Abstract
Aim
To compare clinical presentation, operative management and short- and long-term outcomes of congenital bile duct cysts (BDC) in adults with children.
Methods
Retrospective multi-institutional Association Francaise de Chirurgie study of Todani types I+IVB and IVA BDC.
Results
During the 37-year period to 2011, 33 centers included 314 patients (98 children; 216 adults). The adult population included more high-risk patients, with more active, more frequent prior treatment (47.7% vs 11.2%; p < 0.0001), more complicated presentation (50.5% vs 35.7%; p = 0.015), more synchronous biliary cancer (11.6% vs 0%; p = 0.0118) and more major surgery (23.6% vs 2%; p < 0.0001), but this latter feature was only true for type I+IVB BDC. Compared to children, the postoperative morbidity (48.1% vs 20.4%; p < 0.0001), the need for repeat procedures and the status at follow-up were worse in adults (27% vs 8.8%; p = 0.0009). However, severe postoperative morbidity and fair or poor status at follow-up were not statistically different for type IVA BDC, irrespective of patients' age. Synchronous cancer, prior HBP surgery and Todani type IVA BDC were independent predictive factors of poor or fair long-term outcome.
Conclusion
BDC is a more indolent disease in children compared to adults, except for Todani type IV-A BDC.
Introduction
In contrast to Asian countries, congenital bile duct cysts (BDC) are rare in the United States and Europe.1, 2, 3, 4, 5, 6, 7 This might explain the lack of recent publication of large multicenter or retrospective nationwide series outside Asia. The disease is most frequently discovered in childhood, but 20% of patients are diagnosed in adult life.1 The proportion of presence of bile duct cyst in adults has probably been underestimated. In USA, Europe and India, an increasing number of adult patients have been reported. This trend might reflect an institutional referral bias or the lack of attention to the disease in regions with a low prevalence, but might also possibly be due to improved accuracy of non-invasive imaging studies.8, 9, 10 Only six single-center Western studies with greater than 30 patients have compared results between adult and pediatric populations.2, 3, 4, 5, 6, 7 These series showed that complications at presentation (such as cholangitis, pancreatitis and bile duct cancer) or following surgery were significantly more frequent in adults than in children. However, these comparisons were not made according to the BDC subtype of the classical Todani classification.2, 3, 5, 11 Furthermore, worse short and long-term outcomes were also reported in Todani type IVA BDC with intrahepatic disease involvement.12, 13, 14, 15 There is little available information in the Western literature about the management and long-term results in adult and pediatric populations relating to BDC type I or IVA in the Todani classification. The aim of the present study was to compare clinical presentation, operative management, and short- and long-term outcome in adults and children patients according to the BDC subtype in the Todani classification in a large retrospective multicenter European study.
Methods
Study population and patients' data collection
This multicenter retrospective study was conducted by members of the Association Française de Chirurgie (AFC). Data from patient medical records were included in an online database (http://www.chirurgie-viscerale.org) using a standardized questionnaire. Between 1974 and 2011, 33 centers (including 24 academic centers) from six different European countries included 314 patients (250 females and 64 males; 216 adults and 98 children) operated with Todani type I, IVB and IVA BDC. Recorded data included patient demographics, past history of surgical and/or endoscopic interventions for hepato-biliary and pancreatic (HBP) disease, clinical symptoms (prior to initial surgery), laboratory and imaging studies, details of surgical procedures, pathological data, duration of follow-up, and short- and long-term postoperative outcome. Additional data were obtained from e-mail or phone exchanges with the referral centers. To ensure homogeneous disease classification and to exclude doubtful cases (such as intrahepatic stones secondary to biliary strictures), operative and pathology reports as well as imaging studies were systematically reviewed by the three senior authors (JYM, RK, and JFG). Accordingly, from an initial 600 patients, 95 were excluded, leaving 505 patients with confirmed BDC.
A pediatric patient was defined as being <15 years of age. Patients' operative risk was evaluated by using the American Society of Anesthesiology physical status score (ASA).16
Complicated clinical presentation was defined by the presence of cholangitis or pancreatitis, portal hypertension, biliary peritonitis or HBP carcinoma. Postoperative morbidity and mortality were defined at three months or during hospital stay. Postoperative morbidity was graded according to Dindo-Clavien classification17 and grade III and IV complications were considered as severe morbidity.
505 BDC subtypes were classified according to the Todani classification11 based on imaging studies. These subtypes included type I in 47.3% (n = 239), type II in 3.8% (n = 19), type III in 2.6% (n = 13), type IVA in 14.5% (n = 73), type IVB in 1.2% (n = 6) and type V in 30.7% (n = 155). Due to the similarity of extra-hepatic disease involvement in both subtypes, Todani type I and type IVB were grouped together. Conversely, Todani subtypes II, III and V were excluded from the present study, leaving 318 patients with only extra-hepatic disease (Type I and IVB BDC) and a combined extra- and intrahepatic disease (type IVA BDC) to enable comparison between adult and pediatric populations. Disease involvement of the main biliary convergence (MBC) was classified according to the classification reported previously.18 From these 318 patients, 314 were operated and represented the final cohort of patients of the present study. Surgery was not performed in three patients of the adult group due to carcinomatosis (n = 1, a type IVa BDC patient who died 6 months later) and refusal of intervention (n = 2). In the children group, one patient was considered to be too young for surgery (<3 months). Major surgical procedures included hepatectomy and/or pancreatectomy and/or cancer resection.
Long-term outcome after 3 months was evaluated according to the dedicated clinical evaluation score reported by the Mayo Clinic:6 excellent if the patient remained free of symptoms without further re-intervention; good if the patient had occasional and mild episodes of cholangitis or pancreatitis not impairing the quality of life; fair if the patient had repeated episodes of cholangitis or pancreatitis, or had portal hypertension without further re-intervention; poor if the patient required later biliary or liver-related surgical procedures, developed biliary cirrhosis or complications because of portal hypertension, or died from cyst-related malignancy or liver and biliary-related complications.
Statistical analysis
Comparison between adults and children was performed using the chi-squared test (or the Fisher's exact test when conditions for the chi-square test were not fulfilled) for categorical variables and using the Student t-test (or the Mann–Whitney nonparametric rank sum test in case of non-normality) for continuous variables. Variables significant at the 0.15 level in univariate analysis were introduced in a multivariate logistic regression. A Kaplan–Meier analysis was used to estimate the postoperative survival rate. The log-rank test was used to compare adults and children. Statistical analysis was performed using SAS® version 9.2 (SAS Institute Inc, Cary, North Carolina, USA). A p value <0.05 was considered statistically significant.
Results
Adult and pediatric demographic and imaging data are presented in Table 1, and Fig. 1. Data regarding prior intervention, clinical presentation and coexistent HBP diseases are presented in Table 2. Details of surgical procedures and short-term and long-term postoperative outcome are presented in Table 3. There were significantly more female (p = 0.0354) and high-risk patients in adults (p = 0.0055).
