Abstract
Introduction
The role of systemic chemotherapy in patients with resectable colorectal liver metastases (CRLM) is ambiguous. The aim of this review was to compare the outcomes of regimens using systemic neoadjuvant, adjuvant or perioperative (combination of pre and postoperative) chemotherapy, for the treatment of resectable CRLM.
Methods
MEDLINE was searched for articles investigating the use of chemotherapy for adults with resectable CRLM. Randomized controlled trials reporting overall survival (OS), disease-free survival (DFS) and grade 3–4 adverse events (AEs) were screened for inclusion. PROSPERO record: CRD42015020609.
Results
Four trials met the inclusion criteria (1098 patients). No significant improvement in median OS was achieved with chemotherapy/surgery compared with surgery-alone. Two trials demonstrated a significant improvement in DFS with chemotherapy/surgery compared to surgery-alone (Hazard ratio 0.78 (0.61–0.99) p = 0.04 and HR 0.66 (0.46–0.96) p = 0.03). Fluorouracil/folinic acid alone had a lower incidence of AEs than combination therapies, and the addition of cetuximab shortened DFS in one trial (HR 1.48 (1.04–2.12) p = 0.03).
Conclusion
There is a lack of adequately powered trials of chemotherapy in combination with liver resection for CRLM, partly due to difficulties in recruitment. In an unselected patient group, FOLFOX in combination with liver resection appears to improve DFS compared to surgery-alone, but trials are underpowered for OS. Future trials will require prospective stratification of patients based on biomarkers predictive of response.
Introduction
Approximately half of all colorectal cancer patients will develop liver metastases (CRLM), with 1 in 5 patients having synchronous CRLM at the time of presentation.1 For all patients with CRLM, 20–30% will have operable disease. When possible, liver resection remains the treatment of choice and confers the best prognosis for long-term survival.2 In a retrospective study, participants with untreated resectable CRLM failed to achieve 5-year survival and had a median survival time of 14.2 months, whereas a 5-year survival rate of 31% was seen in patients who underwent resection, with some patients surviving more than 10 years post-resection.3
Despite this, the rate of recurrence following surgery remains high. In one multi-centre study, up to 57% of patients developed recurrence with a median disease-free survival time of 16.3 months.4 Randomized trials have been conducted to assess the benefit of systemic chemotherapy in combination with liver resection, with the aim of improving long-term survival and reducing disease recurrence.5 Chemotherapy may be given before (neoadjuvant), after (adjuvant), or before and after (perioperative) liver resection.
The use of systemic chemotherapy in combination with liver resection for CRLM has become an accepted standard of care: the National Comprehensive Cancer Network (NCCN) in the US recommend 6 months of perioperative systemic chemotherapy and the National Institute for Health and Care Excellence (NICE) in England suggest considering the use of systemic chemotherapy prior to liver resection.6, 7, 8 Despite this, evidence of benefit is controversial, particularly for longer-term survival when compared to liver resection-alone.9, 10
Fluorouracil-based chemotherapy is the mainstay of treatment and is usually administered in combination with other agents such as FOLFOX (folinic acid (FA), 5-FU and oxaliplatin) or FOLFIRI (FA, 5-FU and irinotecan). More recently there has been exploration of the role of monoclonal antibodies such as bevacizumab, cetuximab and panitumumab added to fluorouracil-based regimens.11, 12, 13
Adjuvant chemotherapy following liver resection aims to reduce recurrence of CRLM, while neoadjuvant chemotherapy has an additional advantage of allowing tumour chemoresponsiveness to be evaluated.14 Chemoresponsiveness may aid in differentiating patients who will benefit from liver resection/adjuvant chemotherapy from those with aggressive tumour biology in whom further treatment may not be helpful.15
For patients receiving neoadjuvant chemotherapy, chemotherapy-associated liver injury remains a concern and is associated with a poorer short-term prognosis.16 Although 20–25% of patients receiving neoadjuvant chemotherapy experience liver-related complications post-surgery, this has not been shown to be significantly different to those receiving surgery-alone.17, 18 Other factors, such as obesity, may have a greater influence on liver-related complications.19, 20
Currently, the role of systemic chemotherapy in resectable CRLM remains ambiguous due to a lack of clear evidence of benefit.9 Discrepancies in reported benefits on survival outcomes with systemic chemotherapy in combination with liver resection may be due to the variability of regimens or chemotherapeutic agents used in the respective trials. The aim of the current review was to analyse the outcomes of all randomized controlled trials on systemic adjuvant, neoadjuvant and perioperative chemotherapy regimens in combination with surgery for the treatment of resectable CRLM.
Methods
A systematic review of literature was conducted as described in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.21 The review protocol was registered with the University of York Centre for Reviews and Dissemination International prospective register of systematic reviews (PROSPERO Record: CRD42015020609, http://www.crd.york.ac.uk/PROSPERO/).
The review criteria included randomized controlled trials reporting on the outcomes of overall survival (OS), disease-free survival (DFS) and grade 3–4 complications in patients with resectable CRLM. OS was defined as the interval between the date of randomization or liver resection, and date of death. DFS was defined as the time from randomization or liver resection, to recurrence, disease progression, or death. In this review, DFS was used to represent both DFS and progression-free survival (PFS).
