Abstract
Anticoagulants are commonly used drugs that are frequently encountered during device placement. Deciding when to halt or continue the use of anticoagulants is a balance between the risks of thromboembolism versus bleeding. Patients taking warfarin with a high risk of thromboembolism should continue to take their warfarin without interruption during device placement while ensuring their international normalized ratio remains below 3. For patients who are taking warfarin and have low risk of thromboembolism, either interrupted or continued warfarin may be used, with no evidence to clearly support either strategy. There is little evidence to support continuing direct acting oral anticoagulants (DOACs) for device implantation. The timing of halting these medications depends largely on renal function. If bleeding occurs, warfarin׳s anticoagulation effect is reversible with vitamin K and activated prothrombin complex concentrate. There are no DOAC reversal agents currently available, but some are under development. Regarding antiplatelet agents, aspirin alone can be safely continued while clopidogrel alone may also be continued, but with a slightly higher bleeding risk. Dual antiplatelet therapy for bare-metal stent/drug-eluting stent implanted within 4 weeks/6 months, respectively, should be continued due to high risk of stent thrombosis; however, if they are implanted after this period, then clopidogrel can be halted 5 days before the procedure and resumed soon after, while aspirin is continued. If the patient is taking both aspirin and warfarin, aspirin should be halted 5 days prior to the procedure, while warfarin is continued.
Keywords: Anticoagulant, Antiplatelet, Cardiac implantable device surgery
1. Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia managed in clinical practice and the most common arrhythmia requiring hospitalization [1], [2]. Thromboembolism occurs with similar incidence, regardless of the form of AF [3], [4]. AF management includes anticoagulation to prevent thromboembolic stroke, its most debilitating complication [2], [5]. Anticoagulation with warfarin, at a target international normalized ratio (INR), or with a direct acting oral anticoagulant (DOAC), has consistently been shown to reduce the risk of stroke and is therefore a major goal of therapy for AF [6], [7]. AF is the most common reason for anticoagulation [8]. Anticoagulants are also frequently used for other indications, ranging from venous thromboembolism to mechanical prosthetic heart valves [9]. Indeed, their widespread use in clinical practice leads to a high likelihood of their being encountered in patients undergoing invasive procedures. Cardiac implantable electrophysiological device (CIED) surgeries, which include pacemaker (PM) and implantable cardioverter defibrillator (ICD) placements, are now commonplace worldwide with approximately 1.5 million procedures performed per year. Of patients who undergo such procedures, up to 35% require long-term anticoagulation [10].
When determining who should receive anticoagulation, a risk-stratification model is used. The rationale behind risk stratification is that although anticoagulation has clearly been shown to be more effective than antiplatelet agents or placebos in the prevention of thromboembolic stroke, their use should be restricted to patients whose risk for a thromboembolic event exceeds their risk of hemorrhage [11], [12], [13]. Risk factors for thromboembolic events in nonvalvular AF include a history of stroke, diabetes mellitus, hypertension, heart failure, and age. These were incorporated into the initial score called CHADS2 [6], [14]. The annual risk of stroke increased incrementally from 2%, with a score of 0, to as high as 22%, with a score of 6, in the absence of anticoagulant therapy [11], [15], [16]. A second score known as CHA2DS2-VASc was developed to further delineate the risk in the perceived low-risk groups using additional risk factors [6], [17], [18].
The risk of bleeding also increases substantially with the use of anticoagulants, and this presents a challenge to their clinical use [6], [19]. A problem that arises is how to manage patients on anticoagulation treatment who require an invasive procedure that inherently increases their risk of bleeding. In this review, we will discuss the management of antithrombotic therapy in patients undergoing CIED surgery, including anticoagulants, such as warfarin and the DOACs, and antiplatelet drugs, such as aspirin and clopidogrel.
2. Oral anticoagulants
Warfarin has been the main oral anticoagulant used in clinical practice for nearly fifty years, especially in patients with AF. It inactivates vitamin K in the hepatic microsomes by inhibiting epoxide reductase, which hinders the formation of clotting factors that are dependent on vitamin K, such as factors II (prothrombin), VII, IX, and X [20]. The onset of the therapeutic action of warfarin is delayed by two to seven days while the preformed factors are depleted. Warfarin dosing is targeted to a therapeutic INR, which is usually 2–3 in AF but may be higher for mechanical mitral valves [20], [21]. It has few side effects other than its major and most significant side effect, which is bleeding [22]. In addition, the INR requires monitoring in order to maintain it in a therapeutic range. Numerous medications interact with warfarin and affect its metabolism [20]. Over-anticoagulation leads to a significant risk of bleeding when the INR is greater than 3 [23], [24]. While there is a trend away from warfarin treatment towards use of the newer anticoagulants, most clinicians maintain warfarin treatment in patients who are already taking the drug and have a stable INR [13].
