Abstract
Background
Hepatocellular carcinoma (HCC) with main portal vein tumor thrombus (mPVTT) has a poor prognosis even after surgical resection. Whether neoadjuvant radiotherapy improves surgical outcomes is currently unknown. The aim of this study was to compare the survival of patients with resectable HCC and mPVTT who underwent neoadjuvant therapy to those who underwent surgery alone.
Methods
A non-randomized comparative study was performed. For patients in the neoadjuvant radiotherapy group, three-dimensional conformal radiotherapy was administrated with a daily fraction of 300 cGy in 6 consecutive days. Hepatectomy was carried out 4 weeks after completion of irradiation.
Results
95 patients were enrolled into this study. In the neoadjuvant radiotherapy group (n = 45), 12 patients showed gross radiological reduction in extent of PVTT. In 6 patients, the extent of PVTT was reduced to be within the ipsilateral side of the portal vein. When compared with patients who underwent surgery alone (n = 50), neoadjuvant radiotherapy significantly decreased the rates of HCC recurrence and HCC-related death, with hazard ratios of 0.36 (95% CI, 0.19–0.70) and 0.32 (95% CI, 0.18–0.57), respectively.
Conclusion
For patients with HCC with mPVTT, neoadjuvant radiotherapy before partial hepatectomy provided better postoperative survival outcomes than partial hepatectomy alone.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related death in the world.1 It has a great propensity to invade the portal venous system, resulting in portal vein tumor thrombus (PVTT), which is a significant poor prognostic factor.2, 3 Surgical treatment may offer a chance of cure, but the extent and location of PVTT influence post-operative long-term survival outcomes. For patients with HCC and PVTT in segmental, sectoral, and right or left portal veins, post-operative 5-year overall survival (OS) have been reported to range from 10% to 59%.4, 5, 6 Once PVTT invades the main portal vein (mPVTT), post-operative outcomes become poor, with a peri-operative mortality of 0–28%, and a 5-year OS of 0–26.4%.7, 8, 9
Radiotherapy has been reported to “downstage” HCC, making unresectable HCC eligible for surgical resection and untransplantable HCC transplantable.10, 11 However, whether neoadjuvant radiotherapy improves post-operative survival outcomes of patients with HCC with mPVTT is unknown. The aim of this study was to compare the survival outcomes of neoadjuvant radiotherapy before partial hepatectomy with partial hepatectomy alone in a non-randomized study on patients with resectable HCC with mPVTT.
Methods
Study design
The study protocol which conformed to the 1975 Declaration of Helsinki was approved by the Ethics Committee of the Eastern Hepatobiliary Surgery Hospital (Shanghai, China). All participants provided written consent for their data to be used for research. No patients who were enrolled in this study were included in any previous studies or clinical trials.
The study was carried out at the Department of Hepatic Surgery VI, the Eastern Hepatobiliary Surgery Hospital (EHBH). After hospital admission, the patients underwent a routine three-phrase dynamic CT examination. A diagnosis of PVTT was made when there was presence of low-attenuation intraluminal masses that expanded the portal vein, or when there were filling defects in the portal venous system on CT images. The extent of PVTT was classified using the Cheng's classification12, 13: type I, tumor thrombus involving segmental or sectoral branches of the portal vein, or more proximal; type II, involvement of the right or left portal vein; type III, invasion of the main trunk of portal vein (mPVTT); and type IV, involvement of the superior mesenteric vein.
If there were multiple and disseminated intrahepatic nodules (>3) on imagings, patients were not considered for surgical treatment. The liver functional reserve was assessed by the Child-Pugh grading. Liver functional reserves of Child-Pugh class C were considered as a contraindication for surgical treatment. Major hepatectomy, which was defined as resection of 3 or more Couinaud's liver segments, was only carried out on patients with Child-Pugh class A liver function. Selected patients with Child-Pugh class B liver function underwent hepatectomy if the tumor could be removed by minor hepatectomy, which was defined as resection of 2 or fewer Couinaud's liver segments.
The inclusion criteria for this study were: (i) patients aged between 20 and 70 years; (ii) HCC with PVTT as shown on preoperative imagings; (iii) the PVTT had invaded the main portal vein (mPVTT, Cheng's type III); (iv) resectable lesion(s) in the liver with adequate hepatic functional reserve. The exclusion criteria were: (i) patients with a previous history of other cancer; (ii) severe co-existing systemic disease; (iii) Child-Pugh class C liver function; (iv) previous radiotherapy, chemotherapy or other invasive treatments for cancer.
