Abstract
Background
While traditional survival analyses focus on factors determined at the time of surgery, conditional survival (CS) estimates prognosis relative to time following treatment. We sought to compare actuarial and CS among patients undergoing curative intent surgery for hilar cholangiocarcinoma.
Methods
242 patients undergoing surgery between 2000 and 2014 were identified using a multi-institutional database. CS was calculated as the probability of surviving an additional 3 years, given that the patient had already survived “x” years from surgery.
Results
Median patient age was 67 years (IQR: 57–73) and most patients were male (n = 140, 57.9%). Lymph node metastases were noted in 79 (32.6%) patients while an R0 margin was obtained in 66.1% (n = 160). Median OS was 22.3 months. Actuarial survival decreased over time from 46.3% at 2 years following surgery to 18.2% at 5 years; in contrast, the 3-year CS (CS3) increased with time (CS3 at 2 years was 39.3% versus 54.4% at 5 years). CS3 exceeded actuarial survival for high-risk patients with patients with perineural invasion demonstrating an actuarial survival of 15.4% at 5 years versus CS3 of 37.6% at 2 years following surgery (Δ = 22.2%).
Conclusions
CS provides a more accurate, dynamic estimate for survival, especially among high-risk patients.
Introduction
Hilar cholangiocarcinoma (HC) is the most common malignancy arising from the biliary tract accounting for 50–67% of all cases of cholangiocarcinoma.1, 2 Despite only 25% of patients presenting with resectable disease at the time of diagnosis, complete surgical resection remains the only option for cure with an estimated 5-year survival ranging from 11 to 42%.3, 4 Given the poor prognosis associated with HC, appropriate risk-stratification of patients is important to inform decisions pertaining to cure, surveillance and palliation. Currently, there exist two commonly used prognostic classification tools for patients with HC: the Bismuth-Corlette classification and the American Joint Committee on Cancer (AJCC) classification system.5, 6 While these prognostic classification schemes have identified important risk factors for HC including nodal disease and margin status, their ability to predict long-term survival remains limited. Specifically, constructed using traditional survival estimates, these prognostic classification systems are unable to account for changes in survival probability relative to the time elapsed from diagnosis.7 Under such circumstances, conditional survival (CS), which accounts for the changing probability of survival over time, has been identified as a more clinically relevant measure to predict long-term survival.8, 9
CS is based on the underlying premise that as a patient survives past a given time point, the survival probability changes compared to the time of initial diagnosis.10 As such, CS has been proposed as a more clinically useful measure to predict long-term survival compared with traditional survival estimates. Furthermore, the use of CS estimates to predict long-term survival may help facilitate appropriate risk-stratification of patients, as well as determine more accurate end-points for future randomized, controlled trials. Specifically, as the risk of death changes with time elapsed from surgery, certain populations may benefit from an extended follow-up or increased surveillance in clinical trials. To this point, previous reports on patients undergoing surgery for lung, pancreatic and breast cancer have demonstrated more accurate estimates for disease-free and overall survival using CS.8, 9, 11, 12 However, to our knowledge, no previous research has assessed CS among patients undergoing surgery for HC. Given this, the aim of the current study was to define conditional survival among patients undergoing curative intent surgery for HC. Additionally, we sought to assess the prognostic ability of previously established risk factors relative to the time elapsed after surgery.
Methods
Data sources and patient population
Patients undergoing surgery for hilar cholangiocarcinoma between January 1, 2000 and December 31, 2014 at one of ten institutions participating in the Extrahepatic Biliary Malignancy Consortium were identified (Johns Hopkins University, Baltimore, Maryland; Emory University, Atlanta, Georgia; Stanford University, Stanford, California; University of Wisconsin, Milwaukee, Wisconsin; Ohio State University, Columbus, Ohio; Washington University, St. Louis, Missouri; Vanderbilt University, Nashville, Tennessee; New York University, New York, New York; University of Louisville, Louisville, Kentucky; Wake Forest University, Winston-Salem, North Carolina). Only patients with histologically confirmed hilar cholangiocarcinoma and patients who underwent curative intent surgery for their primary tumor were included. Patients who died within 30 days of surgery were excluded.
