Abstract
Marine Protected Areas (MPAs) are often established to mitigate the effects of overfishing and other human disturbances. In Fiji these are locally managed and, where enforced, have significantly higher coral cover, higher fish biomass, and lower seaweed cover than in the adjacent, unprotected reefs (non-MPAs). We investigated how the isotopic signatures of a common, mid-level consumer, Epinephelus merra, differed among three small (0.5- 0.8km2) MPAs versus adjacent, unprotected reefs. Isotopic ratios suggested that the fish in the MPAs fed higher in the food chain than those in the adjacent non-MPAs, despite being slightly smaller in size. Calculations using a brown alga as representative of the basal level of the food chain estimate this difference to be about half a trophic level. Thus, the isotopic ratio of a mid-level consumer can be noticeably altered over scales of only a few hundred meters. This may result from more complete food webs and hence greater prey choice and availability in the MPAs and implies that MPAs affect not only species’ abundance and diversity, but also diet composition and trophic biology of member individuals. Our findings suggest E. merra exhibits considerable site fidelity in its feeding biology and thus provides a localized isotopic signal of its reef of residence. If the isotopic signal of this mid-level carnivore is reflective of the composition of the food web beneath it, the signal might provide an easily obtained indication of reef conditions in that area.
Keywords: Nitrogen, carbon, isotope, grouper, Turbinaria conoides, trophic position, Phaeophyte
Introduction
Overfishing has pervasive impacts on marine ecosystems, ranging from species extinctions to fundamental alterations of ecosystem processes (Jackson et al. 2001; Worm et al. 2006). A common strategy for protecting marine communities from overfishing is the establishment of no-take marine protected areas (MPAs). Their effectiveness has been debated (Roberts & Polunin 1993, Bruno & Selig 2007), but where they are well enforced, MPAs can facilitate recovery of enclosed communities (Lester et al. 2009), enhancing abundance and diversity of fishes, as well as the ecosystem’s mean trophic level (Libralato et al. 2010; Rasher et al. 2013; Bonaldo et al. submitted). Hence, MPAs can alter the composition of species assemblages, but their impacts on the feeding behavior and trophic biology of individual species are relatively unexplored.
By protecting larger consumers, MPAs may facilitate trophic cascades that alter lower trophic level species’ behavior and access to resources. In the Caribbean, Stallings (2008) found the presence of large grouper caused smaller grouper to spend less time foraging and to have lower growth rates than when the larger grouper were absent. Recruitment of lower trophic level species was also higher when the larger grouper were present. Through such interactions, MPAs might alter fishes’ feeding biology and potentially their trophic position within the food web. This possible effect of MPAs on fish behavior and resource use has rarely been investigated.
Here we use stable isotope analysis to ask if the trophic biology of a mid-level consumer (the small grouper Epinephelus merra) differed depending on whether the individual was living in the no-take MPA or in the adjacent fished area, only a few hundred meters away. Stable isotope analysis has been widely used to elucidate connections in food webs and species’ trophic positions. This technique has the advantage that it need not be destructive (one can use fin clips as opposed to gut content analysis) and it integrates the sources of nitrogen assimilated (Cocheret de la Moriniere et al. 2003) over periods of weeks to years (Hobson 1999, MacNeil et al. 2006); rather than producing a ‘snapshot’ view as provided by stomach content analysis (Harmelin-Vivien & Bouchon 1976). It is also able to give an accurate representation of energy flow (Post 2002) and the relative importance of differing food sources or feeding strategies, such as omnivory (Post 2002). As a result, analyses of carbon and nitrogen isotopic ratios have been able to answer some questions at greater resolution, or beyond the scope of other methods.
Here we chose the grouper Epinephelus merra as our focus species because it is one of the only site attached predatory fish that is common in both MPAs and non-MPAs along the coast of Fiji (Clements et al. 2012), making it a possible integrator of the food chain up to its level in both MPAs and fished areas.