Trials were limited to adult human subjects and availability in the English language. Trials were not restricted by publication status or date of publication. Publications which did not contain OS or DFS outcomes, or pertained to transarterial chemotherapy or initially unresectable liver metastases, were excluded. Resectable CRLM was defined as the realistic prospect of surgical removal of all metastatic disease in the liver with clear margins while maintaining adequate liver reserve.
Potentially relevant trials were identified by searching the Ovid MEDLINE database (1946 to April week 2 2015) using exploded Medical Subject Headings (MeSH) terms and specific text-words terms. Search terms were grouped as follows: group 1 – ‘liver neoplasm’ AND “Colorectal liver metastases” ti,ab., group 2 – ‘Neoadjuvant Therapy’ AND (neoadjuvant adj2 (chemotherapy* or therap*)). ti,ab. AND (pre adj1 (operative or surg*)). ti,ab., group 3 – ‘Chemotherapy, Adjuvant’ AND (adjuvant adj2 (chemotherapy* or therap*)). ti,ab. Search results were extracted from the combination of groups 1 AND 2, OR groups 2 AND 3. The search was conducted by EK according to the agreed protocol and relevant studies were identified by reviewing the titles and when necessary, abstracts.
Two authors (SON and EK) independently extracted data from all identified reports. Discrepancies in obtained data were resolved by consensus among the authors (SON, EK), and by consultation with the senior author (EMH). Investigators of included trials and trials lacking specific data which are otherwise eligible for inclusion were contacted as appropriate.
Extracted data items include: trial year, trial period, treatment protocols, trial population size, number of female and male participants, median ages of participants, median months of follow-up, number of resected patients, patients with 1–3 liver metastases and synchronous metastases, plasma CEA of >30 ng/mL at diagnosis, WHO or ECOG performance status, median months of OS and DFS, as well as the incidence and number of grade 3–4 complications from chemotherapy. Collected data were compiled into tables accordingly. The TNM staging system was used for staging assessment of the primary tumour in all papers except Ychou 2009, in which it was not specified.5, 22, 23, 24
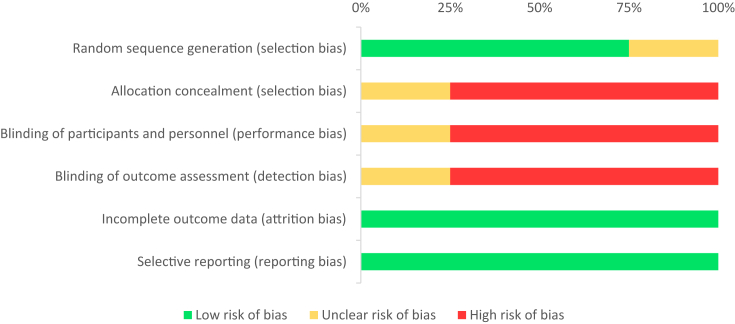
Participants were analysed on an intention-to-treat basis to allow unbiased comparisons, as excluding patients in whom decisions were made resulting in the discontinuation of chemotherapy or non-performance of liver resection may inadvertently skew results. Assumptions were made in trials whereby a variety of chemotherapy agents were used, as a known chemotherapy regimen was designated according to the majority of users to allow for comparisons (see Discussion). The risk of bias in individual trials was independently assessed using the Cochrane Collaboration's tool25 (see Appendix A1 and A2, Appendix A1).
Results
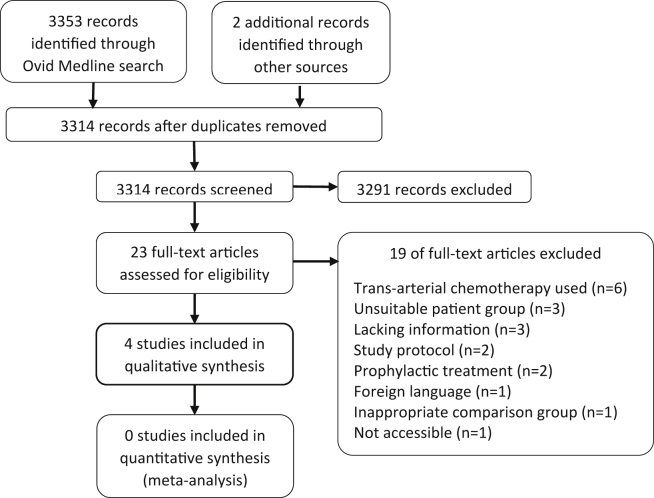
The search returned 3353 articles on the 18th April 2015 (Fig. 1). Four randomized controlled trials were included with a total of 1098 participants (Table 1, Table 2).5, 22, 23, 24
Figure 1.
PRISMA flow diagram
Table 1.
