionally, PL-targeted Bdnf knockdown, which would presumably interfere with cocaine-induced increases in mPFC BDNF, failed in one report to block habits caused by adolescent cocaine exposure (Hinton et al., 2014). The suggestion that drug-related mPFC BDNF overexpression induces reward-seeking habits is also at odds with evidence that stressor exposure blocks cocaine-induced Bdnf up-regulation (Fumagalli et al., 2009) and at the same time facilitates habit formation in both rodents and humans (Dias-Ferreira et al., 2009; Schwabe, 2013; see also Gourley et al., 2012a). Further, the presence of the met allele at codon 66 of the BDNF gene in humans increases, rather than decreases, the likelihood that individuals will rely on habit-based strategies in spatial navigation tasks (Banner et al., 2011). These findings challenge a model in which cocaine-induced BDNF over-expression in the mPFC induces biases towards habit-based decision making.
Effects of trkB stimulation
To summarize, mPFC BDNF-trkB significantly impacts behavioral sensitivity to cocaine, and in ways that can appear contradictory. For example, mPFC BDNF-trkB appears to enhance locomotor sensitivity to cocaine (Lu et al., 2010), but also facilitate the extinction of a cocaine-reinforced response (Berglind et al., 2007) [even while interfering with the extinction of food-reinforced responding (Gourley et al., 2009a)]. Adding to this already complicated picture is evidence that drug-induced BDNF over-expression in certain subcortical structures is implicated in drug-seeking behaviors (reviewed Li & Wolf, 2015).
These properties may suggest that BDNF-trkB has limited utility as a therapeutic target. Nonetheless, a bioactive, high-affinity trkB agonist that causes receptor dimerization and auto-phosphorylation was recently characterized (Jang et al., 2010), resulting in studies assessing the behavioral effects of this putative trkB agonist, 7,8-dihydroxyflavone (7,8-DHF). Systemic administration of 7,8-DHF dose-dependently attenuates methamphetamine-induced locomotor sensitization (Ren et al., 2014) and normalizes drug-induced impairments in prepulse inhibition (Ren et al., 2013). 7,8-DHF additionally interferes with cocaine seeking in mice that self-administered cocaine, were then subject to forced abstinence, and finally, were re-exposed to the cocaine-associated context (DePoy et al., in review). 7,8-DHF also blocks stimulus-response habits induced by response over-training (Zimmermann et al., 2015). This effect is reversed by co-administration of a trkB antagonist, raising the possibility that trkB-targeting manipulations could mitigate habits caused by drugs of abuse. Additionally, local infusions of 7,8-DHF into the IL enhance the extinction of cocaine-CPP (Otis et al., 2014), while systemic 7,8-DHF treatment has apparently no effects on the acquisition of cocaine-CPP in typical rodents (Tzeng et al., 2013). Another trkB agonist, LM22A-4, decreases compulsive-like alcohol consumption in mice (Warnault et al., 2015; see for further discussion, Logrip et al., 2015). These findings together highlight the possible utility of pairing trkB-based interventions with therapies for drug use disorders, though further research is certainly necessary.
oPFC BDNF regulates reward-related and goal-directed decision making
Studies utilizing viral-mediated gene silencing strategies to assess the role of Bdnf in the oPFC in complex decision making and cocaine-related behaviors indicate that Bdnf knockdown enhances the acquisition, and impairs the extinction, of cocaine-CPP (Gourley et al., 2013a). Additionally, oPFC-selective Bdnf knockdown induces stimulus-response habits that occur at the expense of goal-directed decision making (Gourley et al., 2013a; Zimmermann et al., 2015), mimicking the effects of cocaine exposure (discussed above).
Despite these and other findings, whether the oPFC regulates goal-directed action selection vs. habit behavior remains a contentious topic. Ostlund and Balleine (2007) generated large lesions encompassing the lateral and ventrolateral oPFC in rats and reported that oPFC damage did not impact behavioral sensitivity to reinforcer devaluation. In other words, oPFC damage apparently did not cause habits. Meanwhile, certain forms of Pavlovian (stimulus-outcome) conditioning were impaired, consistent with an historical focus on oPFC involvement in stimulus-outcome learning and memory (discussed Ostlund & Balleine, 2007). In 2013, however, Gremel and Costa placed lesions in the ventrolateral oPFC of the mouse, causing behavioral insensitivity to reinforcer devaluation, suggesting that oPFC damage causes habit-based decision making. They also found, using multi-site multi-electrode recordings, neural ensembles in the oPFC that encoded action-value information (Gremel & Costa, 2013). In the same year, the present authors reported that oPFC-selective knockdown of Bdnf and lesions disconnecting the oPFC from the dorsal striatum also induce habits (Gourley et al., 2013a). We additionally find that habit biases can be attributed to failures in consolidating or retaining action-outcome memory (Zimmermann et al., 2015), and we have reported that viral-mediated knockdown of Gabra1 and Fmr1 in the oPFC also induce habit-based responding (Swanson et al., 2015; Gross et al., 2015). These findings suggest that the healthy oPFC is important for goal-directed (action-outcome) response selection.