Methods
Study Site & Species
Along the Coral Coast of Fiji’s main island, local villages have established and enforced no-take MPAs. Thus multiple, small MPAs occur scattered within the unprotected back-reef (non-MPA) which is subject to artisanal fishing using hand lines, nets and spears. The MPAs and non-MPAs we investigated are 1-1.5m deep at low tide, occupy an 11km stretch of continuous coastline (Fig. 1) and thus are impacted by the same oceanic waters and similar terrestrial influences. Our study focused on three pairs of protected (MPA) and fished reefs associated with the villages of Votua, Vatu-o-lailai and Namada. The MPAs cover areas of ~0.8km2, ~0.5km2 and ~0.5km2 respectively and are located between ~300m and ~1km from the adjacent fished site (Fig. 1). The MPAs were established in 2002-2003 (Simpson 2010) and now differ greatly from their associated unprotected reefs. Live coral cover in the MPAs is 38-56% on hard substrates, but only 4-16% in the non-MPAs, while macroalgal cover is 1-3% in the MPAs but 49-91% in the non-MPAs (Rasher et al. 2013). Fish diversity, density, biomass and recruitment are also suppressed in the non-MPAs relative to the MPAs of these villages (Rasher et al. 2013, Bonaldo and Hay 2014, Dixson et al. 2014, Bonaldo et al. submitted).
Figure 1.
Map of sampling locations on Fiji’s Coral Coast. MPAs are in grey, the fished area is in white and distances between the protected and fished collection sites are shown for each village. The pairs of numbers are the sample size for each site: the number of algal samples is below the number of fish samples.
We chose the brown macroalga Turbinaria conoides to give an indication of ambient nitrogen conditions at each location because it is longer-lived and thus integrates fluctuating conditions. It is also one of the few macroalgal species found in both the MPAs and non-MPAs at most locations. We selected the small grouper Epinephelus merra as the consumer of focus because it is a mid-level, generalist carnivore, common in both the protected and fished habitats of the reef-flat (Clements et al. 2012). It is reported to have a limited home range (47.7±11 m2; To 2009) and therefore should feed predominantly - if not exclusively – in the area of collection, thus providing a localized dietary signal. Its diet consists of small fishes, crabs and a small percentage of shrimps and cephalopods, the proportions of which vary with ontogeny and feeding period (Harmelin-Vivien & Bouchon 1976). Harmelin-Vivien & Bouchon (1976) found that diets contained a higher proportion of crabs after nocturnal feeding periods and a higher proportion of small fishes during diurnal feeding periods. They also reported that smaller individuals consumed more crustaceans, while the larger ate more fish.
Sample Collection
As the MPAs were no-take reserves, we non-lethally sampled individuals by clipping fins, which has been shown to be a viable alternative to muscle sampling and correlates strongly with results from muscle tissue (Suzuki et al. 2005; Sanderson et al. 2009). Fin tissue has the additional benefit that it contains collagen which integrates dietary signal over the individual’s lifetime and thus provides a representation of E. merra’s overall trophic history, rather than sampling only the preceding few days or weeks (as some tissues such as liver or whole blood would; Hobson 1999). The outer 0.5cm of the pectoral fin margin was cut so that the total size of each sample was <1cm2 meaning that samples were composed of webbing tissue, fin rays and skin covering the fin. Samples were shaken vigorously in seawater to remove any particulates (none noted) prior to being stored at −20°C until processing in the lab. Between four and 15 fish were caught ~100m from shore using baited hand lines at a depth of ~1m at each site in May-June 2012.
The top 2cm of T. conoides were collected in May-June 2011 from seven to ten randomly collected replicates from each site except Namada’s MPA where T. conoides was not found, potentially due to heavy grazing (Rasher et al. 2013). Like the fin clips, samples were shaken vigorously in seawater to remove epibionts or other surface-attached particulates (none noted). All samples were collected ~100m from shore at ~1m depth in each site and frozen at −20°C until processing. Collecting only the uppermost sections meant all samples were of recent growth, so minimizing temporal differences and avoiding the potentially confounding effects of fouling organisms that are found on older growth. Phaeophytes such as T. conoides have previously been used in calculations of trophic position and Carassou et al. (2008) found close agreement between estimates of E. merra’s trophic position based on brown macroalgae versus particulate organic matter.
Constraints of field time resulted in collections of algae occurring one year prior to the collections of fish. Since collagen integrates the isotopic signature over the individual’s life (Stenhouse & Baxter 1976, Hobson 1999), fish samples will include the time period represented by the algae. Moreover, both seaweed and fish were sampled at the same time of year, so limiting seasonal differences.
Sample Preparation and Lab Analyses
No lipid treatment was performed on the fin clips as mean C:N ratios from all sites were all around 4 so correction from lipid-normalization would be minimal (McConnaughey and McRoy 1979; Sanderson et al. 2009). Fin clips were not acid treated because fin rays are composed primarily of collagen fibers (Nagai and Suzuki 2000).