Table 1.
Comparison between adults and children of patients' and disease' characteristics according to BDC subtype in the Todani classification
| Whole series |
p value |
Type I-IVB BDC |
p value |
Type IVA BDC |
p value |
||||
|---|---|---|---|---|---|---|---|---|---|
| Children n = 98 | Adults n = 216 | Children n = 79 | Adults n = 164 | Children n = 19 | Adults n = 52 | ||||
| Median age (years) | 3 [0–15] | 41.5 [16–81] | <0.0001 | 3 [0–15] | 43 [16–81] | <0.0001 | 2.7 [0–15] | 36.9 [16–72] | <0.0001 |
| Sex ratio (F/M) | 2.6 | 4.8 | 0.0354 | 3.43 | 5.07 | 0.0230 | 3.75 | 4.1 | 1.0000 |
| Ethnical origin | |||||||||
| • Caucasian | 74 (75.5%) | 178 (82.4%) | 0.2325 | 60 (75.9%) | 136 (82.9%) | 0.4946 | 14 (73.7%) | 42 (80.8%) | 0.2386 |
| • West and south African | 7 (7.1%) | 5 (2.3%) | 6 (7.6%) | 5 (3%) | 1 (5.3%) | 0 | |||
| • North African | 9 (9.2%) | 13 (6.0%) | 6 (7.6%) | 10 (6.1%) | 3 (15.8%) | 3 (5.8%) | |||
| • Asian | 4 (4.1%) | 9 (4.2%) | 4 (5.1%) | 6 (3.7%) | 0 | 3 (5.8%) | |||
| • Others | 4 (4.1%) | 11 (5.1%) | 3 (3.8%) | 7 (4.3% | 1 (5.3%) | 4 (7.7%) | |||
| ASA score | |||||||||
| • 1 or 2 | 98 (100%) | 201 (93.1%) | 0.0039 | 79 (100%) | 153 (93.3%) | 0.0181 | 19 (100%) | 48 (92.3%) | 0.5678 |
| • ≥3 | 0 | 15 (6.9%) | 0 | 11 (6.7%) | 0 | 4 (7.7%) | |||
| Todani BDC types | |||||||||
| • Type I | 78 (79.6%) | 159 (73.6%) | 0.2537 | – | – | – | – | – | – |
| • Subtype Ia | 50 (64.1%) | 67 (42.1%) | 0.0112 | 50 (64.1% | 67 (42.1%) | 0.0112 | – | – | – |
| • Subtype Ib | 6 (7.7%) | 22 (13.8%) | 6 (7.7%) | 22 (13.8%) | – | – | – | ||
| • Subtype Ic | 22 (28.2%) | 67 (42.1%) | 22 (28.2%) | 67 (42.1%) | – | – | – | ||
| • Not reported | 0 | 3 (1.9%) | 0 | 3 (1.9%) | – | – | – | ||
| • Type IVB | 1 (1%) | 5 (2.3%) | 0.3577 | 1 (1.2%) | 5 (3%) | 0.6669 | – | – | – |
| • Type IVA | 19 (19.4%) | 52 (24.1%) | 0.6696 | – | – | – | – | – | – |
| Main biliary convergence (MBC) adequately analyzed | 83 (84.6%) | 183 (84.7%) | 0.3541 | 68 (86.1%) | 140 (85.3%) | 1.0000 | 15 (78.9%) | 43 (82.7%) | 0.7362 |
| Cyst Involvement of MBC | 23/83 (27.7%) | 63/183 (34.4%) | 0.2780 | 12/68 (17.6%) | 40/140 (28.6%) | 0.0879 | 11/15 (73.3%) | 23/43 (53.5%) | 0.1790 |
| • MBC-1 | 20 | 41 | 0.932 | 11 | 31 | 0.1540 | 9 | 10 | 0.0440 |
| • MBC-2 | 3 | 22 | 1 | 9 | 2 | 13 | |||
| Presence of pancreatobiliary malunion (PBM) adequately explored | 71 (72.4%) | 170 (78.7%) | 0.7568 | 56 (70.8%) | 134 (81.7%) | 0.0038 | 15 (78.9%) | 35 (67.3%) | 0.3948 |
| Anomalous PBM | 64/71 (90.1%) | 119/170 (70%) | 0.0008 | 51 (91%) | 98 (73.1%) | 0.0063 | 13 (86.6%) | 21 (48.8%) | 0.0597 |
Abbreviations: ASA score, The American Society of Anesthesiology physical status score.
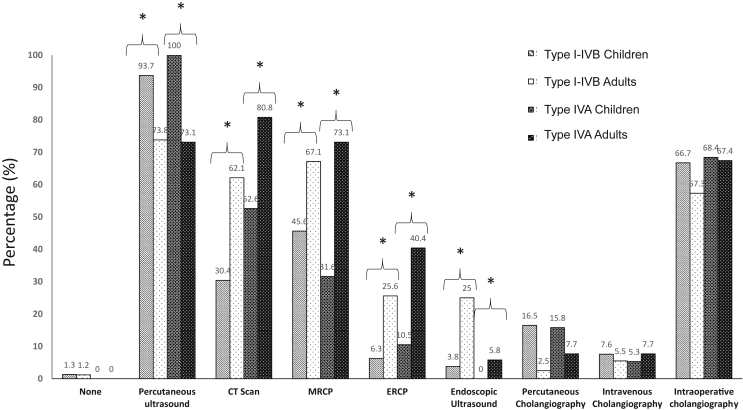
Figure 1.
Comparison between adults and children of Imaging according to BDC subtype in the Todani classification (MRCP: Magnetic resonace cholangio-pancreatography; ERCP: Endoscopic retrograde cholangio-pancreatography)
Table 2.
