Abstract
Terminal alkenes are readily available functional groups that appear in α-olefins produced by chemical industry, and they appear in the products of many contemporary synthetic reactions. While the organic transformations that apply to such alkenes are amongst the most studied reactions in all of chemical synthesis, the number of reactions that apply to nonactivated terminal alkenes in a catalytic enantioselective fashion is small in number. This Review highlights the cases where stereocontrol in catalytic reactions of 1-alkenes is high enough to be useful for asymmetric synthesis.
Keywords: alkenes, enantioselective catalysis, asymmetric synthesis, chirality, transition metals
1. Introduction
The catalytic enantioselective reactions of monosubstituted alkenes offer singular opportunities for strategic chemical synthesis. These reactions can facilitate both hydrocarbon chain-extension and functional group installation simultaneously, and in a stereoselective fashion. As substrates, terminal alkenes are amongst the most attractive of starting materials for chemical synthesis. They are readily available from large scale industrial processes and they may be accessed on smaller scale by a number of efficient, catalytic and highly selective processes. In addition to ready availability, the reactivity characteristics of alkenes render them ideal functional groups for strategic chemical synthesis. They are relatively non-polar and hence inert to all but the strongest of bases. Moreover, their reluctance to engage with many common oxidants, reductants, and nucleophiles allows polar functionality (i.e. halides, alcohols, ketones, esters, etc.) to be manipulated in their presence. Coupled with this general inertness, however, is a crucial feature: under specialized reaction conditions, alkenes may be transformed, often with exquisite levels of chemoselectivity, into a range of functionalized products.
Considering the remarkable properties of terminal alkenes and the utility of their asymmetric transformations, it may seem surprising that so little progress has been charted in regards to their efficient catalytic asymmetric transformation. However, there are well-founded reasons that selective transformations that apply to this functional group have been slow to emerge. This review surveys the challenges associated with stereocontrol in reactions of α-olefins and documents the techniques that are currently available to synthetic chemists for the asymmetric transformation of this substrate class in useful levels of selectivity (> ca. 80% ee). For reasons that will be delineated below, we restrict the discussion to transformations that apply to aliphatic alkenes and do not include the many reactions that apply only to electronically biased alkenes (i.e. styrenes, dienes, enynes, enones), or that require the presence of chelating groups, directing groups, or other auxiliary functionality.
Enantioselective transformations of unactivated alkenes are most often accomplished with transition metal catalysts. For many of these reactions, quadrant diagrams can simplify stereochemical analysis and provide insight into the stereochemical outcome. In these diagrams, a chiral ligand attached to a reaction center provides a non-symmetric environment where steric encumbrance (shaded quadrant) guides reaction of the substrate. In the example below, stereochemistry-determining olefin insertion into an M-A bond is depicted as a prototypical elementary catalytic step that might control enantioselectivity. As depicted in Scheme 1, a significant obstacle to controlling the selectivity in some reactions of terminal alkenes can arise because in many olefin insertion reactions, bond formation occurs by 1,2-insertion of the alkene into the M-A bond. In this mode, in contrast to reactions of cis, trans, and trisubstituted alkenes, the steric bias provided by the ligand framework is remote from the prochiral carbon atom of the substrate and prospects for effective stereocontrol are diminished. In contrast, asymmetric reactions of functionalized terminal alkenes are often more successful, in part because the substrate functionality turns over the regioselectivity of insertion reactions thereby positioning the prochiral carbon of the alkene much closer to the chiral ligand (2,1-insertion). For example, π-benzyl and π-allyl stabilization of organometallics turns over the insertion regiochemistry of styrenes and dienes and hence highly selective hydro- and difunctionalizations of these substrates are well developed. Similarly, chelation can turn over the regiochemistry of vinyl acetate and allyl alcohol insertions. The end result of these features is that the many excellent enantioselective reactions that apply to styrenes, dienes, and heteroatom-functionalized terminal alkenes, often give achiral linear products or are less selective with common aliphatic terminal olefins.