How might we reconcile these findings with the early findings of Ostlund and Balleine (2007)? Key differences include species and sex, since Ostlund and Balleine (2007) utilized female rats, while Costa, Gremel, and the present authors have primarily utilized male mice. Another possible factor is the training history of the experimental animals. The rats used in the report of Ostlund and Balleine (2007) were first trained for 8 days to associate distinct auditory stimuli with two different outcomes (pellets vs. sucrose). Then, 11 days of instrumental conditioning followed, in which rats were trained to respond for the same two outcomes. Thus, rats had ample opportunity to form multiple stimulus-outcome and action-outcome associations prior to test. By contrast, the mice used by Gremel and Costa (2013) and Gourley et al. (2013a) responded for a single outcome and were not subject to explicit reward-associated stimuli. Further, the mice in the Gourley report also generated much lower response rates overall, which could minimize the opportunity to strongly encode or retain action-outcome information.
It may be that the oPFC is involved in early phases of forming or retaining action-outcome associations, but that with sufficient task experience, this information can be encoded and retained in the absence of a healthy oPFC. Consistent with this notion, mice with unilateral oPFC Bdnf knockdown and contralateral amygdala lesions “disconnecting” these structures are insensitive to instrumental contingency degradation, deferring to habit-based response strategies (Zimmermann et al., 2015). These same mice can, however, ultimately develop sensitivity to changes in instrumental contingencies with repeated training, suggesting that oPFC insult delays, but does not fully block, learning or retaining new information about action-outcome contingencies. This may also account for instances in which mice with oPFC damage fail to develop sensitivity to instrumental contingency degradation, but when tested in a reinforcer devaluation task following additional training, responding is intact (Gourley et al., 2013a; Swanson et al., 2015). Additional experiments would, however, be required to explicitly test this model.
Experiments using inhibitory Gi-coupled Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) in the ventrolateral oPFC further suggest that the oPFC is involved in behavioral sensitivity to action-outcome relationships. Gremel and Costa (2013) found that activating Gi-DREADDs in the oPFC before outcome revaluation testing induced habit-like responding. Additionally, in a recent experiment, Gi-DREADDs were stimulated immediately following modifications in familiar action-outcome contingencies, during the presumptive consolidation of new learning. During a subsequent drug-free probe test, all mice were initially able to select responses that were more, vs. less, likely to be reinforced, a goal-directed response strategy. However, response preference rapidly decayed in mice expressing Gi-DREADDs in the oPFC, such that these mice ultimately deferred to habit-based strategies (Zimmermann et al., 2015). These studies further suggest that the oPFC is involved in retaining action-outcome-based memory.
Does cocaine impact BDNF expression in the oPFC?
As discussed, mPFC BDNF levels increase following repeated cocaine exposure, including cocaine exposure during early-life development (Lu et al., 2010; Giannotti et al., 2014). In contrast, early-life exposure to atomoxetine, which, like cocaine, inhibits the norepinephrine transporter, decreases Bdnf expression in the adult oPFC (Sun et al., 2012). In mature rodents, Bdnf in the oPFC increased following repeated cocaine self-administration in one report, and interestingly, this up-regulation was only detectable in rats that were re-exposed to the cocaine self-administration context prior to euthanasia (Hearing et al., 2008). Context-specific changes in oPFC Bdnf are consistent with the existence of projections from hippocampal structures to the oPFC (e.g., Morecraft et al., 1992). Given that oPFC Bdnf knockdown degrades goal-directed response selection (Gourley et al., 2013a; Zimmermann et al., 2015), it is tempting to speculate that drug-related oPFC Bdnf deficiency could drive habit-bsaed drug seeking, while oPFC Bdnf overexpression could conversely drive goal-oriented drug seeking in drug-related contexts, though additional studies are necessary.
