Abstract
Introduction
Anti-arrhythmic medications (AAMs) are known to increase cardiac mortality significantly due to their pro-arrhythmic effects. However, the effect of AAMs on non-cardiac mortality has not been evaluated.
Methods
Trials published in English language journals from 1990 to 2015 were thoroughly retrieved by searching websites such as PubMed, Medline, Cochrane Library, and Google Scholar. Randomized controlled trials reporting non-cardiac deaths as primary or secondary outcomes were used to compare AAMs to non-arrhythmic therapy (AV nodal blocking agents, implantable cardiovascular defibrillation (ICD), or placebo). Information regarding the sample size, treatment type, baseline characteristics, and outcomes was obtained by using a standardized protocol. The fixed effect model was used to perform meta-analysis, and results were expressed in terms of odds ratio (OR) with confidence interval (CI) of 95%, inter study heterogeneity was assessed using I2. Intention to treat principle was applied to extract data.
Results
Total of 18,728 patients were enrolled in 15 trials; 9359 patients received AAMs and 9369 received non-arrhythmic therapy. AAMs were associated with an increased risk of non-cardiac mortality (OR=1.30, [95% CI: 1.12, 1.50], p=0.0005, I2 index=24%) and all-cause mortality (OR=1.09, [95% CI: 1.01, 1.18], p=0.04, I2=54%) as compared to non-arrhythmic therapy. There was no difference in the cardiac mortality (OR=1.01, [95% CI: 0.92, 1.11], p=0.82, I2=53%) or arrhythmic mortality (OR=1.00, [95% CI: 0.89, 1.13], p=0.94, I2=64%) between the two groups.
Conclusion
AAMs are associated with an increased risk of non-cardiac and all-cause mortality. The effect of AAMs, especially amiodarone, on non-cardiac mortality requires further evaluation.
Keywords: Anti-arrhythmic medications, Non-cardiac mortality, ICD, Meta-analysis
1. Introduction
Anti-arrhythmic medications (AAMs) are the most commonly used treatment for medical management of cardiac arrhythmias [1], [2]. The use of AAMs in treatment of both atrial and ventricular arrhythmias is limited owing to their limited efficacy and long-term toxicity. Numerous randomized trials had compared the all-cause mortality or other arrhythmic outcomes of AAMs with either placebo treatment or invasive interventions such as implantable cardioverter defibrillator or ablation [3], [4], [5], [6], [7], [8], [9], [10], [11]. Considering the potential side effects of different anti-arrhythmic agents, none of the trials had reported the effect of AAMs on non-cardiac mortality. The primary aim of this meta-analysis was to analyze the effect of anti-arrhythmic medications (AAMs) on non-cardiac mortality.
2. Methods
Two researchers identified all the published randomized trials that had compared any AAM with non-arrhythmic therapy (AV nodal blocking agents, implantable cardiovascular defibrillation (ICD), or placebo) and assessed the eligibility of each trial. Each trial was required to report non-cardiac deaths as primary or secondary outcomes.
2.1. Search strategy
Two authors performed thorough literature search on Medline, Google Scholar, and the Cochrane Library for the relevant articles published from 1990 to April 2015. Following keywords were used for the search: randomized, trial, antiarrhythmic, cardiac, non-cardiac, mortality, outcomes, survival, ventricular arrhythmias, atrial fibrillation, treatment, rhythm control, amiodarone, dofetilide, sotolol, propafenone, flecainide, and procainamide. For a comprehensive search abstracts from the annual meetings of the American Heart Association, American College of Cardiology and European Society of Cardiology were investigated for the same time. References of all the selected journal and review articles were reviewed so that any additional randomized trials could be identified. The search was limited to humans and to articles reported in English language.
Inclusion criteria for the retrieved studies were: (a) controlled comparison of AAMs to atrial ventricular nodal blocking agents, ICD or placebo, (b) randomized treatment allocation, (c) intention to treat analysis, (d) reports of cardiovascular and non-cardiovascular mortality. Studies were excluded if they had the following criteria (a) vague treatment allocation process, (b) significant variability amongst study population, (c) studies comparing two different AAMs. Standardized protocol was used to obtain information on sample size, treatment type, medications, baseline characteristics, and outcomes.