Comparison between adults and children of previous surgical and/or endoscopic procedures, clinical presentation, coexistent hepatobiliary and pancreatic (HBP) disease according to BDC Todani subtype (please refer to Supplementary Table 2 for full results)
| Whole series |
p value |
Type I+IVB BDC |
p value |
Type IVA BDC |
p value |
||||
|---|---|---|---|---|---|---|---|---|---|
| Children n = 98 | Adults n = 216 | Children n = 79 | Adults n = 164 | Children n = 19 | Adults n = 52 | ||||
| Prior surgical and/or radiological and/or endoscopic procedures | |||||||||
| • Prior biliary tract surgery and/or endoscopic procedures | 11 (11.2%) | 103 (47.7%) | <0.0001 | 10 (12.7%) | 72 (43.9%) | <0.0001 | 1 (5.3%) | 31 (59.6%) | <0.0001 |
| • Surgery alone | 4 (4.1%) | 62 (28.7%) | <0.0001 | 4 (5.1%) | 45 (27.4%) | <0.0001 | 0 | 17 (32.7%) | 0.0035 |
| • Cyst-enterostomy | 0 | 22 (10.2%) | 0.0011 | 0 | 16 (9.8%) | 0.0041 | 0 | 6 (11.5%) | 0.1824 |
| Clinical presentation | |||||||||
| • No symptom | 8 (8.2%) | 33 (15.3%) | 0.0803 | 6 (7.6%) | 27 (16.5%) | 0.0587 | 2 (10.5%) | 6 (11.5%) | 1.0000 |
| • Isolated pain | 47 (48%) | 86 (39.8%) | 0.1760 | 36 (45.6%) | 70 (42.7%) | 0.6708 | 11 (57.9%) | 16 (30.8%) | 0.0371 |
| • Cholangitis | 17 (17.3%) | 78 (36.1%) | 0.0008 | 15 (19.0%) | 49 (29.9%) | 0.0710 | 2 (10.5%) | 29 (55.8%) | 0.0007 |
| • Pancreatitis | 25 (25.5%) | 38 (17.6%) | 01045 | 18 (22.8%) | 33 (20.1%) | 0.6330 | 7 (36.8%) | 5 (9.6%) | 0.0120 |
| • Abdominal mass | 30 (30.6%) | 3 (1.4%) | <0.0001 | 22 (27.8%) | 2 (1.2%) | <0.0001 | 8 (42.1%) | 1 (1.9%) | <0.0001 |
| • Jaundice | 42 (42.9%) | 46 (21.3%) | <0.0001 | 34 (43.0%) | 31 (18.9%) | <0.0001 | 8 (42.1%) | 15 (28.8%) | 0.2906 |
| • Complicated presentation | 35 (35.7%) | 109 (50.5%) | 0.0151 | 27 (34.2%) | 77 (47.0%) | 0.0594 | 8 (42.1%) | 32 (61.5%) | 0.1438 |
| Coexistent hepatobiliary and pancreatic (HBP) diseases | |||||||||
| • Liver disease | 2(2%) | 23 (10.6%) | 0.0090 | 2 (2.5%) | 8 (4.9%) | 0.5061 | 0 | 15 (28.8%) | 0.0072 |
| • Biliary disease | 14 (14.3%) | 77 (35.6%) | 0.0001 | 12 (15.2%) | 49 (29.8%) | 0.0134 | 2 (10.5%) | 28 (53.4%) | 0.0011 |
| ○ stones | 13 (13.3%) | 51 (23.6%) | 0.0350 | 11 (13.9%) | 30 (18.3%) | 0.3943 | 2 (10.5%) | 21 (40.4%) | 0.0173 |
| ○ Synchronous cancer | 0 | 25 (11.6%) | 0.0118 | 0 | 19 (11.6%) | 0.0016 | 0 | 6 (11.5%) | 0.1777 |
| • Pancreatic disease | 5 (5.1%) | 15 (6.9%) | 0.5356 | 4 (5.1%) | 10 (6.1%) | 1.0000 | 1 (5.3%) | 5 (9.6%) | 1.0000 |
Table 3.
Comparison between adults and children of short- and long-term outcome and preoperative treatment and types of surgery according to BDC Todani subtype (please refer to Supplementary Table 3 for full results)
| Whole series |
p value |
Type I+VB BDC |
p value |
Type IVA BDC |
p value |
||||
|---|---|---|---|---|---|---|---|---|---|
| Children n = 98 | Adults n = 216 | Children n = 79 | Adults n = 164 | Children n = 19 | Adults n = 52 | ||||
| TYPES OF SURGICAL PROCEDURES | |||||||||
| ○ Incomplete cyst excision | 10 (10.2%) | 29/209 (13.8%) | 0.4630 | 4 (5.0%) | 21/158 (13.3%) | 0.0702 | 6 (31.6%) | 8/51 (15.7%) | 0.1393 |
| ASSOCIATED PROCEDURES | |||||||||
| ○ Hepatectomy | 1 (1%)* | 28 (12.9%) | 0.0002 | 0 | 6 (3.7%) | 0.4327 | 1 (5.2%) | 22***(42.3%) | 0.0031 |
| ○ Pancreatoduodenectomy | 1 (4%) | 12 (5.5%) | 0.0707 | 0 | 8 (4.9%) | 0.0564 | 1 (5.3%) | 4 (7.7%) | 1.0000 |
| ○ Cancer excision | 0 | 23 (10.6%) | 0.0002 | 0 | 18 (10.9%) $ | 0.0003 | 0 | 5 (9.6%) | 0.329 |
| • Combined resection° | 2 (2%) | 51 (23.6%) | <0.0001 | 0 | 25(15%) | <0.0001 | 2 (10%) | 26 (50%) | 0.0509 |
| EARLY POSTOPERATIVE OUTCOME | |||||||||
| • Mortality rate (%) | 0 | 1 (0.5%) | 1.0000 | 0 | 1 (0.6%) | 1.0000 | 0 | 0 | 1.0000 |
| • Complications rate (%) | 20 (20.4%) | 104 (48.1%) | <0.0001 | 15 (19%) | 72 (43.9%) | 0.0001 | 5 (26.3%) | 32 (61.5%) | 0.0085 |
| o Grade III-IV | 4 (4.1%) | 49 (22.7%) | <0.0001 | 2 (2.5%) | 33 (20.1%) | 0.0003 | 2 (10.5%) | 16 (30.7%) | 0.1238 |
| LONG-TERM OUTCOME | n=80 (82%) | n=178 (82.4%) | n=66 (83.5%) | n=132 (80.5%) | n=14 (74%) | n=46 (88.5%) | |||
| • Median follow-up (months) | 42 (4-732) | 36 (4-292) | 0.0619 | 39.5 (4-372) | 36.5 (4-292) | 0.2060 | 45 (8-351) | 24.5 (4-228) | 0.1592 |
| • Mayo clinic score | |||||||||
| ○ Excellent + Good | 73 (91.2%) | 130 (73%) | 0.0009 | 61 (92.4%) | 102 (77.3%) | 0.0084 | 12 (85.7%) | 27 (58.7%) | 0.1080 |
| ○ Fair + Poor | 7 (8.8%) | 48 (27%) | 5 (7.6%) | 30 (22.7%) | 2 (14.3%) | 19 (41.3%) | |||
| • Repeat procedures | 5/78 | 39/161 | <0.0001 | 3/64 | 25/118 | 0.0066 | 2/14 | 14/43 | 0.3347 |
*One total hepatectomy for transplantation in a child.
**Two total hepatectomy for transplantation in adults.
***Two patients underwent biliary stone extraction.
$ A secondary central hepatectomy was performed after good response of chemotherapy.
