Abstract
Background
To evaluate the efficacy of perioperative atorvastatin administration for prophylaxis of postoperative atrial fibrillation (POAF) after heart valve surgery.
Methods
Our study included 90 patients with heart valve disease who were scheduled to undergo elective cardiac surgery. Cases with previous AF or preoperative beta-blocker therapy were excluded. Patients were randomized into the atorvastatin group, which included 47 patients who received 40 mg/day of atorvastatin 7 days before and after the surgery and the control group, which included 43 patients. Primary endpoint was the occurrence of POAF. Secondary endpoints included modifications in the preoperative and postoperative levels of the markers of inflammation (C-reactive protein [CRP]), myocardial injury (ultrasensitive troponin T and creatinine phosphokinase MB [CPK-MB]), and cardiac dysfunction (pro-brain natriuretic peptide [proBNP]) related to POAF and changes in the echocardiographic parameters, such as atrial electromechanical interval, A wave, E/A ratio, and Doppler imaging systolic velocity wave amplitude, related to POAF.
Results
No relationship between atorvastatin administration and reduction in the incidence of POAF was observed (42.6% in the atorvastatin vs. 30.2% in the control group) (p=0.226). No difference in the levels of CPK-MB, ultrasensitive troponin T, CRP, or proBNP and in the analyzed echocardiographic parameter was detected between both groups.
Conclusions
Atorvastatin in the described dose, was not adequate for the prophylaxis of POAF after heart valve surgery. It was ineffective in controlling the inflammatory phenomena, myocardial injury, and echocardiographic predictors of POAF.
Keywords: Atrial fibrillation, Atorvastatin, Inflammation, Surgery, Trials
1. Introduction
Atrial fibrillation (AF) is a common finding in the postoperative course after cardiac surgery. It occurs in 20–40% of the patients after coronary artery bypass graft (CABG) and in about 50% of those who undergo cardiac valve surgeries [1], [2], [3]. Clinical manifestations of postoperative AF (POAF) range from asymptomatic forms to heart failure and/or embolic events, which increase the cost and in-hospital stay [4], [5]. POAF etiology involves electrical, metabolic, neurohumoral, and inflammatory disorders that modify the atrial geometry and electrophysiology. Effective therapies preventing POAF can cause hypotension and symptomatic bradycardia, both undesirable side effects after cardiac surgery and unfortunately, its incidence has not been reduced significantly during last decade. Pleiotropic effects of atorvastatin have been considered protective against POAF [6], [7], [8]. Atorvastatin reduces postoperative Systemic Inflammatory Response Syndrome (SIRS); prevents atrial remodeling and modulates thrombogenesis, oxidation and atrial fibrosis. A direct electrophysiological effect inhibiting calcium increase in the atrial myocyte, thus favoring anti-sympathetic action of the autonomic nervous system, has also been proposed. Randomized studies regarding atorvastatin use [6] have reported a 40–60% reduction in POAF incidence avoiding undesirable side effects. Nevertheless, all these studies were biased in sample selection (predominantly included CABG procedures), drug dosing, and concomitant administration of beta-blockers (recognized as prophylactic therapy for POAF).
PROFACE aimed to investigate the efficacy of atorvastatin in preventing POAF and SIRS. Atorvastatin, 40 mg/day, was administered 7 days before and after elective heart valve surgery in a randomized patient population with no preoperative beta-blocker treatment or previous episodes of AF. The justification for atorvastatin dosage was based on the increased prevalence of POAF following valve surgery and on the proposed superior prophylactic effect of moderate vs. low doses of atorvastatin [9], [10]. A high-dose regimen (80 mg) was not considered because of the higher risk of serious adverse events and no demonstrated significant POAF incidence reduction compared to a low-dose regimen [11].
2. Materials and methods
2.1. Study population and trial design
PROFACE was an interventional, open-label, randomized, unicenter, prospective, and parallel-assignment trial authorized by the Spanish Agency of Medicine and Sanitary Products (Number EudraCT "2009-011964-12"). The trial population included patients who were scheduled to undergo heart valve surgery at the University Hospital of Valladolid (Spain). The study was conducted in accordance with the Declaration of Helsinki, the protocol was approved by the locally appointed ethics committee, and informed consent was obtained from all the subjects.
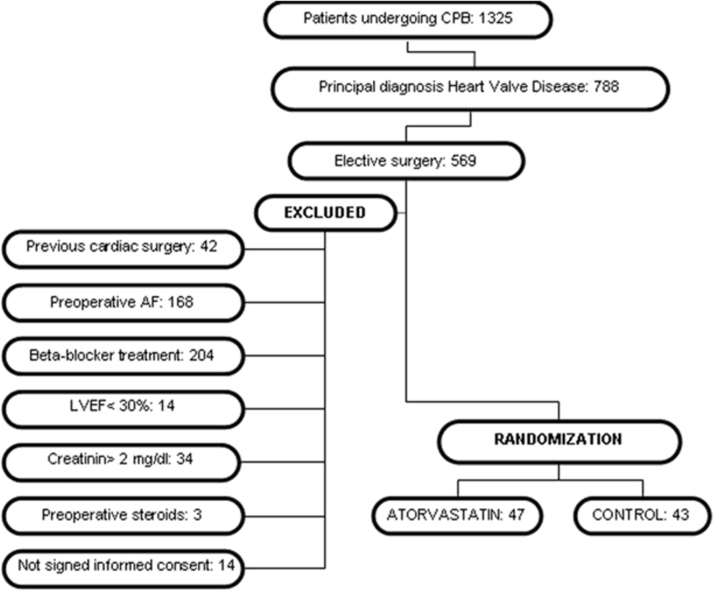
The study included men and women over 18 years of age with sinus rhythm and primary diagnosis of heart valve disease (isolated or associated with coronary artery disease) satisfying the requirements for heart surgery under cardiopulmonary bypass (CPB); women of childbearing potential were asked to use effective contraception and commit to maintain it throughout the study. The exclusion criteria were as follows: urgent surgery; endocarditis; patients with previous episodes of AF; beta-blocker therapy at randomization; severe left ventricular dysfunction (left ventricular ejection fraction<30%); chronic use of nonsteroidal anti-inflammatory drugs and/or corticosteroids; uncontrolled thyroid disease; previous statin therapy; active liver disease and/or history of previous chronic liver disease; alcoholism; predisposing factors to statin side effects such as increased transaminase levels at baseline (×3 normal value), renal failure (creatinine levels>2 mg/dL), previous diagnosis of myopathy of any etiology, and known hypersensitivity to calcium atorvastatin and/or lactose monohydrate; women with positive pregnancy test on the day of inclusion in the study; unsigned informed consent form; inability to understand study objectives. The exclusion criteria after study initiation were consent withdrawal and changes in liver function laboratory parameters (transaminases>×3 the normal value) and/or creatine-phosphokinase (CPK) level suggesting adverse effects of statins. A detailed diagram of the patient selection process is shown in Fig. 1.