2.2. Outcomes
The primary outcomes of interest were total mortality, cardiovascular mortality, non-cardiovascular mortality, and arrhythmic mortality. Our research committee classified the causes of death reported in the selected studies into cardiac mortality, arrhythmic mortality, non-cardiac mortality, and all-cause mortality. The studies classified events as non-cardiovascular death when the event committee classified the events based on supportive material emergency room reports, hospital notes, discharge summaries, autopsy reports, laboratory tests, ECG, etc. The non-cardiovascular mortality mainly included cancer, sepsis, trauma, pulmonary disease, non-cardiac surgery, suicide, and the illness or treatment complications resulting in death. Vascular deaths, which included hemorrhage, vascular catastrophe, systemic embolism, pulmonary embolism, and central nervous system (CNS) events, were also considered non-cardiovascular deaths. In instances where non-cardiovascular mortality was not reported, the difference of total mortalities and cardiovascular mortalities was taken as non-cardiovascular mortalities.
Cardiovascular mortalities were divided into arrhythmic and non-arrhythmic cardiac mortalities. The studies in general classified mortality as cardiac non-arrhythmic, when death occurred due to congestive heart failure (CHF), shock, or myocardial ischemia with evidence of ischemic symptoms, ECG changes, or enzyme abnormalities. Deaths following collapse, spontaneous and rapid blood loss, and unexplained death during sleep were classified as cardiac arrhythmic mortalities.
2.3. Statistical analysis
An intention-to-treat analysis was used to obtain results for each trial. Odds ratios (OR) were used as a summary measure of efficacy for dichotomous data and mean difference (MD) with 95% confidence interval (CI) was used for continuous variables. Fixed-effect model was used for the analysis since studies showed low heterogeneity, which was measured by I2 that describes the percentage of total variation across trials due to heterogeneity rather than chance. I2 can be calculated as I2=100%×(Qv−df)/Q, where Q is Cochran׳s heterogeneity statistics and df the degrees of freedom. I2 values less than 25% were considered as low heterogeneity, 25–50% as moderate, and greater than 75% as high heterogeneity. The summary estimators of treatment effect were calculated using the DerSimonian and Laird fixed-effect method. A p value of 0.05 or less was regarded as significant. The number of patients needed to treat to prevent one end point was calculated using the overall weight risk difference (NNT=1/[absolute risk difference]). All analyses were performed using Review Manager (RevMan) Version 5.3 for Windows.
3. Results
The initial search yielded 1588 potential literature citations (Fig. 1). Out of these, we identified 15 randomized controlled trials that compared antiarrhythmic medications to AV nodal blocking agents, ICD or placebo and fulfilled our inclusion criteria. The main reasons for exclusion were that the selected studies did not report non-cardiac mortality, were not randomized, or were randomized trials that compared two different AAMs.
Fig. 1.
Search strategy.
3.1. Trial characteristics
The combined search of Medline, Google Scholar, and references identified 1588 articles, 1573 of which were excluded. The studies that were not randomized or did not report non-cardiac mortality were excluded (Table 1).
Table 1.
Baseline characteristics of trials.
