Abstract
Human organic anion transporter 1 (hOAT1) belongs to a family of organic anion transporters that play critical roles in the body disposition of clinically important drugs, including anti-viral therapeutics, anti-cancer drugs, antibiotics, antihypertensives, and anti-inflammatories. hOAT1 is abundantly expressed in the kidney and brain. In the current study, we examined the regulation of hOAT1 by serum- and glucocorticoid-inducible kinase 2 (sgk2) in the kidney COS-7 cells. We showed that sgk2 stimulated hOAT1 transport activity. Such stimulation mainly resulted from an increased cell surface expression of the transporter, kinetically revealed as an increased maximal transport velocity V max without significant change in substrate-binding affinity K m. We further showed that stimulation of hOAT1 activity by sgk2 was achieved by preventing hOAT1 degradation. Our co-immunoprecipitation experiment revealed that the effect of sgk2 on hOAT1 was through a direct interaction between these two proteins. In conclusion, our study demonstrated that sgk2 stimulates hOAT1 transport activity by enhancing the stability of the transporter. This study provides the insights into sgk2 regulation of hOAT1-mediated transport in normal physiology and disease.
Keywords: Organic anion transporter, drug transport, regulation, serum and glucocorticoid-inducible kinase
Introduction
The organic anion transporter (OAT) family mediates the body disposition of a diverse array of environmental toxins and clinically important drugs, including anti-HIV therapeutics, anti-tumor drugs, antibiotics, anti-hypertensives, and anti-inflammatories [1-5]. Therefore, understanding the regulation of these transporters has profound clinical significance.
Multiple OAT isoforms have been identified in distinct tissues and cell membranes. OAT1 is highly expressed at the basolateral membrane of the kidney proximal tubule cells and utilizes a tertiary transport mechanism to move organic anions across the basolateral membrane into the proximal tubule cells for subsequent exit across the apical membrane into the urine for elimination. Through this tertiary transport mechanism, Na+/K+-ATPase maintains an inwardly directed (blood-to-cell) Na+ gradient. The Na+ gradient then drives a sodium dicarboxylate cotransporter, sustaining an outwardly directed dicarboxylate gradient that is utilized by a dicarboxylate/organic anion exchanger, namely OAT, to move the organic anion substrates into the cell. This cascade of events indirectly links organic anion transport to metabolic energy and the Na+ gradient, allowing the entry of a negatively charged substrate against both its chemical concentration gradient and the electrical potential of the cell [1-5].
The serum- and glucocorticoid-inducible kinases (sgk) are involved in controlling diverse cellular processes including sodium homeostasis, osmoregulation, cell survival, and cell proliferation [6-11]. The sgk family of protein kinases has three isoforms: sgk1, sgk2 and sgk3. It has been shown that the expression, regulation, and role of sgk2 within the mammalian kidney are distinct from sgk1 and sgk3 [12]. Sgk1 and sgk3 are expressed in every tissue, whereas sgk2 seems to be present primarily in the liver, kidney, pancreas, and brain. Unlike sgk1 and sgk3, sgk2 expression in the kidney was not subjected to the regulation by aldosterone. Immunochemical characterization localized sgk1 protein to distal convoluted tubule, cortical and medullary collecting duct, whereas sgk2 protein is highly expressed in kidney proximal tubule cells, where it modulates the function of membrane proteins such as Na+/H+ exchanger [12]. Based on the distinct characteristics of sgk2, we investigated whether hOAT1, also highly expressed in kidney proximal tubule cells, is regulated by sgk2. We demonstrated a new regulatory mechanism that sgk2 modulates hOAT1 expression and function through controlling the stability of the transporter.
Materials and methods
Materials
COS-7 cells were purchased from American Type Culture Collection (Manassas, VA). [3H]-labeled p-aminohippuric acid (PAH) was purchased from PerkinElmer (Waltham, MA). Membrane-impermeable biotinylation reagent NHS-SS-biotin, streptavidin-agarose beads and protein G-agarose beads were purchased from Pierce (Rockford, IL). cDNAs for mouse sgk2 (wild-type sgk2 and constitutive active sgk2 (CA-sgk2)) were generously provided by Dr. Alan C. Pao from Department of Medicine, Stanford University (Stanford, CA). Mouse anti-myc antibody (9E10) was purchased from Roche (Indianapolis, IN). Rabbit anti-sgk2 antibody was purchased from Cell signaling (Danvers, MA). Mouse anti-β-actin was purchased from Santa Cruz (Santa Cruz, CA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture and transfection
Parental COS-7 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum at 37°C in 5% CO2. Transfection with plasmids was carried out for 48 hours using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Transfected cells were starved with serum free medium for 12 hours for further experiments.