The imaging studies used to diagnose BDC differed between the two groups. More non-invasive studies were performed in children, including mostly percutaneous ultrasound (US) (93 of 98 children versus 159 of 216 adults; p < 0.0001) as well as percutaneous transhepatic cholangiography (16/98 children and 8/216 adults; p < 0.0001) in the early period of the study. Significantly more contrast imaging studies and endoscopic techniques were performed in adults. Magnetic resonance (MR)-cholangiography was increasingly used over time both in adults and in children. In three patients, occult BDC was discovered during other abdominal surgery and no preoperative imaging study was thus performed in these patients (Fig. 1).
There was no difference between the Todani subtypes of BDC between adults and children. However, in Todani type I, there were significantly more subtype Ia (cystic subtype) in children and more subtype Ib (cylindrical subtype) in adults. There was no difference between the two groups regarding main biliary convergence (MBC) disease involvement while an anomalous pancreato-biliary malunion (PBM) was significantly more frequently detected in children. Similar observations were made in children for the type I+IVB but not for type IVA.
Prior surgical and/or endoscopic biliary procedures (Table 2)
Prior surgical and/or endoscopic and/or radiological biliary procedures were significantly more frequently performed in adults both in BDC types I+IVB and IVA. Adult patients underwent significantly more cholecystectomy and cyst-enterostomy. However, regarding cyst-enterostomy, the difference was significant in BDC type I+IVB for adults compared to children but not for BDC type IVA. There was significantly more incomplete BDC resection in adults.
Clinical presentation (Table 2)
Forty-one patients (13.1%) were asymptomatic at the time of diagnosis and the median delay between disease first symptoms and diagnosis was three months, being significantly longer in adults. Pain was the most common presenting symptom and children presented more often with an abdominal mass and jaundice. Adults presented more often with cholangitis, weight loss and a complicated presentation. The median delay between first symptom and diagnosis was significantly longer in adults in both subtypes I+IVB and IVA BDC. Similarly, abdominal mass was more frequently encountered in children in both BDC subtypes. However, in subtype IVA BDC, there was more pain and cholangitis but less pancreatitis in adults.
Coexistent hepato-biliary and pancreatic (HBP) diseases (Table 2)
Both liver and biliary disease was significantly more frequent in adults compared to children. Coexistent biliary stones were more common in adults in the whole group of patients but only in BDC subtype IVA. Pancreatic disease rate was similar in adults and children. Synchronous biliary cancer was not encountered in children.
In covariate analysis, there was a significant correlation between occurrence of cancer and prior history of cyst-enterostomy (p = 0.0002). There was a significantly greater rate of synchronous cancer in adult patients with previous cyst-enterostomy (6 out of 22; 27%) compared to patients without previous cyst-enterostomy (19 out of 194; 9.7%) (p = 0.0270).
Preoperative treatment and surgery (Table 3)
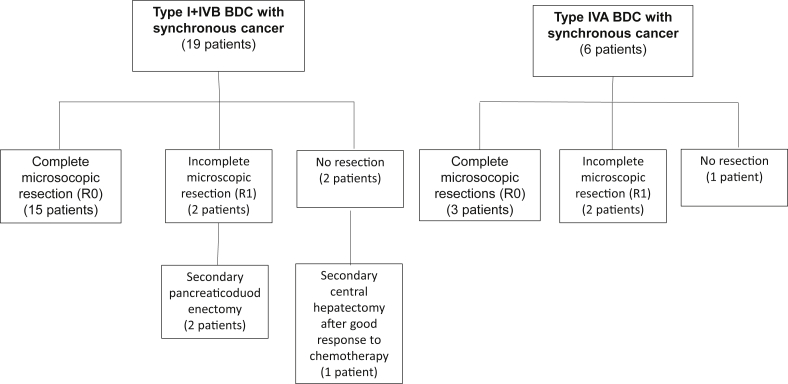
Preoperative biliary drainage was performed in 29 patients, 25 of whom were adults. All patients underwent surgical exploration. However, seven adult patients did not undergo BDC resection because of locally advanced gallbladder or intrahepatic cancer in two patients, peritoneal carcinomatosis in another and severe inflammation of hepatic pedicle in two patients. Cholecystectomy alone was performed in one patient and intervention on the gallbladder was not known in the remaining patient. Repeat surgery was more frequent in adults. Concomitant cyst excision and hepatectomy was performed more frequently in adults but only in Todani type IVA BDC (Table 3). Bile duct stone extraction was required more often in adults. A significant difference was also observed for type I+IVb BDC between adults and children but not for type IVA BDC. Bile duct anastomosis was performed more frequently on the main biliary convergence (MBC) in adults in total and in subtype I+IVB BDC. Major surgical procedures were more frequent in adults compared to children group for type I+IVB BDC. Management and postoperative morbidity and mortality of BDC complicated by synchronous cancer was detailed in Fig. 2 and Table 4.
Figure 2.
Flow chart of management of patients suffering from bile duct cysts with synchronous cancer
Table 4.
Presentation of synchronous cancer according to Todani subtype of bile duct cyst (BDC)
| Type I+IVb BDC | Type IVA BDC | p value | |
|---|---|---|---|
| Patients with cancer | 19 | 6 | 0.8072 |
| Median age (years) | 47 (22–70) | 51 (30–67) | 0.8622 |
| Previous cyst enterostomy | n = 5 | n = 1 | 1.0000 |
| Location of cancer | 0.8915 | ||
| • Gallbladder | n = 7 | n = 0 | |
| • Extrahepatic bile duct | n = 5 | n = 3 | |
| • Intrahepatic bile duct | n = 0 | n = 3 | |
| • BDC | n = 6 | n = 0 | |
| • Pancreas | n = 1 | n = 0 | |
| Presence of pancreatobiliary malunion | 0.9796 | ||
| • Not explored | n = 6 | n = 3 | |
| • Abnormal | n = 10 | n = 1 | |
| • Normal | n = 3 | n = 2 | |
| Type of surgery | 0.5996 | ||
| • No resection (carcinomatosis) | n = 1 | n = 1 | |
| • BDC resection | n = 7 | n = 0 | |
| • BDC resection + hepatectomy | n = 6a | n = 3 | |
| • BDC resection + pancreaticoduodenectomy | n = 5 |
n = 2b |
|
| Mortality and Morbidity | 0.8859 | ||
| • Grade 1 | n = 3 | n = 1 | |
| • Grade 2 | n = 2 | n = 1 | |
| • Grade 3 | n = 4 | n = 1 | |
| • Grade 4 | n = 0 | n = 1 | |
| • Grade 5 (death) | n = 1 | n = 0 | |
| Staging of tumour (UICC)c | – | 0.9144 | |
| • Stage 0 | n = 2 | n = 1 | |
| • Stage IA | n = 3 | n = 1 | |
| • Stage IB | n = 5 | n = 1 | |
| • Stage IIA | n = 4 | n = 1 | |
| • Stage IIB | n = 3 | n = 1 | |
| • Stage III | n = 0 | n = 0 | |
| • Stage IV | n = 2 | n = 1 | |
| Median follow-up time (months) (range) | 12.5 (3–34) | 12 (6–88) | 1.0000 |
| Status at final follow-up | 0.7472 | ||
| • Alive without complications | n = 9 | n = 3 | |
| • Alive with complications | n = 1 | n = 1 | |
| • Alive with cancer recurrence | n = 1 | n = 0 | |
| • Death related to cancer | n = 6 | n = 2 | |
| • Death related to disease complications | n = 1 | n = 0 | |
| • Death not related to disease complications | n = 0 | n = 0 |
One patient underwent central hepatectomy 6 months after chemotherapy.