Regulation of neuron structure
In addition to synaptic plasticity, BDNF-trkB interactions regulate the shape and structure of neurons. For example, stimulation of trkB promotes neurite outgrowth in several biological systems and acts at several steps to suppress p75 signaling (Reichardt, 2006). This is relevant because p75 activity can otherwise inhibit neural outgrowth via activation of the RhoA GTPase and substrates such as Rho-kinase. Accordingly, we have discovered that systemic administration of a Rho-kinase inhibitor corrects impulsive-like food-reinforced responding following oPFC Bdnf knockdown in female mice (DePoy et al., 2013) and habit-based responding following oPFC Bdnf knockdown in male mice (Zimmermann et al., 2015). These findings highlight another possible point of intervention in combatting cocaine seeking – that is, the regulation of cell shape and structure. This idea is reinforced by evidence that cocaine cue-induced neuroplasticity in the PL regulates changes in dendritic spine head size – a metric of synaptic strength – in the NAC following the presentation of cocaine-related cues (Gipson et al., 2013), and that inhibiting the activity of the cytoskeletal regulatory elements Arg kinase and β1-integrin in the oPFC and forebrain, respectively, greatly exaggerates cocaine-induced locomotor sensitization (Gourley et al., 2009b; Warren et al., 2012).
Determining whether BDNF-trkB influences cocaine-induced cellular structural modifications throughout the cortico-limbic structures implicated in drug abuse and addiction may be a fruitful topic of future research. Under certain circumstances, the putative trkB agonist 7,8-DHF can induce dendritic spine proliferation in the hippocampus and oPFC (Zeng et al., 2012; Zhang et al., 2014a,b; Zimmermann et al., 2015), while cocaine decreases dendrite complexity and dendritic spine density in the oPFC (Gourley et al., 2012b; DePoy et al., 2014; Radley et al., 2015). Notably, one study found no changes in oPFC dendritic spine density following extended-access cocaine self-administration (Ferrario et al., 2005). It is possible that different methods in imaging and dendritic branch selection could explain the different results between studies, given that the Ferrario report (2005) utilized Golgi-Cox staining and sampled spines from third-order terminal tips or greater, while other studies used fluorescence imaging and examined segments within 150 μm of the soma (Gourley et al., 2012b; Radley et al., 2015). Whether 7,8-DHF can block cocaine-induced spine loss in the oPFC has not, to our knowledge, been tested.
In another study using mice lacking Fmr1, dendritic spines aberrantly proliferated in the hippocampus, and 7,8-DHF reduced densities to typical levels (Tian et al., 2015). Together, these findings suggest that 7,8-DHF promotes homeostatic dendritic spine plasticity, rather than simply increasing or decreasing spine numbers. This is notable given that cocaine and other psychostimulants can both increase and decrease dendritic spine densities, depending on the brain region sampled (reviewed Kolb & Muhammad, 2014; DePoy & Gourley, 2015). Strategies that normalize structural and synaptic plasticity throughout multiple regions may be particularly attractive strategies for treating drug use disorders.
Conclusions
BDNF is involved in a wide range of brain functions, including neuronal differentiation and neurite outgrowth during development and synapse structure and plasticity throughout development and adulthood (Binder & Scharfman, 2004; Park & Poo, 2013). BDNF is also crucial for multiple forms of learning and memory (Yamada & Nabeshima, 2003; Lu et al., 2008) and is implicated in several psychiatric disorders, including depression, addiction, and obsessive-compulsive disorder (Binder & Sharfman, 2004; Autry & Monteggia, 2012). Studies reviewed here indicate that mPFC and oPFC BDNF systems are dynamically regulated by cocaine exposure, and in turn impact cocaine-related learning and memory and decision making. There is interest in treating substance use disorders with therapies that enhance flexible goal-directed decision-making processes, as opposed to, for example, mitigating the reinforcing effects of, or craving for, cocaine (see Everitt & Robbins, 2005; Pierce & Vanderschuren, 2010). The effects of BDNF on synapse formation and learning and memory could conceivably complement therapies aimed at strengthening goal-directed decision making or extinguishing connections between drug cues and craving. The complex effects of cocaine exposure on BDNF, as well as the complicated role of BDNF in reward-related decision making in general, may limit its therapeutic potential, but preliminary studies with systemic administration of 7,8-DHF (Ren et al., 2013,2014; Zimmermann et al., 2015) provide some evidence of utility. Further understanding the intricacies of how site-specific, and global, stimulation of BDNF-trkB activity affect behavior could open avenues for the development of novel pharmacotherapies.
Acknowledgments
This work was supported by PHS DA011717, DA027844 (JRT), MH101477, DA034808 and DA036737 (SLG), and the Connecticut Department of Mental Health and Addiction Services (JRT). The Yerkes National Primate Research Center is supported by the Office of Research Infrastructure Programs/OD P51OD011132.
