Abstract
Background
Exposure to traffic pollution during fetal development has been associated with reduced fetal growth, and there is evidence to suggest that epigenetic mechanisms in the placenta in the form of variant DNA methylation may be a potential mechanism of this effect.
Objectives
To examine the association between residential proximity to nearest major roadway, as a marker of traffic-related pollution, fetal growth and placental DNA methylation.
Methods
We obtained residential addresses, placenta samples, and demographic data from 471 women following delivery. Using generalized linear models we evaluated the association between living close to a major roadway (defined as living ≤150 m from a primary highway or primary road or ≤50 m from a secondary road) and fetal growth and DNA methylation of repetitive elements (LINE-1 and AluYb8). We evaluated epigenome-wide methylation in a subset of 215 women to further investigate specific variation in DNA methylation associated with proximity to major roadways.
Results
Living close to a major roadway was associated with a 175.9 g (95% CI: -319.4, -32.5; p=0.016) lower birth weight, 1.8 (95% CI: 0.9, 3.8; p=0.09) times the odds of being small for gestational age, and 0.82 percentage points (95% CI: −1.57, −0.07; p=0.03) lower mean placental LINE-1 methylation levels in fully adjusted models. In epigenome-wide analyses, 7 CpG sites were significantly associated with residential proximity to major roadways. Additional adjustment for placental methylation did not attenuate the association between roadway proximity and birth weight.
Conclusions
Living close to major roadways was associated with lower fetal growth and significant placental epigenetic changes. However, the observed epigenetic changes appear insufficient to explain the observed association between roadway proximity and fetal growth.
Keywords: traffic, pollution, epigenetics, placenta, birthweight
1.0 Introduction
Environmental exposures during fetal development can have short-term (e.g. reduced fetal growth) and long-term (e.g. impaired neurodevelopment) impacts on the health of the child (Ballester et al. 2010; Jirtle and Skinner 2007; Li et al. 2003; Marsit et al. 2012; Nelissen et al. 2011). The placenta can play an important role in this developmental programming as it responds to these exposures and regulates the growth and development of the fetus by controlling nutrient, gas, and waste exchange, as well as growth factor and hormone production (Sood et al. 2006). Epigenetic mechanisms, such as DNA methylation, play an important role in placental development and function by affecting gene expression (Nelissen et al. 2011).
A number of previous studies have reported an association between prenatal exposure to traffic-related air pollution and reduced fetal growth, recently reviewed by Stieb et al. (2012). However, the pathophysiologic mechanisms underlying these associations remain uncertain. Based on some prior evidence, we hypothesize that one possible mechanism is through epigenetic alterations of the placenta, in utero. Indeed, one prior study reported that in utero exposures to particulate air pollution was associated with a marker of global DNA methylation in placenta (Janssen et al. 2013) but to our knowledge the findings from this study have not been replicated. Other evidence indicates that placental DNA methylation patterns, assessed epigenome-wide and at specific repetitive elements (e.g. LINE-1 and AluYb8), are associated with fetal growth (Banister et al. 2011; Wilhelm-Benartzi et al. 2012). More specifically these prior studies found that decreased placental methylation at repetitive elements and patterns of distinct methylation (hypo- or hyper- methylation) at specific loci are associated with reduced fetal growth. This raises the possibility that the observed associations between traffic pollution and reduced fetal growth are mediated through placental epigenetic changes. However, we are not aware of any published studies evaluating this hypothesis.
Accordingly, we examined the association between proximity to major roadways, as a marker of residential exposure to traffic pollution, markers of fetal growth, and placental DNA methylation in a cohort of 471 mother-infant pairs from the Rhode Island Child Health Study (RICHS). We hypothesized that living close to major roadways would be associated with: (1) lower birth weight, (2) higher odds of being small for gestational age (SGA), (3) lower levels of placental DNA methylation of repetitive elements as assessed by LINE-1 and AluYb8, and (4) altered patterns of placental DNA methylation at specific loci.
2.0 Methods
2.1 Study Population
This study included 471 mother-infant pairs who were enrolled in the RICHS cohort following delivery at Women and Infants Hospital in Providence, Rhode Island between March 2009 and May 2013, as previously described (Marsit et al. 2012). Briefly, inclusion criteria for this study included singleton, viable, full term births to mothers 18 years or older and exclusion criteria were a life-threatening complication for the mother or a congenital or chromosomal abnormality of the infant. About 63% of mother-infant pairs who were identified as eligible and invited to participate consented and joined the study. Infants born SGA or large for gestational age (LGA) were selected and matched one-to-one on sex, gestational age (±3 days), and maternal age (±2 years) to infants appropriate for gestational age (AGA). However, the number of SGA and LGA infants is not equal because not everyone consented to participate and as a result of excluding preterm babies there were more LGA than SGA infants in the study. Information, including residential address, was collected from a structured medical chart review and lifestyle, demographic, and exposure histories were collected from a structured questionnaire administered by an interviewer in person.
2.2 Exposure Assessment
To estimate residential exposure to traffic pollution we used ArcMap 10.1 (ESRI; Redlands, CA) to geocode participant addresses and calculate the Euclidean distance to nearest major roadway (Supplemental Material Figure 1). We obtained residential addresses from maternal inpatient medical records at the time of delivery, but did not identify address changes throughout pregnancy.
Major roadways were defined as those with census feature class codes A1 (primary highway with limited access), A2 (primary road without limited access), or A3 (secondary and connecting roads). A1 and A2 roadways include interstate highways and US highways, which typically contain a mix of car and truck traffic moving at higher speeds, and A3 roadways include state highways and other major arteries, which typically have lower traffic counts moving at slower average speeds. The particle number concentration exponentially decays as distance from a major roadway increases and reaches a plateau after 150 m (Zhu et al. 2002a; Zhu et al. 2002b). Therefore, to combine exposures from different road types using a single metric as in previous studies (Gan et al. 2010; Gan et al. 2014; Hart et al. 2013), we considered participants living ≤150 m of an A1 or A2 roadway or ≤50 m of an A3 roadway as “exposed” and unexposed otherwise.