Patients who met the inclusion criteria were interviewed by a multidisciplinary team of clinicians. The pros and cons of the two treatment plans were explained to the patients in detail. If the patients refused surgical treatment, they were excluded from further analysis. The remaining patients were then asked to select to enter either into the neoadjuvant radiotherapy group or the surgery only group. The sole difference between the two groups was determined by the patient's willingness to undergo neoadjuvant radiotherapy. The rest of the treatment was the same for the two groups throughout the study period.
Neoadjuvant radiotherapy
Preoperative radiotherapy was given within 3 days after completion of all preoperative investigations. Three-dimensional conformal radiotherapy was carried out using a 10-mV linear accelerator (Aowo International Technology Development, Shenzhen, China). The irradiation regimen was based on authors' previous unpublished data (in submission). Furthermore, the authors have previously identified (unpublished data) the optimal interval between partial hepatectomy and irradiation to be 4 weeks in order to minimize liver injury, intraoperative blood loss and postoperative liver failure. As a consequence, a daily fraction of 300 cGy was administered in 6 consecutive days for patients in the neoadjuvant radiotherapy group, to deliver a total irradiation dose of 1800 cGy. The dose was prescribed to the 50–80% isodose distribution areas. 3D view techniques were used to minimize the irradiation dose delivered to organs other than the tumor and the PVTT by designing the irradiated volumes and angles. Four weeks after completion of irradiation, the liver function and CT imagings were carried out before surgery. The revised response evaluation criteria in solid tumors (mRECIST) guideline14 was used to evaluate the therapeutic effects of radiotherapy on PVTT and tumor. At least a 30% decrease in the sum of diameters (partial response, PR) was considered as an effective reduction of the extent of PVTT. The PVTT type was also re-evaluated according to the Cheng's classification. If patients developed any contraindications to surgery as described previously, they were treated with transcatheter arterial chemoembolization (TACE), Sorafenib or supportive care.
Surgical procedure
The operation was carried out using a right subcostal incision with a midline extension. The abdominal cavity was then carefully searched for extent of disease, and to rule out any extrahepatic metastases and/or peritoneal seedings. After mobilization of the liver, intraoperative ultrasound was performed to assess the number and size of the lesion(s), the relation of the tumor(s) to vascular structures, as well as the extent of portal vein tumor thrombus. Before transection of liver parenchyma using the anterior approach, the Pringle's maneuver was applied to occlude the blood inflow of the liver using cycles of clamp/unclamp times of 15/5 min. The liver hanging technique was not used as a routine. Liver transection was carried out by a clamp crushing method.
For patients with mPVTT which had protruded into the main portal vein beyond the liver parenchymal transection plane, the thrombus was extracted from the opened stump of the portal vein. Occasionally, if the thrombus could not be extracted because of its size, the main portal vein was clamped proximally to the thrombus. The portal vein was then incised at the bifurcation, and the PVTT was then extracted. After flushing with normal saline and confirming that no residual PVTT remained, the stump was closed with a continuous 4.0 nylon suture. All patients received the same postoperative care by the same team of surgeons and nurses in the intensive care unit during the early postoperative period. Subsequent need for stay in the intensive care unit was determined by the patient's condition. Liver function tests and clotting profiles were monitored.
Follow-up
All patients had postoperative follow-up by the same team of surgeons. The follow-up protocol was the same for all the patients. The patients were followed-up at the outpatient clinic once every month in the first year and then with increasing intervals. At each visit to the clinic, any adverse events were documented and blood tests were taken for blood counts, coagulation profile, and renal and liver functions. Serum alpha-fetoprotein (AFP) and abdominal US were performed once every monthly. Contrast CT scan and chest X-ray were performed once every 3 monthly for surveillance of recurrence. When tumor recurrences or metastases were suspected, further investigations with CT scan, magnetic resonance imaging (MRI), or positron emission tomography-CT scan were performed. Fine needle aspiration/biopsies were performed when necessary. The diagnosis of tumor recurrence was based on histologic/oncologic evidences or by the noninvasive diagnostic criteria for HCC as used by the European Association for the Study of the Liver.15 The study was censored on June 10, 2015.
Patients with intrahepatic recurrence were treated with surgery, local ablative therapy, or systemic therapy, depending on location, size, and number of recurrent tumors, presence or absence of tumor thrombus in the portal vein, liver function status, and any extrahepatic disease. Palliative treatment was given to patients with advanced diseases, poor general status, or poor liver function.