Footnotes
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, Gordon JA, Hen R. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340(6137):1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389(6653):856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27(2):265–277. doi: 10.1016/s0896-6273(00)00035-0. [DOI] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-Derived Neurotrophic Factor and Neuropsychiatric Disorders. Pharmacol Rev. 2012;64(2):238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banner H, Bhat V, Etchamendy N, Joober R, Bohbot VD. The brain-derived neurotrophic factor Val66Met polymorphism is associated with reduced functional magnetic resonance imaging activity in the hippocampus and increased use of caudate nucleus-dependent strategies in a human virtual navigation task. Eur J Neurosci. 2011;33(5):968–977. doi: 10.1111/j.1460-9568.2010.07550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Taylor JR, De Vries TJ, Peters J. Brain-derived neurotrophic factor and addiction: Pathological versus therapeutic effects on drug seeking. Brain Res. 2014 doi: 10.1016/j.brainres.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26(3):757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr, LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29(12):3715–3719. doi: 10.1523/jneurosci.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22(3):123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield LA, Dezfouli A, van Holstein M, Chieng B, Balleine BW. Medial Orbitofrontal Cortex Mediates Outcome Retrieval in Partially Observable Task Situations. Neuron. 2015;88(6):1268–1280. doi: 10.1016/j.neuron.2015.10.044. [DOI] [PubMed] [Google Scholar]
- Butkovich LM, DePoy LM, Allen AG, Shapiro LP, Swanson AM, Gourley SL. Adolescent-onset GABA alpha1 silencing regulates reward-related decision making. Eur J Neurosci. 2015 doi: 10.1111/ejn.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Hong CJ, Yu YW, Chen TJ, Wu HC, Tsai SJ. Brain-derived neurotrophic factor (Val66Met) genetic polymorphism is associated with substance abuse in males. Brain Res Mol Brain Res. 2005;140(1–2):86–90. doi: 10.1016/j.molbrainres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Choi DC, Gourley SL, Ressler KJ. Prelimbic BDNF and TrkB signaling regulates consolidation of both appetitive and aversive emotional learning. Transl Psychiatry. 2012;2:e205. doi: 10.1038/tp.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Chieng BC, Balleine BW. Effects of repeated cocaine exposure on habit learning and reversal by N-acetylcysteine. Neuropsychopharmacology. 2014;39(8):1893–1901. doi: 10.1038/npp.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corominas-Roso M, Roncero C, Daigre C, Grau-Lopez L, Ros-Cucurull E, Rodríguez-Cintas L, … Casas M. Changes in brain-derived neurotrophic factor (BDNF) during abstinence could be associated with relapse in cocaine-dependent patients. Psychiatry Research. 2015;225(3):309–314. doi: 10.1016/j.psychres.2014.12.019. http://dx.doi.org/10.1016/j.psychres.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Corominas-Roso M, Roncero C, Eiroa-Orosa FJ, Gonzalvo B, Grau-Lopez L, Ribases M, … Casas M. Brain-derived neurotrophic factor serum levels in cocaine-dependent patients during early abstinence. European Neuropsychopharmacology. 2013;23(9):1078–1084. doi: 10.1016/j.euroneuro.2012.08.016. http://dx.doi.org/10.1016/j.euroneuro.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Corominas-Roso M, Roncero C, Eiroa-Orosa FJ, Ribases M, Barral C, Daigre C, … Casas M. Serum brain-derived neurotrophic factor levels and cocaine-induced transient psychotic symptoms. Neuropsychobiology. 2013;68(3):146–155. doi: 10.1159/000353259. [DOI] [PubMed] [Google Scholar]
- DePoy LM, Allen AG, Gourley SL. Adolescent cocaine self-administration induces habit behavior in adulthood: Sex differences and structural consequences. doi: 10.1038/tp.2016.150. In review. [DOI] [PMC free article] [PubMed]
- DePoy LM, Perszyk RE, Zimmermann KS, Koleske AJ, Gourley SL. Adolescent cocaine exposure simplifies orbitofrontal cortical dendritic arbors. Front Pharmacol. 2014;5:228. doi: 10.3389/fphar.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy LM, Gourley SL. Synaptic cytoskeletal plasticity in the prefrontal cortex following psychostimulant exposure. Traffic. 2015 doi: 10.1111/tra.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy LM, Noble B, Allen AG, Gourley SL. Developmentally divergent effects of Rho-kinase inhibition on cocaine- and BDNF-induced behavioral plasticity. Behav Brain Res. 2013;243:171–175. doi: 10.1016/j.bbr.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325(5940):621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nestler EJ. The neural rejuvenation hypothesis of cocaine addiction. Trends in Pharmacological Sciences. 2014;35(8):374–383. doi: 10.1016/j.tips.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories - indications for novel treatments of addiction. Eur J Neurosci. 