Fig. 1.
Patient selection process for inclusion/exclusion in PROFACE clinical trial. CPB: cardiopulmonary bypass; AF: atrial fibrillation; LVEF: left ventricular ejection fraction.
Among the patients who fulfilled the inclusion criteria and authorized their participation, 47 were randomized to the atorvastatin group (atorvastatin, 40 mg/day, administered 7 days prior and after the surgery) and 43 to the control group (without added treatment or placebo administration). We administered atorvastatin until the 7th postoperative day (POD). Long-term treatment was not considered necessary, as maximum occurrence of POAF and SIRS has been observed during the 2nd and 3rd PODs.
2.2. Sample size
POAF incidence after heart valve surgery ranges between 40% and 50%. If we hypothesize a 50% reduction in the treatment arm, a sample size of 246 patients would provide 90% statistical power to detect with an α-level between 0.05 and 0.01 and a β-error of 0.20. The sample size was calculated using the C4-Study Design packaging program (Glaxo Wellcome, v 1.1). Randomization was performed (at the time of patient inclusion) by an independent statistician, who was blinded about the treatment or clinical decisions affecting the patients. Clinical and echocardiographic variables were measured preoperatively. After the surgery, all the patients were continuously monitored using ECG until the 7th POD (inclusive). Events related to cardiac rhythm disturbances were recorded daily and analyzed by a cardiologist, who was blinded to the treatment group and the clinical management received by the patients. Before hospital discharge (on the 6th POD), echocardiography control was achieved in all the patients.
Additionally, a biochemical study, including high-sensitive cardiac troponin T (hs-cTnT), C-reactive protein (CRP), pro-brain natriuretic peptide (proBNP), renal and hepatic profiles, hemogram, and coagulation profile, was obtained after study randomization, before surgical incision, and at 6, 24, 48, and 72 h after the surgery. Concentrations of proBNP were measured using electrochemiluminescence immunoassay on an Elecsys 2010 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Hs-cTnT was determined using a highly sensitive electrochemiluminescence immunoassay (Elecsys Troponin T hs) on an Elecsys 2010 analyzer. All the CRP concentrations were determined by turbidometry using a high-sensitivity commercial kit (Hitachi 911; Boehringer Mannheim).
2.3. Echocardiographic methodology
The Atrial electromechanical interval (AEI), which is the time in milliseconds from the onset of electrocardiographic P wave to the beginning of atrial systole (backward movement in the atrioventricular plane), was measured on the lateral surface of the left atrium. Measurements from the four chambers were obtained in apical view from the mitral lateral ring in transthoracic echocardiography.
A-wave, the late atrial filling velocity (cm/s), was measured using pulse-wave Doppler across the mitral valve. It was calculated from the average speed of 5 consecutive beats in M-Mode.
2.4. End points
The primary endpoint of the trial was the efficacy of atorvastatin in preventing POAF after valve surgery. For evaluation, we considered POAF episodes lasting ≥5 min and those lasting <5 min accompanied with hemodynamic disturbances.
The secondary endpoints included correlation between variation of the inflammatory markers during CPB and POAF incidence, indicating a possible atorvastatin modulator effect; modifications in echocardiographic parameters (owing to atorvastatin) conventionally related to POAF; frequency, duration, and clinical characteristics of AF after heart valve surgery; clinical and hemodynamic consequences of POAF; and economic repercussion, depending on hospital stay prolongation.
2.5. Statistical methods
Statistical analysis was performed using the Statistical Package for the Social Sciences software, version 22.0. Quantitative variables were expressed as mean±standard deviation or as median, for asymmetric distributions. Qualitative variables were expressed as absolute value and percentage. The association between variables was identified using the chi-square or Fisher exact test for qualitative variables and the Student’s t-test or Mann Whitney U test for quantitative variables. The association between the risk factors and analyzed events in univariable analysis (p<0.2) was introduced in logistic multivariable regression. p<0.05 (2-tailed) was considered significant.
3. Results
3.1. Study population
The patients were recruited between February 2011 and October 2013. A lack of economic support forced early termination of the clinical trial in May 2014. A total of 47 patients were randomized to the atorvastatin group and 43 to the control group. Neither adverse reactions nor dropouts were observed in the atorvastatin group. The clinical, demographic, and surgical variables are detailed in Table 1, Table 2.
Table 1.
