Abstract
In this study, the genotype-phenotype correlations in four unrelated families with a PKP2 c.2146-1G>C gene variant were studied. Our primary aim was to determine the carriers that fulfilled the arrhythmogenic right ventricular cardiomyopathy (ARVC) diagnostic criteria of 2010. Our secondary aim was to investigate whether any specific clinical characteristics can be attributed to this particular gene variant. Index patients were assessed using next generation ARVC panel sequencing technique and their family members were assessed by Sanger sequencing targeted at the PKP2 c.2146-1G>C variant. The gene variant carriers were offered a clinical follow-up, with evaluation based on the patient’s history and a standard set of non-invasive testing. The PKP2 c.2146-1G>C gene variant was found in 23 of 41 patients who underwent the examination. Twelve of the 19 family members showed “possible ARVC”. One with “borderline ARVC” and the rest with “definite ARVC” demonstrated re-polarization disturbances, but arrhythmia was uncommon. A lethal event occurred in a 14-year-old boy. In the present study, no definitive genotype-phenotype correlations were found, where the majority of the family members carrying the PKP2 c.2146-1G>C gene variant were diagnosed with “possible ARVC”. These individuals should be offered a long-term follow-up since they are frequently symptomless but still at risk for insidious sudden cardiac death due to ventricular arrhythmia.
Keywords: ARVC, heredity, PKP2, sudden cardiac death
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) was first clinically described in adults in 1982. It is characterized by progressive fibro-fatty replacement of the right ventricular myocardium, creating a substrate for ventricular arrhythmia [1]. Ventricular fibrillation and sudden cardiac death (SCD) may occur, and the increased risk is potentiated by physical activity. The prevalence of ARVC is estimated to be 1:5000, which probably is an underestimate [2]. Its diagnosis is based on the presence of major and minor criteria as developed in the modified Task Force Criteria of 2010 [3]. However, the application of these diagnostic criteria may be difficult due to the sparsely observed symptoms and signs in the early stages of the disease. Therefore, the progression of the disease is still not completely understood. Although the manifestations of the disease are present mainly in the right ventricle (RV), there is also a left ventricular (LV) involvement, which has been shown earlier [4,5].
The heterogeneity of the clinical course in ARVC may be related to the involvement of several genes, mechanisms, and variable pathogenicity of mutations [6]. The causative role of desmosomal gene mutations is speculative to a certain extent. Impaired desmosomes function under the conditions of mechanical stress, which is believed to induce detachment of myocytes from one another, leading to cell death. This injury may be accompanied by an inflammatory response leading to limited regeneration of cardiac myocytes, followed by its repair through fibro-fatty replacement mechanism, resulting in ventricular remodeling. The most commonly mutated ARVC-related gene is PKP2 (10-45%), followed by DSP (10-15%), DSG2 (7-10%), and DSC2 (2%). The use of genetic testing in individuals with clinical manifestation of ARVC helps in identifying the causative gene mutation in up to 50% of ARVC families [7-9].
Presymptomatic testing for the identification of pathogenic mutations known to cause ARVC is important when estimating the risk of the disease in asymptomatic family members. However, it may be difficult to manage such asymptomatic carrier individuals. Many mutation carriers have a normal life span and continue to be asymptomatic throughout their lifetime due to the highly variable penetrance of the genetic abnormality. Therefore, the assessment of the risk for lethal arrhythmia, e.g., during exercise, has been prioritized [3].
Thus, the aim of this study was to investigate four Swedish ARVC families where a PKP2 c.2146-1G>C mutation was previously detected so as to determine 1) the extent to which the mutation carriers fulfilled the revised Task Force Criteria of 2010, and 2) whether specific clinical characteristics can be attributed to carriers of this particular gene variant.
Materials and methods
Subjects
In the South-East region of Sweden, we have performed a systematic clinical follow-up of patients and their relatives with ARVC since the late 1980s. The present genetic investigation revealed that four unrelated families (Families A, B, C, and D) carried the pathogenic PKP2 c.2146-1G>C gene variant. The family members, defined by 1st to 4th-degree relatives of the index patients, were included in the study. The details of each family are presented below:
Family A
In the early 1980s, the physically-active index patient (a female from the South of Sweden presented with palpitations due to ventricular premature beats. In 1982, she had a cardiac arrest associated with physical exercise. The mechanism was believed to be ventricular tachycardia or primary ventricular fibrillation. Resuscitation started immediately, and she showed no physical sequelae. The patient was treated with a combination of sotalol and disopyramide, which could not provoke any arrhythmia, and underwent an invasive electrophysiological examination in 1984. In 2000, she was presented to the emergency room (ER) with dizzy spells. The electrocardiograph (ECG) showed ventricular tachycardia originating from the RV. The ARVC diagnosis was based on the classical criteria that included a positive endomyocardial biopsy. Shortly after that, the patient was implanted with an implantable cardioverter defibrillator (ICD) sented with palpitations due to ventricular premature beats.