2014;40(1):2163–2182. doi: 10.1111/ejn.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58(9):751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24(29):6600–6610. doi: 10.1523/jneurosci.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Caffino L, Racagni G, Riva MA. Repeated stress prevents cocaine-induced activation of BDNF signaling in rat prefrontal cortex. Eur Neuropsychopharmacol. 2009;19(6):402–408. doi: 10.1016/j.euroneuro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA. Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. Eur J Neurosci. 2007;26(10):2756–2763. doi: 10.1111/j.1460-9568.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Moro F, Caffino L, Orru A, Cassina C, Giannotti G, Di Celmente A, Racagni G, Riva MA, Cervo L. Region-specific effects on BDNF expression after contingent or non-contingent cocaine i.v. self-administration in rats. Int J Neuropsychopharmacol. 2013;16(4):913–918. doi: 10.1017/s146114571200096x. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience. 2006;139(3):1039–1048. doi: 10.1016/j.neuroscience.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Gabriele A, Setlow B, Packard MG. Cocaine self-administration alters the relative effectiveness of multiple memory systems during extinction. Learn Mem. 2009;16(5):296–299. doi: 10.1101/lm.1253409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010;35(2):157–171. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotti G, Caffino L, Calabrese F, Racagni G, Riva MA, Fumagalli F. Prolonged abstinence from developmental cocaine exposure dysregulates BDNF and its signaling network in the medial prefrontal cortex of adult rats. Int J Neuropsychopharmacol. 2014;17(4):625–634. doi: 10.1017/s1461145713001454. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77(5):867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Howell JL, Rios M, DiLeone RJ, Taylor JR. Prelimbic cortex bdnf knock-down reduces instrumental responding in extinction. Learn Mem. 2009a;16(12):756–760. doi: 10.1101/lm.1547909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Koleske AJ, Taylor JR. Loss of dendrite stabilization by the Abl-related gene (Arg) kinase regulates behavioral flexibility and sensitivity to cocaine. Proc Natl Acad Sci U S A. 2009b;106(39):16859–16864. doi: 10.1073/pnas.0902286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur J Neurosci. 2010;32(10):1726–1734. doi: 10.1111/j.1460-9568.2010.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Jacobs AM, Howell JL, Mo M, Dileone RJ, Koleske AJ, Taylor JR. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc Natl Acad Sci U S A. 2012a;109(50):20714–20719. doi: 10.1073/pnas.1208342109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Olevska A, Warren MS, Taylor JR, Koleske AJ. Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. J Neurosci. 2012b;32(7):2314–2323. doi: 10.1523/jneurosci.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Olevska A, Zimmermann KS, Ressler KJ, Dileone RJ, Taylor JR. The orbitofrontal cortex regulates outcome-based decision-making via the lateral striatum. Eur J Neurosci. 2013a;38(3):2382–2388. doi: 10.1111/ejn.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Koleske AJ. Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. J Neurosci. 2013b;33(7):3107–3112. doi: 10.1523/jneurosci.2138-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat Neurosci. 2011;14(12):1507–1509. doi: 10.1038/nn.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Costa RM. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun. 2013;4:2264. doi: 10.1038/ncomms3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23(3):742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Raj N, Molinaro G, Allen AG, Whyte AJ, Gibson JR, … Bassell GJ. Selective role of the catalytic PI3K subunit p110beta in impaired higher order cognition in fragile X syndrome. Cell Rep. 2015;11(5):681–688. doi: 10.1016/j.celrep.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Miller SW, See RE, McGinty JF. Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacology (Berl) 2008;198(1):77–91. doi: 10.1007/s00213-008-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27(6):555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Zimmermann K, Parker K, Gaval M, Weinshenker D, Ressler KJ. BDNF deletion or TrkB impairment in amygdala inhibits both appetitive and aversive learning. J Neurosci. 2014;34(7):2444–2450. doi: 10.1523/jneurosci.4085-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton EA, Wheeler MG, Gourley SL. Early-life cocaine interferes with BDNF-mediated behavioral plasticity. Learn Mem. 2014;21(5):253–257. doi: 10.1101/lm.033290.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Projections of the medial orbital and ventral orbital cortex in the rat. J Comp Neurol. 2011;519(18):3766–3801. doi: 10.1002/cne.22733. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19(10):4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107(6):2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313(4):574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146(4):373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kabir ZD, Katzman AC, Kosofsky BE. Molecular mechanisms mediating a deficit in recall of fear extinction in adult mice exposed to cocaine in utero. PLoS One. 2013;8(12):e84165. doi: 10.1371/journal.pone.0084165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kolb B, Muhammad A. Harnessing the power of neuroplasticity for intervention. Front Hum Neurosci. 2014;8:377. doi: 10.3389/fnhum.2014.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. A single cocaine exposure increases BDNF and D3 receptor expression: implications for drug-conditioning. Neuroreport. 2005;16(2):175–178. doi: 10.1097/00001756-200502080-00022. [DOI] [PubMed] [Google Scholar]
- LeBlanc KH, Maidment NT, Ostlund SB. Repeated cocaine exposure facilitates the expression of incentive motivation and induces habitual control in rats. PLoS One. 2013;8(4):e61355. doi: 10.1371/journal.pone.0061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, DeJoseph MR, Urban JH, Bahi A, Dreyer JL, Meredith GE, Ford KA, Ferrario CR, Loweth JA, Wolf ME. Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance. J Neurosci. 2013;33(3):1130–1142. doi: 10.1523/jneurosci.3082-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wolf ME. Multiple faces of BDNF in cocaine addiction. Behav Brain Res. 2015;279:240–254. doi: 10.1016/j.bbr.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Lu L, Zhu XG, Gong JP, Shaham Y, Uhl GR. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res. 2006;1067(1):1–12. doi: 10.1016/j.brainres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Barak S, Warnault V, Ron D. Corticostriatal BDNF and alcohol addiction. Brain Res. 2015 doi: 10.1016/j.brainres.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2014;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron. 2010;67(5):821–833. doi: 10.1016/j.neuron.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89(3):312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci. 2012;15(3):358–366. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macenski MJ, Meisch RA. Ratio size and cocaine concentration effects on oral cocaine-reinforced behavior. J Exp Anal Behav. 1998;70(2):185–201. doi: 10.1901/jeab.1998.70-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Curr Opin Neurobiol. 2013;23(4):675–683. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mátyás F, Lee J, Shin HS, Acsady L. The fear circuit of the mouse forebrain: connections between the mediodorsal thalamus, frontal cortices and basolateral amygdala. Eur J Neurosci. 2014;39(11):1810–1823. doi: 10.1111/ejn.12610. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44(1):1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71(1):55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles FJ, Everitt BJ, Dickinson A. Oral cocaine seeking by rats: action or habit? Behav Neurosci. 2003;117(5):927–938. doi: 10.1037/0735-7044.117.5.927. [DOI] [PubMed] [Google Scholar]
- Miller TR, Hendrie D. Substance abuse dollars and cents: A cost-benefit analysis. Rockville, MD: Center for Substance Abuse Prevention, Substance Abuse and Mental Health Services Administration; 2008. DHHS Publication No. (SMA) 07-4298. [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, Aston-Jones G. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 2014 doi: 10.1016/j.brainres.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323(3):341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Saunders BT, Maren S, Robinson TE. Sign-tracking to an appetitive cue predicts incubation of conditioned fear in rats. Behav Brain Res. 2015;276:59–66. doi: 10.1016/j.bbr.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A, Killcross S. Amphetamine exposure enhances habit formation. J Neurosci. 2006;26(14):3805–3812. doi: 10.1523/jneurosci.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJ, Killcross S. Accelerated habit formation following amphetamine exposure is reversed by D1, but enhanced by D2, receptor antagonists. Front Neurosci. 2013;7:76. doi: 10.3389/fnins.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordquist RE, Voorn P, de Mooij-van Malsen JG, Joosten RN, Pennartz CM, Vanderschuren LJ. Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. Eur Neuropsychopharmacol. 2007;17(8):532–540. doi: 10.1016/j.euroneuro.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci. 2007;27(18):4819–4825. doi: 10.1523/jneurosci.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Fitzgerald MK, Mueller D. Infralimbic BDNF/TrkB enhancement of GluN2B currents facilitates extinction of a cocaine-conditioned place preference. J Neurosci. 2014;34(17):6057–6064. doi: 10.1523/jneurosci.4980-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14(1):7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16(5):279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AB, Abel JM, Lynch WJ. Dose-dependent effects of wheel running on cocaine-seeking and prefrontal cortex Bdnf exon IV expression in rats. Psychopharmacology (Berl) 2014;231(7):1305–1314. doi: 10.1007/s00213-013-3321-4. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Vanderschuren LJ. Kicking the habit: the neural basis of ingrained behaviors in cocaine addiction. Neurosci Biobehav Rev. 2010;35(2):212–219. doi: 10.1016/j.neubiorev.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Anderson RM, Cosme CV, Glanz RM, Miller MC, Romig-Martin SA, LaLumiere RT. The Contingency of Cocaine Administration Accounts for Structural and Functional Medial Prefrontal Deficits and Increased Adrenocortical Activation. J Neurosci. 2015;35(34):11897–11910. doi: 10.1523/jneurosci.4961-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapanelli M, Lew SE, Frick LR, Zanutto BS. Plasticity in the rat prefrontal cortex: linking gene expression and an operant learning with a computational theory. PLoS One. 2010;5(1):e8656. doi: 10.1371/journal.pone.0008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q, Zhang JC, Fujita Y, Ma M, Wu J, Hashimoto K. Effects of TrkB agonist 7,8-dihydroxyflavone on sensory gating deficits in mice after administration of methamphetamine. Pharmacol Biochem Behav. 2013;106:124–127. doi: 10.1016/j.pbb.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Ren Q, Zhang JC, Ma M, Fujita Y, Wu J, Hashimoto K. 7,8-Dihydroxyflavone, a TrkB agonist, attenuates behavioral abnormalities and neurotoxicity in mice after administration of methamphetamine. Psychopharmacology (Berl) 2014;231(1):159–166. doi: 10.1007/s00213-013-3221-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Do-Monte FH, Tanimura Y, Quirk GJ, Haber SN. Enhancement of fear extinction with deep brain stimulation: evidence for medial orbitofrontal involvement. Neuropsychopharmacology. 2015;40(7):1726–1733. doi: 10.1038/npp.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, et al. [Accessed 2-May-2011];The mouse brain library. 2000 www.mbl.org.
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56(Suppl 1):73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bass CE, Terwilliger EF, Pierce RC, Cha JH. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30(35):11735–11744. doi: 10.1523/jneurosci.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67(8):730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011;36(8):1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett. 2008;432(1):40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, McGinty JF, West AE, Sadri-Vakili G. Epigenetics and psychostimulant addiction. Cold Spring Harb Perspect Med. 2013;3(3):a012047. doi: 10.1101/cshperspect.a012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Apostolidis S, Amoa R, O’Rear C, Kaster M, Stowers J, Ritz R. Post-training cocaine administration facilitates habit learning and requires the infralimbic cortex and dorsolateral striatum. Neurobiol Learn Mem. 2015;118:105–112. doi: 10.1016/j.nlm.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cereb Cortex. 2005;15(8):1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- Schwabe L. Stress and the engagement of multiple memory systems: integration of animal and human studies. Hippocampus. 2013;23(11):1035–1043. doi: 10.1002/hipo.22175. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Dickinson A, Wolf OT. Stress, habits, and drug addiction: a psychoneuroendocrinological perspective. Exp Clin Psychopharmacol. 2011;19(1):53–63. doi: 10.1037/a0022212. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290(2):213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Simchon-Tenenbaum Y, Weizman A, Rehavi M. The Impact of Chronic Early Administration of Psychostimulants on Brain Expression of BDNF and Other Neuroplasticity-Relevant Proteins. J Mol Neurosci. 2015 doi: 10.1007/s12031-015-0611-9. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Cooch NK, Schoenbaum G. What the orbitofrontal cortex does not do. Nat Neurosci. 2015;18(5):620–627. doi: 10.1038/nn.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Tao J, Zhang J, Xie Y, Sun Y, Li L, Xu K, Han B, Lu Y, Sun H, Wei Y, Wang Y, Zhang Y, Zou S, Wu W, Zhang J, Zhang X, He J. An association between BDNF Val66Met polymorphism and impulsivity in methamphetamine abusers. Neurosci Lett. 2014;582:16–20. doi: 10.1016/j.neulet.2014.08.030. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Mental health findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2014. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2010 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. Rockville, MD: 2012. [PubMed] [Google Scholar]
- Sun H, Cocker PJ, Zeeb FD, Winstanley CA. Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology (Berl) 2012;219(2):285–301. doi: 10.1007/s00213-011-2419-9. [DOI] [PubMed] [Google Scholar]