Demographic profile and clinical characteristics.
| Variable | Atorvastatin group, n (%) | Control group, n (%) | p |
|---|---|---|---|
| Female sex | 19 (40.4) | 12 (27.9) | 0.212 |
| Smokers | 7 (14.9) | 5 (11.6) | 0.649 |
| Arterial hypertension | 31 (66) | 21 (48.8) | 0.1 |
| Peripheral arteriopathy | 0 (0) | 0 (0) | |
| Diabetes | 13 (27.7) | 5 (11.6) | 0.058 |
| Dyslipidemia | 19 (40.4) | 18 (41.9) | 0.89 |
| Renal failure | 0 (0) | 0 (0) | |
| COPD | 7 (14.9) | 4 (9.3) | 0.419 |
| Previous AMI | 0 (0) | 0 (0) | |
| Obesity (BMI>30) | 16 (34) | 9 (21) | 0.165 |
| Cerebrovascular accident | 2 (4.3) | 0 (0) | 0.495 |
| Left main coronary disease | 0 (0) | 0 (0) | – |
| Angina | 18 (38.3) | 15 (34.9) | 0.737 |
| NYHA III–IV | 6 (12.8) | 10 (23.3) | 0.194 |
| Moderate left ventricular dysfunction | 3 (6.4) | 3 (7) | 1 |
| Preoperative treatment: | 38 (80.9) | 32 (76.2) | 0.592 |
| Antiplatelet drugs | 3 (6.3) | 4 (9.3) | 0.605 |
| Digoxin | 0 (0) | 1 (2.4) | 0.478 |
| ACE inhibitors | 29 (59.6) | 17 (39.5) | 0.058 |
| Calcium antagonists | 2 (4.3) | 1 (2.3) | 1 |
| Mean (SD) | Mean (SD) | ||
| Age (years) | 67.4 (11.2) | 65.5 (12.0) | 0.489 |
| Weight (kg) | 73.3 (12.7) | 73.9 (13.9) | 0.997 |
| Height (cm) | 162 (10.9) | 165 (9.2) | 0.241 |
COPD: chronic obstructive pulmonary disease; AMI: acute myocardial infarction; BMI: body mass index; ACE: angiotensin-converting enzyme; SD: standard deviation; NYHA: New York Heart Association.
Table 2.
Surgical data.
| Perioperative variables | Atorvastatin group, n (%) | Control group, n (%) | p |
|---|---|---|---|
| Valve surgery+CABG | 5 (10.6) | 4 (9.3) | 1 |
| Mitral valve replacement | 3 (6.4) | 2 (4.7) | 0.483 |
| Aortic valve replacement | 43 (91.5) | 41 (95.3) | |
| Mitral and aortic valve replacement | 1 (2.1) | 0 (0) | |
| Prosthesis | |||
| Mechanical | 19 (40.4) | 21 (48.8) | 0.702 |
| Bioprosthesis | 26 (55.3) | 20 (46.5) | |
| Valve repair | 2 (4.3) | 2 (4.7) | |
| Drugs after surgery | |||
| No | 33 (70.2) | 33 (76.7) | 0.147 |
| Inotropics (0.5–10 mcg/kg/min) | 7 (14.9) | 7 (16.3) | |
| Inotropics (>10 mcg/kg/min) | 4 (8.5) | 0 (0) | |
| Vasodilators | 3 (6.4) | 3 (7) | |
| Heart rhythm after declamping | |||
| Sinus | 46 (97.6) | 41 (95.3) | 0.473 |
| Atrial fibrillation | 3 (6.4) | 1 (2.3) | |
| Atrioventricular block | 1 (2.1) | 1 (2.3) | |
| Mean (SD) | Mean (SD) | ||
| CBP time (min) | 103.94 (40.3) | 99.44 (38.99) | 0.541 |
| Aortic clamp time (min) | 79.89 (35.64) | 75.53 (33.99) | 0.625 |
| Electrical cardioversion | 1.93 (2.07) | 2.34 (2.89) | 0.443 |
| Coronary bypass graft | 0.093 (0.29) | 0.19 (0.68) | 0.786 |
| RBC transfusion (units) | 1.19 (1.34) | 1.49 (3.41) | 0.395 |
CABG: coronary artery bypass graft; CBP: cardiopulmonary bypass; RBC: red blood cell; SD: standard deviation.
3.2. Primary endpoint
The prevalence of POAF was 36.7%; 42.6% in the atorvastatin group vs. 30.2% in the control group (p=0.226). In all the patients, POAF developed on the 2nd POD, with a median of 45 h after surgery. Although the median number of episodes was 3 in both groups, the median duration in the atorvastatin group (22 min) was superior compared to that in the control group (7 min). During electrocardiographic monitoring, no significant differences in cardiac frequency were observed between the groups.