Brief summary of included studies
| Study | Study period | Regimen | Treatment | N | Follow-up regimen after treatment | Recurrence | Trial name |
|---|---|---|---|---|---|---|---|
| Primrose 2014 | Feb 2007–Nov 2012 | Perioperativea | FOLFOX/Surgery/FOLFOXe vs. FOLFOXe+CET/Surgery/FOLFOXe+CET | 257 | CT or MRI every 3 months for 2 years, then every 6 months for 3 years, until progression or death | Disease progression established by MDT | New EPOC |
| Nordlinger 2013 | Oct 2000–July 2004 | Perioperativeb | Surgery vs. FOLFOX/Surgery/FOLFOX | 364 | CXR, abdominal USS or CT, and CEA every 3 months for 2 years, then every 6 months thereafter | Imaging, cytology or histology | EPOC/ EORTC 40983 |
| Ychou 2009 | Dec 2001–July 2006 | Adjuvantc | Surgery/FUFA vs. Surgery/FOLFORI | 306 | Physical, haematological and biochemical evaluation, CEA and CT every 3 months for 2 years, then every 6 months for 2 years | NR | – |
| Portier 2006 | Dec 1991–Dec 2001 | Adjuvantd | Surgery vs. Surgery/FUFA | 171 | Clinical examination, abdominal USS, CXR, CEA, ±CT every 3 months for 2 years, then yearly until death or end of study | NR | FFCD ACHBTH AURC 9002 |
Abbreviations: CET, cetuximab; 5-FU, 5-fluorouracil; FUFA, folinic acid and 5-FU; FOLFORI, folinic acid, 5-FU and irinotecan; FOLFOX, folinic acid, 5-FU and oxaliplatin; CT, computed tomography scan; MRI, magnetic resonance imaging; USS, ultrasound scan; CEA, carcinoembryonic antigen; MDT, multidisciplinary team; NR, not reported; EORTC, European Organisation for Research and Treatment of Cancer; EPOC, the Cochrane Effective Practice and Organization of Care Group: FFCD, Fédération Francophone de Carcinologie Digestive.
4–6 Cycles over 12 weeks pre-operatively with a minimum break of 4 weeks prior to surgery and 4–6 cycles over 12 weeks post-operatively.
Six 14-day cycles pre-operatively with liver resection performed 2–5 weeks after the last administration of preoperative chemotherapy, and six 14-day cycles post-operatively.
Twelve cycles over 6 months.
Six cycles over 6 months.
Three alternative regimens were allowed. Regime 1, oxaliplatin and fluorouracil (n = 156); regimen 2, oxaliplatin and oral capecitabine (n = 51); regimen 3, patients who had received adjuvant oxaliplatin could receive irinotecan with fluorouracil instead of oxaliplatin (n = 26).
Table 2.
Study details and characteristics of randomised patients at baseline
| Study | Treatment | Total participants | Males | Median age (range) | Median follow-up in months | No. of resected patients (%) | No. of patients with 1–3 liver metastases (%) | Synchronous metastases (%) | Plasma CEA >30 ng/mL at diagnosis (%) | WHO performance status 0 or 1 (%) | Previous adjuvant chemotherapy for primary cancer (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Primrose 2014 | FOLFOX/Surgery/FOLFOX | 128 | 80 | 64 (59–70) | 21 | 93 (93) | 102 (80) | 60 (47) | 31 (24) | 128 (100) | NRf |
| FOLFOX + CET/Surgery/FOLFOX + CET | 129 | 92 | 63 (59–69) | 85 (87) | 97 (75) | 68 (53) | 33 (26) | 126 (98) | NRf | ||
| Nordlinger 2013 | Surgery | 182a | 114 | 64 (25–78) | 102 | 152 (84) | 166 (92) | 67 (37) | 54 (30) | 181 (99) | 76 (42)c |
| FOLFOX/Surgery/FOLFOX | 182b | 127 | 62 (29–79) | 151 (83) | 170 (93) | 61 (34) | 61 (34) | 180 (99) | 78 (43)c | ||
| Ychou 2009 | Surgery/FUFA | 153 | 100 | 64 (34–76) | 42 | NR | 149 (97)d | NR | NR | 148 (97) | 54 (35)e |
| Surgery/FOLFIRI | 153 | 90 | 63 (27–75) | NR | 148 (97)d | NR | NR | 149 (97) | 61 (40)e | ||
| Portier 2006 | Surgery | 85 | 53 | NR | 87 | NR | 81 (95) | NR | NR | NR | NRg |
| Surgery/FUFA | 86 | 46 | NR | NR | 82 (95) | NR | NR | NR | NRg |
Nordlinger 2013 – 3 no data.
Nordlinger 2013 – 1 no data.
Nordlinger 2013 – excluding oxaliplatin chemotherapy.
Ychou 2009 – no. of patients with 1–4 liver metastases.
Ychou 2009 – excluding irinotecan-based chemotherapy and completed 3 months or more before first trial treatment.
Primrose 2014 – previous adjuvant chemotherapy permitted if completed 6 months or more before trial entry, previous rectal chemoradiotherapy permitted if completed 1 month or more before trial entry. No previous systemic chemotherapy for metastatic disease allowed.
Portier 2006 – patients receiving chemotherapy in the year preceding liver surgery excluded.
