Abstract
HIV infection can cause direct and indirect damage to the brain and is consistently associated with neurocognitive disorders, including impairments in decision‐making capacities. The tendency to devalue rewards that are delayed (temporal discounting) is relevant to a range of health risk behaviors. Making choices about delayed rewards engages the executive control network of the brain, which has been found to be affected by HIV. In this case–control study of 18 HIV‐positive and 17 HIV‐negative adults, we examined the effects of HIV on brain activation during a temporal discounting task. Functional MRI (fMRI) data were collected while participants made choices between smaller, sooner rewards and larger, delayed rewards. Choices were individualized based on participants' unique discount functions, so each participant experienced hard (similarly valued), easy (disparately valued), and control choices. fMRI data were analyzed using a mixed‐effects model to identify group‐related differences associated with choice difficulty. While there was no difference between groups in behavioral performance, the HIV‐positive group demonstrated significantly larger increases in activation within left parietal regions and bilateral prefrontal regions during easy trials and within the right prefrontal cortex and anterior cingulate during hard trials. Increasing activation within the prefrontal regions was associated with lower nadir CD4 cell count and risk‐taking propensity. These results support the hypothesis that HIV infection can alter brain functioning in regions that support decision making, providing further evidence for HIV‐associated compensatory activation within fronto‐parietal cortices. A history of immunosuppression may contribute to these brain changes. Hum Brain Mapp 37:2455–2467, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: functional magnetic resonance imaging, decision making, temporal discounting, executive function, HIV
Abbreviations
- DOF
Degrees of freedom
- fMRI
Functional MRI
- GDS
Global deficit score
- ICT
Intertemporal Choice Task
- MCQ
Monetary Choice Questionnaire
- ROI
Region‐of‐interest
INTRODUCTION
Early in the course of infection, HIV can infiltrate the central nervous system, causing direct and indirect damage to the brain [Valcour et al., 2011]. The HIV virus can trigger axonal degeneration and neuronal damage by shedding neurotoxic viral proteins, such as GP120 and Tat [Mocchetti et al., 2014], and can initiate neuroinflammatory cascades involving infected monocytes and microglia that lead to pathogenesis [Burdo et al., 2013]. The concentration of HIV virions is particularly high in the frontal and temporal lobes, basal ganglia, and brainstem [Nath, 2015]. HIV neuropathology is characterized by atrophy of subcortical nuclei and decreased integrity of white matter tracts within the corpus callosum, cingulum, and frontal and parietal lobes [Archibald et al., 2004; Chen et al., 2009; Cloak et al., 2004; Cohen et al., 2010; Gongvatana et al., 2009; Jahanshad et al., 2012; Pfefferbaum et al., 2009; Zhu et al., 2013]. These neuronal changes have been linked to HIV‐associated neurocognitive impairment [Chang et al., 2008a; Chen et al., 2009; Gongvatana et al., 2009; Zhu et al., 2013]. Even in the era of combination antiretroviral therapy, HIV‐associated neurocognitive disorders are common [Cysique et al., 2014; Heaton et al., 2010; Heaton et al., 2011; Simioni et al., 2010], including impairments in decision‐making capacities [Hardy et al., 2006; Iudicello et al., 2013; Martin et al., 2013; Thames et al., 2012; Vassileva et al., 2013].
A core decision‐making process relevant to everyday behaviors is temporal discounting, which describes individuals' tendency to devalue a reward as a function of the delay until its receipt [Rachlin and Green, 1972]. An exaggerated preference for immediate rewards has been associated with a range of health risk behaviors, such as substance abuse, problem gambling, and sexual risk taking [Appelhans et al., 2011; Brevers et al., 2012; Johnson et al., 2015; Jones and Sullivan, 2015]. Thus, excessive discounting may be a trans‐disease process [Bickel et al., 2012], which is also specifically relevant for HIV and its co‐morbid disorders. Functional MRI (fMRI) research has implicated lateral and medial prefrontal cortices, ventral striatum, posterior cingulate, and insula in reward valuation and choice during temporal discounting [Kable and Glimcher, 2007; McClure et al., 2004; Tanaka et al., 2004]. Regions of the executive control network, including the lateral prefrontal, posterior parietal, and anterior cingulate cortices, are increasingly recruited during hard choices (in which the subjective values of immediate and delayed rewards are similar) compared to easy choices (in which the values between immediate and delayed rewards are highly discrepant) [Hoffman et al., 2008; Meade et al., 2011; Monterosso et al., 2007].
A 2014 meta‐analysis of fMRI studies concluded that HIV has particular effects upon the executive control network across a variety of tasks [Plessis et al., 2014]. In these studies, HIV‐infected participants showed larger increases in activation compared to HIV‐negative participants [Melrose et al., 2008; Schweinsburg et al., 2012], and some studies also demonstrate greater activation with increased attentional load [Chang et al., 2001; Chang et al., 2004, 2008b). Moreover, these load‐dependent differences in activation increased over 1 year in HIV‐positive compared to HIV‐negative participants [Ernst et al., 2009]. The studies in this meta‐analysis focused on attention, memory, motor functioning, and semantic and spatial processing. Since then, one published study has examined the effects of HIV on neural processing during a risky decision‐making task [Connolly et al., 2014]. HIV‐positive participants showed increased activation compared to HIV‐negative participants in the basal ganglia, anterior cingulate, dorsolateral prefrontal cortex, and insula during risky choices, but reduced activation in the anterior cingulate and dorsolateral prefrontal cortices during safe choices [Connolly et al., 2014]. This pattern of hyperactivity within task‐relevant regions has been interpreted as a compensatory mechanism for maintaining cognitive performance in the context of decreased efficiency due to neural injury or healthy aging [Barulli and Stern, 2013; Cabeza and Dennis, 2012; Stern, 2002], and it has been applied to HIV‐associated increases in brain activation [Chang et al., 2004; Connolly et al., 2014; Ernst et al., 2009; Melrose et al., 2008].
To further investigate the theory of compensation, the present study examined the effects of HIV on brain activation during a temporal discounting task. To assess the effects of increasing cognitive load, we chose a task that included easy (disparately valued) and hard (similarly valued) choices. Given prior evidence that HIV is associated with altered functioning in frontal, parietal, and striatal regions, we hypothesized the following differences in HIV‐positive compared to HIV‐negative participants: (1) during easy choices, greater activation within the prefrontal cortex and striatum (regions implicated in reward valuation and choice); and (2) during hard choices, greater activation within the prefrontal cortex and striatum, as well as additional activation within the anterior cingulate and posterior parietal cortices (regions of the executive control system).