Transport measurements
Cells were plated in 48-well plates. For each well, uptake solution was added. The uptake solution consisted of phosphate-buffered saline (PBS)/Ca2+/Mg2+ (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, 0.1 mM CaCl2, and 1 mM MgCl2, pH 7.3) and [3H] PAH (20 µM). At the times indicated, uptake process was stopped by aspirating the uptake solution and rapidly washing the cells with ice-cold PBS solution. The cells were then solubilized in 0.2 N NaOH, neutralized in 0.2 N HCl, and aliquotted for liquid scintillation counting.
Cell surface biotinylation
Cell surface expression level of hOAT1 was examined using the membrane-impermeable biotinylation reagent, NHS-SS-biotin. Cells were seeded in 6-well plates. Each well of cells was incubated with 1 ml of freshly made NHS-SS-biotin (0.5 mg/ml in PBS/CM) in two successive 20 min incubations on ice with very gentle shaking. After biotinylation, each well was briefly rinsed with 3 ml of PBS/CM containing 100 mM glycine then incubated with the same solution for 20 min on ice, to ensure complete quenching of the unreacted NHS-SS-biotin. The cells were then lysed on ice for 30 min in 400 ml of lysis buffer (10 mM Tris/HCl, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton X-100 with 1/100 protease inhibitor mixture). The cell lysates were cleared by centrifugation at 16,000 g at 4°C. 40 µl of streptavidin-agarose beads was then added to the supernatant to isolate cell membrane proteins. hOAT1 was detected in the pool of surface proteins by SDS-PAGE and immunoblotting using an anti-myc antibody 9E10.
Degradation assay
We followed the procedure previously established in our laboratory [13]. Cells, co-transfected with hOAT1 and control vector, or with hOAT1 and CA-sgk2, underwent biotinylation with 0.5 mg/ml sulfo-NHS-SS-biotin at 4°C. After biotinylation, each dish was rinsed with 2 ml PBS containing 100 mM glycine and then incubated with the same solution for 20 minutes on ice, to ensure complete quenching of the unreacted NHS-SS-biotin. The biotin-labeled cells were incubated in DMEM at 37°C. Cells were collected at 0, 2, 4, and 6 hours and lysed in lysis buffer with protease inhibitor cocktail. The cell lysates were cleared by centrifugation at 16,000 × g for 30 minutes at 4°C. 40 μl of streptavidin agarose beads were then added to the supernatant to isolate cell membrane proteins, followed by immunoblotting with anti-myc antibody.
Immunoprecipitation
Cells were lysed with lysis buffer (20 mM Tris/HCl, pH 7.5, 1% Triton X-100, 2 mM EDTA, and 25 mM NaF), freshly added with 1% of protease inhibitor cocktail. Cell lysates were precleared with protein G-agarose beads to reduce nonspecific binding at 4°C for 1.5 hours. Anti-myc antibody (1:100) was incubated with appropriate volume of protein G-agarose beads at 4°C for 1.5 hours. The precleared protein sample was then mixed with antibody-bound protein G-agarose beads and underwent end-over-end rotating at 4°C overnight. Proteins bound to the protein G-agarose beads were eluted with Urea buffer containing β-mecaptoethanol and analyzed by immunoblotting with indicated antibodies.
Electrophoresis and immunoblotting
Protein samples were resolved on 7.5% SDS-PAGE minigels and electroblotted on to polyvinylidene difluoride membranes. The blots were blocked for 1 hour with 5% nonfat dry milk in PBS-0.05% Tween 20, washed, and incubated overnight at 4°C with appropriate primary antibodies, followed by horseradish peroxidase-conjugated secondary antibodies. The signals were detected by SuperSignal West Dura Extended Duration Substrate kit (Pierce). Nonsaturating, immunoreactive protein bands were quantified by scanning densitometry with the FluorChem 8000 imaging system (Alpha Innotech Corp., San Leandro, CA).
Data analysis
Each experiment was repeated a minimum of three times. The statistical analysis was from multiple experiments. Statistical analysis was performed using Student’s paired t-tests. A p-value of < 0.05 was considered significant.