Statistical analysis
Demographics, intra- and post-operative variables and outcomes were collected prospectively. The primary outcome measures included tumor recurrence and overall survival. Secondary outcome measures included liver function changes, procedure-related complications which were graded by Clavien–Dindo Classification16 and hospital mortality which included 30- and 90-day mortalities. The data were analyzed on an intention-to-treat basis.
Continuous data were expressed as median and range. Categorical variables were compared by the Chi-square test or Fisher's exact test, and continuous variables were compared by the Mann–Whitney U test. The Pearson product-moment correlation coefficient was used to analyze the relationship between two variables. Survival analysis was estimated by the Kaplan–Meier survival method. Any statistical comparison of survival distributions was analyzed by the log-rank test. The overall survival (OS) was measured from the date of radiotherapy to the time of death for the neoadjuvant group, and from the date of operation to the time of death for the surgery alone group, respectively. The time to tumor recurrence was measured from the date of operation to the time when recurrent tumor was first diagnosed.
Statistical analyses were performed using univariable tests to test for the differences in variables with regard to survival. Factors that appeared to be significantly (P < 0.1) associated with survival were entered into a Cox. A proportional hazards model to test for significant effects while adjusting for multiple factors simultaneously. A P < 0.05 was considered to be statistically significant.
Results
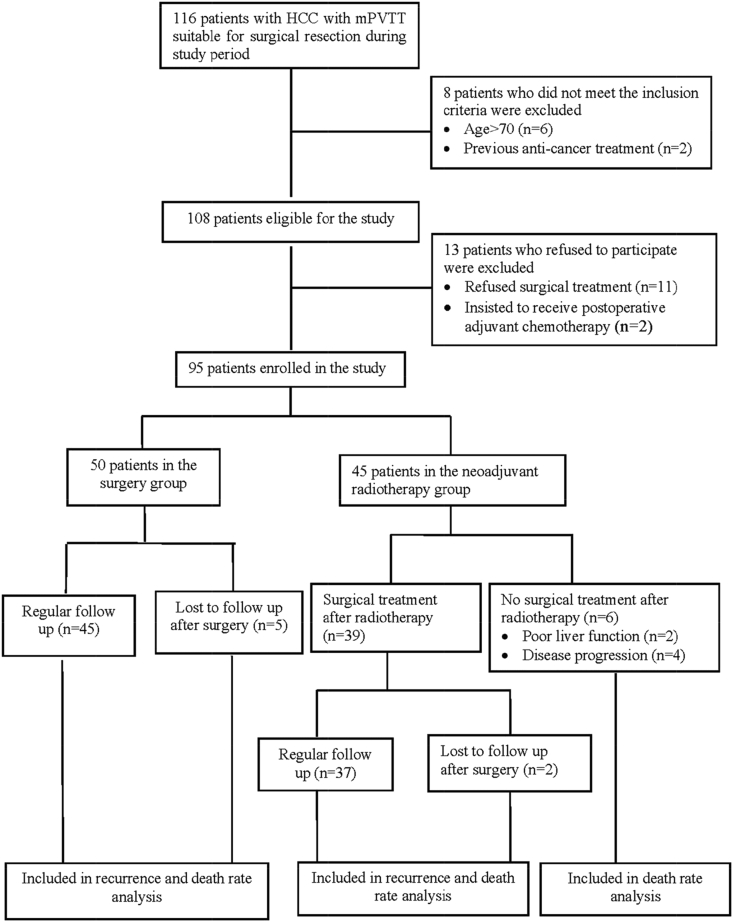
From January 2010 to December 2013, of 116 consecutive patients with HCC with mPVTT eligible for surgical treatment, 95 (82%) patients met the inclusion criteria and agreed to enroll into the study (Fig. 1). Table 1 shows the comparison of clinical features between the patients in the neoadjuvant radiotherapy group and the surgery only group. There were no significant differences in the baseline characteristics. Table 2 shows the comparison of surgical and pathological features between the two groups, revealing the neoadjuvant radiotherapy group to have a significantly higher rate of perioperative blood transfusion (P = 0.049).
Figure 1.
Patients' flow chart
Table 1.