METHOD
Participants
This study enrolled HIV‐positive and HIV‐negative adults aged 18‐55 years who were fluent and literate in English. For individuals with documented HIV infection, HIV status was verified by medical record review. For others, an OraQuick© rapid HIV test was conducted to verify HIV‐negative status. Exclusion criteria were: <9th grade education; severe learning disability; serious neurological disorders; acute opportunistic brain infections or a history of such infections without return to normal cognition; severe head trauma with loss of consciousness >30 minutes and persistent functional decline; severe mental illness (psychotic or mood disorders that caused serious distress and persistent interference in social or occupational functioning, such as schizophrenia or bipolar I); current use of antipsychotic or mood stabilizing medications; any substance use in the past month or history of regular substance use, with the exception of alcohol, marijuana, or nicotine; current alcohol or marijuana use disorder; MRI contraindications; and impaired mental status.
Procedures
Participants were recruited via advertisements in local newspapers, websites, community‐based organizations, and infectious diseases clinics. After completing a telephone screen, potentially eligible individuals were invited for an in‐person screening. Eligible participants returned on another day for an assessment that included an MRI scan. All procedures were approved by the institutional review boards at Duke University Health System and University of North Carolina at Chapel Hill.
Measures
Screening
Participants completed clinical interviews and a computerized survey and provided a urine sample for drug and pregnancy testing. The Wechsler Test of Adult Reading was used to estimate premorbid verbal IQ [Wechsler, 2001]. The Mini International Neuropsychiatric Interview identified serious psychiatric disorders, including bipolar and psychotic disorders [Sheehan et al., 1998]. Module E of the Structured Clinical Interview for DSM‐IV‐TR identified current and past substance use disorders [First et al., 1996]. The Addiction Severity Index‐Lite assessed lifetime substance use and associated impairments, as well as socio‐economic factors (e.g., past month income) [McLellan et al., 1992]. The timeline follow‐back assessed frequency of substance use in the past 30 days [Robinson et al., 2014; Sobell and Sobell, 1996]. The computerized survey assessed demographics and smoking history. Current smokers also completed the Fagerstrom Test for Nicotine Dependence [Heatherton et al., 1991]. Participants provided a release for their medical records, which were used to determine HIV and hepatitis C status and, if applicable, HIV disease indicators (e.g., CD4 cell counts, opportunistic infections) and treatment. For historical indicators of HIV disease, self‐reported interview data was used to supplement incomplete medical record data for four participants who had been diagnosed with HIV for many years and had since switched clinics (one case for years since diagnosis, one case for nadir CD4 cell count, and two cases for AIDS diagnosis).
Assessment
Participants repeated the timeline follow‐back for substance use in the 30 days prior to the MRI and the Barratt Impulsivity Scale for trait impulsivity [Patton et al., 1995]. A neuropsychological testing battery assessed functioning in seven cognitive domains, as described previously [Meade et al., 2015]. After T‐scoring using up‐to‐date published norms, a global deficit score (GDS) was computed, with scores ranging from 0 (no impairment) to 5 (severe impairment) [Carey et al., 2004]. Finally, participants completed the Balloon Analogue Risk Task, a risk‐taking measure that involves inflating 30 virtual balloons that pop after an unpredictable number of pumps [Lejuez et al., 2002]. Risk‐taking propensity is defined as the average pumps on unpopped balloons.
fMRI Task
The Intertemporal Choice Task (ICT) is a temporal discounting task that was designed to probe executive control during fMRI [Clewett et al., 2014; Meade et al., 2011; Monterosso et al., 2007]. It consists of three types of monetary choices characterized by degree of difficulty: “hard,” “easy,” and “control”. Choices are individualized based on participants' unique discount rate (k‐value), which were determined prior to the scan using a modified Monetary Choice Questionnaire (MCQ) [Kirby et al., 1999]. During the MCQ, participants made 38 choices between immediate rewards ($3‐$53) and delayed rewards ($10‐$55) delayed 1–30 days. K‐values associated with each trial were computed using the hyperbolic discounting equation: V immediate= V delayed/(1 + kD), where V is reward value in dollars and D is delay in days. Possible k‐values associated with each of these trials ranged from 0.001 to 3.108, with higher values indicating greater discounting [Kirby et al., 1999]. Then, k‐values for each participant were determined based on their “indifference point,” which is inferred by identifying the pair of trials where choices shifted from the delayed to the immediate options when trials were ordered in ascending k‐values [Kirby, 2000]. The geometric midpoint between the k‐values associated with those two trials was computed to estimate the participant's k‐value.
Figure 1 illustrates the ICT. The delayed options (ranging $10–55 with 1–30 day delays) remained constant across participants. The sooner reward (available in 0–6 days) was calculated for each participant using the discounting equation. For hard choices, participants' k‐value was used, resulting in sooner values that were subjectively similar to the delayed value. For easy choices, the k‐value was multiplied by 10 or 0.1, resulting in sooner values that were subjectively much less or much more valuable than the delayed choice, respectively. For control choices, the sooner reward was $0 (in which case the delayed option was preferable) or equivalent to the delayed reward (in which case the sooner option was preferable). The ICT was programmed using MATLAB R2012a (MathWorks, Natick, MA) with PsychoPhysics Toolbox extensions [Brainard, 1997; Pelli, 1997]. Participants completed two runs of 60 trials each for a total of 120 trials (48 hard, 48 easy, and 24 control). The order in which the runs were administered was randomized across participants. The order of trials was randomized once and fixed across participants. Participants' choice on a randomly selected trial was honored (scaled to a maximum of $10) by adding the monetary reward to a gift card on the specified day.
Figure 1.
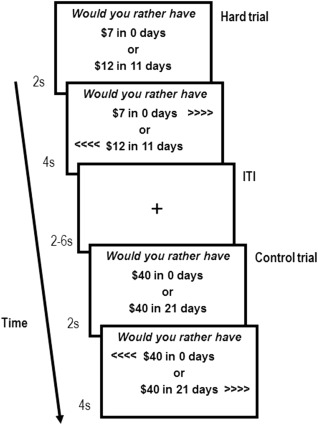
Illustration of the Intertemporal Choice Task (ICT). For each trial, there was a 2s presentation period, followed by a 4s response period. To signal the beginning of the response period, left and right arrows appeared on the screen to indicate which button on the response pad corresponded to each of the two options. The left/right location of the sooner and delayed options were randomized across trials within individuals to minimize lateralized motion preparation to the choice period. Once a choice was made, that choice was underlined. During the inter‐trial interval (ITI), which ranged 2‐8 s (M = 3.31), a cross hair appeared. The choices presented in the example hard trial are for an individual with a k‐value of 0.055.