3.3. Secondary endpoints
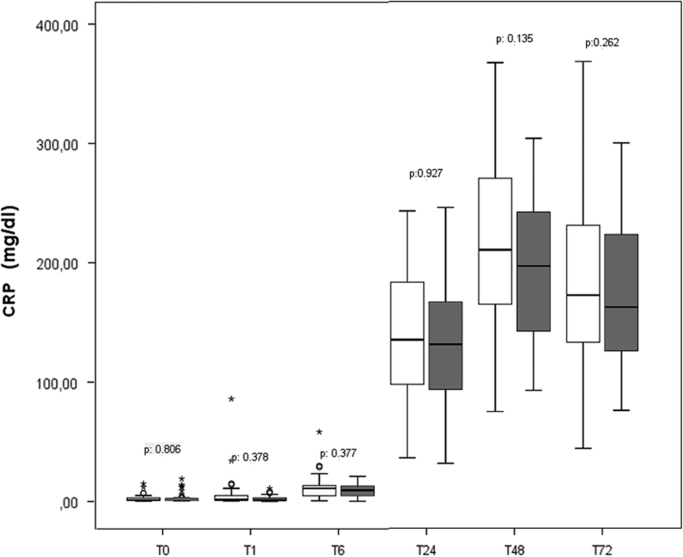
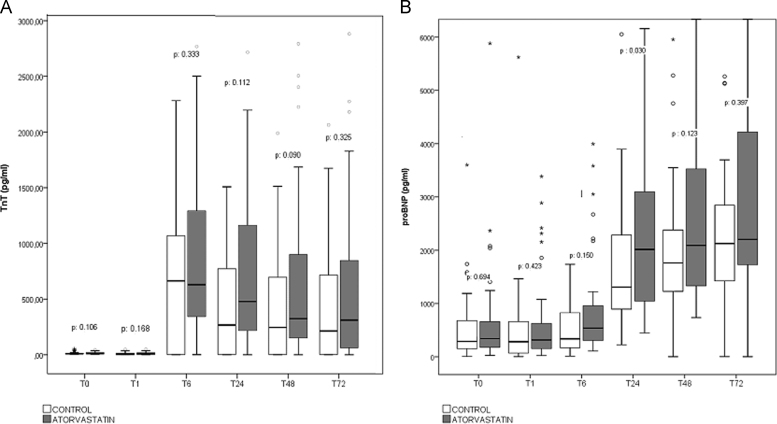
The CRP values were slightly lower in the atorvastatin group (Fig. 2); however, no significant difference was observed during the first 72 h after surgery. We did not find differences in the platelet and leukocyte counts during the same period. As shown in Fig. 3A, no cardio-protective effect attributable to atorvastatin was identified; hs-cTnT levels were slightly higher, but not statistically significant, in the atorvastatin group. No difference was observed in the CPK or CPK-MB levels. Preoperative proBNP values were increased in all the participants (median of 288 pg/mL and 342 pg/mL in the control and atorvastatin group, respectively); the variation in the values during the monitoring did not show differences related to atorvastatin treatment. However, a significant increase in proBNP level in the atorvastatin group was observed 24 h after the surgery (2277.61±13399.78 pg/mL vs. 2006.95±2237.67 pg/mL in the atorvastatin vs. the control group, respectively) (p=0.030), but no significant differences were identified in subsequent evaluations (Fig. 3B) (Supplementary materials).
Fig. 2.
Monitored levels of C-reactive protein (CRP) before and during the first 72 h after surgery.
Fig. 3.
(A) Monitored levels of cardiac high-sensitive cardiac troponin T (TnT) and (B) pro-brain natriuretic peptide (proBNP) before and during the first 72 h after surgery.
All the patients included in the PROFACE trial showed moderate pulmonary hypertension, mild diastolic dysfunction, and impaired left ventricular relaxation in preoperative echocardiography. Consequently, a longer A-wave and normal or pseudonormal Doppler E-wave filling were observed. The preoperative values of left atrial diameter and left atrial volume index with mild or moderate preoperative dilatation correlated in both groups. No differences attributable to atorvastatin treatment were identified in the postoperative echocardiographic parameters associated with POAF in previous studies (AEI, A-wave, left atrial volume and diameter, and E/A ratio) or in the other analyzed parameters (Table 3).
Table 3.
Preoperative and postoperative echocardiographic variables.
| Variable |
Preoperative |
Postoperative |
||||
|---|---|---|---|---|---|---|
| Atorvastatin group, n (%) | Control group, n (%) | p | Atorvastatin group, n (%) | Control group, n (%) | p | |
| LVEF (%) | 64.38 (7.09) | 62.88 (8.02) | 0.300 | 63.43 (8.55) | 61.98 (8.63) | 0.434 |
| PASP (mmHg) | 34.62 (5.23) | 36.07 (5.69) | 0.314 | 36.89 (6.44) | 36.13 (5.79) | 0.633 |
| TAMG (mmHg) | 56.78 (21.08) | 54.08 (24.22) | 0.651 | 21.29 (7.92) | 20.26 (8.2) | 0.767 |
| TMMG (mmHg) | 3.63 (1.73) | 6.23 (3.7) | 0.302 | 5.28 (2) | 3.95 (2.07) | 0.286 |
| Aortic area (cm2) | 0.75 (0.29) | 0.97 (0.74) | 0.235 | 1.53 (0.57) | 1.43 (0.24) | 0.923 |
| Mitral area (cm2) | 2.2 (0.25) | 3.09 (1.61) | 0.286 | 2.43 (0.52) | 3.2 (1.25) | 0.402 |
| LAD (mm) | 42.89 (5.6) | 43.23 (6.1) | 0.785 | 43.21 (5.49) | 43.06 (6) | 0.911 |
| A wave (m/s) | 1.04 (0.34) | 0.94 (0.34) | 0.179 | 1.35 (2.05) | 0.92 (0.32) | 0.136 |
| AEI (ms) | 67.89 (18.1) | 68.93 (17.85) | 0.791 | 55.58 (20.73) | 59.4 (17.47) | 0.390 |
| LAV (mL) | 74.3 (27.95) | 75.42 (27.48) | 0.850 | 70.72 (22.57) | 69.2 (20.5) | 0.932 |
| LAVI (mL/m2) | 41.54 (14.96) | 42.36 (16.82) | 0.809 | 39.72 (11.53) | 38.8.(12.42) | 0.760 |
| E/A ratio | 0.92 (0.43) | 0.92 (0.40) | 0.997 | 1.08 (0.34) | 1.21 (0.49) | 0.173 |
| TAPSE | 21.84 (3.29) | 20.88 (3.48) | 0.197 | 12.31 (2.72) | 12.52 (2.84) | 0.655 |
LVEF: left ventricular ejection fraction; PASP: pulmonary artery systolic pressure; TAMG: transaortic mean gradient; TMMG: transmitral mean gradient; LAD: left atrial diameter; AEI: atrial electromechanical interval; LAV: left atrial volume; LAVI: left atrial volume index; TAPSE; tricuspid annular plane systolic excursion.