Results
Effect of sgk2 on hOAT1 transport activity
To explore the role of sgk2 in hOAT1 function, we co-transfected COS-7 cells with hOAT1 and wild type sgk2 (WT-sgk2), or with hOAT1 and its constitutive active form CA-sgk2. hOAT1-mediated uptake of [3H]-PAH was then measured. As shown in Figure 1, wild type sgk2 stimulated ~42% increase in the uptake as compared to that in control cells; and CA-sgk2 significantly augmented the effect of sgk2, resulting in an additional ~85% increase in the uptake. To examine the mechanism of sgk2-induced stimulation of hOAT1 activity, we determined hOAT1-mediated uptake of [3H]-PAH at different substrate concentrations. An Eadie-Hofstee analysis of the derived data (Figure 2) showed that the transfection of CA-sgk2 resulted in an increase in maximal transport velocity Vmax of hOAT1 (208.32 ± 19.48 pmol·mg-1·3 min-1 with control cells and 328.20 ± 22.30 pmol·mg-1·3 min-1 with cells transfected with CA-sgk2) with no significant change in the substrate-binding affinity Km of the transporter (87.88 ± 5.40 µM with control cells and 86.78 ± 6.46 µM with cells transfected with CA-sgk2).
Figure 1.
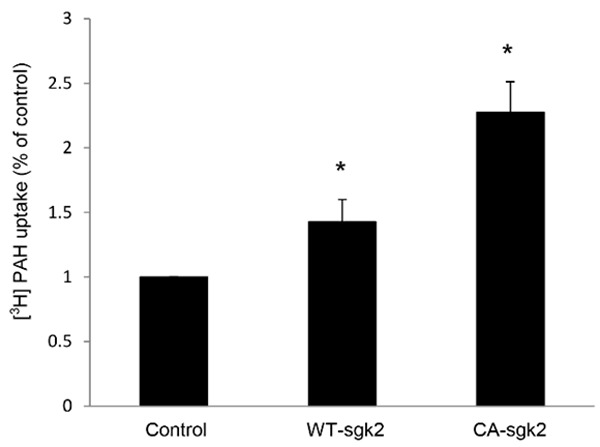
Effect of sgk2 on hOAT1 transport activity. COS-7 cells were co-transfected with hOAT1 and control vector, or with hOAT1 and wild type sgk2 (WT-sgk2), or with hOAT1 and the constitutive active form of sgk2 (CA-sgk2). 3-min uptake of [3H]-PAH (20 µM) was then measured. Uptake activity was expressed as a percentage of the uptake measured in control cells. The data represent uptake into hOAT1-transfected cells minus uptake into mock cells (parental COS-7 cells). Values are mean ± S.E. (n = 3). *P < 0.05.
Figure 2.
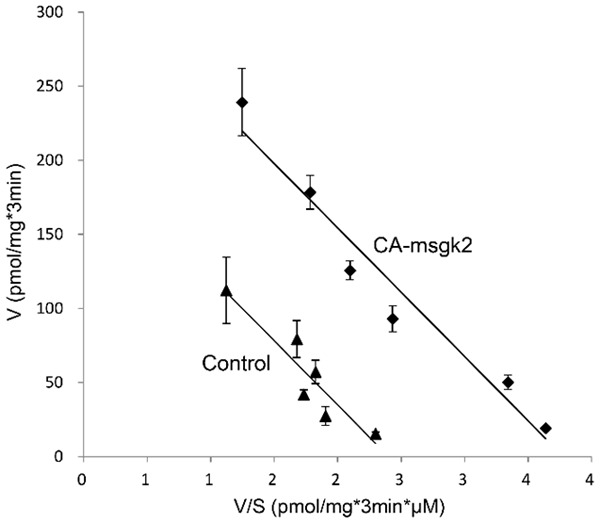
Effect of sgk2 on the kinetics of hOAT1-mediated PAH transport. COS-7 cells were co-transfected with hOAT1 and the constitutive active form of sgk2 (CA-sgk2), or with hOAT1 and control vector. Initial uptake (3 min) of [3H] PAH was measured at the concentration of 5-200 µM. The data represent uptake into hOAT1-transfected cells minus uptake into mock cells (parental COS-7 cells). Values are means ± S.E. (n = 3). V, velocity; S, substrate concentration.
Effect of sgk2 on hOAT1 expression
An increase in maximal transport velocity V max of hOAT1 shown above could be affected by either an increased number of the transporter at the cell surface or an increased transporter turnover rate (the rate at which membrane proteins transport their substrates across the plasma membrane per unit time [14]). We performed experiments that differentiated between these possibilities by measuring transporter expression both at the cell surface and in the total cell lysates. We showed that overexpression of CA-sgk2 resulted in an increase in hOAT1 expression both at cell surface (Figure 3A) and in total cell lysate (Figure 3C). Such a change in hOAT1 expression was not due to the general perturbation of cellular proteins as the expression of the house-keeping protein β-actin was not affected under these conditions (Figure 3E).