Scheme 1.

Quadrant diagrams describe pathways for migratory insertion of a prochiral substituted alkene into the M-A bond in a representative chiral transition metal complex. The shaded quadrant indicates and area of steric encumbrance established by the ligand framework.
2. Hydrosilylation
The catalytic hydrosilylation of 1-alkenes is well-studied, and many transition metals have been utilized to date, including platinum, palladium, nickel, rhodium, and even a number of lanthanides.1 In almost all cases, hydrosilylation of unactivated 1-alkenes proceeds with high anti-Markovnikov selectivity via a Chalk-Harrod-type mechanism (or closely related variants thereof) to provide achiral 1-silylalkanes (Scheme 2, cycle I).2 In such a mechanism, oxidative addition with a hydrosilane furnishes coordinatively unsaturated complex A, followed by coordination of an alkene substrate to generate intermediate B. Subsequently, hydrometallation provides alkyl-silyl complex C after a 1,2-insertion. Finally, reductive elimination delivers 1-silylalkane E and regenerates the active catalyst. Alternatively, an alkene insertion into the M-Si bond (i.e. silylmetallation) has been suggested, most notably under Rh(I) or Co(III) catalyst systems (cycle II).2b,3 In this variant, a 2,1-migratory insertion mode results in generation of complex D, followed by reductive elimination to form the hydrosilylation product E.
Scheme 2.
Mechanisms for transition-metal-catalyzed hydrosilylation.
In 1991, Hayashi and co-workers reported the first and only catalytic asymmetric hydrosilylation of aliphatic terminal alkenes (Scheme 3).4 With only 0.2 mol % of a palladium salt in conjunction with the chiral monodentate phosphine MeO-MOP, various terminal alkenes underwent hydrosilylation with trichlorosilane. These reactions provide 2-silylalkanes in high yields and >80:20 regioselectivity in most cases (hindered terminal alkenes resulted in lower regioisomeric ratios). The silylalkanes generated could be oxidized in situ under Tamao-Fleming oxidation5 conditions (hydrogen peroxide in the presence of an anionic fluoride source) to generate 2-alkanols in >94% ee.
Scheme 3.
Catalytic hydrosilylation of terminal alkenes developed by Hayashi.
While the authors do not speculate about the origin of the unusual regioselectivity, they suggest that the high reactivity arises because the monodentate ligand allows access to a 16-electron palladium(II) intermediate [PdH(SiCl3)L(CH2=CHR)], bearing a coordination site for olefin binding and activation. Indeed, reactions conducted in the presence of chelating bis(phosphine) ligands such as BINAP did not provide hydrosilylation products even when conducted at elevated temperatures. Furthermore, the authors suggest that the MOP ligand accelerates reductive elimination relative to β-hydride elimination when compared to other monodentate ligands such as triphenylphosphine or tri-o-tolylphosphine; this feature minimizes olefin isomerization during the course of the hydrosilylation. Lastly, it was found that the methoxy group at the 2'-position of the binaphthyl ligand framework is not imperative for high regioselectivity, since varying this group (Et-MOP, i-PrO-MOP) provided similar levels of regio- and enantioselectivity (Scheme 4).6 Consisteny with these findings. a crystal structure of [PdCl2{(R)-MeO-MOP}2] indicates that the substituent at the 2' position is far removed from the palladium center (Figure 1).
Scheme 4.
A. Comparison of substituted MOP ligands with varying substitution at the 2'-napthyl position. B. Crystal structure of [PdCl2(R-MeO-MOP)2].
Figure 1.

X-ray structure of [PdCl2{(R)-MeO-MOP}2]
3. Hydroformylation
Due to its superb atom economy, fast reaction rates, and high turnover numbers, transition metal-catalyzed hydroformylation of alkenes has become one of the largest and most successful catalytic commodity chemical manufacturing processes, producing millions of tons of achiral aldehydes annually.7 Despite many decades of research to efficiently produce linear aldehydes from inexpensive petroleum feedstocks, methods of generating the chiral α-branched isomer with high regio- and enantioselectivity have not been well developed. Considering the abundance of biologically active compounds that may be derived from nonracemic chiral aldehydes, the ability to directly provide α-chiral aldehydes from terminal alkenes in an enantioselective manner would be an appealing transformation.
