Abstract
Cell-extracellular matrix (ECM) interactions are essential for tissue development, homeostasis, and response to injury. Basement membranes (BMs) are specialized ECMs that separate epithelial or endothelial cells from stromal components and interact with cells via cellular receptors, including integrins and discoidin domain receptors. Disruption of cell-BM interactions due to either injury or genetic defects in either the ECM components or cellular receptors often lead to irreversible tissue injury and loss of organ function. Animal models that lack specific BM components or receptors either globally or in selective tissues have been used to help with our understanding of the molecular mechanisms whereby cell-BM interactions regulate organ function in physiological and pathological conditions. We review recently published work on animal models that explore how cell-BM interactions regulate kidney homeostasis in both health and disease.
Keywords: Extracellular matrix, basement membrane, mouse models, kidney, Alport syndrome, Pierson syndrome, peroxidasin, tubular cells, integrins, discoidin domain receptors
Basement membranes (BM) are specialized sheet-like extracellular matrix structures which lie beneath epithelial or endothelial cells. In addition to providing mechanical stability, BMs regulate essential cell functions, including cell polarity, proliferation, apoptosis, and matrix synthesis/remodeling. These effects are mediated by the integrin, discoidin, and dystroglycan transmembrane family receptors.
The kidney is formed by functional units called nephrons, which consist of the glomerular filtering unit and specialized tubules that reabsorbs and secretes the filtrate. In the glomerulus, there is a specialized BM called the glomerular BM (GBM) that separates endothelial cells from podocytes and is an essential component of the glomerular filtration barrier. In the tubules, there is a BM that separates monolayer of tubular epithelial cells from the stroma. Defects of these BM components as well as the cellular receptors required for cells to interact with these BMs have been associated with kidney diseases. This review highlights recent findings on animal models with perturbations in BM components or cellular receptors that have significantly contributed to our understanding of kidney disease.
Basement membrane components in healthy and diseased kidney
The main BM components are collagen IV, laminins, nidogen, and heparan sulfate proteoglycans (see below for details on their structure). BMs in the glomerulus provide support for mesangial cells and the GBM is a physical separation between endothelial cells and podocytes. The GBM contains specific isoforms of BM components, such as the α3α4α5 collagen IV network, laminin-521, and agrin (reviewed in (Borza and Pozzi, 2012; Miner, 2012; Pozzi et al., 2009; Suh and Miner, 2013)). Mutations in genes encoding some of the key GBM components cause severe kidney abnormalities, which underscore their importance for tissue development, homeostasis, and response to injury. In this regard, mutations in collagen IV or laminin cause Alport and Pierson syndromes in humans, respectively. The availability of mice either lacking or carrying mutated BM components has allowed investigation of the molecular mechanisms whereby these matrices regulate glomerular and tubular kidney function. We will review only the most recent findings related to these mouse models as the renal phenotype of some of these mice has already been extensively reviewed (Abrahamson, 2012; Cosgrove et al., 2007; Gross and Kashtan, 2009; Kashtan and Segal, 2011; Suh and Miner, 2013).
The Alport mouse models of kidney disease
Collagen IV, the major component of BMs, is a triple helical protein which contains a short 7S domain at the N-terminal, a long collagenous domain that occupies the midsection of the molecule; and a non-collagenous (NC) domain positioned at the C-terminal (Hudson et al., 2003). There are six genetically distinct α chains (α1–α6), which assemble into 3 specific hetero-trimeric molecules; the α1α1α2, α3α4α5 and α5α5α6 protomers. These protomers form three distinct networks by dimerization via NC1-to-NC1 interactions and by tetramerization via 7S-to-7S domain interactions (Hudson et al., 2003). In the adult kidney, the α1α1α2 network is found primarily in the mesangium of the glomerulus and in the tubular BM; the α3α4α5 network is mainly present in the GBM; and the α5α5α6 network in the Bowmans capsule (Hudson et al., 2003).
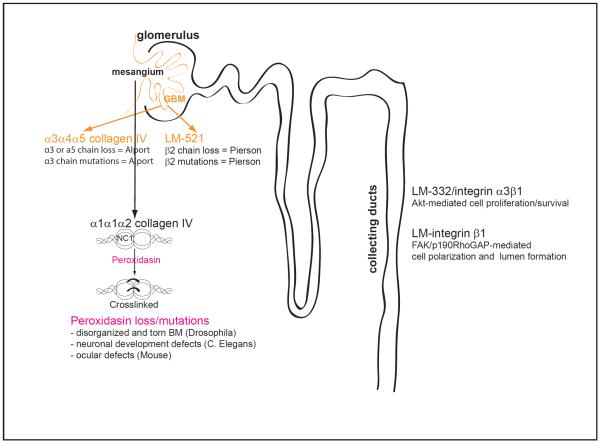
Mutations in either COL4A3, COL4A4 or COL4A5 chains that result in absence of α3α4α5(IV) network and persistence α1α1α2(IV) networks in the GBM cause Alport syndrome (Figure 1). The α1α1α2(IV) network which is not as highly cross-linked or resistant to proteases as α3α4α5(IV) network provides less mechanical stability and is insufficient to maintain normal kidney function. Patients present with either macroscopic or microscopic hematuria, thickening and splitting of the GBM and many will ultimately develop end stage glomerulosclerosis. Mice deficient in COL4A3 (Cosgrove et al., 1996; Miner and Sanes, 1996), COL4A4 (Arnold et al., 2011), COL4A5 (Rheault et al., 2004) and COL4A3/COL4A4 (Lu et al., 1999) recapitulate human pathology, but the disease penetrance is highly strain-dependent (Cosgrove et al., 2007). For instance, COL4A3-null mice reach end-stage renal failure (ESRF) around 66 days of age on the 129X1/SvJ background, while on the C57BL/6J background, the mean age at ESRF was 194 days of age, which suggests the existence of modifier genes that influence disease progression (Andrews et al., 2002). Strain-specific ectopic expression of the α5α5α6(IV) network in the GBM of C57/BL6 but not 129X1/SvJ COL4A3-null mice may also contribute to the milder Alport renal phenotype on the C57Bl/6 genetic background (Kang et al., 2006).
Figure 1.
Schematic representation of the nephron highlighting the contribution of some basement membrane components in glomerular and tubular homeostasis.
GBM, glomerular basement membrane; NC, non-collagenous.
Although the defects in Alport syndrome are attributed largely to a defective GBM, it has been proposed that the unfolded protein response of ER stress in podocytes induced by defective collagen IV chains might also contribute to the pathogenesis. In support of this, a COL4A3 chain carrying the G1332E mutation overexpressed in podocytes in vitro or expressed in vivo in mice caused chain retention in the endoplasmic reticulum (ER). This resulted in activation of unfolded protein response-related markers of ER stress and the development of an Alport syndrome phenotype (Pieri et al., 2014). Interestingly, heterozygous COL4A3-G1334E mutations are seen in patients with thin GBM disease (Pieri et al., 2014).
Recently, a mouse model for Alport was reported where the collagen α3α4α5(IV) network is synthetized and incorporated into the GBM but is abnormal and produced at low levels resulting in Alport like lesions (Korstanje et al., 2014). These mice, identified in a colony of NONcNZO recombinant inbred mice, arose through a spontaneous mutation, localized to chromosome 1, which results in skipping of the COL4A4 exon 30 but maintains the mRNA reading frame and generates of a shorter α4(IV) chain. As abnormal collagen α3α4α5(IV) is also found in a subset of Alport patients, this mouse represents an excellent model to analyze how abnormal collagen IV structure and assembly leads to Alport syndrome.
