Abstract
Estrogen-related Receptors (ERR) are members of the steroid hormone receptor superfamily of transcription factors that regulate expression of genes required for energy metabolism including mitochondrial biogenesis, fatty acid oxidation and oxidative phosphorylation. While ERRα and EPPγ isoforms are known to share a wide array of target genes in the adult myocardium, the function of ERRβ has not been characterized in cardiomyocytes. The purpose of this study was to determine the role of ERRβ in regulating energy metabolism in adult cardiomyocytes in primary culture. Adult feline cardiomyocytes were electrically stimulated to contract in either hypoxia (0.5% O2) or normoxia (21% O2). As compared to baseline values measured in normoxia, ERRβ mRNA levels increased significantly after 8 hours of hypoxia and remained elevated over 24 h. Conversely, ERRβ mRNA decreased to normoxic levels after 4 hours of reoxygenation. Hypoxia increased expression of the α and β isoforms of Peroxisome Proliferator-Activated Receptor γ Coactivator-1 (PGC-1) mRNA by 6-fold and 3-fold, respectively. Knockdown of ERRβ expression via adenoviral-mediated delivery of ERRβ shRNA blocked hypoxia-induced increases in PGC-1β mRNA, but not PGC-1α mRNA. Loss of ERRβ had no effect on mtDNA content as measured after 24 h of hypoxia. To determine whether loss of ERRβ affected mitochondrial function, oxygen consumption rates (OCR) were measured in contracting versus quiescent cardiomyocytes in normoxia. OCR was significantly lower in contracting cardiomyocytes expressing ERRβ shRNA than scrambled shRNA controls. Maximal OCR also was reduced by ERRβ knockdown. In conclusion: 1) hypoxia increases in ERRβ mRNA expression in contracting cardiomyocytes; 2) ERRβ is required for induction of the PGC-1β isoform in response to hypoxia; 3) ERRβ expression is required to sustain OCR in normoxic conditions.
Keywords: Cardiomyocyte, hypoxia, estrogen-related receptor, estrogen-related receptor β, peroxisome proliferator-activated receptor γ coactivator-1
Introduction
Estrogen-related receptors (ERR) are unique members of the nuclear hormone receptor superfamily that do not require a ligand for activation [1]. The three known isoforms of ERR (α, β, γ) function by regulating transcription of a wide spectrum of target genes that are essential for glycolysis, β-oxidation of fatty acids, mitochondrial electron transport and oxidative phosphorylation [2,3]. Instead of ligand binding, ERR isoforms are activated mainly through interactions with coactivator or corepressor proteins, in particular, the α and β isoforms of the peroxisome proliferator-activated receptor γ coactivator-1 (PGC-1). Thus, gene expression is controlled largely by fluctuations in PGC-1α and PGC-1β isoforms, both of which bind directly to ERRs and coactivate transcription via epigenetic mechanisms such as catalyzing histone acetylation in the regulatory elements of target genes [4]. PGC-1 isoforms increase in response to stimuli that create greater energy demands including exercise, cold exposure and hypoxia [5-8]. Conversely, decreases in PGC-1 activity have been implicated in the pathophysiology of adverse myocardial remodeling as demonstrated by down regulation of ERRα and numerous ERRα target genes in experimental models of LV pressure overload hypertrophy and in end-stage human heart failure [9,10].
All three ERR isoforms are expressed constitutively in adult myocardium [1]. ERRα expression increases perinatally in parallel with PGC-1α, which enables the developmental shift in myocardial energy metabolism from glycolysis to the predominant postnatal pathway of fatty acid oxidation [11]. Further evidence indicates that ERRγ functions coordinately with ERRα in the myocardium to regulate transcription through formation of heterodimers and by binding to a common set of sequence motifs within regulatory elements of target genes [12,13]. In contrast, the functions of the ERRβ isoform have been described mainly in tissues other than myocardium. For example, ERRβ is essential for proper development of the chorion during placentation [14]. Consequently, homozygous knockout of ERRβ alleles is embryonic lethal in mice. In sensory organs, ERRβ regulates expression of genes encoding ion channels and transporters in specialized cells in the cochlea and expression of multiple target genes involved in energy metabolism in photoreceptors of the retina [15,16]. Another study indicates that ERRβ has a role in the physiological response to stress, possibly through modulation of neuropeptide secretion by the hypothalamic-hypophyseal-adrenal axis [17]. Lastly, as shown in breast cancer cells, ERRβ suppresses proliferation and stimulates apoptosis by mechanisms that inhibit estrogen-dependent signaling [18,19].