2.3 DNA Methylation Analysis
Within 2 hours of birth, full-thickness sections were taken from the maternal side of the placenta, 2 cm from the umbilical cord-insertion site and free of maternal decidua. These sections were immediately placed in RNAlater™ (Applied Biosystems, Inc., AM7020). Following ≥72 hours at 4°C, samples were blotted dry, snap-frozen in liquid nitrogen, homogenized via pulverization and stored at −80°C until analysis. DNA was extracted, quantified and bisulfite modified via QIAmp DNA Mini Kit (Qiagen, 51304), ND-1000 spectrophotometer (NanoDrop), and EZ DNA Methylation Kit (Zymo Research, D5008).
The LINE-1 and AluYb8 methylation analysis was completed as previously described for all 471 participants (Wilhelm-Benartzi et al. 2012). Briefly, bisulfite pyrosequencing of LINE-1 and AluYb8 was used to determine DNA methylation. LINE-1 methylation extent is defined as the mean percent methylation of four CpG sites on the LINE-1 element. AluYb8 methylation extent is defined as the mean percent methylation of five CpG sites on the AluYb8 element.
For a subset of 215 women, we previously reported epigenome-wide methylation of CpG sites using the Infinium HumanMethylation450 (450K) Bead Chip (Illumina, San Diego, CA) (Maccani et al. 2015). For our analysis, we removed all probes that were present on either the X or Y chromosome, have previously been identified as cross-hybridizing with other genomic locations (Chen et al. 2013), or contained a single nucleotide polymorphism. In addition, probes identified as having detection P values >0.01 in at least one sample were removed. The final data set included 336,484 autosomal probes from 215 samples.
Data was normalized using the functional normalization (funNorm) protocol within the ‘minfi’ package as specified by the software authors (Aryee et al. 2014). In order to correct for any batch to batch variation, we adjusted our data using the ‘ComBat’ method (Johnson et al. 2007), and a principle component analysis (PCA) was used for appropriate normalization and batch effect control.
2.4 Covariate Data
We used data collected from in-person interviewer-administered questionnaires to adjust for potential confounders. Maternal age in years was considered as a continuous variable. We defined maternal education as the highest self-reported level of education attained by the mother and categorized into three groups: high school graduate or less, some college, and college graduate or more. Maternal race was dichotomized as white or other. Maternal alcohol and tobacco use during pregnancy were each dichotomized as yes or no. Maternal prenatal vitamin use during pregnancy was defined as regularly taking prenatal vitamins and was dichotomized as yes or no. Parity was categorized as having 1 child, 2 children, or 3 or more children. Household income (9.5% missing) was categorized in the interviewer-administered survey, but categories were further collapsed into 5 groups: <$25,000; $25,000 - <$50,000; $50,000 - <$80,000; $80,000 - < $100,000; and ≥$100,000. Maternal health insurance was categorized as no insurance/self-pay, private insurance, or public insurance/other. Marital status was defined as married or not married, which includes single, separated, and divorced. Maternal BMI before pregnancy was considered in the analyses as a continuous variable, and was previously calculated from self-reported height and weight.
Covariate data for the infant include sex, gestational age at birth in weeks, birth weight, and infant growth status. SGA was defined as the lowest 10th percentile and LGA was defined as the highest 10th percentile based on birth weight and gestational age and calculated from the Fenton growth chart (Fenton and Kim 2013).
Neighborhood socioeconomic status (SES) was assessed by 6 census-level variables (Diez Roux et al. 2001): median household income; percent of households with interests, dividends, or rent income; percent of residents with high school diploma; percent with college degree; percent with professional occupation; and median value of owner-occupied housing units. We calculated a z-score for each of these variables and summed the scores to create a zsum, which we used to control for potential confounding by neighborhood SES.
2.5 Statistical Analysis
We examined unadjusted bivariate associations of all potential confounders with distance to roadway and with LINE-1 and AluYb8 methylation levels using one-way ANOVA tests, t-tests, and chi-square tests, as appropriate. We used linear regression to evaluate the association between proximity to major roadways and continuous outcomes (birth weight, LINE-1 methylation, AluYb8 methylation). We used logistic regression to evaluate the association between proximity to major roadways and infant growth status (SGA versus LGA and AGA). We drew a causal diagram (Supplemental Material Figure 2) to determine the selection of variables to include in the models. Models were adjusted for age, education level, tobacco use during pregnancy, prenatal vitamin use during pregnancy, BMI before pregnancy, parity, annual household income, health insurance status, marital status, race, infant gestational age at birth, infant sex, and neighborhood SES as a sum z-score. Birthweight adjusted for gestational age provides a marker of fetal growth (Oken et al. 2003; Vidal et al. 2015), which was our primary health outcome of interest. For the analyses of infant growth status, SGA, AGA, and LGA are categorized by percentiles that are based on gestational age so we do not include gestational age as a covariate in our models.
We performed secondary analyses to better understand potential mediation effects. We examined the association between proximity to major roadway and birth weight additionally adjusted for mean LINE-1 methylation levels and mean AluYb8 methylation levels to test the hypothesis that the effects of traffic pollution on fetal growth are mediated at least in part through changes in global methylation levels as assessed by LINE-1 and AluYb8. If some or all of the effect on birth weight is mediated through placental methylation, under certain unverifiable assumptions (Cole and Hernan 2002; VanderWeele and Vansteelandt 2009, 2010) we would expect that further adjusting by LINE-1 and AluYb8 should attenuate the observed association between living close to a major roadway and birth weight.
For the epigenome-wide analysis, we used a linear regression model in the Limma package (R version 3.0.0) to compare the methylation beta values of those living close vs further from a major roadway. Our primary model adjusted for the same covariates as the previous models for LINE-1 and AluYb8, with the exception of health insurance status and parity because there was virtually no variation in these variables in the subset with epigenome-wide methylation data.