To minimize the impact of epibionts in the analysis of T. conoides, only the newest growth - the uppermost 1cm of the algal ramet - was prepared for isotopic analysis. Both the fin clips and algal samples were dried to a constant weight at 70°C and ground with a pestle and mortar into a fine powder before analysis.
Samples were analyzed in triplicate by continuous-flow isotope ratio mass spectrometry (CF-IRMS) using a Carlo Erba NC2500 elemental analyzer interfaced to a Micromass Optima mass spectrometer. Each analytical run included a series of elemental (methionine) and isotope (peptone) standards to correct for blanks and instrumental drift. We conservatively estimate an analytical precision of ±0.2‰ for our isotopic measurements. Isotope abundances are expressed as δ15N and δ13C values relative to atmospheric N2 and Vienna Pee Dee Belemnite (VPDB) respectively.
Statistical Analysis
Statistical analyses were performed using SPSS version 16.0. Contrasts of isotopic signature between the MPA and non-MPA areas were assessed with an ANOVA blocked by ‘village’ where ‘protection status’ was the main effect. No post-hoc tests were necessary because there were only two levels of ‘Protection Status’. Within village MPA/non-MPA differences were analyzed with Independent Samples T-tests. Assumptions of normality and homogeneity of variance were examined using the Shapiro-Wilk test and the Levene’s Test respectively, with α = 0.05. No data sets violated these assumptions (algal δ13C p= 0.179; algal δ15N p= 0.574; fish δ13C p= 0.943; fish δ15N p= 0.672; fish total length p= 0.685).
Linear regression analysis evaluated whether there were correlations between δ15N and total length, as well as between δ13C and total length for the fish E. merra.
Fish trophic position (TP) was calculated using the following equation (Post 2002, Carassou et al. 2008):
Where:
δ15Nalgae in this equation refers to the mean algal δ15N ratio for each site. This was subtracted from each individual fish δ15N value to give an estimate of trophic position.
Results
From the non-MPAs, 25 pieces of T. conoides and 29 E. merra fin clips were collected and analyzed. Twenty algal samples and 20 fin clips were collected and analyzed from the MPAs. Mean variation between our duplicate samples was only 0.2‰ for both δ13C and δ15N. Jardine & Cunjak (2005) proposed a limit of 0.5‰, so this indicates that our samples were well homogenized and the potential difference in isotopic signature between fin webbing and fin ray would not confound our analyses.
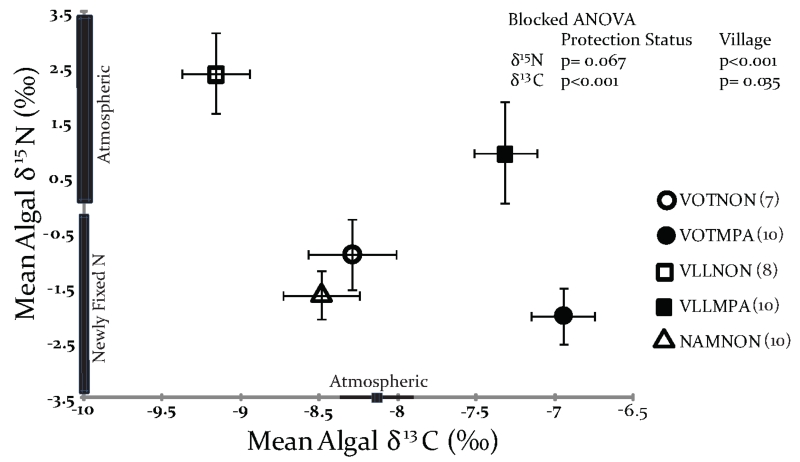
Algal δ13C was significantly higher in the MPAs versus the fished sites (Blocked ANOVA, p<0.001), while we detected no significant differences in the δ15N signatures between the MPAs and non-MPAs (p = 0.067), although the trend was towards higher values in the fished areas. Individual values of δ13C ranged from−8.3‰ to−6.1‰ (mean−7.1‰ ±0.14) in the MPAs and from−9.8‰ to−7.3‰ (mean−8.7‰ ±0.16) in the non-MPAs, while algal δ15N ranged from−4.2‰ to 5.7‰ (mean−0.6‰ ±0.62) in the MPAs and from−4.5‰ to 5.3‰ (mean−0.2‰ ±0.49) in the non-MPAs (Fig. 2).