- Sun WL, Eisenstein SA, Zelek-Molik A, McGinty JF. A single brain-derived neurotrophic factor infusion into the dorsomedial prefrontal cortex attenuates cocaine self-administration-induced phosphorylation of synapsin in the nucleus accumbens during early withdrawal. Int J Neuropsychopharmacol. 2014;18(1) doi: 10.1093/ijnp/pyu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson AM, Allen AG, Shapiro LP, Gourley SL. GABAAalpha1-mediated plasticity in the orbitofrontal cortex regulates context-dependent action selection. Neuropsychopharmacology. 2015;40(4):1027–1036. doi: 10.1038/npp.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Zeng Y, Hu Y, Yuan X, Liu S, Li J, Lu P, Sun Y, Gao L, Fu D, Li Y, Wang S, McClintock SM. 7, 8-Dihydroxyflavone induces synapse expression of AMPA GluA1 and ameliorates cognitive and spine abnormalities in a mouse model of fragile X syndrome. Neuropharmacology. 2015;89:43–53. doi: 10.1016/j.neuropharm.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol Learn Mem. 2011;96(4):609–623. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Quinn JJ, Taylor JR. Impulsivity, compulsivity, and habit: the role of orbitofrontal cortex revisited. Biol Psychiatry. 2008;63(3):253–255. doi: 10.1016/j.biopsych.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng WY, Chuang JY, Lin LC, Cherng CG, Lin KY, Chen LH, Su CC, Yu L. Companions reverse stressor-induced decreases in neurogenesis and cocaine conditioning possibly by restoring BDNF and NGF levels in dentate gyrus. Psychoneuroendocrinology. 2013;38(3):425–437. doi: 10.1016/j.psyneuen.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci. 2013;16(1):42–47. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola TW, Tractenberg SG, Levandowski ML, Pezzi JC, Bauer ME, Teixeira AL, Grassi-Oliveira R. Neurotrophic factors in women with crack cocaine dependence during early abstinence: the role of early life stress. Journal of Psychiatry & Neuroscience : JPN. 2014;39(3):206–214. doi: 10.1503/jpn.130027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Diemen L, Kapczinski F, Sordi AO, de Magalhães Narvaez JC, Guimarães LSP, Kessler FHP, … Pechansky F. Increase in brain-derived neurotrophic factor expression in early crack cocaine withdrawal. The International Journal of Neuropsychopharmacology. 2014;17(1):33–40. doi: 10.1111/j.1369-1600.2012.00444.x. [DOI] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Morisot N, Phamluong K, Wilbrecht L, Massa SM, … Ron D. The BDNF Valine 68 to Methionine Polymorphism Increases Compulsive Alcohol Drinking in Mice that Is Reversed by Tropomyosin Receptor Kinase B Activation. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren MS, Bradley WD, Gourley SL, Lin YC, Simpson MA, Reichardt LF, … Koleske AJ. Integrin beta1 signals through Arg to regulate postnatal dendritic arborization, synapse density, and behavior. J Neurosci. 2012;32(8):2824–2834. doi: 10.1523/jneurosci.3942-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield TW, Jr, Shi X, Sun WL, McGinty JF. The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. J Neurosci. 2011;31(3):834–842. doi: 10.1523/jneurosci.4986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 2013;226(2):217–228. doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91(4):267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- Yan QS, Feng MJ, Yan SE. RNA interference-mediated inhibition of brain-derived neurotrophic factor expression increases cocaine’s cytotoxicity in cultured cells. Neurosci Lett. 2007;414(2):165–169. doi: 10.1016/j.neulet.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci. 2008;28(8):1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30(46):15457–15463. doi: 10.1523/jneurosci.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Lv F, Li L, Yu H, Dong M, Fu Q. 7,8-dihydroxyflavone rescues spatial memory and synaptic plasticity in cognitively impaired aged rats. J Neurochem. 2012;122(4):800–811. doi: 10.1111/j.1471-4159.2012.07830.x. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Wu J, Fujita Y, Yao W, Ren Q, Yang C, Li SX, Shirayama Y, Hashimoto K. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol. 2014a;18(4) doi: 10.1093/ijnp/pyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liu X, Schroeder JP, Chan CB, Song M, Yu SP, Weinshenker D, Ye K. 7,8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2014b;39(3):638–650. doi: 10.1038/npp.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhu X, Huang C, Zhang X. Molecular changes in the medial prefrontal cortex and nucleus accumbens are associated with blocking the behavioral sensitization to cocaine. Scientific Reports. 2015;5 doi: 10.1038/srep16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann KS, Yamin J, Rainnie DG, Ressler KJ, Gourley SL. Connections of the mouse orbitofrontal cortex and regulation of goal-directed action selection by BDNF-TrkB. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.10.026. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg B, Hintiryan H, Gou L, Song MY, Bay M, Bienkowski MS, … Dong HW. Neural networks of the mouse neocortex. Cell. 2014;156(5):1096–1111. doi: 10.1016/j.cell.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]