| Trial name | Age (years) | Female (%) | HTN (%) | DM (%) | CAD (%) | LVEF (%) | |
|---|---|---|---|---|---|---|---|
| AFFIRM [9] | AAMa | 69.7±9 | 39.3 | 50.8 | N/A | 26.1 | 54±13 |
| Control | 69.8±9 | 40.6 | 51.6 | N/A | 24.5 | 54.9±13 | |
| AMIOVIRT [6] | AAM | 60±12 | 26 | 67 | 36 | 11 | 23±8 |
| Control | 58±11 | 33 | 58 | 31 | 4.9 | 22±10 | |
| AVID [3] | AAM | 65.3 | 19 | 44 | 76 | 18 | 30.7 |
| Control | 64.8 | 22 | 44 | 75 | 18 | 32.2 | |
| CAMIAT [7] | AAM | 64 | 17.5 | 41 | 15 | N/A | N/A |
| Control | 64 | 18 | 43 | 18 | N/A | N/A | |
| CAST-2 [12] | AAM | 61.7±9.9 | 17.8 | 33.6 | 21.5 | N/A | 32.3±8.4 |
| Control | 62.0±10 | 16.4 | 35 | 21.5 | N/A | 32.9±7.9 | |
| CEREMUZYNSKI [8] | AAM | 59.4±12.3 | 28.9 | 43.3 | 19.7 | N/A | N/A |
| Control | 58.6±11.8 | 31.8 | 48.1 | 18.8 | N/A | N/A | |
| CHF-STAT [17] | AAM | 65±8.5 | 0.9 | N/A | N/A | 72 | N/A |
| Control | 66.1±8.1 | 1.2 | N/A | N/A | 70.7 | N/A | |
| CIDS [4] | AAM | 63.8±9.9 | 16.3 | N/A | N/A | 82.2 | 33±14 |
| Control | 63.3±9.2 | 14.6 | N/A | N/A | 82.9 | 34±14 | |
| DIAMOND [16] | AAM | 68 | 28 | 16 | 13 | 36 | N/A |
| Control | 69 | 25 | 17 | 13 | 37 | N/A | |
| EMIAT [15] | AAM | 60.2 | 15.1 | N/A | 17 | 26 | 30±8 |
| Control | 59.6 | 16.1 | N/A | 17 | 32 | 30±7 | |
| GEMICA [14] | AAM | 60±11 | 19.4 | 54.8 | 19 | 15.7 | N/A |
| Control | 60±12 | 24.9 | 51.6 | 13.4 | 13.7 | N/A | |
| RACE [10] | AAM | 69±8 | 35 | 56 | 11 | 34 | N/A |
| Control | 69±9 | 35 | 42 | 13 | 38 | N/A | |
| ROY [24] | AAM | 66±11 | 22 | 49 | 22 | 48 | 27±6 |
| Control | 67±11 | 15 | 46 | 20 | 48 | 27±6 | |
| STAF [11] | AAM | 65.3±9.4 | 41 | 63 | N/A | 34 | N/A |
| Control | 66.2±7.6 | 32 | 62 | N/A | 53 | N/A | |
| SWORD [13] | AAM | 60.4±10 | 14 | 37 | N/A | 34 | 31±6.8 |
| Control | 59.9±9.8 | 14 | 35 | N/A | 32 | 30±7 |
Anti-arrhythmic medication.
Fifteen trials were selected based on the inclusion and the exclusion criteria. The trial names, acronyms, details of patient characteristics and AAMs inclusion are shown in Table 1. Four trials compared treatment strategies of rhythm-control with AAMs and rate-control with atrial fibrillation [9], [10], [11]. Seven trials evaluated the effect of AAMs on mortality in patients who had survived myocardial infarction [7], [8], [12], [13], [14], [15], [16]. Three of these trials [13], [15], [16] enrolled patients with left ventricular dysfunction and one trial enrolled patients with frequent or repetitive ventricular premature depolarization [7]. Two trials evaluated the role of AAMs in patients with ventricular tachycardia [6], [17] and one of the trials included patients with heart failure [17]. ICD where used in two of the trials as one of the therapies for ventricular tachycardia (VT) [3], [4]. One of these two trials included only patients with non-ischemic cardiomyopathy [6]. The trials included mostly males between the average mean ages of 60–65 years (Table 1). The average follow up for all the studies was 2.3 years.
3.2. Trial results
3.2.1. Non-cardiac mortality
Nine out of the 13 trials showed that treatment with AAMs resulted in non-cardiac mortality; however, only one trial (AFFIRM) showed a significant difference between the treatment groups. The combined OR of non-cardiac-mortality with AAMs was associated with an increased risk of non-cardiac deaths by 30% (OR=1.30, [95% CI: 1.12, 1.50], p=0.0005, I2=24%), when compared to non-arrhythmic therapy (Fig. 2). The analysis was dominated by the AFFIRM trial [9]. There was no heterogeneity across the trials (heterogeneity χ2=17.15, df=13 (p=0.19), I2=24%). These findings did not change after excluding the two trials that included ICD as the placebo arm (OR=1.30, [1.11, 1.51], p=0.001). Three of the trials (AVID, SWORD, DOMOND) included patients with heart failure or LV dysfunction. When combined, these trials also showed significant increase in non-cardiac mortality (OR=1.44 [1.00, 2.09] p=0.050).