One patient underwent pancreatectomy+hepatectomy.
UICC staging (www.uicc.org) 7eme edition distal extrahepatic duct BDC bile duct cyst.
Postoperative mortality and morbidity (Table 3)
Only one adult patient died postoperatively. Median postoperative hospital stay, overall morbidity and severe complications rates was greater in adults. Biliary and pancreatic complication rates were greater in adults. The median hospital stay and overall complications rate were significantly greater in adults for type I+IVB and type IVA. However, severe morbidity rate (Dindo-Clavien grade III and IV) was significantly greater in adults only for types I+IVB BDC. The postoperative morbidity rate in patients without major surgical procedures was greater in adults compared to children regardless of BDC Todani subtype and type IVA BDC.
Long-term outcome (Table 3)
Two hundred fifty-eight patients (82%) were followed-up for more than 3 months, during a median duration of 36 months (range: 4–372 months). Patient status at follow-up was worse in adults (Table 3). When comparison between BDC subtypes was made (Table 3), worse status at follow-up was observed in adults for subtype I+IVB BDC while for subtype IVA BDC long-term outcome was not significantly different between adults and children. Only one adult patient suffering from subtype IVA BDC with MBC disease involvement presented with metachronous intrahepatic cholangiocarcinoma, 70 months after primary BDC resection and 57 months after revision of an anastomotic stricture. Chemotherapy was initiated for metastatic disease but the patient died a few months after diagnosis.15 Previous cyst-enterostomy signified a worse long-term outcome (Table 5).
Table 5.
Comparison of long-term outcome of adult patients suffering from bile duct cyst according to previous cyst-enterostomy (CE)
| Patients with previous CE | Patients without previous CE | p value | |
|---|---|---|---|
| Patients | 19 | 159 | |
| Median follow-up (months) (range) | 36 (12–175) | 36 (4–292) | 0.9283 |
| Mayo clinic score | |||
| • Excellent | 7 (36.8%) | 104 (65.4%) | 0.0077 |
| • Good | 2 (10.5%) | 16 (10%) | |
| • Fair | 0 | 9 (5.6%) | |
| • Poor | 10 (52.6%) | 30 (18.8%) | |
| Mayo clinic score | |||
| • Excellent + Good | 9 (47.4%) | 120 (75.5%) | 0.0143 |
| • Fair + Poor | 10 (52.6%) | 39 (24.5%) | |
Univariate and multivariate statistical analysis of poor long-term outcome according to Mayo clinical score (Table 6)
Table 6.
Predictive factors of fair or poor long-term outcome according to the Mayo clinical score in the whole series of 258 patients (including cancer patients) suffering from Bile Duct Cyst (BDC)
| Covariate | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Odd Ratio | 95% confidence interval | p value | Odd Ratio | 95% confidence interval | p-value | |
| • Adults (≥15y), Adults vs Children | 3.960 | 1.706–9.192 | 0.0014 | 1.859 | 0.739–4.679 | 0.1878 |
| • Todani BDC subtypes, Type IV-A versus Type I +IV-B | 2.508 | 1.317–4.775 | 0.0051 | 2.075 | 1.025–4.199 | 0.0425 |
| • Prior hepatobiliary surgery, Yes vs No | 3.486 | 1.888–6.435 | <0.0001 | 2.640 | 1.327–5.252 | 0.0057 |
| • Prior cyst-enterostomy, Yes vs No | 4.662 | 1.792–12.129 | 0.0016 | |||
| • Cholangitis, Yes vs No | 2.152 | 1.172–3.949 | 0.0134 | |||
| • Pancreatitis, Yes vs No | 3.620 | 1.241–10.559 | 0.0185 | 2.895 | 0.911–9.200 | 0.0715 |
| • Coexistent biliary disease, Yes vs No | 2.368 | 1.291–4.346 | 0.0054 | |||
| • Coexistent biliary stones, Yes vs No | 2.351 | 1.075–5.138 | 0.0322 | |||
| • Coexistent synchronous cancer, Yes vs No | 8.909 | 3.171–25.032 | <0.0001 | 6.354 | 2.148–18.798 | 0.0008 |
| • MBC involvement, Yes vs No | 2.391 | 1.23–4.651 | 0.0102 | |||
| • Repeat treatment, Yes vs No | 3.920 | 1.212–12.673 | 0.0255 | |||
| • Post-operative complications, Yes vs No | 2.145 | 1.178–3.907 | 0.0126 | |||
Univariate analysis in the 258 patients identified 12 predictive features of fair or poor long-term outcome; adult patients, subtype IVA BDC, prior HBP surgery, cyst-enterostomy, cholangitis and pancreatitis at initial clinical presentation, coexistent biliary disease, stones and synchronous cancer, MBC disease involvement, repeat treatment and occurrence of post-operative complications. Multivariate analysis identified only coexistent synchronous cancer, prior HBP surgery, Todani type IVA BDC as independent predictive factors of fair or poor long-term outcome. Independent predictive factors of synchronous cancer included previous cyst-enterostomy (Odd ratio: 5.3 [1.583–17.746]; p < 0.0001), patient age (OR: 1.047 [1.021–1.073]; p < 0.0001), and weight loss at the time of diagnosis (OR: 12.683 [3.604–44.616]; p < 0.0001).
Survival
The overall 1 and 3 year survival rate of adult patients (97% and 91%, respectively) was significantly lower than in children (both 100%, p = 0.0238) after exclusion of cancer patients, the overall survival at 1 and 3 years for adult patients (98.6% and 98.6%, respectively) was not different from children. Similar results were observed for Todani types I+IVB BDC. In contrast, for type IVA BDC, the overall survival at 1 and 3 years was similar between adults and children with (95% and 92% vs both 100%, p = 0.6016) or without inclusion of cancer patients (97% and 97% vs 100% and 100%, respectively, p = 0.5385). Finally, the overall survival rate for Todani type I+IVB and IVA BDC at 1 and 5 years was significantly worse in type IVA BDC (100% and 83% vs 100% and 100%) (p = 0.0339) but there was no significant difference after exclusion of cancer patients (p = 0.5385).
