Abstract
Resistance (RE) and aerobic exercise (AE) can promote hemodynamic, physiologic and clinical modifications in coronary artery disease (CAD) patients. The aim of the study is to assess key physiologic and clinical responses during RE at 30% and 60% of 1-RM on a 45° leg press and to compare responses during AE. We evaluated fifteen male subjects with coronary artery disease (60.8±4.7 years) that performed the following tests: (1) incremental AE test on cycle ergometer; (2) 1-RM test on a leg press at 45°; (3) and RE at 30% and 60% of 1-RM for 24 repetitions. Peak cardiac output (CO), heart rate (HR), oxygen consumption (VO2), carbon dioxide production (VCO2) and the minute ventilation (VE, L/min)/VCO2 ration were measured. We found that both AE and RE at 60% of aerobic and resistance capacity elicited similar hemodynamic and ventilatory responses (p>0.05). However, RE at 30% 1-RM showed more attenuated responses of VO2, VE/VCO2, HR and CO when compared with 60% of aerobic and resistance capacity. Interestingly, the number, percentage and the severity of arrhythmias were higher at 60% 1-RM (P<0.05). Our data suggest that high repetition sets of RE at 60% 1-RM appears to result in hemodynamic, ventilatory, and metabolic changes equivalent to those observed during AE at a comparable intensity.
Keywords: Cardiac rehabilitation, resistance exercise, coronary artery disease
Introduction
Resistance exercise (RE) is now a core component of multi-disciplinary cardiac rehabilitation [1]. Previous studies have shown that RE enhances muscular strength and endurance, functional capacity, insulin sensitivity [2] and reduces disability [3-5]. Recent RE guidelines recommend 1 set of 8-15 repetitions (3 s concentric, 3 s eccentric) for 8-10 different exercises. For coronary artery disease (CAD) patients, an initial intensity corresponding to 30% to 40% of 1-repetition maximum (RM) for the upper extremity and 50% to 60% of 1-RM for lower extremity RE has been recommended [1].
The physiological responses [i.e., oxygen consumption (VO2), cardiac output (CO), heart rate (HR) and ventricular premature contractions] to aerobic exercise (AE) testing are well established and typically used to guide AE prescription [7-9], this is not the case for RE. The limited research related to RE has found, key initial insights, for example, VO2 and HR are significantly higher during dynamic RE when compared to static contractions on a leg press [6]. These results are relevant and may indicate that RE may also result in improved aerobic performance when a high repetition-low intensity scheme is prescribed, a potentially important benefit in patients with CAD undergoing rehabilitation. However, there are obvious knowledge gaps in the literature regarding physiologic and symptomatic responses during this type of dynamic RE, which requires investigation before clinical recommendations can be made. However, to our knowledge, we not found previous studies that compared (dynamic and resistance) in CAD patients, in anaerobic threshold (30%) of 1-RM and above anaerobic threshold (60%) evaluating hemodynamic, metabolic and cardiovascular responses and symptoms and safety during incremental dynamic exercise (IDE), during resistance exercise and recovery. Therefore, in this cross-sectional study, we hypothesized that hemodynamic, metabolic responses, as well as the symptoms, in high intensity of RE resemble the responses during the IDE. In this sense, the aim of this study was to compare the ventilatory responses obtained during the RE loads (30% and 60%) with the maximum incremental test on a cycle ergometer response. Secondly, we aimed to evaluate the hemodynamic, ventilatory, cardiovascular responses and electrocardiographic changes during RE on leg press 45°, contrasting intensities of 30% (at the anaerobic threshold) and 60% of 1-RM (above of anaerobic threshold) and the recovery condition of exercise in CAD patients.
Main outcomes
The primary endpoint of the study were hemodynamic evaluate (CO and HR) during the RE. Additionally, the secondary endpoint of the study were presented of electrocardiographic signals during the RE.
Methods
Participants and procedures
This was a cross-sectional study defined on the basis of the STROBE statement. Patients were recruited and evaluated in the period of April to December 2011. Volunteers recruited to participate in this study previously participated in a cardiovascular rehabilitation program (CR) for at least 1 year, basically composed of aerobic exercises. Methods for the selection of volunteers was through disclosure in CR program, pamphlets and printed disclosure. Only men with clinically stable CAD were included in this study.
Twenty male CAD subjects were initially assessed for study; fifteen were enrolled and completed the protocol. Inclusion criteria consisted of: 1) An established diagnosis of CAD for at least one year; 2) Optimal medical management for at least one year according to AHA/ACC recommendations [9]; and 3) Participation in a cardiac rehabilitation program comprised of AE only for at least one year. Exclusion criteria consisted of documented ongoing angina, electrocardiographic evidence of exertional ischemia, atrial fibrillation, lung disease, peripheral vascular disease, and/or orthopedic, neurologic and musculoskeletal disorders that could limit performance during RE or AE. This study was approved by the local Institutional Research Ethics Committee and each subject signed a written informed consent (n°. 397/2011). This cross-section study was registered at ClinicalTrials.gov (RBR-63kf95).
