Abstract
Pulmonary arterial hypertension (PAH) secondary to pulmonary Langerhans cell histiocytosis (PLCH) is known to be a relatively common complication and is associated with a poor prognosis. However, the optimal therapeutic approach for these cases remains to be established. A 57-year-old man visited our hospital because of a progressive dry cough. A thoracic computed tomography examination showed a combination of diffuse thick-walled cysts and reticulonodular shadows that were predominant in bilateral upper lobes of the lungs. He was diagnosed as having PLCH based on the results of video-assisted thoracoscopic lung biopsies. During a 3-year clinical course, his condition deteriorated despite smoking cessation. A systemic evaluation demonstrated precapillary PAH caused by PLCH (PAH-PLCH), and treatment with tadalafil, a phosphodiesterase-5 inhibitor, was started. During a 50-month period of treatment with tadalafil, improvements in his dyspnea, 6-min walking distance, and hemodynamics were maintained without either overt hypoxemia or pulmonary edema. We considered that tadalafil therapy may be a useful option in the treatment of patients with PAH-PLCH.
Keywords: Pulmonary arterial hypertension, Pulmonary langerhans cell histiocytosis, Phosphodiesterase-5 inhibitor, Tadalafil
1. Introduction
Pulmonary Langerhans cell histiocytosis (PLCH) is characterized by the infiltration of large numbers of Langerhans cells to the involved tissues and is a rare lung disease that occurs predominantly in young smokers [1]. The clinical course and prognosis of PLCH are unpredictable. Although most patients with PLCH recover spontaneously or remain in a stable condition without treatment after smoking cessation, some patients experience progressive respiratory impairment related to a deleterious change in lung function or to the development of pulmonary arterial hypertension (PAH) [1]. Precapillary PAH is an important complication in patients with PLCH, since PAH is associated with a poor prognosis [2]. However, little information on the optimal treatment of PAH caused by PLCH (PAH-PLCH) is available [3], [4], [5], [6]. Indeed, the limited data available for PAH-PLCH suggests that PAH-specific therapies are associated with a long term improvement in hemodynamics [4], [5], [6].
Here, we present a patient with PAH-PLCH who has maintained a long-term improvement by undergoing treatment with tadalafil, a phosphodiesterase-5 inhibitor.
2. Case report
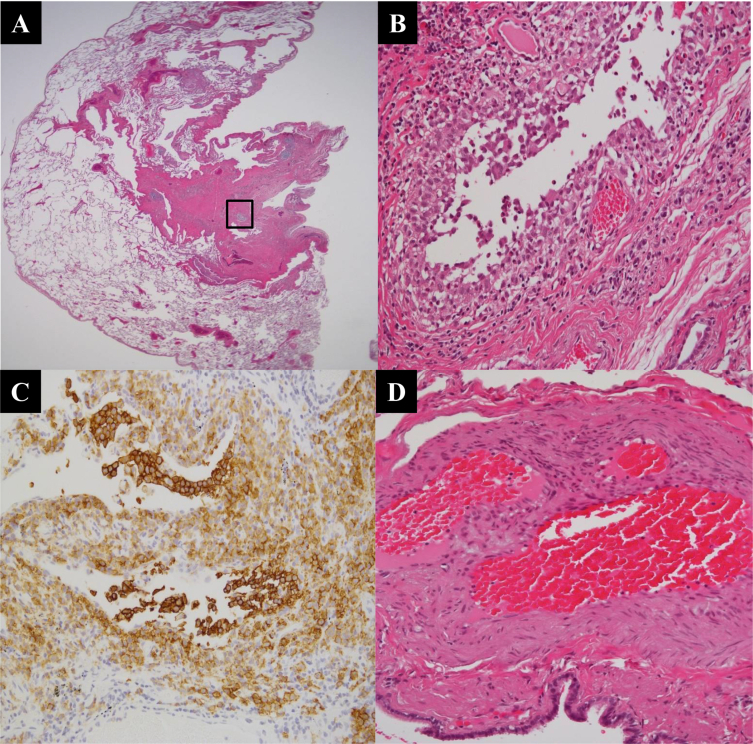
A 57-year-old man who had no significant past medical history visited our hospital in 2007 because of a progressive dry cough. He was a current smoker (60 pack-years) and had worked as a foundry worker with respiratory protection until hospital admission. A chest radiography (Fig. 1A) and computed tomography (Fig. 1B and C) showed a combination of diffuse thick-walled cysts and reticulonodular shadows that were predominant in the upper lobes of the lungs. Pulmonary function tests demonstrated an almost normal pattern, except for a decrease in the carbon monoxide diffusion