Our recent studies found that ERRα is required to increase PGC-1 mRNA levels in adult cardiomyocytes maintained in hypoxic conditions [20]. Here we investigated whether the ERRβ isoform plays a role in the adaptive response to sustained hypoxia and reoxygenation using an adult feline cardiomyocyte model of continuous electrically stimulated contraction in vitro. The utility of the electrical stimulation model is the generation of increased workload in the form of active tension development during contraction of adherent cardiomyocytes in primary cell culture [21]. Thus, energy demands required for excitation-contraction coupling are reestablished. These studies demonstrate that ERRβ increases in contracting cardiomyocytes in hypoxic conditions. Furthermore, ERRβ may function as part of the adaptive response to sustained hypoxia by facilitating an increase in the PGC-1β isoform. Lastly, in normoxia, loss of ERRβ expression in contracting cardiomyocytes results in decreased oxygen consumption rates.
Material and methods
Adult feline cardiomyocytes
The procedure conforms to the Guide for the Care and Use of Laboratory Animals, Eighth Edition and was approved by the Animal Care and Use Committee of the Medical University of South Carolina. Male or female cats were anesthetized with 10-20 mg/kg ketamine, im.; 0.1-0.33 mg/kg acepromazine, im; 2.2 mg/kg meperidine, im., and placed on isoflurane anesthesia (1-3% in 100% oxygen at 1-1.5 L/min) delivered via nose cone. Prior to removal of the heart, a ketamine overdose of 20 mg/kg was administered iv., a left thoracotomy was performed and euthanasia occurred by exsanguination. Adult feline cardiomyocytes were isolated from the left ventricular myocardium, plated onto 4-well, laminin-coated culture trays (Nunc International) at an initial density of 1.25 x 104 cells/cm2, and maintained in serum-free media [21,22].
Cardiomyocytes were electrically stimulated to contract at a frequency of 1 Hz by delivering 5 msec pulses of alternating polarity (120-130 V) through the culture medium via carbon electrodes [22]. Non-stimulated feline cardiomyocytes, which do not contract in vitro, were used as quiescent controls. Hypoxia was produced using compressed gas (5% CO2, balanced N2) and a Proox (BioSpherix, Ltd.) oxygen control system with an incubation chamber that was retrofitted for continuous electrical stimulation of cardiomyocyte contraction. Cardiomyocytes were maintained under either normoxic (21% O2) or hypoxic (0.5% O2) conditions. Hypoxia reduces pO2 in the culture medium from approximately 120 mmHg to 60 mmHg after 60 min, and pO2 recovers to normoxic values within 30 min of reoxygenation [20].
Quantitation of cardiomyocyte RNA
RNA was extracted using Trizol Reagent (Ambion) and treated with DNase I prior to quantitation of mRNA levels using a one-step reverse transcription-PCR kit (QuantiTect SYBR Green RT-PCR Kit, Qiagen Sciences) and a CFX96 Real-Time System C1000 Thermal Cycler (Bio-Rad). The amount of each specific mRNA was extrapolated from a standard curve using a dilution series derived from a reference sample of cardiomyocyte RNA. Relative levels of mRNA were calculated by normalizing to the corresponding amount of 18S rRNA in each sample. The primer sequences used for these studies are listed in Table 1.
Table 1.
Primer sets used for quantitative real-time RT-PCR
| Target RNA Sequence | Primer Set |
|---|---|
| Estrogen-related Receptor α (ERRα) | F: 5’-GGCCCTTGCCAATTCAGA-3’ |
| R: 5’-GGCCTCGTGCAGAGCTTCT-3’ | |
| Estrogen-related Receptor β (ERRβ) | F: 5’-CATGAAATGCCTGAAAGTGG-3’ |
| R: 5’-CACAGAGAGTGGTCAGGGCCT-3’ | |
| Peroxisome Proliferator-Activated Receptor γ Coactivator-1α (PGC-1α) | F: 5’-CAAGCCAAACCAACAACTTTATCTCT-3’ |
| R: 5’-CACACTTAAGGTGCGTTCAATAGTC-3’ | |
| Peroxisome Proliferator-Activated Receptor γ Coactivator-1β (PGC-1β) | F: 5’-CATCTGGGAAAAGCAAGTACGA-3’ |
| R: 5’-CCTCGAAGGTTAAGGCTGATATCA-3’ | |
| 18S rRNA | F: 5’-TATGGTTCCTTTGGTCGCTC-3’ |
| R: 5’-GGTTGGTTTTGATCTGATAAATG-3’ | |
| NADH Dehydrogenase Subunit 1 (ND1) | F: 5’-GCCATAATATGATTTATCTC-3’ |
| R: 5’-AGAAAGTTTTTTCATAGGAG-3’ | |
| Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) | F: 5’-AGGTCATCCCAGAGCTGAAC-3’ |
| R: 5’-CCTGCTTCACCACCTTCTTG-3’ |
Adenoviral-mediated knockdown of ERRβ expression
Human ERRβ shRNA sequence from Openbiosystems (Clone ID TRCN0000022205) was provided by Jian Feng, State University of New York, Buffalo, NY. The sequence was subcloned into DUAL-BASIC-EGFP plasmid and used for construction of recombinant adenovirus Ad-ERRβ shRNA (Vector Biolabs). Approximately 16 h after plating, cardiomyocytes were infected with Ad-ERRβ shRNA at a multiplicity of infection of 500. The efficiency of infection was 100% as monitored by visual inspection of the cardiomyocytes for eGFP expression using a fluorescence microscope. The cardiomyocytes were incubated for 48 h prior to initiation of electrical stimulation in either normoxia or hypoxia to allow for knockdown of endogenous ERRβ mRNA. Controls were infected in parallel with an adenovirus expressing a scrambled shRNA sequence (Ad-scrambshRNA, Vector Biolabs).