All subjects were evaluated during the same period of the day and each of the exercise protocols included in the current study was performed on different days separated by a 7 day interval. Clinical evaluations consisted of: 1) health history screening; 2) anthropometric measurements (height and body weight); and 3) symptom limited AE testing. All subjects were instructed to maintain their pharmacologic regimen during the study protocol.
Cardiopulmonary exercise testing
The ramp protocol was performed in a semi-recumbent position on a calibrated cycle ergometer (Lode Corival, The Netherlands). The AE test consisted of the following steps: 1) 5 minutes at rest in a seated position; 2) 4 minutes at “zero” workload, obtained through an electrical system which moves the ergometer free wheel at 60 rpm; 3) An ramp phase of 15 W/min while maintaining a constant cadence of 60 rpm. The ramp phase was terminated when the designated workload or cadence could no longer be maintained or volitional exhaustion was reached; 4) Immediately following the ramp phase, the subjects were instructed to maintain pedaling for 1 minute at “zero load” (active recovery); and 5) Monitoring at rest in the seated position on the ergometer for 5 min (passive recovery). Test procedures followed recommendations provided by Neder et al [10].
1-repetition maximum test
To determine the protocol loads for RE, the 1-RM test was applied by gradually increasing the resistance until the volunteer succeeded in performing no more than 1 repetition of the exercise on a leg press 45° (Pro-Fitness, Sao Paulo, Brazil) [11]. Subjects maintained a seated position on the equipment with the knees and hips flexed at 90°. During the maneuver, the knees and hips were extended and returned to the initial position after flexion. Before the execution of the test, subjects oriented to avoid the isometric component and exhale during the extension of the knees and hips to avoid the Valsalva maneuver [12]. The resistance load for 1-RM was estimated (1RM-E) before the test by multiplying the body weight of the volunteer by 4, based on previous studies [11-14]. The initial resistance load applied to determine 1-RM was 80% of the 1RM-E, and if the volunteer was able to perform more than 1 complete movement, the load was increased 10% of the 1RM-E, after a 5-min rest interval between trials. When the first attempt was unsuccessful due to overestimation of the resistance load, the load was reduced by 10% 1RM-E. When the pre-1-RM was determined, a second attempt with an additional 10% above the load was performed to verify the load value and in cases where the individual was not successful on this second attempt, the previous load was considered as their 1-RM. If the subject was successful, a new load was added until the 1-RM was determined. Based on the estimated loads of 1-RM, it was expected that 1-RM would be determined within 6 attempts [11].
Resistance exercise tests at 30% 1-RM and 60% 1-RM
The RE tests were performed at 30% 1-RM (first test), and after 30 min of rest, a second test at 60% 1-RM (second test) was performed. During the test, subjects underwent 2 min of exercise at a movement rhythm of 12 repetitions/min, maintaining a respiratory cadence, with each repetition performed in 5 seconds (2 seconds of extension and 3 seconds of knee and hip flexion), with the rhythm controlled by verbal commands. In the present study, we selected 24 repetitions in order to produce sustained dynamic RE contractions, analyzing metabolic and hemodynamic responses. Subjects underwent a 30 min recovery period at the end of the 2 min exercise maneuver. The electrocardiograph (ECG) signal was monitored throughout the test. Blood pressure (BP) was recorded during the exercise maneuver. Pain in the lower limbs was evaluated by the Borg Scale (0-10) [15] at the end of each exercise set.
Measurements during aerobic and resistance exercise testing
During both tests, the following variables were determined: 1) VO2 (ml•kg-1•min-1); 2) carbon dioxide production (VCO2, ml•min-1); and 3) minute ventilation (VE, L•min-1). The VE/VCO2 ratio was also determined. Data was collected using a portable ventilatory gas analysis system (Oxycon Mobile; Jaeger, Hoechberg, Germany®). Cardiac output and heart rate (HR) were continuously measured by impedance cardiograph using a Physio-Flow device (Manatec Biomedical, Paris, France®). The rate pressure product (RPP) was calculated by multiplying systolic BP by HR [16]. During the entire test period, subjects were monitored continuously by 12-lead ECG (Wincardio System®, Micromed, Brasilia, Brazil). Ectopic beats were identified 5 minutes before RE (rest position), during 30% and 60% of 1-RM and the recovery period following each load (5 minutes). The number and percentage of supraventricular and ventricular beats were computed in subjects.