MRI Data Acquisition
Functional and structural MRI data were acquired using a 3.0T GE scanner with an 8‐channel head coil. Whole‐brain BOLD images were collected using high‐throughput T2*‐weighted echo‐planar imaging with the following parameters: TR= 2,000 ms; TE = 27 ms; FOV= 24.0 cm; flip angle= 77°; in‐plane matrix size= 64 × 64; and slice thickness= 3.80 mm. This resulted in functional data from 39 axial slices with voxels of 3.75 mm × 3.75 mm × 3.80 mm. High‐resolution T1‐weighted structural images were acquired with the following parameters: TR= 8.096ms; TE= 3.18ms; FOV= 25.6cm; flip angle= 12°; in‐plane matrix size= 256 × 256; slice thickness= 1mm; and number of slices= 166.
Quality control
All 41 study completers responded to >90% of trials, but two HIV‐positive and three HIV‐negative participants were excluded due to >25% incorrect choices on control trials. Following visual inspection of MCFLIRT motion correction plots, one additional participant was excluded for head motion >3mm across a full run. Among the final sample of 35 participants, relative mean displacement was <0.03mm, with no group differences across runs [t(33) = 0.32, P = 0. 75].
fMRI Data Analysis
All imaging data were processed using FSL 5.0.1 (FMRIB Software Library, Oxford, UK) [Smith et al., 2004]. Functional images were corrected for motion (MCFLIRT) and slice timing, spatially smoothed using a 5‐mm FWHM Gaussian kernel, and highpass temporal filtered. Functional images were first aligned to the high‐resolution T1‐weighted main structural image [full search linear registration, 12 degrees of freedom (DOF)] and then to MNI152 standard space (normal search nonlinear registration, 12 DOF, 10mm warp resolution) [Jenkinson et al., 2002; Jenkinson and Smith, 2001]. Data from individual runs for each participant were subjected to a general linear model using FILM prewhitening, a temporal derivative, and temporal filtering. Binary regressors (EVs) were derived from choice type and convolved with a double‐gamma HRF function. From the resulting parameter estimates, three contrasts were examined: easy (easy > control), hard (hard > control) and hard versus easy (hard > easy).
For each participant, data from individual runs were combined using a fixed‐effects analysis [Beckmann et al., 2003]. These results were combined across participants using mixed‐effects (FLAME 1 + 2) models for each contrast to examine overall task activation by choice type. Next, group comparisons were examined using two‐sample unpaired t‐tests. All group‐level results were cluster thresholded at Z > 2.3 and P < 0.05, corrected over the entire brain using Gaussian random field theory [Worsley et al., 1992]. The Harvard‐Oxford cortical and subcortical structural atlases were used to identify neuroanatomical locations of activation peaks [Desikan et al., 2006; Frazier et al., 2005; Goldstein et al., 2007; Makris et al., 2006]. Finally, a functional region‐of‐interest (ROI) approach was used to identify the magnitude of group differences. Binarized masks were created for clusters with significant group differences for the easy and hard contrasts, and Featquery was used to extract mean percent signal change for each participant's data in standard space. These values were then compared across groups using t‐tests, and Cohen's d was computed as a measure of effect size. Within the HIV‐positive group, we examined the relationship between percent signal change within the ROIs and nadir and current CD4 cell count using Pearson correlation and current HIV viral suppression using t‐tests. A natural log transformation was used to improve the normality of the nadir CD4 cell count data. Within the full sample, we examined the relationship of percent signal change to trait impulsivity and risk‐taking propensity using Pearson correlation.
RESULTS
Participant Characteristics
The sample included 18 HIV‐positive and 17 HIV‐negative adults. Table 1 summarizes the sample characteristics and group comparisons. Overall, participants were 57% male and ranged in age from 23 to 54 years (M = 41.00). The majority were non‐Hispanic (94%) and Black (77%). Most had at least a high school diploma (89%), with a mean of 14.26 years of education. HIV‐positive participants had significantly lower monthly income than HIV‐negative participants (Median= $1,000 vs. $2,000, respectively). The groups had similar premorbid IQ, current neurocognitive functioning, and trait impulsivity. However, the HIV‐positive group had significantly greater risk‐taking propensity. A third (37%) of participants smoked cigarettes, but frequency of substance use in the past month was low for both alcohol (M = 1.27) and marijuana (M = 1.00).
Table 1.
Sample characteristics
| Full Sample | HIV‐positive | HIV‐negative | Statistic | |
|---|---|---|---|---|
| N = 35 | N = 18 | N = 17 | ||
| Demographics | ||||
| Male gender, n (%) | 20 (57%) | 11 (61%) | 9 (53%) | χ2(1) = 0.24 |
| Age in years, M (SD) | 41.00 (9.51) | 40.78 (9.27) | 41.24 (10.03) | t(33) = 0.14 |
| Race, n (%) | χ2(2) = 0.68 | |||
| Black | 27 (77%) | 13 (72%) | 14 (82%) | |
| White | 6 (17%) | 4 (22%) | 2 (12%) | |
| Mixed/Other | 2 (6%) | 1 (6%) | 1 (6%) | |
| Hispanic ethnicity, n (%) | 2 (6%) | 1 (6%) | 1 (6%) | FET = 1.00 |
| Education in years, M (SD) | 14.26 (2.08) | 14.17 (2.23) | 14.35 (1.97) | t(33) = 0.26 |
| Monthly income, Median (IQR) | $1,586 (1800) | $1,000 (1377) | $2,000 (2000) | U(1) = 87.50a |
| Hepatitis C diagnosis, n (%) | 1 (3%) | 1 (6%) | 0 (0%) | FET = 1.00 |
| Neurocognitive factors | ||||
| Premorbid verbal IQ, M (SD) | 91.63 (17.87) | 88.11 (18.05) | 95.35 (17.43) | t(33) = 1.21 |
| Neurocognitive impairment (GDS), M (SD) | 0.34 (0.30) | 0.39 (0.33) | 0.28 (0.26) | t(33) = 1.10 |
| Trait impulsivity, M (SD) | 56.06 (10.84) | 58.39 (12.17) | 53.59 (8.92) | t(33) = 1.32 |
| Risk‐taking propensity, M (SD) | 39.72 (14.79) | 45.14 (12.64) | 33.98 (15.07) | t(33) = 2.38a |
| Current psychiatric disorders | ||||
| Major depression, n (%) | 2 (6%) | 2 (11%) | 0 (0%) | FET = 0.49 |
| Posttraumatic stress, n (%) | 1 (3%) | 1 (6%) | 0 (0%) | FET = 1.00 |
| Other anxiety, n (%) | 0 (0%) | 0 (%) | 0 (%) | NA |
| Substance use | ||||
| Past substance dependence | ||||
| Alcohol | 2 (6%) | 1 (6%) | 1 (6%) | FET = 1.00 |
| Marijuana | 3 (9%) | 2 (11%) | 1 (6%) | FET = 1.00 |
| Days of use in 30 days prior to MRI, M (SD) | ||||
| Alcohol | 1.57 (3.14) | 0.50 (1.04) | 2.71 (4.14) | t(33) = 2.19a |
| Marijuana | 1.00 (5.06) | 1.83 (7.04) | 0.12 (0.49) | t(33) = 1.00 |
| Current smoker, n (%) | 13 (37%) | 7 (39%) | 6 (35%) | χ2(1) = 0.05 |
| Nicotine dependence (Fagerstrom score) | 2.92 (2.29) | 2.14 (1.95) | 3.83 (2.48) | t(11) = 1.38 |
P < 0.05; FET = Fisher's Exact Test; MDD= major depressive disorder; PTSD = posttraumatic stress disorder.