Measurement of oxygen consumption rates
Oxygen consumption rates (OCR) in cardiomyocytes were measured using a Seahorse Bioscience XF24 analyzer with laminin-coated V28 cell culture microplates as described before [20]. The microplates were modified for electrical stimulation by inserting platinum electrodes in a subset of the wells. A measurement cycle consisted of 1 min periods each of mix and incubation followed by a 2 min measurement period. The first 3 measurement cycles were made in quiescent cardiomyocytes to obtain basal OCR, followed by 3 cycles in which the cardiomyocytes were electrically stimulated to contract. Maximal OCR was measured following injection of 20 μM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) dissolved in cell media. OCR was calculated from the continuous slope of O2 decrease using a compartmentalization model that accounts for O2 partitioning between plastic, atmosphere, and cellular uptake [23]. For each experimental group, the OCR measurements were made in 6-8 individual wells and averaged over 3 cycles. Maximal OCR was measured in the first cycle after FCCP injection.
Quantification of mitochondrial DNA
DNA was extracted from cardiomyocytes using the DNeasy Blood and Tissue kit (Qiagen). Real-time PCR measurements were performed using SsoAdvanced SYBR Green supermix (Bio-Rad). The amount of mitochondrial DNA (mtDNA) was measured using primers for the mitochondrial gene NADH dehydrogenase 1 (MT-ND1) and normalized to the amount of genomic DNA in the same samples using primers for the nuclear gene Glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primer sequences are listed in Table 1.
Statistical analysis
Data were analyzed using SigmaStat 4.0 (Systat Software, Inc, San Jose, CA). Differences between means in Figure 2 were determined by a Students T-test. In Figures 3 and 4, statistical comparisons between individual means were performed by ANOVA followed by the Fisher’s LSD test for comparisons between individual means. A p value <0.05 was considered to be a statistically significant difference.
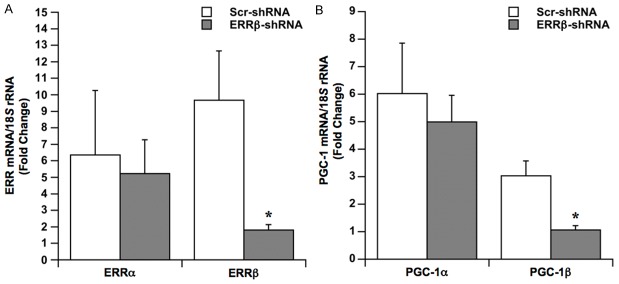
Figure 2.
Effects of ERRβ knockdown on gene expression. Cardiomyocytes were infected with adenovirus expressing either ERRβ-shRNA or Scr-shRNA. After 48 h of incubation, cardiomyocytes were electrically stimulated to contract in hypoxia over 24 h. Contracting cardiomyocytes in normoxia were used as baseline controls. A. Fold changes in levels of ERR mRNA isoforms. Values are the mean ± SE, *p<.05, n=4 experiments. B. Fold changes in levels of PGC-1 mRNA isoforms. Values are the mean ± SE, *p<.05, n=5 experiments.
Figure 3.
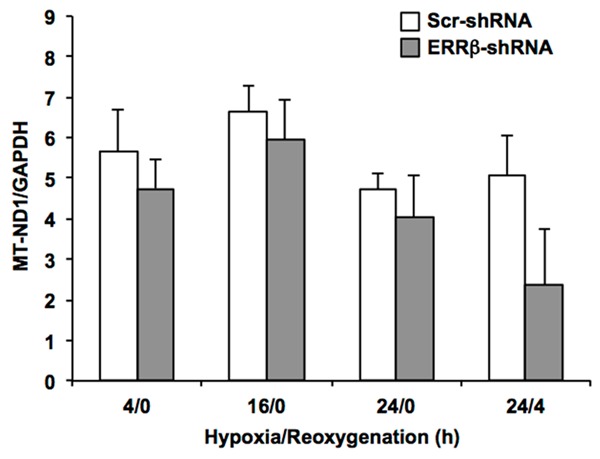
Effects of ERRβ knockdown on mtDNA content. Cardiomyocytes were infected with adenovirus expressing either ERRβ-shRNA or Scr-shRNA. After a 48 h incubation period, cardiomyocytes were electrically stimulated to contract and DNA was extracted at the indicated hours of hypoxia/reoxygenation. Mitochondrial NADH dehydrogenase 1 (MT-ND1) was measured by real-time PCR and normalized to the nuclear gene GAPDH to correct for differences in genomic DNA. Values are the mean + SE, n=5 experiments. There were no significant differences between cardiomyocytes expressing Scr-shRNA versus ERRβ-shRNA.