Statistical analysis
The data distribution was verified by the Shapiro-Wilk test, and when the normality was confirmed, the data were expressed as mean and SD. Repeated measures ANOVA compared variables of interest obtained during AE and RE. Borg scale values were compared using the Friedman test. Statistical analyses of ectopic beats values were performed for Chi-Square test using Minitab software (version 17 for Windows).
All other data were analyzed using STATISTICA 5.5 software package (Stat Soft®). The level of significance was set at p<0.05 for all statistical tests.
Results
Initially were recruited twenty patients to participate in this study. After the first visit (IDE), 2 individuals were excluded because they have given up voluntarily. Later the second evaluation (1-RM test), 3 patients were excluded, 1 voluntarily gave up and 2 were excluded due to joint pain. In this sense, 15 other volunteers successfully completed the exercise protocol without abnormalities that indicate against the continuation in this study. The flowchart of the study is summarized in Figure 1.
Figure 1.
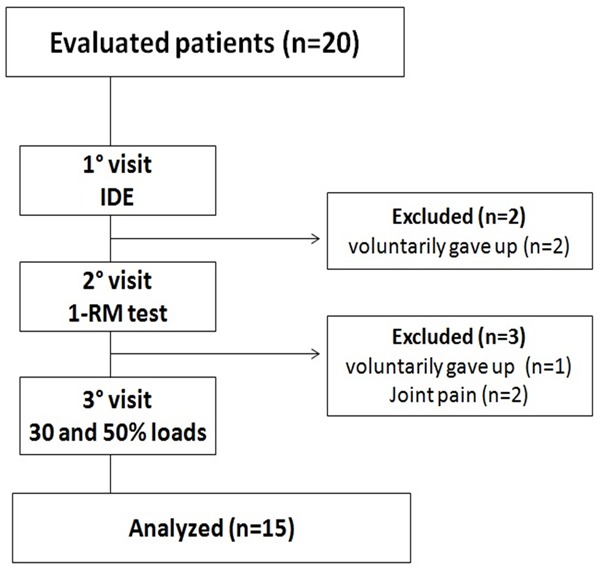
Flowchart of the study.
Anthropometric characteristics, risk factor, cardiac events and medications of the 15 subjects completing the protocol are summarized in Table 1. Of the 15 patients (100%) were elderly, six were I degree obese and all patients presented normal ventricular function. In addition, 9 patients used beta-blocker and 7 used angiotensin-converting enzyme inhibitors. Furthermore, 14 patients used simvastatin and twelve AAS.
Table 1.
Anthropometric characteristics, risk factor, cardiac events and medications of the 15 subjects completing the protocol are summarized in Table 1
| Variables | n = 15 |
|---|---|
| Age (years) | 60.8±4.7 |
| Body Mass (Kg) | 77.2±12.6 |
| Height (m) | 1.6±0.6 |
| Body Mass Index (Kg∙m2) | 27±3.1 |
| Ejection Fraction (%) | 62±3.2 |
| Risk Factor (n) | |
| Tabagism | 6 |
| Diabetes | 5 |
| Hypertention | 10 |
| Cardiac event (n) | |
| AMI | 15 |
| CABG | 12 |
| Medication (n) | |
| Carvedilol | 2 |
| Atenolol | 5 |
| Metoprolol | 2 |
| Enalapril | 3 |
| Captopril | 2 |
| Simvastatin | 14 |
| AAS | 12 |
Abbreviations: n = number of sample; AMI = acute myocardial infarction; SRCABG = coronary artery bypass surgery; AAS = acid acetilsalicilic. Data are reported as mean ± SD.
During CPX, all 15 subjects ceased activity secondary to symptoms consistent with maximal exertion. In this study, no ST-segment changes suggestive of ischemia or atrioventricular and/or intraventricular blocks were observed during RE. The descriptive analysis representing the type and severity of arrhythmia during rest, 30% and 60% 1-RM RE, and recovery from RE is listed in Table 2. Isolated extrasystoles were prominent during 30% 1-RM RE and recovery from 60% 1-RM RE (p<0.05). Multifocal ventricular extrasystoles were detected during 60% 1-RM RE when contrasted with other situations (p<0.05). The severity of arrhythmias was also higher during 60% 1-RM RE when compared with 30% 1-RM RE, rest and recovery (p<0.05).
Table 2.