During repeated visits to the outpatient clinic, the ICD registered several episodes of non-sustained ventricular arrhythmias, but no shock therapy was given by the internal defibrillator. There was no history of SCD or arrhythmia in the family.
Family B
Here, the index patient is a physically active male from the South of Sweden. The pedigree analysis is presented in Figure 1. In 2008, some months before his first medical contact, he noticed chest discomfort while exercising. The patient was presented to the ER on the day after syncope when he had been playing floorball. After an extensive workup (see Table 1), he received an ICD the same week. The syncope was believed to be due to ventricular tachycardia. Later on, he was presented with recurrent ventricular tachycardia, which was related to physical activities. The ICD delivered both ATP (Anti-Tachycardia Pacing) and shocks. He has now been forced to limit his athletic activities and is being treated with a combination of metoprolol and flecainide.
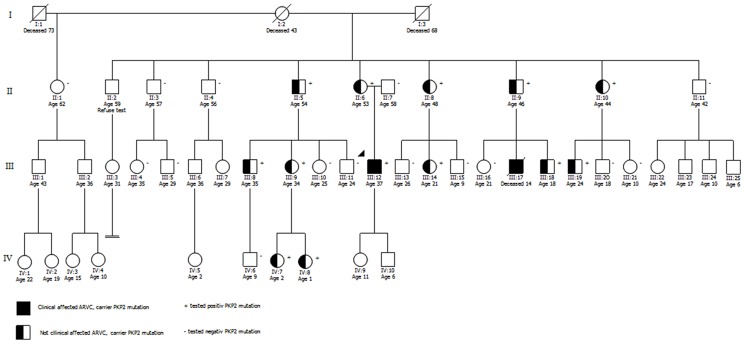
Figure 1.
Pedigree analysis of family B. Circles in the pedigree denote females, squares males. A crossed-over symbol indicates that this particular individual has died. The arrow points out the index patient for genetic screening. The ages given in the pedigree are from first contact and/or screening.
Table 1.
Task Force Criteria, revised of 2010 [3] includes the following parts: Group I: Global or regional dysfunction and structural alterations; Group II: Tissue characterization of wall (biopsies); Group III: Repolarization abnormalities; Group IV: Depolarization/conduction abnormalities; Group V: Arrhytmias; Group VI: Family history (including mutations). The diagnosis of ARVC relies on the demonstration of structural, functional, and electrophysiological abnormalities. Depending on the severity, the changes can fulfill minor or major criteria. Carriership of a known pathogenic mutation fulfills a major criterion in group IV. Definite diagnosis: 2 major or 1 major plus 2 minor or 4 minor criteria from different groups. Borderline: 1 major plus 1 minor or 3 minor criteria from different categories. Possible: 1 major or 2 minor criterion from different groups
| Patient no/sex | Age at genetic screening (yrs) | Age at first symptom and diagnosis (yrs) | Revised Task Force Criteria, 2010 | Category of Diagnosis | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Group I | Group II | Group III | Group IV | Group V | Group VI | ||||
| Family A | |||||||||
| II:1*/F | - | 22/41 | X | X | X | - | I | G | Definite |
| Family B | |||||||||
| II:5/M | 53 | - | - | NA | - | - | - | G | Possible |
| III:8/M | 34 | - | - | NA | - | - | - | G | Possible |
| III:9/F | 33 | - | - | NA | - | - | - | G | Possible |
| IV:7/F | 2 | NA | NA | NA | NA | NA | NA | G | Possible |
| IV:8/F | 1 | NA | NA | NA | NA | NA | NA | G | Possible |
| II:6/F | 53 | ? | ? | NA | - | ? | ? | X+G | Possible |
| III:12*/M | - | 32/32 | X | NA | X | - | X+I | G | Definite |
| II:8/F | 47 | - | - | NA | - | - | - | G | Possible |
| III:14/F | 19 | - | - | NA | - | NA | - | G | Possible |
| II:9/M | 45 | - | - | NA | I | - | - | X+G | Borderline |
| III:18/M | 14 | 16 | X | NA | X | I | I | X+G | Definite |
| II:10/F | 42 | - | - | NA | - | I | - | G | Borderline |
| III:19/M | 18 | - | - | NA | - | NA | - | G | Possible |
| Family C | |||||||||
| II:1*/F | - | -/65 | X | NA | X | I | I | G | Definite |
| Family D | |||||||||
| I:2/M | - | - | ? | NA | ? | ? | ? | X+G | Possible |
| II:2/F | 51 | -/63 | - | NA | X | - | - | X+G | Definite |
| III:1/M | - | 25/26 | X | X | X | I | I | X+G | Definite |
| III:2*/F | 29 | 29/30 | X | X | X | - | I | X+G | Definite |
| III:3/M | 39 | - | - | NA | - | I | NA | X+G | Borderline |
| IV:5/M | 8 | - | - | NA | - | I | - | X+G | Borderline |
| IV:6/M | 10 | - | - | NA | - | - | - | G | Possible |
| IV:7/M | 10 | - | - | NA | - | - | - | G | Possible |
Abbreviations: M = Male; F = Female; RV = right ventricular; - = normal results; X = major criteria; I = minor criteria; G = mutation positive; NA = not applicable/not performed; * = proband; ? = performed elsewhere and result not known.