Figure 3.
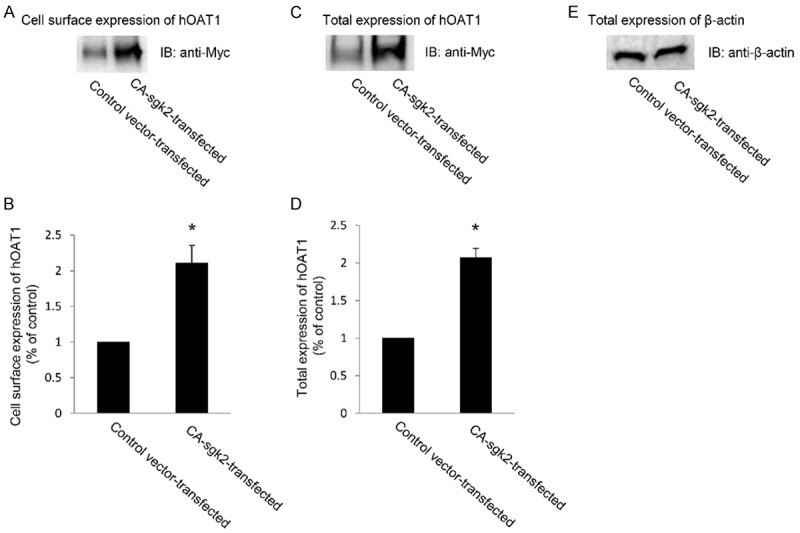
Effect of sgk2 on hOAT1 expression. (A) Cell surface expression of hOAT1. COS-7 cells were co-transfected with hOAT1 and control vector or with hOAT1 and the constitutive active form of sgk2 (CA-sgk2). Transfected cells were labeled with membrane-impermeable biotin. Biotinylated/cell surface proteins were separated with streptavidin beads, followed by immunoblotting (IB) with an anti-myc antibody. (B) Densitometry plot of results from (A) as well as from other repeat experiments. The values are mean ± S.E. (n = 3). *P < 0.05. (C) Total cell expression of hOAT1. COS-7 cells were co-transfected with hOAT1 and control vector or with hOAT1 and the constitutive active form of sgk2 (CA-sgk2). Transfected cells were lysed, followed by immunoblotting (IB) with an anti-myc antibody. (D) Densitometry plot of results from (C) as well as from other repeat experiments. The values are mean ± S.E. (n = 3). *P < 0.05. (E) Total cell expression of β-actin. COS-7 cells were co-transfected with hOAT1 and control vector or with hOAT1 and the constitutive active form of sgk2 (CA-sgk2). Transfected cells were lysed, followed by immunoblotting (IB) with an anti-β-actin antibody.
Effect of sgk2 on hOAT1 stability
Sgk2-induced increase in hOAT1 expression may reflect an increased stability of the transporter. In this experiment, we examined such a possibility by measuring the degradation rate of cell surface hOAT1 in the presence and absence of CA-sgk2. COS-7 cells were co-transfected with hOAT1 and control vector or with hOAT1 and CA-sgk2 for 48 hours. Cell membrane proteins were then biotinylated with membrane impermeable biotinylation reagent sulfo-NHS-SS-biotin. Biotin-labeled cells were lysed at 2-, 4-, and 6-hour time points after the biotinylation, and cell surface proteins were isolated using streptavidin-agarose beads, followed by immunoblotting with anti-myc antibody (myc was tagged to hOAT1). Our results (Figure 4A and 4B) showed that the degradation rate of cell surface hOAT1 decreased significantly in the presence of CA-sgk2 as compared to that in control cells, suggesting that CA-sgk2 enhanced the stability of hOAT1. Furthermore, both CA-sgk2 (Figure 4C, top panel) and house-keeping protein/cellular protein marker β-actin (Figure 4C, bottom panel) were equally expressed in all samples tested, indicating that the change in the degradation rate was not due to the differences in the amount of CA-sgk2 transfected or due to general perturbation of the cellular proteins.
Figure 4.