Electrocardiografy response: number of arrhythmias at rest, exercise and recovery of resistance exercise
| Electrocardiografy Analysis | Rest | 30% | R30 | 60% | R60 | P |
|---|---|---|---|---|---|---|
| N° of VES (% Patients) | 4 (13) | 14 (47) | 5 (33) | 30 (73) | 4 (40) | P<0.05 |
| N° of VES monofocal isolated (% Patients) | 4 (13) | 14 (47) | 4 (27) | 17 (60) | 3 (33) | P<0.05 |
| N° of VES multifocal (% Patients) | 0 | 0 | 1 (7) | 13 (13) | 1 (7) | P<0.01 |
| Severity of VES | ||||||
| N° of patients without event | 13 | 8 | 10 | 4 | 9 | P<0.01 |
| N° of patients with VES monofocal isolated | 2 | 7 | 4 | 9 | 5 | P<0.03 |
| N° of patients VES multifocal | 0 | 0 | 1 | 2 | 1 | P<0.05 |
VES = ventricular extrasystole; N° = number of ventricular extrasystole; (%) percentage at number of patients (% of total); 30% = resistance exercise load; r30 = recovery of 30% of 1RM; 60% = resistance exercise load; r60 = recovery of 60% 1RM. Were not found bigeminism, VES paired, ventricular tachycardia not sustained or ventricular tachycardia sustained. Chi Square test p<0.05.
As demonstrated in Figure 2, 60% 1-RM RE elicited an equivalent ventilatory and metabolic demand when compared with AE (p>0.05). However, 60% 1-RM RE as well as AE induced significantly higher values of VO2 when compared with 30% 1-RM RE (p<0.05). In addition, VE/VCO2 differed only during 30% of maximal AE capacity when compared to 60% of maximal AE capacity (p<0.05).
Figure 2.
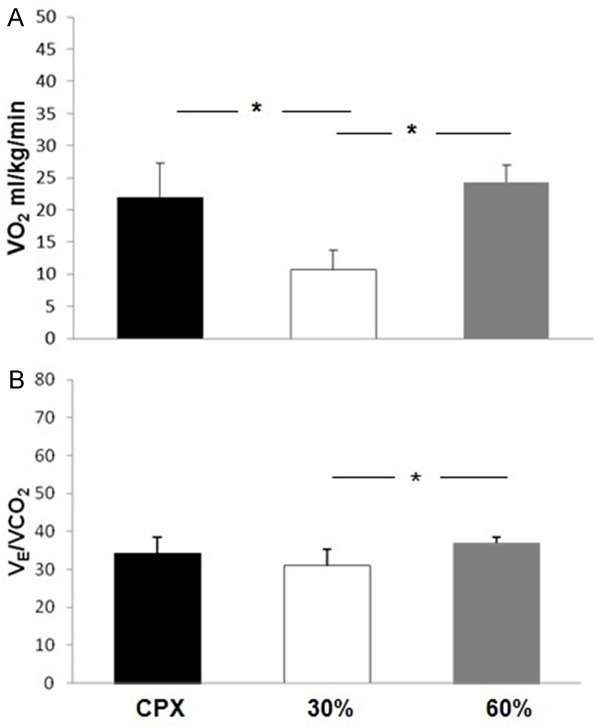
VO2, oxygen uptake; VE/VCO2, ventilation to carbon dioxide production. Contrasting of peak VO2 (A) and VE/VCO2 in (B) on CPX, in 30% and 60% of 1-RM on leg press 45°. Data are reported as means ± SD. *P<0.05 comparing CPX and 30%, #P<0.05 comparing 30% and 60% loads (one-way ANOVA).
Hemodynamic responses at 30 and 60% of 1-RM RE contrasted with AE are illustrated in Figure 3. Cardiac output at 60% 1-RM RE was similar to AE (p>0.05). However, 60% of 1-RM RE as well as AE induced significantly higher CO values compared to 30% 1-RM RE (p<0.05). However, HR during 60% 1-RM elicited a response that was approximately 93% of the maximal HR obtained during AE. In addition, 60% 1-RM RE induced a significantly higher HR when compared to 30% 1-RM RE and 60% maximal AE capacity.
Figure 3.
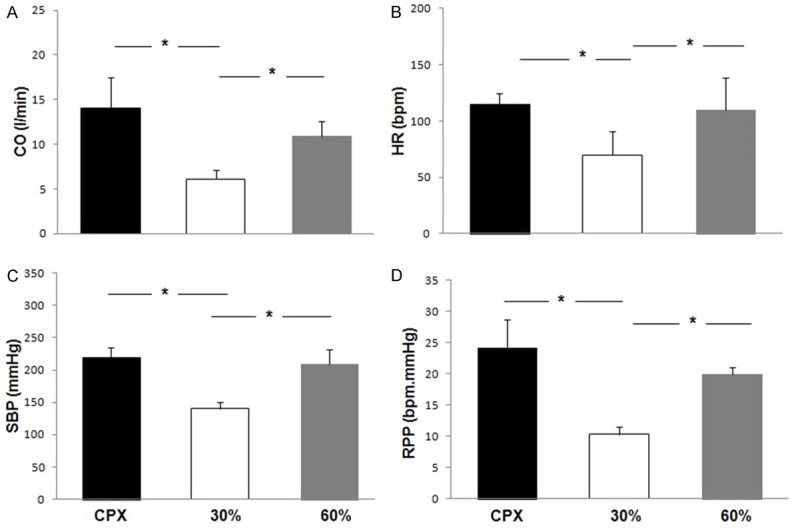
Contrasting of peak CO, cardiac output (A), HR, heart rate (B), SBP, systolic blood pressure (C), RPP, rate pressure product (D) on CPX, in 30% and 60% of 1-RM on leg press 45°. Data are reported as means ± SD. *P<0.05 comparing CPX and 30%, #P<0.05 comparing 30% and 60% loads (one-way ANOVA).