Comparison of clinical features between patients in the neoadjuvant radiotherapy group and the surgery alone group
| Clinicopathologic features | Neoadjuvant radiotherapy (n = 45) | Surgery (n = 50) | P value |
|---|---|---|---|
| Age (years, median), rangea | 50 (33–66) | 47 (25–69) | 0.255 |
| Gender (male) | 43 | 42 | 0.096 |
| ECOG score | |||
| 0 | 38 | 37 | 0.497 |
| 1 | 5 | 10 | |
| 2 | 2 | 3 | |
| Viral serology | |||
| Positive for HBsAg | 37 | 44 | 0.428 |
| Positive for HBeAg | 12 | 6 | 0.185 |
| Positive HBV DNA load (>50 IU/ml) | 20 | 31 | 0.087 |
| Anti-virus treatment | 9 | 12 | 0.639 |
| Liver functional status (n) | |||
| Child-Pugh grade A | 40 | 42 | 0.560 |
| Child-Pugh grade B | 5 | 8 | |
| AFP level (ng/ml, median), rangea | 160 (3–1210) | 262 (2–1210) | 0.826 |
| Tumor diameter (cm, median), rangea | 10 (3–18) | 9 (3–15) | 0.370 |
| Tumor number | 0.370 | ||
| Single | 42 | 40 | 0.076 |
| Multiple | 3 | 10 | |
| Liver cirrhosisb | 29 | 30 | 0.155 |
| Microvascular invasionb | 30 | 33 | 0.261 |
| Tumor differentiationb | |||
| I | 0 | 1 | 0.175 |
| II | 30 | 44 | |
| III | 9 | 5 | |
| Presence of satellite nodulesb | 20 | 28 | 0.658 |
| Tumor capsuleb | |||
| Complete | 13 | 17 | 0.947 |
| Incomplete/absence | 26 | 33 | |
AFP, α-fetoprotein; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus.
Continuous parameters were calculated using Mann–Whitney U test.
In the neoadjuvant radiotherapy group, parameters were based on the pathological findings in 39 patients who finally underwent surgical treatment.
Table 2.
Comparison of surgical and pathological characteristics between patients in the neoadjuvant radiotherapy group and the surgery alone group
| Data of surgical procedure | Neoadjuvant radiotherapy (n = 39) | Surgery (n = 50) | P value |
|---|---|---|---|
| Type of hepatectomy | |||
| Major | 23 | 22 | 0.161 |
| Minor | 16 | 28 | |
| Anatomical resection | |||
| Yes | 18 | 24 | 0.863 |
| No | 21 | 26 | |
| Hilar clamping time (min, median), range | 20 (12–32) | 18 (0–38) | 0.065 |
| Intraoperative blood loss (ml, median),range | 600 (300–3500) | 500 (100–1900) | 0.377 |
| Perioperative blood transfusion (n) | |||
| Yes | 23 | 19 | 0.049∗ |
| No | 16 | 31 | |
| Postoperative complicationa | 0.240 | ||
| None | 21 | 36 | |
| Grade I | 13 | 11 | |
| Grade II | 5 | 3 | |
| In-hospital mortality (30-day) | 0 | 0 | – |
∗P < 0.05.
Graded by Clavien–Dindo Classification.
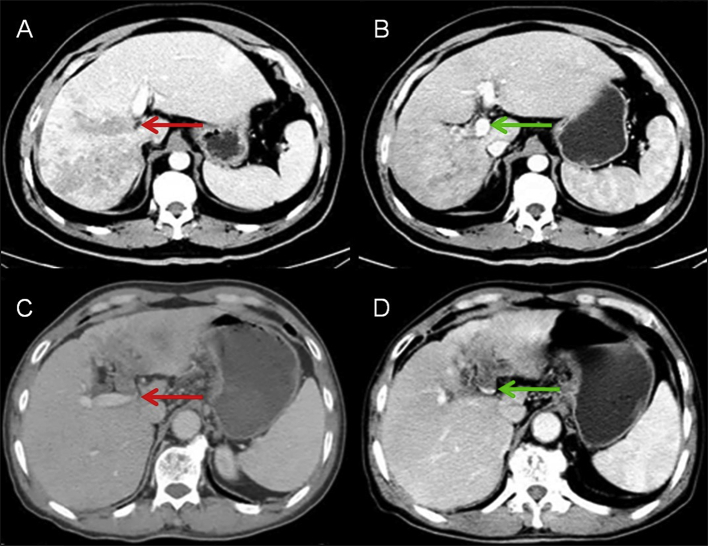
In the neoadjuvant radiotherapy group, 12 of 45 patients had significant reduction in extent of PVTT following radiotherapy, i.e. partial response (PR) according to the mRECIST guideline. Of these 12 patients, the mPVTT was downstaged from Cheng's type III to type II (i.e. the extent of PVTT was reduced from the main PV to be within the ipsilateral side of the portal vein) in 6 patients (Fig. 3). For all the remaining patients in this study, the number of patients with complete response (CR), stable disease (SD) and progressive disease (PD) for PVTT were 0, 31 and 2, respectively. For the response of HCC, the number of patients with CR, PR, SD and PD were 0, 6, 35 and 4.