Standard demographic and clinicopathologic data were collected for each patient including age, sex, race, primary tumor size, AJCC 7th edition stage, AJCC T-stage, histologic grade, presence of nodal disease, final resection margin, and the presence of vascular and/or perineural invasion. Tumor size was defined as the largest diameter of the tumor in the resected specimen. If multiple tumors were resected, the largest diameter was used to define tumor size. Histologic grade was defined as either well, moderate, or poorly differentiated with the highest histologic grade used to define tumor grade among patients with multiple resected specimens. Margin and nodal status were determined using the final postoperative pathologic report. R1 resection was defined by the presence of microscopic disease at the resection margin, while R0 resection was defined by the absence of both microscopic and macroscopic disease at the resection margin. The presence of lymph node metastases was defined by the presence of disease in one or more resected lymph nodes. Additionally, treatment specific information including the extent of surgery, receipt of lymphadenectomy, as well as the receipt of pre or postoperative chemo or radiotherapy were also collected for each patient. A major liver resection was defined as the removal of three or more Couinaud segments. Postoperative complications were graded according to the Clavien–Dindo classification. The institutional review board of each participating institutional approved this study.
Statistical analysis
Summary statistics were provided as whole numbers and percentages for categorical variables and medians with interquartile range (IQR) for continuous variables. The primary outcome of interest was overall survival (OS), defined as the time interval between the date of surgery and the date of death (all-cause mortality) or last available follow-up, as appropriate. Estimates for OS were calculated using the Kaplan–Meier method. Differences in survival between patient groups were assessed using the Mantel–Haenszel test. A Cox proportional hazards model was built to identify potential risk factors for overall survival. Specifically, patient and disease factors evaluated included age, sex, primary tumor size, T-stage, histologic grade, lymph node metastases, margin status, and tumor invasion (vascular and/or perineural). Results from the Cox models were subsequently reported as hazard ratios (HR) and corresponding 95% confidence intervals (CI).
Conditional survival was defined as the probability of surviving an additional number of “y” years given that a patient had already survived for “x” years and was calculated as CS(x|y) = S(x+y)/S(x), with S(x) representing the OS at x years estimated using the Kaplan–Meier method. For example, the CS for surviving another year among patients who had already survived 4 years, CS(1|4), was calculated by dividing the 5-year Kaplan–Meier survival estimate S(5) by the 4-year Kaplan–Meier survival estimate S(4).8, 9, 13, 14 Differences in CS were assessed using linear regression and standardized differences. Standardized differences (d) can be used as the index to contrast 2 rates such that d < 0.1 represents very small differences; 0.1 ≤ d < 0.3, small differences; 0.3 ≤ d < 0.5, moderate differences, and d ≥ 0.5, considerable differences. All analyses were performed using SPSS 22.0 (IBM, New York). All tests were 2-sided and p < 0.05 defined statistical significance.
Results
Demographic and clinicopathologic characteristics
A total of 242 patients who underwent curative intent surgery for HC and met inclusion criteria were identified (Table 1). The median patient age was 67 years (IQR 57–73) with a majority of patients being male (n = 140, 57.9%) and Caucasian (n = 184, 78.3%). Neoadjuvant chemotherapy was administered to 4.1% (n = 10) of patients, whereas only 2.5% (n = 6) of patients received neoadjuvant radiotherapy. At the time of surgery, the majority (n = 179, 74.9%) of patients underwent a major hepatic resection; 239 (98.8%) patients underwent an open resection, whereas 1 patient underwent a laparoscopic resection and 2 patients underwent a laparoscopic resection that was converted to an open procedure. A portal vein resection was performed in 19 (7.9%) patients; 4 patients had a formal segmental portal vein resection and reconstruction. On final histopathology, 82 (33.9%) patients had a positive (R1) surgical margin. The median tumor size was 2.5 cm (1.8–3.9) and the majority of tumors were classified as moderately differentiated (n = 131, 58.2%). Lymph node metastases were observed in 79 (32.6%) patients while 160 (77.3%) patients had tumors with perineural invasion.
Table 1.