DOACs are drugs that directly inhibit either thrombin or activated factor X and were designed in response to the need for an oral anticoagulant that did not require frequent monitoring and was less likely to have dietary and medication interactions. Three drugs gained approval in rapid succession: dabigatran etexilate, rivaroxaban, and apixaban [12]. A fourth followed soon after: edoxaban [25], [26] (awaiting approval in Canada). All four are oral medications that do not require anticoagulation monitoring via blood tests. All four DOACs have rapid time to peak plasma concentrations of 2 (rivaroxaban/edoxaban) to 6 h (dabigatran); therefore, immediately after the first dose, patients are well anticoagulated [27]. The first of these drugs, dabigatran, is a potent direct competitive inhibitor of thrombin. The RE-LY trial demonstrated that dabigatran was comparable to warfarin in terms of stroke prevention with lesser rates of major hemorrhage in the 110 mg group, and superior to warfarin with equal rates of hemorrhage in the 150 mg group [28]. Rivaroxaban is an oral, direct factor Xa inhibitor and was shown in the ROCKET-AF trial to be similar to warfarin for the prevention of stroke in nonvalvular AF with no significant difference in the risk of major bleeding [29]. Rivaroxaban is used at a dose of 20 mg once daily or 15 mg daily if the creatinine clearance (CrCl) is between 30 and 49 mL/min. Apixaban, also an oral direct factor Xa inhibitor, was demonstrated to be superior to warfarin in preventing stroke or systemic embolism in nonvalvular AF in the ARISTOTLE trial [30]. The dose of apixaban is 5 mg twice daily with a dose reduction to 2.5 mg twice daily if two of the following three conditions are present: age 80 years or older, body weight of 60 kg or less, and a serum creatinine level of 133 µmol/L or greater [27], [30]. Edoxaban is another oral direct factor Xa inhibitor that was shown to be noninferior to warfarin with respect to the prevention of stroke or systemic embolism in nonvalvular AF and was associated with significantly lower rates of bleeding in the ENGAGE AF-TIMI 48 trial [31]. The recommended dose of edoxaban is 60 mg once daily with a reduced dose of 30 mg once daily if CrCl is between 15 and 50 mL/min [32]. DOACs may be useful for use in elderly patients on a multitude of medications that may interact with warfarin; indeed, DOACs have been shown to be safe in the elderly [33].
3. Management of warfarin during pacemaker/implantable cardioverter defibrillator insertion
Until recently, warfarin use was halted before PM/ICD insertion and the patient was bridged with intravenous (IV) unfractionated heparin or subcutaneous low-molecular-weight heparin (LMWH) if the thromboembolic risk was considered high (usually CHADS2 ≥3) [34], [35], [36]. This was usually performed by discontinuing warfarin use five days before the procedure and initiating IV heparin or LMWH 3 days before the procedure, once the INR decreased below 2; the procedure was usually performed when the INR was less than 1.5 and after halting IV heparin 4 h prior to the procedure or after halting the last dose of LMWH 24 h prior [36]. This process is marred by several difficulties and logistical issues. To start IV heparin, the patient must be a hospital inpatient for several days before the procedure. In addition, heparin requires frequent checking of the PTT with dose adjustments to ensure a therapeutic time; LMWH is less problematic in terms of monitoring, but still requires daily injections and CrCl above 30 mL/min [34]. Sometimes, the INR does not decrease below 1.5 on the day of the procedure, leading to the administration of vitamin K to decrease the INR, which results in restoring the INR to the therapeutic range after post-procedural reinitiation of warfarin and a further increase in hospital stay. Furthermore, several studies that this bridging strategy leads to significantly increased rate of device-pocket hematoma, ranging from 17% to 31% [37]. A recent systematic review demonstrated that compared to no heparin, heparin bridging was associated with a combined odds ratio of 4.47 for the development of pocket hematoma and prolonged hospital stay [38]. The consequence of a device-pocket hematoma is substantial, including the need for a prolonged period of time off anticoagulation with a risk of thromboembolism, the need for further procedures to clear the hematoma, an increased risk of infection, and an increase in hospital stay; when a hematoma occurs, the increased risk of infection can be as much as 22-fold higher, as demonstrated in the REPLACE registry [39].
A major change has recently occurred in the management of warfarin during pacemaker insertion because of the results of the large trial, BRUISE CONTROL, in which 661 patients with an annual thromboembolism risk greater than 5% per year (CHADS2≥3) were randomized to continued warfarin therapy versus bridging therapy during elective pacemaker/ICD implantation [10]. The trial was performed after several case series and smaller trials had shown a benefit to continued warfarin therapy and several centers had shifted their clinical practice to continued warfarin therapy; a meta-analysis of these trials showed a benefit of continued oral anticoagulation versus bridging in terms of reduced device-pocket hematoma and hematoma drainage/revision [37]. The aforementioned bridging strategy was used in this trial. The trial was stopped early because the results showing 16% of the bridging group to have clinically significant hematoma versus just 3.5% of the continued warfarin group [10]. Furthermore, there was significantly more hematoma causing prolonged hospitalization, hematoma requiring interruption of anticoagulation and hematoma requiring an evacuation [10]. This study represents the main body of evidence for switching to a continued warfarin strategy in those at higher risk of thromboembolism. An algorithm for the peri-procedural management of warfarin is shown in Fig. 1. To provide evidence on whether a bridging strategy is beneficial in high risk patients, the recent BRIDGE study randomized 1884 patients to bridging with initiation of low-molecular-weight heparin three days before the procedure versus no bridging. The study showed an incidence of arterial thromboembolism of 0.4% in the no-bridging group versus 0.3% in the bridging group (95% confidence interval [CI], −0.6 to 0.8; P=0.01 for noninferiority), while the incidence of major bleeding was 1.3% in the no-bridging group and 3.2% in the bridging group (relative risk, 0.41; 95% CI, 0.20–0.78; P=0.005 for superiority) [40]. Several additional studies have shown an increased risk of bleeding with bridging [35], [36]. Indeed, a recent meta-analysis showed that compared with heparin bridge, continued oral anticoagulation at the time of CIED surgery was associated with a significantly lower risk of postoperative bleeding (OR=0.25, 95% CI 0.17–0.36, P<0.0000), but there was no difference noted in the risk of thromboembolic events between the 2 strategies (OR=1.86, 95% CI 0.29–12.17, P=0.57) [41]. In addition, continued warfarin therapy is much more cost effective than bridging therapy [42]. When performing device surgery without interruption of warfarin, the INR should be checked 3–7 days prior to the procedure to provide time for dose adjustment, and then once more on the day of the procedure. The INR on the day of the procedure should be less than or equal to the upper limit of the therapeutic range prescribed for the patient, usually ≤3 (≤3.5 for some prosthetic heart valves) [25].