Figure 4.
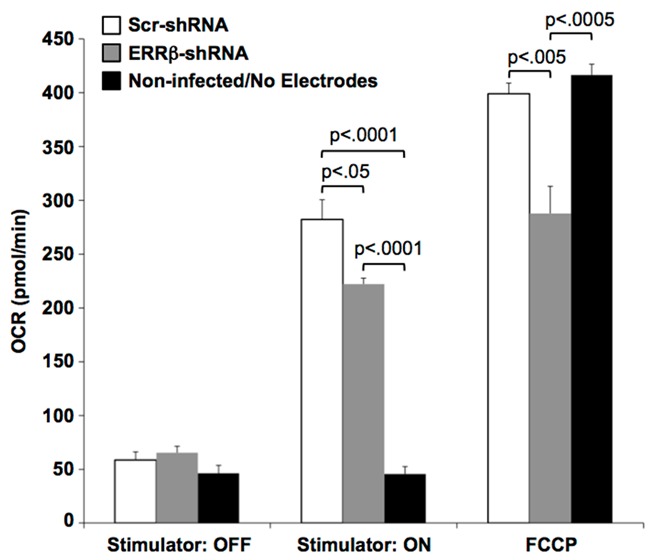
Effects of ERRβ knockdown on oxygen consumption rate (OCR). Cardiomyocytes were infected with adenovirus expressing either ERRβ-shRNA or Scr-shRNA. OCR measurements were obtained after 48 h of incubation. Non-infected cardiomyocytes were maintained in wells without electrodes and remained quiescent. Values are the mean + SE; n=5 experiments. Statistical comparisons between individual means were performed by ANOVA followed by the Fisher’s LSD test for comparisons between individual means. Although not indicated in the figure, electrically stimulated contraction (Stimulator: ON) significantly increased OCRs in cardiomyocytes expressing either ERRβ-shRNA or Scr-shRNA as compared to their quiescent state (Stimulator: OFF); p<0.0001.
Results
Previous studies in adult feline cardiomyocytes demonstrated that ERRα mRNA levels increased 3-fold after 24 h of hypoxia with no detectable changes in ERRγ mRNA [20]. Therefore, to determine whether expression of the ERRβ isoform is sensitive to changes in oxygen availability, the effects of hypoxia (0.5% O2) on ERRβ mRNA levels were measured in contracting cardiomyocytes as a function of time (Figure 1). Fold changes in ERRβ mRNA levels were calculated relative to contracting cardiomyocytes in normoxia, thereby establishing a baseline control for gene expression in each experimental isolation of cardiomyocytes. ERRβ mRNA levels were increased after 8 h of hypoxia in all experiments and remained elevated over 24 h of hypoxia. When contracting cardiomyocytes were returned to normoxia, ERRβ mRNA levels decreased during the initial 4 h period of reoxygenation. As a result, ERRβ mRNA levels were increased 2-fold or less over normoxic controls. Taken together, these data demonstrate an inverse relationship between oxygen availability and expression of the ERRβ isoform in adult cardiomyocytes.
Figure 1.
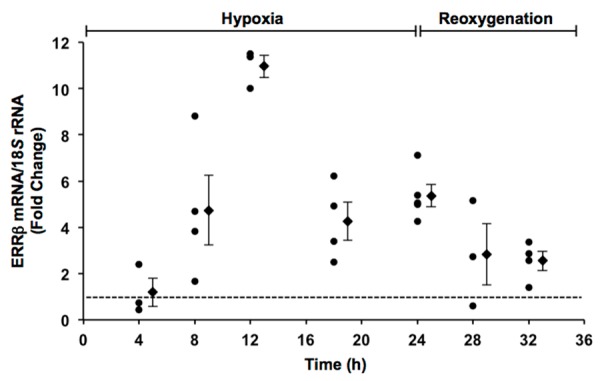
Scatter plot showing the effects of hypoxia and reoxygenation on ERRβ mRNA levels in contracting cardiomyocytes as a function of time. Fold changes in ERRβ mRNA levels were calculated relative to contracting cardiomyocytes in normoxia as indicated by the dotted line. Also shown is the mean ± SE of individual experiments at each time point.