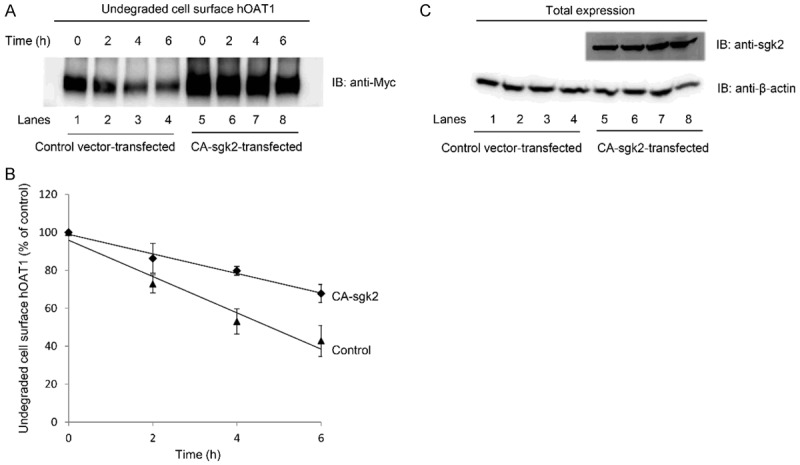
Effect of sgk2 on the degradation of cell surface hOAT1. (A) COS-7 cells were co-transfected with hOAT1 and control vector or with hOAT1 and the constitutive active form of sgk2 (CA-sgk2). The degradation of cell surface hOAT1 was then analyzed as described in “Materials and Methods” section followed by immunoblotting (IB) using anti-myc antibody. (B) Densitometry plot of results from (A) as well as from other repeat experiments. The amount of undegraded cell surface hOAT1 was expressed as % of total initial cell surface hOAT1 pool. Values are mean ± S.E. (n = 3). (C) Total expression of sgk2 and house-keeping protein β-actin. COS-7 cells were co-transfected with hOAT1 and control vector or with hOAT1 and the constitutive active form of sgk2 (CA-sgk2). Transfected cells were lysed, followed by immunoblotting (IB) with anti-sgk2 antibody (top panel) or anti-β-actin antibody (bottom panel).
Interaction of sgk2 with hOAT1
We assessed whether there was an association between sgk2 and hOAT1 through co-immunoprecipitation assay. hOAT1-expressing cells were transfected with control vector or with CA-sgk2. hOAT1 was then immunoprecipitated with anti-myc antibody (myc was tagged to hOAT1) or with control IgG (as negative control), followed by immunoblotting with anti-sgk2 antibody. As shown in Figure 5A, significant amount of CA-sgk2 co-immunoprecipitated with hOAT1 in cells transfected with CA-sgk2 (lane 2) as compared to that in cells transfected with control vector (lane 1), suggesting a direct association between hOAT1 and CA-sgk2. No amount of CA-sgk2 was detected when hOAT1 was immunoprecipitated with negative control IgG instead of hOAT1-specific anti-myc antibody (lane 3). Furthermore, the differences in the amount of hOAT1-associated CA-sgk2 were not due to the differences in the amount of hOAT1 immunoprecipitated as equal hOAT1 abundance could be observed when the same immunoblot was reprobed with anti-myc antibody (Figure 5B, top panel, lanes 1 and 2). The differences in the amount of CA-sgk2 detected in the co-immunoprecipitation assay (Figure 5A) were also not due to differences in transfection efficiency of CA-sgk2 or due to general perturbation of cellular proteins as a similar expression of CA-sgk2 (Figure 5C, top panel) and a similar expression of the cellular protein marker β-actin (Figure 5C, bottom panel) were shown. These data suggest that sgk2 regulates hOAT1 expression and activity through direct interaction with the transporter.
Figure 5.