Unpublished or updated data were obtained by contacting authors directly, with new data received for three of the trials. These included updates on median OS in the Primrose trial and Ychou trial, as well as further detail on chemotherapy related AEs in the Nordlinger trial.22, 23, 24 Authors of trials excluded for lacking information on OS and DFS were contacted to obtain this data.26, 27, 28 No response was received from two26, 27 and another responded but was unable to provide the required data.28
Based on the heterogeneity of the included trials, a decision was made not to report a formal synthesis of outcome data. A planned Bayesian network analysis was not undertaken due to an insufficient number of relationships within the network.
Survival outcomes
Median OS and DFS were evaluated to allow standardization of results, as determination of disease-free survival endpoints can be biased by subjectivity and are dependent on the length of follow-up. OS and DFS were calculated from the time of randomization to the aforementioned endpoints in all studies22, 23, 24 except Portier,5 in which the time interval from liver resection to an established endpoint was used. While DFS was used to represent both DFS and PFS in this review, DFS was the original endpoint in the Portier and Ychou trials as eligible participants had histologically proven (R0) resections, and PFS was used in the Nordlinger and Primrose trials, where participants were deemed to have resectable disease on pre-operative assessment but may later be found to be suboptimal (still treated with curative intent). Overall survival figures as of October 2014 are shown in Table 3.
Table 3.
Overall survival and disease-free survival
| Trial | Treatment | N | Overall survival |
Disease-free survival |
||
|---|---|---|---|---|---|---|
| Median OS (95% CI) (months) | Hazard ratio (95% CI) | Median DFS (95% CI) (months) | Hazard ratio (95% CI) | |||
| Primrose 2014 | FOLFOX/Surgery/FOLFOX | 128 | NM | – | 20.5 (16.8–26.7) | – |
| FOLFOX + CET/Surgery/FOLFOX + CET | 129 | 39.1 (23.6-NM) | 1.49 (0.86–2.60) p = 0.16 | 14.1 (11.8–15.9) | 1.48 (1.04–2.12) p = 0.03 | |
| Nordlinger 2013 (Randomised) | Surgery | 182 | 54.3 (41.9–79.4) | – | 12.5 (9.7–17.7) | – |
| FOLFOX/Surgery/FOLFOX | 182 | 61.3 (51.0–83.4) | 0.88 (0.68–1.14) p = 0.34 | 20.0 (15.9–27.6) | 0.81 (0.64–1.02) p = 0.07 | |
| Nordlinger 2013 (Eligible) | Surgery | 171 | 55.0 (41.9–79.4) | – | 12.5 (9.7–18.2) | – |
| FOLFOX/Surgery/FOLFOX | 171 | 63.7 (52.7–87.3) | 0.87 (0.66–1.14) p = 0.30 | 20.9 (17.1–28.9) | 0.78 (0.61–0.99) p = 0.04 | |
| Ychou 2009 | Surgery/FUFA | 153 | NR | – | 21.6 (14.6–30.4) | – |
| Surgery/FOLFIRI | 153 | NR | 1.09 (0.72–1.64) p = 0.69* | 24.7 (18.7–38.9) | 0.89 (0.66–1.19) p = 0.44* | |
| Portier 2006 | Surgery | 85 | 46.4 (37.4–55.4) | – | 17.6 (12.3–22.9) | – |
| Surgery/FUFA | 86 | 62.1 (41.1–83.1) | 0.73 (0.48–1.10) p = 0.13* | 24.4 (17.3–31.5) | 0.66 (0.46–0.96) p = 0.03* | |
Abbreviations: CI, confidence interval; DFS, disease-free survival; NA, not applicable; NM, not met; NR, not reported; OS, overall survival. * From multivariable analysis correcting for other prognostic factors.
Adverse events
Adverse events (AEs) were graded according to the National Cancer Institute Common Toxicity Criteria, except in the trial by Portier in which the WHO Toxicity Criteria29 was used. The numbers of patients with grade 3–4 AEs and the total number of grade 3–4 AEs were extracted for each trial. After contacting the authors of the Nordlinger trial, grade 3–4 AEs for the report were obtained from the preceding publication of the same trial in 2008.30
Complications from pre-operative chemotherapy were available in two trials (439 patients), while data for post-op complications could be obtained from all four trials (831 patients) (Table 4). Neutropenia was the most frequent grade 3–4 AE observed. FUFA was associated with a lower incidence of complications (25% of patients in Portier5 and 30% in Ychou22) when compared to regimens involving an additional chemotherapeutic agents (oxaliplatin, irinotecan and cetuximab), which ranged from 40% to 59%.22, 23, 24 These observed differences do not account for variation in regimen intensity and only in the Ychou trial was an additional agent (irinotecan) used with the same FUFA backbone.22
Table 4.