Figure 3.
Reduction of PVTT extents after neoadjuvant radiotherapy CT imaging data of HCC with mPVTT before and 4 weeks after completion of irradiation. A. A 47-year-old man with a 10 × 9 cm mass in the right liver with PVTT in the main portal vein (red arrow). B. The extent of PVTT was reduced to be within the right portal vein (green arrow). C. CT imaging shows a 6 × 5 cm tumor (red yellow) with PVTT at the bifurcation of the main portal vein (red arrow) in a 33-year-old man. D. Blood flow resumed in the bifurcation after completion of irradiation (green arrow). Abbreviations: CT: computed tomography; HCC: hepatocellular carcinoma; PVTT: portal vein tumor thrombus
There was no severe adverse effect relating to radiotherapy, except in 2 patients who showed deterioration in liver function. A total of 6 patients developed contraindications to partial hepatectomy (2 with deteriorating liver function and 4 with disease progression/extrahepatic metastasis). These patients were treated with TACE (n = 5) or supportive treatment (n = 1). Finally, 39 patients underwent partial hepatectomy in the neoadjuvant radiotherapy group.
Follow-up data
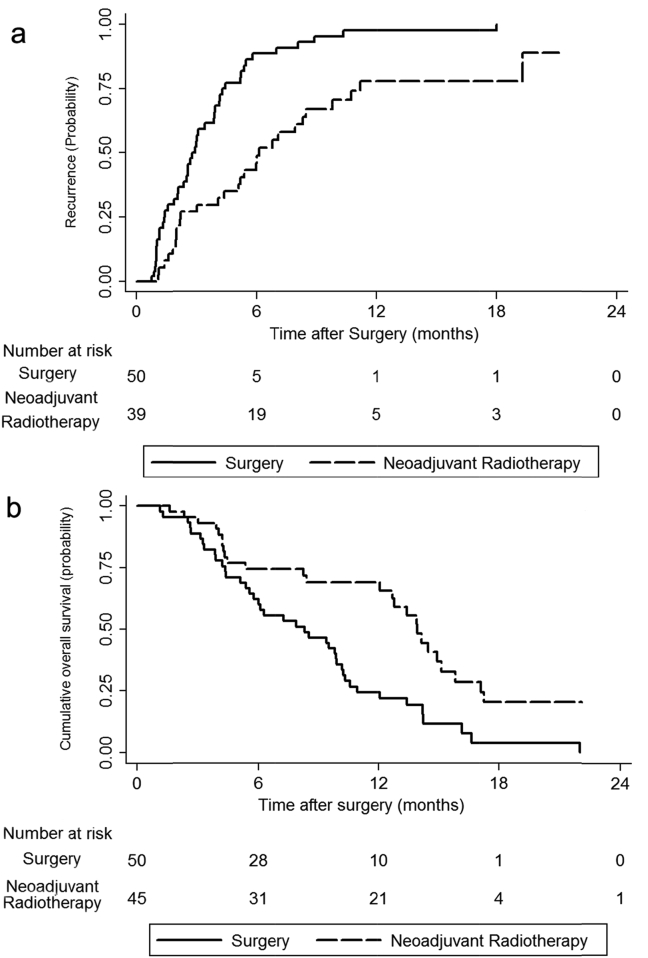
The follow-up periods ranged from 1 to 26 months, with a median of 8.4 months. Of 89 patients who finally received partial hepatectomy, 73 patients (82.0%, 73/89) had developed recurrent HCC and 67 patients (70.5%, 67/95) had died. The recurrent rates at 6 and 12 months were 49.0% and 77.0% for the neoadjuvant radiotherapy group vs 88.7% and 97.7% for the surgery only group (P < 0.01, Fig. 2a). The 1- and 2-year OS rates were 69.0% and 20.4% for the neoadjuvant radiotherapy group vs 35.6% and 0% for the surgery only group (P < 0.01, Fig. 2b).