In multivariate analysis, we observed no differences between the groups regarding independent predictors of POAF, morbidity, or length of hospital stay (Table 4). In all the participants, age >65 years (p=0.007; odds ratio [OR], 4.80; 95% confidence interval [CI], 1.52–15.14) and a longer preoperative AEI (p=0.042; OR, 1.029; 95% CI, 1.001–1.059) were independent predictors of POAF. One patient in each group died because of cardiogenic and hemorrhagic shock, respectively.
Table 4.
Postoperative complications and characteristics of POAF.
| Variable | Atorvastatin group n (%) | Control group n (%) | p |
|---|---|---|---|
| POAF | 20 (42.6) | 13 (30.2) | 0.226 |
| Arterial hypotension | 3 (6.4) | 2 (4.7) | 1 |
| Low cardiac output | 3 (6.4) | 0 (0) | 0.243 |
| Perioperative myocardial infarction | 2 (4.3) | 0 (0) | 0.495 |
| Cerebrovascular accident | 1 (2.1) | 1 (2.3) | 1 |
| Coma | 0 (0) | 1 (2.3) | 0.478 |
| Dialysis | 3 (6.4) | 0 (0) | 0.243 |
| Respiratory failure | 2 (4.3) | 0 (0) | 0.495 |
| Mean (SD) | Mean (SD) | ||
| ICU-stay (d) | 3.34 (4.75) | 2.79 (2.49) | 0.444 |
| Hospital stay (d) | 10.22 (7.22) | 8.28 (3.28) | 0.262 |
| POAF longer than 5 min | 1.89 (3) | 1.62 (5.07) | 0.128 |
| Duration of POAF (min) | 156.78 (306.84) | 52.31 (10.26) | 0.447 |
| POD | 2.46 (1.61) | 2.45 (1.28) | 0.789 |
| Time (h) before the first episode of POAF | 52.9 (35.8) | 52 (40.57) | 0.854 |
| Mechanical ventilation (h) | 16.9 (44.44) | 8.13 (8.14) | 0.577 |
POAF: postoperative atrial fibrillation; ICU: intensive care unit; POD: postoperative day; SD: standard deviation.
Follow-up was conducted in all the surviving patients (median, 30 months; range: 13–45 months). Only one patient died during this period due to late prosthetic valve endocarditis. About 20% of the patients suffered at least 1 episode of AF recurrence (23.9% and 14.3% in the atorvastatin and control group, respectively) (p=0.383). Embolic phenomena related to AF were diagnosed in 2 patients, 1 patient had transient ischemic attack (32 d after hospital discharge) and 1 had peripheral embolism.
4. Discussion
Data regarding the efficacy of atorvastatin in prevention of POAF [6], [9], [12], [13.] are controversial. Although reduction in POAF incidence was identified in some studies, statistical relevance was not observed in most of them [10], [11], [14], [15]. Kuhn et al. included >90,000 patients in their meta-analysis; however, only 2661 (2.9%) (3 studies) had undergone isolated valve surgery procedures [16]. Among these 3 studies, only 1 [17] considered POAF reduction as an endpoint, and no decrease in the incidence related to statin therapy was observed. New-onset POAF was analyzed only in 30.8% of the patients (6 studies) [16]. All the 6 studies evaluated patients who had undergone combined (valve+CABG) procedures and none of them analyzed new-onset POAF prevalence after isolated valve procedures. Among these 6 studies, the study conducted by Borger et al. [18] did not reveal any benefit of preoperative statin administration in valve surgery patients, in spite of including an increased number of patients on combined treatment (beta-blockers+statins). In the Kourliouros et al. study [10], 78% of the included patients (including patients on beta-blocker therapy) had undergone CABG procedures; the study revealed that the incidence of POAF reduction was significantly increased among patients who received high-dose statin treatment. Only 18% patients with isolated valve procedures were preoperatively under statin treatment. Therefore, their conclusions were not applicable to this group of patients. The Lertsburapa et al. study [19] included 24% of the patients who had undergone isolated valve procedures and only 9% of them were under preoperative statin treatment. New-onset POAF prevalence was significantly higher among patients who had undergone valve procedures and no preventive effect of statins was observed. In the Patti et al. study [9], atorvastatin was effective in POAF prevention after CABG surgery, but not in isolated valve procedures (16%); 66% of the patients had also received preoperative beta-blocker treatment. Although they concluded that high-dose statin (but not low-dose) combined with beta-blocker treatment might influence POAF prevention, neither perioperative beta-blocker therapy nor previous episodes of AF were excluded in this study. Similar populations have been observed and similar results have been showed in a more recent meta-analysis [20].
Thus, we believe that our hypothesis has not been consistently explored before, and our results and data can be considered as original and useful in clinical practice. Although it seems that perioperative statin treatment prevents POAF in CABG patients and potentiates the effect of beta-blocker therapy, the results are questionable in valve procedure patients, as shown in the abovementioned studies. The novelty and objective of our study was based on the fact that so far, no report had analyzed the prophylactic efficacy of perioperative statin administration in preventing new-onset POAF in patients with predominant or isolated valve pathology excluding the confounding factor represented by concomitant beta-blocker treatment.