Fig. 2.
Non-cardiac mortality.
Ten of the trials used amiodarone as one of the AAMs. In the GEMICA trial, the most common cause of death was stroke and cancer. Of the 13 trials, only four trials reported significant increase in non-cardiac mortality between the groups. The most common causes included stroke, cancer, and pulmonary causes. The statistically significant heterogeneity found among the trials by I2, is a reflection in part of the wide range of sample size of the individual trials.
3.2.2. All-cause mortality, cardiac mortality, and arrhythmic mortality
There was also increase in all-cause mortality between patients taking placebo and AAMs (OR=1.09 [95% CI: 1.01, 1.18] p=0.04) (Fig. 3). There was no difference in cardiac (OR=1.01 [95% CI: 0.92, 1.11] p=0.82) or arrhythmic mortality (OR=1.00 [95% CI: 0.89, 1.13] p=0.94). Trials were significantly heterogeneous for all-cause mortality, cardiac, and arrhythmic mortality (Fig. 3, Fig. 4).
Fig. 3.
All-cause mortality.
Fig. 4.
Cardiac mortality.
4. Discussion
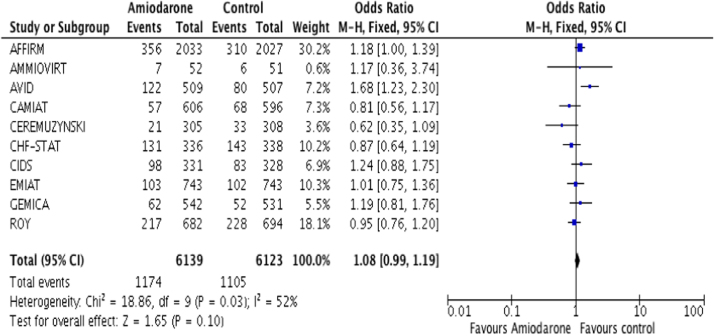
The present meta-analysis of randomized trials demonstrated that treatment with AAMs is associated with an increase in non-cardiac and all-cause mortality as compared to non-arrhythmic therapy or control group. There was no significant reduction in cardiac or arrhythmic mortality between the two groups. The finding was independent of the ICD or AV nodal blocking agents use in the control group. Amiodarone was the most commonly used drug in these trials. Current evidences show that amiodarone is the most effective form of treatment for life threatening arrhythmias [18], [19]. Singh et al. reported that prolonged use of amiodarone increases the left ventricular ejection fraction possibly by prolonging repolarization period [20]. Amiodarone primarily acts by blocking potassium channels, leading to a prolonged repolarization phase; however, it also has inhibitory effects on sodium and calcium channels, as well as beta and alpha adrenergic receptors. Heart Rhythm Society (HRS) has provided class 1C evidence for amiodarone use in atrial fibrillation after considering its potential toxicities and when other agents have failed or are contraindicated [21]. Amiodarone has number of side effects and can affect the lungs, liver, and thyroid [19]. Multiple larger population-based trials have shown that AAMs are proarrhythmic and are capable of increasing cardiac mortality due to their proarrhythmic effects [4], [13], [14]. To our knowledge, there are no reports where primary endpoint was increase in non-cardiac mortality caused by AAMs (Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9).
Fig. 5.
Arrhythmic mortality (M–H: Mantel–Haenszel, CI: confidence interval, AAM: antiarrhythmic medication).
Fig. 6.
Total mortality amiodarone.
Fig. 7.
Amiodarone cardiac mortality.
Fig. 8.
Amiodarone non-cardiovascular mortality.
Fig. 9.
Amiodarone arrhythmic mortality. (M–H: Mantel–Haenszel, CI: confidence interval, AAM: antiarrhythmic medication).