We used the Houseman reference-free method to calculate and adjust for cell type proportions as described previously (Houseman et al. 2014). Briefly, methylation and covariate data are used to infer the number of latent variables (number of cell types) in placenta samples without a reference set. Then a linear regression model is fit and includes the latent variables and potential confounders to obtain adjusted estimates. DNA methylation profiles are cell-specific, thus we need to adjust for cell type proportions to control for this potential confounding bias. This method has been used previously to adjust for confounding by cell type proportion and also to reduce standard errors (Green et al. 2016). To calculate standard errors and p-values we ran 100 Bootstraps. Finally, to adjust for multiple comparisons, we calculated q-values to protect the false discovery rate (FDR) using the Benjamini-Hochberg method (FDR<0.05). In a secondary analysis, we performed the main analysis additionally adjusting for the statistically significant CpGs to evaluate potential mediation. Using the annotation files from the GenomeStudio from Illumina, we mapped the statistically significant CpGs its gene of origin. Analyses were performed in SAS 9.3 (SAS Institutes, Inc.; Cary, NC) and in R (v3.0.0) with packages: Limma (Linear Models for Microarray Analysis), qvalue, RefFreeEWAS, and qqman (Team 2013).
3.0 Results
Most of the 471 participants were white (76.7%), married (63.9%), and multiparous (73.7%) with a mean age of 30.0 (standard deviation, SD=5.6) years (Table 1). About 21% of mothers lived near a major roadway (Supplemental Figure 1) and tended to be slightly younger, slightly more obese, and reported slightly lower annual household income compared to mothers living further away from major roadways.
Table 1.
Population characteristics by residential distance to nearest major roadway (within 150 m of A1 or A2 roadway or within 50 m of A3 roadway)
| Total (n = 471) | Near major roadway (n = 98) | Further from major roadway (n = 373) | p-valuea | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age, years, mean +− SD | 30.0 ± 5.6 | 29.2 ± 5.1 | 30.3 ± 5.8 | 0.091 |
| BMI before pregnancy, kg/m2 | 26.9 ± 7.1 | 27.9 ± 7.5 | 26.7 ± 7.0 | 0.12 |
| Tobacco use during pregnancy | 23 (4.9%) | 3 (3.1%) | 20 (5.4%) | 0.24 |
| Alcohol use during pregnancy | 4 (0.9%) | 1 (1.0%) | 3 (0.8%) | 0.44 |
| Prenatal care during pregnancy, No | 458 (97.2%) | 96 (98.0%) | 362 (97.1%) | 0.84 |
| Prenatal vitamin use, No | 434 (92.1%) | 89 (90.8%) | 345 (92.5%) | 0.66 |
| Annual household income | 0.11 | |||
| <$25,000 | 101 (21.4%) | 27 (27.6%) | 74 (19.8%) | |
| $25,000-49,999 | 71 (15.1%) | 19 (19.4%) | 52 (13.9%) | |
| $50,000-79,999 | 82 (17.4%) | 19 (19.4%) | 63 (16.9%) | |
| $80,000-99,999 | 55 (11.7%) | 6 (6.1%) | 49 (13.1%) | |
| ≥$100,000 | 117 (24.8%) | 19 (19.4%) | 98 (26.3%) | |
| Married | 301 (63.9%) | 63 (64.3%) | 238 (63.8%) | 0.91 |
| Race, Caucasian | 361 (76.7%) | 75 (76.5%) | 286 (76.7%) | 0.87 |
| Education | 0.74 | |||
| < High school | 102 (21.7%) | 24 (24.5%) | 78 (20.9%) | |
| Some college | 115 (24.4%) | 26 (26.5%) | 89 (23.9%) | |
| College or more | 249 (52.9%) | 47 (48.0%) | 202 (54.2%) | |
| Health insurance | 0.18 | |||
| None/self-pay | 8 (1.7%) | 2 (2.0%) | 6 (1.6%) | |
| Private | 282 (59.9%) | 52 (53.1%) | 230 (61.7%) | |
| Public/other | 175 (37.2%) | 41 (41.8%) | 134 (35.9%) | |
| Parity | 0.79 | |||
| 1 | 123 (26.1%) | 24 (24.5%) | 99 (26.5%) | |
| 2 | 164 (34.8%) | 32 (32.7%) | 132 (35.4%) | |
| ≥3 | 183 (38.9%) | 42 (42.9%) | 141 (37.8%) | |
| Newborn characteristics | ||||
| Gestational age (weeks), mean ± SD | 39.0 ± 0.9 | 39.1 ± 0.9 | 39.0 ± 0.9 | 0.47 |
| Infant sex, Male | 244 (51.8%) | 49 (50.0%) | 195 (52.3%) | 0.69 |
| Neighborhood SES, z-score | 0.0 ± 5.3 | 0.3 ± 5.0 | −0.1 ± 5.4 | 0.57 |
| Outcomes | ||||
| Birth weight (g), mean ± SD | 3568.3 ± 664.2 | 3483.4 ± 688.3 | 3590.6 ± 656.9 | 0.15 |
| Growth status | 0.50 | |||
| SGA | 77 (16.4%) | 19 (19.4%) | 58 (15.6%) | |
| AGA | 260 (55.2%) | 55 (56.1%) | 205 (55.0%) | |
| LGA | 134 (28.5%) | 24 (24.5%) | 110 (29.5%) | |
| Mean LINE-1 | 41.7 ± 3.1 | 41.1 ± 3.5 | 41.9 ± 3.0 | 0.023 |
| Mean AluYb8 | 70.5 ± 3.7 | 71.1 ± 4.7 | 70.3 ± 3.5 | 0.066 |
Note: Near major roadway defined as ≤150 m of A1/A2 or ≤50 m of A3 and far from major roadways defined as >150 m of A1/A2 or >50 m of A3. Not all N values equal 471 because of missing values. Neighborhood SES z-sum is the sum of the z-scores for median household income, percent of households with interests, dividends, or rent income, percent of residents with high school diploma, percent with college degree, percent with professional occupation, and median value of owner-occupied housing units.