Figure 2.
a. Kaplan–Meier analysis of tumor recurrence rate in the neoadjuvant radiotherapy (dashed line) and surgery (black line) groups (P < 0.01). b. Kaplan–Meier analysis of overall survival in the neoadjuvant radiotherapy (dashed line) and surgery (black line) groups (P < 0.01)
The factors which were significantly (P < 0.1) associated with HCC recurrence and HCC-related death on univariable Cox regression analysis (Supplementary table) were introduced into the multivariable Cox model. The results showed neoadjuvant radiotherapy significantly reduced both HCC recurrence and HCC-related death when compared with partial hepatectomy only [HR 0.36 (95% CI, 0.19–0.70) and 0.32 (95% CI, 0.18–0.57)] (Table 3). A long duration of hilar clamping (≥18 min) was significantly associated with tumor recurrence (HR 1.69, 95% CI, 1.03–2.75).
Table 3.
Factors associated with postoperative hepatocellular carcinoma recurrence and hepatocellular carcinoma-related death using multivariate Cox regression analysis
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Risk of tumor recurrence | |||
| HBV DNA load (≥50 IU/ml vs <50 IU/ml) | 1.11 | 0.56–2.21 | 0.757 |
| Anti-virus treatment (yes vs no) | 0.70 | 0.38–1.31 | 0.267 |
| Tumor diameter (≥10 cm vs <10 cm) | 1.22 | 0.69–2.17 | 0.482 |
| Tumor number (multiple vs single) | 1.58 | 0.79–3.17 | 0.196 |
| Type of hepatectomy (major vs minor) | 1.48 | 0.84–2.63 | 0.178 |
| Hilar clamping time (≥18 min vs <18 min) | 1.69 | 1.03–2.75 | 0.036* |
| Neoadjuvant radiotherapy (yes vs no) | 0.36 | 0.19–0.70 | 0.002* |
| Risk of tumor-related death | |||
| Tumor diameter (≥10 cm vs <10 cm) | 1.65 | 0.81–3.36 | 0.164 |
| Type of hepatectomy (major vs minor) | 1.45 | 0.71–2.99 | 0.311 |
| Neoadjuvant radiotherapy (yes vs no) | 0.32 | 0.18–0.57 | 0.000* |
HR: hazard ratio; CI: confidential interval; HBV, hepatitis B virus.
*P < 0.05.
Discussion
Although Sorafenib is recommended in the Barcelona Clinic Liver Cancer (BCLC) guideline as the only therapy for HCC patients with PVTT, several studies showed partial hepatectomy in selected patients could result in better overall survival than sorafenib.4, 13 However, once the tumor thrombus extends to the main portal vein, the postoperative survival becomes unsatisfactory.12, 13 Radiotherapy, a commonly used therapeutic modality in cancer treatment, has seldomly been used in HCC as this tumor has long been considered to be radio-resistant.17 With new developments in external radiotherapy, for example by using three-dimensional conformal radiotherapy, concentrating irradiation to tumor and PVTT becomes possible.18 This increases the tumor response rate while at the same time spares normal liver tissues from irradiation.
Recent clinical studies showed conformal radiotherapy, when combined with TACE or hepatic arterial infusion chemotherapy, shrunk tumor and provided promising survival outcomes in HCC patients with PVTT.17, 19, 20 Pracht et al. reported transarterial radioembolization using Yttrium-90 glass microspheres was efficient in downstaging HCC with ipsilateral PVTT with a disease control rate of 88.9%.20 Lau et al. reported salvage resectional surgery following downstaging of unresectable HCC resulted in long-term survival in selected patients.10, 11 The current non-randomized comparative study showed that neoadjuvant external radiation followed by partial hepatectomy resulted in better survival outcomes in patients with HCC with PVTT than partial hepatectomy only. In this study, the PVTT responded well to external radiotherapy with a significant proportion of lesions showing significant reduction in extent of PVTT (PR) (12/45). Meanwhile, the adverse effects of radiation were acceptable. There are several possible explanations for neoadjuvant radiotherapy to improve surgical and long-time survival outcomes of HCC with mPVTT:
First, advanced irradiation techniques such as three-dimensional conformal radiotherapy have been reported to improve the radiological response rate of patients with HCC and PVTT significantly. Seong et al.21 reported a response rate of 67.1% in 158 patients with unresectable HCC. Another study which used high dose radiotherapy to treat 27 patients with small HCCs unsuitable for curative liver resection reported a response rate of 92%.22 In this study, a strategy to use a low dose irradiation over a long duration to minimize radiation injury to the non-tumorous liver and to ensure safety of the subsequent surgical treatment. The response rate of PVTT was satisfactory (27%), while the response rate of HCC was lower (13%). This result showed PVTT responded better to irradiation than HCC.