Premature termination and reduction in the sample size of the PROFACE trial make its conclusions about atorvastatin efficacy in preventing POAF after heart valve surgery questionable. Nevertheless, no POAF incidence reduction trend was identified in this group of patients. Our results are similar to those observed in the subgroup of heart valve surgery patients in the “Atorvastatin for reduction of myocardial dysrhythmia after cardiac surgery” (ARMYDA-3) trial [9] and contradict relevant findings observed in the clinical trials including CABG patients [6], [9], [12], [21] in which atorvastatin, even in a low-dose regimen, reduced POAF prevalence by 40–60%. Population, timing, and different methods of quantification of POAF make comparison of various studies difficult [22]. Compared to previous studies, several factors influenced the different conclusions observed in our clinical trial. First, valve pathology and not coronary artery disease was the main diagnosis. Beneficial effect of POAF prevention attributed to atorvastatin therapy was previously observed only in patients who had undergone CABG surgery [6], [9], [12]. Besides, PROFACE trial design prevented bias related to direct or synergistic POAF prevention due to combined effect of beta-blocker and statin therapy (common treatment in coronary artery disease patients) [7], [11], [12], [15], [21], [22], [23], [24].
Effect of atorvastatin in modulation and protection of cardiomyocyte ischemia and inflammatory phenomena (identified as a decline in perioperative CRP, hs-cTnT, and CPK-MB levels) [15], [25], [26], [27] was not confirmed in the PROFACE trial. Because of no significant differences in the clinical preoperative or intraoperative variables between the treatment groups, we observed that preoperative characteristics of heart valve surgery population could lead to a different physiological response to atorvastatin administration. Although not significant, diabetic patients were more frequently randomized to the atorvastatin group. Association between diabetes, inflammation, and pharmacological response variability following administration of atorvastatin in diabetic patients [28] could explain atorvastatin failure in decreasing serum CRP levels in this group. However, no significant differences in the CRP values were observed on comparing the diabetic patients randomized to the atorvastatin or control group. The percentage of diabetic patients in our study was significantly lower than that in previous studies including CABG patients, in whom peak CRP levels were significantly lower than those observed in the PROFACE trial [9], [15], [21]. In our study population, the lack of effectiveness of atorvastatin in reducing inflammatory biomarkers was significantly related to heart valve disease and preoperative variables, such as old age [29], increased preoperative left atrial volume index, and preoperative pulmonary hypertension, associated with increased circulating CRP levels [15], [30], [31]. Such a conclusion may also be applied to myocardial damage biomarkers, such as Hs-cTnT and CPK-MB, that increased proportional to the degree of surgical trauma, and were higher in patients undergoing heart valve procedure than in those undergoing CABG procedures. Atrial remodeling was infrequent in patients with isolated coronary disease and this might explain the more effective protection against POAF observed in this group. This hypothesis was confirmed after analyzing the clinical features of the ARMYDA-3 trial population. In this group of patients, arterial hypertension and diastolic dysfunction were the most significant risk factors for POAF [9]. AEI, the elongation of which is correlated with POAF [30], [32], was not significantly modified in PROFACE patients. AEI length was shorter in patients of the PROFACE trial than that in CABG patients of previous trials (probably because beta-blockers were excluded), and it was not modified by atorvastatin therapy. Prophylactic atorvastatin effect in atrial remodeling observed in CABG patients is probably minimized due to a summative effect of advanced age, atrial dilatation, diastolic dysfunction, and pulmonary hypertension, evidenced in both the groups of patients in PROFACE trial.
Left ventricular diastolic dysfunction and increased left atrial volume were associated with higher prevalence of POAF [33], [34]. Neurohumoral expression in both phenomena correlated with the serum levels of proBNP [30], [31]. The proBNP levels in patients with valvular AF increased proportional to the progression of heart valve disease [33], [34]. In patients with aortic stenosis, a high preoperative serum proBNP level was associated with poor surgical prognosis [35]. The purpose of preoperative statin therapy in patients with valve disease was to regulate remodeling of myocardial hypertrophy, limit isoprenoid production, and consequently, reduce serum proBNP levels [7]. Proportional to the type of patients included in our clinical trial, the median value of preoperative proBNP was, at least, twice the normal reference values. The significantly higher values of proBNP observed in the atorvastatin group at 24 h disappeared when the patients requiring higher inotropic support (>10 mcg/kg/min) during this period were excluded from the analysis. The rise in proBNP level was due to a certain grade of postoperative ventricular dysfunction. In the analyzed population, increase in the proBNP level was more related to heart valve disease progression and grade of ventricular dysfunction than to atrial enlargement leading to POAF. This would explain the ineffectiveness of atorvastatin in POAF prevention and the conflicting results with previous studies about the influence of atorvastatin in reducing N-terminal-proBNP in patients affected by dilated cardiomyopathy [31], [35].
4.1. Limitations
This was a single-center clinical trial conducted in a small group of patients. The findings may have been affected by mandatory reduction of the sample size due to premature closure of the trial for economic reasons. There were no data related to prolonged follow-up and possible effects of atorvastatin on the incidence of AF in medium and long-term periods, as suggested by some authors. The specific demographic and clinical features of the PROFACE trial population are difficult to compare with those of previous trial population. Current results do not exclude the fact that the expected effect could be obtained with other statins or with a different dose and time of administration.
Although of no statistical significance, the number of patients under preoperative angiotensin-converting enzyme (ACE) inhibitors therapy and with comorbid diabetes was greater in the atorvastatin group than in the control group (Table 1). ACE inhibitors (identified by some studies as potential POAF prophylactic treatment) [7] might have contributed in decreasing the prevalence of POAF among this group of patients. Regarding diabetes, the variability of atorvastatin pharmacological response among diabetic patients might have modified the POAF incidence. Therefore, both situations could be proposed as confounding factors in our study.