Figure 2.
Isotopic cross plot showing the relationship between algal δ15N and δ13C (mean± 1SE) by village and protection status. Villages are abbreviated as: Votua (VOT), Vatu-o-lailai (VLL) and Namada (NAM) with protected area (MPA) and fished area (NON) in each village. T. conoides was absent from Namada’s protected site (NAMMPA). N for each location is indicated in parentheses in the legend. Analysis by Blocked ANOVA found no significant difference in δ15N (p=0.067), while algae from the MPA were significantly enriched in 13C (p<0.001).
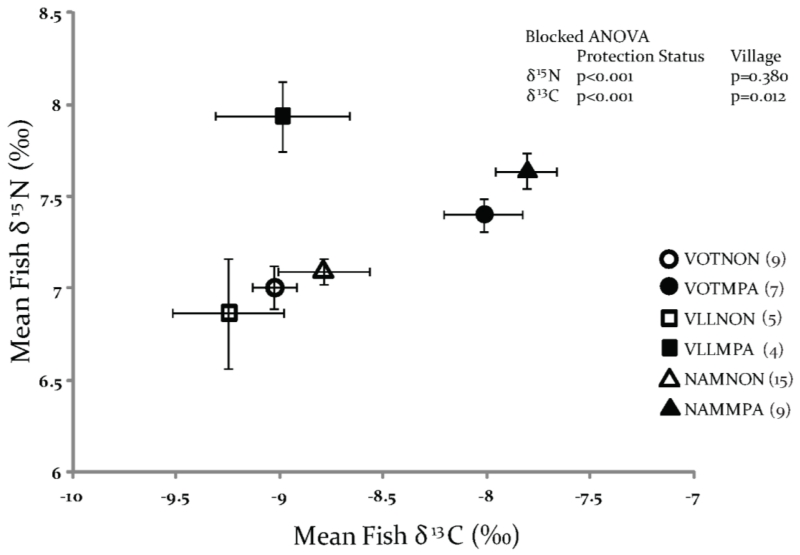
For the fish E. merra, individuals from the MPAs were significantly enriched in both 13C (Blocked ANOVA, p<0.001) and 15N (p<0.001) compared to individuals from the adjacent non-MPAs. The mean fish δ13C values from the MPAs were greater than those from the non-MPAs by 1.0‰, 0.3‰ and 1.0‰ in Votua, Vatu-o-lailai and Namada respectively, which gave a mean difference of 0.8‰. The mean fish δ15N values from the MPAs were greater than those from the non-MPAs by 0.4‰, 1.1‰ and 0.5‰ in Votua, Vatu-o-lailai and Namada respectively, which gave a mean difference of 0.7‰. δ13C values in individual fin clips ranged from−9.9‰ to−7.0‰ (mean−8.1‰ ±0.15) in the MPAs and from−11.4‰ to−7.9‰ (mean−8.9‰ ±0.13) in the non-MPAs. Individual values for δ15N ranged from 6.9‰ to 8.4‰ (mean 7.6‰ ±0.08) in the MPAs and from 6.1‰ to 7.8‰ (mean of 7.0‰ ±0.07) in the non-MPAs (Fig. 3).
Figure 3.
Isotopic cross plot showing the relationship between fish δ15N and δ13C (mean± 1SE) by village and protection status.
Symbols, analyses and site abbreviations as in Figure 2; analysis by Blocked ANOVA found fish from the MPA were significantly enriched in 13C (p<0.001) and 15N (p<0.001).