The first study to show an increase in non-cardiac mortality was the mode-of-death analysis of the AFFIRM study that showed a significant increase in fatal non-cardiovascular events in the rhythm-control arm [9]. The most commonly used drug in the AFFIRM trial was amiodarone, prescribed in approximately 60% of patients. In the study, after adjusting for other significant covariates, the risk of non-cardiovascular death was increased 1.5 fold (p=0.0007) if the assignment in AFFIRM was to the rhythm-control arm. It is evident from Fig. 1, that six studies showed an increased trend in non-cardiac mortality with AAMs; however, none of them were statistically significant. Similar findings were also observed by Gronefeld et al., showing that significant quality of life at 1 year follow up was better with rate control strategy as compared to rhythm control strategy [22]. The reasons for increase in non-cardiac mortality with AAMs are unclear at this time. In our meta-analysis, the increase in AAMs has been seen in patients with and without LV dysfunction. Waldo et al. showed increased mortality with anti-arrhythmic medications in patients with low ejection fraction [13]; however, subsequent studies did not report any confirming evidence [13], [15], [16]. In our meta-analysis AFFIRM is the only study, which had included patients with normal ejection fraction, all other studies had patients with low ejection fraction and seven studies did not specify LV function (Table 2).
Table 2.
Trials with amiodarone as the main anti-arrhythmic medication.
Despite its clinical efficacy, amiodarone was found to be associated with increased non-cardiac mortality. Its long turn accumulation leads to serious end organ toxicities, mainly manifested in the lung, liver, and thyroid. A recent retrospective cohort analysis studying the mortality risk of amiodarone therapy in atrial fibrillation revealed a higher risk of non-cardiac death in patients treated with amiodarone compared to other AAMs [23]. Lung toxicity is considered the most serious adverse event, as it can lead to non-reversible damage and fatal outcomes [24]. Lung toxicity correlates with the dose and the duration of amiodarone use, and it can present as early as few days to years after the treatment is started. Fatal outcomes range from 10% in patients who develop pneumonitis, up to 50% in patient presenting with acute respiratory distress syndrome (ARDS) [25]. Amiodarone exposure was associated with an increased likelihood of interstitial pneumonitis, which can be explained by its immunologic mechanism of hypersensitivity [26]. Liver toxicity is a well-known adverse effect of amiodarone and is related to its cumulative doses. Symptomatic events are seen in less than 3% of the cases. Most patients have reversible liver damage; however, death secondary to cirrhosis and liver failure had been reported in several cases [27]. Several mechanisms had been attributed to thyroid dysfunction after amiodarone use, leading to hypo and hyperthyroidism. However, fatal outcomes had been reported in the literature in few cases and meta-analysis [28], [29], [30], [31]. Optic neuropathy and corneal deposits had been reported with prolonged use of amiodarone due to its effect on endothelial and vascular smooth muscles [32]; few cases of leucocytoclastic vasculitis following treatment with amiodarone had also been reported explaining its cutaneous side effects [33].
Amongst other anti-arrhythmic medications, disopyramide (class 1A AAM) has anticholinergic side effects such as dry mouth, urinary hesitancy, constipation and exacerbation of conditions like glaucoma and myasthenia gravis. Disopyramide acts by targeting the fast sodium channels in the cardiac tissue. However, it also blocks potassium channels in pancreatic cells, which increases insulin secretion, leading to episodes of hypoglycemia, resulting in coma and neurological damage. This side effect is seen at normal therapeutic levels [34], therefore it should be avoided in patients taking potassium (ATP) channel inhibitor such as glimepiride [35]. Procainamide was widely used in the past; however, its use has been limited as its chronic administration leads to frequent side effects manifested mainly by lupus like syndrome in around 30% of patients [36]. On the other hand, bone marrow toxicity is a serious but less frequent side effect, manifested in less than 0.22% of the patients. It should be considered in any patient who develops pancytopenia once procainamide treatment is stopped [37]. Mexelitine is a well-tolerated class 1B antiarrhythmic drug. Its side effects are limited to gastrointestinal (GI) and neurological manifestation such as dizziness and numbness [38]. However more serious effects like thrombocytopenia are rare, and are based on case reports [39]. Propafenone is a Class 1C antiarrhythmic drug. It has been reported to be associated with suppressive sympathetic effects such as dizziness, nausea, visual disturbances [40]. A negative inotropic effect of propafenone significantly increases pulmonary capillary wedge pressure, systemic and pulmonary vascular resistance, and cardiac output especially in patients with low ejection fraction, leading to increase in non-cardiac mortality and morbidity [41]. Although GI side effects such as nausea and metallic taste are frequent and mild, propafenone has been implicated in acute cholestatic hepatitis as described in a case report [42].