Second, selective internal radiotherapy followed by salvage liver resection have been shown to be effective in achieving better long-term survival outcomes in selected patients with unresectable HCC.10, 11, 23, 24 Lau et al.11 reported 49 patients with unresectable HCC, of whom 7 had PVTT in the main portal vein. After tumor downstaging and salvage surgery, the 5-year OS was 57%. In this study, the therapies used for downstaging included systemic chemotherapy (81.6%) and internal radiotherapy using intra-arterial yttrium-90 microspheres (8.2%). Tang et al.19 reported in patients with advanced HCC, with their tumors downstaged with radiotherapy, subsequent surgical resection produced much better survival outcomes than TACE. Therefore, neoadjuvant radiotherapy followed by partial hepatectomy are expected to produce good post-operative survival outcomes.
Third, the importance of downstaging the extent of PVTT from Cheng's Classification from III (PVTT involving the main portal vein) to II (PVTT involving the right or the left portal vein). When PVTT invades the main portal vein, thrombectomy has to be combined with either a right or a left hepatectomy to remove all tumor tissues. During the operation, it would be difficult to avoid squeezing or fragmenting the tumor thrombus, thus increasing the chance of tumor spread. If the extent of PVTT could be downstaged using irradiation, the HCC and the PVTT can be resected within the transection plane of either a right or a left hepatectomy with total removal of all the HCC and the PVTT, thus minimizing the chance of tumor spread.
Although high dose external radiotherapy of HCC can produce adverse effects such as anorexia, leukopenia, thrombocytopenia, and deterioration in liver function,17, 22, 25 the current used a strategy of a low dose with a long duration of irradiation. This treatment was well-tolerated by the patients with severe adverse effect only observed in 2 patients who presented with deterioration of liver function.
The current study has limitations. This is a non-randomized study with a small sample size. Well-designed prospective studies are needed to document the true impact of the effect of neoadjuvant radiotherapy on patients with HCC with PVTT. Furthermore, the strategy of external radiotherapy can be improved further to increase its effectiveness and safety, and to decrease its adverse effects.
In conclusion, neoadjuvant radiotherapy followed by surgery resulted in better survival outcomes than surgery alone for patients with HCC with mPVTT.
Conflict of interest
There was no conflicts of interest.
Footnotes
This work was supported by the grants of the Science Fund for Creative Research Groups (No: 81221061); The State Key Project on Diseases of China (2012zx10002016016003); The China National Funds for Distinguished Young Scientists (No: 81125018); Changjiang Scholar Program (2013) of Chinese Ministry of Education; The National Key Basic Research Program “973 project” (No: 2015CB554000); National Natural Science Foundation of China (No: 81472282, 81101511, 81101831); The New Excellent Talents Program (No: XBR2011025) and General program (No: 20124301) from Shanghai Municipal Health Bureau; Shanghai Rising-star Program (No: 13QA1404900) from Shanghai Science and Technology Committee; SMMU Innovation Alliance for Liver Cancer Diagnosis and Treatment (2012); there are no financial disclosures, conflicts of interest, and/or acknowledgments.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.hpb.2016.04.003.
Contributor Information
Shu-Qun Cheng, Email: chengshuqun@aliyun.com.