The HIV‐positive group had been diagnosed with HIV for an average of 8.00 years (SD= 7.79, range: 5 months to 25 years), and 50% had an AIDS diagnosis. All participants were currently in HIV care and receiving antiretroviral therapy. Nadir CD4 cell counts ranged from 21 to 718 (M = 272.94, SD = 239.57). Current CD4 cell counts ranged from 36 to 894 (M = 507.50, SD = 311.04), and 33% had a detectable viral load at ≥50 copies/mL (range: 120–933,000 copies/mL).
Performance on the ICT
The k‐value estimates were similar across the HIV‐positive and HIV‐negative groups for both smaller delayed rewards ($10‐30; geometric M = 0.052 vs. 0.061) and larger delayed rewards ($35‐55; geometric M = 0.028 vs. 0.030). After normalizing the k‐values using a natural log transformation, there were no group differences for small [t(33) = 0.367, P = 0.72] or large [t(33) = 0.172, P = 0.86] trials. There was no difference between participants with low versus high income (based on median split) on k‐values for small [t(33) = 0.580, P = 0.57] or large [t(33) = 1.059, P = 0.30] trials. Participants with a current diagnosis of major depressive disorder or posttraumatic stress disorder had k‐values for small and large trials within 1 SD of the sample mean.
Latency to respond following the 2s presentation period was longest for hard trials (M = 1.48s, SD = 0.37), intermediate for easy trials (M = 1.24s, SD = 0.31), and shortest for control trials (M = 1.07s, SD = 0.30). In a repeated‐measures ANOVA, trial type was a strong predictor of latency [F(2,33) = 25.85, P < 0.001]. There was no trial type by group interaction effect [F(2,33) = 0.12, P = .89]. On average, participants responded as predicted on 93% (SD = 7%) of control trials and 84% (SD= 17%) of easy trials, with no group differences [t(33) = 0.78, P = 0.440 and t(33) = 0.40, P = 0.69, respectively]. For the hard trials, the sooner and delayed choices were designed to be of equivalent value for each participant; as expected, participants chose the options similarly often (sooner option, 53%; SD= 25%), with no group difference [t(33) = 0.72, P = 0.48].
Task‐Related Brain Activation
Figure 2 shows clusters with significant task‐related activation in the full sample. During easy trials, increased activation was observed in two clusters encompassing the paracingulate gyrus and the anterior cingulate, bilaterally in the inferior and precentral gyri, and in the right frontal pole and middle frontal gyrus. As expected, there were broader increases in activation during hard trials. The hard versus easy contrast shows that this increased activation was observed in the paracingulate and anterior cingulate gyri; bilaterally in the superior frontal, middle frontal, inferior frontal, and precentral gyri; bilaterally in the superior parietal lobule, lateral occipital cortex, and angular gyrus; the right occipital pole, lateral inferior occipital cortex, and occipital fusiform gyrus; the left posterior supramarginal gyrus; bilaterally in the cerebellum; and in the brainstem. A detailed description of these clusters is available in Table 2.
Figure 2.
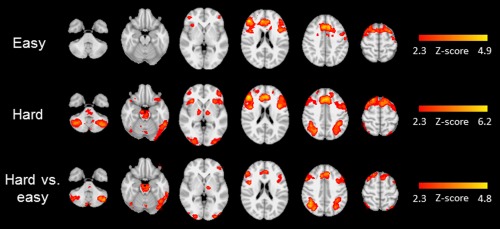
Radiological presentation of clusters with significantly greater activation in the full sample during easy choices (top row), hard choices (middle row), and hard versus easy choices (bottom row). These clusters are characterized further in Table II.
Table 2.
Task‐related activation during the ICT by choice difficulty (full sample, N = 35)
| Number of voxels | Max Z‐score | MNI coordinates (x, y, z) at peak | Anatomical region at peak | Anatomical regions at local maxiuma |
|---|---|---|---|---|
| Easy‐control | ||||
| 9016 | 4.95 | −6, 28, 32 | Paracingulate gyrus | Cingulate gyrus (anterior), L inferior frontal gyrus, L precentral gyrus |
| 2659 | 4.84 | 46, 36, 18 | R frontal pole | R middle frontal gyrus, R precentral gyrus, R inferior frontal gyrus |
| Hard‐control | ||||
| 18,010 | 6.26 | 4, 32, 30 | Paracingulate gyrus | Cingulate gyrus (anterior), L/R precentral gyrus, L/R inferior frontal gyrus, L/R frontal pole, L/R middle frontal gyrus, L/R superior frontal gyrus, L frontal orbital cortex, L insular cortex, L temporal pole |
| 4523 | 4.97 | −42, −68, −48 | L cerebellum | – |
| 2460 | 5.37 | 30, −68, 48 | R lateral occipital cortex (superior) | R angular gyrus, R supramarginal gyrus (posterior), superior parietal lobule |
| 2368 | 4.75 | 40, −62, −38 | R cerebellum | – |
| 2183 | 4.76 | −46, −42, 48 | L supramarginal gyrus (posterior) | L supramarginal gyrus (anterior), L superior parietal lobule, L postcentral gyrus, L lateral occipital cortex (superior) |
| 1995 | 3.88 | 0, −28, −22 | Brain stem | |
| 1312 | 4.32 | 30, −96, −8 | R occipital pole | R lateral occipital cortex (inferior), R occipital fusiform gyrus |
| 913 | 4.66 | 34, 24, −6 | R frontal orbital cortex | R insular cortex, R temporal pole, |
| Hard‐easy | ||||
| 5,346 | 4.06 | 10, 20, 46 | Paracingulate gyrus | R/L superior frontal gyrus, R middle frontal gyrus, R inferior frontal gyrus, R precentral gyrus, cingulate gyrus (anterior) |
| 4,281 | 4.89 | −42, −68, −50 | L cerebellum | – |
| 2,455 | 4.02 | −56, 20, 32 | L middle frontal gyrus | L inferior frontal gyrus, L precentral gyrus, L middle frontal gyrus |
| 2,276 | 4.26 | 42, −54, 44 | R angular gyrus | R superior parietal lobule, R lateral occipital cortex (superior) |
| 2,002 | 3.90 | 32, −68, −54 | R cerebellum | – |
| 1,660 | 4.34 | −30, −68, 45 | L lateral occipital cortex (superior) | L supramarginal gyrus (posterior), L superior parietal lobule, L angular gyrus |
| 1,140 | 3.40 | −2, −18, −22 | Brain stem | – |
| 856 | 3.67 | 26, −92, −12 | R occipital pole | R lateral occipital cortex (inferior), R occipital fusiform gyrus |
Note: R = right hemisphere, L= left hemisphere.