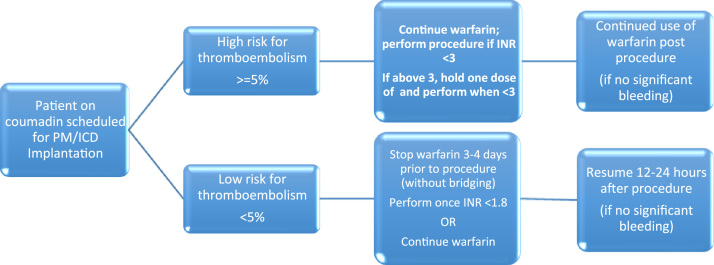
Fig. 1.
Peri-device surgery warfarin management [30], [39], [58]. Low risk for thromboembolism (annual risk of thromboembolic events <5%) includes patients with AF and a CHA2DS2-VASc score of ≤2. High risk for thromboembolism (annual risk of thromboembolic events >=5%) includes patients with AF and a CHA2DS2-VASc score of ≥3, a CHA2DS2-VASc of 2 due to stroke or TIA within 3 months, patients planned for cardioversion or defibrillation testing at device implantation, AF and rheumatic valve disease prosthetic mitral valve, caged ball or tilting disc aortic valve, a combination of bileaflet aortic valve prosthesis with atrial fibrillation and a CHA2DS2-VASc of 2, recent venous thromboembolism (within 3 months) or an intra-cardiac thrombus.
Regarding patients with a lower annual risk of thromboembolism (<5%), there is no clear evidence for continued warfarin versus discontinuing warfarin. Clearly, a bridging strategy should not be used, given the low risk of thromboembolism and higher risk of device-pocket hematoma with bridging. Current guidelines suggest to stop warfarin treatment 3–4 days before the procedure, to perform the procedure once the INR is <1.8, and resume warfarin 12–24 h later [25], [34]. However, as suggested in a recent guideline and in keeping with evidence from two recent meta-analyses, continued warfarin use may be considered, especially if the patient has a history of previous transient ischemic attack or embolic stroke [25].
4. Management of direct acting oral anticoagulants during pacemaker insertion
There are less data available regarding management of DOACs peri-procedurally. The general principle is that PM/ICD implantation is considered a low bleeding risk procedure [10], [34]. Because of the predictable waning anticoagulant effects of DOACs, bridging therapy is not required and management involves timing the cessation and re-initiation of the drug based on the predicted clearance, which takes renal function into account [27], [43]. A recent Canadian study showed that device implantation performed with DOAC interruption, but without bridging [44]. In patients with normal renal function undergoing a low bleeding risk procedure, the usual practice is to discontinue the DOAC 24 h (i.e. last dose 2 days before) prior to the procedure and to reinstate the drug 24–48 h after, provided no significant bleeding has occurred [25], [34], [45] (Fig. 2). A large randomized control trial (BRUISECONTROL2: ClinicalTrials.gov NCT01675076) is underway, in patients undergoing CIED procedures, to test the safety of continuing DOACs—its results are eagerly awaited [46]. The details for each separate DOAC are discussed below.
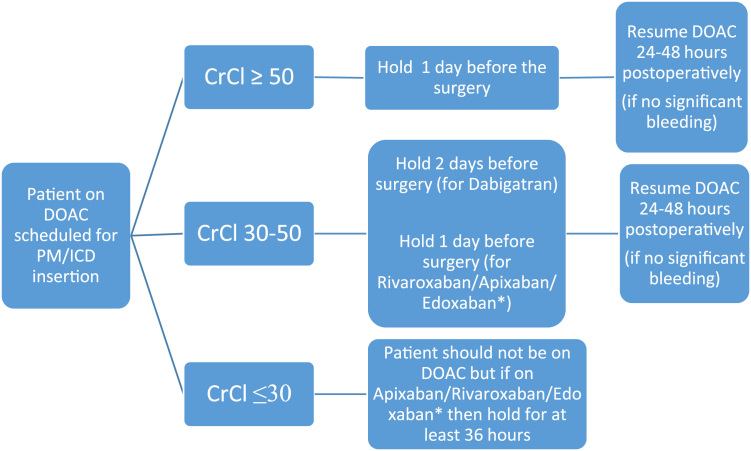
Fig. 2.
