Abstract
Few therapeutic options are available for T790M-negative non-small cell lung cancer (NSCLC) after failure of primary epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) and chemotherapy. This report presents the case of a 71-year-old Asian female never smoker with EGFR mutated T790M negative non squamous cell lung cancer (NSCLC) pre-treated with the experimental epi-immunotherapeutic agent, RRx-001, that re-responded to single agent carboplatin after failure of platinum doublets, TKIs, pemetrexed and nivolumab. The management of advanced EGFR mutation-positive NSCLC is briefly reviewed herein and the emerging paradigm of episensitization, which contradicts the long-standing and widely accepted tenet about the immutability of resistance and the futility of therapeutic rechallenge, is introduced as a strategy to avert treatment failure and thereby stave off deterioration and death.
Keywords: NSCLC, T790 mutation, EGFR, RRx-001, Episensitization
1. Introduction
Up to 85% of all lung tumors, the leading cause of cancer mortality worldwide, greater than the combined mortality from cancers of the breast, prostate and colon, are classified as non–small-cell lung cancer (NSCLC) [1].
The identification of targetable driver mutations such as EGFR, KRAS, EML4-ALK and MET has dramatically changed the treatment landscape for NSCLC. As one of the most commonly altered genes in lung cancer, epidermal growth factor receptor (EGFR) is mutated in approximately 17% of tumors in patients with pulmonary adenocarcinoma, leading to constitutive activation of the EGFR tyrosine kinase [2]. Upregulation of EGFR signaling, due to deletions or amino acid substitutions clustered around the ATP-binding pocket of the tyrosine kinase domain of EGFR, and present in more than 50% of East Asians, never smokers and women [3] with adenocarcinoma histology is correlated with poor responses to PD-1 checkpoint inhibition [4], [5]. Despite strikingly high efficacy rates with EGFR tyrosine kinase inhibitors (TKIs), erlotinib, gefitinib, and afatinib, especially in patients with an activating mutation in exons 18–21 of the EGFR gene, (including an exon 19-deletion and L858R substitution collectively referred to as EGFRm) the development of resistance [6] after 10–12 months is nearly inevitable in all tumors [7].
In about 50% of non-squamous cell patients steric hindrance from the replacement of a threonine by the bulkier methionine at the gatekeeper position of the kinase domain (T790M) is thought to significantly decrease the inhibitory activity of gefitinib, erlotinib and afatinib, leading to resistance [8]. T790M mutation is a double-edged sword because, on the one hand, its emergence limits long-term treatment with TKIs but, on the other, the mutation is associated with indolent progression and a more favorable prognosis than its mutation negative counterpart [9]. Moreover, in the case of resistance due to a T790M mutation the third generation T790M mutant-specific tyrosine kinase inhibitor, osimertinib, based on data from the Phase II AURA 2 trial and the AURA extension cohort, is indicated irrespective of previous exposure to an EGFR TKI (The third generation TKI, rociletinib, is in phase III clinical development) [10], [11], [12]. At progression on osimertinib treatment is generally platinum doublets for 4–6 cycles with or without bevacizumab followed by the option of single agent pemetrexed or docetaxel or erlotinib.