capacity (DLco) (Table 1). Routine hematologic, biochemical, and serologic tests were within the normal ranges. Transthoracic echocardiography showed a normal cardiac image. A bronchoalveolar lavage analysis and transbronchial lung biopsy did not reveal a definitive diagnosis. Therefore, video-assisted thoracoscopic lung biopsies of the right upper and lower lobes were performed, and multiple cystic lesions and nodular infiltrates with fibrosis formed from various types of cells, but mostly histiocytes and eosinophils, were observed (Fig. 2A and B). These histiocytes had a convoluted irregular nucleus and a pale eosinophilic cytoplasm that stained strongly for anti-CD1a and were identified as Langerhans cells (Fig. 2C). Based on these histological characteristics, a diagnosis of PLCH was established. The patient stopped smoking and changed his occupational environment after the surgical biopsy.
Fig. 1.
(A) Chest X-ray showing diffuse cysts with reticular shadows in bilateral lung fields. (B, C) Chest CT showing diffuse irregular- and thick-walled cysts with reticulonodular shadows that were predominant in the upper lobes of the lungs.
Table 1.
Overview of clinical, function and haemodynamic features before and after tadalafil therapy.
| Variables | 2007 | 2009 | 2010 (Baseline) | 1 month | 15 months | 30 months | 40 months | 50 months |
|---|---|---|---|---|---|---|---|---|
| NYHA class | I | II | IV | III | II | II | II | II |
| NT pro BNP (pg/ml) | ND | 61.6 | 63.3 | 43.3 | 25.7 | 21.5 | 39.1 | 26.3 |
| 6MWT distance (m) | ND | 265a | 255a | 200a | ND | 230a | 305a | 310a |
| 6MWT SpO2, percentage of decrease (%) | ND | 2a | 5a | 9a | ND | 2a | 5a | 7a |
| PaO2 at rest (mmHg) | 83.1 | 74.2b | 56.6b | 52.9b | ND | 70.3b | 74.2b | 83.8b |
| PaCO2 at rest (mmHg) | 41.8 | 44.7b | 42.8b | 46.5b | ND | 39.7b | 41.5b | 46.9b |
| VC, % pred (%) | 106.9 | 97.4 | 64.5 | 61.9 | 75.1 | 72.8 | 66.2 | 73.5 |
| FEV1, % pred (%) | 103.3 | 72.9 | 49.8 | 49.8 | 50.4 | 52.6 | 48.4 | 52.4 |
| DLco, % pred (%) | 70.2 | 49.1 | 34.6 | 37.3 | 44.2 | 38.4 | 34.9 | 50.0 |
| PAP systolic/diastolic (mmHg) | ND | ND | 51/24 | 51/27 | ND | 34/18 | 34/16 | 32/13 |
| Mean PAP (mmHg) | ND | ND | 34 | 34 | ND | 25 | 24 | 21 |
| Mean PCW (mmHg) | ND | ND | 6 | 13 | ND | 8 | 9 | 5 |
| PVR (dynes/s/cm5) | ND | ND | 638.3 | 342.5 | ND | 376.1 | 321.1 | 387.7 |
| CI (L/min/m2) | ND | ND | 2.02 | 2.97 | ND | 2.23 | 2.33 | 2.00 |
| Therapy | Smoking cessation | Nasal oxygen | Nasal oxygen Tadalafil commenced | Nasal oxygen plus Tadalafil | Nasal oxygen plus Tadalafil | Nasal oxygen plus Tadalafil | Nasal oxygen plus Tadalafil | Nasal oxygen plus Tadalafil |
NYHA: New York Heart Association; NT pro BNP: N-terminal pro-brain naturiuretic peptide; 6MWT: 6-min walk test; PaO2: arterial oxygen tension; PaCO2: arterial carbon dioxide tension; FVC: forced vital capacity; FEV1: forced expiratory volume in1 second; DLco: transfer factor for carbon monoxide; PAP: pulmonary artery pressure; PCW: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance; CI: cardiac index; ND: not determined.