Abbreviations: SD is standard deviation, BMI is body mass index, SGA is small for gestational age, AGA is average for gestational age, LGA is large for gestational age, and SES is socioeconomic status.
p-values obtained from chi square tests for categorical variables and one-way ANOVA tests for continuous variables
In crude analyses, the association between proximity to major roadways and birth weight did not reach statistical significance (p=0.15). However, after adjusting for a number of individual and area-level sociodemographic variables, living near a major roadway was associated with a 175.9 g (95% CI: −318.9, −32.9; p=0.016) lower birth weight and 1.8 (95% CI: 0.9, 3.8; p=0.09) times the odds of being SGA, compared to those living farther from a major roadway (Figure 1). Living near a major roadway was not associated with odds of being LGA (OR=0.63; 95% CI: 0.36, 1.12; p=0.12).
Figure 1.
Association between distance to roadway (≤150m of A1/A2 or ≤50m of A3) and difference in birth weight (filled circles indicate mean difference, bars indicate 95% confidence intervals) (A) and infant growth status (B, filled circles indicate odds ratio, bars indicate 95% confidence intervals). Models are adjusted for maternal age, BMI before pregnancy, maternal education, tobacco use during pregnancy, prenatal vitamin use, annual household income, health insurance, maternal ethnicity, parity, gestational age, infant sex, and neighborhood SES z-sum.
LINE-1 and AluYb8 methylation data were available in 469 (99.6%) and 467 (99.2%) participants, respectively. Mean LINE-1 methylation levels were marginally associated only with gestational age (p=0.071), while AluYb8 methylation levels were associated with infant sex (p<0.001) and marginally associated with maternal education (p=0.06) (Supplemental Material Table 1). In crude analyses, mean LINE-1 methylation levels were significantly lower (p=0.03) among those living near a major roadway, while AluYb8 levels tended to be slightly higher (p=0.07) among participants living near a major roadway. After adjusting for a number of individual and area-level sociodemographic variables, living near a major roadway was associated with a mean LINE-1 methylation that was 0.82 percentage points lower (95% CI: −1.57, −0.07; p=0.03) versus participants living further from a major roadway. We found no associations between residential proximity to nearest major roadway and mean placental AluYb8 methylation levels in fully adjusted models. In fully adjusted models the associations between LINE-1 and AluYb8 methylation levels and birth weight did not reach statistical significance (data not shown).
To examine the association between proximity to nearest major roadway and birth weight not mediated through placental DNA methylation, we additionally adjusted for mean LINE-1 and AluYb8 methylation levels and found that living near a major roadway was associated with a 172.0 (95% CI: −316.7, −27.3; p=0.02) and 179.8 (95% CI: −324.1, −35.6; p=0.015) lower birth weight, respectively (Table 2), which is essentially unchanged from the 175.9 g difference found in the main analyses not adjusted for LINE-1 or AluYb8.
Table 2.
Association between residential distance to nearest major roadway (within 150 m of A1 or A2 roadway or within 50 m of A3 roadway) and birth weight (g), with and without adjustment for LINE-1 and/orAluYb8 methylation levels
| Adjustment for Potential Intermediate | Difference in birth weight (95% CI) | p-value |
|---|---|---|
| None | −175.9 (−318.9, −32.9) | 0.016 |
| LINE-1 | −172.0 (−316.7, −27.3) | 0.020 |
| AluYb8 | −179.8 (−324.1, −35.6) | 0.015 |
| LINE-1 + AluYb8 | −175.7 (−321.4, −30.1) | 0.018 |
All models adjusted for maternal age, BMI before pregnancy, maternal education level, tobacco use during pregnancy, prenatal vitamin use, annual household income, health insurance status, maternal ethnicity, gestational weeks, infant sex, parity, and neighborhood SES z-sum (the sum of the z-scores for median household income, percent of households with interests, dividends, or rent income, percent of residents with high school diploma, percent with college degree, percent with professional occupation, and median value of owner-occupied housing units). The LINE-1 model additionally adjusted for mean LINE-1 methylation levels. The AluYb8 model additionally adjusted for mean AluYb8 methylation levels. The LINE-1+AluYb8 model additionally adjusted for mean LINE-1 methylation levels and AluYb8 methylation levels.
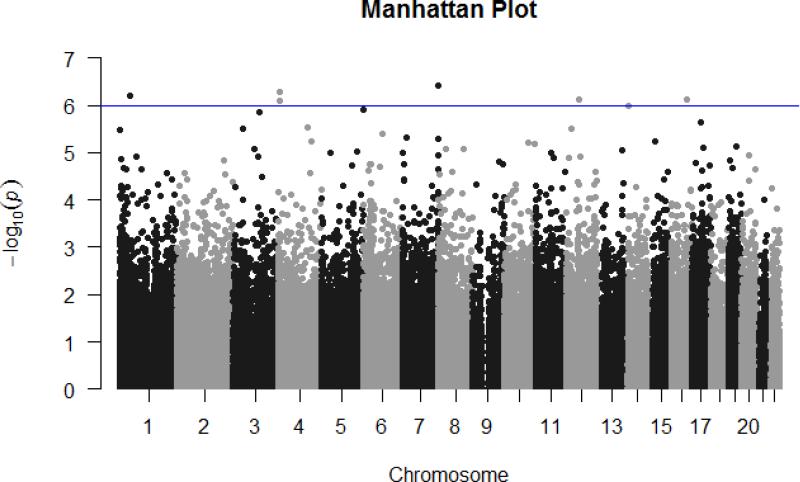
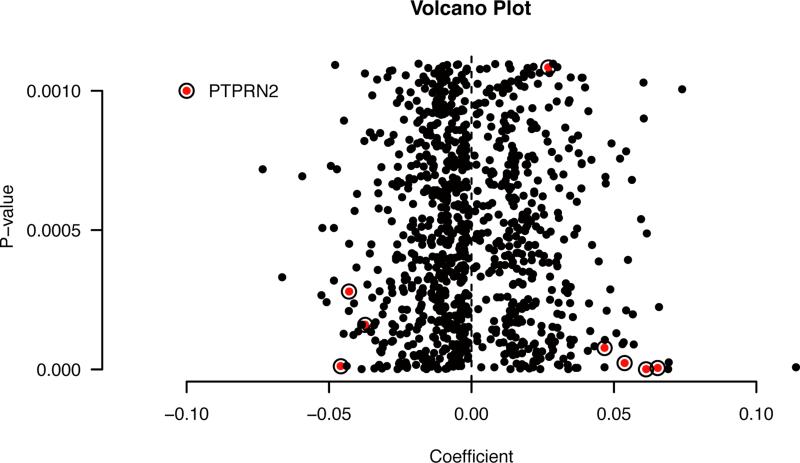
After finding a statistically significant association between residential proximity to major roadways and LINE-1 indicative of epigenetic activity, we performed an epigenome-wide analysis in a subset of the study population. There was no statistically significant difference between the full cohort (n=471) and the 215 participants with epigenome-wide data (Supplemental Material Table 2). Among this sub-cohort, we found 7 CpG sites that were differentially methylated (at the FDR<0.05 level) in mothers living close to a major roadway versus further away (Table 3 and Figure 2). Three of these CpG sites were mapped to genes PTPRN2, TMEM125, and VPS4A, but the other 4 sites map to non-genic regions. Figure 3 plots the coefficient for each CpG site versus its p-value. Loci on the PTPRN2 gene, highlighted in red, appear many times in the top 1000 sites. It also has the most significant p-value, as shown in Table 3.