Discussion
The present series represents the largest multicenter series on BDC from European centers with the aim of comparing presentation, treatment and outcome between adult and pediatric populations as well as BDC subtype based on the Todani classification. A previous small study has already been reported under the auspices of the Association Française de Chirurgie but the results did not compare outcomes specifically for adults and children.9 Several comparative studies have been reported according to age population but there were retrospective with small patient numbers.2, 3, 4, 5, 6, 7, 19, 20, 21, 22 The recent large Western multi-institutional comparative study reported by Soares et al.23 mostly included American surgical departments with very few European centers of which none (except one for 20 patients) participated in the present study.
The present study has to be critically analyzed for its strengths and weaknesses. First, it should emphasized that there is an obvious selection bias related to the predominant participation of HBP centers treating only adult patients, with only 3 centers managing both pediatric and adult patients. For this reason, children represent only one third of the study population. Furthermore, 73% of the participating centers were highly-specialized academic HBP teams representing 97.5% of patient inclusion. This series thus reports expert centers' experience with BDC, a feature that might explain the low reported mortality rate. Additionally, a selection bias of recruitment may also explain the overrepresentation of rare type V BDC.
Potential study weakness include the retrospective design, the long time span of diagnostic and treatment strategies, the substantial number of centers having included patients and the number of patients without available long-term follow-up. The comparison between adults and children for subtype BDC type IVA should also be considered with caution due to the small number of pediatric patients suffering from such a subtype. Strengths include the large number of patients and the central revision of diagnostic criteria, disease classification and details of surgical treatment by three senior experts. Additionally, Todani types II, III, and V BDC were excluded due to their lower incidence, different clinical presentation and surgical approach, and to achieve homogeneous disease comparison between adults and children.
Jaundice, abdominal mass, pancreatitis and pain symptoms were the most common presenting symptoms in children while in contrast, complicated clinical presentation (cholangitis, biliary lithiasis) was predominant in adults.2, 3, 4, 5, 6, 7, 15, 19, 20, 21, 22, 23 Our stratification according to BDC subtype demonstrated that cholangitis and pancreatitis were more often associated to subtype IVA BDC. In studies comparing clinical presentation between subtype I and IVA BDC, an increased rate of cholangitis has already been reported in the latter group.7, 12, 24, 25 Additionally, the rate of previous HPB surgical and/or endoscopic procedures was significantly greater in adults whatever the BDC subtype in the Todani classification.2, 3, 4, 5, 6, 7, 19, 20, 21, 22 However stratification according to BDC subtype shows in the present series that cyst-enterostomy was more often associated to subtype I+IVB BDC. Treatment was also reported to be different with significantly more pancreatic and hepatic resections in adult patients.15, 23 This was confirmed in the present study with also more hepatectomies performed for subtype IVA BDC. Such an aggressive surgical approach is clearly related to a greater rate of coexistent biliary disease (especially intrahepatic lithiasis), sometimes complicated by liver atrophy or abscess26 and by coexistent synchronous cancer in adults. Zheng and colleagues have recently reported that hepatectomy brings better long-term outcome than extrahepatic cyst resection for adult patients suffering from Todani subtype IVA BDC.15 The low mortality rate encountered in the present study in both adults and children is in line with that reported in the literature.2, 3, 5, 19, 21 The significant mortality (6.9% in adults vs 3% in children) reported in the multi-institutional series of Soares et al. is surprising and might be related to inclusion bias of end-stage BDC disease and transplanted patients. However, the morbidity rate related to BDC resection remains significant, despite issued from expert HBP surgical departments, being increased in adults compared to children (range: 6–41% vs 0–16%, respectively)2, 3, 5, 19, 21, 23 and significantly greater in two studies.5, 23 Biliary complications represents the most common complications.2, 3, 19, 21, 23 According to BDC subtypes stratification, the present study found that severe morbidity and pancreatic complications rates were significantly increased in adults only for subtype I+IVB BDC. On the other hand, Lal et al.24 reported a significantly greater morbidity rate for subtype IV BDC (17% versus 3%, p = 0.01) when comparing postoperative morbidity of subtypes I and IV BDC.
Several comparative retrospective studies reported a greater incidence of long-term complications in adults (32.8%) than in children (13%).2, 21, 27 The present series confirmed a higher rate of repeat procedures and worse clinical outcome according the Mayo clinic clinical scoring system in adult patients. Most of the long-term complications (stenosis and stones) with consecutive cholangitis are reported for patients with type IVA BDC of the Todani classification.12, 13, 14 However subgroup analysis in the present study confirmed that worse outcome results were encountered in subtype IVA BDC in the whole series whatever age stratification (data not shown) but that when BDC subtype and age stratification are combined, worse outcome results exist only for subtype I+IVB in the adult population. In other words, the results of subtype IVA are similar independent of age stratification. It might be considered that subtype IVA BDC represent a more complex disease form. However, these results might be influenced by the small number of subtype IVA in children. Previous cyst-enterostomy clearly had a worse impact on the long-term outcome of patients. More complex surgical procedures were used for patients with a previous cyst-enterostomy, mainly due to an increased need for pancreaticoduodenectomy especially for type I+IVB BDC and hepatectomy for type IVa BDC. Such extensive procedures were related in half of these patients to the presence of coexistent synchronous carcinoma, necessitating tumor resection with a wide tumor-free margin.
As already reported,23, 28 survival is mostly impacted by the presence of synchronous cancer at the time of surgery. This was confirmed in our series with a better survival in children for subtype I+IVB and for the whole series when cancer patients are included while survival was not affected by cancer in subtype IVA BDC. However, the short median time of follow-up may have influenced these results. Interestingly, some disease features (BDC subtype IVA), coexistent cancer and prior HBP surgery were identified by multivariate analysis in the present study as independent predictive factors of poor or fair outcome.
Conclusion
BDC in children is associated with a less complicated presentation, less coexistent HPB disease and synchronous cancer rate, and better short and long-term outcome following resection. Type IVA BDC represents a more aggressive disease, a feature illustrated by the lack of difference regarding early and late outcome between adults and children.
Conflicts of interest
None declared.
Authors contributions
Concept and design of the study: OUAÏSSI Mehdi, KIANMANESH Reza, MABRUT Jean-Yves, GIGOT Jean-François.
Statistical analysis: ROUX Adeline.
Acquisition of data: Barbara WIDHABER, NUZZO Gennaro, DUBOIS Remi, REVILLON Yann, CHERQUI Daniel, AZOULAY Daniel, LETOUBLON Christian, PRUVOT François-René.
Data analysis: OUAÏSSI Mehdi, KIANMANESH Reza, MABRUT Jean-Yves, GIGOT Jean-François, RAGOT Emilia, BELGHITI Jacques.
Drafting of the manuscript: OUAÏSSI Mehdi, GIGOT Jean-François.
Supervision of the manuscript: OUAÏSSI Mehdi, KIANMANESH Reza, MABRUT Jean-Yves, GIGOT Jean-François.