Nasaloxygen, 5 L/min.
Nasaloxygen, 2 L/min.
Fig. 2.
(A, B) Histological findings show multiple cystic lesions and nodular infiltrates with fibrosis formed from various types of cells, but mostly histiocytes and eosinophils (hematoxylin and eosin, A; panoramic view, B; in box, ×400). (C) An immunostaining study revealed the histiocytes to be Langerhans cells (anti-CD1a antibody, ×400). (D) Histological findings of the pulmonary arteries away from the LCH lesions show mild medial thickening and intimal fibrosis.
In 2009, despite the complete cessation of smoking, he began to experience exertional dyspnea (New York Heart Association [NYHA] functional class II) with hypoxemia. A transthoracic echocardiography showed no evidence of right arterial overloading, but pulmonary function testing showed an obstructive impairment and a decline in DLco (Table 1). Therefore, long-term nasal oxygen therapy was started.
In 2010, he presented with increased dyspnea (NYHA functional class IV) and marked hypoxemia. Pulmonary function tests demonstrated the progression of obstructive impairment and a reduction in vital capacity (VC) and Dlco (Table 1). The radiographic findings also showed the progression of emphysematous change. Right heart catheterization (RHC) was performed for the first time during his clinical course, and the results demonstrated precapillary PAH with a mean pulmonary arterial pressure (mPAP) of 34 mmHg, a cardiac index of 2.02 L/min/m2, and a pulmonary vascular resistance (PVR) of 638.3 dyne/s/cm5. Since other causes of PAH, such as pulmonary embolism, systemic disease, HIV infection, and portal hypertension, seemed to be negative, the patient was diagnosed as having PAH-PLCH. A retrospective analysis of the previous specimens of lung biopsies revealed mild medial thickening and intimal fibrosis in the pulmonary arteries away from the LCH lesions; however, it was not related to histological features of advanced PAH (Fig. 2D). He was treated with corticosteroid therapy (prednisolone, 0.5 mg/kg/day) for 1 month, but his symptoms did not improve. Therefore, tadalafil therapy was started.
After one month of tadalafil therapy, his dyspnea decreased (NYHA functional class III) and his hemodynamics improved (cardiac index, 2.97 L/min/m2; PVR, 342.5 dyne/s/cm5), although a temporal worsening of hypoxemia and the 6-min walking distance (6MWD) were confirmed (Table 1). During a 50-month period of treatment with tadalafil, the improvements in his dyspnea (NYHA functional class II), hypoxemia, and 6MWD were maintained. In addition, RHC findings showed sustained improvements in pulmonary arterial pressure and pulmonary vascular resistance (Table 1).
3. Discussion
This case was diagnosed as PLCH based on a lung biopsy, and the patient developed precapillary PAH three years after the initial diagnosis. The long-term use of tadalafil in this case was well tolerated and safe, leading to sustained improvements in dyspnea, exercise capacity and hemodynamics.