Yan Meng, Email: yanmeng_ehbh@163.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.McGlynn K.A., London W.T. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223–243. doi: 10.1016/j.cld.2011.03.006. vii–x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuqun C., Mengchao W., Han C., Feng S., Jiahe Y., Guanghui D. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology. 2007;54:499–502. [PubMed] [Google Scholar]
- 3.Thomas M.B., Jaffe D., Choti M.M., Belghiti J., Curley S., Fong Y. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994–4005. doi: 10.1200/JCO.2010.28.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X.P., Qiu F.Z., Wu Z.D., Zhang Z.W., Huang Z.Y., Chen Y.F. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol. 2006;13:940–946. doi: 10.1245/ASO.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Inoue Y., Hasegawa K., Ishizawa T., Aoki T., Sano K., Beck Y. Is there any difference in survival according to the portal tumor thrombectomy method in patients with hepatocellular carcinoma? Surgery. 2009;145:9–19. doi: 10.1016/j.surg.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Liu P.H., Lee Y.H., Hsia C.Y., Hsu C.Y., Huang Y.H., Chiou Y.Y. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann Surg Oncol. 2014;21:1825–1833. doi: 10.1245/s10434-014-3510-3. [DOI] [PubMed] [Google Scholar]
- 7.Wu C.C., Hsieh S.R., Chen J.T., Ho W.L., Lin M.C., Yeh D.C. An appraisal of liver and portal vein resection for hepatocellular carcinoma with tumor thrombi extending to portal bifurcation. Arch Surg. 2000;135:1273–1279. doi: 10.1001/archsurg.135.11.1273. [DOI] [PubMed] [Google Scholar]
- 8.Ikai I., Hatano E., Hasegawa S., Fujii H., Taura K., Uyama N. Prognostic index for patients with hepatocellular carcinoma combined with tumor thrombosis in the major portal vein. J Am Coll Surg. 2006;202:431–438. doi: 10.1016/j.jamcollsurg.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Poon R.T., Fan S.T., Ng I.O., Wong J. Prognosis after hepatic resection for stage IVA hepatocellular carcinoma: a need for reclassification. Ann Surg. 2003;237:376–383. doi: 10.1097/01.SLA.0000055224.68432.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau W.Y., Lai E.C. Salvage surgery following downstaging of unresectable hepatocellular carcinoma–a strategy to increase resectability. Ann Surg Oncol. 2007;14:3301–3309. doi: 10.1245/s10434-007-9549-7. [DOI] [PubMed] [Google Scholar]
- 11.Lau W.Y., Ho S.K., Yu S.C., Lai E.C., Liew C.T., Leung T.W. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004;240:299–305. doi: 10.1097/01.sla.0000133123.11932.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J., Lai E.C., Li N., Guo W.X., Xue J., Lau W.Y. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci. 2011;18:74–80. doi: 10.1007/s00534-010-0314-0. [DOI] [PubMed] [Google Scholar]
- 13.Shi J., Lai E.C., Li N., Guo W.X., Xue J., Lau W.Y. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073–2080. doi: 10.1245/s10434-010-0940-4. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujino H., Kimura T., Aikata H., Miyaki D., Kawaoka T., Kan H. Role of 3-D conformal radiotherapy for major portal vein tumor thrombosis combined with hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Hepatol Res. 2015;45:607–617. doi: 10.1111/hepr.12392. [DOI] [PubMed] [Google Scholar]
- 18.Cheng S.H., Lin Y.M., Chuang V.P., Yang P.S., Cheng J.C., Huang A.T. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14:1025–1033. doi: 10.1046/j.1440-1746.1999.01994.x. [DOI] [PubMed] [Google Scholar]
- 19.Tang Q.H., Li A.J., Yang G.M., Lai E.C., Zhou W.P., Jiang Z.H. Surgical resection versus conformal radiotherapy combined with TACE for resectable hepatocellular carcinoma with portal vein tumor thrombus: a comparative study. World J Surg. 2013;37:1362–1370. doi: 10.1007/s00268-013-1969-x. [DOI] [PubMed] [Google Scholar]
- 20.Pracht M., Edeline J., Lenoir L., Latournerie M., Mesbah H., Audrain O. Lobar hepatocellular carcinoma with ipsilateral portal vein tumor thrombosis treated with yttrium-90 glass microsphere radioembolization: preliminary results. Int J Hepatol. 2013;2013:827649. doi: 10.1155/2013/827649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seong J., Park H.C., Han K.H., Chon C.Y. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys. 2003;55:329–336. doi: 10.1016/s0360-3016(02)03929-9. [DOI] [PubMed] [Google Scholar]
- 22.Mornex F., Girard N., Beziat C., Kubas A., Khodri M., Trepo C. Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies–mature results of the French Phase II RTF-1 trial. Int J Radiat Oncol Biol Phys. 2006;66:1152–1158. doi: 10.1016/j.ijrobp.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Lau W.Y., Yu S.C., Lai E.C., Leung T.W. Transarterial chemoembolization for hepatocellular carcinoma. J Am Coll Surg. 2006;202:155–168. doi: 10.1016/j.jamcollsurg.2005.06.263. [DOI] [PubMed] [Google Scholar]
- 24.Lau W.Y., Lai E.C. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249:20–25. doi: 10.1097/SLA.0b013e31818eec29. [DOI] [PubMed] [Google Scholar]
- 25.Ishikura S., Ogino T., Furuse J., Satake M., Baba S., Kawashima M. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol. 2002;25:189–193. doi: 10.1097/00000421-200204000-00019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.