In this family, we found one case of SCD in a physically active 14-year old boy (III:17 in Figure 1). The death occurred in the same year of diagnosis of the index patient. The autopsy showed histological signs of ARVC. DNA testing could not be performed on this boy, who was a cousin of the index patient.
The father (II:9) of the deceased boy showed a right bundle branch block (RBBB) in the ECG report, and a dilated RV on the echocardiogram. However, he did not show any clinical criterion for ARVC.
A physically active brother of the deceased boy (III:18) had an ICD implanted because of the family history. Only one month after the implantation, the ICD delivered an adequate shock discharge due to fast ventricular tachycardia associated with pre-syncope. It should also be noted that the pedigree of this family shows three cases of leukemia (not marked here) that were of at least two different kinds.
Family C
The index patient, a woman of British origin, was shifted to the cardiology ward from the ER in 2012 because of syncope. The ECG of the patient was abnormal with negative T-waves in V1-V3 present since at least 1981. An echocardiogram performed in 1998 showed normal LV function. However, the RV showed minor changes, and a follow-up with echo was suggested but was never performed. The presenting syncopal episode was not considered to be caused by arrhythmia, but a comprehensive evaluation for ARVC was thought to be appropriate, which resulted in the diagnosis of ARVC. Since the patient showed no further symptoms, she did not receive an ICD but was monitored via the outpatient clinic.
Genetic testing has not been performed on her children or her brother for various reasons. There was no history of SCD or arrhythmia in this family.
Family D
The index patient was the daughter of a man from Southern Sweden, who died in 1992 due to ARVC that caused ventricular arrhythmia. Her first clinical screening in 1994 showed subtle morphological abnormalities, but she was not diagnosed with ARVC until 1996. She had palpitations due to non-sustained ventricular tachycardia but did not experience syncope or pre-syncope. When the echocardiography and magnetic resonance imaging (MRI) of the patient showed the progression of the abnormalities, an ICD was implanted in the index patient in 2010. Telemetry of stored rhythm strips revealed both non-sustained and sustained ventricular tachycardia, of which the latter was treated with ATP but without shocks.
In 1994, her brother was also screened for the disease, and he has been regularly followed-up since then. After discussions with the patient, the subtle progress of echocardiographic abnormalities led to the implantation of a primary preventive ICD in 2009. Interrogations with the patient later revealed non-sustained as well as sustained ventricular tachycardia on several occasions that were treated with ATP but without shocks. He is presently completely free of symptoms.
Clinical evaluation
The evaluation of patients and relatives involved their detailed medical history and their thorough physical examination. A local algorithm was used for the clinical follow-up of patients suspected of ARVC. The study was approved by the regional ethical review board in Linköping (Decision number M68-09), and informed consent was obtained from each subject.
Electrocardiography
A standard 12-lead ECG was recorded and analyzed with regard to T-wave inversion beyond V1, in the absence of RBBB, QRS-duration exceeding 110 ms in V1-V3, and the presence of epsilon wave, defined as a distinct low-amplitude deflection after the QRS complex.
A signal-averaged ECG (SAECG) was recorded, and band pass was filtered at 40-250 Hz. The SAECG was considered positive for late potentials when at least one of the three following criteria was fulfilled: (a) QRS-filtered duration >114 ms, (b) low amplitude signal duration >38 ms, and (c) the root mean square amplitude of the last 40 ms of the QRS <20 uV. The frequency and morphology of the ventricular premature complexes were assessed both from the resting 12-lead ECG and 24-hour LTER. Ventricular tachycardia was defined by three or more consecutive premature ventricular complexes, and if it lasted longer than 30 s, it was defined as sustained ventricular tachycardia.