To specifically block expression of the ERRβ isoform, cardiomyocytes were infected prior to hypoxia with adenovirus expressing either ERRβ-shRNA or scrambled shRNA (Scr-shRNA) as a control. Cardiomyocytes were then electrically stimulated to contract in hypoxia for 24 h and changes in mRNA levels were calculated by normalization to contracting cardiomyocytes in normoxia. ERRα mRNA levels increased after 24 h of hypoxia in cardiomyocytes expressing Scr-shRNA (Figure 2A). Although the magnitude of the response varied, hypoxia increased ERRα mRNA levels in all experiments relative to baseline normoxic controls. Consistent with the results obtained in non-infected cardiomyocytes (Figure 1), ERRβ mRNA levels were increased in the Scr-shRNA group after 24 of hypoxia. Figure 2A further shows that expression of ERRβ-shRNA in contracting cardiomyocytes had no effect on hypoxia-induced increases in ERRα mRNA, while increases in ERRβ mRNA were blocked. These results confirm the specificity of ERRβ-shRNA for the ERRβ isoform.
Prior studies demonstrated that hypoxia-induced increases in PGC-1 mRNA are dependent on expression of the ERRα isoform [20]. Therefore, the effects of ERRβ knockdown on expression of PGC-1 isoforms were examined in contracting cardiomyocytes after 24 of hypoxia (Figure 2B). In Scr-shRNA controls, PGC-1α and PGC-1β mRNA were increased by 6-fold and 3-fold, respectively. ERRβ-shRNA had no effect on PGC-1α mRNA levels produced in response to hypoxia, however, it completely blocked the increase in PGC-1β mRNA levels.
Chronic hypoxia stimulates mitochondrial biogenesis in the adult myocardium in vivo, a process which is controlled by increasing the activity of PGC-1 isoforms [24]. Therefore, we examined whether changes in ERRβ expression affected mitochondrial content in contracting cardiomyocytes maintained in hypoxia (Figure 3). Cardiomyocytes were infected with adenovirus expressing either ERRβ-shRNA or Scr-shRNA and incubated for 48 h. Subsequently, the effects of hypoxia and hypoxia/reoxygenation on mtDNA content were measured in contracting cardiomyocytes. These data show that mtDNA did not change significantly over time in hypoxia and that loss of ERRβ expression did not affect mtDNA content as compared to Scr-shRNA controls. After 4 h of reoxygenation, mtDNA decreased in cardiomyocytes expressing ERRβ-shRNA. Although not statistically significant, this reduction in mtDNA could indicate some possible mitochondrial damage during reoxygenation.
Mitochondria are the primary determinant of oxidative capacity in cardiac muscle [25]. To determine how expression of ERRβ contributes to mitochondrial function, the electrical stimulation system was adapted to measure oxygen consumption rates (OCR). Cardiomyocytes were infected with adenovirus expressing either ERRβ-shRNA or Scr-shRNA and maintained for 48 h prior to measuring the effects of electrically stimulated contraction on OCR. Non-infected, quiescent cardiomyocytes (no electrodes) were included as a baseline control. As shown in Figure 4, there were no significant differences in OCRs between the ERRβ-shRNA and Scr-shRNA groups of quiescent cardiomyocytes (Stimulator: OFF). When the same groups of cardiomyocytes were electrically stimulated to contract (Stimulator: ON), OCRs increased significantly as compared to their corresponding quiescent state. However, OCRs in contracting cardiomyocytes expressing ERRβ-shRNA were significantly lower than Scr-shRNA shRNA controls. At the end of each experiment, maximal OCRs were measured by injection of the mitochondrial uncoupling agent FCCP. Maximal OCRs in cardiomyocytes expressing ERRβ-shRNA were significantly lower than Scr-shRNA controls. These data indicate that ATP synthesis was reduced by loss of ERRβ in contracting cardiomyocytes.
Discussion
It is well established that ERR isoforms function coordinately to regulate transcription of an extensive network of genes that are vital for maintaining oxidative capacity, particularly in tissues with high metabolic demands such as the myocardium [1]. During excitation-contraction coupling, myosin ATPase accounts for approximately two-thirds of myocardial energy expenditure with most of the remaining energy consumed by ATPases coupled to ion channels [26]. This increased demand for ATP is underscored by findings that OCR was approximately 3-fold higher in cardiomyocytes that were electrically stimulated to contract as compared to non-stimulated, quiescent cardiomyocytes [20]. Thus, continuous electrical stimulation serves as an ideal model for examining the role of ERR isoforms in maintaining energy metabolism in contracting cardiomyocytes in vitro. These studies demonstrate that ERRβ is required for optimal mitochondrial function as measured by OCR in contracting cardiomyocytes. Furthermore, while ERR isoforms coordinately control mitochondrial gene expression, the reduction in OCR caused by ERRβ knockdown suggests that ERRα and ERRγ can not fully compensate for loss of ERRβ in adult cardiomyocytes.