Generation of COL4A3-null mice has allowed not only investigation of the mechanisms responsible for Alport syndrome but also testing of potential therapies. In this context, COL4A3-null mice engineered to express an inducible human α3(IV) chain in the podocytes formed an α3α4α5(IV) network in the GBM with intact podocyte foot process architecture, reduced glomerulosclerosis, albuminuria, and a longer lifespan (Lin et al., 2014). Thus, the α3(IV) chain produced by podocytes is sufficient to promote proper collagen network formation in the GBM. In other studies, intracardial injection of amniotic fluid stem cells in COL4A5-null mice before the onset of proteinuria delayed the progression of glomerular sclerosis and prolonged animal survival (Garcia et al., 2013). This protective effect was due to recruitment and activation of anti-fibrotic and pro-tissue remodeling M2 macrophages, rather than stem cells differentiating into podocyte-like cells or collagen α5(IV) chain production. Finally, miR-21 silencing in COL4A3-null mice has been shown to improve survival and to reduce glomerulosclerosis, interstitial fibrosis, tubular injury and inflammation (Gomez et al., 2015). This protective effect was due to reduced TGF-β-induced fibrogenesis and inflammation in glomerular and interstitial cells as well as improved mitochondrial function in both glomerular and tubular cells. This study suggests that inhibition of miR-21 represents a potential therapeutic strategy for chronic kidney diseases including Alport nephropathy.
Laminins and mouse models of glomerular kidney disease
Laminins (LM) are large heterotrimeric glycoproteins that are essential for BM assembly. Each trimer is composed of one α, one β, and one γ chain (Colognato and Yurchenco, 2000) and there are currently five α, four β, and three γ chain genes described in vertebrates which can assemble into 15 different heterotrimers (reviewed in (Miner, 2008)). Many of the laminin chains are expressed during kidney development under strict temporal control. For instance, LM-111 is expressed in the presumptive GBM, LM-511 and LM-521 in the semi-mature GBM, and LM-521 is the sole trimer present in mature GBM (reviewed in (Miner, 2008)).
The importance of LM-521 in the GBM is demonstrated by the fact that patients carrying null mutations in the LAMB2 gene develop Pierson syndrome characterized by mesangial sclerosis and diffuse alterations of the GBM (Zenker et al., 2004). Consistent with this finding, mice lacking the LAMβ2 chain develop massive proteinuria and glomerular sclerosis (Noakes et al., 1995) (Figure 1).
In addition to LAMB2 null mutations, certain LAMB2 missense mutations, including C321R cause congenital nephrotic syndrome. To determine how this mutation leads to glomerular disease, a Lamβ2-null mice expressing the rat C321R-LAMB2 was generated (Chen et al., 2013). During the first postnatal month, C321R-LAMB2 attenuated proteinuria in LAMB2 null mice in a dose-dependent fashion, however as the mice aged they developed proteinuria and renal failure. This phenotype occurs because the C321R mutation leads to improper secretion of LM-521, podocyte ER stress and apoptosis. The finding that in vitro treatment with chemicals that facilitate protein folding and trafficking increased the secretion of the mutant LAMB2 (Chen et al., 2013), suggests that therapies which improve protein folding might be beneficial for the treatment of mild forms of Pierson syndrome. These data are very similar to those described for mice harboring the COL4A3-G1334E mutation and support the hypothesis that ER stress is a general mechanism of podocyte injury in mice harboring point mutations in BM proteins that cannot be properly secreted.
One feature of patients with Pierson syndrome and the Lamb2-null mice is ectopic expression of the LAMβ1 chain in the GBM. However, the expression of this chain is only marginally increased and fails to compensate for the loss of LAMβ2. Interestingly, LamB2-null mice engineered to express high levels of LAMβ1 selectively in podocytes are spared from the development of nephrotic syndrome and show a greatly extended lifespan (Suh et al., 2011). The finding that the levels of LAMβ1 inversely correlates to albuminuria and defects in the GBM, suggest that maneuvers to increase LAMβ1 expression in patients with LAMβ2 null mutations could ameliorate the severity of nephrotic syndrome.
Laminins and renal epithelial cell homeostasis
In addition to its function in BM assembly, laminins interact with the cellular receptor integrins to provide polarity cues and to control cell function. We recently reported that kidney collecting duct cells interact with LM-332, a major component of kidney tubular BMs, via integrin α3β1. This interaction is key in promoting integrin α3β1-dependent Akt activation and tubular cell function. Interestingly, K63-linked polyubiquitination, but not the classical PI3K, is necessary for promoting LM-322/integrin α3β1-dependent cell signaling required for the proper development of the collecting system (Yazlovitskaya et al., 2015). Using MDCK cells grown as hollow cysts in Matrigel, Bryant and colleagues has recently identified a molecular switch mechanism controlling polarity orientation whereby ECM signals through a integrin β1/FAK/p190RhoGAP complex to promote trafficking of podocalyxin from a basal to an apical membrane position thus allowing lumen formation (Bryant et al., 2014). Thus, interactions between BM components and tubular cells play a key role in governing the proper development, polarization, and lumen formation of kidney tubules (Figure 1).
Nidogens and heparan sulfate proteoglycans in kidney homeostasis
Nidogen-1 and nidogen-2 are widely expressed in BMs, interact with both laminins and collagen IV, and are hypothesized to function as a bridge between the two networks. However, nidogen-1 or nidogen-2 deficient mice are normal with no obvious kidney defects. Interestingly, deletion of both nidogen-1 and nidogen-2 results in mice that die shortly after birth, although their BMs only show mild abnormalities (Bader et al., 2005).
Proteoglycans like agrin and perlecan contain heparan sulfate polysaccharide side chains covalently attached to a core protein. Because heparan sulfate side chains are negatively charged, proteoglycans serve in conferring the GBM a net negative charge. Surprisingly, podocyte-specific deletion of agrin alone or in combination with loss of perlecan, the two predominant proteoglycans in GBM, does not affect the GBM structure (Goldberg et al., 2009). These studies indicate that unlike collagen IV and laminins these proteins do not play a role in the maintenance of the kidney BMs at both physiological and pathological levels.
Basement membrane modifying enzymes in health and disease
In addition to mutation and/or loss of key BM components, loss of enzymes required for posttranslational modification of BM components affects tissue mechanical stability and are implicated in tissue biogenesis and maintenance. Collagen IV forms a network that is stabilized by a sulfilimine bond between the alpha chains (Vanacore et al., 2009). This bond is catalyzed by peroxidasin, a BM-bound extracellular heme-peroxidase that requires bromine as a key cofactor for its activity (Bhave et al., 2012; Fidler et al., 2014; McCall et al., 2014). Loss or mutation in peroxidasin has been associated with disorganized collagen IV networks and torn BMs in drosophila (Bhave et al., 2012), neuronal developmental defects in C. Elegans (Lee et al., 2015) and severe ocular defects in mice (Yan et al., 2014) (Figure 1). The finding that peroxidasin expression is increased in a murine model of kidney fibrosis and is organized into a fibril-like network suggests that it promotes matrix formation in response to injury (Peterfi et al., 2009). Whether increased peroxidasin expression contributes to physiological or pathological fibrogenic response is unclear. Thus, the development of a mouse lacking peroxidasin in selective part of the kidney is needed to determine the role of this crosslinking enzyme in kidney repair following injury.
Integrins in healthy and diseased kidney
Integrins are transmembrane receptors for extracellular matrix components which consist of non-covalently associated α and β subunits. There are 18α and 8β subunits in mammals, which form 24 unique heterodimers (Fu et al., 2012) with distinct specificities for the ECM. In this chapter will focus on integrins that function as receptors for major BMs components namely collagen IV and laminins.