In 10 out of the 15 trials included in the analysis, amiodarone was the major antiarrhythmic prescribed. Moreover, in 9 out of the 15 trials, there was an increase in non-cardiac mortality and in 8 out of 15 trials, there was an increase in all-cause mortality with AAMs while cardiac or arrhythmic mortalities remained the same. The increase in non-cardiac mortality was mainly attributed to increased incidence of all-cause mortality
4.1. Strengths and limitations
Our meta-analysis had several strengths that are as follows: a comprehensive search for relevant studies was conducted, all the included trials were randomized controlled, the eligibility criteria were applied systematically and explicitly, study quality was considered carefully, and a rigorous analytical approach was used. However, there were some limitations of the analysis. First, the sample sizes were different in various studies, affecting their statistical power in the analyses. Second, the studies that were included showed significant heterogeneity as evidenced by high I2 value; however, that might be expected as we had included studies with different protocols. Thirdly, the amiodarone dose was not similar in all the studies.
5. Conclusion
Non-cardiac and all-cause mortality rates are reportedly higher with AAMs compared to non-arrhythmic therapy. This effect of AAMs especially amiodarone, on non-cardiac mortality requires further evaluation.
Conflict of interest
All authors declare no conflict of interest related to this study.
References
- 1.Randomized antiarrhythmic drug therapy in survivors of cardiac arrest (the CASCADE Study). The CASCADE Investigators. Am J Cardiol 1993;72:280–7.
- 2.Mason J.W. A comparison of seven antiarrhythmic drugs in patients with ventricular tachyarrhythmias. Electrophysiologic Study versus Electrocardiographic Monitoring Investigators. N Engl J Med. 1993;329:452–458. doi: 10.1056/NEJM199308123290702. [DOI] [PubMed] [Google Scholar]
- 3.A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med 1997;337:1576–83.
- 4.Connolly S.J., Gent M., Roberts R.S. Canadian implantable defibrillator study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297–1302. doi: 10.1161/01.cir.101.11.1297. [DOI] [PubMed] [Google Scholar]
- 5.Kuck K.H., Cappato R., Siebels J. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH) Circulation. 2000;102:748–754. doi: 10.1161/01.cir.102.7.748. [DOI] [PubMed] [Google Scholar]
- 6.Strickberger S.A., Hummel J.D., Bartlett T.G. Amiodarone versus implantable cardioverter-defibrillator: randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia—AMIOVIRT. J Am Coll Cardiol. 2003;41:1707–1712. doi: 10.1016/s0735-1097(03)00297-3. [DOI] [PubMed] [Google Scholar]
- 7.Cairns J.A., Connolly S.J., Roberts R. Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial Investigators. Lancet. 1997;349:675–682. doi: 10.1016/s0140-6736(96)08171-8. [DOI] [PubMed] [Google Scholar]
- 8.Ceremuzynski L., Kleczar E., Krzeminska-Pakula M. Effect of amiodarone on mortality after myocardial infarction: a double-blind, placebo-controlled, pilot study. J Am Coll Cardiol. 1992;20:1056–1062. doi: 10.1016/0735-1097(92)90357-s. [DOI] [PubMed] [Google Scholar]
