Abstract
Malignant struma ovarii (SO) is a rare tumor, and as a consequence, treatments and follow-up procedures are not clearly established. Presented in this study are two cases of suspicious ovarian masses, resected and corresponding to malignant SO on histopathology. Similar to thyroid cancer, we proposed complementary radioiodine therapy (131I) after total thyroidectomy (no malignancy was observed at this level in our two patients). Patients underwent treatment with 3.7 GBq 131I followed by post-therapy whole-body scintigraphy, which can detect residual disease or occult metastases. Thyroid remnant ablation increases the sensitivity and specificity of follow-up testing using serum thyroglobulin levels as a tumor marker. Our two patients remained disease-free for 3 and 5 years, respectively, after treatment.
Keywords: Malignant struma ovarii, Radioactive iodine therapy, Thyroid carcinoma
Highlights
-
•
Malignant struma ovarii is a rare ovarian tumor, corresponding to thyroid carcinoma.
-
•
It is generally diagnosed after surgical resection of an ovarian mass.
-
•
Iodine 131 therapy can be proposed for adjuvant therapy, after total thyroidectomy.
-
•
Follow-up must be extended for 20 years, using thyroglobulin level as a tumor marker.
1. Introduction
Struma ovarii (SO) is a rare tumor defined as a mature ovarian teratoma containing 50% or more thyroid tissue, and it accounts for approximately 5% of all ovarian teratomas that are mostly benign. Malignant transformation of SO is reported to occur in less than 5% of all cases, and it is even less likely to lead to metastatic disease (5–6%) (Dardik et al., 1999). Due to the rarity of this type of tumor, there has been a paucity of data in the literature pertaining to treatments and follow-up procedures for this tumor. After local resection of the ovarian tumor, no consensus exists between surveillance alone and adjuvant treatment (De Simone et al., 2003). Similar to thyroid cancers, we proposed thyroidectomy, required before radioactive iodine ablation (131I) and thyroxine suppressive therapy. We report two cases of malignant SO treated in our institution: one patient with localized disease and the other with lymph node and peritoneal extension.
2. Clinical cases
2.1. Case 1
A 49-year-old woman presented for evaluation of an asymptomatic left ovarian mass, which was incidentally discovered during a routine check of an intrauterine device (2012, October). Ultrasonography and pelvic MRI exhibited an 8-cm-diameter left ovarian mass, mixed tumor with gadolinium enhancement of the posterior solid component and low abundance intraperitoneal effusion. The appearance was consistent with an ovarian mucinous adenocarcinoma.
The CA-125 level was elevated to 111 U/ml (normal < 35), while CEA and CA19-9 levels were normal. In December 2012, this patient underwent laparoscopy with a total hysterectomy, bilateral salpingo-oophorectomy with omentectomy, and pelvic-aortic lymphadenectomy. On pathological examination, the ovarian mass was a malignant SO, consisting of follicular variants of papillary thyroid carcinoma (7 × 5.5 × 3 cm) and no other histological features of malignancy. For adjuvant therapy, it was proposed to treat this patient with procedures similar to thyroid cancer, rather than other malignant ovarian cancers. In February 2013, she underwent total thyroidectomy with the intention of administering 131I therapy as an adjuvant setting. On histological examination, the thyroid consisted of normal tissue. Two months later, the patient received a treatment dose of iodine 131 (3.7 GBq) after thyroxine withdrawal for four weeks. The thyroglobulin level was increased to 19 ng/mL (TSH = 27 mIU/l), and serum thyroglobulin antibodies were negative. Two days after treatment, the whole-body 131 I scintigraphy showed high uptake of cervical remnants (Fig. 1A and B). The patient was maintained on thyroid hormone suppression with thyroxine. Six months later, a diagnostic 131I scintigraphy (185 MBq) was conducted after four weeks of thyroxine withdrawal, which demonstrated complete remnant thyroid ablation (Fig. 1C and D). Clinical examination, neck ultrasonography and thyroglobulin level measurements were also negative (thyroglobulin < 1 ng/ml, TSH = 43 mIU/l), confirming remission. Clinical and biological (TSH and thyroglobulin) examination was planned every 6 months for two years and annually thereafter. This patient has completed 3 years of follow-up with no evidence of recurrence.
Fig. 1.
A 49-year-old woman presented a left ovarian malignant struma ovarii resected followed two months later by a total thyroidectomy.
(A and B) Whole-body scan (anterior and posterior views) performed two days after 131I therapy (3.7 GBq) showed remnant thyroid tissue but no evidence of distant functioning metastasis. (C and D) Diagnostic whole body scan (anterior and posterior views) of 131I (185 MBq) performed six months later, showing complete ablation of remnant tissues.