Demographic and clinicopathologic characteristics
| Variable | N, (%)/Median (IQR) |
|---|---|
| Age, y | 67 (57–73) |
| <65 | 110 (45.5) |
| ≥65 | 132 (54.5) |
| Sex | |
| Male | 140 (57.9) |
| Female | 102 (42.1) |
| Race | |
| Caucasian | 184 (78.3) |
| African American | 17 (7.2) |
| Other | 34 (14.5) |
| BMI | 25.1 (22.2–28.6) |
| Functionally independent | 217 (98.2) |
| Comorbidities | |
| Hypertension | 99 (43.2) |
| History of cardiac disease | 26 (11.4) |
| Diabetes | 36 (15.7) |
| Preoperative jaundice | 190 (79.2) |
| Type of resection | |
| Minor resection (<3 Couinaud segments) | 60 (25.1) |
| Major resection (≥3 Couinaud segments) | 179 (74.9) |
| Margin status | |
| R0 | 160 (66.1) |
| R1 | 82 (33.9) |
| Tumor size | 2.5 (1.8–3.9) |
| ≤2.5 cm | 116 (51.1) |
| >2.5 cm | 111 (48.9) |
| Grade | |
| Well differentiated | 43 (19.1) |
| Moderately/poorly differentiated | 182 (80.9) |
| Bismuth-Corlette class | |
| I | 28 (12.6) |
| II | 37 (16.6) |
| IIIa | 58 (26.0) |
| IIIb | 47 (21.1) |
| IV | 53 (23.8) |
| AJCC 7th edition stage | |
| Stage I & II | 114 (58.2) |
| Stage III & IV | 82 (41.8) |
| AJCC T-stage | |
| T1–T2 | 157 (80.1) |
| T3–T4 | 39 (19.9) |
| Lymph node metastases | |
| No | 163 (67.4) |
| Yes | 79 (32.6) |
| Lymphovascular invasion | |
| No | 118 (60.8) |
| Yes | 76 (39.2) |
| Perineural invasion | |
| No | 47 (22.7) |
| Yes | 160 (77.3) |
Following surgery, 150 (64.4%) patients developed a postoperative complication, 83 (56.4%) of which were classified as grade III or higher. The most common complications were intra-abdominal abscesses and fluid collections requiring percutaneous drainage, which were observed in 18.6% (n = 43) and 21.8% (n = 51) of patients, respectively.
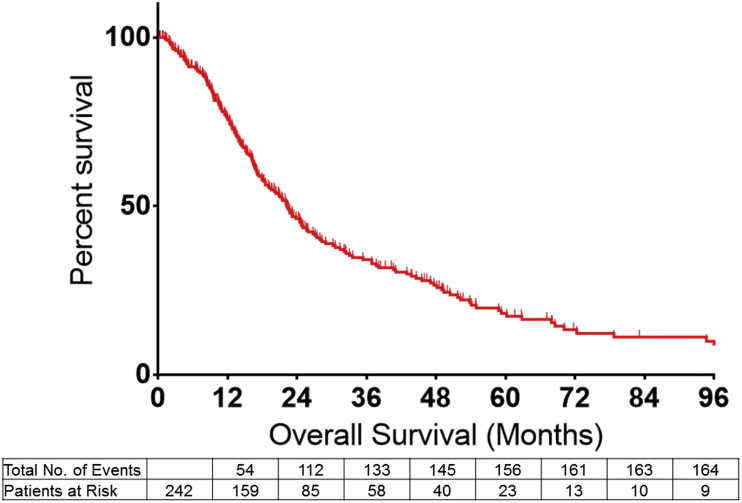
Factors associated with overall survival
The median actuarial OS among all patients was 22.3 months with an estimated 1-, 3- and 5-year OS of 75.6% (95%CI 69.9–81.2), 46.3% (95%CI 39.3–53.0) and 18.2% (95%CI 12.6–24.6), respectively (Fig. 1). On Cox regression analyses, several patient and disease specific characteristics were associated with a worse OS. Specifically, age at diagnosis >65 years (HR 1.36; 95%CI 1.01–1.85, p = 0.04), a greater T-stage (AJCC 7th T-Stage III or IV; HR 1.73; 95%CI 1.14–2.61, p = 0.009), presence of nodal metastasis (HR 1.71; 95%CI 1.24–2.36, p = 0.001), and overall advanced disease stage (AJCC Stage III–IV; HR 1.81; 95%CI 1.27–2.58, p = 0.001) were each associated with worse OS.