Cardiac imaging
The two-dimensional echocardiographic examination was performed using a 3.5 MHz or 4 MHz transducer (Vivid 7 and E9, GE Healthcare, Milwaukee, USA). Images from different standard views were recorded. Dimensional and functional parameters of the RV and LV were analyzed. Cardiac magnetic resonance imaging (CMR) was requested at the discretion of the attending physician and performed using the Philips 1.5-T Achieva Nova Dual scanner (Philips Healthcare, Best, the Netherlands). Left and right ventricular volumes and ejection fraction were calculated. In most cases, the CMR was performed immediately after echocardiography. We have applied the modified ARVC criteria of 2010, presented by Marcus et al [3], to all studies.
Genetic analyses
Genetic sequencing analyses were performed regarding PKP2, DSP, DSG2, DSC2, JUP, DES, TGFB3, and TMEM43.
DNA extraction, quantification, and quality control
DNA extraction from whole blood samples was performed using either EZ1 (Qiagen) or Prepito (Techtum). DNA concentration and quality were determined using NanoDrop spectrophotometer. Samples with A260/A280 ratios between 1.8 and 2.0, and A260/A230 ratios above 1.5 were accepted for further sequencing.
Next generation sequencing
Next Generation Sequencing (NGS) was performed using HaloPlex PCR target enrichment system (Agilent) and MiSeq (Illumina) as described by Greén et al [10]. The ARVC panel probes were generated to cover the exons of the genes DES (CCDS33383.1), DSC2 (CCDS11892.1, CCDS11893.1), DSG2 (CCDS42423.1), DSP (CCDS47368.1, CCDS4501.1), JUP (CCDS11407.1), PKP2 (CCDS31771.1, CCDS8731.1), TGFB3 (CCDS9846.1), and TMEM43 (CCDS2618.1). Library preparation, sequencing, and bioinformatic analyses were carried out as described by Greén et al [10].
Sanger sequencing
Sanger sequencing was performed for all exons of PKP2 in the probands according to earlier studies performed in our lab [10]. Testing for the c.2146-1G>C gene variant in family members was performed using the Sanger sequencing method.
Results
The families A, B, C, and D were selected for this study because the probands had previously been diagnosed with a heterozygous PKP2 c.2146-1 C>T mutation. No other pathogenic mutation in other ARVC-related genes, i.e., DES (CCDS33383.1), DSC2 (CCDS11892.1, CCDS11893.1), DSG2 (CCDS42423.1), DSP (CCDS47368.1, CCDS4501.1), JUP (CCDS11407.1), PKP2 (CCDS31771.1, CCDS8731.1), TGFB3 (CCDS9846.1), TMEM43 (CCDS2618.1), TTN (NM_133432, NM_133437, NM_003319, NM_133378, NM_133379), was found in any of the index patients. The PKP2 c.2146-1G>C result was confirmed by Sanger sequencing. This variant was predicted to be pathogenic by several bioinformatic tools (Polyphen, Mutation taster), and the mutation has been reported earlier as pathogenic (ARVC/D database).
In family A, two first-degree relatives were examined, but none of them tested positive for the PKP2 c.2146-1G>C mutation.
In family B, 27 relatives were examined; of which, only 12 relatives showed positive results (Figure 1).
In family C, no relatives were examined.
In family D, 12 relatives were examined; of which 7 showed positive results.
Thus, in total, 41 family members were examined, and 19 (~46%) were detected to be mutation carriers.
The 23 subjects with a PKP2 c.2146-1G>C mutation in the heterozygous form underwent clinical examinations as outlined in Table 1. Two children were excluded from the follow-up program due to their young age, as presented in Table 1.
Seven of 23 mutation carriers established a definite ARVC diagnosis. This included the index patients from the four families. However, since the father of the index patient in family D had died, his genetic analysis could not be performed. Four of the mutation carriers are categorized as “borderline ARVC”, and the remaining 12 as “possible ARVC” - in several cases due to detected genetic abnormality. None of the probands had additional mutations in the remaining ARVC-related genes.
One of the family members with borderline ARVC, and others with definite ARVC exhibited repolarization disturbances on 12-lead ECG, but clinical arrhythmia was uncommon.
Involvement of the LV in ARVC is well documented but is not a part of the diagnostic criteria. In our study, three index patients and one family member in family D had LV-dysfunction.