In addition to their function of anchoring cells to ECM, integrins are signaling molecules which regulate cell migration, differentiation, proliferation, and survival under both physiological and pathological conditions (Askari et al., 2009). Integrins modulate these diverse cellular functions by interacting with the cytoskeleton of the cell and by mediating bi-directional cell signaling from the outside of the cell inwards and from the inside of the cell outwards (Fu et al., 2012). Thus, integrins act as a bridge for cells to bind to and transduce signals from the ECM into the cell as well as for cells to modify the extracellular environment. The observation that the expression of some of the integrin family members is altered in the course of kidney diseases, has initiated studies to analyze the contribution of these matrix receptors in kidney function in physiological and pathological conditions. Generation of mice lacking integrin subunits in selective kidney cells has contributed to our understanding of the role these matrix receptors play in kidney homeostasis in health and disease. Collagen receptors, α1β1 and α2β1, and laminin receptors α3β1 and α6β1 are highly expressed in kidney (Mathew et al., 2012). As global deletion of the integrin β1 subunit leads to embryonic lethality at peri-implantation stage (Fassler and Meyer, 1995), the role of this subunit in kidney homeostasis has been made possible only by the recent generation of the integrin β1fl/fl mice. This, together with Cre technology, has enabled specific deletion of the β1 integrin in various kidney cells at early and late stages of development.
Integrin β1 in glomerular homeostasis
Selective deletion of the β1 subunit in the podocytes by crossing the β1fl/fl mice with the podocin-Cre mice has resulted in mice with podocyte abnormalities and proteinuria at birth, despite a grossly normal GBM (Pozzi et al., 2008) (Figure 2). Following the advent of glomerular filtration, these mice show progressive podocyte loss as well as capillary loop and mesangium degeneration with little evidence glomerulosclerosis (Pozzi et al., 2008). By 3 weeks of age the mice develop severe end stage renal failure characterized by both tubulointerstitial and glomerular pathology (Pozzi et al., 2008). In contrast to this data, Kanasaki and colleagues showed that deleting the β1 subunit in the podocytes by crossing the β1fl/fl mice with the nephrin-Cre mice results in detectable proteinuria on day 1 and death within a week of birth (Kanasaki et al., 2008). The kidneys of these mice exhibit normal glomerular endothelium, but show severe GBM defects with multilaminations and splitting including podocyte foot process effacement (Pozzi et al., 2008). The difference in phenotype in these two studies may be because: 1) the nephrin-Cre promoter is stronger than the podocin-Cre; 2) the use of different mouse backgrounds; 3) partial deletion of the β1 subunit due to incomplete efficiency of the Cre; 4) long half-life of the β1 subunit protein, despite complete efficiency of the Cre; and 4) compensation by non β1 containing integrins. Despite these differences, these two studies demonstrate that podocyte β1 integrin is critical for postnatal development and maintaining the structural integrity of the glomerulus, especially the filtration barrier.
Figure 2.
Schematic representation of the nephron highlighting the contribution of some basement membrane-binding integrins in glomerular and tubular homeostasis.
Integrin β1 in tubular cell homeostasis
Integrin β1 is also required for the development of the ureteric bud. Deletion of the integrin β1 subunit using a hoxb7-cre mouse, expressed in the UB at E10.5, results in a severe branching morphogenesis with decreased nephron formation and death at 4–6 weeks of age (Zhang et al., 2009). Interestingly, deleting the β1 subunit in the collecting ducts at E18.5 using an aquaporin-2-cre mouse did not result in developmental defects although the mice were more susceptible to injury (Zhang et al., 2009). This results show that integrin β1 is required for governing cell growth and branching during the early stages of UB development and structural integrity of the collecting duct at later stages of UB development (Zhang et al., 2009) (Figure 2). Recently, we showed that the integrin β1 controls the fate of kidney proximal and distal epithelial cells by regulating the composition and function of tight and adherent junctions. Deletion of the integrin β1 subunit in proximal tubules using the γGT-cre mouse (expressed in proximal tubules at P10) has minimal impact in kidney morphology, but results in isosmolar diuresis under basal conditions and an inability to concentrate urine following water deprivation (Elias et al., 2014). This defect is due to the fact that deleting the integrin β1 subunit in proximal tubular cells converts them from a “loose” to a “tight” epithelium with features similar to those seen in distal tubular cells (Figure 2). Thus, this study suggests that cell-matrix interactions might regulate terminal differentiation and function of polarized epithelial cells.
Integrins α1β1 and α2β1 in glomerular homeostasis
While deletion of integrin β1 subunit eliminates multiple integrin heterodimers, availability of mice lacking specific integrin α subunits has allowed investigation of the contribution of specific alpha subunits to kidney function in health and disease. Integrins α1β1 and α2β1 are the two major collagen binding receptors in the kidney. Both integrins can bind collagen IV; however deleting these two major receptors does not affect normal glomerular development (Chen et al., 2002; Gardner et al., 1996; Holtkotter et al., 2002). By contras both integrins play an important role in regulating ECM production and degradation in the course of kidney fibrosis(Borza and Pozzi, 2012; Pozzi et al., 2009) (Figure 2).
Integrin α1β1, which binds with high affinity to collagen IV, is expressed by podocytes, endothelial cells and mesangial cells of the glomerulus (Korhonen et al., 1990; Voigt et al., 1995). This receptor is associated with renal disease and is overexpressed in the proliferating mesangium in glomerulonephritis (Kuhara et al., 1997; Shikata et al., 1995). In addition, integrin α1 antibodies reduced scarring in rat models of glomerular injury by inhibiting integrin α1β1-dependent (VLA-1) leukocyte function (Cook et al., 2002). This is likely due to the inability of leukocytes to traffic to the sites of injury. Despite this beneficial effect of inhibiting VLA-1 in a model of glomerulonephritis, mice lacking the integrin α1 subunit appear to develop more glomerulosclerosis than wild type mice in multiple models of glomerular injury (Chen et al., 2004; Yu et al., 2012; Zent et al., 2006). These results can be explained by the fact that integrin α1β1 is a negative regulator of collagen synthesis as this receptor is required to sense extracellular collagen levels and downregulate both endogenous collagen I and collagen IV synthesis (Gardner et al., 1999). Consistent with these findings, integrin α1-null mice develop worse glomerulosclerosis than wild type mice. Interestingly, integrin α1-null mesangial cells produce more pro-fibrotic ROS than wild type cells which lead to decreased cell proliferation and increased glomerular collagen IV accumulation (Chen et al., 2007). Thus, integrin α1β1 negatively modulates glomerulosclerosis by either directly altering collagen production or by negatively regulating the production of ROS, which in turn control collagen turnover and ultimately fibrosis. In vitro studies have demonstrated that integrin α1β1 negatively controls ROS production by downregulating the activation state of the pro-fibrotic epithelial growth factor (EGF) receptor (Chen et al., 2007), and it does so by controlling the levels and phosphorylation state of caveolin-1, a scaffolding protein involved in receptor signaling and localization (Borza et al., 2010; Chen et al., 2010).
Thus, integrin α1β1 is a negative regulator of collagen production and its engagement is beneficial in the setting of fibrosis.