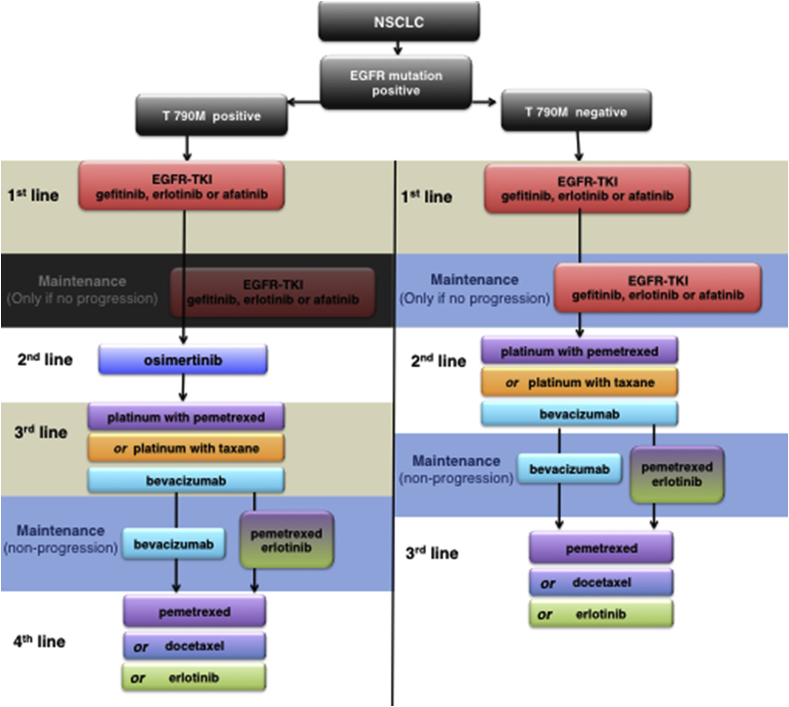
T790M negative status, however, correlates with a more aggressive tumor phenotype and a worse prognosis; second-line chemotherapy is the current standard of care [13] (Fig. 1).
Fig. 1.
Proposed treatment algorithm for patients with EGFRm non-squamous NSCLC and T790M positive and T790M negative mutation status.
This report presents the case of a 71-year-old Asian female never smoker with TKI, platinum, pemetrexed, and PD-1-resistant EGFR positive T790M negative NSCLC who received the epi-immunotherapeutic priming agent RRx-001 in the context of a clinical trial called TRIPLE THREAT (NCT02489903) [14]; per protocol on progression of RRx-001 platinum doublets were reintroduced. However due to the development of peripheral neuropathy from nab-paclitaxel, the patient received carboplatin only, resulting in a partial response after 4 cycles.
2. Case report
This case concerns a 71-year-old Asian female never smoker diagnosed in 2008 with stage 1B NSCLC adenocarcinoma located in the left upper lobe for which she underwent a left lobectomy. In 2010 recurrence to the right hila was treated with a wedge resection and adjuvant carboplatin/taxol for 2 cycles followed by cisplatin/etoposide and concurrent radiotherapy. In January 2012, she was diagnosed with metastatic adenocarcinoma of the lung, epidermal growth factor receptor mutation positive with a deletion found on exon 19; erlotinib was started with a partial response. In August 2013 after progression on erlotinib, she began pemetrexed, which was continued for over a year until September 2014, when progression was documented. In November 2014 she began nivolumab but progressed after only 3 doses (6 weeks).
On rebiopsy of the tumor in January 2015 the activating deletion mutation in exon 19 was still present but T790M mutation was not, which led to treatment with the second generation TKI, afatinib, in February 2015. Due to multiple episodes of poorly tolerated Grade 3 diarrhea, several dose reductions and interruptions were required, leading to permanent discontinuation of afatinib in August 2015.