- 9.Van Gelder I.C., Hagens V.E., Bosker H.A. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 10.Hagens V.E., Crijns H.J., Veldhuisen D.J. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J. 2005;149:1106–1111. doi: 10.1016/j.ahj.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson J., Miketic S., Windeler J. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:690–696. doi: 10.1016/s0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 12.Trial C.A.S., Investigators I. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N Engl J Med. 1992;327:227–233. doi: 10.1056/NEJM199207233270403. [DOI] [PubMed] [Google Scholar]
- 13.Waldo A.L., Camm A.J., deRuyter H. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival with oral d-sotalol. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- 14.Elizari M.V., Martinez J.M., Belziti C. Vol. 21. 2000. Morbidity and mortality following early administration of amiodarone in acute myocardial infarction. GEMICA study investigators, GEMA Group, Buenos Aires, Argentina. Grupo de Estudios Multicentricos en Argentina; pp. 198–205. (Eur Heart J). [DOI] [PubMed] [Google Scholar]
- 15.Julian D.G., Camm A.J., Frangin G. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. European Myocardial Infarct Amiodarone Trial Investigators. Lancet. 1997;349:667–674. doi: 10.1016/s0140-6736(96)09145-3. [DOI] [PubMed] [Google Scholar]
- 16.Kober L., Bloch Thomsen P.E., Møller M. Effect of dofetilide in patients with recent myocardial infarction and left-ventricular dysfunction: a randomised trial. Lancet. 2000;356:2052–2058. doi: 10.1016/s0140-6736(00)03402-4. [DOI] [PubMed] [Google Scholar]
- 17.Singh S.N., Fletcher R.D., Fisher S.G. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med. 1995;333:77–82. doi: 10.1056/NEJM199507133330201. [DOI] [PubMed] [Google Scholar]
- 18.Herre J.M., Sauve M.J., Malone P. Long-term results of amiodarone therapy in patients with recurrent sustained ventricular tachycardia or ventricular fibrillation. J Am Coll Cardiol. 1989;13:442–449. doi: 10.1016/0735-1097(89)90525-1. [DOI] [PubMed] [Google Scholar]
- 19.Greene H.L., Graham E.L., Werner J.A. Toxic and therapeutic effects of amiodarone in the treatment of cardiac arrhythmias. J Am Coll Cardiol. 1983;2:1114–1128. doi: 10.1016/s0735-1097(83)80338-6. [DOI] [PubMed] [Google Scholar]
- 20.Singh B.N. Historical development of the concept of controlling cardiac arrhythmias by lengthening repolarization: particular reference to sotalol. Am J Cardiol. 1990;65:3A–11A. doi: 10.1016/0002-9149(90)90195-7. [discussion 35A–36A] [DOI] [PubMed] [Google Scholar]
- 21.January C.T., Wann L.S., Alpert J.S. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;2014(130):2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 22.Grönefeld G.C., Lilienthal J., Kuch K.H. Impact of rate versus rhythm control on quality of life in patients with persistent atrial fibrillation. Results from a prospective randomized study. Eur Heart J. 2003;24:1430–1436. doi: 10.1016/s0195-668x(03)00261-6. [DOI] [PubMed] [Google Scholar]
- 23.Qin D., Leef G., Alam M.B. Mortality risk of long-term amiodarone therapy for atrial fibrillation patients without structural heart disease. Cardiol J. 2015;22:622–629. doi: 10.5603/CJ.a2015.0055. [DOI] [PubMed] [Google Scholar]
- 24.Dusman R.E., Stanton M.S., Miles W.M. Clinical features of amiodarone-induced pulmonary toxicity. Circulation. 1990;82:51–59. doi: 10.1161/01.cir.82.1.51. [DOI] [PubMed] [Google Scholar]
- 25.Papiris S.A., Triantafillidou C., Kolilekas L. Amiodarone: review of pulmonary effects and toxicity. Drug Saf. 2010;33:539–558. doi: 10.2165/11532320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Akoun G.M., Gauthier-Rahman S., Milleron B.J. Amiodarone-induced hypersensitivity pneumonitis. Evidence of an immunological cell-mediated mechanism. Chest. 1984;85:133–135. doi: 10.1378/chest.85.1.133. [DOI] [PubMed] [Google Scholar]