For systolic BP [Figure 3C], 60% 1-RM RE elicited a response that was approximately 98% of that obtained at maximal AE, differing only between 30% 1-RM RE and 60% 1-RM RE (p<0.05). Rate pressure product at 60% of 1-RM RE was similar to AE. Furthermore, 60% of 1-RM RE and AE induced significantly higher values in contrast to 30% 1-RM RE (p<0.05). As illustrated in Figure 4, CO and VO2 response were higher during 60% of 1-RM RE in contrast to 30% 1-RM RE. As demonstrated in Figure 5, leg fatigue was lower during 30% 1-RM RE when contrasted with maximal AE and 60% 1-RM RE (p<0.05).
Figure 4.
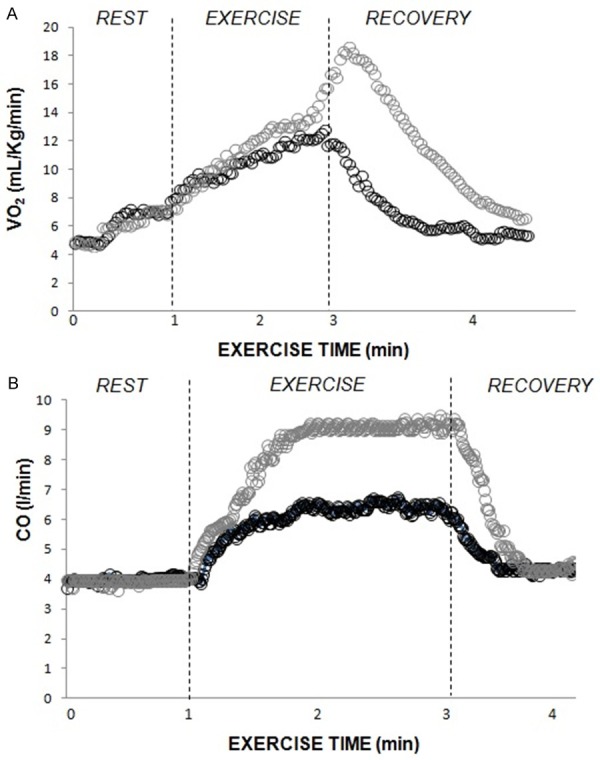
Illustration of VO2, oxygen uptake and CO, cardiac output during rest, exercise and recovery in 30% and 60% of 1-RM on leg press 45°.
Figure 5.
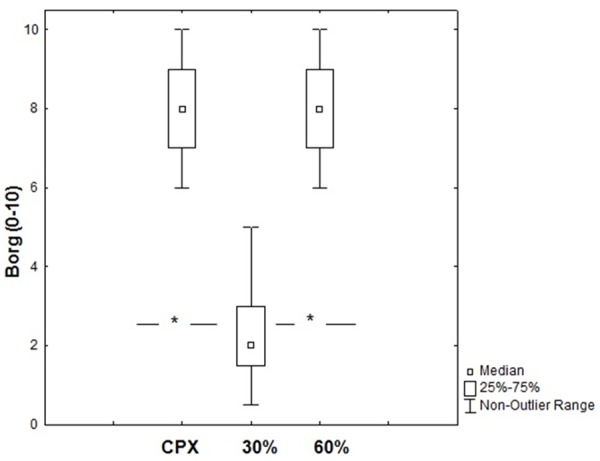
Leg fatigue (0-10) on CPX, 30% and 60% RM. *P<0.01 contrasting CPX, 30% and 60% loads (Friedman test). Data are reported as means ± SD.
Discussion
The present study investigated the physiologic and symptom responses at two RE intensities, at the low and high end-range of what is recommended for CAD patients, comparing findings to responses obtained during AE. The main findings of this study were: 1) 60% 1-RM RE induced similar hemodynamic, metabolic and perceptual responses when are compared with maximal AE; and 2) a higher number of complex extrasystoles were observed during 60% 1-RM RE when contrasted with 30% 1-RM RE. These results have meaningful implications for the clinical implementation of RE prescription in CAD patients.