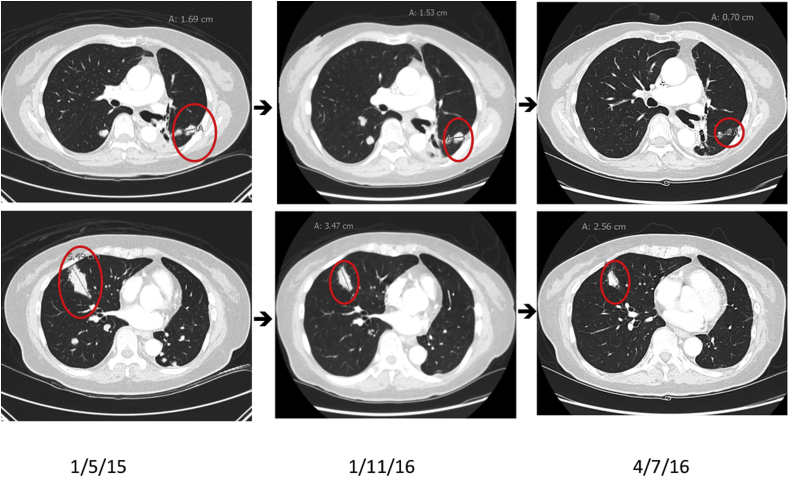
In October 2015 the patient was enrolled on the TRIPLE THREAT clinical trial (NCT02489903), so-named because patients with NSCLC, high grade neuroendocrine carcinoma and SCLC previously treated with platinum doublets are eligible, and received her first dose of weekly 4mg RRx-001 on December 08, 2015. The first CT scan at six weeks demonstrated stable disease (increase of approximately 8%). By twelve weeks she progressed and was started per protocol on carboplatin and nab-paclitaxel. However, due to the recurrence of sensory neuropathy, nab-paclitaxel was discontinued and only carboplatin was administered at a reduced dose. On 4/7/16, at twelve weeks (4 cycles) the CT scan showed a partial response with a tumor reduction of 34.8%. Serial CT scans over an approximately 1-year timespan demonstrate that a significant decrease in tumor size occurred as a result of RRx-001 pretreatment prior to sequentially reintroduced carboplatin (Fig. 2).
Fig. 2.
The evolution of tumor burden is shown over 3 serial CT scans: 1) on 1/5/15 before starting afatinib 2) after stopping RRx-001 on 1/11/16 due to progression and 3) at the nadir of tumor regression (34.8% compared to 1/11/16) on 4/7/16 after 4 cycles of reintroduced carboplatin. Taken together the net percentage of tumor reduction from 1/5/15 to 4/7/16 was 54.6%. Target lesions are circled in red.
At this time, no further cycles of carboplatin are planned and the PI is planning to start the patient on gefitinib.
3. Discussion & conclusion
Episensitization is a hybrid term coined by Oronsky et al. [15], [16] for a hybrid strategy that involves administration of an epigenetic therapy such as RRx-001 or hypomethylator, decitabine, or the histone deacetylase inhibitor (HDAC), vorinostat, to prime tumors to respond better (or anew) to sequential or concurrent immuno- or chemotherapy. The current treatment guidelines in the palliative-intent setting in NSCLC (and most other tumor types) involve a switch to a different therapy at progression until all lines are exhausted and a clinical trial or hospice are the only remaining options. The switch at progression is mandated by the development of resistance and cross-resistance, which generally prevents a retrial of formerly tried or similar therapies.
While the literature describes retreatment with TKI inhibitors after a drug-free interval and/or treatment past RECIST-defined progression, no clear evidence supports these strategies [17]; in fact, recent data from the IMPRESS trial [18] (A Study of IRESSA Treatment Beyond Progression in Addition to Chemotherapy Versus Chemotherapy Alone), which randomly assigned 265 patients with disease progression on first-line gefitinib to cisplatin/pemetrexed plus either gefitinib or placebo suggests inferior survival in the arm combining chemotherapy with gefitinib (survival from time of randomization, 14.8 months for the chemotherapy/gefitinib arm vs. 17.2 months for the chemotherapy/placebo arm).
Episensitization, a strategy intended to reverse the multidrug resistant phenotype in tumors after a priming period with epigenetic-based therapies, has the potential to render the term “treatment failure” irrelevant (or at the very least less definitive) since patients may still benefit from reintroduced first or second line therapies even after their initial cessation due to progression [19], [20]. In this and other journals, episensitization with RRx-001 has been documented in the context of Phase I and Phase II clinical trials on multiple occasions and in several different metastatic tumor types such as colorectal, neuroendocrine, SCLC and NSCLC, which suggests that priming refractory cancer cells with RRx-001 may globally change patterns of resistance and treatment responses [21], [22], [23]. As evidence that the episensitization strategy may successfully “turn back the clock” on cancer cells, serial CT scans over 11 months in the patient of this case report demonstrated a marked reduction in tumor size.