A key finding of these studies is expression of the ERRβ isoform is required to increase PGC-1β mRNA levels in response to hypoxia. PGC-1 isoforms function as master regulators of gene networks involved in fatty acid oxidation, the electron transport chain and mitochondrial DNA synthesis [27]. The observed rise in PGC-1β would be expected to stimulate mitochondrial biogenesis in cardiomyocytes as demonstrated in response to cardiac-specific overexpression of PGC-1α [28]. However, mitochondrial content did not change significantly in contracting cardiomyocytes over 24 h of sustained hypoxia. This duration time of hypoxia was likely too short to generate significant increases in mitochondria considering that chronic hypoxia for 7 days or longer was required to increase mitochondrial density in adult myocardium of rodents [29,30]. It is also possible that mitochondrial number remained stable because the increases in PGC-1 mRNA levels after 24 h of hypoxia did not produce corresponding changes in PGC-1 protein. This possibility is questionable because PGC-1α mRNA and ERRα mRNA are translated efficiently into protein as reflected by proportional increases in free and polyribosome-bound mRNA pools after 24 h of hypoxia in contracting cardiomyocytes [20].
In previous studies, we showed that hypoxia-induced increases in PGC-1α and PGC-1β mRNA levels were blocked in contracting cardiomyocytes by treatment with XCT-790, a selective inverse agonist for ERRα [20]. The inhibitory effects of XCT-790 on expression of PGC-1 isoforms were caused by knockdown of ERRα levels. Here we show that increases in PGC-1β mRNA in response to hypoxia were also dependent on ERRβ. ERR isoforms are nuclear receptors that bind as a monomer to a half-site in an ERR response element (ERRE), but remain capable of forming either homodimers or heterodimers during DNA binding [31]. Dimerization of individual ERR isoforms modulates transcriptional activity as measured using a consensus ERRE found in target genes [32]. For example, formation of a ERRα-ERRγ heterodimer inhibits transcriptional activity as compared to homodimers consisting of either isoform. Our studies have shown that PGC-1 mRNA levels increase during the initial 24 h of hypoxia without significant increases in the levels of ERRα or ERRγ mRNA [20]. Therefore, the rise in ERRβ levels in response to hypoxia could modulate transcription of the PGC-1β gene through formation or stabilization of heterodimers with either the ERRα or ERRγ isoform.
In addition to regulating synthesis of mitochondrial components, ERRβ could function via mechanisms that impact the activity of existent mitochondria in the cardiomyocyte. ERR isoforms have been shown to recruit PGC-1α to the promoter of the pyruvate dehydrogenase kinase 4 (PDK4) gene to enhance its transcription [33]. PDK isoforms negatively regulate pyruvate dehydrogenase (PDH) activity, thereby decreasing conversion of pyruvate into acetyl-CoA for utilization as an energy source. Another possible mechanism involves fatty acid binding protein (Fabp3) that is essential for uptake and oxidation of fatty acids in cardiac muscle cells [34,35]. Hypoxia-induced expression of Fabp3 mRNA is blocked by inhibition of ERRα in contracting cardiomyocytes, which is consistent with the presence of an ERR response element (ERRE) in the promoter of the Fabp3 gene [13,20]. Lastly, the increase in ERRβ may function as part of an adaptive response to hypoxia, possibly by modifying the molecular properties of the ATP synthase complex in order to sustain efficient synthesis of ATP [36].
In general, ERR expression is greater in tissues with higher energy demands such as the myocardium [37]. Despite overlapping functions of ERR isoforms, contracting cardiomyocytes display distinct differences in expression patterns during hypoxia. ERRα mRNA increased after 24 h of hypoxia while ERRγ mRNA levels were unchanged [20]. The mechanisms responsible for differential expression of individual ERR isoforms have not been determined, but they appear to be linked to PGC-1. For example, PGC-1α can regulate expression of the ERRα isoform by a positive feedback loop [38]. The promoter region of the ERRα gene contains an ERRE that is activated through interactions of ERRα with PGC-1α [39]. Other studies have shown that ERRα expression is coupled to increased energy demands via AMP kinase (AMPK) [40]. It remains to be determined whether AMPK activation causes the rise in ERRβ mRNA during hypoxia, possibly by a mechanism involving a sustained increase in the AMP:ATP ratio in contracting cardiomyocytes [41].
In summary, these studies demonstrate that sustained hypoxia increases ERRβ mRNA levels in adult cardiomyocytes undergoing electrically stimulated contraction. There is a corresponding rise in PGC-1β mRNA that is dependent on expression of ERRβ. Loss of ERRβ does not affect mitochondrial content over 24 h of hypoxia in contracting cardiomyocytes, but OCR is reduced. Thus, ERRβ is involved in maintaining maximal ATP generation in contracting cardiomyocytes. Despite functional overlap with other ERR isoforms, these findings indicate that ERRβ has distinct functions in regulating energy metabolism in the adult cardiomyocyte.