Incidence and total of adverse events and post-operative complications
| Trial | Treatment | N | Patients with grade 3–4 AEs pre-op | Total grade 3–4 AEs pre-op | Patients with grade 3–4 AEs post-op | Total grade 3–4 AEs post-op | Post-operative complications |
|---|---|---|---|---|---|---|---|
| Primrose 2014 | FOLFOX/Surgery/FOLFOX | 128 | 54/134 (40%) | 64 | 22/104 (21%) | 25 | 23/100 (23%) |
| FOLFOX + CET/Surgery/FOLFOX + CET | 129 | 64/137 (47%) | 83 | 29/105 (28%) | 37 | 16/98 (16%) | |
| Nordlinger 2013 | Surgery | 182 | NA | NA | NA | NA | 27/170 (16%)* |
| FOLFOX/Surgery/FOLFOX | 182 | 71/141 (42%) | 116 | 68/115 (59%) | 132 | 40/159 (25%)* | |
| Ychou 2009 | Surgery/FUFA | 153 | NA | NA | 45/152 (30%)* | 43 | NA |
| Surgery/FOLFIRI | 153 | NA | NA | 73/154 (47%)* | 92 | NA | |
| Portier 2006 | Surgery | 85 | NA | NA | NA | NA | NA |
| Surgery/FUFA | 86 | NA | NA | 20 (25%) | 27 | NA |
Abbreviations: AEs, adverse events; pre-op, pre-operative; post-op, post-operative; NA, not applicable; NR, not reported.
*p < 0.05.
The completion rate of the planned chemotherapy regimen varied between trials (65–82%) but did not appear to relate to the regimen used. In the Primrose trial, similar rates of completion are seen in both the chemotherapy and chemotherapy/cetuximab groups at 73% and 76%, respectively.24 However, about 30–40% patients were yet to complete post-op chemotherapy at time of analysis of the trial, so the completion rates of post-op chemotherapy cannot be reliably evaluated. In the Nordlinger trial, 79% completed the planned 6 cycles of pre-op chemotherapy, but the number of participants starting post-op chemotherapy was only 63% of the original cohort, with only 70% (80 of 115 patients) of this group completing post-op chemotherapy.30 The completion rates in the trial by Ychou et al. are 82% and 75% for the FUFA and FOLFORI regimes, respectively.22 In the Portier trial, a relatively low completion rate of 67% (54 of 81 participants) was observed.5
Discussion
This systematic review collates currently available data and includes unpublished data obtained from authors for RCTs involving chemotherapy in patients with resectable CRLM. In trials that compared surgery-alone to surgery with 5-FU based chemotherapy (adjuvant and perioperative), there was a significant increase in DFS after correction of prognostic factors, but not OS.5, 23 In trials comparing variations of combination 5-FU based chemotherapy in adjuvant or perioperative setting, no significant improvements in survival were observed.22, 24
Despite the lack of convincing data in support of chemotherapy for patients undergoing liver resection for resectable CRCLM generated from these RCTs and a previous meta-analysis, its use and endorsement remains widespread.7, 10 All of the relevant trials had difficulties in recruitment, perhaps due to a lack of equipoise amongst both patients and clinicians.
As compared to the previous meta-analysis, this current systematic review includes longer-term outcomes, and newer trials published up to April 2015 and excluded meeting abstracts not reported as full papers given the clear risk of bias.10, 31 It was elected not to perform a meta-analysis due to the significant heterogeneity seen in the trials identified. Factors influencing this included between-trial differences in chemotherapeutic agents, dosing regimens, and inclusion criteria, for example, the Primrose trial targeted KRAS exon 2 wild-type patients after a protocol amendment, given the mode of action of cetuximab.24
A further useful study combined data from Langer31 and Portier5 to perform a patient-level pooled analysis.35 Although a rational approach given the similarities between these two trials, the Langer study has not to the current authors' knowledge, been published in full.31 A significant difference in progression-free survival (PFS) between surgery-alone vs. chemotherapy/surgery was not seen in the univariable analysis (log-rank test, p = 0.058). In a multivariable analysis accounting for other prognostic factors, a benefit of chemotherapy was seen (PFS, HR 1.39, 1.04–1.85, p = 0.026; overall survival, HR 1.39, 1.00–1.93, p = 0.046). Again, in a sufficiently powered trial achieving robust randomisation, a multivariable approach should not be required in the analysis. This reflects the difficulties in recruiting to these trials with the authors stating that a definitive demonstration of the benefit of chemotherapy remains to be shown.