Acknowledgements
The AFC Working Group: Jean De Ville de Goyet1, Catherine Hubert1, Jan Lerut1, Jean-Bernard Otte1, Raymond Reding1, Olivier Farges2, Alain Sauvanet2, Oulhaci Wassila3, Pietro Majno3, Felice Giulante4, Francesco Ardito4, Maria De rose Agostino4, Thomas Gelas5, Pierre-Yves Mure5, Jacques Baulieux6, Christian Gouillat6, Christian Ducerf6, Sabine Irtan7, Sabine Sarnacki7, Alexis Laurent8, Philippe Compagnon8, Chady Salloum8, Roger Lebeau9, Olivier Risse9, Stéphanie Truant10, Emmanuel Boleslawski10, François Corfiotti10, Patrick Rat11, Alexandre Doussot11, Pablo Ortega-Deballon11, François Paye12, Pierre Balladur12, Mustapha Adham13, Christian Partensky13, Taore Alhassane13, Karim Boudjema14, Catelin Tiuca Dane14, Yves-Patrice Le Treut15, Mathieu Rinaudo15, Jean Hardwigsen15, Hélène Martelli16, Frédéric Gauthier16, Sophie Branchereau16, Simon Msika17, Daniel Sommacale18, Jean-Pierre Palot 18, Ahmet Ayav19, Charles-Alexandre Laurain19, Massimo Falconi20, Denis Castaing21, Oriana Ciacio21, René Adam21, Eric Vibert21, Jean-Marc Régimbeau22, Roberto Troisi23, Aude Vanlander23, Stéphane Geiss24, Gilles De Taffin24, Denis Collet25, Antonio Sa Cunha25, Laurent Duguet26, Bouzid Chafik27, Kamal Bentabak27, Abdelaziz Graba27, Nicolas Meurisse28, Jacques Pirenne28, Serena Langelle29, Nermin Halkic30, Nicolas Demartines30, Alessandra Cristaudi30, Gaëtan Molle31, Baudouin Mansvelt31, Massimo Saviano32, Gelmini Roberta32, Ousema Baraket33, Samy Bouchoucha33, Bernard Sastre34.
From: 1Cliniques Universitaires Saint- Luc, Brussels, Belgium, 2Beaujon Hospital, Clichy, France, 3Geneva University Hospital, Geneva, Switzerland, 4Gemelli University Hospital, Roma, Italy, 5Mother and Children Hospital, Lyon, France, 6La Croix-Rousse Hospital, Lyon, France, 7Necker Hospital, Paris, France, 8Henri Mondor Hospital, Creteil, France, 9Michallon Hospital, Grenoble, France, 10Claude Huriez Hospital, Lille, France, 11Dijon University Hospital, Dijon, France, 12Saint Antoine Hospital, Paris, France, 13Edouard-Herriot Hospital, Lyon, France, 14Rennes University Hospital, Rennes, France, 15Conception Hospital, Marseille, France, 16Bicetre Hospital, Paris, France, 17Louis Mourier Hospital, Colombes, France, 18Robert Debré Hospital, Reims, France, 19Nancy University Hospital, Nancy, France, 20Negrar University Hospital, Verona, Italy, 21Paul-Brousse Hospital, Paris, France, 22Amiens University Hospital, Amiens, France, 23Ghent University Hospital, Ghent, Belgium, 24Le Parc Hospital, Colmar, France, 25Bordeaux University Hospital, Bordeaux, France, 26Sainte Camille Hospital, Bry-sur-Marne, France, 27Pierre et Marie Curie Hospital, Alger, Algeria, 28UZ Leuven University Hospital, Leuven, Belgium, 29Mauriziano University Hospital, Torino, Italy, 30Vaudois University Hospital, Lausanne, Switzerland, 31Jolimont Hospital, La Louvière, Belgium, 32Modena University Hospital, Modena, Italy, 33Habib Boughefta Hospital, Bizertz, Tunisia, 34La Timone Hospital, Marseille, France.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.hpb.2016.04.005.
Contributor Information
Jean-François Gigot, Email: jfgigot@hotmail.com.
French Surgical Association:
Jean De Ville de Goyet, Catherine Hubert, Jan Lerut, Jean-Bernard Otte, Raymond Reding, Olivier Farges, Alain Sauvanet, Oulhaci Wassila, Barbara Wildhaber, Felice Giulante, Francesco Ardito, Maria De Rose Agostino, Thomas Gelas, Pierre-Yves Mure, Jacques Baulieux, Christian Gouillat, Christian Ducerf, Sabine Irtan, Sabine Sarnacki, Alexis Laurent, Philippe Compagnon, Chady Salloum, Roger Lebeau, Olivier Risse, Stéphanie Truant, Emmanuel Boleslawski, François Corfiotti, Patrick Rat, Alexandre Doussot, Pablo Ortega-Deballon, François Paye, Pierre Balladur, Mustapha Adham, Christian Partensky, Taore Alhassane, Karim Boudjema, Catelin Tiuca Dane, Yves-Patrice Le Treut, Mathieu Rinaudo, Jean Hardwigsen, Hélène Martelli, Frédéric Gauthier, Sophie Branchereau, Simon Msika, Daniel Sommacale, Jean-Pierre Palot, Ahmet Ayav, Charles-Alexandre Laurain, Massimo Falconi, Denis Castaing, Oriana Ciacio, René Adam, Eric Vibert, Roberto Troisi, Aude Vanlander, Stéphane Geiss, Gilles De Taffin, Denis Collet, Antonio Sa Cunha, Laurent Duguet, Bouzid Chafik, Kamal Bentabak, Abdelaziz Graba, Nicolas Meurisse, Jacques Pirenne, Lorenzo Capussotti, Serena Langelle, Nermin Halkic, Nicolas Demartines, Alessandra Cristaudi, Gaëtan Molle, Baudouin Mansvelt, Massimo Saviano, Gelmini Roberta, Ousema Baraket, Samy Bouchoucha, and Bernard Sastre
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Soreide K., Korner H., Havnen J., Soreide J.A. Bile duct cysts in adults. Br J Surg. 2004;91:1538–1548. doi: 10.1002/bjs.4815. [DOI] [PubMed] [Google Scholar]
- 2.Nicholl M., Pitt H.A., Wolf P., Cooney J., Kalayoglu M., Shilyansky J. Choledochal cysts in western adults: complexities compared to children. J Gastrointest Surg. 2004;8:245–252. doi: 10.1016/j.gassur.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Lipsett P.A., Pitt H.A., Colombani P.M., Boitnott J.K., Cameron J.L. Choledochal cyst disease. A changing pattern of presentation. Ann Surg. 1994;220:644–652. doi: 10.1097/00000658-199411000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singham J., Schaeffer D., Yoshida E., Scudamore C. Choledochal cysts: analysis of disease pattern and optimal treatment in adult and paediatric patients. HPB Oxf. 2007;9:383–387. doi: 10.1080/13651820701646198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edil B.H., Cameron J.L., Reddy S., Lum Y., Lipsett P.A., Nathan H. Choledochal cyst disease in children and adults: a 30-year single-institution experience. J Am Coll Surg. 2008;206:1000–1005. doi: 10.1016/j.jamcollsurg.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 6.Gigot J.F., Nagorney D.M., Farnell M.B., Moir C., Ilstrup D. Bile duct cysts: a changing spectrum of presentation. J Hep Bil Pancr Surg. 1996;3:405–411. [Google Scholar]