Peri-device surgery DOAC management [39], [42]. A strategy of continued DOAC for patients at high risk of arterial thromboembolism is currently under investigation [43] .* Based on pharmacokinetics but no recommendation in guidelines if CrCl <80.
Dabigatran is a twice daily drug with a half-life of 14 h, if the CrCl is above 50 mL/min. Its use should be halted the day before surgery, which translates to missing 2 doses before the day of surgery. This usually results in a 12–25% residual anticoagulant effect at the time of surgery, which is acceptable given the low risk of bleeding [25], [27], [45]. If the CrCl is between 30 and 50 mL/min, signifying moderate renal impairment and a likely half-life of 15–18 h, then dabigatran use should be halted 2 days before surgery, i.e., 4 missed doses to give the same residual anticoagulant effect. Dabigatran should not be used in patients with a CrCl of less than 30 mL/min. The drug should then be resumed 24 h postoperatively [25], [34], [45], [47], [48]. An observational study showed that continued dabigatran treatment did not result in increased bleeding; only one episode of minor bleeding occurred and that was in a patient on dual antiplatelet therapy [49]. This finding has been replicated in other studies [50]; thus, the results of the randomized control trial [46] are awaited before final recommendations can be made regarding continuation of DOACs.
Rivaroxaban is a once daily drug with a half-life of 9 h if the CrCl is above 30 mL/min. Its use should halted the day before surgery, which translates to missing one dose before the day of surgery and results in a 12–25% residual anticoagulant effect at the time of surgery, which is acceptable given the low risk of bleeding [27], [47], [48]. Again, rivaroxaban should not be used in patients with a CrCl less than 30 mL/min, but if used in patients with a CrCl between 15 and 30 mL/min, at least 36 h of drug interruption is advised [25]. Use of the drug should then be resumed 24–48 h postoperatively [34], [45]. Apixaban is similar to rivaroxaban in its mechanism of action and half-life of 9 h if CrCl is above 30 mL/min [27], [45]. Therefore, use of the drug may be halted the day before surgery and resumed 24–48 h postoperatively, if renal function is >30 mL/min [34], [45], [47], [48]. If apixaban is used in patients with a CrCl between 15 and 30 mL/min then at least 36 h of drug interruption is advised [25]. Edoxaban is a once daily drug with a half-life of 10–14 h if the CrCl is above 30 mL/min [32]. However, there is less guidance available regarding edoxaban and the current recommendation is for use of the drug to be halted the day before surgery and resumed 24–48 h postoperatively, if renal function is >80 mL/min [25], [32]. Given the pharmacokinetics of edoxaban, these recommendations will most likely extend to a CrCl of greater than 30, but guidance is currently lacking.
5. Anticoagulation reversal
Anticoagulation may require elective (as described above) or emergent reversal. Vitamin K may be administered, which substantially reduces INR within 24 h; the rapidity of INR decrease depends on the route of administration and the dose [51], [52]. Oral administration is preferred if there is no need for very rapid reversal because it is effective, safe, convenient, and predictable [52], [53]. Doses required may range from 1 mg to 10 mg, keeping in mind that although the INR will decrease faster and to lower levels with higher doses, returning to therapeutic INR will be delayed and more difficult due to warfarin resistance, which may last up to a week [51], [52]. Intravenous administration is the fastest route and lowers the INR within 12 h. If rapid reversal is required, then this is easily achieved using prothrombin complex concentrate (PCC), which is the current preferred method of rapid reversal of warfarin and its use is preferable to fresh frozen plasma [52].
To date, there is no reversal agent for DOACs, although they are currently in development and may soon be available for clinical use [54]. First, administration of the drug should be halted and an investigation into the cause of bleeding carried out with treatment if indicated [55]. Neither vitamin K, fresh frozen plasma, nor prothrombin complex concentrate fully reverse the anticoagulant effects of DOACs, but they are used in clinical practice because of the lack of an alternative [27]. Dabigatran may be cleared relatively efficiently by dialysis [56]. Despite the absence of reversal agents, in comparative studies DOACS (except dabigatran at the higher dose) had lesser rates of bleeding than did warfarin [28], [29], [30]. In addition, DOACs are cleared reliably and quickly, and by the time a bleed occurs, their levels may already be waning [27]. There are several blood tests that when positive indicate the presence of DOACs in the blood and when normal do not infer their absence. For dabigatran, the thrombin time is a very sensitive indicator of the drug׳s presence in the blood. In addition, the PTT may be prolonged if dabigatran is present. For rivaroxaban, a prolonged PT/INR may indicate the presence of the drug. Currently, there is no reliable method to detect the presence of apixaban or edoxaban in coagulation studies. Anti-Xa levels may be used to detect the presence of the Xa inhibitors, apixaban, rivaroxaban, or edoxaban, in the blood [55]. Non-specific blood thinners, such as DDAVP and tranexamic acid, should be used because even though their efficacy is not proven, they cause little harm and may add benefit [57]. Reversal agents the focus of ongoing trials that show promise, and they may soon be available in clinical practice. Idarucizumab is a specific reversal agent for dabigatran and a recent phase III trial showed rapid and durable complete reversal [58]. Andexanet alpha is a reversal agent for factor Xa inhibitors, such as apixaban, rivaroxaban, or edoxaban, and phase two studies of this compound have also shown rapid complete reversal [59]. Aripazine is a reversal agent that targets all DOACs and has shown complete reversal [54].