Integrin α2β1 is another collagen IV receptor, although it binds this ligand with lower affinity than integrin α1β1. Like integrin α1β1, integrin α2β1 is expressed by mesangial cells and podocytes (Borza et al., 2008; Chen et al., 2004). Expression of integrin α2β1 increases in the kidneys of patients with diabetic nephropathy (Jin et al., 1996) and rapidly progressive glomerulonephritis (Baraldi et al., 1995). However, whether increased expression of this collagen receptor contributes to or counteracts the development of glomerulosclerosis, is unclear. Integrin α2-null mice develop mild proteinuria at 6 months of age and mild glomerular damage due to increased expression of the pro-fibrotic transforming growth factor (TGF)-β and connective tissue growth factor (CTGF) (Girgert et al., 2010). Although this result suggests that integrin α2β1 is a negative regulator of glomerulosclerosis, in vitro studies with non-renal cells suggest that integrin α2β1 is a positive regulator collagen I and ROS synthesis (Honore et al., 2003; Ivaska et al., 1999). Furthermore, crossing the COL4A3-null mice, a mouse model of Alport disease, with the integrin α2-null mouse results in increased survival, improved renal function and decreased glomerular matrix deposition and scarring (Rubel et al., 2014). We investigated the role of integrin α2β1 in glomerulosclerosis and found that integrin α2-null mice developed significantly less proteinuria and glomerulosclerosis than wild type mice following adriamycin-mediated injury (Borza et al., 2012). In agreement with the observation that loss of integrin α2β1 plays a protective role in glomerular injury, treatment of wild type mice with a selective integrin α2β1 inhibitor (Miller et al., 2009) decreases albuminuria and glomerular injury following adriamycin injection (Borza et al., 2012). The pro-fibrotic role of integrin α2β1 can be explained by the fact that binding of this receptor to collagen I induces activation of Stat3 a latent transcription factor involved fibrotic diseases (Chuang and He, 2010; Pechkovsky et al., 2012). Consistent with this finding, genetic deletion or inhibition of integrin α2β1 blocks integrin/collagen interactions thus resulting in decreased Stat3 activation with consequent decreased collagen IV deposition and amelioration of glomerular damage (Borza et al., 2012). Thus, integrin α2β1 positively regulates collagen IV synthesis thus contributing to glomerular injury and its inhibition is beneficial in the setting of fibrosis.
Integrin α1β1 in tubular homeostasis
As mentioned above, selective deletion of the integrin β1 subunit in proximal or distal tubules of the nephron identified a role for this subunit in cell fate, proliferation, branching, and response to injury (Zhang et al., 2009). In addition, the laminin receptor integrin α3β1 has been shown to play a role in collecting system development of the kidney (Yazlovitskaya et al., 2015). The role of the BM collagen binding integrins integrins to tubular homeostasis is unknown. We recently found that integrin α1-null mice develop more tubulointerstitial fibrosis than wild type mice after unilateral ureter obstruction-mediated injury (Chen et al., 2014) (Figure 2). This effect was due to increased activation of pro-fibrotic signaling downstream of TGF-β receptor II in integrin α1-null mice or collecting duct cells. Interestingly, we found that integrin α1β1 counteracts activation of TGF-β receptor II-mediated pro-fibrotic signaling by negatively regulating the tyrosine phosphorylation levels of TGF-β receptor II (Chen et al., 2014). This protective effect is mediated by selective integrin α1β1-dependent recruitment and activation of the tyrosine phosphatase TCPTP. Although this study was focused on kidney injury, given the widely expression of integrin α1β1, TCPTP, and TGF- β receptor II, this finding suggests that integrin α1β1/TCPTP-mediated prevention of tyrosine phosphorylation of TGF-β receptor II might be viewed as a valid tool to control unwanted activation of TGF-β signaling, in situations such as inflammation cancer and fibrotic diseases.
Integrin α3β1 and glomerular homeostasis
Integrin α3β1 is highly expressed by podocytes and facilitates tight binding to the GBM in order to maintain a functional filtration barrier (Sachs et al., 2012; Sachs et al., 2006). Global deletion of the integrin α3 subunit in mice results in abnormalities in glomerular development and alterations in the GBM and integrin α3-null mice die soon after birth (Kreidberg et al., 1996). In contrast, deletion of the same subunit specifically in podocytes leads to massive proteinuria caused by focal glomerulosclerosis and disorganization of the GBM (Sachs et al., 2006) (Figure 2). Together with the kidney phenotype of the mice lacking the integrin β1 subunit in podocytes (see above), these studies indicate that integrin α3β1 is the major receptor required to maintain the glomerular filtration barrier. More importantly, the phenotypes of α3-deficient mice have recently been validated in humans where severe renal abnormalities and premature death are associated with absence or mutations of the integrin α3 subunit (Has et al., 2012; Nicolaou et al., 2012; Shukrun et al., 2014; Yalcin et al., 2015). The importance of integrin α3β1 in the maintenance of podocyte stability is also demonstrated by the finding that podocyte expression of the integrin α3 subunit in patients with primary focal segmental glomerulosclerosis is significantly lower than in normal controls, and the expression of this subunit negatively correlates with the degree of glomerular sclerosis score (Chen et al., 2006). Thus, analysis of patients with mutations in integrin α3 which result in lethality and α3-deficient mice indicate that integrin α3β1 is crucial for basement membrane organization and kidney function.
A possible mechanism whereby integrin α3β1 controls podocyte stability is via its interaction with the tetraspanin protein CD151 (Sachs et al., 2012), which mediates integrin α3β1-dependent adhesion. Interestingly, CD151-null mice show severe alterations of the GBM consisting of massive thickening and splitting and consequent kidney failure (Baleato et al., 2008; Sachs et al., 2006), thus mimicking the phenotype of mice lacking the integrin α3 subunit in podocytes (Sachs et al., 2006). Selective deletion of CD151 in mouse podocytes results in redistribution of integrin α3β1 from diffuse/strong focal adhesions to large/weak focal adhesions thus decreasing binding to laminin substrata (Sachs et al., 2012). Consistent with this in vitro finding, in vivo podocyte-specific deletion of CD151 results in proteinuria, podocyte loss, and glomerular nephropathy (Sachs et al., 2012). Thus, CD151 is a crucial modifier of integrin-mediated adhesion of podocytes to the GBM and plays a critical role of tight adhesion of podocytes to the GBM for maintaining glomerular integrity (Pozzi and Zent, 2012). Overall, diseases associated with mutations in integrin α3β1, CD151 and the laminin β2 suggest a key role for laminins and their principal receptors in normal glomerular function.
Non-integrin receptors in kidney homeostasis
In addition to integrins, cells interact with BMs through non-integrin receptors like dystroglycan, syndecans and discoidin domain receptors (DDR).
Dystroglycan is a at transmembrane receptor that consists of two subunits: the α subunit which binds BM components like laminin and the β subunit which binds to cytoskeletal proteins. This receptor is highly expressed in the muscle and skeletal muscle-targeted deletion of dystroglycan or fukutin, an enzyme required for dystroglycan processing, results in muscular dystrophy in mice (Beedle et al., 2012; Cohn et al., 2002). Interestingly, dystroglycan is also expressed at high levels in podocytes; however, podocytes-specific deletion of this receptor does not result in significant renal abnormalities either at baseline or following injury (Jarad et al., 2011). This study clearly indicates that, unlike the muscle, dystroglycan is not a primary receptor in kidney glomerular cells, and other BM receptors contribute to glomerular homeostasis.
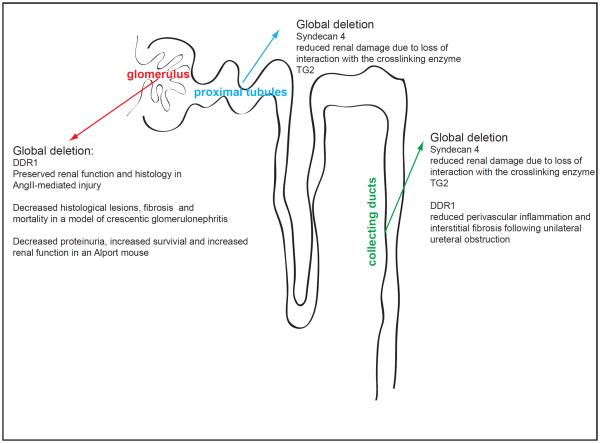
In contrast to the studies described above, a role for the transmembrane heparan sulfate proteoglycan syndecan-4 in promoting injury of proximal and collecting tubular cells was recently described. In tubular cells, syndecan-4 interacts with the extracellular matrix crosslinking enzyme transglutaminase type 2 (TG2). This interaction is necessary for cell surface trafficking, localization, and activity of TG2 (Scarpellini et al., 2009). Interestingly, loss of syndecan-4 protects mice from injury-induced tubular interstitial fibrosis due to reduced TG2 activation and excessive crosslinking and accumulation of extracellular matrix (Scarpellini et al., 2014) (Figure 3). Although this study suggests that preventing syndecan-4/TG2 interaction or inhibitingTG2 action might be beneficial for the treatment of kidney fibrosis, the picture is complicated by the multiple-functional properties of TG2. In addition to its role in promoting matrix crosslink, TG2 promotes clearance of necrotic cells and plays a protective role in promoting hepatic repair following injury (Nardacci et al., 2003). Thus, targeting TG2 in fibrosis and/or injury seems to be highly tissue specific.