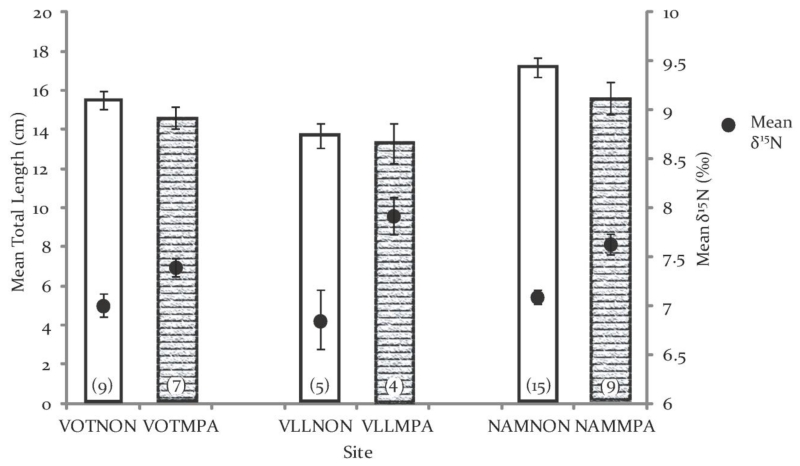
Although the range of fish total length was similar in the non-MPAs and MPAs (12.6cm to 19.7cm and 10.6cm to 20.1cm, respectively) and comparisons by Independent Samples T-tests were not significant for any of the villages, when data were pooled across all villages, the mean total length of fish from the non-MPAs was a significant 7.5% greater (mean= 16.0cm ±0.39 for the non-MPAs and 14.8cm ±0.48 for MPAs; p=0.036, blocked ANOVA, Fig. 3). Nevertheless, this difference in length would not have confounded isotopic values because there were no correlations between fish total length and either δ13C or δ15N (r2=0.001, p=0.823 and r2=0.005, p=0.622, respectively), in addition the Beta coefficients were low (−0.033 and−0.072, respectively; Figs. S1 and S2).
Analyses of algal δ13C for MPA versus non-MPA samples by Independent Samples T-tests detected significant differences in Votua (p= 0.001) and Vatu-o-lailai (p< 0.001), but not δ15N (Votua p= 0.180, Vatu-o-lailai p= 0.260). The comparison could not be made in Namada due to the absence of T. conoides in its MPA. For the fish, Independent Samples T-tests were possible for all three villages and found the difference between MPA and non-MPA δ13C significant for Votua (p<0.001) and Namada (p= 0.004), but not Vatu-o-lailai (p= 0.550). For δ15N, MPA non-MPA differences were significant for all three villages (Votua p= 0.022, Vatu-o-lailai p= 0.026, Namada p<0.001).
Calculated trophic position of E. merra was about half a trophic level higher in the MPAs of each village than in the corresponding fished areas. Trophic position was calculated to be 3.3, 2.3 and 3.6 in the non-MPAs and 3.8, 3.1 and 4 in the MPAs of Votua, Vatu-o-lailai and Namada respectively. This gives a mean trophic position of 3.1 for fish from the non-MPAs and 3.6 for those from the MPAs.
Discussion
We documented isotopic signatures for the grouper E. merra that indicate individuals from three different MPAs are feeding higher in the food chain than individuals collected from spatially-paired non-MPAs located 300-1000m away. Thus, in addition to altering fish density, biomass, and species composition (Clements et al. 2012; Rasher et al. 2013; Bonaldo et al. submitted), MPAs can also alter a species’ trophic biology relative to conspecifics living in nearby fished reefs. Both the δ13C and the δ15N values of the grouper were significantly higher in individuals from the MPAs (Blocked ANOVA, p<0.001 for both δ13C and δ15N; Fig. 3). This difference was not caused by a difference in ambient nitrogen conditions, since the algal samples indicated there were no significant differences between the MPA and non-MPA within each village.
If the algal samples are used as representative of autotrophs at the base of the food web, calculations show the fish from the MPAs fed about half a trophic level higher than those from the corresponding non-MPAs: these estimates of trophic position ranged from 2.3 to 3.6 in the non-MPAs and 3.1 to 4 in the MPAs. Following the values used by Bozec et al. (2004), this reflects a shift in the diet of E. merra from primarily invertebrates in the non-MPAs to primarily fishes in the MPAs. This seems reasonable given that the density of small recruiting fishes (prey for E. merra) is 5-8 times higher in the MPAs than non-MPAs (Dixson et al. 2014). The enriched isotopic signal in fish from the MPAs may result from greater prey choice and availability in those areas. This is despite the likelihood of higher competition and predation in the MPAs, as densities of Epinephelus spp. specifically (Clements et al. 2012; Bonaldo et al. submitted) and piscivores generally (Bonaldo et al. submitted) were significantly higher in the MPAs.
A study of 7 individuals in New Caledonia (also using brown macroalgae as indicative of the basal trophic level) estimated E. merra’s trophic position to be 2.5 to 2.9 (Carassou et al. 2008). No mention was made regarding protection status or fishing pressure in their sites, but our data suggest they may have been sourced from reefs where their diet was more reliant on invertebrates than fishes.