Chronic lung diseases such as COPD and chronic interstitial lung disease, particularly those combined with pulmonary fibrosis and emphysema, are known to be associated with a high incidence of PAH [7]. Although PLCH is an uncommon lung disease in comparison with the above-mentioned diseases, there have been some reports of PAH during the course of PLCH. In a retrospective study of 39 patients with PLCH who underwent lung transplantation, PAH was detected in 92% of the cases and was moderate-to-severe (mPAP >35 mmHg) in 72.5% [8]. Therefore, this study suggested that PAH is a common feature among patients with end-stage PLCH [8]. On the other hand, Chaowalit et al. reported that PAH is not limited to patients with end-stage PLCH [9]. This study also suggested that the development of PAH in patients with PLCH may be associated with an increased mortality (hazard ratio, 22.8; 95% confidence interval, 7.6 to >68.9; P < 0.001) [9]. These findings indicate that PAH is a relatively common and important complication of PLCH.
In general, vasodilator therapy may worsen gas exchange in patients with chronic lung disease because of an increased imbalance in ventilation/perfusion caused by the inhibition of hypoxic pulmonary vasoconstriction [10]. Indeed, the present case revealed the progression of hypoxemia after a month of tadalafil therapy, despite the improvement in his pulmonary hemodynamics. Although the pathophysiology of PAH associated with PLCH is not well understood, this clinical course seems to indicate that pulmonary vasoconstriction caused by chronic hypoxemia is an important mechanism in the pathogenesis of PAH-PLCH. On the other hand, our case exhibited sustained improvements in dyspnea, exercise capacity, hemodynamics, and hypoxemia during long term treatment with tadalafil. These results indicate that mechanisms other than hypoxic vasoconstriction may be responsible for PAH-PLCH. In the present case, the lung tissues specimen revealed mild medial thickening and intimal fibrosis in pulmonary vessels unrelated to the LCH lesions, though it was not a direct evidence of PAH. Since the blood flow at the lesions have been predicted to decrease as a result of disease progression of the LCH, the excessive blood flow in the remaining vessels away from the LCH lesions may have induced the PAH. Therefore, the effects of tadalafil therapy on PAH-PLCH in our case may have been induced by the dilation of vessels unrelated to the LCH lesions. In addition to these mechanisms, Fortoukh et al. [2] reported that the cause of PAH-PLCH may be related to an intrinsic pulmonary vascular disease independent of the involvement of small airways and lung parenchyma injury. In this report, these vascular abnormalities were observed in areas away from PLCH lesions in half of the patients, therefore, Fortoukh et al. speculated that the PLCH may play a certain role in the genesis of vascular abnormalities through the production of cytokines and growth factors [2]. However, we cannot address whether good responsiveness to treatment with tadalafil in our case support Fortoukh's hypothesis. Therefore, further clinical studies are needed to clarify the mechanisms of PAH-PLCH.
The efficacy of PAH-specific therapies for PAH-PLCH has not been well discussed in medical literature. Some previous reports have indicated that the pulmonary venules are involved in the disease process that is responsible for PAH-PLCH [2], [11]. Therefore, the use of PAH-specific therapies has long been considered hazardous in PAH-PLCH patients because of the risk of pulmonary edema. However, Kiakouma et al. reported a patient with PAH-PLCH who was treated using the dual endothelin receptor antagonist bosentan and exhibited clinical, functional, and hemodynamic improvements, and these benefits were maintained for a total of 7 years [5]. In addition, Pavec et al.'s study evaluated patients with PAH-PLCH who underwent short-term and long-term treatment (5.5 ± 2.5 months and 16 ± 4 months after baseline in 12 and 10 patients, respectively) and, suggested that PAH-specific therapy may improve the WHO function class and hemodynamics without oxygen worsening or pulmonary edema [4]. Recently, May et al. reported that a patient with PAH-PLCH responded well to PAH-specific therapies included a tadalafil, and echocardiography and WHO function class improvement were sustained over a 10 year follow-up period [6]. In our case, tadalafil therapy was associated with an improvement in the WHO function class, 6MWD, and hemodynamics without oxygen worsening. Furthermore, these improvements were sustained over a 50-month follow-up period.