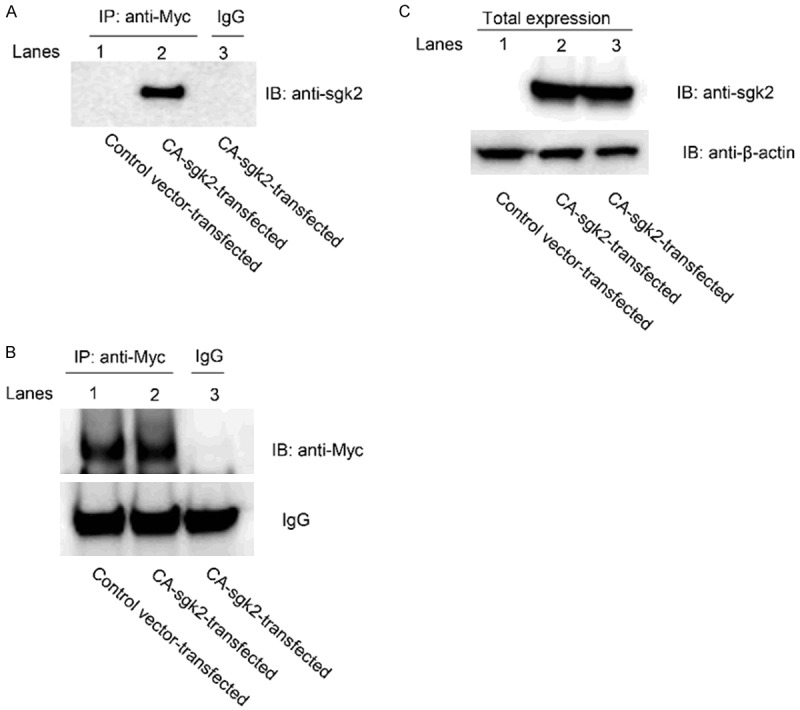
The Interaction of sgk2 with hOAT1. (A) COS-7 cells were co-transfected with hOAT1 and control vector or with hOAT1 and the constitutive active form of sgk2 (CA-sgk2). Transfected cells were then lysed, and hOAT1 was immunoprecipitated (IP) with anti-myc antibody or with control IgG (as negative control), followed by immunoblotting (IB) with anti-sgk2 antibody. (B) Top panel: The same immunoblot from (A) was reprobed by anti-myc antibody to determine the amount of hOAT1 immunoprecipitated. Bottom panel: IgG signals. (C) Total expression of CA-sgk2 and β-actin. COS-7 cells were co-transfected with hOAT1 and control vector or with hOAT1 and CA-sgk2. Transfected cells were then lysed. The expression of CA-sgk2 and β-actin was detected by anti-sgk2 antibody (top panel) and anti-β-actin antibody (bottom panel), respectively.
Discussion
Active organic anion/drug transport mediated by organic anion transporters (OATs) is a major determinant of the effects of therapeutics and toxic chemicals. Therefore, understanding the molecular and cellular mechanisms underlying OAT regulation is of clinical and pharmacological importance. The present study revealed a new regulatory mechanism for hOAT1-mediated organic anion/drug transport, namely, sgk2 regulated hOAT1 transport activity through modulating the stability of the transporter.
Our current studies were carried out in a heterologous cell system - COS-7 cells. COS-7 cells offer several useful advantages for the study of the cloned organic anion transporter. (i) These cells are kidney origin. Studies in these cells have led to the understanding of other renal transport processes, including organic cation transport [15,16]. (ii) This cell line does not express endogenous OATs. Therefore, expression of hOAT1 in these cells will allow us to dissect the transport characteristics of hOAT1 without the interference of other organic anion transporters. (iii) The multiple signaling pathways in these cells provide a good experimental model system for delineating the underlying regulatory mechanisms of many transport processes [17,18]. (iv) The previously published work showed that the transport characteristics of OATs in these cells were in a good agreement with that observed in other systems [19-23]. Our studies in COS-7 cells pave the way for the future work focusing on determining whether the same mechanisms are operative in native epithelia.
In our current study, we established that sgk2 stimulated hOAT1-mediated PAH uptake (Figure 1) as a result of an enhanced maximum transport velocity (V max) (Figure 2), an increased hOAT1 expression (Figure 3), and a decreased hOAT1 degradation rate (Figure 4). We cannot exclude the possibility that sgk2 also regulates the transcriptional level of hOAT1 in addition to its stability. However, since the hOAT1 expression vector for the current study does not contain the promoter region of hOAT1, the long-term regulation at the transcriptional level cannot be investigated in this study.
Sgks, like other protein kinases, exert their effects through phosphorylating their target substrates. Sgks (sgk1, sgk2, sgk3) preferentially phosphorylate serine or threonine residues within the RXRXXS/T motifs [24,25], which overlaps with the protein kinase A (PKA) consensus RXXS/T motifs [26]. hOAT1 does not bear SGK consensus motifs. Instead, it contains two PKA consensus motifs within its 3rd intracellular loop between the sixth and seventh trans-membrane domains (S276 and T334), which could possibly serve as the potential phosphorylation site/sites by sgk2. It is also possible that sgk2 modulates hOAT1 expression and function by phosphorylating an unconventional site(s) in hOAT1 sequence. On the other hand, it has been reported that several transporters and channels are modulated by sgk1, an isoform of sgk2, not directly through phosphorylating the transporters themselves but rather indirectly through phosphorylating intermediate partners such as the E3 ubiquitin ligase Nedd4-2 [27-29]. The work aiming at identifying the sgk2-specific phosphorylation sites in hOAT1 and/or at identifying the intermediate partner is currently being carried out in our lab.