There are alternative, but improbable, explanations for the higher δ15N, and consequent estimate of trophic position, in E. merra from the MPAs. Firstly, this difference in isotopic signature could result from differing physical and chemical environments. This seems unlikely because these reef flat sites are all of similar depth, distance from shore and are interspersed within an 11km stretch of continuous coastline that is subject to the same oceanic waters and similar terrestrial inputs. Furthermore, analysis of algal δ15N by blocked ANOVA failed to find any significant differences among these sites and intra-village comparisons were also not significant (Independent Samples T-tests p= 0.180 for Votua and p= 0.260 for Vatu-o-lailai, Fig. 1). In addition, the MPAs were far smaller in area than the corresponding non-MPAs, so a positive relationship between trophic position and volume, as reported by Post et al. (2000), would not have produced our results.
Secondly, E. merra has been reported to shift from a crustacean-rich to a fish-rich diet as it grows (Harmelin-Vivien & Bouchon 1976). Thus, a higher δ15N may be expected from larger individuals. However in our study, the fish from the non-MPAs were slightly larger (blocked ANOVA p=0.036) and were significantly less enriched in 15N. Indeed the site with the largest fish; Namada’s non-MPA; also had the lowest δ15N of all 6 sites; thus differences in fish size within the range we investigated, cannot have generated our findings and indeed may have reduced the magnitude of isotopic difference we documented between MPA and non-MPA sites. Nevertheless, it was surprising to find the fish from the MPAs were smaller in length and this may be related to lower growth rate in those areas with higher trophic level consumers and greater risk of predation, as reported by Stallings (2008).
Harmelin-Vivien & Bouchon (1976) found the diet of E. merra between 6 & 9cm in length contained only 35% fish, while individuals between 10 & 24cm consumed fish as 68% of the diet. Every fish in our study was from the latter size class, so we would expect a comparable diet, and thus isotopic signature, in all individuals if they had equal access to prey. This makes the significant difference in isotopic ratio all the more interesting, as it suggests that diet is not determined by consumer length, within the size range we sampled. Moreover, regressions of fish total length against isotopic signatures found low Beta coefficients that were not significantly different from zero. In addition, the r2 values of 0.001 and 0.005 suggest no relationship, as do plots of fish total length versus isotopic signature (Figs. S1 & S2).
Finally, although we did not examine gender of the fish, it is unlikely this would have confounded our results because E. merra is a protogynous hermaphrodite. Pothin et al. (2004) found 75% of individuals smaller than 23.5cm to be female and since our largest specimen was 20.1cm long, it is probable that most, if not all, specimens in our study were female.
Thus, it seems that human activities like fishing can simplify habitats and communities and in this case, limit trophic options and lower the isotopic signature of the mid-level consumer E. merra. The establishment and enforcement of MPAs can cause dramatic changes in species diversity, abundance, biomass (Lester et al. 2009, Russ et al. 2008, Rasher et al. 2013), growth rate (Stallings 2008), recruitment (Dixson et al. 2014), longevity and age at sexual maturity (McClanahan & Omukoto 2011). Here we see that feeding biology and trophic level are also impacted. The greater prey choice and availability for E. merra in the MPAs may indicate more complete food webs there. This is in agreement with other reports of a return of trophic links in response to protecting large predators (Shears et al. 2002). Briand & Cohen (1987) found ecosystem dimensionality to be a significant factor in food chain length, with longer food chains found in ecosystems of greater topographic complexity. The coral dominated MPAs are more topographically complex than the fished areas, so MPAs in these villages may enhance community integrity and food chain length as well as preventing direct removal of species through over-harvesting. More research is necessary to support this hypothesis, but it is an exciting possibility. Stable isotope analysis of a relatively sessile, mid-level generalist carnivore (such as small grouper or possibly lizardfish) may thus be able to provide a simple, integrative means of assessing food web integrity and efficacy of MPAs. Extension of this approach to additional species and locations will provide a critical test of this hypothesis.