2.2. Case 2
A 67-year-old woman with a history of right nephrectomy for clear cell carcinoma in 1986 presented with post menopausal bleeding in December 2010. Abdomino-pelvic ultrasonography showed uterine fibroma, a 4-cm left ovarian mass associated with a right retrocaval adenopathy, without ascites. Pelvic MRI showed a left latero-uterine lesion (3.5 × 4 × 2.5 cm), hypo signal T1 and T2, without gadolinium enhancement. Appearance was consistent with a fibroma or fibrothecoma. Further exploration by 18FDG PET showed a retrocaval lymph node and left ovarian mass hypermetabolisms (Fig. 2). Bone scintigraphy and thoracic computed tomography were negative. Tumor markers were normal (CEA = 2.4 μg/l, CA125 = 28.2 U/ml, CA15–3 = 13 U/ml and CA19-9 = 23 U/ml). In February 2011, she underwent a laparotomy with total hysterectomy, bilateral salpingo-oophorectomy with omentectomy, and paraaortic, retrocave and pelvic lymphadenectomy. The left ovarian tumor measured 5.5 × 4 cm. Histological examination showed malignant SO (papillary carcinoma type), associated with a metastatic retrocaval lymph node (5 × 2.5 × 4 cm) and omental nodules (Fig. 3).
Fig. 2.
18FDG PET was performed in a 67-year-old woman with a left ovarian lesion and a history of right nephrectomy for clear cell carcinoma. (A) Coronal MIP image showed two pathological 18FDG uptake (arrows). (B and C) Axial fused PET/CT images showed retrocaval lymph node (B) and left ovarian mass (C) hypermetabolisms. (D) Six months after treatment, 18FDG PET coronal MIP image exhibited no pathological uptake.
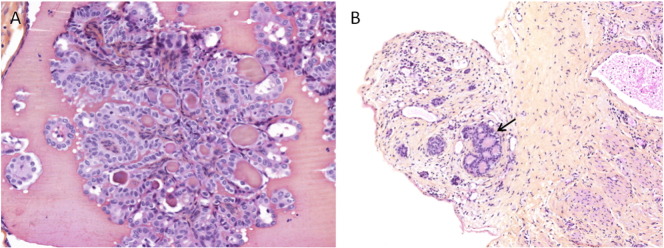
Fig. 3.
(A) Microscopic focus of papillary thyroid carcinoma in struma ovarii (× 200). (B) Omental nodule showing similar thyroid follicles (arrow) (× 100).
After the diagnosis of thyroid-type malignancy was established, she had a total thyroidectomy (May 2011) with negative histology, to optimize uptake of radioactive iodine 131 (3.7 GBq) six weeks later. The 131 I post-therapeutic scintigraphy only showed thyroid remnant uptake without abnormal abdominal uptake. The stimulated serum thyroglobulin was lower than 1 ng/mL (TSH > 100 mIU/ml).
Six months later, stimulated serum thyroglobulin, 18FDG PET, diagnostic 131I scintigraphy and neck ultrasonography were normal. The subsequent follow-up consisted of a clinical examination and thyroglobulin measurements performed every six months for two years and annually thereafter. Pelvic MRIs performed 1 and 2 years after treatment showed no evidence of recurrent disease. She remains free of disease 5 years later.
3. Discussion
Most cases of malignant SO are incidentally discovered. The typical presenting symptoms are pelvic pain, abnormal uterine bleeding and abdominal or pelvic masses (De Simone et al., 2003, Yoo et al., 2008 Jun). It is rare for patients to present signs of hyperthyroidism (approximately 7% of SO) (Matsuda et al., 2001 Sep).
DeSimone et al. (De Simone et al., 2003) reviewed approximately 24 cases of malignant SO: lesion sizes ranged from 5 to 20 cm, more commonly on the left side (63%), and most frequently with follicular variants of papillary thyroid carcinoma (54%) and papillary thyroid carcinoma (21%). Our two clinical cases presented the same primary characteristics.
CA125 is widely accepted as tumor marker of ovarian cancers and is also elevated in other tumor lesions. Moreover, this marker can increase in non-malignant related gynecologic conditions (endometriosis, pregnancy) or as a secondary effect due to the presence of ascites (Leung and Hammond, 1993, Jiang et al., 2010 Jul 29). In our two reported malignant SO cases, the first with ascites had increased CA125, while the second patient (with peritoneal involvement but without ascites) had a normal CA125 rate at diagnosis.