- 27.Richer M., Robert S. Fatal hepatotoxicity following oral administration of amiodarone. Ann Pharmacother. 1995;29:582–586. doi: 10.1177/106002809502900605. [DOI] [PubMed] [Google Scholar]
- 28.Lima J., Carvalho P., Molina M.A. Thyroid dysfunction and amiodarone. Arq Bras Endocrinol Metabol. 2013;57:71–78. doi: 10.1590/s0004-27302013000100010. [DOI] [PubMed] [Google Scholar]
- 29.Fideler F.J., Dieterich H.J., Schroeder T.H. Fatal outcome during anaesthesia induction in a patient with amiodarone-induced thyrotoxicosis. Eur J Anaesthesiol. 2008;25:337–339. doi: 10.1017/S0265021507002864. [DOI] [PubMed] [Google Scholar]
- 30.Peche R., Abramowicz M., Unger J. Failure to respond to dexamethasone with fatal consequences, after initial response to multidrug treatment in a case of amiodarone-associated thyrotoxicosis. Am J Med. 1992;93:702–703. doi: 10.1016/0002-9343(92)90208-s. [DOI] [PubMed] [Google Scholar]
- 31.Vorperian V.R., Havighurst T.C., Miller S. Adverse effects of low dose amiodarone: a meta-analysis. J Am Coll Cardiol. 1997;30:791–798. doi: 10.1016/s0735-1097(97)00220-9. [DOI] [PubMed] [Google Scholar]
- 32.Mäntyjärvi M., Tuppurainen K., Ikäheimo K. Ocular side effects of amiodarone. Surv Ophthalmol. 1998;42:360–366. doi: 10.1016/s0039-6257(97)00118-5. [DOI] [PubMed] [Google Scholar]
- 33.Dootson G., Byatt C. Amiodarone-induced vasculitis and a review of the cutaneous side-effects of amiodarone. Clin Exp Dermatol. 1994;19:422–424. doi: 10.1111/j.1365-2230.1994.tb02701.x. [DOI] [PubMed] [Google Scholar]
- 34.Abe M., Maruyama T., Fujii Y. Disopyramide-induced hypoglycemia in a non-diabetic hemodialysis patient: a case report and review of the literature. Clin Nephrol. 2011;76:401–406. doi: 10.5414/cn106622. [DOI] [PubMed] [Google Scholar]
- 35.Negishi M., Shimomura K., Proks P. Mechanism of disopyramide-induced hypoglycaemia in a patient with Type 2 diabetes. Diabet Med. 2009;26:76–78. doi: 10.1111/j.1464-5491.2008.02619.x. [DOI] [PubMed] [Google Scholar]
- 36.Reidenberg M.M., Drayer D.E. Procainamide, N-acetylprocainamide, antinuclear antibody and systemic lupus erythematosus. Angiology. 1986;37:968–971. [PubMed] [Google Scholar]
- 37.Danielly J., DeJong R., Radke-Mitchell L.C. Procainamide-associated blood dyscrasias. Am J Cardiol. 1994;74:1179–1180. doi: 10.1016/0002-9149(94)90478-2. [DOI] [PubMed] [Google Scholar]
- 38.Johansson B.W., Stavenow L., Hanson A. Long-term clinical experience with mexiletine. Am Heart J. 1984;107:1099–1102. doi: 10.1016/0002-8703(84)90181-9. [DOI] [PubMed] [Google Scholar]
- 39.Fasola G.P., D’Osualdo F., de Pangher V. Thrombocytopenia and mexiletine. Ann Intern Med. 1984;100:162. doi: 10.7326/0003-4819-100-1-162_1. [DOI] [PubMed] [Google Scholar]
- 40.Odeh M., Seligmann H., Oliven A. Propafenone-induced ataxia: report of three cases. Am J Med Sci. 2000;320:151–153. doi: 10.1097/00000441-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 41.Baker B.J., Dinh H., Kroskey D. Effect of propafenone on left ventricular ejection fraction. Am J Cardiol. 1984;54:20D–22D. doi: 10.1016/s0002-9149(84)80280-5. [DOI] [PubMed] [Google Scholar]
- 42.Gandolfi A., Rota E., Zanghieri G. Acute cholestatic hepatitis caused by propafenone. Report of a case and review of the literature. Recent Prog Med. 2001;92:197–199. [PubMed] [Google Scholar]