Table 3.
Statistically significant (FDR q<0.05) CpG sites out of 336,484 total sites
| Name | P value | P value adjusted* | Q value | Gene name | Chromosome | Difference in Mean Methylation Percentage |
|---|---|---|---|---|---|---|
| cg22517801 | 2.904e-06 | 3.729e-07 | 0.046 | PTPRN2 | 7 | 0.061 |
| cg02757456 | 1.359e-03 | 5.122e-07 | 0.046 | unknown | 4 | −0.039 |
| cg05375878 | 1.077e-03 | 6.242e-07 | 0.046 | TMEM125 | 1 | −0.012 |
| cg26468152 | 3.151e-05 | 7.573e-07 | 0.046 | VPS4A | 16 | −0.016 |
| cg13839439 | 2.508e-04 | 7.658e-07 | 0.046 | unknown | 12 | 0.024 |
| cg03838806 | 4.209e-04 | 8.289e-07 | 0.046 | unknown | 4 | 0.034 |
| cg21884122 | 1.418e-04 | 1.019e-06 | 0.049 | unknown | 14 | 0.014 |
All models are adjusted for maternal age, BMI before pregnancy, maternal education level, prenatal vitamin use, annual household income, maternal ethnicity, gestational age, infant sex, and neighborhood SES z-sum (the sum of the z-scores for median household income, percent of households with interests, dividends, or rent income, percent of residents with high school diploma, percent with college degree, percent with professional occupation, and median value of owner-occupied housing units).
Adjusted p-values additionally adjusted for cell type using the Reference-free method. Q-values were calculated using the Benjamini-Hochberg method
Figure 2.
Manhattan plot showing the association between distance to roadway (≤150m of A1/A2 or ≤50m of A3) and DNA methylation of CpGs. Each dot represents an individual CpG site, and the horizontal blue line the level of statistical significance (q < 0.05, P < 1×10−6). Models adjusted for maternal age, BMI before pregnancy, maternal education, prenatal vitamin use, annual household income, maternal ethnicity, gestational age, infant sex, neighborhood SES z-sum and cell type.
Figure 3.
Volcano plot showing the regression coefficient (x-axis) of the association between living near a major roadway (≤ 150m of A1/A2 or ≤ 50m of A3) and DNA methylation of each individual CpG site for the top 1000 loci, plotted versus its p-value (y-axis). All models adjusted for maternal age, BMI before pregnancy, maternal education, prenatal vitamin use, annual household income, maternal ethnicity, gestational age, infant sex, neighborhood SES z-sum and cell type.
To gain further insights into the potential mediating effect of DNA methylation, we examined the association between distance to roadway and birth weight with and without additional adjustment for the 7 CpG sites that reached statistical significance. In this subset of participants, living close to a major roadway was associated with a 297.6 g (95% CI: −507.9, −87.2; p=0.006) lower birth weight and 1.6 (95% CI: 0.6, 4.0; p=0.33) times the odds of being SGA, compared to those living farther from a major roadway (Figure 1). After additional adjustment for methylation levels of one or all of the 7 CpG sites, the association between roadway proximity and birth weight was essentially unchanged both in magnitude and statistical significance (Table 4). Accordingly, the associations between each CpG site and birth weight did not reach statistical significance (data not shown).
Table 4.
Association between distance to nearest major roadway (≤150m of A1/A2 or ≤50m of A3) and birth weight with and without adjustment for differentially methylated loci
| CpG site | Change in birth weight (95% CI) | P-value |
|---|---|---|
| None | −297.6 (−507.9, −87.2) | 0.006 |
| cg22517801 | −286.8 (−496.9, −76.6) | 0.008 |
| cg02757456 | −288.3 (−499.2, −77.4) | 0.008 |
| cg05375878 | −284.5 (−495.4, −73.6) | 0.008 |
| cg26468152 | −285.6 (−496.3, −74.8) | 0.008 |
| cg13839439 | −286.3 (−496.9, −75.6) | 0.008 |
| cg03838806 | −288.3 (−499.2, −77.4) | 0.008 |
| cg21884122 | −281.7 (−493.0, −70.4) | 0.009 |
| All 7 CpGs | −285.4 (−500.3, −70.4) | 0.010 |
All models are adjusted for maternal age, BMI before pregnancy, maternal education level, prenatal vitamin use, annual household income, maternal ethnicity, gestational age, infant sex, and neighborhood SES z-sum (the sum of the z-scores for median household income, percent of households with interests, dividends, or rent income, percent of residents with high school diploma, percent with college degree, percent with professional occupation, and median value of owner-occupied housing units).
4.0 Discussion
Among 471 mother-infant pairs, we found that living close to a major roadway was associated with statistically significant lower birth weight, lower levels of placental LINE-1 DNA methylation, and differential methylation of DNA at 7 loci, compared to those living farther from major roadways. We did not find an association between residential proximity to nearest major roadway and mean AluYb8 methylation levels.