Figure 3.
Schematic representation of the nephron highlighting the contribution of non-integrin binding receptor in glomerular and tubular homeostasis.
DDR1 and DDR2 are receptor tyrosine kinases that bind to and are activated by collagen. While both DDRs bind to fibrillar collagens, only DDR1 binds collagen IV and will be discussed further in this chapter. DDR1 is composed of an extracellular Discoidin (DS) homology domain which contains the collagens binding site, a DS-like domain, an extracellular juxtamembrane region which contains N- and O-glycosylation sites and matrix metalloproteinase cleavage sites, a transmembrane domain which mediates collagen-independent receptor dimerization, a large intracellular juxtamembrane region which contains tyrosines that may serve as docking sites upon phosphorylation and an intracellular tyrosine kinase domain (reviewed in (Borza and Pozzi, 2013)). DDR1 is expressed at low levels in healthy adult kidney but DDR1 expression increases in patients with lupus nephritis and Goodpasture's syndrome as well as in a mouse model of crescentic glomerulonephritis (Kerroch et al., 2012). Similarly, DDR1 expression increases in the glomeruli of rats that have undergone partial renal ablation (Lee et al., 2004) and in tubules of mice undergone unilateral ureteral obstruction (Guerrot et al., 2011) suggesting that DDR1 plays a role in renal injury. Older DDR1-null mice show focal swelling of the GBM and mild proteinuria (Gross et al., 2004) suggesting that DDR1 might play a protective role in the maintenance of kidney homeostasis. However, extensive analysis of DDR1-null mice in several mouse models of kidney injury indicated that compared to wild type mice DDR1-null mice have increased survival, improved renal function, as well as reduced fibrosis and inflammation (Flamant et al., 2006; Gross et al., 2010; Guerrot et al., 2011; Kerroch et al., 2012). Moreover, DDR1-null mice show reduced macrophage infiltration following kidney injury suggesting that DDR1 contributes to kidney damage and fibrosis by promoting inflammatory responses (Guerrot et al., 2011; Kerroch et al., 2012) (Figure 3).
Conclusions
In this review, we highlighted recent findings supporting the role of ECM components and integrins in regulating kidney function. The availability of transgenic mice has allowed us to recapitulate the features of some human kidney diseases and, in some cases, diseases were identified because phenotypes were initially identified in animals.
Selective molecular targeting of matrix components and their receptors has proven to be problematic in kidney disease. In this regard, although forced expression of LAMβ1 in podocytes ameliorates feature of Pierson syndrome in LAMβ2-null mice (Suh et al., 2011) and injection of amniotic stem cells or silencing microRNA ameliorates renal damage in a mouse model of Alport syndrome (Garcia et al., 2013), whether forced expression of LAMβ1 or stem cell therapy can be achieved in humans to treat these devastating diseases is unclear. Finally, although we provide evidence that activation of the integrin α1β1/TCPTP plays a key role in protecting from glomerular and tubular fibrosis, the generation and tissue-targeted delivery of integrin α1β1 and TCPTP activators might not be easy to achieve. Despite these difficulties, the current mouse models available have clearly strengthened our understanding of how integrins and BM components not only control kidney function, but also can be targeted to selectively reduce and ideally prevent kidney diseases.
Acknowledgments
This work was in part supported by the Veterans Affairs Merit Reviews 1I01BX002025-01 (A.P.) and 1I01BX002196-01 (R.Z.); the National Institutes of Health grants DK095761 (A.P.), DK075594 (R.Z.), DK069221 (R.Z.), DK083187 (R.Z.).
References
- Abrahamson DR. Role of the podocyte (and glomerular endothelium) in building the GBM. Semin Nephrol. 2012;32:342–349. doi: 10.1016/j.semnephrol.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews KL, Mudd JL, Li C, Miner JH. Quantitative trait loci influence renal disease progression in a mouse model of Alport syndrome. Am J Pathol. 2002;160:721–730. doi: 10.1016/S0002-9440(10)64892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold CN, Xia Y, Lin P, Ross C, Schwander M, Smart NG, Muller U, Beutler B. Rapid identification of a disease allele in mouse through whole genome sequencing and bulk segregation analysis. Genetics. 2011;187:633–641. doi: 10.1534/genetics.110.124586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari JA, Buckley PA, Mould AP, Humphries MJ. Linking integrin conformation to function. J Cell Sci. 2009;122:165–170. doi: 10.1242/jcs.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader BL, Smyth N, Nedbal S, Miosge N, Baranowsky A, Mokkapati S, Murshed M, Nischt R. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol. 2005;25:6846–6856. doi: 10.1128/MCB.25.15.6846-6856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baleato RM, Guthrie PL, Gubler MC, Ashman LK, Roselli S. Deletion of CD151 results in a strain-dependent glomerular disease due to severe alterations of the glomerular basement membrane. Am J Pathol. 2008;173:927–937. doi: 10.2353/ajpath.2008.071149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraldi A, Zambruno G, Furci L, Ballestri M, Tombesi A, Ottani D, Lucchi L, Lusvarghi E. Beta 1 and beta 3 integrin upregulation in rapidly progressive glomerulonephritis. Nephrol Dial Transplant. 1995;10:1155–1161. [PubMed] [Google Scholar]
- Beedle AM, Turner AJ, Saito Y, Lueck JD, Foltz SJ, Fortunato MJ, Nienaber PM, Campbell KP. Mouse fukutin deletion impairs dystroglycan processing and recapitulates muscular dystrophy. J Clin Invest. 2012;122:3330–3342. doi: 10.1172/JCI63004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Cummings CF, Vanacore RM, Kumagai-Cresse C, Ero-Tolliver IA, Rafi M, Kang JS, Pedchenko V, Fessler LI, Fessler JH, Hudson BG. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nature chemical biology. 2012;8:784–790. doi: 10.1038/nchembio.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borza CM, Borza DB, Pedchenko V, Saleem MA, Mathieson PW, Sado Y, Hudson HM, Pozzi A, Saus J, Abrahamson DR, Zent R, Hudson BG. Human podocytes adhere to the KRGDS motif of the alpha3alpha4alpha5 collagen IV network. J Am Soc Nephrol. 2008;19:677–684. doi: 10.1681/ASN.2007070793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borza CM, Chen X, Mathew S, Mont S, Sanders CR, Zent R, Pozzi A. Integrin {alpha}1{beta}1 promotes caveolin-1 dephosphorylation by activating T cell protein-tyrosine phosphatase. J Biol Chem. 2010;285:40114–40124. doi: 10.1074/jbc.M110.156729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borza CM, Pozzi A. The role of cell-extracellular matrix interactions in glomerular injury. Exp Cell Res. 2012;318:1001–1010. doi: 10.1016/j.yexcr.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borza CM, Pozzi A. Discoidin domain receptors in disease. Matrix Biol. 2013 doi: 10.1016/j.matbio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borza CM, Su Y, Chen X, Yu L, Mont S, Chetyrkin S, Voziyan P, Hudson BG, Billings PC, Jo H, Bennett JS, Degrado WF, Eckes B, Zent R, Pozzi A. Inhibition of integrin alpha2beta1 ameliorates glomerular injury. J Am Soc Nephrol. 