ECG alterations during resistance exercise and implications for safety
Regarding the safety of RE, no atrioventricular and/or intraventricular blocks were observed during either RE intensity studied. However, we observed the emergence of increased ectopic events during 60% 1-RM RE. In this context, monitoring during RE in cardiac patients requires careful evaluation. In contrast, Faigenbaum et al [17] studied RE in cardiac patients that consisted of two sets of seven repetitions at 75% of maximal voluntary contraction, determined on seven upper-body Nautilus machines, and noticed no significant ECG changes. The possible explanation between the differences found in the current and previously cited investigation is the significant difference in number of repetitions (7 vs. 24). Thus, our extended number of repetitions may have produced myocardial irritability in certain subjects. However, in our study, despite an increased frequency of ventricular ectopic at 60% 1-RM RE (7 patients, 47% of total), no angina or ischemia were detected and in general, ECG disturbances were still below thresholds considered for exercise termination [5]. Rather, this particular finding may provide guidance on what exercises and intensities require more diligent monitoring in some patients. It should be noted that the cohort assessed in the current study had a lower level of disease burden and severity. Thus, additional caution and monitoring should be considered when prescribing RE to cardiac patients with higher levels of disease severity. Moreover, the current study only assessed male patients during a single lower extremity RE maneuver. Transferability and clinical application of results is therefore limited and should be addressed by future investigations.
Other studies suggest that strength testing (i.e., 1-RM) does not produce anginal symptoms, ischemic ST-segment depression, complex ventricular arrhythmias, and cardiovascular complications for clinically stable CAD patients [17,18]. The findings of this study are consistent with previous investigations in this context; RE using high repetitions at the higher range of the recommended intensity can produce ECG changes without symptoms or ischemia. The relevance of this finding in this patient population is novel given the literature lacks studies on the safety and feasibility of high repetition RE.
Ventilatory, metabolic and hemodynamic responses during dynamic and resistance exercise
Interestingly, the VO2 increase during 60% 1-RM RE was proportional to AE, but significantly differed from 30% 1-RM RE. Also, the VE/VCO2 ratio was higher during 60% 1-RM RE when compared with 30% 1-RM RE. In accordance with our findings, Marzolini et al [19] showed that higher volumes of RE can produce a significant increase in VO2. These results are interesting, since RE at the higher range of moderate intensity, using a higher number of repetitions may produce higher ventilatory and metabolic demands. Consistent with our results, Arimoto et al [6] observed that during the dynamic leg press exercise, VE, VO2, HR and systolic BP reached steady-state after 3 minutes of exercise. However, during static leg press, the hemodynamic responses continued to increase at the end of exercise while VO2 did not change during these contractions. In our study, only the 60% 1-RM RE maneuver elicited increases in VO2, HR, systolic BP and RPP that were comparable to maximal AE responses. In addition, we clearly showed that VO2 continued to increase after 60% 1-RM RE (Figure 3), as previously shown [20], despite relative stability of hemodynamic responses when contrasted with 30% 1-RM RE.
A previous study showed that low-intensity RE with slow movement and tonic force generation is an effective method for increasing muscle mass and strength as well as basal femoral blood flow [21]. However, it is unknown if this specific RE training paradigm also leads to ventilatory and metabolic improvement.
In the present study, 60% of maximal AE capacity induced a significant increase in VE/VCO2 when compared to 30% 1-RM RE. The VE/VCO2 ratio reflects ventilatory efficiency, more specifically matching of pulmonary ventilation and perfusion and is related to the intensity of exercise. In addition, as previously demonstrated, blood lactate release is consistently increased during RE intensities higher than 30% 1-RM using the 45° leg press and bench press [11,22]. We therefore posit the higher VE/VCO2 ratio observed in the current study also reflects increased anaerobic metabolism in skeletal muscle.
Our study found that, high-repetition sets of 60% 1-RM RE increased hemodynamic variables; CO, BP, HR and RPP as well as leg fatigue in a comparable fashion compared to maximal AE. It is known that high repetition RE influences the magnitude of hemodynamic responses [11,12,22,23]. However, when contrasting 30% of maximal AE capacity with 60% 1-RM RE, we observed the time of exercise as well as intensity influenced HR, BP and RPP increases. Similar results were observed by Karlsdottir et al [24] when comparing moderate-intensity RE with constant dynamic work rate in patients with CAD and reduced ejection fraction.