The absence of the T790M mutation in EGFR+ non-squamous NSCLC is a harbinger for a more rapid progression of disease and shorter survival. Given the dearth of viable treatment options after failure of first and second generation EGFR TKIs and platinum doublets in this EGFR+ subset, RRx-001 priming may lead to renewed sensitivity to chemotherapy (and possibly immunotherapy) with partial responses and increased overall survival, all of which occurred in the pan-refractory patient of this case report.
Conflict of interest
The authors disclose that the clinical trial in which this case was observed is funded by EpicentRx, Inc.
References
- 1.D'Addario G., Früh M., Reck M., Baumann P., Klepetko W., Felip E. ESMO guidelines working group. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010 May;21(Suppl. 5):v116–119. doi: 10.1093/annonc/mdq189. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R., Moran T., Queralt C. Spanish lung cancer group. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2009;361(10):958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd F.A., Rodrigues Pereira J., Ciuleanu T., Tan E.H., Hirsh V., Thongprasert S. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 4.Paz-Ares L., Horn L., Borghaei H. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC) [ASCO abstract LBA109] J. Clin. Oncol. 2015;33 [Google Scholar]
- 5.Melosky B., Chu Q., Juergens R., Leighl N., McLeod D., Hirsh V. Pointed progress in second-line advanced non-small-cell lung cancer: the rapidly evolving field of checkpoint inhibition. J. Clin. Oncol. 2016 Feb 16 doi: 10.1200/JCO.2015.63.8049. JCO638049. [DOI] [PubMed] [Google Scholar]
- 6.Sequist L.V., von Pawel J., Garmey E.G. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J. Clin. Oncol. 2011;29:3307–3315. doi: 10.1200/JCO.2010.34.0570. [DOI] [PubMed] [Google Scholar]
- 7.Michalczyk A., Klüter S., Rode H.B., Simard J.R., Grütter C., Rabiller M., Rauh D. Structural insights into how irreversible inhibitors can overcome drug resistance in EGFR. Bioorg. Med. Chem. 2008;16:3482–3488. doi: 10.1016/j.bmc.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 8.Engel J., Lategahn J., Rauh D. Hope and disappointment: covalent inhibitors to overcome drug resistance in non-small cell lung cancer. ACS Med. Chem. Lett. 2016;7(1):2–5. doi: 10.1021/acsmedchemlett.5b00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hata A., Katakami N., Yoshioka H., Takeshita J., Tanaka K., Nanjo S., Fujita S., Kaji R., Imai Y., Monden K., Matsumoto T., Nagata K., Otsuka K., Tachikawa R., Tomii K., Kunimasa K., Iwasaku M., Nishiyama A., Ishida T., Nishimura Y. Rebiopsy of non-small cell lung cancer patients with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor: comparison between T790M mutation-positive and mutation-negative populations. Cancer. 2013 Dec 15;119(24):4325–4332. doi: 10.1002/cncr.28364. [DOI] [PubMed] [Google Scholar]
- 10.Yver A. Osimertinib (AZD9291)—a science-driven, collaborative approach to rapid drug design and development. Ann. Oncol. March 8, 2016:1–6. doi: 10.1093/annonc/mdw129. [DOI] [PubMed] [Google Scholar]
- 11.Park K, Tan E, Zhang L, et al. Afatinib (A) vs gefitinib (G) as first-line treatment for patients (pts) with advanced non-small cell lung cancer (NSCLC) harboring activating EGFR mutations: results of the global, randomized, open-label, Phase IIb trial LUX-Lung 7 (LL7). Presented at: 2015 ESMO Asia Congress; Singapore; December 18-21, 2015. Abstract LBA2.