2012;23:1027–1038. doi: 10.1681/ASN.2011040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Roignot J, Datta A, Overeem AW, Kim M, Yu W, Peng X, Eastburn DJ, Ewald AJ, Werb Z, Mostov KE. A molecular switch for the orientation of epithelial cell polarization. Dev Cell. 2014;31:171–187. doi: 10.1016/j.devcel.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CA, Hwang JC, Guh JY, Chang JM, Lai YH, Chen HC. Reduced podocyte expression of alpha3beta1 integrins and podocyte depletion in patients with primary focal segmental glomerulosclerosis and chronic PAN-treated rats. J Lab Clin Med. 2006;147:74–82. doi: 10.1016/j.lab.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Abair TD, Ibanez MR, Su Y, Frey MR, Dise RS, Polk DB, Singh AB, Harris RC, Zent R, Pozzi A. Integrin {alpha}1{beta}1 Controls Reactive Oxygen Species Synthesis by Negatively Regulating Epidermal Growth Factor Receptor-Mediated Rac Activation. Mol Cell Biol. 2007;27:3313–3326. doi: 10.1128/MCB.01476-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Moeckel G, Morrow JD, Cosgrove D, Harris RC, Fogo AB, Zent R, Pozzi A. Lack of integrin alpha1beta1 leads to severe glomerulosclerosis after glomerular injury. Am J Pathol. 2004;165:617–630. doi: 10.1016/s0002-9440(10)63326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang H, Liao HJ, Hu W, Gewin L, Mernaugh G, Zhang S, Zhang ZY, Vega-Montoto L, Vanacore RM, Fassler R, Zent R, Pozzi A. Integrin-mediated type II TGF-beta receptor tyrosine dephosphorylation controls SMAD-dependent profibrotic signaling. J Clin Invest. 2014;124:3295–3310. doi: 10.1172/JCI71668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Whiting C, Borza C, Hu W, Mont S, Bulus N, Zhang MZ, Harris RC, Zent R, Pozzi A. Integrin alpha1beta1 regulates epidermal growth factor receptor activation by controlling peroxisome proliferator-activated receptor gamma-dependent caveolin-1 expression. Mol Cell Biol. 2010;30::3048–3058. doi: 10.1128/MCB.00892-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YM, Zhou Y, Go G, Marmerstein JT, Kikkawa Y, Miner JH. Laminin beta2 gene missense mutation produces endoplasmic reticulum stress in podocytes. J Am Soc Nephrol. 2013;24:1223–1233. doi: 10.1681/ASN.2012121149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang PY, He JC. JAK/STAT signaling in renal diseases. Kidney Int. 2010;78:231–234. doi: 10.1038/ki.2010.158. [DOI] [PubMed] [Google Scholar]
- Cohn RD, Henry MD, Michele DE, Barresi R, Saito F, Moore SA, Flanagan JD, Skwarchuk MW, Robbins ME, Mendell JR, Williamson RA, Campbell KP. Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell. 2002;110:639–648. doi: 10.1016/s0092-8674(02)00907-8. [DOI] [PubMed] [Google Scholar]
- Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev.Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Cook HT, Khan SB, Allen A, Bhangal G, Smith J, Lobb RR, Pusey CD. Treatment with an antibody to VLA-1 integrin reduces glomerular and tubulointerstitial scarring in a rat model of crescentic glomerulonephritis. Am J Pathol. 2002;161:1265–1272. doi: 10.1016/S0002-9440(10)64403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D, Kalluri R, Miner JH, Segal Y, Borza DB. Choosing a mouse model to study the molecular pathobiology of Alport glomerulonephritis. Kidney Int. 2007;71:615–618. doi: 10.1038/sj.ki.5002115. [DOI] [PubMed] [Google Scholar]
- Cosgrove D, Meehan DT, Grunkemeyer JA, Kornak JM, Sayers R, Hunter WJ, Samuelson GC. Collagen COL4A3 knockout: a mouse model for autosomal Alport syndrome. Genes Dev. 1996;10:2981–2992. doi: 10.1101/gad.10.23.2981. [DOI] [PubMed] [Google Scholar]
- Elias BC, Mathew S, Srichai MB, Palamuttam R, Bulus N, Mernaugh G, Singh AB, Sanders CR, Harris RC, Pozzi A, Zent R. The integrin beta1 subunit regulates paracellular permeability of kidney proximal tubule cells. J Biol Chem. 2014;289:8532–8544. doi: 10.1074/jbc.M113.526509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler R, Meyer M. Consequences of lack of β1 integrin gene expression in mice. Genes & Development. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Fidler AL, Vanacore RM, Chetyrkin SV, Pedchenko VK, Bhave G, Yin VP, Stothers CL, Rose KL, McDonald WH, Clark TA, Borza DB, Steele RE, Ivy MT, Aspirnauts, Hudson JK, Hudson BG. A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc Natl Acad Sci U S A. 2014;111:331–336. doi: 10.1073/pnas.1318499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamant M, Placier S, Rodenas A, Curat CA, Vogel WF, Chatziantoniou C, Dussaule JC. Discoidin domain receptor 1 null mice are protected against hypertension-induced renal disease. J Am Soc Nephrol. 2006;17:3374–3381. doi: 10.1681/ASN.2006060677. [DOI] [PubMed] [Google Scholar]
- Fu G, Wang W, Luo BH. Overview: structural biology of integrins. Methods Mol Biol. 2012;757:81–99. doi: 10.1007/978-1-61779-166-6_7. [DOI] [PubMed] [Google Scholar]
- Garcia O, Carraro G, Turcatel G, Hall M, Sedrakyan S, Roche T, Buckley S, Driscoll B, Perin L, Warburton D. Amniotic fluid stem cells inhibit the progression of bleomycin-induced pulmonary fibrosis via CCL2 modulation in bronchoalveolar lavage. PLoS ONE. 2013;8:e71679. doi: 10.1371/journal.pone.0071679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner H, Broberg A, Pozzi A, Laato M, Heino J. Absence of integrin alpha1beta1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci. 1999;112:263–272. doi: 10.1242/jcs.112.3.263. [DOI] [PubMed] [Google Scholar]
- Gardner H, Kreidberg J, Koteliansky V, Jaenisch R. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- Girgert R, Martin M, Kruegel J, Miosge N, Temme J, Eckes B, Muller GA, Gross O. Integrin alpha2-deficient mice provide insights into specific functions of collagen receptors in the kidney. Fibrogenesis Tissue Repair. 2010;3:19. doi: 10.1186/1755-1536-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S, Harvey SJ, Cunningham J, Tryggvason K, Miner JH. Glomerular filtration is normal in the absence of both agrin and perlecan-heparan sulfate from the glomerular basement membrane. Nephrol Dial Transplant. 2009;24:2044–2051. doi: 10.1093/ndt/gfn758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, Nakagawa N, Xin C, Newitt R, Pandya S, Xia TH, Liu X, Borza DB, Grafals M, Shankland SJ, Himmelfarb J, Portilla D, Liu S, Chau BN, Duffield JS. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest. 2015;125:141–156. doi: 10.1172/JCI75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross O, Beirowski B, Harvey SJ, McFadden C, Chen D, Tam S, Thorner PS, Smyth N, Addicks K, Bloch W, Ninomiya Y, Sado Y, Weber M, Vogel WF. DDR1-deficient mice show localized subepithelial GBM thickening with focal loss of slit diaphragms and proteinuria. Kidney Int. 2004;66:102–111. doi: 10.1111/j.1523-1755.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- Gross O, Girgert R, Beirowski B, Kretzler M, Kang HG, Kruegel J, Miosge N, Busse AC, Segerer S, Vogel WF, Muller GA, Weber M. Loss of collagen-receptor DDR1 delays renal fibrosis in hereditary type IV collagen disease. Matrix Biol. 2010;29:346–356. doi: 10.1016/j.matbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Gross O, Kashtan CE. Treatment of Alport syndrome: beyond animal models. Kidney Int. 2009;76:599–603. doi: 10.1038/ki.2009.223. [DOI] [PubMed] [Google Scholar]