Figure 1.
Overall survival among all patients undergoing curative intent surgery for hilar cholangiocarcinoma
Comparison of overall and conditional survival
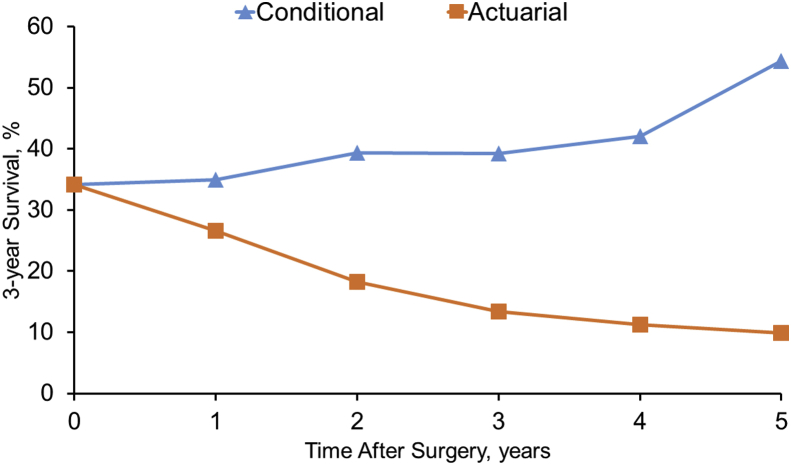
In contrast to actuarial OS, which was observed to decrease from the time of surgery, estimates for CS increased over time as surviving patients accrued more survival time (Fig. 2). For example, while actuarial OS decreased from 26.6% at 4 years to 9.9% at 8 years following surgery, 3-year conditional survival (CS3) was noted to increase from 35.0% at 1-year versus 54.4% at 5-years following surgery. Of note, the 3-year CS estimates given the patient had already survived for 1-, 2-, and 5-years were 35.0%, 39.3%, and 54.4%, respectively (Table 2).
Figure 2.
A comparison of 3-year actuarial survival and 3-year conditional survival among all patients undergoing a curative intent surgery for hilar cholangiocarcinoma
Table 2.
Conditional survival
| Total survival time, y | If the patient has survived. % |
||||||
|---|---|---|---|---|---|---|---|
| 1 y | 2 y | 3 y | 4 y | 5 y | 6 y | 7 y | |
| 1 | |||||||
| 2 | 60.8 | ||||||
| 3 | 44.9 | 73.9 | |||||
| 4 | 35.0 | 57.5 | 77.8 | ||||
| 5 | 23.9 | 39.3 | 53.2 | 68.4 | |||
| 6 | 17.6 | 28.9 | 39.2 | 50.4 | 73.6 | ||
| 7 | 14.7 | 24.2 | 32.7 | 42.1 | 61.5 | 83.6 | |
| 8 | 13.0 | 21.4 | 28.9 | 37.2 | 54.4 | 73.9 | 88.4 |
Each column represents the actual time survived/elapsed from the date of surgery, while each row represents the survival estimates for surviving an additional time. For example, when looking at survival estimates from the first column from the left (if the patient has survived 1 year), one can note that the probability of surviving to year 2 if 60.8% whereas the probability of surviving to year 8 is 13%. Similarly, if a patient has already survived to 3 years (third column from left), the probability of surviving to 5 years is 53.2%.