Highly successful asymmetric and branch-selective hydroformylations of electronically activated terminal olefins or those containing chelating groups have been reported with a number of transition metals,8 but most often with Rh(I).9 As stated above, generation of the branched isomer from aliphatic terminal alkenes with similar catalyst systems has met with limited success, a result which can be explained by considering the mechanism for hydroformylation (Scheme 5).10 During regioselectivity-determining olefin insertion, Rh-complex B or B' is generated from precursor Rh-alkene complex A. Coordination of CO and migratory insertion provides acyl complex C or C'. Finally, oxidative addition of H2 followed by reductive elimination provides branched product D or linear product D' with concomitant regeneration of the rhodium catalyst. When intermediate B is stabilized by the substitution pattern on the alkene (for example, when R= Ph, a stable π-benzyl intermediate is formed), branched product D is preferred and unfavorable steric interactions between the substrate and the metal center are overridden. However, with aliphatic alkenes, little to no stabilization effect exists and intermediate B' predominates. Thus, linear product D' is favored so as to avoid steric interactions that are created in B.
Scheme 5.
Catalytic cycle for Rh-catalyzed olefin hydroformylation.
Developed by Takaya, Nozaki, and co-workers in 1993, phosphine-phosphite derived BINAPHOS 1 was the first ligand used for a highly enantioselective hydroformylation of aliphatic terminal alkenes (Scheme 6).11 Although synthetically useful levels of enantioselectivity were achieved for 1-hexene (82% ee) and 1-butene (83% ee), the undesired linear isomer predominated (1:3 branched:linear), a problem that was compounded by the fact that similar chemical and physical properties of the two isomers render purification difficult. Through extensive theoretical studies, it was later determined that facial selectivity of the olefin addition is mainly dictated by the absolute configuration of the phosphine group (occupying the equatorial site), while the phosphite unit (apical site) has a significant impact on the degree of enantioselectivity that is observed.7b, 12, 13 The favored conformation of the intermediate RhH(CO)2[(R,S)-BINAPHOS] along with quadrant representations of the two possible hydroformylation paths are provided in Figure 2. Calculations suggest that pathway II is highly favored over pathway I since the catalyst conformation in path I does not have an unimpeded quadrant. Reaction through path II is highly selective with the alkene substituent placed in the unhindered site. It is concluded that for high stereoselectivity, Rh-diphosphane catalysts require (1) equatorial-apical specific coordination containing chirality at the apical position to enhance diastereoisomeric ligand-substrate interactions, (2) two stereogenic centers which discriminate against competing equatorial-apical pathways, and (3) a rigid catalyst that enhances stereoinduction on the substrate.12
Scheme 6.
Rh-Catalyzed hydroformylation of alkenes developed by Takaya.
Figure 2.

Favored catalyst conformation and quadrant diagrams showing preferred olefin binding modes.
In 2012, Clarke and Cobey developed the first example of asymmetric hydroformylation in which electronically unbiased alkenes favored the branched isomer in high levels of enantioselectivity (Scheme 7).14 For example, in the presence of bobphos and Rh(acac)(CO)2 as the precatalyst, 1-hexene underwent hydroformylation in 93% ee and in a 3:1 branched:linear ratio. While low reaction temperatures resulted in low turnover numbers resulted and incomplete conversion even after long reaction times (21–66 hours), the Clark system is a fundamentally important advance likely to lead to refined systems.
Scheme 7.
Branched-selective Rh-catalyzed hydroformylation of alkenes reported by Clarke and Cobey.