- 12.Yang JC, Ahn M, Ramalingam SS, et al. AZD9291 in pre-treated T790M positive advanced NSCLC: AURA study Phase II extension cohort. Presented at: 16th World Conference on Lung Cancer; September 6-9; Denver, CO. Abstract 943.
- 13.Piotrowska Z., Sequist L.V. Continued EGFR inhibition with postprogression chemotherapy: where do we stand? Oncologist. 2015 Nov;20(11):1230–1232. doi: 10.1634/theoncologist.2015-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao H., Ning S., Scicinski J., Oronsky B., Knox S.J., Peehl D.M. Epigenetic effects of RRx-001: a possible unifying mechanism of anticancer activity. Oncotarget. 2015 Dec 22;6(41):43172–43181. doi: 10.18632/oncotarget.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oronsky B.T., Oronsky A.L., Lybeck M., Oronsky N.C., Scicinski J.J., Carter C., Fanger G.R., Reid T.R. In: Episensitization: a New Word for a New Concept, in Drug Discovery in Cancer Epigenetics. Egger G., editor. 2016. [Google Scholar]
- 16.Oronsky B., Oronsky N., Knox S., Fanger G., Scicinski J. Episensitization: therapeutic tumor resensitization by epigenetic agents: a review and reassessment. Anticancer Agents Med. Chem. 2014;14(8):1121–1127. doi: 10.2174/1871520614666140418144610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuczynski E.A., Sargent D.J., Grothey A., Kerbel R.S. Drug rechallenge and treatment beyond progression—implications for drug resistance. Nat. Rev. Clin. Oncol. 2013;10:571–587. doi: 10.1038/nrclinonc.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soria J.C., Wu Y.L., Nakagawa K., Kim S.W., Yang J.J., Ahn M.J., Wang J., Yang J.C., Lu Y., Atagi S., Ponce S., Lee D.H., Liu Y., Yoh K., Zhou J.Y., Shi X., Webster A., Jiang H., Mok T.S. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015 Aug;16(8):990–998. doi: 10.1016/S1470-2045(15)00121-7. [DOI] [PubMed] [Google Scholar]
- 19.Oronsky B.T., Oronsky A.L., Lybeck M., Oronsky N.C., Scicinski J.J., Carter C., Day R.M., Rodriguez Orengo J.F., Rodriguez-Torres M., Fanger G.F., Reid T.R. Episensitization: defying time's arrow. Front. Oncol. 2015 Jun 11;5:134. doi: 10.3389/fonc.2015.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strauss J., Figg W.D. Epigenetic approaches to overcoming chemotherapy resistance. Lancet Oncol. 2015;16(9):1013–1015. doi: 10.1016/S1470-2045(15)00231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter C.A., Oronsky B.T., Caroen S.Z., Scicinski J.J., Degesys A., Kim M.M., Oronsky A.L., Lybeck H., Cabrales P., Oronsky N., Reid T., Roswarski J., Brzezniak C. RRx-001 in refractory small-cell lung carcinoma: a case report of a partial response after a third reintroduction of platinum doublets. Case Rep. Oncol. 2016 Mar 11;9(1):171–176. doi: 10.1159/000444631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reid T., Dad S., Korn R., Oronsky B., Knox S., Scicinski J. Two Case reports of resensitization to previous chemotherapy with the novel hypoxia-activated hypomethylating anticancer agent RRx-001 in metastatic colorectal cancer patients. Case Rep. Oncol. 2014 Jan 24;7(1):79–85. doi: 10.1159/000358382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter C.A., Oronsky B.T., Caroen S.Z., Scicinski J.J., Cabrales P., Reid T., Degesys A., Jenkins J., Brzezniak C. Partial response to platinum doublets in refractory EGFR-positive non-small cell lung cancer patients after RRx-001: evidence of episensitization. Case Rep. Oncol. 2016 Jan 28;9(1):62–67. doi: 10.1159/000443725. [DOI] [PMC free article] [PubMed] [Google Scholar]