Therein lies the difficulty. Nordlinger has demonstrated a benefit of FOLFOX before and after surgery compared with surgery-alone, albeit for disease-free/progression-free survival alone and not for overall survival. Taken together with the benefit of adjuvant chemotherapy shown in resected stage III colon cancer, is it then ethical to randomise to surgery-alone for stage IV disease? Perhaps it would be if, in the neoadjuvant setting, sufficient information existed that chemotherapy-associated liver damage made surgery more dangerous and thus less successful. Nordlinger reported specifically on reversible postoperative complications and these were significantly more frequent in the chemotherapy/surgery group (40/159, 25%) compared with surgery-alone (27/170, 16%). But as has been shown, this did not translate into a worse progression-free survival in the chemotherapy/surgery group. This fits with the argument that a complication may be “worth it” if it is reversible and the treatment prolongs life. Regimen intensity and timing of chemotherapy may be important in this regard.32 Surgeons are familiar with the sinusoidal obstructive syndrome and steatohepatitis commonly seen following neoadjuvant chemotherapy. Restricting the number of cycles of neoadjuvant chemotherapy may reduce the incidence of postoperative complications, but the optimal regimen remains to be determined.33
In this review, variations in patient characteristics between studies (Table 2) may reflect differences in definitions of resectability. In the Portier and Ychou trials, eligible patients have already undergone histologically proven R0 resection of liver metastases prior to randomization, while the selection of eligible patients may be influenced by the experience of the multidisciplinary team in the Nordlinger and Primrose trials.5, 22, 23, 24, 34 Although existing guidelines allow standardization of diagnostic and therapeutic work-up, the availability of new treatments and improvements in imaging contributes to the evolving definition of resectability.35, 36, 37
The most recently published study reported a shorter progression-free survival with the addition of cetuximab to chemotherapy/surgery in KRAS exon 2 wild-type patients.24 This was unexpected and the interpretation of the results has attracted robust debate.38, 39, 40, 41, 42 Discussion points have included quality assurance of the surgery, data completeness, and the apparent higher response rate in the cetuximab arm and yet the poorer PFS with a higher number of earlier recurrences. Longer-term outcome data may contribute further to this debate. Additionally, it would be useful to determine cetuximab use worldwide in patients with resectable disease and whether patterns of treatment have changed since the publication of this trial. Further basic science research will help here; there are already suggestions of patient subgroups predicted to have a better response to cetuximab43 with an increase of information in this area expected.
Some systemic chemotherapy regimens in combination with liver resection appear to improve DFS compared to surgery-alone. No improvement in overall survival has been demonstrated to date, and the addition of cetuximab to standard regimens for unselected KRAS wild-type patients may be detrimental. Future trials will require prospective stratification of patients based on biomarkers predictive of response. The medical community must commit to providing information in an unbiased manner with equipoise, so patients can make informed choices. Trial design for these precision medicine studies will be challenging and is currently a research area of high priority. Current patient selection for neoadjuvant chemotherapy should be thoughtful, with an assumption that proceeding directly to surgery will be the best option for a subgroup.
Funding sources
None.
Conflicts of interest
None declared.
Author contribution
Each author has made a substantial contribution to the conception, design, drafting and critical revision of this article for important intellectual content; and has given final approval of the version to be published. Specific roles are summarised below:
EK – search, data extraction, analysis, writing of manuscript.
SON – design, data extraction, analysis, drafting of manuscript.
EB – analysis, drafting of manuscript.
SJW – analysis, drafting of manuscript.
EMH – conception, design, analysis, drafting of manuscript.
Abbreviations
- 5-FU
5-fluorouracil
- AEs
adverse events
- CALI
chemotherapy-associated liver injury
- CAPOX
capecitabine and oxaliplatin CET: Cetuximab
- CRLM
colorectal-liver metastases
- DFS
disease-free survival
- EORTC
European Organisation for Research and Treatment of Cancer
- EPOC
The Cochrane Effective Practice and Organization of Care Group
- FA
folinic acid
- FFCD
Fédération Francophone de Carcinologie Digestive
- FUFA
FA and 5-FU
- FOLFIRI
FA, 5-FU and irinotecan
- FOLFOX
FA, 5-FU and oxaliplatin
- NCCN
National Comprehensive Cancer Network
- NICE
National Institute for Health and Care Excellence
- OS
overall survival
- RCT
randomised controlled trial
Appendix A1. Risk of bias summary (as per Cochrane Collaboration's tool for assessing risk of bias)
| Random sequence generation (selection bias) | Allocation concealment (Selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (Detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | |
|---|---|---|---|---|---|---|
| Primrose 2014 |  |
 |
 |
 |
 |
 |
| Nordlinger 2013 |  |
 |
 |
 |
 |
 |
| Ychou 2009 |  |
 |
 |
 |
 |
 |
| Portier 2006 |  |
 |
 |
 |
 |
 |
Appendix A2. Risk of bias graph
References
- 1.Leporrier J., Maurel J., Chiche L., Bara S., Segol P., Launoy G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93:465–474. doi: 10.1002/bjs.5278. [DOI] [PubMed] [Google Scholar]
- 2.Simmonds P.C., Primrose J.N., Colquitt J.L., Garden O.J., Poston G.J., Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheele J., Stangl R., Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 4.de Jong M.C., Pulitano C., Ribero D., Strub J., Mentha G., Schulick R.D. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 5.Portier G., Elias D., Bouche O., Rougier P., Bosset J.F., Saric J. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976–4982. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 6.NICE. Colorectal cancer: The diagnosis and management of colorectal cancer. Available from: https://www.nice.org.uk/guidance/cg131/resources/guidance-colorectal-cancer-pdf.
- 7.NCCN. Colon cancer guidelines v. 3 2012; (28/9/2014). Available from: http://www.nccn.org/professionals/physician_gls/pdf/colorectal_screening.pdf.