5. Conclusion
A POAF incidence reduction trend was not identified in heart valve patients under prophylactic atorvastatin treatment at described doses. Atorvastatin was ineffective in reducing inflammatory phenomena and myocardial ischemic injury or in controlling echocardiographic POAF predictors. There is no evidence to recommend routine atorvastatin therapy in heart valve surgery patients without concomitant dyslipidemia or coronary disease.
Financial support
This research was supported by a grant from the ׳Gerencia de Salud, Consejería de Sanidad, Junta de Castilla y Leon׳ [GRS 308/A/08] and ‘Caja Burgos Foundation’ [Convocatoria 2007].The funding organization had no involvement in the study.
Clinical trial registration
https://clinicaltrials.gov/ct2/results?term=proface&Search=Search
Number EudraCT "2009-011964-12"
ClinicalTrials.gov identifier: NCT01570530
Conflict of interest
The authors declare no conflict of interest related to this study.
Acknowledgments
We would like to acknowledge Pedro Mota, Fe Muñoz, and all the postoperative cardiac surgery nurses for their work in this trial.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.joa.2016.01.010.
Contributor Information
Yolanda Carrascal, Email: ycarrascal@hotmail.com.
Roman J. Arnold, Email: roman.arnold@hotmail.com.
Luis De la Fuente, Email: lfuentegalan@gmail.com.
Ana Revilla, Email: arevillaorodea@gemail.com.
Teresa Sevilla, Email: tereseru@gmail.com.
Nuria Arce, Email: nuriaarce@yahoo.es.
Gregorio Laguna, Email: goyotxmed@hotmail.com.
Pilar Pareja, Email: pilarparejapelaez@gmail.com.
Miriam Blanco, Email: maomyriam@hotmail.com.
Appendix A. Supplementary material
Supplementary material
References
- 1.Mariscalco G., Biancari F., Zanobini M. Bedside tool for predicting the risk of postoperative atrial fibrillation after cardiac surgery: the POAF score. J Am Heart Assoc. 2014;3:e000752. doi: 10.1161/JAHA.113.000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen J., Lall S., Zheng V. The persistent problem of new-onset postoperative atrial fibrillation: a single-institution experience over two decades. J Thorac Cardiovasc Surg. 2011;141:559–570. doi: 10.1016/j.jtcvs.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mariscalco G., Engström K.G. Atrial fibrillation after cardiac surgery: risk factors and their temporal relationship in prophylactic drug strategy decision. Int J Cardiol. 2008;129:354–362. doi: 10.1016/j.ijcard.2007.07.123. [DOI] [PubMed] [Google Scholar]
- 4.Sareh S., Toppen W., Mukdad L. CHADS2 score predicts atrial fibrillation following cardiac surgery. J Surg Res. 2014;190:407–412. doi: 10.1016/j.jss.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 5.McKeown P.P., Gutterman D. American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128(2 Suppl):1S–5S. doi: 10.1378/chest.128.2_suppl.1s. [DOI] [PubMed] [Google Scholar]
- 6.Liakopoulos O., Kuhn E., Slottosch I. Preoperative statin therapy for patients undergoing cardiac surgery. Cochrane Database Syst Rev. 2012;4:CD008493. doi: 10.1002/14651858.CD008493.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Pinho-Gomes A.C., Reilly S., Brandes R.P. Targeting inflammation and oxidative stress in atrial fibrillation: role of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibition with statins. Antioxid Redox Signal. 2014;20:1268–1285. doi: 10.1089/ars.2013.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marín F., Pascual D.A., Roldán V. Statins and postoperative risk of atrial fibrillation following coronary artery bypass grafting. Am J Cardiol. 2006;97:55–60. doi: 10.1016/j.amjcard.2005.07.124. [DOI] [PubMed] [Google Scholar]
- 9.Patti G., Chello M., Candura D. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for reduction of myocardial dysrhythmia after cardiac surgery) study. Circulation. 2006;114:1455–1461. doi: 10.1161/CIRCULATIONAHA.106.621763. [DOI] [PubMed] [Google Scholar]
- 10.Kourliouros A., De Souza A., Roberts N. Dose-related effect of statins on atrial fibrillation after cardiac surgery. Ann Thorac Surg. 2008;85:1515–1520. doi: 10.1016/j.athoracsur.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Kourliouros A., De Souza A., Roberts N. Preoperative high-dose atorvastatin for prevention of atrial fibrillation after cardiac surgery: a randomized controlled trial. J Thorac Cardiovasc Surg. 2011;141:244–248. doi: 10.1016/j.jtcvs.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Chello M., Patti G., Candura D. Effects of atorvastatin on systemic inflammatory response after coronary bypass surgery. Crit Care Med. 2006;34:660–667. doi: 10.1097/01.CCM.0000201407.89977.EA. [DOI] [PubMed] [Google Scholar]
- 13.Saso S., Vecht J.A., Rao C. Statin therapy may influence the incidence of postoperative atrial fibrillation what is the evidence? Tex Heart Inst J. 2009;36:521–529. [PMC free article] [PubMed] [Google Scholar]
- 14.Liakopoulos O.J., Choi Y., Haldenwang P.L. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: a meta-analysis of over 30,000 patients. Eur Heart J. 2008;29:1548–1559. doi: 10.1093/eurheartj/ehn198. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y., Ji Q., Mei Y. Role of preoperative atorvastatin administration in protection against postoperative atrial fibrillation following conventional coronary artery bypass grafting. Int Heart J. 2011;52:7–11. doi: 10.1536/ihj.52.7. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn E.W., Liakopoulos O.J., Stange S. Preoperative statin therapy in cardiac surgery: a meta-analysis of 90,000 patients. Eur J Cardiothorac Surg. 2014;45:17–26. doi: 10.1093/ejcts/ezt181. [DOI] [PubMed] [Google Scholar]