In summary, stable isotope analysis indicates that a common mid-level predator on Pacific coral reefs fed higher in the food chain when living in well-enforced MPAs than when living in fished areas only a few hundred meters away. This did not appear to be related to environmental differences or to ontogenetic shifts in diet, as the fish in the MPAs were slightly, but significantly, smaller in size. Fish collected from the three MPAs we investigated were feeding about half a trophic level higher than conspecifics in the adjacent non-MPAs. Establishment of MPAs may thus not only enhance fish biomass and species richness, but may also impact the trophic function of some fishes. Here we investigated only one mid-level consumer, but if the values we documented for E. merra are a reflection of the local food web, then it is possible that this is not just a species trait, but one generated by altered food-web structure. Stable isotope analysis could thus provide a rough measure of community integrity in such scenarios. Investigations of more species and locations will be needed to rigorously evaluate this possibility.
Supplementary Material
Figure 4.
Mean (± 1SE) fish total length and mean δ15N in each site; N is shown in parentheses at the base of each bar. The dashed columns are the protected sites in each village; site abbreviations as in Figure 2. Analysis by blocked ANOVA found individuals from the MPA were significantly smaller (p=0.036) and significantly enriched in 15N (p<0.001).
Acknowledgements
We thank the Fijian government and the Korolevu-i-wai district elders for granting research permission. Thanks to V. Bonito for logistical and field support, A. Warneke & D. Gibbs for assistance in the field and S. Weber for assistance in the laboratory. Support came from the National Science Foundation (OCE 0929199), the National Institute of Health (U01-TW007401) and the Teasley Endowment to the Georgia Institute of Technology. We also thank three anonymous reviewers for constructive comments on an earlier draft of the manuscript.
IACUC Permit A12043
(3206ABA (Doc ID 111091), 32066HH/H K (Doc ID 100567), 3206534)
References
- Bonaldo RM, Hay ME. Seaweed-Coral Interactions: Variance in seaweed allelopathy, coral susceptibility, and potential effects on coral resilience. PLoS One. 2014;9(1):e85786. doi: 10.1371/journal.pone.0085786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaldo RM, Pires MM, Bonito V, Hay ME, Roberto-Guimarães P. Small no-take areas benefit fish biomass, species diversity of fish schools, and coral recruitment: evidence from replicated protected and fished coral reefs in Fiji. Submitted to Biodiversity & Conservation. [Google Scholar]
- Bozec Y, Gascuel D, Kulbicki M. Trophic model of lagoonal communities in a large open atoll (Uvea, Loyalty islands, New Caledonia) Aquat Living Resour. 2004;17:151–162. [Google Scholar]
- Briand F, Cohen JE. Environmental correlates of food chain length. Science, New Series. 1987;238:956–960. doi: 10.1126/science.3672136. [DOI] [PubMed] [Google Scholar]
- Bruno JF, Selig ER. Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS One. 2007;2(8):e711. doi: 10.1371/journal.pone.0000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carassou L, Kulbicki M, Nicola TJR, Polunin NVC. Assessment of fish trophic status and relationships by stable isotope data in the coral reef lagoon of New Caledonia, southwest Pacific. Aquat Living Resour. 2008;21:1–12. [Google Scholar]
- Clements C, Bonito V, Grober-Dunsmore R, Sobey M. Effects of small, Fijian community-based marine protected areas on exploited reef fish. Mar Ecol Prog Ser. 2012;449:233–243. [Google Scholar]
- Cocheret de la Morinière E, Pollux BJA, Nagelkerken I, Van der Velde G. Diet shifts of Caribbean grunts (Haemulidae) and snappers (Lutjanidae) and the relation with nursery-to-coral reef migrations. Estuar, Coast Shelf S. 2003;57:1079–1089. [Google Scholar]
- Dixson DL, Abrego D, Hay ME. Chemically mediated behavior of recruiting corals and fish: a tipping point that may limit reef recovery. Science. 2014;345:892–897. doi: 10.1126/science.1255057. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Harmelin-Vivien ML, Bouchon C. Feeding behavior of some carnivorous fish (Serranidae and Scorpaenidae) from Tuléar (Madagascar) Mar Biol. 1976;37:329–340. [Google Scholar]