6. Management of antiplatelet drugs during pacemaker/implantable cardioverter defibrillator insertion
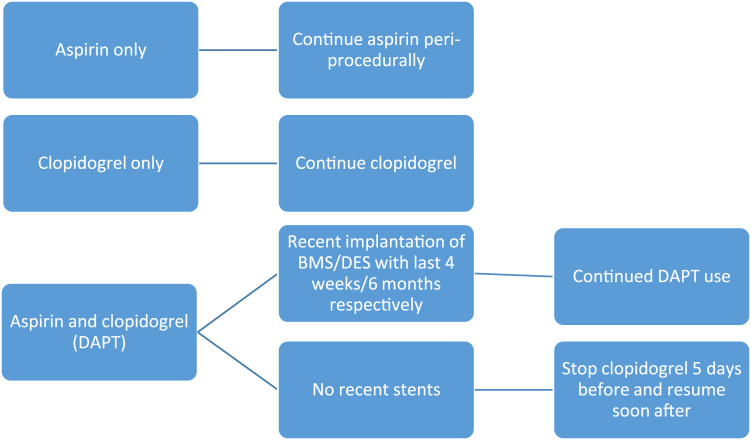
Antiplatelet drugs are commonly used in patients who require PM/ICD insertion. Aspirin and clopidogrel are the most widely used antiplatelet drugs. A study by Tomkins et al. in patients who underwent CIED surgery showed that the risk of developing a pocket hematoma doubled when using aspirin alone, as compared to no antiplatelet medication, and was four-fold higher when comparing dual antiplatelet therapy (DAPT) to no antiplatelet [60]. A smaller study showed that approximately 18% of patients on clopidogrel developed a hematoma after PM/ICD insertion, but none of those who had clopidogrel treatment halted held for 4 days or more developed a hematoma. The risk further increased if aspirin and clopidogrel treatment were combined. Other studies have shown that clopidogrel is associated with a hematoma formation rate of 25%, compared to just 4% when its use is discontinued prior to the procedure. In addition, there was no significant difference in hematoma formation if aspirin was continued or discontinued [37]. The main necessity for dual-antiplatelet therapy arises post-insertion of coronary stents to decrease the risk of stent thrombosis and after acute coronary syndrome. The PARIS study was a two-year prospective observational study that enrolled 5018 patients to examine the effect of DAPT cessation after PCI on major adverse cardiovascular events (MACE), which is a composite of cardiac death, definite or probable stent thrombosis, myocardial infarction, or target-lesion revascularization. The results of this study demonstrated that 30-days, post-stent insertion, DAPT could be safely interrupted and reinitiated 14 days post procedure with no increase in MACE, including no increase in stent thrombosis [61]. However, there is still no clear consensus regarding DAPT management. An easy-to-use summary is as follows (Fig. 3): if the patient is only taking aspirin, then aspirin can be safely continued. If the patient is only on clopidogrel, then continue clopidogrel. For patients on DAPT, though the Paris study demonstrated that DAPT can be safely interrupted after 30 days, more evidence would be needed in the form of a randomized controlled trial before such a recommendation can be made. Given current evidence, the following is recommended: continue DAPT if a bare-metal stent (BMS) was inserted in the last 4 weeks and if a drug-eluting stent (DES) was implanted in the last 6 months. If more than 4 weeks/6 months have elapsed after BMS/DES implantation, respectively, then clopidogrel can be halted 5–7 days prior to the procedure and resumed as soon as possible after, while continuing aspirin [25], [61], [62]. Current guidelines also suggest deferring elective device implantation, if possible, until the completion of DAPT. Recommendations from recent guidelines are summarized in Table 1. There is no reliable way to reverse the effects of antiplatelet medications, other than administering platelet transfusions, and the efficacy of this approach remains under debate [63].
Fig. 3.
Peri-device surgery management of antiplatelets [39].
Table 1.
Peri-procedural (PM/ICD) management of antiplatelets—guideline recommendations.
| ACCP 2012a[30] | ACC/AHA 2014a[60] | CCS 2012a[61] | ESC/ESA 2014a, b[62] | EHRA 2015a[39] | |
|---|---|---|---|---|---|
| Aspirin | Continue aspirin given low-risk procedure | Continue aspirin given low-risk procedure | N/A | Continue aspirin given low-risk procedure | Continue aspirin |
| DAPT- BMS | Continue DAPT if <6 weeks post-insertion | Continue DAPT if <30 days post-insertion | Minimum of 1-month duration of DAPT | Minimum of 1-month duration of DAPT | Minimum of 1-month duration of DAPT |
| DAPT- DES | Continue DAPT if <6 months post-insertion | Continue DAPT if <12 months post-insertion | Minimum 3-month duration of DAPT | Minimum 6-month duration of DAPT | Minimum 6-month duration of DAPT (3 months if new-generation DES) |
Abbreviations: ACCP, American College of Chest Physicians; ACC/AHA, American College of Cardiology/American Heart Association; CCS, Canadian Cardiovascular Society; ESC/ESA, European Society of Cardiology; DAPT, Dual Antiplatelet Therapy; BMS, Bare Metal Stent; DES, Drug Eluting Stent; N/A, Not Available.