Because ovarian malignancies were suspected from the images, our patients were first treated with surgery, enabling histological diagnosis and locoregional extent.
The cytological diagnostic criteria for papillary carcinoma are similar to those described for the cervical thyroid gland, which include mitotic activity, appearance of the cores (irregular, ground-glass) or vascular invasion (Devaney et al., 1993 Oct). SO with metastasis is also classified as a malignant disease.
Therapy for benign SO is surgical resection (Kunstmann & Fénichel, 2007 Jan). In malignant SO, surgery is also the first phase of treatment. A conservative surgery (unilateral salpingo-oophorectomy) may be acceptable in fertility-desiring patients if they have a localized tumor (Salomon et al., 2003). A total hysterectomy with bilateral salpingo-oophorectomy is a reasonable option in postmenopausal women or in the case of extra ovarian extent (Makani et al., 2004 Sep). Currently, no consensus exists on the postoperative treatment of patients with malignant SO. It is commonly managed based on the treatments for thyroid carcinoma (De Simone et al., 2003, Willemse et al., 1987), taking into account the stage and the aggressiveness of the disease.
Malignant SO measuring over 2 cm, disease outside the ovary, or aggressive histological features should be treated by 131I ablation therapy. Because normal thyroid concentrates 131I, total thyroidectomy is necessary before radioiodine therapy (Luo et al., 2014 Nov). It generally confirms normal thyroid pathology and excludes a primary thyroid carcinoma with subsequent metastasis to the ovary (10% of cases) or a second thyroid cancer (Leong et al., 2013 Dec). Similar to thyroid cancer, the administration of 131I can treat residual or metastatic disease and facilitates subsequent biological monitoring by serum thyroglobulin. Indeed, monitoring serum thyroglobulin can be performed in malignant SO in a manner analogous to patients with thyroid carcinoma. Thyroglobulin is a protein precursor of thyroid hormones (T3 and T4), synthesized and secreted by the thyroid follicular cells. Levels of thyroglobulin are detectable in the serum and indicative of thyroid function. In thyroid carcinoma, after thyroid suppression, thyroglobulin levels must be undetectable (< 1 ng/ml). Increased thyroglobulin levels are indicative of persistent or recurrent thyroid carcinoma (Rose et al., 1998). Post-therapeutic 131I whole-body scintigraphy specifies the extent of the disease.
For low-risk malignant SO (thyroid carcinoma confined to the ovarii, measuring less than 2 cm with no problematic histological features), adjuvant therapy should be discussed. Suppressive thyroxine therapy is then recommended, to reduce TSH secretion (serum TSH between 0.1 and 0.5 mIU/l). The disadvantage is that it is unlikely, due to the thyroid still in place, that thyroglobulin becomes undetectable. Recurrence will be suspected in cases with increasing thyroglobulin levels.
For monitoring after 131I therapy, we recommend:
-
-
Six months after the end of treatment: clinical examination, serum thyroglobulin measurements and diagnostic 131I scintigraphy (after rhTSH stimulation or withdrawal).
-
-
Every 6 months for 18 months and every year thereafter: the mainstay follow-up of clinical examination and thyroglobulin measurements with TSH suppression (thyroglobulin must be < 1 ng/ml). In the case of abnormal results, additional imaging must be performed (131I scintigraphy, 18FDG PET, ultrasonography, CT and/or MRI). Pelvic locations can justify complementary imaging, adapted to the initial stage of the disease. For our two clinical cases, pelvic MRIs were performed 1 and 2 years after therapy only for the patient with the metastatic disease.
The prognosis of malignant SO is not well-characterized, given the rarity of this disease. Localized forms have very good prognosis. However, metastatic forms are also compatible with prolonged survival. In the literature, recurrence occurs in approximately 15–35% of malignant SO cases (De Simone et al., 2003, Makani et al., 2004 Sep). The average time to recurrence is approximately 4–6 years, mostly in patients without adjuvant therapy (De Simone et al., 2003, Jean et al., 2012 Apr). The clinico-biological monitoring of SO (malignant and even benign) that we recommend is for at least 20 years, cases of late recurrence having been described (De Simone et al., 2003).
4. Conclusions
Malignant SO is a rare gynecologic tumor that is most often diagnosed by histological analysis after surgery for a suspicious ovarian mass. After surgical resection of malignant SO, we suggest adjuvant therapy with thyroidectomy followed by radioactive 131 I therapy and suppressive thyroxine therapy. We recommend the same follow-up as thyroid cancer associated with abdomino-pelvic evaluation. This monitoring should be extended for 20 years.