Discussion
The PKP2 c.2146-1G>C mutation detected in the four Swedish families selected for this study has been described earlier in individuals with a clinical diagnosis of ARVC [8,11,12]. However, to the best of our knowledge, the present study is the first to report extensive clinical follow-up and genetic testing of 1st to 4th degree relatives with an identical PKP2 c.2146-1G>C mutation (ascertained through cascade screening of first-degree relatives).
The diagnostic criteria for ARVC include genetic diagnosis. The progress in molecular genetics has facilitated the genetic diagnosis of this potentially lethal disease in asymptomatic family members. New categories of patients or more accurately, potential patients, are defined in accordance with the subtle findings in ECG and ventricular premature beats rather than full blown arrhythmia or sudden death that characterizes those who qualify for a definitive ARVC diagnosis. An even larger group consists of those diagnosed with “possible” or “probable” disease through cascade screening, where the mere presence of a mutation means “possible ARVC”. This presents a challenge to the dissemination of information. The follow-up unveils low predictive value since we do not possess the tools that are required for the accurate prediction of the disease development and arrhythmia predisposition in the individual mutation carrier. As per the study, the clinical phenotype varied due to the variable penetrance and expressivity. This means that a clearly pathogenic mutation can have a high diagnostic value but may have a low prognostic utility. An advanced challenge lies in distinguishing the pathogenicity of a mutation from normal genetic variation.
The PKP2 c.2146-1G>C variant found in our study patients is a splice site mutation predicted to activate a cryptic splice acceptor site in intron 12 or, alternatively, another cryptic splice acceptor site in PKP2 exon 13. Gerull et al [8] could not detect this variant among 500 healthy controls, and it was earlier described as pathogenic [11,12]. Both the bioinformatic tools, Polyphen and Mutation taster, suggested this variant to be non-tolerable [13,14].
Three families in this study come from different regions of Sweden, and one family is of British origin. This suggests that the mutation is not of pure Swedish origin, even though our material is too small to be certain. Recent publications have claimed that mutations in ARVC-related genes are rather common among healthy populations [15]. Kapplinger et al [15] showed that a supposedly pathogenic variant can be found in 16% of a normal population. However, they described missense mutations that are less serious than splice site mutations. This emphasizes the importance of cautiousness when giving missense mutations clinical value, especially during the screening of first-degree relatives. It has also been suggested that compound and digenic heterozygosity may be of great importance with regard to the severity of the disease in individuals from the same family [16,17].
In the present study, the patients have been screened for mutations in eight genes that have relevance in the prediction of such disease, but undoubtedly future studies may determine new genes pertaining to ARVC that will add to our understanding of the disease. Marcus et al [18] reported that as many as 48% of people with ARVC showed at least two different mutations. In contrary to these reports, the patient population from the South-east region of Sweden exhibited 43 probands that fulfilled the ARVC-related Task Force Criteria, and none of the probands had a known pathogenic mutation in more than one of the eight genes investigated (data not shown). It has been described earlier that gender is of importance for the development of ARVC and arrhythmic symptoms as well as SCD [19]. The only death due to arrhythmia was observed in the 14-year-old boy belonging to family B, who was also the cousin of the athletic proband. The non-invasive evaluation did not reveal additional severe pathological findings in male mutation carriers than in female mutation carriers and thus did not lead to more positive diagnoses in any of the two sexes.
Earlier studies have indicated that the patients with one or more family members with a history of SCD were at an elevated risk [11]. However, markers predicting SCD in patients with ARVC have not been fully defined in large prospective studies [20,21] as such studies are hard to perform in case of rare diseases. Several subgroups of patients with ARVC have an increased risk for SCD or, in those with an ICD, appropriate device intervention. Such patients are of a lower age and with previous syncope [21]. Patients with two or more disease-causing mutations as well as patients with LV involvement [20,22-25] are also considered to be at a higher risk. In this study, three index cases and one family member with definite diagnosis had LV involvement.
In our study, all the mutation carriers were considered as “possible ARVC” based on the carriership alone. In most cases, “borderline ARVC” was based on ECG changes with repolarization or depolarization disturbances. Three of the first-degree relatives were diagnosed with “definite ARVC” with significant arrhythmias, which was confirmed from their ICD report.