In conclusion, this is the first demonstration of a new regulatory mechanism of hOAT1 transport activity: sgk2 stimulates hOAT1 transport activity by enhancing the stability of the transporter. Our study provides important insights into the understanding of the molecular and cellular basis of hOAT1 regulation in normal physiology and diseases.
Acknowledgements
This work was supported by grants (to Dr. Guofeng You) from the National Institute of General Medical Sciences (R01-GM079123 and R01-GM097000).
Disclosure of conflict of interest
None.
References
- 1.Ahn SY, Nigam SK. Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol. 2009;76:481–490. doi: 10.1124/mol.109.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dantzler WH, Wright SH. The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules. Biochim Biophys Acta. 2003;1618:185–193. doi: 10.1016/j.bbamem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Srimaroeng C, Perry JL, Pritchard JB. Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica. 2008;38:889–935. doi: 10.1080/00498250801927435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos. 2009;31:1–71. doi: 10.1002/bdd.693. [DOI] [PubMed] [Google Scholar]
- 5.You G. Structure, function, and regulation of renal organic anion transporters. Med Res Rev. 2002;22:602–616. doi: 10.1002/med.10019. [DOI] [PubMed] [Google Scholar]
- 6.Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci U S A. 1999;96:2514–2519. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naray-Fejes-Toth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Toth G. sgk is an aldosterone-induced kinase in the renal collecting duct. Effects on epithelial na+ channels. J Biol Chem. 1999;274:16973–16978. doi: 10.1074/jbc.274.24.16973. [DOI] [PubMed] [Google Scholar]
- 8.Rozansky DJ, Wang J, Doan N, Purdy T, Faulk T, Bhargava A, Dawson K, Pearce D. Hypotonic induction of SGK1 and Na+ transport in A6 cells. Am J Physiol Renal Physiol. 2002;283:F105–113. doi: 10.1152/ajprenal.00176.2001. [DOI] [PubMed] [Google Scholar]
- 9.Waldegger S, Barth P, Forrest JN Jr, Greger R, Lang F. Cloning of sgk serine-threonine protein kinase from shark rectal gland - a gene induced by hypertonicity and secretagogues. Pflugers Arch. 1998;436:575–580. doi: 10.1007/s004240050674. [DOI] [PubMed] [Google Scholar]
- 10.Leong ML, Maiyar AC, Kim B, O’Keeffe BA, Firestone GL. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J Biol Chem. 2003;278:5871–5882. doi: 10.1074/jbc.M211649200. [DOI] [PubMed] [Google Scholar]
- 11.Buse P, Tran SH, Luther E, Phu PT, Aponte GW, Firestone GL. Cell cycle and hormonal control of nuclear-cytoplasmic localization of the serum- and glucocorticoid-inducible protein kinase, Sgk, in mammary tumor cells. A novel convergence point of anti-proliferative and proliferative cell signaling pathways. J Biol Chem. 1999;274:7253–7263. doi: 10.1074/jbc.274.11.7253. [DOI] [PubMed] [Google Scholar]
- 12.Pao AC, Bhargava A, Di Sole F, Quigley R, Shao X, Wang J, Thomas S, Zhang J, Shi M, Funder JW, Moe OW, Pearce D. Expression and role of serum and glucocorticoid-regulated kinase 2 in the regulation of Na+/H+ exchanger 3 in the mammalian kidney. Am J Physiol Renal Physiol. 2010;299:F1496–1506. doi: 10.1152/ajprenal.00075.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Suh W, Pan Z, You G. Short-term and long-term effects of protein kinase C on the trafficking and stability of human organic anion transporter 3. Int J Biochem Mol Biol. 2012;3:242–249. [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzales AL, Lee W, Spencer SR, Oropeza RA, Chapman JV, Ku JY, Eskandari S. Turnover rate of the gamma-aminobutyric acid transporter GAT1. J Membr Biol. 2007;220:33–51. doi: 10.1007/s00232-007-9073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Evans KK, Wright SH. Molecular cloning of rabbit organic cation transporter rbOCT2 and functional comparisons with rbOCT1. Am J Physiol Renal Physiol. 2002;283:F124–133. doi: 10.1152/ajprenal.00367.2001. [DOI] [PubMed] [Google Scholar]