Group Differences in Brain Activation
The HIV‐positive group demonstrated broader increases in activation when making both easy and hard choices, with effects more pronounced during easy trials (see Fig. 3). Figure 4 shows the clusters with significant group differences. The easy contrast yielded two clusters. The first (“Easy Parietal”) included left parietal regions (intraparietal sulcus, angular gyrus, and supramarginal gyrus). The second (“Easy PFC”) was a larger cluster that included multiple bilateral regions of the prefrontal cortex (middle and superior frontal gyri and frontal pole). For the hard contrast, there was a single cluster (“Hard PFC/ACC”) confined to the prefrontal cortex (right frontal pole and paracingulate gyrus) and the anterior cingulate. A detailed description of these clusters is available in Table 3. Figure 4 also illustrates the percent signal change (relative to the control condition) for the two easy clusters and the one hard cluster. In all three cases, the HIV‐positive group had larger signal change compared to the HIV‐negative group, with large effect sizes [Easy Parietal cluster: t(33) = 3.12, P = 0.004, d = 1.05; Easy PFC cluster: t(33) = 3.50, P = 0.001, d = 1.18; Hard PFC/ACC cluster: t(33) = 4.53, P < 0.001, d = 2.63].
Figure 3.
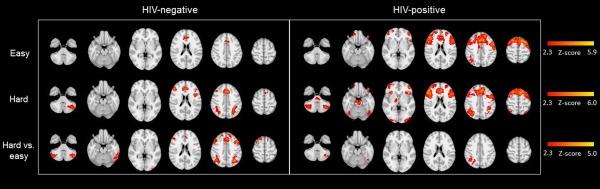
Radiological presentation of clusters with significantly greater activation for each of the 3 contrasts presented separately for HIV‐negative and HIV‐positive groups.
Figure 4.
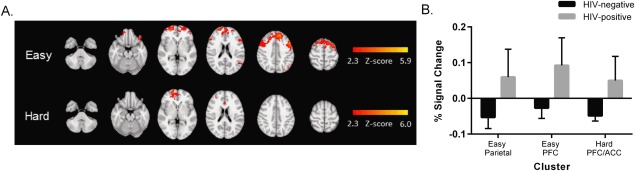
Differential activation between groups during the ICT. A. Radiological presentation of clusters with significantly greater activation for the HIV‐positive group compared to the HIV‐negative group for easy (top row) and hard (bottom row) choices. B. Comparison of percent signal change (mean ± standard error) for the HIV‐positive and HIV‐negative groups in the clusters in which group differences were identified (two clusters in the easy contrast and one cluster in the hard contrast).
Table 3.
Description of clusters with increased activation in the HIV‐positive compared to the HIV‐negative group for the easy and hard trials
| Number of voxels | Max Z‐score | MNI coordinates (x, y, z) at peak | Anatomical region at peak | Anatomical regions at local maxima |
|---|---|---|---|---|
| Easy‐control | ||||
| 11,763 | 5.07 | −46, 10, 48 | L Middle frontal gyrus | L superior frontal gyrus, L frontal pole, R frontal pole (all bilateral) |
| 957 | 3.96 | −42, −68, 46, | L lateral occipital cortex | L angular gyrus, L supramarginal gyrus |
| Hard‐control | ||||
| 1194 | 4.25 | 20, 60, −2 | R frontal pole | R paracingulate gyrus, bilateral anterior cingulate |
Note: R = right hemisphere, L= left hemisphere.
Correlation of Brain Activation to HIV Disease Markers and Behavioral Variables
There were negative correlations between nadir CD4 cell count and percent signal change within the Easy PFC cluster (r = −0.54, P = 0.021) and the Hard PFC/ACC cluster (r = −0.48, P = 0.043) (Fig. 5). Current CD4 cell count and HIV viral load suppression were not significantly associated with percent signal change. In the full sample, there were positive correlations between risk‐taking propensity and percent signal change within the Easy PFC cluster (r = 0.38, P = 0.023) and the Hard PFC/ACC cluster (r = 0.38, P = 0.024) (Fig. 5). Trait impulsivity was unrelated to percent signal change.
Figure 5.

Correlations of brain activation to nadir CD4 cell count and risk‐taking propensity for each of the clusters in which group differences were identified. Risk‐taking propensity was measured using the Balloon Analogue Risk Task (BART). ‡ Nadir CD4 cell count was natural log transformed to improve normality. *P < 0.05.
DISCUSSION
The results of this study support the hypothesis that HIV infection can alter brain functioning within regions involved in decision making. Using BOLD fMRI, we found that HIV‐infected adults had larger increases in activation within the executive control network when making temporal discounting choices. Previous studies have found a similar pattern of increased activation in fronto‐parietal cortices during visual attention and memory tasks among individuals with HIV [Chang et al., 2001; Ernst et al., 2009; Melrose et al., 2008; Schweinsburg et al., 2012]. This study extends that pattern to decision making, a key cognitive process that is relevant to everyday functioning. Specifically, we found that increased activity in the prefrontal cortex during temporal discounting was associated with risk‐taking propensity. Among HIV‐infected participants, prefrontal activity was correlated with nadir CD4 cell count, suggesting that a history of immunosuppression contributes to alterations in brain functioning.
Findings of HIV‐associated increases in neural activation have been previously conceptualized using compensatory models, which suggest that brain injury can decrease efficiency and neural capacity, such that greater recruitment of neural resources is necessary to preserve cognitive function [Barulli and Stern, 2013; Stern, 2009]. As chronic inflammatory damage in HIV‐infected brain regions accumulates, the additive burden of supplying blood to task‐relevant regions may lead to increases in BOLD signal [Ances et al., 2010]. In this study, during easy trials in which the subjectively greater valued reward was obvious, the HIV‐positive group had larger increases in brain activation within prefrontal and parietal regions, potentially due to decreased neural efficiency. In contrast, the HIV‐negative group had small increases in activation, resulting in a pronounced difference between the groups. This increased activity within the HIV‐positive group may have allowed participants to effectively evaluate the two options, as there was no difference between the groups on response time or choice on the temporal discounting task. During the hard trials that required greater executive control [Massar et al., 2015; McClure et al., 2004], the HIV‐negative group demonstrated the expected pattern of increased activation within the executive control network. In contrast, the HIV‐positive group had minimal additional increases in activation within fronto‐parietal regions, with peak activation observed on easy trials, a pattern often seen in the context of reduced neural capacity [Barulli and Stern, 2013]. These results mirror findings observed in healthy aging and neurological disorders, such as Huntington's disease, systemic lupus erythematosus, and Alzheimer's disease [Barraclough et al., 2015; Hagen et al., 2014; Li et al., 2015; Martins et al., 2012; Muller et al., 2014; Papoutsi et al., 2014].