Our findings of associations between residential proximity to major roadways, acting as a marker of residential exposure to traffic-related air and noise pollution, lower birth weight and higher odds of being SGA are consistent with prior studies (Dadvand et al. 2013; Dadvand et al. 2014; Fleisch et al. 2015; Stieb et al. 2012). For example, Fleisch et al. (2015) found that those living <50 m from a major roadway had reduced fetal growth compared to those living ≥200 m, and that maternal exposure to traffic-related air pollutants was also associated with reduced fetal growth. Similarly, Dadvand et al. (2014) examined maternal residential proximity to major roadways and found that living within 200 m of a major roadway was associated with increased risk of low birth weight and that this association was partially mediated through higher exposure to traffic-related air pollutants. A number of other studies have reported associations between maternal exposure to either NO2, a marker of traffic-related air pollution, or ambient particulate matter and markers indicative of reduced fetal growth (Ballester et al. 2010; Dadvand et al. 2013).
A number of previous studies have reported associations between traffic-related air pollutants and DNA methylation changes in circulating leukocytes of adults (Baccarelli et al. 2009; Madrigano et al. 2011; Tarantini et al. 2009). However, we are aware of only two prior studies considering the effects of traffic pollutants on placental DNA methylation. Similar to our observation of decreased LINE-1 placental DNA methylation associated with living close to a major roadway, Janssen et al. (2013) found that maternal exposure to ambient fine particulate matter (PM2.5) was associated with lower global placental DNA methylation. In a separate study, Janssen et al. (2015) found that PM2.5 was positively associated with placental mitochondrial DNA (mtDNA) methylation and inversely associated with mtDNA content. Mediation analyses suggest that mtDNA methylation partially mediated the association between PM2.5 and mtDNA content (Janssen et al. 2015).
Based on these prior studies, we hypothesized that those living near a major roadway would have lower global methylation levels compared to those living farther from a major roadway. Previous studies have examined the potential mechanism by which this happens and found that air pollutants were associated with cellular stress and inflammation responses, which results in oxidation and potentially to changes to DNA methylation (Baccarelli et al. 2009; Brook et al. 2004; Donaldson et al. 2001; Valinluck et al. 2004). Our finding that living close to a major roadway was associated with lower mean LINE-1 methylation levels supports this hypothesis.
Among the subset of participates with epigenome-wide data, we found that living close to a major roadway was associated with 7 statistically significant differentially methylated CpGs in placental tissue. Three of the 7 CpG sites were mapped to genes: PTPRN2, TMEM125, and VPS4A. Breton et al. (2009) previously found an association between prenatal smoking and differentially methylated PTPRO, which encodes a protein tyrosine phosphatase receptor and is a candidate tumor suppressor (Motiwala et al. 2004). PTPRN2 also encodes a protein tyrosine phosphatase receptor (Motiwala et al. 2004; NCBI 2015a), potentially suggesting some degree of similarity in the molecular mechanisms by which maternal exposure to smoking and traffic-related pollution impacts placental DNA methylation.
The implications of the differential methylation of the other loci are less clear. The TMEM125 gene encodes a transmembrane protein and the VPS4A gene encodes a protein involved in vacuolar protein sorting and is part of the ATPase family, which is associated with diverse cellular activities (NCBI 2015b, c). To our knowledge, neither of these genes has been studied in the context of prenatal environmental exposures and DNA methylation so we cannot compare our findings.
In secondary analyses we additionally adjusted for methylation levels to explore the hypothesis that the putative effects of living close to a major roadway on birth weight are mediated through placental epigenetic changes. The association between residential proximity to major roadways and birth weight were nearly identical with or without adjustment for LINE-1, AluYb8, or both LINE-1 and AluYb8 methylation levels, suggesting that LINE-1 and AluYb8 methylation are not important mediators of the association between traffic pollution and fetal growth. In the subset of participants with data on epigenome-wide methylation, we found that adjusting for any or all 7 CpGs further strengthened the association between residential proximity to major roadways and birth weight, also suggesting that epigenetic changes at these loci are not important mediators of the observed associations between traffic pollution and birth weight. However, these analyses rely on the unverifiable assumption that there is no residual confounding by other common causes of methylation and fetal growth (Cole and Hernan 2002). Clearly additional studies on the potential mediating influences of epigenetic changes are needed to confirm or refute our findings.
This study has some important limitations. First, residential distance to nearest major roadway is a marker of traffic pollution so the associations observed could be due to traffic-related air pollution, traffic-related noise, or potentially other stressors associated with living very close to a major roadway. However, the use of residential proximity to major roadways has a significant advantage in that it allows consideration of the joint effects of air and noise pollution from traffic and has been used as such in a number of prior studies of both adults and children (Fleisch et al. 2015; Gan et al. 2010; Harris et al. 2015; Hart et al. 2013; Kingsley et al. 2015). Second, although the study area is geographically small there are likely differences in noise and air pollution characteristics at varying distances from major roadways across the study area. Additional studies with high quality data on residential exposure to both air and noise pollution are clearly needed to disentangle these effects. Third, information on residential history throughout pregnancy was unavailable. Between 9-32% of pregnant mothers move during their pregnancies (Bell and Belanger 2012; Saadeh et al. 2013), which likely lead to some exposure misclassification. Fourth, we do not know how much time pregnant mothers spend at home vs other locations. Fifth, we do not have measures of indoor pollution levels or information on potential modifiers of the association such as air conditioning use. Sixth, we do not have measures of wind speed or direction which could impact the level of traffic-related air pollution exposure from living near a major roadway. Seventh, although we expect geocoding errors to be relatively small in an area with high population density such as the northeast, there remains potential for geocoding errors that may also contribute to exposure misclassification (Jacquez 2012). Eighth, there is potential for selection bias if participation in the study was influenced by proximity to major roadway conditional on the demographic and socioeconomic factors included in our models. Finally, the observed epigenetic changes may not represent changes in gene expression, which depend on the CpG site's location on the genome and the promoter's CpG density (Marchal and Miotto 2015). However, DNA methylation reset early in development and is critical in in defining tissue differentiation and function. Thus, environmental exposures, such as traffic pollution, may interfere with this epigenetic programming during this critical period in development and can have long term consequences (Jaenisch and Bird 2003). We acknowledge, though, that we are examining these marks using a terminally differentiated tissue (the placenta) at a single time point, and so cannot make definitive statements regarding persistence, although this is an area for further investigation utilizing longitudinal study designs
Nonetheless, important strengths of this study include a comparatively large sample size for a study of epigenetics, the use of highly quantitative bisulfite pyrosequencing for LINE-1 and AluYb8 methylation profiling, and the use of the high sample throughput Infinium HumanMethylation450 (450K) Bead Chip that spans across the genome. Additionally, we adjusted for the potential confounder of cellular heterogeneity in the placenta when modelling the effect of residential proximity to major roadway and differentially methylated CpGs.