To further compare differences in actuarial OS versus CS, additional analyses were performed stratified by clinicopathologic characteristics such as age, nodal status, depth of invasion, as well as margin status and tumor stage. As expected, older patient age, the presence of nodal metastasis, perineural invasion, T3/T4 disease, as well as advanced overall AJCC stage were all associated with decreased actuarial OS (all p < 0.05, Table 3). For example, patients with nodal metastases had a substantially worse 5-year actuarial OS (8.7%) versus patients without nodal disease (22.7%) (p = 0.001). Similarly, the 5-year actuarial OS for patients with stage I/II disease was 27.7% compared with 5.8% for patients with stage III/IV disease (p = 0.001). Interestingly, CS3 estimates exceeded actuarial OS for patients in high-risk subgroups (Fig. 3a–e). For example, among patients who had an R1 margin at the time of surgery, the “all comer” observed 5-year actuarial OS was 8.4%; however, among patients who had an R1 margin but survived to year 2 following surgery, the chances of being alive an additional 3 years (i.e. CS3, based on being alive for 2 years a cumulative total of 5 years from surgery) was 22.8% (Δ = 14.4%, Table 4). Differences in actuarial versus CS were also noted among patients with tumors characterized by adverse biologic features. For example, among patients with perineural invasion the calculated actuarial 5-year OS was only 15.4% versus an estimated CS3 at 2 years of 37.6% (Δ = 22.2%). Similarly, patients with nodal disease had an estimated 5-year actuarial OS of 8.7% based on actuarial data calculated from the time of their operation. In contrast, among patients with nodal metastasis who had survived 2 years, the estimate that these patients would still be alive in another 3 years was much higher, with an estimated CS3 of 29.7% (Δ = 21.0%).
Table 3.
Overall survival stratified by risk factors
| Variable | Patient survival, % |
p value* | |||
|---|---|---|---|---|---|
| 1 y | 3 y | 5 y | 8 y | ||
| All patients | 75.6 (159) | 34.2 (58) | 18.2 (23) | 9.9 (9) | |
| Age, y | |||||
| <65 | 81.2 (78) | 36.8 (29) | 20.7 (12) | 11.4 (4) | 0.048 |
| ≥65 | 70.7 (80) | 31.9 (28) | 15.9 (10) | 8.7 (4) | |
| Sex | |||||
| Male | 80.8 (96) | 31.8 (32) | 17.8 (12) | 8.3 (4) | 0.687 |
| Female | 68.8 (62) | 37.0 (25) | 18.6 (10) | 12.2 (5) | |
| Margin status | |||||
| R0 | 77.7 (109) | 37.5 (39) | 23.6 (19) | 11.9 (7) | 0.056 |
| R1 | 71.4 (49) | 27.7 (18) | 8.4 (3) | – (1) | |
| Tumor size | |||||
| ≤2.5 cm | 73.2 (77) | 35.4 (30) | 21.3 (13) | 5.0 (2) | 0.744 |
| >2.5 cm | 79.3 (74) | 35.3 (26) | 17.3 (10) | 15.4 (8) | |
| Grade | |||||
| Well differentiated | 77.6 (31) | 42.6 (15) | 17.8 (4) | 0.0 (0) | 0.562 |
| Moderately/poorly differentiated | 73.7 (114) | 30.3 (36) | 17.8 (15) | 12.7 (7) | |
| AJCC stage | |||||
| Stage I & II | 79.3 (75) | 45.6 (32) | 27.7 (15) | 18.2 (7) | 0.001 |
| Stage III & IV | 68.5 (48) | 21.1 (11) | 5.8 (2) | 0.0 (0) | |
| AJCC T-stage | |||||
| T1–T2 | 74.6 (98) | 40.1 (39) | 24.3 (16) | 14.3 (6) | 0.008 |
| T3–T4 | 75.0 (25) | 15.3 (4) | 0.0 (0) | 0.0 (0) | |
| Lymph node metastases | |||||
| No | 80.3 (112) | 40.1 (45) | 22.7 (19) | 13.2 (9) | 0.001 |
| Yes | 66.2 (46) | 22.3 (12) | 8.7 (3) | 0.0 (0) | |
| Lymphovascular invasion | |||||
| No | 82.9 (80) | 41.7 (32) | 24.2 (14) | 6.1 (2) | 0.067 |
| Yes | 67.1 (45) | 26.1 (12) | 15.2 (5) | – (3) | |
| Perineural invasion | |||||
| No | 85.9 (34) | 47.8 (15) | 34.5 (10) | 21.6 (3) | 0.040 |
| Yes | 72.3 (102) | 29.8 (33) | 15.4 (14) | 5.9 (3) | |
Overall survival Kaplan–Meier estimates, the numbers provided are percentages of patients alive. Number of patients at risk is depicted between the parentheses. *p-value is determined using the Mantel–Haenszel test.
Figure 3.
A comparison of 3-year conditional survival by (a) age (b) margin status (c) AJCC 7th edition tumors stage (d) presence of lymph node metastases (e) presence of perineural invasion
Table 4.