Acknowledgements
We thank Daisy Dominick and Dr. Harinath Kasiganesan for their excellent technical assistance. Supported by a Merit Review Award from the Department of Veterans Affairs (P.J.M.); National Institutes of Health Predoctoral Fellowship T32HL07260 (K.F.C.); American Heart Association Predoctoral Fellowship 12PRE120500 (K.F.C.).
Disclosure of conflict of interest
None.
References
- 1.Giguere V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29:677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, Loh YH, Yeo HC, Yeo ZX, Narang V, Govindarajan KR, Leong B, Shahab A, Ruan Y, Bourque G, Sung WK, Clarke ND, Wei CL, Ng HH. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 3.Eichner LJ, Giguere V. Estrogen related receptors (ERRs): a new dawn in transcriptional control of mitochondrial gene networks. Mitochondrion. 2011;11:544–552. doi: 10.1016/j.mito.2011.03.121. [DOI] [PubMed] [Google Scholar]
- 4.Ge K, Cho YW, Guo H, Hong TB, Guermah M, Ito M, Yu H, Kalkum M, Roeder RG. Alternative mechanisms by which mediator subunit MED1/TRAP220 regulates peroxisome proliferator-activated receptor γ-stimulated adipogenesis and target gene expression. Mol Cell Biol. 2008;28:1081–1091. doi: 10.1128/MCB.00967-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 6.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor γ coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 9.Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguere V, Murphy E, Kelly DP. The nuclear receptor ERRα is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ. PGC-1α and ERRα target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol. 2009;46:201–212. doi: 10.1016/j.yjmcc.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leone TC, Kelly DP. Transcriptional control of cardiac fuel metabolism and mitochondrial function. Cold Spring Harb Symp Quant Biol. 2011;76:175–182. doi: 10.1101/sqb.2011.76.011965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, Evans RM. ERRγ directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 2007;6:13–24. doi: 10.1016/j.cmet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguere V. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRα and γ. Cell Metab. 2007;5:345–356. doi: 10.1016/j.cmet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguere V. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-β. Nature. 1997;388:778–782. doi: 10.1038/42022. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Nathans J. Estrogen-related receptor β/NR3B2 controls epithelial cell fate and endolymph production by the stria vascularis. Dev Cell. 2007;13:325–337. doi: 10.1016/j.devcel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Onishi A, Peng GH, Poth EM, Lee DA, Chen J, Alexis U, de Melo J, Chen S, Blackshaw S. The orphan nuclear hormone receptor ERRβ controls rod photoreceptor survival. Proc Natl Acad Sci U S A. 2010;107:11579–11584. doi: 10.1073/pnas.1000102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byerly MS, Swanson RD, Wong GW, Blackshaw S. Estrogen-related receptor β deficiency alters body composition and response to restraint stress. BMC Physiol. 2013;13:10. doi: 10.1186/1472-6793-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sengupta D, Bhargava DK, Dixit A, Sahoo BS, Biswas S, Biswas G, Mishra SK. ERRβ signalling through FST and BCAS2 inhibits cellular proliferation in breast cancer cells. Br J Cancer. 2014;110:2144–2158. doi: 10.1038/bjc.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanida T, Matsuda KI, Yamada S, Hashimoto T, Kawata M. Estrogen-related Receptor β Reduces the Subnuclear Mobility of Estrogen Receptor α and Suppresses Estrogen-dependent Cellular Function. J Biol Chem. 2015;290:12332–12345. doi: 10.1074/jbc.M114.619098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham KF, Beeson GC, Beeson CC, Baicu CF, Zile MR, McDermott PJ. Estrogen-Related Receptor α (ERRα) is required for adaptive increases in PGC-1 isoform expression during electrically stimulated contraction of adult cardiomyocytes in sustained hypoxic conditions. Int J Cardiol. 2015;187:393–400. doi: 10.1016/j.ijcard.2015.03.353. [DOI] [PubMed] [Google Scholar]
- 21.Ivester CT, Kent RL, Tagawa H, Tsutsui H, Imamura T, Cooper G, McDermott PJ. Electrically stimulated contraction accelerates protein synthesis rates in adult feline cardiocytes. Am J Physiol. 1993;265:H666–674. doi: 10.1152/ajpheart.1993.265.2.H666. [DOI] [PubMed] [Google Scholar]
- 22.Kato S, Ivester CT, Cooper G, Zile MR, McDermott PJ. Growth effects of electrically stimulated contraction on adult feline cardiocytes in primary culture. Am J Physiol. 1995;268:H2495–2504. doi: 10.1152/ajpheart.1995.268.6.H2495. [DOI] [PubMed] [Google Scholar]