All guidelines recommend a delay in procedure if possible.
ESC/ESA recommends at least 12 months of DAPT after ACS.
7. Conclusion
Antithrombotic therapy and its peri-procedural management continue to be a rapidly evolving area. There is now clear evidence that continuing warfarin treatment during CIED surgery insertion is safe, without a significant increased rate of bleeding and with continued prevention of thromboembolism. For patients at high thromboembolic risk, continued warfarin therapy should be favored over heparin bridging, which increases bleeding, device pocket hematoma, and health care costs. Presently, the use of DOACs is commonly halted prior to insertion, but new evidence is eagerly awaited to evaluate the risk to benefit ratio of their continuation peri-operatively. The predictability and speed of their waning effect is an advantage and the forthcoming availability of specific- and complete-reversal agents may relieve concerns about the management of potential bleeding complications. Combination antithrombotic therapy at the time of CIED should be avoided when possible (e.g. in the absence of recent stent), with temporary interruption of antiplatelet agents for patients on continued OAC.
Funding
Dr. Essebag is the recipient of a Clinician Scientist award from the Canadian Institutes of Health Research (CIHR).
Conflict of interest
All authors declare no conflict of interest related to this study.
References
- 1.Lip G.Y. Stroke in atrial fibrillation: epidemiology and thromboprophylaxis. J Thromb Haemost. 2011;9(Suppl 1):344–351. doi: 10.1111/j.1538-7836.2011.04302.x. [DOI] [PubMed] [Google Scholar]
- 2.Falk R.H. Atrial fibrillation. N Engl J Med. 2001;344:1067–1078. doi: 10.1056/NEJM200104053441407. [DOI] [PubMed] [Google Scholar]
- 3.Wipf J.E., Lipsky B.A. Atrial fibrillation. Thromboembolic risk and indications for anticoagulation. Arch Int Med. 1990;150:1598–1603. doi: 10.1001/archinte.150.8.1598. [DOI] [PubMed] [Google Scholar]
- 4.Menke J., Luthje L., Kastrup A. Thromboembolism in atrial fibrillation. Am J Cardiol. 2010;105:502–510. doi: 10.1016/j.amjcard.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 5.European Heart Rhythm A, European Association for Cardio-Thoracic S, Camm A.J. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg B.A., Piccini J.P. Anticoagulation in atrial fibrillation. Br Med J. 2014;348:g2116. doi: 10.1136/bmj.g2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds M.W., Fahrbach K., Hauch O. Warfarin anticoagulation and outcomes in patients with atrial fibrillation: a systematic review and metaanalysis. Chest. 2004;126:1938–1945. doi: 10.1378/chest.126.6.1938. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez C., Blanchard D.G. Atrial fibrillation: diagnosis and treatment. Am Fam Phys. 2011;83:61–68. [PubMed] [Google Scholar]
- 9.Schneider D.J., Sobel B.E. Conundrums in the combined use of anticoagulants and antiplatelet drugs. Circulation. 2007;116:305–315. doi: 10.1161/CIRCULATIONAHA.106.655910. [DOI] [PubMed] [Google Scholar]
- 10.Birnie D.H., Healey J.S., Wells G.A. Pacemaker or defibrillator surgery without interruption of anticoagulation. New Engl J Med. 2013;368:2084–2093. doi: 10.1056/NEJMoa1302946. [DOI] [PubMed] [Google Scholar]
- 11.Samardhi H., Santos M., Denman R. Current management of atrial fibrillation. Aust Prescr. 2011;34:100–104. [Google Scholar]
- 12.Mallouppas M., Vassiliou V. Anticoagulation for atrial fibrillation: Is this the end of warfarin? Not just yet. J Angiol. 2013;2013:1–7. [Google Scholar]
- 13.January C.T., Wann L.S., Alpert J.S. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64:2246–2280. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Rietbrock S., Heeley E., Plumb J. Chronic atrial fibrillation: Incidence, prevalence, and prediction of stroke using the Congestive heart failure, hypertension, age >75, diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J. 2008;156:57–64. doi: 10.1016/j.ahj.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Gage B.F., Waterman A.D., Shannon W. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. J Am Med Assoc. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 16.Gage B.F., van Walraven C., Pearce L. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292. doi: 10.1161/01.CIR.0000145172.55640.93. [DOI] [PubMed] [Google Scholar]
- 17.Lip G.Y., Nieuwlaat R., Pisters R. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 18.Olesen J.B., Lip G.Y., Hansen M.L. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. Br Med J. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pisters R., Lane D.A., Nieuwlaat R. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 20.Holford N.H. Clinical pharmacokinetics and pharmacodynamics of warfarin. Understanding the dose-effect relationship. Clin Pharmacokin. 1986;11:483–504. doi: 10.2165/00003088-198611060-00005. [DOI] [PubMed] [Google Scholar]
- 21.Mant J., Hobbs F.D., Fletcher K. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 22.Wysowski D.K., Nourjah P. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Int Med. 2007;167:1414–1419. doi: 10.1001/archinte.167.13.1414. [DOI] [PubMed] [Google Scholar]