4. Hydroamination
Although asymmetric intramolecular hydroaminations of functionalized terminal alkenes have been well-studied, intermolecular variants that involve reaction of unactivated alkenes are lacking.15 While iridium(I),16 gold(I),17 platinum(II)18 and lanthanum19 complexes are known to catalyze intermolecular Markovnikov hydroamination, the low reactivity of unactivated 1-alkenes necessitates use of high temperatures and often occurs with modest selectivity. Such limitations render asymmetric variants difficult to develop. Nonetheless, in 2009, Widenhoefer and co-workers reported an asymmetric gold(I)-catalyzed Markovnikov-selective intermolecular hydroamination of unactivated alkenes with imidazolidin-2-ones (Scheme 8).20 The process employed a bis(gold) phosphine complex bearing chiral bidentate ligand 2 in combination with a catalytic amount of AgOTf to provide good yields of the alkylated ureas in 71–78% ee. While superstoichiometric amounts of olefin were required for complete conversion, the inexpensive nature of many alkenes negates this problem. While the authors do not provide a mechanistic evaluation, enantioselective functionalizations of C=C π-bonds with chiral bis(gold)phosphine complexes have ample precedent.21 Thus, the reaction may be expected to proceed by an outer sphere nucleophilic attack of the external urea on Au-activated alkene, followed by internal proton transfer. Of note, critical control experiments were performed which excluded the possibility of Ag or acid-catalyzed pathways for olefin hydroamination.
Scheme 8.
Widenhoefer's gold(I) catalyzed hydroamination of alkenes wth cyclic ureas.
5. Addition of Pyridine C-H Bonds to Alkenes
The synthesis of chiral compounds containing pyridine moieties is of significant importance for synthetic chemists considering their prevalence in a wide array of natural products, pharmaceutically relevant and biologically active small molecules, and chiral ligands.22 Asymmetric C-H additions of pyridines to alkenes remains underexplored despite a number of successful non-enantioselective variants.23 In 1994, Rodewald and Jordan reported an enantioselective C-H alkylation of pyridines utilizing a chiral zirconocene-based catalyst (S,S)-3.24 However, low yields due to poor catalyst turnover, and only moderate enantioselectivities were reported for unactivated monosubstituted alkenes (Scheme 9).
Scheme 9.
Zr-Catalyzed C-H addition of 2-picoline to 1-octene reported by Rodewald and Jordan.
Improving upon this work, Hou and co-workers recently reported a highly enantioselective C-H bond addition of pyridines to alkenes by employing chiral half-sandwich dialkyl scandium precatalyst 4 (Scheme 10).25 High yields and excellent branched selectivity were obtained in the enantioselective reaction between unactivated α-olefins and 2-substituted pyridines. The novel catalyst retains a monocyclopentadienyl ligand that bears a tethered chiral binaphthyl backbone; the chiral element serves to block one of the two possible olefin binding modes with the major complex positioning the R group away from the Cp ring and results in facially selective 1,2-insertion of the Sc-pyridyl bond to alkene (Figure 3). Unfortunately, reactions of unsubstituted pyridine and quinoline did not occur, most likely due to enhanced coordination to the metal center, which may poison the catalyst by inhibiting olefin coordination.
Scheme 10.
Hou's asymmetric C-H bond addition of pyridines to 1-alkenes.
Figure 3.

Proposed stereochemical model for olefin coordination in asymmetric C-H addition of pyridines to 1-alkenes.