- 23.Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81:6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLeod CJ, Pagel I, Sack MN. The mitochondrial biogenesis regulatory program in cardiac adaptation to ischemia--a putative target for therapeutic intervention. Trends Cardiovasc Med. 2005;15:118–123. doi: 10.1016/j.tcm.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Park SY, Gifford JR, Andtbacka RH, Trinity JD, Hyngstrom JR, Garten RS, Diakos NA, Ives SJ, Dela F, Larsen S, Drakos S, Richardson RS. Cardiac, skeletal, and smooth muscle mitochondrial respiration: are all mitochondria created equal? Am J Physiol Heart Circ Physiol. 2014;307:H346–352. doi: 10.1152/ajpheart.00227.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suga H. Ventricular energetics. Physiol Rev. 1990;70:247–277. doi: 10.1152/physrev.1990.70.2.247. [DOI] [PubMed] [Google Scholar]
- 27.Rowe GC, Jiang A, Arany Z. PGC-1 coactivators in cardiac development and disease. Circ Res. 2010;107:825–838. doi: 10.1161/CIRCRESAHA.110.223818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor γ coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman I, Moravec J, Reichart E, Hatt PY. Subacute myocardial hypoxia in the rat. An electron microscopic study of the left ventricular myocardium. J Mol Cell Cardiol. 1973;5:125–132. doi: 10.1016/0022-2828(73)90045-x. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy J, Lochner A, Opie LH, Sack MN, Essop MF. PKCε promotes cardiac mitochondrial and metabolic adaptation to chronic hypobaric hypoxia by GSK3β inhibition. J Cell Physiol. 2011;226:2457–2468. doi: 10.1002/jcp.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gearhart MD, Holmbeck SM, Evans RM, Dyson HJ, Wright PE. Monomeric complex of human orphan estrogen related receptor-2 with DNA: a pseudo-dimer interface mediates extended half-site recognition. J Mol Biol. 2003;327:819–832. doi: 10.1016/s0022-2836(03)00183-9. [DOI] [PubMed] [Google Scholar]
- 32.Huppunen J, Aarnisalo P. Dimerization modulates the activity of the orphan nuclear receptor ERRγ. Biochem Biophys Res Commun. 2004;314:964–970. doi: 10.1016/j.bbrc.2003.12.194. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Ma K, Sadana P, Chowdhury F, Gaillard S, Wang F, McDonnell DP, Unterman TG, Elam MB, Park EA. Estrogen-related receptors stimulate pyruvate dehydrogenase kinase isoform 4 gene expression. J Biol Chem. 2006;281:39897–39906. doi: 10.1074/jbc.M608657200. [DOI] [PubMed] [Google Scholar]
- 34.Binas B, Danneberg H, McWhir J, Mullins L, Clark AJ. Requirement for the heart-type fatty acid binding protein in cardiac fatty acid utilization. FASEB J. 1999;13:805–812. doi: 10.1096/fasebj.13.8.805. [DOI] [PubMed] [Google Scholar]
- 35.Murphy EJ, Barcelo-Coblijn G, Binas B, Glatz JF. Heart fatty acid uptake is decreased in heart fatty acid-binding protein gene-ablated mice. J Biol Chem. 2004;279:34481–34488. doi: 10.1074/jbc.M314263200. [DOI] [PubMed] [Google Scholar]
- 36.Reynafarje BD, Marticorena E. Bioenergetics of the heart at high altitude: environmental hypoxia imposes profound transformations on the myocardial process of ATP synthesis. J Bioenerg Biomembr. 2002;34:407–412. doi: 10.1023/a:1022597523483. [DOI] [PubMed] [Google Scholar]
- 37.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, Willy PJ, Schulman IG, Heyman RA, Lander ES, Spiegelman BM. Errα and Gabpα/β specify PGC-1α-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci U S A. 2004;101:6570–6575. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laganiere J, Tremblay GB, Dufour CR, Giroux S, Rousseau F, Giguere V. A polymorphic autoregulatory hormone response element in the human estrogen-related receptor α (ERRα) promoter dictates peroxisome proliferator-activated receptor γ coactivator-1α control of ERRα expression. J Biol Chem. 2004;279:18504–18510. doi: 10.1074/jbc.M313543200. [DOI] [PubMed] [Google Scholar]
- 40.Hu X, Xu X, Lu Z, Zhang P, Fassett J, Zhang Y, Xin Y, Hall JL, Viollet B, Bache RJ, Huang Y, Chen Y. AMP activated protein kinase-α2 regulates expression of estrogen-related receptor-α, a metabolic transcription factor related to heart failure development. Hypertension. 2011;58:696–703. doi: 10.1161/HYPERTENSIONAHA.111.174128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan H, Zhang D, Zhang Q, Wang P, Huang Y. The activation of AMPK in cardiomyocytes at the very early stage of hypoxia relies on an adenine nucleotide-independent mechanism. Int J Clin Exp Pathol. 2012;5:770–776. [PMC free article] [PubMed] [Google Scholar]