- 17.Virani S.S., Nambi V., Razavi M. Preoperative statin therapy is not associated with a decrease in the incidence of postoperative atrial fibrillation in patients undergoing cardiac surgery. Am Heart J. 2008;155:541–546. doi: 10.1016/j.ahj.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Borger M.A., Seeburger J., Walther T. Effect of preoperative statin therapy on patients undergoing isolated and combined valvular heart surgery. Ann Thorac Surg. 2010;89:773–779. doi: 10.1016/j.athoracsur.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Lertsburapa K., White C.M., Kluger J. Preoperative statins for the prevention of atrial fibrillation after cardiothoracic surgery. J Thorac Cardiovasc Surg. 2008;135:405–411. doi: 10.1016/j.jtcvs.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 20.Goh S.L., Yap K.H., Chua K.C. Does preoperative statin therapy prevent postoperative atrial fibrillation in patients undergoing cardiac surgery? Interact Cardiovasc. 2015;20:422–428. doi: 10.1093/icvts/ivu402. [DOI] [PubMed] [Google Scholar]
- 21.Song Y.B., On Y.K., Kim J.H. The effects of atorvastatin on the occurrence of postoperative atrial fibrillation after off-pump coronary artery bypass grafting surgery. Am Heart J. 2008;156:373.e9–373.e16. doi: 10.1016/j.ahj.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Ahlsson A. eComment. Postoperative atrial fibrillation: a robust human model of atrial fibrillation genesis? Interact Cardiovasc Thorac Surg. 2013;17:614–615. doi: 10.1093/icvts/ivt324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shroff G.R., Orlandi Q.G. Letter Regarding Article “Randomized Trial of Atorvastatin for Reduction of Postoperative Atrial Fibrillation in Patients Undergoing Cardiac Surgery: Results of the ARMYDA-3 (Atorvastatin for Reduction of Myocardial Dysrhythmia After Cardiac Surgery) study”. Circulation. 2007;115:e404. doi: 10.1161/CIRCULATIONAHA.106.677708. [DOI] [PubMed] [Google Scholar]
- 24.Padfield G.J., Hawkins N.M., MacDonald M.R. Letter by Padfield et al. regarding article “Randomized Trial of Atorvastatin for Reduction of Postoperative Atrial Fibrillation in Patients Undergoing Cardiac Surgery: Results of the ARMYDA-3 (Atorvastatin for Reduction of Myocardial Dysrhythmia After Cardiac Surgery) Study”. Circulation. 2007;115:e403. doi: 10.1161/CIRCULATIONAHA.106.667253. [DOI] [PubMed] [Google Scholar]
- 25.Ramani G., Zahid M., Good C.B. Comparison of frequency of new-onset atrial fibrillation or flutter in patients on statins versus not on statins presenting with suspected acute coronary syndrome. Am J Cardiol. 2007;100:404–405. doi: 10.1016/j.amjcard.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 26.Amar D., Zhang H., Heerdt P.M. Statin use is associated with a reduction in atrial fibrillation after noncardiac thoracic surgery independent of C-reactive protein. Chest. 2005;128:3421–3427. doi: 10.1378/chest.128.5.3421. [DOI] [PubMed] [Google Scholar]
- 27.Sanders R., Nicholson A., Lewis S. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database Syst Rev. 2013:7. doi: 10.1002/14651858.CD009971.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soedamah-Muthu S.S., Livingston S.J., Charlton-Menys V. Effect of atorvastatin on C-reactive protein and benefits for cardiovascular disease in patients with type-2 diabetes: analyses from the Collaborative Atorvastatin Diabetes Trial. Diabetologia. 2015;58:1494–1502. doi: 10.1007/s00125-015-3586-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmadi-Abhari S., Luben R.N., Wareham N. Distribution and determinants of C-reactive protein in the older adult population: European Prospective Investigation into Cancer-Norfolk Study. Eur J Clin Invest. 2013;43:899–911. doi: 10.1111/eci.12116. [DOI] [PubMed] [Google Scholar]
- 30.Roshanali F., Mandegar M.H., Yousefnia M.A. Prediction of atrial fibrillation via atrial electromechanical interval after coronary artery bypass grafting. Circulation. 2007;116:2012–2017. doi: 10.1161/CIRCULATIONAHA.107.727081. [DOI] [PubMed] [Google Scholar]
- 31.Bielecka-Dabrowa A., Goch J.H., Rysz J. Influence of co-existing atrial fibrillation on the efficacy of atorvastatin treatment in patients with dilated cardiomyopathy: a pilot study. Lipids Health Dis. 2010;9:21. doi: 10.1186/1476-511X-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ari H., Ari S., Acalla M. Predictive value of atrial electromechanical delay for atrial fibrillation recurrence. Cardiol J. 2013;20:639–647. doi: 10.5603/CJ.2013.0164. [DOI] [PubMed] [Google Scholar]
- 33.Gasparovic H., Burcar I., Kopjar T. NT-pro-BNP, but not C-reactive protein, is predictive of atrial fibrillation in patients undergoing coronary artery bypass surgery. Eur J Cardiothorac Surg. 2010;37:100–105. doi: 10.1016/j.ejcts.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Gibson P.H., Croal B.L., Cuthbertson B.H. Use of preoperative natriuretic peptides and echocardiographic parameters in predicting new-onset atrial fibrillation after coronary artery bypass grafting: a prospective comparative study. Am Heart J. 2009;158:244–251. doi: 10.1016/j.ahj.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 35.Liao J.K. Isoprenoids as mediators of the biological effects of statins. Clin Invest. 2002;110:285–288. doi: 10.1172/JCI16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material