4. Conclusion
In the present case, although a temporal worsening of hypoxemia occurred during the initial phase of treatment, we confirmed that a long-term improvement was obtained in a patient with PAH-PLCH who was treated with tadalafil, without any apparent complications. Therefore, tadalafil therapy may be a useful option for the treatment of patients with PAH-PLCH.
Conflict of interest
The authors state that they have no Conflict of Interest (COI).
References
- 1.Tazi A. Adult pulmonary langerhans' cell histiocytosis. Eur. Respir. J. 2006;27:1272–1285. doi: 10.1183/09031936.06.00024004. [DOI] [PubMed] [Google Scholar]
- 2.Fortoukh M., Humbert M., Capron F., Maȋtre S., Parent F., Gall C.L. Severe pulmonary hypertension in histiocytosis X. Am. J. Respir. Crit. Care Med. 2000;161:216–223. doi: 10.1164/ajrccm.161.1.9807024. [DOI] [PubMed] [Google Scholar]
- 3.Benyounes B., Crestani B., Couvelard A., Vissuzaine C., Aubier M. Steroid-responsive pulmonary hypertension in a patient with langerhans' cell granulomatosis (histiocytosis X) Chest. 1996;110:284–286. doi: 10.1378/chest.110.1.284. [DOI] [PubMed] [Google Scholar]
- 4.Pavec J.L., Lorillon G., Jaïs X., Tcherakian C., Feuillet S., Dorfmüller P. Pulmonary langerhans cell histiocytosis-associated pulmonary hypertension. Clinical characteristics and impact of pulmonary arterial hypertension therapies. Chest. 2012;142(5):1150–1157. doi: 10.1378/chest.11-2490. [DOI] [PubMed] [Google Scholar]
- 5.Kiakouama L., Cottin V., Etienne-Mastroïanni B., Khouatra C., Humbert M., Cordier J.F. Severe pulmonary hypertension in histiocytosis X: long-term improvement with bosentan. Eur. Respir. J. 2010;36(1):202–204. doi: 10.1183/09031936.00004810. [DOI] [PubMed] [Google Scholar]
- 6.May A., Kane G., Yi E., Frantz R., Vassallo R. Dramatic and sustained responsiveness of pulmonary Langerhans cell histiocytosis-associated pulmonary hypertension to vasodilator. Respir. Med. Case Rep. 2015;14:13–15. doi: 10.1016/j.rmcr.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seeger W., Adir Y., Barberà J.A., Champion H., Coghlan J.G., Cottin V. Pulmonary hypertension in chronic lung diseases. J. Am. Coll. Cardiol. 2013;62(Suppl. 25):D109–D116. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 8.Dauriat G., Mal H., Thabut G., Mornex J.F., Bertocchi M., Tronc F. Lung transplantation for pulmonary langerhans' cell histiocytosis: a multicenter analysis. Transplantation. 2006;81:746–750. doi: 10.1097/01.tp.0000200304.64613.af. [DOI] [PubMed] [Google Scholar]
- 9.Chaowalit N., Pellikka P.A., Decker P.A., Aubry M.C., Krowka M.J., Ryu J.H. Echocardiographic and clinic characteristics of pulmonary hypertension complicating pulmonary langerhans cell histiocytosis. Mayo Clin. Proc. 2004;79(10):1269–1275. doi: 10.4065/79.10.1269. [DOI] [PubMed] [Google Scholar]
- 10.Simonneau G., Escourrtou P., Duroux P., Lockhart A. Inhibition of hypoxic pulmonary vasoconstriction by nifedipine. N. Engl. J. Med. 1981;304(26):1582–1585. doi: 10.1056/NEJM198106253042606. [DOI] [PubMed] [Google Scholar]
- 11.Travis W.D., Borok Z., Roum J.H., Zhang J., Feuestein I., Ferrans V.J. Pulmonary Langerhans cell granulomatosis (histiocytosis). A clinicopathologic study of 48 cases. Am. J. Surg. Pathol. 1993;17(10):971–986. doi: 10.1097/00000478-199310000-00002. [DOI] [PubMed] [Google Scholar]