- 16.Nagai K, Takikawa O, Kawakami N, Fukao M, Soma T, Oda A, Nishiya T, Hayashi M, Lu L, Nakano M, Kajita E, Fujita H, Miwa S. Cloning and functional characterization of a novel up-regulator, cartregulin, of carnitine transporter, OCTN2. Arch Biochem Biophys. 2006;452:29–37. doi: 10.1016/j.abb.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Kazanietz MG, Caloca MJ, Aizman O, Nowicki S. Phosphorylation of the catalytic subunit of rat renal Na+, K+-ATPase by classical PKC isoforms. Arch Biochem Biophys. 2001;388:74–80. doi: 10.1006/abbi.2000.2264. [DOI] [PubMed] [Google Scholar]
- 18.Cobb BR, Ruiz F, King CM, Fortenberry J, Greer H, Kovacs T, Sorscher EJ, Clancy JP. A(2) adenosine receptors regulate CFTR through PKA and PLA(2) Am J Physiol Lung Cell Mol Physiol. 2002;282:L12–25. doi: 10.1152/ajplung.2002.282.1.L12. [DOI] [PubMed] [Google Scholar]
- 19.Wolff NA, Thies K, Kuhnke N, Reid G, Friedrich B, Lang F, Burckhardt G. Protein kinase C activation downregulates human organic anion transporter 1-mediated transport through carrier internalization. J Am Soc Nephrol. 2003;14:1959–1968. doi: 10.1097/01.asn.0000079040.55124.25. [DOI] [PubMed] [Google Scholar]
- 20.Zhou F, Hong M, You G. Regulation of human organic anion transporter 4 by progesterone and protein kinase C in human placental BeWo cells. Am J Physiol Endocrinol Metab. 2007;293:E57–61. doi: 10.1152/ajpendo.00696.2006. [DOI] [PubMed] [Google Scholar]
- 21.Zhou F, Illsley NP, You G. Functional characterization of a human organic anion transporter hOAT4 in placental BeWo cells. Eur J Pharm Sci. 2006;27:518–523. doi: 10.1016/j.ejps.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Shuprisha A, Lynch RM, Wright SH, Dantzler WH. PKC regulation of organic anion secretion in perfused S2 segments of rabbit proximal tubules. Am J Physiol Renal Physiol. 2000;278:F104–109. doi: 10.1152/ajprenal.2000.278.1.F104. [DOI] [PubMed] [Google Scholar]
- 23.Gekle M, Mildenberger S, Sauvant C, Bednarczyk D, Wright SH, Dantzler WH. Inhibition of initial transport rate of basolateral organic anion carrier in renal PT by BK and phenylephrine. Am J Physiol. 1999;277:F251–256. doi: 10.1152/ajprenal.1999.277.2.F251. [DOI] [PubMed] [Google Scholar]
- 24.Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J. 1999;18:3024–3033. doi: 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J. 1999;344:189–197. [PMC free article] [PubMed] [Google Scholar]
- 26.Snyder PM, Olson DR, Kabra R, Zhou R, Steines JC. cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na(+) channel through convergent phosphorylation of Nedd4-2. J Biol Chem. 2004;279:45753–45758. doi: 10.1074/jbc.M407858200. [DOI] [PubMed] [Google Scholar]
- 27.Lamothe SM, Zhang S. The serum- and glucocorticoid-inducible kinases SGK1 and SGK3 regulate hERG channel expression via ubiquitin ligase Nedd4-2 and GTPase Rab11. J Biol Chem. 2013;288:15075–15084. doi: 10.1074/jbc.M113.453670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naray-Fejes-Toth A, Snyder PM, Fejes-Toth G. The kidney-specific WNK1 isoform is induced by aldosterone and stimulates epithelial sodium channel-mediated Na+ transport. Proc Natl Acad Sci U S A. 2004;101:17434–17439. doi: 10.1073/pnas.0408146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boehmer C, Henke G, Schniepp R, Palmada M, Rothstein JD, Broer S, Lang F. Regulation of the glutamate transporter EAAT1 by the ubiquitin ligase Nedd4-2 and the serum and glucocorticoid-inducible kinase isoforms SGK1/3 and protein kinase B. J Neurochem. 2003;86:1181–1188. doi: 10.1046/j.1471-4159.2003.01937.x. [DOI] [PubMed] [Google Scholar]