- 7.de Vries J.S., de Vries S., Aronson D.C., Bosman D.K., Rauws E.A., Bosma A. Choledochal cysts: age of presentation, symptoms, and late complications related to Todani's classification. J Pediatr Surg. 2002;37:1568–1573. doi: 10.1053/jpsu.2002.36186. [DOI] [PubMed] [Google Scholar]
- 8.Mabrut J.Y., Bozio G., Hubert C., Gigot J.F. Management of congenital bile duct cysts. Dig Surg. 2010;27:12–18. doi: 10.1159/000268109. [DOI] [PubMed] [Google Scholar]
- 9.Lenriot J.P., Gigot J.F., Segol P., Fagniez P.L., Fingerhut A., Adloff M. Bile duct cysts in adults: a multi-institutional retrospective study. French Associations for Surgical Research. Ann Surg. 1998;228:159–166. doi: 10.1097/00000658-199808000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler K.M., Pitt H.A., Zyromski N.J. Choledochoceles: are they choledochal cysts? Ann Surg. 2010;252:683–690. doi: 10.1097/SLA.0b013e3181f6931f. [DOI] [PubMed] [Google Scholar]
- 11.Todani T., Watanabe Y., Narusue M., Tabuchi K., Okajima K. Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263–269. doi: 10.1016/0002-9610(77)90359-2. [DOI] [PubMed] [Google Scholar]
- 12.Cheng S.P., Yang T.L., Jeng K.S., Liu C.L., Lee J.J., Liu T.P. Choledochal cyst in adults: aetiological considerations to intrahepatic involvement. ANZ J Surg. 2004;74:964–967. doi: 10.1111/j.1445-1433.2004.03221.x. [DOI] [PubMed] [Google Scholar]
- 13.Takeshita N., Ota T., Yamamoto M. Forty-year experience with flow-diversion surgery for patients with congenital choledochal cyst with pancreaticobiliary maljunction at a single institution. Ann Surg. 2011;254:1050–1053. doi: 10.1097/SLA.0b013e3182243550. [DOI] [PubMed] [Google Scholar]
- 14.Cho M.J., Hwang S., Lee Y.J., Kim K.H., Ahn C.S., Moon D.B. Surgical experience of 204 cases of adult choledochal cyst disease over 14 years. World J Surg. 2011;35:1094–1102. doi: 10.1007/s00268-011-1009-7. [DOI] [PubMed] [Google Scholar]
- 15.Zheng X., Gu W., Xia H., Huang X., Liang B., Yang T. Surgical treatment of type IV-A choledochal cyst in a single institution: children vs. adults. J Pediatr Surg. 2014;48:2061–2066. doi: 10.1016/j.jpedsurg.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Owens W.D., Felts J.A., Spitznagel E.L., Jr. ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49:239–243. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mabrut J.Y., Partensky C., Gouillat C., Oussoultzoglou E., Baulieux J., Boillot O. Cystic involvement of the roof of the main biliary convergence in adult patients with congenital bile duct cysts: a difficult surgical challenge. Surgery. 2007;141:187–195. doi: 10.1016/j.surg.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 19.Tsai M.S., Lin W.H., Hsu W.M., Lai H.S., Lee P.H., Chen W.J. Clinicopathological feature and surgical outcome of choledochal cyst in different age groups: the implication of surgical timing. J Gastrointest Surg. 2008;12:2191–2195. doi: 10.1007/s11605-008-0593-9. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhary A., Dhar P., Sachdev A., Kumar N., Vij J.C., Sarin S.K. Choledochal cysts–differences in children and adults. Br J Surg. 1996;83:186–188. [PubMed] [Google Scholar]
- 21.Shah O.J., Shera A.H., Zargar S.A., Shah P., Robbani I., Dhar S. Choledochal cysts in children and adults with contrasting profiles: 11-year experience at a tertiary care center in Kashmir. World J Surg. 2009;33:2403–2411. doi: 10.1007/s00268-009-0184-2. [DOI] [PubMed] [Google Scholar]
- 22.Huang C.S., Huang C.C., Chen D.F. Choledochal cysts: differences between pediatric and adult patients. J Gastrointest Surg. 2010;14:1105–1110. doi: 10.1007/s11605-010-1209-8. [DOI] [PubMed] [Google Scholar]
- 23.Soares K.C., Kim Y., Spolverato G., Maithel S., Bauer T.W., Marques H. Presentation and clinical outcomes of choledochal cysts in children and adults: a multi-institutional analysis. JAMA Surg. 2015;150:577–584. doi: 10.1001/jamasurg.2015.0226. [DOI] [PubMed] [Google Scholar]
- 24.Lal R., Agarwal S., Shivhare R., Kumar A., Sikora S.S., Saxena R. Type IV-A choledochal cysts: a challenge. J Hepatobiliary Pancreat Surg. 2005;12:129–134. doi: 10.1007/s00534-004-0960-1. [DOI] [PubMed] [Google Scholar]
- 25.Woon C.Y., Tan Y.M., Oei C.L., Chung A.Y., Chow P.K., Ooi L.L. Adult choledochal cysts: an audit of surgical management. ANZ J Surg. 2006;76:981–986. doi: 10.1111/j.1445-2197.2006.03915.x. [DOI] [PubMed] [Google Scholar]
- 26.Dong Q., Jiang B., Zhang H., Jiang Z., Lu H., Yang C. Management strategy for congenital choledochal cyst with co-existing intrahepatic dilation and aberrant bile duct as well as other complicated biliary anomalies. Yonsei Med J. 2006;47:826–832. doi: 10.3349/ymj.2006.47.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamataka A., Ohshiro K., Okada Y., Hosoda Y., Fujiwara T., Kohno S. Complications after cyst excision with hepaticoenterostomy for choledochal cysts and their surgical management in children versus adults. J Pediatr Surg. 1997;32:1097–1102. doi: 10.1016/s0022-3468(97)90407-3. [DOI] [PubMed] [Google Scholar]
- 28.Lee S.E., Jang J.Y., Lee Y.J., Choi D.W., Lee W.J., Cho B.H. Choledochal cyst and associated malignant tumors in adults: a multicenter survey in South Korea. Arch Surg. 2011;146:1178–1184. doi: 10.1001/archsurg.2011.243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.