- Guerrot D, Kerroch M, Placier S, Vandermeersch S, Trivin C, Mael-Ainin M, Chatziantoniou C, Dussaule JC. Discoidin domain receptor 1 is a major mediator of inflammation and fibrosis in obstructive nephropathy. Am J Pathol. 2011;179:83–91. doi: 10.1016/j.ajpath.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Has C, Sparta G, Kiritsi D, Weibel L, Moeller A, Vega-Warner V, Waters A, He Y, Anikster Y, Esser P, Straub BK, Hausser I, Bockenhauer D, Dekel B, Hildebrandt F, Bruckner-Tuderman L, Laube GF. Integrin alpha3 mutations with kidney, lung, and skin disease. N Engl J Med. 2012;366:1508–1514. doi: 10.1056/NEJMoa1110813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtkotter O, Nieswandt B, Smyth N, Muller W, Hafner M, Schulte V, Krieg T, Eckes B. Integrin alpha 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem. 2002;277:10789–10794. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- Honore S, Kovacic H, Pichard V, Briand C, Rognoni JB. Alpha2beta1-integrin signaling by itself controls G1/S transition in a human adenocarcinoma cell line (Caco-2): implication of NADPH oxidase-dependent production of ROS. Exp Cell Res. 2003;285:59–71. doi: 10.1016/s0014-4827(02)00038-1. [DOI] [PubMed] [Google Scholar]
- Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N Engl J Med. 2003;348:2543–2556. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- Ivaska J, Reunanen H, Westermarck J, Koivisto L, Kahari VM, Heino J. Integrin alpha2beta1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the alpha2 cytoplasmic tail. J Cell Biol. 1999;147:401–416. doi: 10.1083/jcb.147.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarad G, Pippin JW, Shankland SJ, Kreidberg JA, Miner JH. Dystroglycan does not contribute significantly to kidney development or function, in health or after injury. Am J Physiol Renal Physiol. 2011;300:F811–820. doi: 10.1152/ajprenal.00725.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DK, Fish AJ, Wayner EA, Mauer M, Setty S, Tsilibary E, Kim Y. Distribution of integrin subunits in human diabetic kidneys. J Am Soc Nephrol. 1996;7:2636–2645. doi: 10.1681/ASN.V7122636. [DOI] [PubMed] [Google Scholar]
- Kanasaki K, Kanda Y, Palmsten K, Tanjore H, Lee SB, Lebleu VS, Gattone VH, Jr., Kalluri R. Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol. 2008;313:584–593. doi: 10.1016/j.ydbio.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Wang XP, Miner JH, Morello R, Sado Y, Abrahamson DR, Borza DB. Loss of alpha3/alpha4(IV) collagen from the glomerular basement membrane induces a strain-dependent isoform switch to alpha5alpha6(IV) collagen associated with longer renal survival in Col4a3−/− Alport mice. J Am Soc Nephrol. 2006;17:1962–1969. doi: 10.1681/ASN.2006020165. [DOI] [PubMed] [Google Scholar]
- Kashtan CE, Segal Y. Glomerular basement membrane disorders in experimental models for renal diseases: impact on understanding pathogenesis and improving diagnosis. Contrib Nephrol. 2011;169:175–182. doi: 10.1159/000313956. [DOI] [PubMed] [Google Scholar]
- Kerroch M, Guerrot D, Vandermeersch S, Placier S, Mesnard L, Jouanneau C, Rondeau E, Ronco P, Boffa JJ, Chatziantoniou C, Dussaule JC. Genetic inhibition of discoidin domain receptor 1 protects mice against crescentic glomerulonephritis. Faseb J. 2012;26:4079–4091. doi: 10.1096/fj.11-194902. [DOI] [PubMed] [Google Scholar]
- Korhonen M, Ylanne J, Laitinen L, Virtanen I. The alpha 1-alpha 6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J Cell Biol. 1990;111:1245–1254. doi: 10.1083/jcb.111.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korstanje R, Caputo CR, Doty RA, Cook SA, Bronson RT, Davisson MT, Miner JH. A mouse Col4a4 mutation causing Alport glomerulosclerosis with abnormal collagen alpha3alpha4alpha5(IV) trimers. Kidney Int. 2014;85:1461–1468. doi: 10.1038/ki.2013.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg J, Donovan M, Goldstein S, Rennke H, Shepherd K, Jones R, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- Kuhara T, Kagami S, Kuroda Y. Expression of beta 1-integrins on activated mesangial cells in human glomerulonephritis. J Am Soc Nephrol. 1997;8:1679–1687. doi: 10.1681/ASN.V8111679. [DOI] [PubMed] [Google Scholar]
- Lee J, Bandyopadhyay J, Lee JI, Cho I, Park D, Cho JH. A role for peroxidasin PXN-1 in aspects of C. elegans development. Mol Cells. 2015;38:51–57. doi: 10.14348/molcells.2015.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Eidman KE, Kren SM, Hostetter TH, Segal Y. Localization of discoidin domain receptors in rat kidney. Nephron Exp Nephrol. 2004;97:e62–70. doi: 10.1159/000078407. [DOI] [PubMed] [Google Scholar]
- Lin X, Suh JH, Go G, Miner JH. Feasibility of repairing glomerular basement membrane defects in Alport syndrome. J Am Soc Nephrol. 2014;25:687–692. doi: 10.1681/ASN.2013070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Phillips CL, Killen PD, Hlaing T, Harrison WR, Elder FF, Miner JH, Overbeek PA, Meisler MH. Insertional mutation of the collagen genes Col4a3 and Col4a4 in a mouse model of Alport syndrome. Genomics. 1999;61:113–124. doi: 10.1006/geno.1999.5943. [DOI] [PubMed] [Google Scholar]
- Mathew S, Chen X, Pozzi A, Zent R. Integrins in renal development. Pediatr Nephrol. 2012;27:891–900. doi: 10.1007/s00467-011-1890-1. [DOI] [PubMed] [Google Scholar]
- McCall AS, Cummings CF, Bhave G, Vanacore R, Page-McCaw A, Hudson BG. Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell. 2014;157:1380–1392. doi: 10.1016/j.cell.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Basra S, Kulp DW, Billings PC, Choi S, Beavers MP, McCarty OJ, Zou Z, Kahn ML, Bennett JS, DeGrado WF. Small-molecule inhibitors of integrin alpha2beta1 that prevent pathological thrombus formation via an allosteric mechanism. Proc Natl Acad Sci U S A. 2009;106:719–724. doi: 10.1073/pnas.0811622106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH. Laminins and their roles in mammals. Microsc Res Tech. 2008;71:349–356. doi: 10.1002/jemt.20563. [DOI] [PubMed] [Google Scholar]
- Miner JH. The glomerular basement membrane. Exp Cell Res. 2012;318:973–978. doi: 10.1016/j.yexcr.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Sanes JR. Molecular and functional defects in kidneys of mice lacking collagen alpha 3(IV): implications for Alport syndrome. J Cell Biol. 1996;135:1403–1413. doi: 10.1083/jcb.135.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardacci R, Lo Iacono O, Ciccosanti F, Falasca L, Addesso M, Amendola A, Antonucci G, Craxi A, Fimia GM, Iadevaia V, Melino G, Ruco L, Tocci G, Ippolito G, Piacentini M. Transglutaminase type II plays a protective role in hepatic injury. Am J Pathol. 2003;162:1293–1303. doi: 10.1016/S0002-9440(10)63925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou N, Margadant C, Kevelam SH, Lilien MR, Oosterveld MJ, Kreft M, van Eerde AM, Pfundt R, Terhal PA, van der Zwaag B, Nikkels PG, Sachs N, Goldschmeding R, Knoers NV, Renkema KY, Sonnenberg A. Gain of glycosylation in integrin alpha3 causes lung disease and nephrotic syndrome. J Clin Invest. 2012;122:4375–4387. doi: 10.1172/JCI64100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP. The renal glomerulus of mice lacking s-laminin/laminin beta 2: nephrosis despite molecular compensation by laminin beta 1. Nat Genet. 1995;10:400–406. doi: 10.1038/ng0895-400. [DOI] [PubMed] [Google Scholar]