- 23.Molteni M., Cimminiello C. Warfarin and atrial fibrillation: from ideal to real the warfarin affaire. Thromb J. 2014;12:5. doi: 10.1186/1477-9560-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia D.A., Regan S., Crowther M. The risk of hemorrhage among patients with warfarin-associated coagulopathy. J Am Coll Cardiol. 2006;47:804–808. doi: 10.1016/j.jacc.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 25.Sticherling C., Marin F., Birnie D. Antithrombotic management in patients undergoing electrophysiological procedures: a European Heart Rhythm Association (EHRA) position document endorsed by the ESC Working Group Thrombosis, Heart Rhythm Society (HRS), and Asia Pacific Heart Rhythm Society (APHRS) Europace. 2015;17:1197–1214. doi: 10.1093/europace/euv190. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa S., Aonuma K., Tse H.-F. The APHRS׳s 2013 statement on antithrombotic therapy of patients with nonvalvular atrial fibrillation. J Arrhythm. 2013;29:190–200. [Google Scholar]
- 27.Harder S., Graff J. Novel oral anticoagulants: clinical pharmacology, indications and practical considerations. Eur J Clin Pharm. 2013;69:1617–1633. doi: 10.1007/s00228-013-1510-z. [DOI] [PubMed] [Google Scholar]
- 28.Connolly S.J., Ezekowitz M.D., Yusuf S. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 29.Patel M.R., Mahaffey K.W., Garg J. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 30.Granger C.B., Alexander J.H., McMurray J.J. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 31.Giugliano R.P., Ruff C.T., Braunwald E. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 32.Heidbuchel H., Verhamme P., Alings M. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17:1467–1507. doi: 10.1093/europace/euv309. [DOI] [PubMed] [Google Scholar]
- 33.Sardar P., Chatterjee S., Chaudhari S. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc. 2014;62:857–864. doi: 10.1111/jgs.12799. [DOI] [PubMed] [Google Scholar]
- 34.Douketis J.D., Spyropoulos A.C., Spencer F.A. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S–e350S. doi: 10.1378/chest.11-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallego P., Apostolakis S., Lip G.Y. Bridging evidence-based practice and practice-based evidence in periprocedural anticoagulation. Circulation. 2012;126:1573–1576. doi: 10.1161/CIRCULATIONAHA.112.135681. [DOI] [PubMed] [Google Scholar]
- 36.Siegal D., Yudin J., Kaatz S. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126:1630–1639. doi: 10.1161/CIRCULATIONAHA.112.105221. [DOI] [PubMed] [Google Scholar]
- 37.Bernard M.L., Shotwell M., Nietert P.J. Meta-analysis of bleeding complications associated with cardiac rhythm device implantation. Circ Arrhythm Electrophysiol. 2012;5:468–474. doi: 10.1161/CIRCEP.111.969105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proietti R., Porto I., Levi M. Risk of pocket hematoma in patients on chronic anticoagulation with warfarin undergoing electrophysiological device implantation: a comparison of different peri-operative management strategies. Eur Rev Med Pharm Sci. 2015;19:1461–1479. [PubMed] [Google Scholar]
- 39.Poole J.E., Gleva M.J., Mela T. Complication rates associated with pacemaker or implantable cardioverter–defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122:1553–1561. doi: 10.1161/CIRCULATIONAHA.110.976076. [DOI] [PubMed] [Google Scholar]
- 40.Douketis J.D., Spyropoulos A.C., Kaatz S. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823–833. doi: 10.1056/NEJMoa1501035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sant׳anna R.T., Leiria T.L., Nascimento T. Meta-analysis of continuous oral anticoagulants versus heparin bridging in patients undergoing CIED surgery: reappraisal after the BRUISE study. Pacing Clin Electrophysiol. 2015;38:417–423. doi: 10.1111/pace.12557. [DOI] [PubMed] [Google Scholar]
- 42.Coyle D., Coyle K., Essebag V. Cost effectiveness of continued-warfarin versus heparin-bridging therapy during pacemaker and defibrillator surgery. J Am Coll Cardiol. 2015;65:957–959. doi: 10.1016/j.jacc.2014.11.060. [DOI] [PubMed] [Google Scholar]
- 43.Spinler S.A., Shafir V. New oral anticoagulants for atrial fibrillation. Circulation. 2012;126:133–137. doi: 10.1161/CIRCULATIONAHA.112.099283. [DOI] [PubMed] [Google Scholar]
- 44.Nascimento T., Birnie D.H., Healey J.S. Managing novel oral anticoagulants in patients with atrial fibrillation undergoing device surgery: Canadian survey. Can J Cardiol. 2014;30:231–236. doi: 10.1016/j.cjca.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 45.Gladstone D.J., Geerts W.H., Douketis J. How to monitor patients receiving direct oral anticoagulants for stroke prevention in atrial fibrillation: a practice tool endorsed by thrombosis Canada, the Canadian Stroke Consortium, the Canadian Cardiovascular Pharmacists Network, and the Canadian Cardiovascular Society. Ann Int Med. 2015;163:382–385. doi: 10.7326/M15-0143. [DOI] [PubMed] [Google Scholar]
- 46.Ottawa Heart Institute Research Corporation. Strategy of Continued Versus Interrupted Novel Oral Anti-coagulant at Time of Device Surgery in Patients With Moderate to High Risk of Arterial Thromboembolic Events. Bethesda (MD): National Library of Medicine (US) 2000 (cited 2015 Jul 18). (Available from: 〈〈http://clinicaltrials.gov/show/NCT01675076〉〉 NLM Identifier: NCT01675076).