Interestingly, during maximal AE and 60% 1-RM RE, the hemodynamic and clinical responses increased substantially, possibly caused by changes in muscle blood flow and peripheral arterial resistance. Such adjustments are dictated by the central nervous system in an attempt to restore metabolism homeostasis at higher exercise intensities [25,26]. During high intensity RE, the partial occlusion of muscle blood flow occurs, decreasing venous return and increasing afterload, negatively affecting stroke volume [28,29]. The central responses via sympathetic activation increase HR to maintain the increase in CO. In this context, we speculate that sympathetic activation, as observed that during RE above 30% 1-RM at high repetitions, particularly in a CAD population, warrants special consideration since neural sympathetic discharge can potentially produce electrical instability and the emergence of ectopic events [28].
Conversely, in the present study that included a male CAD cohort, high-repetition and lower intensity RE (i.e., 30% 1-RM) was well tolerated, increased ventilatory and hemodynamic responses, and did not elicit abnormal responses. Thus, for RE prescriptions that incorporate higher repetitions in patient populations, a lower intensity may be desirable [29]. In this sense, we recently conducted a study that consisted of a high repetition/low load RE program in CAD patients, demonstrating significant improvements in HRV and muscle strength in these patients [30]. Additional work is needed to further define optimal RE training characteristics.
Conclusion
The findings of this study suggest that RE using 60% of 1-RM and high repetitions may increase physiologic responses as well as perceived symptoms at a level equivalent to responses at maximal AE. In addition, RE at 30% 1-RM using the same high repetition scheme elicits a comparatively lower, albeit significant, physiologic response compared to rest and is well tolerated.
Acknowledgements
The authors would like to thank FAPESP (2011/20074-3) and (2014/00530-2) for providing financial support.
Disclosure of conflict of interest
None.
References
- 1.Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, Gulanick M, Laing ST, Stewart KJ American Heart Association Council on Clinical Cardiology; American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–84. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 2.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 6th ed. Baltimore, Md: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 3.Whaley MH, Brubaker PH, Otto RM, Armstrong LE. Acsm’s Guidelines for Exercise Testing and Prescription. Philadelphia, Pa: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 4.American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 4th ed. Champaign, Ill: Human Kinetics; 2004. [Google Scholar]
- 5.American Geriatrics Society Panel on Exercise and Osteoarthritis. Exercise prescription for older adults with osteoarthritis pain: consensus practice recommendations: a supplement to the AGS Clinical Practice Guidelines on the management of chronic pain in older adults. J Am Geriatr Soc. 2001;49:808–823. doi: 10.1046/j.1532-5415.2001.00496.x. [DOI] [PubMed] [Google Scholar]
- 6.Arimoto M, Kijima A, Muramatsu S. Cardiorespiratory response to dynamic and static leg press exercise in humans. J Physiol Anthropol Appl Human Sci. 2005;24:277–83. doi: 10.2114/jpa.24.277. [DOI] [PubMed] [Google Scholar]
- 7.Bjarnason-Wehrens B, Mayer-Berger W, Meister ER, Baum K, Hambrecht R, Gielen S. Recommendations for resistance exercise in cardiac rehabilitation Recommendations of the German Federation for Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2004;11:352–361. doi: 10.1097/01.hjr.0000137692.36013.27. [DOI] [PubMed] [Google Scholar]
- 8.American College of Sports Medicine. Position stand on the appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2002;34:364–380. doi: 10.1097/00005768-200112000-00026. [DOI] [PubMed] [Google Scholar]
- 9.American College Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–91. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 10.Neder JA, Nery LE. Teste de Exercício Cardiopulmonar. J Pneumol. 2002;28:166–206. [Google Scholar]
- 11.Simões RP, Castello-Simões V, Mendes RG, Archiza B, Santos DA, Machado HG, Bonjorno JC Jr, Oliveira CR, Reis MS, Catai AM, Arena R, Borghi-Silva A. Lactate and heart rate variability threshold during resistance exercise in the young and elderly. Int J Sports Med. 2013;34:991–6. doi: 10.1055/s-0033-1337946. [DOI] [PubMed] [Google Scholar]