In conclusion, we found that living near a major roadway was associated with lower birth weight, increased odds of being SGA, and lower mean LINE-1 methylation levels in human placenta and 7 statistically significant differentially methylated CpG sites, after adjusting for important confounders. However, the observed association between residential proximity to major roadways and fetal growth does not appear to be explained by the observed placental epigenetic changes. Additional studies in with improved exposure assessments are needed to understand the functional impacts of the epigenetic changes.
Supplementary Material
Highlights.
Traffic pollution exposure in utero is associated with reduced fetal growth.
This association may be mediated by placental epigenetic mechanisms.
Living close to a major roadway is associated with lower birth weight.
Living close to a major roadway is associated with placental epigenetic changes.
Placental epigenetic changes do not appear to mediate the relationship.
Acknowledgements and Funding Sources
Research reported in the publication was supported by NIH-NIMH R01MH094609, NIH-NIEHS R01ES022223, NIH-NIEHS P01 ES022832, and NIH R21-ES023073 and by US EPA grant RD83544201. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the sponsoring organizations. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the presentation. Ms. Kingsley was supported by a graduate student fellowship from the Institute at Brown for Environment and Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: The authors declare no competing financial interests.
References
- Aryee M, Jaffe A, Corrada-Bravo H, Ladd-Acosta C, Feinberg A, Hansen K, et al. Minfi: A flexible and comprehensive bioconductor package for the analysis of infinium DNA methylation microarrays. Bioinformatics. 2014;33(10):1363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A, Wright RO, Bollati V, Tarantini L, Litonjua AA, Suh HH, et al. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179(7):572–578. doi: 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester F, Estarlich M, Iniguez C, Llop S, Ramon R, Esplugues A, et al. Air pollution exposure during pregnancy and reduced birth size: A prospective birth cohort study in valencia, spain. Environ Health. 2010;9:6. doi: 10.1186/1476-069X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banister CE, Koestler DC, Maccani MA, Padbury JF, Houseman EA, Marsit CJ. Infant growth restriction is associated with distinct patterns of DNA methylation in human placentas. Epigenetics. 2011;6(7):920–927. doi: 10.4161/epi.6.7.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Belanger K. Review of research on residential mobility during pregnancy: Consequences for assessment of prenatal environmental exposures. J Expo Sci Environ Epidemiol. 2012;22(5):429–438. doi: 10.1038/jes.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180(5):462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. Air pollution and cardiovascular disease: A statement for healthcare professionals from the expert panel on population and prevention science of the american heart association. Circulation. 2004;109(21):2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Chen YA, Lemire M, Choufani S, Butcher DT, Grafodatskaya D, Zanke BW, et al. Discovery of cross-reactive probes and polymorphic cpgs in the illumina infinium humanmethylation450 microarray. Epigenetics. 2013;8(2):203–209. doi: 10.4161/epi.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SR, Hernan MA. Fallibility in estimating direct effects. International Journal of Epidemiology. 2002;31:161–165. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA, et al. Maternal exposure to particulate air pollution and term birth weight: A multi-country evaluation of effect and heterogeneity. Environ Health Perspect. 2013;121(3):267–373. doi: 10.1289/ehp.1205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadvand P, Ostro B, Figueras F, Foraster M, Basagana X, Valentin A, et al. Residential proximity to major roads and term low birth weight: The roles of air pollution, heat, noise, and road-adjacent trees. Epidemiology. 2014;25(4):518–525. doi: 10.1097/EDE.0000000000000107. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Stone V, Seaton A, MacNee W. Ambient particle inhalation and the cardiovascular system: Potential mechanisms. Environ Health Perspect. 2001;109(supplement 4):523–527. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton TR, Kim JH. A systematic review and meta-analysis to revise the fenton growth chart for preterm infants. BMC Pediatrics. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, et al. Prenatal exposure to traffic pollution: Associations with reduced fetal growth and rapid infant weight gain. Epidemiology. 2015;26(1):43–50. doi: 10.1097/EDE.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan WQ, Tamburic L, Davies HW, Demers PA, Koehoorn M, Brauer M. Changes in residential proximity to road traffic and the risk of death from coronary heart disease. Epidemiology. 2010;21(5):642–649. doi: 10.1097/EDE.0b013e3181e89f19. [DOI] [PubMed] [Google Scholar]
- Gan WQ, Allen RW, Brauer M, Davies HW, Mancini GBJ, Lear SA. Long-term exposure to traffic-related air pollution and progression of carotid artery atherosclerosis: A prospective cohort study. BMJ Open. 2014;4:e004743. doi: 10.1136/bmjopen-2013-004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BB, Karagas MR, Punshon T, Jackson BP, Robbins DJ, Houseman EA, et al. Epigenome-wide assessment of DNA methylation in the placenta and arsenic exposure in the new hampshire birth cohort study (USA). Environ Health Perspect. 2016 doi: 10.1289/ehp.1510437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MH, Gold DR, Rifas-Shiman SL, Melly SJ, Zanobetti A, Coull BA, et al. Prenatal and childhood traffic-related pollution exposure and childhood cognition in the project viva cohort (massachusetts, USA). Environ Health Perspect. 2015;123(10):1072–1078. doi: 10.1289/ehp.1408803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JE, Rimm EB, Rexrode KM, Laden F. Changes in traffic exposure and the risk of incident myocardial infarction and all-cause mortality. Epidemiology. 