- 47.Levy J.H., Faraoni D., Spring J.L. Managing new oral anticoagulants in the perioperative and intensive care unit setting. Anesthesiology. 2013;118:1466–1474. doi: 10.1097/ALN.0b013e318289bcba. [DOI] [PubMed] [Google Scholar]
- 48.Birnie D.H., Healey J.S., Essebag V. Management of anticoagulation around pacemaker and defibrillator surgery. Circulation. 2014;129:2062–2065. doi: 10.1161/CIRCULATIONAHA.113.006027. [DOI] [PubMed] [Google Scholar]
- 49.Jennings J.M., Robichaux R., McElderry H.T. Cardiovascular implantable electronic device implantation with uninterrupted dabigatran: comparison to uninterrupted warfarin. J Cardiovasc Electrophysiol. 2013;24:1125–1129. doi: 10.1111/jce.12214. [DOI] [PubMed] [Google Scholar]
- 50.Rowley C.P., Bernard M.L., Brabham W.W. Safety of continuous anticoagulation with dabigatran during implantation of cardiac rhythm devices. Am J Cardiol. 2013;111:1165–1168. doi: 10.1016/j.amjcard.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 51.Garcia D.A., Crowther M.A. Reversal of warfarin: case-based practice recommendations. Circulation. 2012;125:2944–2947. doi: 10.1161/CIRCULATIONAHA.111.081489. [DOI] [PubMed] [Google Scholar]
- 52.Woods K., Douketis J.D., Kathirgamanathan K. Low-dose oral vitamin K to normalize the international normalized ratio prior to surgery in patients who require temporary interruption of warfarin. J Thromb Thrombolysis. 2007;24:93–97. doi: 10.1007/s11239-007-0022-z. [DOI] [PubMed] [Google Scholar]
- 53.Crowther M.A., Douketis J.D., Schnurr T. Oral vitamin K lowers the international normalized ratio more rapidly than subcutaneous vitamin K in the treatment of warfarin-associated coagulopathy. A randomized, controlled trial. Ann Intern Med. 2002;137:251–254. doi: 10.7326/0003-4819-137-4-200208200-00009. [DOI] [PubMed] [Google Scholar]
- 54.Lauw M.N., Coppens M., Eikelboom J.W. Recent advances in antidotes for direct oral anticoagulants: their arrival is imminent. Can J Cardiol. 2014;30:381–384. doi: 10.1016/j.cjca.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Siegal D.M., Crowther M.A. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. 2013;34:489b–498b. doi: 10.1093/eurheartj/ehs408. [DOI] [PubMed] [Google Scholar]
- 56.Singh T., Maw T.T., Henry B.L. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol. 2013;8:1533–1539. doi: 10.2215/CJN.01570213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crowther M.A., Warkentin T.E. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood. 2008;111:4871–4879. doi: 10.1182/blood-2007-10-120543. [DOI] [PubMed] [Google Scholar]
- 58.Pollack C.V., Jr., Reilly P.A., Eikelboom J. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373:511–520. doi: 10.1056/NEJMoa1502000. [DOI] [PubMed] [Google Scholar]
- 59.Siegal D.M., Curnutte J.T., Connolly S.J. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015 doi: 10.1056/NEJMoa1510991. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 60.Tompkins C., Cheng A., Dalal D. Dual antiplatelet therapy and heparin "bridging" significantly increase the risk of bleeding complications after pacemaker or implantable cardioverter–defibrillator device implantation. J Am Coll Cardiol. 2010;55:2376–2382. doi: 10.1016/j.jacc.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 61.Mehran R., Baber U., Steg P.G. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382:1714–1722. doi: 10.1016/S0140-6736(13)61720-1. [DOI] [PubMed] [Google Scholar]
- 62.Korantzopoulos P., Letsas K.P., Liu T. Anticoagulation and antiplatelet therapy in implantation of electrophysiological devices. Europace. 2011;13:1669–1680. doi: 10.1093/europace/eur210. [DOI] [PubMed] [Google Scholar]
- 63.Godier A., Taylor G., Gaussem P. Inefficacy of platelet transfusion to reverse ticagrelor. N Engl J Med. 2015;372(196):7. doi: 10.1056/NEJMc1409373. [DOI] [PubMed] [Google Scholar]