The amount and intensity of physical activity in each individual in our group are unfortunately not known. This is a difficult area to investigate, and we could not determine the exact number of hours per week or the intensity of activities. In patient interviews, we found that two of our probands were regularly engaged in athletic activities before the diagnosis of ARVC. It has been shown earlier that athletes have a more structurally severe form of the disease, and this was also seen in an animal study where athletic heterozygous plakoglobin-deficient mice developed RV dysfunction and arrhythmia [25,26]. Human data also revealed that exercise is associated with a higher degree of disease expression and risk of arrhythmia [27-29]. It is possible that other genetic factors could also be relevant for ARVC. Future studies should quantify the amount of exercise in every potential ARVC patient and also consider providing strong advice against intense exercises in patients belonging to families with ARVC history.
Of the 41 tested family members, 19 (46%) were found to be mutation carriers. In a review of 37 families, 9.6% of initial clinically unaffected subjects developed echocardiographic abnormalities, and almost 50% had symptomatic ventricular arrhythmias during a mean follow-up of 8.5 years [30]. The prognosis of ARVC patients who experience ventricular tachycardia is uncertain. Patients with mild disease and non-sustained ventricular tachycardia appeared to have a relatively low risk of arrhythmic death. The long-term outcome was worse in patients with LV involvement due to arrhythmia and clinical heart failure. In patients with advanced disease, ARVC may be difficult to distinguish from dilated cardiomyopathy [28]. Nava et al [30] classified 151 of 365 family members as being affected with ARVC.
In our study, three family members fulfilled the diagnostic criteria for ARVC during the clinical screening. One of them was a middle-aged woman with no clinical symptoms, illustrating the fact that signs of disease may develop slowly, if at all. Another was a physically-active boy, who based on ECG changes and family history (brother with SCD) received an ICD. One month later, he had an appropriate shock therapy for fast ventricular tachycardia. This could suggest that age and physical activity may be of importance in this particular genetic mutation study.
In conclusion, this is the first study based on genetic cascade screening that demonstrates the variability in the clinical presentation among individuals with the PKP2 c.2146-1G>C genetic variant. We have shown that the vast majority of healthy family members carrying the mutation were classified as “possible ARVC” using the present revised Task Force Criteria of 2010, and it was based mainly on the mutation carriership alone. No definite genotype-phenotype correlations were found. However, age and physical activity may be of importance for those carrying this particular gene variant. Family members with borderline or definite diagnoses frequently had ECG disturbances but rarely showed any significant signs of arrhythmia. The clinical presentation was variable and included one case of potentially lethal arrhythmia, illustrating the unpredictable course of the disease. Our investigation supports the idea that family members carrying the PKP2 c.2146-1G>C mutation should be offered long-term follow-up since the clinical presentation is variable and frequently symptomless with an apparent risk for insidious SCD from arrhythmia.
Acknowledgements
We are thankful to the patients as the study would not have been possible without their invaluable assistance. We also thank Annelie Raschperger for genetic counseling and making contact with the families and Lennart Malmqvist and PärHedberg for their help with data collection. We are also grateful to Jan Engvall and Eva Nylander for their valuable discussions and Jon Jonasson for the excellent reviewing of the manuscript’s content as well as its language. This work was supported by the Medical Research Council of Southeast Sweden and Åke Wiberg Foundation. The funders had no role in the study design, data collection, analysis, and decision to publish or in the preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318:129–133. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 2.Azaouagh A, Churzidse S, Konorza T, Erbel R. Arrhytmogenic right ventricular cardiomyopathy/dysplasia: a review and update. Clin Res Cardiol. 2011;100:383–394. doi: 10.1007/s00392-011-0295-2. [DOI] [PubMed] [Google Scholar]