3 year conditional survival stratified by risk factor
| Variables | Time elapsed since operative resection |
|||||
|---|---|---|---|---|---|---|
| 0 y | 1 y | 2 y | 3 y | 4 y | 5 y | |
| All patients | 34.2 | 35.0 | 39.3 | 39.2 | 42.1 | 54.4 |
| Age, y | ||||||
| <65 | 36.8 | 35.7 | 39.9 | 45.1 | 49.0 | 55.1 |
| ≥65 | 31.9 | 34.2 | 38.3 | 34.2 | 35.5 | 54.7 |
| d | 0.10 | 0.03 | 0.03 | 0.23 | 0.27 | 0.01 |
| Margin status | ||||||
| R0 | 37.5 | 42.1 | 46.1 | 44.3 | 41.6 | 50.4 |
| R1 | 27.7 | 21.4 | 22.8 | 30.3 | 53.8 | – |
| d | 0.21 | 0.46 | 0.51 | 0.29 | −0.25 | – |
| AJCC stage | ||||||
| I & II | 45.6 | 41.2 | 48.9 | 51.3 | 55.0 | 65.7 |
| III & IV | 21.1 | 24.5 | 17.3 | 27.5 | 34.5 | – |
| d | 0.54 | 0.36 | 0.71 | 0.50 | 0.42 | – |
| AJCC T-stage | ||||||
| T1–T2 | 40.1 | 39.7 | 48.7 | 52.1 | 62.9 | 58.8 |
| T3–T4 | 15.3 | 15.3 | – | – | – | – |
| d | 0.58 | 0.57 | – | – | – | – |
| Lymph node metastases | ||||||
| No | 40.1 | 40.5 | 41.4 | 40.4 | 40.6 | 58.7 |
| Yes | 22.3 | 21.9 | 29.7 | 39.0 | 60.0 | – |
| d | 0.39 | 0.41 | 0.25 | 0.03 | −0.40 | – |
| Perineural invasion | ||||||
| No | 47.8 | 44.2 | 56.9 | 60.3 | 56.8 | 62.6 |
| Yes | 29.8 | 30.1 | 37.6 | 31.9 | 35.9 | 38.3 |
| d | 0.38 | 0.29 | 0.40 | 0.59 | 0.43 | 0.50 |
Discussion
HC is the most common malignancy of the biliary tract, accounting for up to two-thirds of all cases of cholangiocarcinoma.1, 2 HC has traditionally been associated with a poor prognosis with 5-year survival ranging from 11 to 42% among patients following curative surgical resection. Given this, identifying high-risk patients is important to guide decisions pertaining to treatment, surveillance and/or palliation. Although prognostic classification systems have been proposed for HC, these schemes are limited because data are only derived from the time of surgery.15 As such, these traditional survival estimates do not account for changes in survival probability over time accrued from the initial diagnosis.8, 9 In contrast, CS estimates account for changes in survival probabilities over time and therefore may be more accurate in predicting long-term survival.8, 9 Although previously reported for patients with bladder, gastric and colon cancer, to the best of our knowledge no previous report has assessed conditional survival among patients with HC. The current study is important because we were able to define CS estimates for patients undergoing curative intent surgery for HC using a large, multi-centric cohort of patients. Of note, CS estimates increased over time and were consistently higher than traditional OS estimates. To further explore differences in conditional and actuarial survival estimates, additional stratified analyses were performed using certain demographic and clinicopathologic characteristics. Interestingly, CS estimates were consistently higher among all patient strata with the magnitude of differences between CS and actuarial OS estimates highest among patients with high-risk factors traditionally associated with a worse OS such as perineural invasion and nodal metastasis.