- 8.Alberts S.R. Evolving role of chemotherapy in resected liver metastases. J Clin Oncol – Off J Am Soc Clin Oncol. 2006;24:4952–4953. doi: 10.1200/JCO.2006.07.9236. [DOI] [PubMed] [Google Scholar]
- 9.Nigri G., Petrucciani N., Ferla F., La Torre M., Aurello P., Ramacciato G. Neoadjuvant chemotherapy for resectable colorectal liver metastases: what is the evidence? Results of a systematic review of comparative studies. Surg – J R Coll Surg Edinb Irel. 2015;13:83–90. doi: 10.1016/j.surge.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Ciliberto D., Prati U., Roveda L., Barbieri V., Staropoli N., Abbruzzese A. Role of systemic chemotherapy in the management of resected or resectable colorectal liver metastases: a systematic review and meta-analysis of randomized controlled trials. Oncol Rep. 2012;27:1849–1856. doi: 10.3892/or.2012.1740. [DOI] [PubMed] [Google Scholar]
- 11.Hurwitz H.I., Tebbutt N.C., Kabbinavar F., Giantonio B.J., Guan Z.Z., Mitchell L. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncology. 2013;18:1004–1012. doi: 10.1634/theoncologist.2013-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang N.P., Li H., Qiu Y.L., Zhou G.M., Wang Y., Ma J. Risk/benefit profile of panitumumab-based therapy in patients with metastatic colorectal cancer: evidence from five randomized controlled trials. Tumour Biol – J Int Soc Oncodevelopmental Biol Med. 2014;35:10409–10418. doi: 10.1007/s13277-014-2354-6. [DOI] [PubMed] [Google Scholar]
- 13.Folprecht G., Hamann S., Schutte K., Trarbach T., Stoehlmacher-Williams J., Ehninger G. Dose escalating study of cetuximab and 5-FU/folinic acid (FA)/oxaliplatin/irinotecan (FOLFOXIRI) in first line therapy of patients with metastatic colorectal cancer. BMC Cancer. 2014;14:521. doi: 10.1186/1471-2407-14-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubbia-Brandt L., Giostra E., Brezault C., Roth A.D., Andres A., Audard V. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 15.Vigano L., Capussotti L., De Rosa G., De Saussure W.O., Mentha G., Rubbia-Brandt L. Liver resection for colorectal metastases after chemotherapy: impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann Surg. 2013;258:731–740. doi: 10.1097/SLA.0b013e3182a6183e. discussion 41–2. [DOI] [PubMed] [Google Scholar]
- 16.Khan A.Z., Morris-Stiff G., Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepato Biliary Pancreatic Surg. 2009;16:137–144. doi: 10.1007/s00534-008-0016-z. [DOI] [PubMed] [Google Scholar]
- 17.Wolf P.S., Park J.O., Bao F., Allen P.J., DeMatteo R.P., Fong Y. Preoperative chemotherapy and the risk of hepatotoxicity and morbidity after liver resection for metastatic colorectal cancer: a single institution experience. J Am Coll Surg. 2013;216:41–49. doi: 10.1016/j.jamcollsurg.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Makowiec F., Mohrle S., Neeff H., Drognitz O., Illerhaus G., Opitz O.G. Chemotherapy, liver injury, and postoperative complications in colorectal liver metastases. J Gastrointest Surg – Off J Soc Surg Alimentary Tract. 2011;15:153–164. doi: 10.1007/s11605-010-1368-7. [DOI] [PubMed] [Google Scholar]
- 19.Ryan P., Nanji S., Pollett A., Moore M., Moulton C.A., Gallinger S. Chemotherapy-induced liver injury in metastatic colorectal cancer: semiquantitative histologic analysis of 334 resected liver specimens shows that vascular injury but not steatohepatitis is associated with preoperative chemotherapy. Am J Surg Pathol. 2010;34:784–791. doi: 10.1097/PAS.0b013e3181dc242c. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez F.G., Ritter J., Goodwin J.W., Linehan D.C., Hawkins W.G., Strasberg S.M. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845–853. doi: 10.1016/j.jamcollsurg.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. w64. [DOI] [PubMed] [Google Scholar]
- 22.Ychou M., Hohenberger W., Thezenas S., Navarro M., Maurel J., Bokemeyer C. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol – Off J Eur Soc Med Oncol ESMO. 2009;20:1964–1970. doi: 10.1093/annonc/mdp236. [DOI] [PubMed] [Google Scholar]
- 23.Nordlinger B., Sorbye H., Glimelius B., Poston G.J., Schlag P.M., Rougier P. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 24.Primrose J., Falk S., Finch-Jones M., Valle J., O'Reilly D., Siriwardena A. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601–611. doi: 10.1016/S1470-2045(14)70105-6. [DOI] [PubMed] [Google Scholar]
- 25.Higgins J., Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011] Cochrane Collab. 2011 www.cochrane-handbook.org [Internet]. Available from: [Google Scholar]