- 12.Simões RP, Castello-Simões V, Mendes RG, Archiza B, Dos Santos DA, Bonjorno JC Jr, de Oliveira CR, Catai AM, Arena R, Borghi-Silva A. Identification of anaerobic threshold by analysis of heart rate variability during discontinuous dynamic and resistance exercise protocols in healthy older men. Clin Physiol Funct Imaging. 2014;34:98–108. doi: 10.1111/cpf.12070. [DOI] [PubMed] [Google Scholar]
- 13.Simões RP, Bonjorno JC Jr, Beltrame T, Catai AM, Arena R, Borghi-Silva A. Slower heart rate and oxygen consumption kinetic responses in the on- and off-transient during a discontinuous incremental exercise: effects of aging. Braz J Phys Ther. 2013;17:69–7. doi: 10.1590/s1413-35552012005000056. [DOI] [PubMed] [Google Scholar]
- 14.Kraemer WJ, Fry AC. Strength testing: development and evaluation of methodology. In: Maud PJ, Foster C, editors. Physiological Assessment of Human Fitness. Champaign, IL: Human Kinetics; 1995. pp. 115–138. [Google Scholar]
- 15.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 16.McCartney N. Acute responses to resistance training and safety. Med Sci Sports Exerc. 1999;31:31–37. doi: 10.1097/00005768-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Faigenbaum AD, Skrinar GS, Cesare WF, Kraemer WJ, Thomas HE. Physiologic and symptomatic responses of cardiac patients to resistance exercise. Arch Phys Med Rehabil. 1990;71:395–821. [PubMed] [Google Scholar]
- 18.Jensen-Urstad K, Bouvier F, Saltin B, Jensen-Urstad M. High prevalence of arrhythmias in elderly male athletes with a lifelong history of regular strenuous exercise. Heart. 1998;79:161–4. doi: 10.1136/hrt.79.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marzolini S, Oh PI, Dina Brooks D. Effect of combined aerobic and resistance training versus aerobic training alone in individuals with coronary artery disease: a meta-analysis. Eur J Prev Cardiol. 2012;19:81–94. doi: 10.1177/1741826710393197. [DOI] [PubMed] [Google Scholar]
- 20.Pafili ZK, Bogdanis GC, Maridaki M. Cardiorespiratory characteristics and cholesterol responses to a single session of heavy leg press exercise. J Sports Sci Med. 2010;9:580–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Tanimoto M, Kawano H, Gando Y, Sanada K, Yamamoto K, Ishii N, Tabata I, Miyachi M. Low-intensity resistance training with slow movement and tonic force generation increases basal limb blood flow. Clin Physiol Funct Imaging. 2009;29:128–35. doi: 10.1111/j.1475-097X.2008.00847.x. [DOI] [PubMed] [Google Scholar]
- 22.Machado-Vidotti HG, Mendes RG, Simões RP, Castello-Simões V, Catai AM, Borghi-Silva A. Cardiac autonomic responses during upper versus lower limb resistance exercise in healthy elderly men. Braz J Phys Ther. 2014;18:9–18. doi: 10.1590/S1413-35552012005000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machado HG, Simões RP, Mendes RG, Castello V, Di Thommazo L, Almeida LB, Lopes SL, Catai AM, Borghi-Silva A. Cardiac autonomic modulation during progressive upper limb exercise by patients with coronary artery disease. Braz J Med Biol Res. 2011;44:1276–1284. doi: 10.1590/s0100-879x2011007500134. [DOI] [PubMed] [Google Scholar]
- 24.Karlsdottir AE, Foster C, Porcari JP, Palmer-McLean K, White-Kube R, Backes RC. Hemodynamic responses during aerobic and resistance exercise. J Cardiopulm Rehabil. 2002;22:170–7. doi: 10.1097/00008483-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Taaffe DR, Galvão DA, Sharman JE, Coombes JS. Reduced central blood pressure in older adults following progressive resistance training. J Hum Hypertens. 2007;21:96–98. doi: 10.1038/sj.jhh.1002115. [DOI] [PubMed] [Google Scholar]
- 26.Umpierre D, Stein R. Hemodynamic and vascular effects of resistance training: implications for cardiovascular disease. Arq Bras Cardiol. 2007;89:256–262. doi: 10.1590/s0066-782x2007001600008. [DOI] [PubMed] [Google Scholar]
- 27.Nery SS, Gomides RS, da Silva GV, de Moraes Forjaz CL, Mion D Jr, Tinucci T. Intra-arterial blood pressure response in hypertensive subjects during low- and high-intensity resistance exercise. Clinics. 2010;65:271–7. doi: 10.1590/S1807-59322010000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveira JL, Galvão CM, Rocha SM. Resistance exercises for health promotion in coronary patients: evidence of benefits and risks. Int J Evid Based Healthc. 2008;6:431–9. doi: 10.1111/j.1744-1609.2008.00114.x. [DOI] [PubMed] [Google Scholar]
- 29.Wienbergen H, Hambrecht R. Physical exercise training for cardiovascular diseases. Herz. 2012;37:486–92. doi: 10.1007/s00059-012-3624-y. [DOI] [PubMed] [Google Scholar]
- 30.Caruso FR, Arena R, Phillips SA, Bonjorno JC Jr, Mendes RG, Arakelian VM, Bassi D, Nogi C, Borghi-Silva A. Resistance exercise training improves heart rate variability and muscle performance: a randomized controlled trial in coronary artery disease patients. Eur J Phys Rehabil Med. 2015;51:281–9. [PubMed] [Google Scholar]