Conflict of interest statement
The authors have no conflict of interest to declare.
References
- Dardik R.B., Dardik M., Westra W., Montz F. Malignant struma ovarii: two case reports and a review of the literature. Gynecol. Oncol. 1999;73:447–451. doi: 10.1006/gyno.1999.5355. [DOI] [PubMed] [Google Scholar]
- De Simone C.P., Lele S.M., Modesitt S.C. Malignant struma ovarii: a case report and analysis of cases reported in the literature with focus on survival and I131 therapy. Gynecol. Oncol. 2003;89:543–548. doi: 10.1016/s0090-8258(03)00141-0. [DOI] [PubMed] [Google Scholar]
- Devaney K., Snyder R., Norris H.J., Tavassoli F.A. Proliferative and histologically malignant struma ovarii: a clinicopathologic study of 54 cases. Int. J. Gynecol. Pathol. 1993 Oct;12(4):333–343. doi: 10.1097/00004347-199310000-00008. [DOI] [PubMed] [Google Scholar]
- Jean S., Tanyi J.L., Montone K., McGrath C., Lage-Alvarez M.M., Chu C.S. Papillary thyroid cancer arising in struma ovarii. J. Obstet. Gynaecol. 2012 Apr;32(3):222–226. doi: 10.3109/01443615.2011.645921. [DOI] [PubMed] [Google Scholar]
- Jiang W., Lu X., Zhu Z.L., Liu X.S., Xu C.J. Struma ovarii associated with pseudo-Meigs' syndrome and elevated serum CA 125: a case report and review of the literature. J. Ovarian Res. 2010 Jul 29;3:18. doi: 10.1186/1757-2215-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunstmann L., Finechel P. Struma ovarii, a rare form of ovarian tumor. Gynecol. Obstet. Fertil. 2007 Jan;35(1):49–54. doi: 10.1016/j.gyobfe.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Leong A., Roche P.J., Paliouras M., Rochon L., Trifiro M., Tamilia M. Coexistence of malignant Struma ovarii and cervical papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2013 Dec;98(12):4599–4605. doi: 10.1210/jc.2013-1782. [DOI] [PubMed] [Google Scholar]
- Leung Y.C., Hammond I.G. Limitation of CA 125 in the preoperative evaluation of a pelvic mass: struma ovarii and ascites. Aust. NZ J. Obstet. Gynaecol. 1993;33:216–217. doi: 10.1111/j.1479-828x.1993.tb02400.x. [DOI] [PubMed] [Google Scholar]
- Luo J.R., Xie C.B., Li Z.H. Treatment for malignant struma ovarii in the eyes of thyroid surgeons: a case report and study of Chinese cases reported in the literature. Medicine (Baltimore) 2014 Nov;93(26) doi: 10.1097/MD.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani S., Kim W., Gaba A.R. Struma ovarii with a focus of papillary thyroid cancer: a case report and review of the literature. Gynecol. Oncol. 2004 Sep;94(3):835–839. doi: 10.1016/j.ygyno.2004.06.003. (Review) [DOI] [PubMed] [Google Scholar]
- Matsuda K., Maehama T., Kanazawa K. Malignant struma ovarii with thyrotoxicosis. Gynecol. Oncol. 2001 Sep;82(3):575–577. doi: 10.1006/gyno.2001.6315. [DOI] [PubMed] [Google Scholar]
- Rose P., Arafah B., Abdul-Karim F. Malignant struma ovarii: recurrence and response to treatment monitored by thyroglobulin levels. Gynecol. Oncol. 1998;70:425–427. doi: 10.1006/gyno.1998.5056. [DOI] [PubMed] [Google Scholar]
- Salomon L.J., Lefevre M., Cortez A., Antoine J.M., Uzan S. Goitre ovarien: Une tumeur rare et particulière, à propos d'un cas et revue des modalités de prise en charge. J. Gynecol. Obstet. Biol. Reprod. 2003;32:175–178. [PubMed] [Google Scholar]
- Willemse P.H., Oosterhuis J.W., Aalders J.G., Piers D.A., Sleijfer D.T., Vermey A. Malignant struma ovarii treated by ovariectomy, thyroidectomy, and 131I administration. Cancer. 1987;60:178–182. doi: 10.1002/1097-0142(19870715)60:2<178::aid-cncr2820600210>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Yoo S.C., Chang K.H., Lyu M.O., Chang S.J., Ryu H.S., Kim H.S. Clinical characteristics of struma ovarii. J. Gynecol. Oncol. 2008 Jun;19(2):135–138. doi: 10.3802/jgo.2008.19.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]