2013;24(5):734–742. doi: 10.1097/EDE.0b013e31829d5dae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseman EA, Molitor J, Marsit CJ. Reference-free cell mixture adjustments in analysis of DNA methylation data. Bioinformatics. 2014;30(10):1431–1439. doi: 10.1093/bioinformatics/btu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquez GM. A research agenda: Does geocoding positional error matter in health gis studies? Spat Spatiotemporal Epidemiol. 2012;3(1):7–16. doi: 10.1016/j.sste.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Janssen BG, Godderis L, Pieters N, Poels K, Kiciński M, Cuypers A, et al. Placental DNA hypomethylation in association with particulate air pollution in early life. Particle and Fibre Toxicology. 2013;10(22):1–11. doi: 10.1186/1743-8977-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BG, Byun HM, Gyselaers W, Lefebvre W, Baccarelli AA, Nawrot TS. Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: An environage birth cohort study. Epigenetics. 2015;10(6):536–544. doi: 10.1080/15592294.2015.1048412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4):253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical bayes methods. Biostatistics. 2007;8(1):118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- Kingsley SL, Eliot MN, Whitsel EA, Wang Y, Coull BA, Hou L, et al. Residential proximity to major roadways and incident hypertension in post-menopausal women. Environ Res. 2015;142:522–528. doi: 10.1016/j.envres.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Hursting SD, Davis BJ, McLachlan JA, Barrett JC. Environmental exposure, DNA methylation, and gene regulation. Ann N Y Acad Sci. 2003;983:161–169. doi: 10.1111/j.1749-6632.2003.tb05971.x. [DOI] [PubMed] [Google Scholar]
- Maccani JZ, Koestler DC, Lester B, Houseman EA, Armstrong DA, Kelsey KT, et al. Placental DNA methylation related to both infant toenail mercury and adverse neurobehavioral outcomes. Environ Health Perspect. 2015;123(7):723–729. doi: 10.1289/ehp.1408561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigano J, Baccarelli A, Mittleman MA, Wright RO, Sparrow D, Vokonas PS, et al. Prolonged exposure to particulate pollution, genes associated with glutathione pathways, and DNA methylation in a cohort of older men. Environ Health Perspect. 2011;119(7):977–982. doi: 10.1289/ehp.1002773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal C, Miotto B. Emerging concept in DNA methylation: Role of transcription factors in shaping DNA methylation patterns. J Cell Physiol. 2015;230(4):743–751. doi: 10.1002/jcp.24836. [DOI] [PubMed] [Google Scholar]
- Marsit CJ, Maccani MA, Padbury JF, Lester BM. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS One. 2012;7(3):e33794. doi: 10.1371/journal.pone.0033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiwala T, Ghoshal K, Majumder S, Kutay H, Neuberg DS, Kitada S, et al. Methylation of the tumor suppressor gene ptpro (receptor-type protein tyrosine phosphatase) is associated with expression of important apoptosis-related proteins in chronic lymphocytic leukemia (cll). ASH Annual Meeting Abstracts. 2004;104(11):2804. [Google Scholar]
- NCBI Ptprn2 protein tyrosine phosphatase, receptor type, n polypeptide 2 [ homo sapiens (human) ] 2015a Part April 5, 2015. [Google Scholar]
- NCBI. Tmem125 transmembrane protein 125 [ homo sapiens (human) ] 2015b Part March 23, 2015. [Google Scholar]
- NCBI. Vps4a vacuolar protein sorting 4 homolog a (s. Cerevisiae) [ homo sapiens (human) ] 2015c Part April 5, 2015. [Google Scholar]
- Nelissen EC, van Montfoort AP, Dumoulin JC, Evers JL. Epigenetics and the placenta. Hum Reprod Update. 2011;17(3):397–417. doi: 10.1093/humupd/dmq052. [DOI] [PubMed] [Google Scholar]
- Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a united states national reference. BMC Pediatrics. 2003;3(6) doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadeh FB, Clark MA, Rogers ML, Linkletter CD, Phipps MG, Padbury JF, et al. Pregnant and moving: Understanding residential mobility during pregnancy and in the first year of life using a prospective birth cohort. Matern Child Health J. 2013;17(2):330–343. doi: 10.1007/s10995-012-0978-y. [DOI] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ Res. 2012;117:100–111. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Tarantini L, Bonzini M, Apostoli P, Pegoraro V, Bollati V, Marinelli B, et al. Effects of particulate matter on genomic DNA methylation content and inos promoter methylation. Environ Health Perspect. 2009;117(2):217–222. doi: 10.1289/ehp.11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RDC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- Valinluck V, Tsai HH, Rogstad DK, Burdzy A, Bird A, Sowers LC. Oxidative damage to methyl-cpg sequences inhibits the binding of the methyl-cpg binding domain (mbd) of methyl-cpg binding protein 2 (mecp2). Nucleic Acids Res. 2004;32(14):4100–4108. doi: 10.1093/nar/gkh739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ, Vansteelandt S. Conceptual issues concerning mediation, interventions and composition. Statistics and Its Interface. 2009;2:457–468. [Google Scholar]
- VanderWeele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172(12):1339–1348. doi: 10.1093/aje/kwq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal AC, Semenova V, Darrah T, Vengosh A, Huang Z, King K, et al. Maternal cadmium, iron and zinc levels, DNA methylation and birth weight. BMC Pharmacol Toxicol. 2015;16:20. doi: 10.1186/s40360-015-0020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm-Benartzi CS, Houseman EA, Maccani MA, Poage GM, Koestler DC, Langevin SM, et al. In utero exposures, infant growth, and DNA methylation of repetitive elements and developmentally related genes in human placenta. Environ Health Perspect. 2012;120(2):296–302. doi: 10.1289/ehp.1103927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hinds WC, Kim S, Shen S, Sioutas C. Study of ultrafine particles near a major highway with heavy-duty diesel traffic. Atmospheric Environment. 2002a;36:4323–4335. [Google Scholar]
- Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. Journal of the Air & Waste Management Association. 2002b;52:1032–1042. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.