While compensatory models are a useful framework for interpreting HIV‐associated alterations in neural activation, there is increasing recognition that fMRI does not directly test hypotheses related to efficiency and neural capacity [Poldrack, 2015; Stern, 2009]. Our design attempted to match task demand across study groups by using personalized k‐values to create a fixed number of hard and easy choices for each participant. Increasing task demand was verified by longer response times on hard versus easy choices across both study groups. Assuming task demand was indeed similar across study groups, decreased efficiency is a possible explanation for differential activation. However, as outlined by Poldrack [2015], there are alternative explanations, such as differences in neural computation or neuronal information coding. The small additional increases in activation during hard relative to easy choices suggest that the HIV‐positive group reached neural capacity on easy choices. However, since our task did not include a wide range of cognitive loads, it is possible that participants in the HIV‐negative group may not have reached neural capacity. That is, HIV‐negative participants may have had even larger increases in activation if task demand had continued to increase. We were also unable to determine the effect of the smaller increases in neural activation during hard choices on the quality of decision making, as by design there was no “wrong” choice on hard trials. Future studies in this area should include decision‐making tasks with a wider range of cognitive demands and measures to assess the quality of decision making.
In the current sample, nadir CD4 cell count was negatively correlated with activation within the prefrontal cortex, which suggests that severity of HIV‐related immunosuppression may contribute to altered neural activity. This is consistent with two previous fMRI studies that found nadir CD4 count to be associated with increased brain activation in fronto‐parietal regions during risky choices and semantic event sequencing, respectively [Connolly et al., 2014; Melrose et al., 2008]. Our sample was diverse with respect to HIV disease progression, which may help to explain the greater variability in BOLD signal change observed within regions of interest among the HIV‐positive group (see Fig. 4). Although there is evidence that antiretroviral therapy assists in normalizing brain metabolites in frontal regions of the brain [Chang et al., 1999], low nadir CD4 cell count is a risk factor for HIV‐associated neurocognitive impairment, despite rebound of plasma CD4 t‐cells and viral suppression [Ellis et al., 2011; Munoz‐Moreno et al., 2008; Robertson et al., 2007; Valcour et al., 2006]. This suggests that nadir CD4 count may be a marker of irreversible neural injury. This effect is prominent in the central nervous system, where HIV initiates a cascade of monocyte and macrophage‐mediated destruction of the glia that is not well controlled by antiretroviral therapy [Burdo et al., 2013].
The following limitations of our study should be noted, with implications for future research. First, due to the cross‐sectional design, it is not possible to determine the cause of the differences observed between the HIV‐positive and HIV‐negative groups. Ernst and colleagues have provided evidence for progressive HIV‐associated increases in brain activation over 1 year during a visual attention task [Ernst et al., 2009]. However, additional research with longitudinal cohorts is needed to confirm that increases in brain activation within fronto‐parietal regions is a direct result of HIV infection and disease progression. Second, while our study was adequately powered to identify group differences in task‐related brain activation, the within‐group correlational analyses may have been underpowered. Future studies with larger samples might also conduct sub‐group analyses to determine the roles of gender/sex and aging. In addition, since all HIV‐positive participants were currently receiving HIV care, it was not possible to determine the relative effects of HIV infection versus the potential neurotoxic or neuroprotective effects of antiretroviral therapy on BOLD signal change [Ances et al., 2008; Chang et al., 2008b]. Finally, our sample overall had low socio‐economic status, and the HIV‐positive group had significantly lower monthly income. Although income was unrelated to temporal discounting, other studies with much larger variability in socio‐economic status have suggested that lower income is associated with greater temporal discounting of delayed rewards [Green et al., 1996; Reimers et al., 2009].
In conclusion, this fMRI study provides evidence for HIV‐associated alterations in brain activation during temporal discounting that may have relevance for everyday decision making, such as adherence to complex antiretroviral regimens and return to work. While neuropsychological testing can establish cognitive impairments, functional neuroimaging may identify alterations in brain functioning before they manifest as outright cognitive impairments. The identification of subclinical markers of HIV‐associated neurocognitive change are critical for early detection and treatment to prevent further impairments. Our results also reinforce the importance of developing interventions, such as cognitive training, that aim to strengthen neuropsychological functioning in HIV‐infected persons.
ACKNOWLEDGMENTS
The authors thank Laura Barnes for programming the Intertemporal Choice Task used in this study. The NIH had no further role in study design, data collection, analysis and interpretation of data, writing of the report, or the decision to submit the paper for publication.