- 26.Saiura A., Yamamoto J., Hasegawa K., Oba M., Takayama T., Miyagawa S. A combination of oral uracil-tegafur plus leucovorin (UFT + LV) is a safe regimen for adjuvant chemotherapy after hepatectomy in patients with colorectal cancer: safety report of the UFT/LV study. Drug Discov Ther. 2014;8:48–56. doi: 10.5582/ddt.8.48. [DOI] [PubMed] [Google Scholar]
- 27.Kemeny N.E., Jarnagin W.R., Capanu M., Fong Y., Gewirtz A.N., Dematteo R.P. Randomized phase II trial of adjuvant hepatic arterial infusion and systemic chemotherapy with or without bevacizumab in patients with resected hepatic metastases from colorectal cancer. J Clin Oncol. 2011;29:884–889. doi: 10.1200/JCO.2010.32.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemeny M.M., Adak S., Gray B., Macdonald J.S., Smith T., Lipsitz S. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy – an intergroup study. J Clin Oncol. 2002;20:1499–1505. doi: 10.1200/JCO.2002.20.6.1499. [DOI] [PubMed] [Google Scholar]
- 29.WHO handbook for reporting results of cancer treatment. WHO Offset Publ No 48 Neoplasma. 1980;20:37–46. [Google Scholar]
- 30.Nordlinger B., Sorbye H., Glimelius B., Poston G.J., Schlag P.M., Rougier P. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langer B., Bleiberg H., Labianca R., Shepherd L. Fluorouracil (FU) plus l-leucovorin (l-LV) versus observation after potentially curative resection of liver or lung metastases from colorectal cancer (CRC): results of the ENG (EORTC/NCIC CTG/GIVIO) randomized trial. Proc Am Soc Clin Oncol. 2002;21:592. [Google Scholar]
- 32.Rees M., Elias D., Coimbra F.J., Orloff S.L. Selection for hepatic resection: expert consensus conference. HPB – Off J Int Hepato Pancreato Biliary Assoc. 2013;15:104–105. doi: 10.1111/j.1477-2574.2012.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zorzi D., Laurent A., Pawlik T.M., Lauwers G.Y., Vauthey J.N., Abdalla E.K. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 34.Mohammad W.M., Martel G., Mimeault R., Fairfull-Smith R.J., Auer R.C., Balaa F.K. Evaluating agreement regarding the resectability of colorectal liver metastases: a national case-based survey of hepatic surgeons. HPB. 2012;14:291–297. doi: 10.1111/j.1477-2574.2012.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bipat S., van Leeuwen M.S., Ijzermans J.N., Bossuyt P.M., Greve J.W., Stoker J. Imaging and treatment of patients with colorectal liver metastases in the Netherlands: a survey. Neth J Med. 2006;64:147–151. [PubMed] [Google Scholar]
- 36.Bipat S., van Leeuwen M.S., Ijzermans J.N., Comans E.F., Planting A.S., Bossuyt P.M. Evidence-base guideline on management of colorectal liver metastases in the Netherlands. Neth J Med. 2007;65:5–14. [PubMed] [Google Scholar]
- 37.Garden O.J., Rees M., Poston G.J., Mirza D., Saunders M., Ledermann J. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55(Suppl. 3):iii1–iii8. doi: 10.1136/gut.2006.098053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nordlinger B.M., Poston G.J., Goldberg R.M. Reply to J.N. Primrose et al and C.-H. Kohne. J Clin Oncol – Off J Am Soc Clin Oncol. 2015;33:2408–2409. doi: 10.1200/JCO.2014.60.4751. [DOI] [PubMed] [Google Scholar]
- 39.Kohne C.H. Is progression-free survival the right end point in trials of patients with clearly resectable, borderline resectable, and unresectable liver-limited colorectal cancer? J Clin Oncol – Off J Am Soc Clin Oncol. 2015;33:2406–2407. doi: 10.1200/JCO.2014.60.7044. [DOI] [PubMed] [Google Scholar]
- 40.Primrose J.N., Cunningham D., Garden O.J., Maughan T.S., Pugh S.A., Stanton L. Cetuximab is contraindicated in the perioperative treatment of colorectal liver metastases. J Clin Oncol – Off J Am Soc Clin Oncol. 2015;33:2405–2406. doi: 10.1200/JCO.2014.60.1344. [DOI] [PubMed] [Google Scholar]
- 41.Nordlinger B., Poston G.J., Goldberg R.M. Should the results of the new EPOC trial change practice in the management of patients with resectable metastatic colorectal cancer confined to the liver? J Clin Oncol – Off J Am Soc Clin Oncol. 2015;33:241–243. doi: 10.1200/JCO.2014.58.3989. [DOI] [PubMed] [Google Scholar]
- 42.Hasegawa K., Oba M., Kokudo N. Cetuximab for resectable colorectal liver metastasis: new EPOC trial. Lancet Oncol. 2014;15:e305–e306. doi: 10.1016/S1470-2045(14)70216-5. [DOI] [PubMed] [Google Scholar]
- 43.Manceau G., Imbeaud S., Thiebaut R., Liebaert F., Fontaine K., Rousseau F. Hsa-miR-31-3p expression is linked to progression-free survival in patients with KRAS wild-type metastatic colorectal cancer treated with anti-EGFR therapy. Clin Cancer Res. 2014;20:3338–3347. doi: 10.1158/1078-0432.CCR-13-2750. [DOI] [PubMed] [Google Scholar]