- 3.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31:806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aneq MA, Fluur C, Rehnberg M, Söderkvist P, Engvall J, Nylander E, Gunnarsson C. Novel plakophilin2 mutation: three-generation family with arrhythmogenic right ventricular cardiomyopathy. Scand Cardiovasc J. 2012;46:72–75. doi: 10.3109/14017431.2011.636068. [DOI] [PubMed] [Google Scholar]
- 5.Lindström L, Nylander E, Larsson H, Wranne B. Left ventricular involvement in arrhythmogenic right ventricular cardiomyopathy- a scintigraphic and echocardiographic study. Clin Physiol Funct Imaging. 2005;25:171–7. doi: 10.1111/j.1475-097X.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 6.Basso C, Czarnowska E, Della Barbera M, Bauce B, Beffagna G, Wlodarska EK, Pilichou K, Ramondo A, Lorenzon A, Wozniek O, Corrado D, Daliento L, Danieli GA, Valente M, Nava A, Thiene G, Rampazzo A. Ultrastructural evidence of intercalated disc remodelling in arrhythmogenic right ventricular cardiomyopathy: an electron microscopy investigation on endomyocardial biopsies. Eur Heart J. 2006;27:1847–1854. doi: 10.1093/eurheartj/ehl095. [DOI] [PubMed] [Google Scholar]
- 7.van Tintelen JP, Entius MM, Bhuiyan ZA, Jongbloed R, Wiesfeld AC, Wilde AA, van der Smagt J, Boven LG, Mannens MM, van Langen IM, Hofstra RM, Otterspoor LC, Doevendans PA, Rodriguez LM, van Gelder IC, Hauer RN. Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2006;113:1650–1658. doi: 10.1161/CIRCULATIONAHA.105.609719. [DOI] [PubMed] [Google Scholar]
- 8.Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, Lerman BB, Markowitz SM, Ellinor PT, MacRae CA, Peters S, Grossmann KS, Drenckhahn J, Michely B, Sasse-Klaassen S, Birchmeier W, Dietz R, Breithardt G, Schulze-Bahr E, Thierfelder L. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–1164. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 9.Antoniades L, Tsatsopoulou A, Anastasakis A, Syrris P, Asimaki A, Panagiotakos D, Zambartas C, Stefanadis C, McKenna WJ, Protonotarios N. Arrhythmogenic right ventricular cardiomyopathy caused by deletions in plakophilin-2 and plakoglobin (Naxos disease) in families from Greece and Cyprus: genotype-phenotype relations, diagnostic features and prognosis. Eur Heart J. 2006;27:2208–2216. doi: 10.1093/eurheartj/ehl184. [DOI] [PubMed] [Google Scholar]
- 10.Gréen A, Gréen H, Rehnberg M, Svensson A, Gunnarsson C, Jonasson J. Assessment of HaloPlex amplification for sequence capture and massively parallel sequencing of arrhythmogenic right ventricular cardiomyopathy associated genes. J Mol Diagn. 2015;17:31–42. doi: 10.1016/j.jmoldx.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Syrris P, Ward D, Asimaki A, Sen-Chowdhry S, Ebrahim HY, Evans A, Hitomi N, Norman M, Pantazis A, Shaw AL, Elliott PM, McKenna WJ. Clinical expression of plakophilin-2 mutations in familial arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:356–364. doi: 10.1161/CIRCULATIONAHA.105.561654. [DOI] [PubMed] [Google Scholar]
- 12.Watkins DA, Hendricks N, Shaboodien G, Mbele M, Parker M, Vezi BZ, Latib A, Chin A, Little F, Badri M, Moolman-Smook JC, Okreglicki A, Mayosi BM ARVC Registry of the Cardiac Arrhythmia Society of Southern Africa (CASSA) Clinical features, survival experience, and profile of plakophylin-2 gene mutations in participants of the arrhythmogenic right ventricular cardiomyopathy registry of South Africa. Heart Rhythm. 2009;6:S10–17. doi: 10.1016/j.hrthm.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 14.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;Chapter 7 doi: 10.1002/0471142905.hg0720s76. Unit7.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapplinger JD, Landstrom AP, Salisbury BA, Callis TE, Pollevick GD, Tester DJ, Cox MG, Bhuiyan Z, Bikker H, Wiesfeld AC, Hauer RN, van Tintelen JP, Jongbloed JD, Calkins H, Judge DP, Wilde AA, Ackerman MJ. Distinguishing arrhythmogenic right ventricular cardiomyopathy/dysplasia-associated mutations from background genetic noise. J Am Coll Cardiol. 2011;57:2317–2327. doi: 10.1016/j.jacc.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauce B, Nava A, Beffagna G, Basso C, Lorenzon A, Smaniotto G, De Bortoli M, Rigato I, Mazzotti E, Steriotis A, Marra MP, Towbin JA, Thiene G, Danieli GA, Rampazzo A. Multiple mutations in desmosomal proteins encoding genes in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2010;7:22–29. doi: 10.1016/j.hrthm.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 17.Rigato I, Bauce B, Rampazzo A, Zorzi A, Pilichou K, Mazzotti E, Migliore F, Marra MP, Lorenzon A, De Bortoli M, Calore M, Nava A, Daliento L, Gregori D, Iliceto S, Thiene G, Basso C, Corrado D. Compound and digenic heterozygosity predicts lifetime arrhythmic outcome and sudden cardiac death in desmosomal gene-related arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Genet. 2013;6:533–542. doi: 10.1161/CIRCGENETICS.113.000288. [DOI] [PubMed] [Google Scholar]