- Hobson KA. Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia. 1999;120:314–326. doi: 10.1007/s004420050865. [DOI] [PubMed] [Google Scholar]
- Jackson JB, Kirby MX, Berger WH, Bjorndal KA. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–637. doi: 10.1126/science.1059199. others. [DOI] [PubMed] [Google Scholar]
- Jardine TD, Cunjak RA. Analytical error in stable isotope ecology. Oecologia. 2005;144(4):528–533. doi: 10.1007/s00442-005-0013-8. [DOI] [PubMed] [Google Scholar]
- Lester SE, Halpern BS, Grorud-Colvert K, Lubchenco J, Ruttenberg BI, Gaines SD, Warner RR. Biological effects within no-take marine reserves: a global synthesis. Mar Ecol Prog-Ser. 2009;384(2):33–46. [Google Scholar]
- Libralato S, Coll M, Tempesta M, Santojanni A. Food-web traits of protected and exploited areas of the Adriatic Sea. Biol Conser. 2010;143:2182–2194. others. [Google Scholar]
- MacNeil MA, Drouillard KG, Fisk AT. Variable uptake and elimination of stable nitrogen isotopes between tissues in fish. Can J Fish Aquat Sci. 2006;63:345–353. [Google Scholar]
- McClanahan TR, Omukoto JO. Comparison of modern and historical fish catches (AD 750–1400) to inform goals for marine protected areas and sustainable fisheries. Conser Biol. 2011;25:945–955. doi: 10.1111/j.1523-1739.2011.01694.x. [DOI] [PubMed] [Google Scholar]
- McConnaughey T, McRoy CP. Food-web structure and the fractionation of carbon isotopes in the Bering Sea. Mar Biol. 1979;53(3):257–262. [Google Scholar]
- Nagai T, Suzuki N. Isolation of collagen from fish waste material—skin, bone and fins. Food Chem. 2000;68(3):277–281. [Google Scholar]
- Post DM. The long and short of food-chain length. TREE. 2002;17:269–277. [Google Scholar]
- Post DM, Pace ML, Hairston NG. Ecosystem size determines food-chain length in lakes. Nature. 2000;405:1047–1049. doi: 10.1038/35016565. [DOI] [PubMed] [Google Scholar]
- Pothin K, Letourneur Y, Lecomte-Finiger R. Age, growth and mortality of the tropical grouper Epinephelus merra Pisces, Serranidae on Reunion Island, SW Indian Ocean. Vie et milieu. 2004;54:193–202. [Google Scholar]
- Rasher DB, Hoey AS, Hay ME. Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology. 2013;94:1347–1358. doi: 10.1890/12-0389.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CM, Polunin NVC. Marine reserves: simple solutions to managing complex fisheries? Ambio. 1993:363–368. [Google Scholar]
- Russ GR, Cheal AJ, Dolman AM, Emslie MJ. Rapid increase in fish numbers follows creation of world’s largest marine reserve network. Curr Biol. 2008;18:R514–R515. doi: 10.1016/j.cub.2008.04.016. others. [DOI] [PubMed] [Google Scholar]
- Sanderson BL, Tran CD, Coe HJ, Pelekis V, Steel EA, Reichert WL. Nonlethal sampling of fish caudal fins yields valuable stable isotope data for threatened and endangered fishes. T Am Fish Soc. 2009;138(5):1166–1177. [Google Scholar]
- Shears NT, Babcock RC. Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia. 2002;132(1):131–142. doi: 10.1007/s00442-002-0920-x. [DOI] [PubMed] [Google Scholar]
- Simpson R. Master’s thesis. University of the South Pacific; Fiji: 2010. Assessing MPA effectiveness through observing the relative abundances of community-selected indicator populations over time. [Google Scholar]
- Stenhouse MJ, Baxter MS. The uptake of bomb 14°C in humans. In: Berger R, Suess HE, editors. Radiocarbon dating. University of California Press; Los Angeles: 1976. pp. 324–341. [Google Scholar]
- Stallings CD. Indirect effects of an exploited predator on recruitment of coral-reef fish. Ecology. 2008;89:2090–2095. doi: 10.1890/07-1671.1. [DOI] [PubMed] [Google Scholar]
- Suzuki KW, Kasai A, Nakayama K, Tanaka M. Differential isotopic enrichment and half-life among tissues in Japanese temperate bass (Lateolabrax japonicus) juveniles: implications for analyzing migration. Can J Fish Aquat Sci. 2005;62(3):671–678. [Google Scholar]
- To W. Ph.D Dissertation. University of Hong Kong; China: 2009. The biology, fishery of groupers (family: serranidae) in Hong Kong and adjacent waters, and implications for management. [Google Scholar]
- Worm B, Barbier EB, Beaumont N, Duffy JE, Folke C, Halpern BS, Watson R. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314(5800):787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.