In describing prognosis following surgery, most studies report survival based on a Kaplan Meier survival curve determined from factors derived at the time of surgery. While traditionally used to estimate survival, such curves have been criticized as being inaccurate due to their “static” nature.7 Rather than being fixed, in reality, the odds that a patient survives for an additional future period of time changes as the patient accrues survival time.8, 9 As such, in order to be more clinically meaningful to patients and providers, survival data should chart the way the odds of survival change over time. Unlike traditional survival estimates, CS estimates a patient's survival odds given the pre-condition of having already survived a certain length of time. In fact, for a wide range of advanced cancers, including cancers with a particularly poor prognosis, there is evidence that the odds actually do improve with time.9, 13, 14, 16, 17 Our group has previously demonstrated that CS estimates may provide critical quantitative information about the changing probability of survival over time among patients with gastric cancer, gastrointestinal stromal tumors, pancreatic cancer, as well as intrahepatic cholangiocarcinoma.9, 14, 16, 17 For example, among patients with intrahepatic cholangiocarcinoma, we previously noted that, while actuarial OS decreased over time from 39% at 3 years to 16% at 8 years, the 3-year CS increased over time among those patients who survived.14 Specifically, the CS3 at 5 years (i.e. the probability of surviving to postoperative year 8 after having already survived to postoperative year 5) was 65% compared with a predicted 8-year actuarial OS of 16%. Of course, time since diagnosis is not the only factor that can affect prognosis, as clinical and tumor specific factors can also be drivers of long-term outcome.
In the current analysis of 242 patients, the median actuarial overall survival was 22.3 months. Consistent with previous reports, the current study identified patient age, T-stage, nodal status, presence of perineural invasion, margin status, as well as overall AJCC stage as important adverse prognostic factors.18, 19, 20 Perhaps of greater interest, data from the current study demonstrated that these clinical and tumor characteristics were not “universally” associated with a prohibitively poor prognosis. In fact, while a lower actuarial survival was noted among patients demonstrating these adverse prognostic factors, the greatest improvement in CS was also noted among these subgroups of patients. For example, patients with nodal disease demonstrated an increase in CS of over 20% compared with patients who did not have nodal metastases. The reasons for differences in actuarial versus CS survival estimates is likely related to the fact that traditional actuarial estimates of survival are disproportionately influenced by high-risk patients many of whom may die within the first few years of surgery. However, those patients with high risk features who do live longer have in a sense “out-lived” some of the prognostic impact of these initial adverse factors.14 In turn, the prognosis of patients who initially had the worse prognostic factors, but who are still alive after a period of time, are the most likely to have their future long-term prognosis inaccurately predicted using traditional actuarial survival estimates. As such, the use of CS to provide information on long-term prognosis is likely to be valuable in estimating long-term survival for patients with diseases that traditionally have a poor prognosis, such as HC.
Several limitations should be considered when interpreting results from the current study. Inherent to all retrospective analyses, there may have been a selection bias regarding the diagnosis and treatment of patients. In addition, the use of data from a multi-institutional cohort did not allow for the standardization of operative and/or perioperative clinical practices between centers. However, given the rarity of HC, the use of data from multiple centers ensured a uniquely large sample size while also making our results more generalizable.
In conclusion, using a large, multi-institutional cohort of patients, we observed that overall survival following curative intent surgery for HC varied as a function of the survival time accrued since surgical resection. The relative improvement in CS estimates was greatest among that subgroup of patients with high risk factors. CS estimates can be used to provide important quantitative information regarding the changing probability of survival over time among patients with HC. In turn, CS may facilitate more accurate prognostication while also aiding in clinical decision making.
Funds
None.
Conflict of interest
None declared.
Footnotes
This study was presented at the 12th World IHPBA Congress, 20–23 April 2016, Sao Paulo, Brazil.