REFERENCES
- Ances BM, Roc AC, Korczykowski M, Wolf RL, Kolson DL (2008): Combination antiretroviral therapy modulates the blood oxygen level‐dependent amplitude in human immunodeficiency virus‐seropositive patients. J. Neurovirol. 14:418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S, McCutchan JA, Ellis RJ (2010): HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. J Infect Dis 201:336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R (2011): Inhibiting food reward: Delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity 19:2175–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald SL, Masliah E, Fennema‐Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, Heaton RK, Grant I, Mallory M, Miller A, Jernigan TL (2004): Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol 61:369–376. [DOI] [PubMed] [Google Scholar]
- Barraclough M, Elliott R, McKie S, Parker B, Bruce IN (2015): Cognitive dysfunction and functional magnetic resonance imaging in systemic lupus erythematosus. LUPUS 24:1239–1247. [DOI] [PubMed] [Google Scholar]
- Barulli D, Stern Y (2013): Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trend Cognit Sci 17:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM (2003): General multilevel linear modeling for group analysis in FMRI. NeuroImage 20:1052–1063. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM (2012): Excessive discounting of delayed reinforcers as a trans‐disease process contributing to addiction and other disease‐related vulnerabilities: Emerging evidence. Pharmacol Ther 134:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH (1997): The psychophysics toolbox. Spat Vis 10:433–436. [PubMed] [Google Scholar]
- Brevers D, Cleeremans A, Verbruggen F, Bechara A, Kornreich C, Verbanck P, Noel X (2012): Impulsive action but not impulsive choice determines problem gambling severity. PLoS One 7:e50647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Lackner A, Williams KC (2013): Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev 254:102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Dennis NA (2012) Frontal lobes and aging: Deterioration and compensation In: Stuss D, Knight RT, editors. Principles of Frontal Lobe Function, 2nd ed. New York: Oxford University Press; pp 628–652. [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK (2004): Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 26:307–319. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Leonido‐Yee M, Witt M, Speck O, Walot I, Miller EN (1999): Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild dementia. Neurology 53:782–789. [DOI] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, Itti L, Ernst T (2001): Neural correlates of attention and working memory deficits in HIV patients. Neurology 57:1001–1007. [DOI] [PubMed] [Google Scholar]
- Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E, Ernst T (2004): Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol 56:259–272. [DOI] [PubMed] [Google Scholar]
- Chang L, Wong V, Nakama H, Watters M, Ramones D, Miller EN, Cloak C, Ernst T (2008a): Greater than age‐related changes in brain diffusion of HIV patients after 1 year. J Neuroimmune Pharmacol 3:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Nakama H, Stokes B, Ernst T (2008b): Antiretroviral treatment is associated with increased attentional load‐dependent brain activation in HIV patients. J Neuroimmune Pharmacol 3:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, An H, Zhu H, Stone T, Smith JK, Hall C, Bullitt E, Shen D, Lin W (2009): White matter abnormalities revealed by diffusion tensor imaging in non‐demented and demented HIV+ patients. NeuroImage 47:1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett D, Luo S, Hsu E, Ainslie G, Mather M, Monterosso J (2014): Increased functional coupling between the left fronto‐parietal network and anterior insula predicts steeper delay discounting in smokers. Hum Brain Mapp 35:3774–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloak CC, Chang L, Ernst T (2004): Increased frontal white matter diffusion is associated with glial metabolites and psychomotor slowing in HIV. J Neuroimmunol 157:147–152. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B (2010): Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol 16:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Bischoff‐Grethe A, Jordan SJ, Woods SP, Ellis RJ, Paulus MP, Grant I, Translational Methamphetamine Aids Research Center (TMARC) Group (2014): Altered functional response to risky choice in HIV infection. PLoS One 9:e111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Heaton RK, Kamminga J, Lane T, Gates TM, Moore DM, Hubner E, Carr A, Brew BJ (2014): HIV‐associated neurocognitive disorder in Australia: A case of a high‐functioning and optimally treated cohort and implications for international neuroHIV research. J Neurovirol 20:258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, Collier AC, Gelman B, McArthur J, Morgello S, McCutchan JA, Grant I (2011): Nadir CD4 is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. Aids 25:1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, Ricardo‐Dukelow ML, Chang L (2009): Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann Neurol 65:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M.B. , Spitzer, R.L. , Gibbon, M. , Williams, J.B.W. (1996) Structured Clinical Interview for DSM‐IV Axis I Disorders, Research Version, Patient/Non‐patient Edition. New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J (2005): Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry 162:1256–1265. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Makris N, Ahern T, O'Brien LM, Caviness Jr VS, Kennedy DN, Faraone SV, Tsuang MT (2007): Hypothalamic abnormalities in schizophrenia: Sex effects and genetic vulnerability. Biol Psychiatry 61:935–945. [DOI] [PubMed] [Google Scholar]
- Gongvatana A, Schweinsburg BC, Taylor MJ, Theilmann RJ, Letendre SL, Alhassoon OM, Jacobus J, Woods SP, Jernigan TL, Ellis RJ, Frank LR, Grant I (2009): White matter tract injury and cognitive impairment in human immunodeficiency virus‐infected individuals. J Neurovirol 15:187–195., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Lichtman D, Rosen S, Fry A (1996): Temporal discounting in choice between delayed rewards: The role of age and income. Psychol Aging 11:79–84. [DOI] [PubMed] [Google Scholar]
- Hagen K, Ehlis AC, Haeussinger FB, Heinzel S, Dresler T, Mueller LD, Herrmann MJ, Fallgatter AJ, Metzger FG (2014): Activation during the Trail Making Test measured with functional near‐infrared spectroscopy in healthy elderly subjects. NeuroImage 85:583–591. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH, Levine AJ, Castellon SA, Lam MN (2006): Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology 20:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991): The fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. Br J Addict 86:1119–1127. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DRJ, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera‐Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema‐Notestine C, Jernigan TL, Wong J, Grant I (2010): HIV‐associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin D, Ellis R, McCutchan J, Letendre S, LeBlanc S, Corkran S, Duarte N, Clifford D, Woods S, Collier A, Marra C, Morgello S, Mindt M, Taylor M, Marcotte T, Atkinson J, Wolfson T, Gelman B, McArthur J, Simpson D, Abramson I, Gamst A, Fennema‐Notestine C, Jernigan T, Wong J, Grant I (2011): HIV‐associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J Neurovirol 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, Mitchell SH (2008): Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology 201:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Cattie JE, Doyle K, Grant I, The H. I. V. Neurobehavioral Research Program Group (2013): Risky decision‐making in HIV‐associated neurocognitive disorders (HAND). Clin Neuropsychol 2:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshad N, Valcour VG, Nir TM, Kohannim O, Busovaca E, Nicolas K, Thompson P (2012): Disrupted brain networks in the aging HIV+ population. Brain Connect 2:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Johnson PS, Herrmann ES, Sweeney MM (2015): Delay and probability discounting of sexual and monetary outcomes in individuals with cocaine use disorders and matched controls. PLoS One 10:e0128641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J, Sullivan PS (2015): Impulsivity as a risk factor for HIV transmission in men who have sex with men: A delay discounting approach. J Homosexuality 62:588–603. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW (2007): The neural correlates of subjective value during intertemporal choice. Nat Neurosci 10:1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN (2000) Instructions for inferring discount rates from choices between immediate and delayed rewards. Unpublished manuscript, Williams College.