- 18.Marcus FI, Edson S, Towbin JA. Genetics of arrhythmogenic right ventricular cardiomyopathy: a practical guide for physicians. J Am Coll Cardiol. 2013;61:1945–1948. doi: 10.1016/j.jacc.2013.01.073. [DOI] [PubMed] [Google Scholar]
- 19.Bauce B, Frigo G, Marcus FI, Basso C, Rampazzo A, Maddalena F, Corrado D, Winnicki M, Daliento L, Rigato I, Steriotis A, Mazzotti E, Thiene G, Nava A. Comparison of clinical features of arrhytmogenic right ventrucular cardiomyopathy in men versus women. Am J Cardiol. 2008;102:1252–1257. doi: 10.1016/j.amjcard.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 20.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 21.Corrado D, Calkins H, Link MS, Leoni L, Favale S, Bevilacqua M, Basso C, Ward D, Boriani G, Ricci R, Piccini JP, Dalal D, Santini M, Buja G, Iliceto S, Estes NAr 3rd, Wichter T, McKenna WJ, Thiene G, Marcus FI. Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillation or sustained ventricular tachycardia. Circulation. 2010;122:1144–1152. doi: 10.1161/CIRCULATIONAHA.109.913871. [DOI] [PubMed] [Google Scholar]
- 22.Corrado D, Leoni L, Link MS, Della Bella P, Gaita F, Curnis A, Salerno JU, Igidbashian D, Raviele A, Disertori M, Zanotto G, Verlato R, Vergara G, Delise P, Turrini P, Basso C, Naccarella F, Maddalena F, Estes NA 3rd, Buja G, Thiene G. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108:3084–3091. doi: 10.1161/01.CIR.0000103130.33451.D2. [DOI] [PubMed] [Google Scholar]
- 23.Quarta G, Muir A, Pantazis A, Syrris P, Gehmlich K, Garcia-Pavia P, Ward D, Sen-Chowdhry S, Elliott PM, McKenna WJ. Familial evaluation in arrhythmogenic right ventricular cardiomyopathy: impact of genetics and revised task force criteria. Circulation. 2011;123:2701–2709. doi: 10.1161/CIRCULATIONAHA.110.976936. [DOI] [PubMed] [Google Scholar]
- 24.Le Guludec D, Gauthier H, Porcher R, Frank R, Daou D, Benelhadj S, Leenhardt A, Lavergne T, Faraggi M, Slama MS. Prognostic value of radionuclide angiography in patients with right ventricular arrhythmias. Circulation. 2001;103:1972–1976. doi: 10.1161/01.cir.103.15.1972. [DOI] [PubMed] [Google Scholar]
- 25.Cox MG, van der Zwaag PA, van der Werf C, van der Smagt JJ, Noorman M, Bhuiyan ZA, Wiesfeld AC, Volders PG, van Langen IM, Atsma DE, Dooijes D, van den Wijngaard A, Houweling AC, Jongbloed JD, Jordaens L, Cramer MJ, Doevendans PA, de Bakker JM, Wilde AA, van Tintelen JP, Hauer RN. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: pathogenic desmosome mutations in index-patients predict outcome of family screening: Dutch arrhythmogenic right ventricular dysplasia/cardiomyopathy genotype-phenotype follow-up study. Circulation. 2011;123:2690–2700. doi: 10.1161/CIRCULATIONAHA.110.988287. [DOI] [PubMed] [Google Scholar]
- 26.Kirchhof P, Fabritz L, Zwiener M, Witt H, Schäfers M, Zellerhoff S, Paul M, Athai T, Hiller KH, Baba HA, Breithardt G, Ruiz P, Wichter T, Levkau B. Age- and training-dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin-deficient mice. Circulation. 2006;114:1799–1806. doi: 10.1161/CIRCULATIONAHA.106.624502. [DOI] [PubMed] [Google Scholar]
- 27.James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol. 2013;62:1290–1297. doi: 10.1016/j.jacc.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbüchel H, Prior DL. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33:998–1006. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- 29.La Gerche A, Robberecht C, Kuiperi C, Nuyens D, Willems R, de Ravel T, Matthijs G, Heidbüchel H. Lower than expected desmosomal gene mutation prevalence in endurance athletes with complex ventricular arrhythmias of right ventricular origin. Heart. 2010;96:1268–1274. doi: 10.1136/hrt.2009.189621. [DOI] [PubMed] [Google Scholar]
- 30.Nava A, Bauce B, Basso C, Muriago M, Rampazzo A, Villanova C, Daliento L, Buja G, Corrado D, Danieli GA, Thiene G. Clinical profile and long-term follow-up of 37 families with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2000;36:2226–2233. doi: 10.1016/s0735-1097(00)00997-9. [DOI] [PubMed] [Google Scholar]