References
- 1.DeOliveira M.L., Cunningham S.C., Cameron J.L., Kamangar F., Winter J.M., Lillemoe K.D. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke E.C., Jarnagin W.R., Hochwald S.N., Pisters P.W., Fong Y., Blumgart L.H. Hilar cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228:385–394. doi: 10.1097/00000658-199809000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W., Yan L.N. Perihilar cholangiocarcinoma: current therapy. World J Gastrointest Pathophysiol. 2014;5:344–354. doi: 10.4291/wjgp.v5.i3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakeeb A., Tran K.Q., Black M.J., Erickson B.A., Ritch P.S., Quebbeman E.J. Improved survival in resected biliary malignancies. Surgery. 2002;132:555–563. doi: 10.1067/msy.2002.127555. discussion 563–554. [DOI] [PubMed] [Google Scholar]
- 5.Bismuth H., Corlette M.B. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170–178. [PubMed] [Google Scholar]
- 6.Jarnagin W.R., Fong Y., DeMatteo R.P., Gonen M., Burke E.C., Bodniewicz B.J. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. discussion 517–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mertens A.C., Yong J., Dietz A.C., Kreiter E., Yasui Y., Bleyer A. Conditional survival in pediatric malignancies: analysis of data from the childhood cancer survivor study and the surveillance, epidemiology, and end results program. Cancer. 2015;121:1108–1117. doi: 10.1002/cncr.29170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan H., de Jong M.C., Pulitano C., Ribero D., Strub J., Mentha G. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210:755–764. doi: 10.1016/j.jamcollsurg.2009.12.041. 764–756. [DOI] [PubMed] [Google Scholar]
- 9.Mayo S.C., Nathan H., Cameron J.L., Olino K., Edil B.H., Herman J.M. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer. 2012;118:2674–2681. doi: 10.1002/cncr.26553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurta M.L., Edwards R.P., Moysich K.B., McDonough K., Bertolet M., Weissfeld J.L. Prognosis and conditional disease-free survival among patients with ovarian cancer. J Clin Oncol. 2014;32:4102–4112. doi: 10.1200/JCO.2014.55.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skuladottir H., Olsen J.H. Conditional survival of patients with the four major histologic subgroups of lung cancer in Denmark. J Clin Oncol. 2003;21:3035–3040. doi: 10.1200/JCO.2003.04.521. [DOI] [PubMed] [Google Scholar]
- 12.Ploussard G., Shariat S.F., Dragomir A., Kluth L.A., Xylinas E., Masson-Lecomte A. Conditional survival after radical cystectomy for bladder cancer: evidence for a patient changing risk profile over time. Eur Urol. 2014;66:361–370. doi: 10.1016/j.eururo.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 13.Cucchetti A., Piscaglia F., Cescon M., Ercolani G., Terzi E., Bolondi L. Conditional survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Clin Cancer Res. 2012;18:4397–4405. doi: 10.1158/1078-0432.CCR-11-2663. [DOI] [PubMed] [Google Scholar]
- 14.Spolverato G., Kim Y., Ejaz A., Alexandrescu S., Marques H., Aldrighetti L. Conditional probability of long-term survival after liver resection for intrahepatic cholangiocarcinoma: a multi-institutional analysis of 535 patients. JAMA Surg. 2015;150:538–545. doi: 10.1001/jamasurg.2015.0219. [DOI] [PubMed] [Google Scholar]
- 15.Chaiteerakij R., Harmsen W.S., Marrero C.R., Aboelsoud M.M., Ndzengue A., Kaiya J. A new clinically based staging system for perihilar cholangiocarcinoma. Am J Gastroenterol. 2014;109:1881–1890. doi: 10.1038/ajg.2014.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bischof D.A., Kim Y., Dodson R., Jimenez M.C., Behman R., Cocieru A. Conditional disease-free survival after surgical resection of gastrointestinal stromal tumors: a multi-institutional analysis of 502 patients. JAMA Surg. 2015;150:299–306. doi: 10.1001/jamasurg.2014.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y., Ejaz A., Spolverato G., Squires M.H., Poultsides G., Fields R.C. Conditional survival after surgical resection of gastric cancer: a multi-institutional analysis of the us gastric cancer collaborative. Ann Surg Oncol. 2015;22:557–564. doi: 10.1245/s10434-014-4116-5. [DOI] [PubMed] [Google Scholar]
- 18.Groot Koerkamp B., Wiggers J.K., Gonen M., Doussot A., Allen P.J., Besselink M.G. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann Oncol. 2015;26:1930–1935. doi: 10.1093/annonc/mdv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valero V., 3rd, Cosgrove D., Herman J.M., Pawlik T.M. Management of perihilar cholangiocarcinoma in the era of multimodal therapy. Expert Rev Gastroenterol Hepatol. 2012;6:481–495. doi: 10.1586/egh.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao K., Liu J., Sun J., Zhang J., Chen J., Pawlik T.M. Patterns and prognostic value of lymph node dissection for resected perihilar cholangiocarcinoma. J Gastroenterol Hepatol. 2016;31:417–426. doi: 10.1111/jgh.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]