- Pechkovsky DV, Prele CM, Wong J, Hogaboam CM, McAnulty RJ, Laurent GJ, Zhang SS, Selman M, Mutsaers SE, Knight DA. STAT3-mediated signaling dysregulates lung fibroblast-myofibroblast activation and differentiation in UIP/IPF. Am J Pathol. 2012;180:1398–1412. doi: 10.1016/j.ajpath.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Peterfi Z, Donko A, Orient A, Sum A, Prokai A, Molnar B, Vereb Z, Rajnavolgyi E, Kovacs KJ, Muller V, Szabo AJ, Geiszt M. Peroxidasin is secreted and incorporated into the extracellular matrix of myofibroblasts and fibrotic kidney. Am J Pathol. 2009;175:725–735. doi: 10.2353/ajpath.2009.080693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri M, Stefanou C, Zaravinos A, Erguler K, Stylianou K, Lapathitis G, Karaiskos C, Savva I, Paraskeva R, Dweep H, Sticht C, Anastasiadou N, Zouvani I, Goumenos D, Felekkis K, Saleem M, Voskarides K, Gretz N, Deltas C. Evidence for activation of the unfolded protein response in collagen IV nephropathies. J Am Soc Nephrol. 2014;25:260–275. doi: 10.1681/ASN.2012121217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, Holzman LB, Phillips CL, Fassler R, Quaggin SE, Miner JH, Zent R. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol. 2008;316:288–301. doi: 10.1016/j.ydbio.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi A, Voziyan PA, Hudson BG, Zent R. Regulation of matrix synthesis, remodeling and accumulation in glomerulosclerosis. Curr Pharm Des. 2009;15:1318–1333. doi: 10.2174/138161209787846748. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Zent R. Hold tight or you'll fall off: CD151 helps podocytes stick in high-pressure situations. J Clin Invest. 2012;122:13–16. doi: 10.1172/JCI61858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheault MN, Kren SM, Thielen BK, Mesa HA, Crosson JT, Thomas W, Sado Y, Kashtan CE, Segal Y. Mouse model of X-linked Alport syndrome. J Am Soc Nephrol. 2004;15:1466–1474. doi: 10.1097/01.asn.0000130562.90255.8f. [DOI] [PubMed] [Google Scholar]
- Rubel D, Frese J, Martin M, Leibnitz A, Girgert R, Miosge N, Eckes B, Muller GA, Gross O. Collagen receptors integrin alpha2beta1 and discoidin domain receptor 1 regulate maturation of the glomerular basement membrane and loss of integrin alpha2beta1 delays kidney fibrosis in COL4A3 knockout mice. Matrix Biol. 2014;34:13–21. doi: 10.1016/j.matbio.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Sachs N, Claessen N, Aten J, Kreft M, Teske GJ, Koeman A, Zuurbier CJ, Janssen H, Sonnenberg A. Blood pressure influences end-stage renal disease of Cd151 knockout mice. J Clin Invest. 2012;122:348–358. doi: 10.1172/JCI58878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, Sonnenberg A. Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol. 2006;175:33–39. doi: 10.1083/jcb.200603073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpellini A, Germack R, Lortat-Jacob H, Muramatsu T, Billett E, Johnson T, Verderio EA. Heparan sulfate proteoglycans are receptors for the cell-surface trafficking and biological activity of transglutaminase-2. J Biol Chem. 2009;284:18411–18423. doi: 10.1074/jbc.M109.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpellini A, Huang L, Burhan I, Schroeder N, Funck M, Johnson TS, Verderio EA. Syndecan-4 knockout leads to reduced extracellular transglutaminase-2 and protects against tubulointerstitial fibrosis. J Am Soc Nephrol. 2014;25:1013–1027. doi: 10.1681/ASN.2013050563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata K, Makino H, Morioka S, Kashitani T, Hirata K, Ota Z, Wada J, Kanwar YS. Distribution of extracellular matrix receptors in various forms of glomerulonephritis. Am J Kidney Dis. 1995;25:680–688. doi: 10.1016/0272-6386(95)90542-1. [DOI] [PubMed] [Google Scholar]
- Shukrun R, Vivante A, Pleniceanu O, Vax E, Anikster Y, Dekel B, Lotan D. A human integrin-alpha3 mutation confers major renal developmental defects. PLoS ONE. 2014;9:e90879. doi: 10.1371/journal.pone.0090879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JH, Jarad G, VanDeVoorde RG, Miner JH. Forced expression of laminin beta1 in podocytes prevents nephrotic syndrome in mice lacking laminin beta2, a model for Pierson syndrome. Proc Natl Acad Sci U S A. 2011;108:15348–15353. doi: 10.1073/pnas.1108269108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JH, Miner JH. The glomerular basement membrane as a barrier to albumin. Nature reviews. Nephrology. 2013;9:470–477. doi: 10.1038/nrneph.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacore R, Ham AJ, Voehler M, Sanders CR, Conrads TP, Veenstra TD, Sharpless KB, Dawson PE, Hudson BG. A sulfilimine bond identified in collagen IV. Science. 2009;325:1230–1234. doi: 10.1126/science.1176811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt S, Gossrau R, Baum O, Loster K, Hofmann W, Reutter W. Distribution and quantification of alpha 1-integrin subunit in rat organs. Histochem J. 1995;27:123–132. doi: 10.1007/BF00243907. [DOI] [PubMed] [Google Scholar]
- Yalcin EG, He Y, Orhan D, Pazzagli C, Emiralioglu N, Has C. Crucial role of posttranslational modifications of integrin alpha3 in interstitial lung disease and nephrotic syndrome. Hum Mol Genet. 2015 doi: 10.1093/hmg/ddv111. [DOI] [PubMed] [Google Scholar]
- Yan X, Sabrautzki S, Horsch M, Fuchs H, Gailus-Durner V, Beckers J, Hrabe de Angelis M, Graw J. Peroxidasin is essential for eye development in the mouse. Hum Mol Genet. 2014;23:5597–5614. doi: 10.1093/hmg/ddu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazlovitskaya EM, Tseng HY, Viquez O, Tu T, Mernaugh G, McKee KK, Riggins K, Quaranta V, Pathak A, Carter BD, Yurchenco P, Sonnenberg A, Bottcher RT, Pozzi A, Zent R. Integrin alpha3beta1 regulates kidney collecting duct development via TRAF6-dependent K63-linked polyubiquitination of Akt. Mol Biol Cell. 2015 doi: 10.1091/mbc.E14-07-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Su Y, Paueksakon P, Cheng H, Chen X, Wang H, Harris RC, Zent R, Pozzi A. Integrin alpha1/Akita double-knockout mice on a Balb/c background develop advanced features of human diabetic nephropathy. Kidney Int. 2012;81:1086–1097. doi: 10.1038/ki.2011.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dotsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13:2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- Zent R, Yan X, Su Y, Hudson BG, Borza DB, Moeckel GW, Qi Z, Sado Y, Breyer MD, Voziyan P, Pozzi A. Glomerular injury is exacerbated in diabetic integrin alpha1-null mice. Kidney Int. 2006;70:460–470. doi: 10.1038/sj.ki.5000359. [DOI] [PubMed] [Google Scholar]
- Zhang X, Mernaugh G, Yang DH, Gewin L, Srichai MB, Harris RC, Iturregui JM, Nelson RD, Kohan DE, Abrahamson D, Fassler R, Yurchenco P, Pozzi A, Zent R. beta1 integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity. Development. 2009;136:3357–3366. doi: 10.1242/dev.036269. [DOI] [PMC free article] [PubMed] [Google Scholar]