- Kirby KN, Petry NM, Bickel WK (1999): Heroin addicts have higher discount rates for delayed rewards than non‐drug‐using controls. J Exp Psychol Gen 128:78–87. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA (2002): Evaluation of a behavioral measure of risk taking: The balloon analogue risk task (BART). J Exp Psychol Appl 8:75–84. [DOI] [PubMed] [Google Scholar]
- Li HJ, Hou XH, Liu HH, Yue CL, Lu GM, Zuo XN (2015): Putting age‐related task activation into large‐scale brain networks: A meta‐analysis of 114 fMRI studies on healthy aging. Neurosci Biobehav Rev 57:156–174. [DOI] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ (2006): Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res 83:155–171. [DOI] [PubMed] [Google Scholar]
- Martin EM, DeHaan S, Vassileva J, Gonzalez R, Weller J, Bechara A (2013): Decision making among HIV+ drug using men who have sex with men: A preliminary report from the Chicago Multicenter AIDS Cohort Study. J Clin Exp Neuropsychol 35:573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins R, Simard F, Provost JS, Monchi O (2012): Changes in regional and temporal patterns of activity associated with aging during the performance of a lexical set‐shifting task. Cereb Cortex 22:1395–1406. [DOI] [PubMed] [Google Scholar]
- Massar SAA, Libedinsky C, Weiyan C, Huettel SA, Chee MWL (2015): Separate and overlapping brain areas encode subjective value during delay and effort discounting. NeuroImage 120:104–113. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD (2004): Separate neural systems value immediate and delayed monetary rewards. Science 306:503–507. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M (1992): The fifth edition of the addiction severity index. J Subst Abuse Treat 9:199–213. [DOI] [PubMed] [Google Scholar]
- Meade CS, Lowen SB, Maclean RR, Key MD, Lukas SE (2011): fMRI brain activation during a delay discounting task in HIV‐positive adults with and without cocaine dependence. Psychiatry Res 192:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Towe SL, Skalski LM, Robertson KR (2015): Independent effects of HIV infection and cocaine dependence on neurocognitive impairment in a community sample living in the southern United States. Drug Alcohol Depend 149:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose RJ, Tinaz S, Castelo JM, Courtney MG, Stern CE (2008): Compromised fronto‐striatal functioning in HIV: An fMRI investigation of semantic event sequencing. Behav Brain Res 188:337–347. [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Bachis A, Esposito G, Turner SR, Taraballi F, Tasciotti E, Paige M, Avdoshina V (2014): Human immunodeficiency virus‐associated dementia: A link between accumulation of viral proteins and neuronal degeneration. Curr Trend Neurol 8:71–85. [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED (2007): Frontoparietal cortical activity of methamphetamine‐dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp 28:383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller LD, Guhn A, Zeller JB, Biehl SC, Dresler T, Hahn T, Fallgatter AJ, Polak T, Deckert J, Herrmann MJ (2014): Neural correlates of a standardized version of the trail making test in young and elderly adults: A functional near‐infrared spectroscopy study. Neuropsychologia 56:271–279. [DOI] [PubMed] [Google Scholar]
- Munoz‐Moreno JA, Fumaz CR, Ferrer MJ, Prats A, Negredo E, Garolera M, Perez‐Alvarez N, Molto J, Gomez G, Clotet B (2008): Nadir CD4 cell count predicts neurocognitive impairment in HIV‐infected patients. AIDS Res Hum Retrovirus 24:1301–1307. [DOI] [PubMed] [Google Scholar]
- Nath A (2015): Eradication of human immunodeficiency virus from brain reservoirs. J Neurovirol 21:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsi M, Labuschagne I, Tabrizi SJ, Stout JC (2014): The cognitive burden in Huntington's disease: Pathology, phenotype, and mechanisms of compensation. Mov Disord 29:673–683. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES (1995): Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51:768–774. [DOI] [PubMed] [Google Scholar]
- Pelli DG (1997): The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat Vis 10:437–442. [PubMed] [Google Scholar]
- Pfefferbaum a, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV (2009): Frontostriatal fiber bundle compromise in HIV infection without dementia. Aids 23:1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessis SD, Vink M, Joska JA, Koutsilieri E, Stein DJ, Emsley R (2014): HIV infection and the fronto‐striatal system: A systematic review and meta‐analysis of Fmri studies. Aids 28:803–811. [DOI] [PubMed] [Google Scholar]
- Poldrack RA (2015): Is “efficiency” a useful concept in cognitive neuroscience? Dev Cognit Neurosci 11:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Green L (1972): Commitment, choice and self‐control. J Exp Anal Behav 17:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers S, Maylor EA, Stewart N, Chater N (2009): Associations between a one‐shot delay discounting measure and age, income, education and real‐world impulsive behavior. Person Ind Diff 47:973–978. [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, Mcarthur JC, Collier aC, Evans SR, Ellis RJ (2007): The prevalence and incidence of neurocognitive impairment in the Haart Era. Aids 21:1915–1921. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI (2014): Reliability of the timeline followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav 28:154–162. [DOI] [PubMed] [Google Scholar]
- Schweinsburg BC, Scott JC, Schweinsburg AD, Jacobus J, Theilmann RJ, Frank LR, Weber E, Grant I, Woods SP (2012): Altered prefronto‐striato‐parietal network response to mental rotation in HIV. J Neurovirol 18:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998): The Mini International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatr 59:22–33. [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA (2010): Cognitive dysfunction in HIV patients despite long‐standing suppression of viremia. Aids 24:1243–1250. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23 Suppl 1:S208–S219. [DOI] [PubMed] [Google Scholar]
- Sobell, L.C. , Sobell, M.B. (1996) Timeline Follow‐back User's Guide: A Calendar Method for Assessing Alcohol and Drug Use. Toronto: Addiction Research Foundation. [Google Scholar]
- Stern Y (2002): What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 8:448–460. [PubMed] [Google Scholar]
- Stern Y (2009): Cognitive reserve. Neuropsychologia 47:2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S (2004): Prediction of immediate and future rewards differentially recruits cortico‐basal ganglia loops. Nat Neurosci 7:887–893. [DOI] [PubMed] [Google Scholar]
- Thames AD, Streiff V, Patel SM, Panos SE, Castellon SA, Hinkin CH (2012): The role of HIV infection, cognition, and depression in risky decision‐making. J Neuropsychiatry Clin Neurosci 24:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Yee P, Williams AE, Shiramizu B, Watters M, Selnes O, Paul R, Shikuma C, Sacktor N (2006): Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection–The Hawaii Aging with HIV Cohort. J Neurovirol 12:387–391. [DOI] [PubMed] [Google Scholar]
- Valcour V, Sithinamsuwan P, Letendre S, Ances B (2011): Pathogenesis of HIV in the central nervous system. Curr HIV AIDS Rep 8:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva J, Ahn WY, Weber KM, Busemeyer JR, Stout JC, Gonzalez R, Cohen MH (2013): Computational modeling reveals distinct effects of HIV and history of drug use on decision‐making processes in women. PLoS One 8:e68962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (2001) Wechsler Test of Adult Reading (WTAR) Manual. San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P (1992): A three‐dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12:900–918. [DOI] [PubMed] [Google Scholar]
- Zhu T, Zhong J, Hu R, Tivarus M, Ekholm S, Harezlak J, Ombao H, Navia B, Cohen R, Schifitto G (2013): Patterns of white matter injury in HIV infection after partial immune reconstitution: A DTI tract‐based spatial statistics study. J Neurovirol 19:10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