6. Dihydroxylation
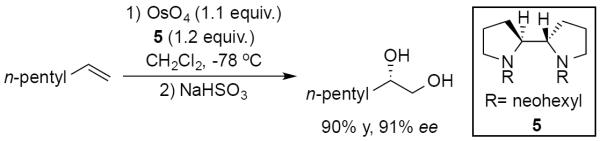
Developed in 1980, the Sharpless asymmetric dihydroxylation (SAD) is one of the most broadly used and well-studied olefin difunctionalizations in organic synthesis.26 Early variants by Sharpless and co-workers necessitated stoichiometric amounts of both a chiral quinuclidine-based ligand and expensive and toxic OsO4. Furthermore, initial dihydroxylation conditions provided diminished enantioselectivities for unactivated terminal olefins. Building on early findings by Sharpless that chiral quinuclidine- and amine-based ligands can both accelerate reaction rate and control facial selectivity during oxidation of the olefin,27 Hirama reported the first highly enantioselective dihydroxylation of unactivated terminal olefins by utilizing chiral N,N'-dineohexyl-2,2'-bypyrrolidine 5.28 For example, 1-heptene could be dihydroxylated to generate the vicinal 1,2-diol in 90% yield and 91% ee (Scheme 11). However, the strongly binding bidentate nature of the ligand appeared to inhibit hydrolysis of the osmium(VI) glycolate product thereby inhibiting ligand and osmium turnover. Thus, the Hirama example necessitates stoichiometric amounts of both OsO4 and chiral ligand, in addition to an external oxidant.
Scheme 11.
Early examples of a highly enantioselective dihydroxylation of terminal alkenes reported by Hirama.
In 1988, Sharpless demonstrated catalytic enantioselective dihydroxylation of alkenes by employing N-methylmorpholine N-oxide as a co-oxidant.29 In this process, lower enantioselectivities were observed compared to the stoichiometric variant due to a secondary competing and nonselective catalytic cycle.30 This alternate mechanism could be avoided by performing the reaction with K3Fe(CN)6 as a stoichiometric reoxidant under biphasic conditions.31 By 1996, with an effective and reliable catalytic system in hand, the Sharpless lab had tested over 500 different ligands, ultimately discovering that easily accessible dihydroquinidinyl- and dihydroquininyl-based ligands, (DHQD)2AQN and (DHQ)2AQN, provided high enantioselectivities for a broad range of olefin substrates. Importantly, terminal unactivated olefins were found to undergo dihydroxylation with moderate to good levels of enantioinduction (Scheme 12).32 Diphenylpyrimidine-based ligand, [(DHQD)2PYR], was also found to be highly selective with unactivated terminal alkenes, especially those with α-branching. Important to note, is that while “AD-mix” catalysts derived from phthalazine-based ligands [(DHQD)2PHAL or (DHQ)2PHAL] outperform most catalyst/ligand combinations for most alkene classes, “AD-mix” underperforms for unactivated terminal alkenes and the pyrazine catalysts are generally superior.
Scheme 12.
Sharpless' asymmetric dihydroxylation applied to 1-alkenes.
7. Epoxidation
Although many highly stereoselective epoxidation methods have been developed, the majority of these require electronically-biased or functionalized substrates.33 Selective epoxidation of unfunctionalized substrates are generally limited to alkenes that are di- and trisubstituted with selective reactions of unfunctionalized terminal alkenes being quite scarce. In addition to the recurring challenge associated with poor facial selectivity with terminal olefins, the lower HOMO energy of monosubstitued alkenes relative to their more substituted counterparts often leads to diminished reactivity of 1-alkenes towards electrophilic oxidants. Without practical and scalable methodology in hand for this challenging substrate class, production processes for nonracemic terminal epoxides rely on nonselective olefin epoxidation followed by Co(III)/salen-catalyzed hydrolytic kinetic resolution.34 While this process is highly practical with inexpensive substrates, for more precious alkene starting materials resolution processes may be too costly.
In 2006, Strukul reported that use of 2 mol % of Pt-complex (S,S)-6 along with environmentally benign hydrogen peroxide furnished enantiomerically enriched terminal epoxides in good yields and moderate levels of enantioselectivity (Scheme 13).35 Reactions of 1,4-dienes resulted in complete regioselectivity for the terminal double bond with excellent enantioinduction—an important breakthrough considering most chiral metal-based catalysts favor epoxidation of the more electron-rich substituted double bond. Kinetic analysis35b shows the reaction rate is first order in alkene concentration and independent of peroxide concentration. Further analysis of stoichiometric reactions of the Pt catalysts shows the process is complex and does not operate by either outer-sphere attack of peroxide on a Pt-alkene complex, or by oxo-transfer from a Pt-activated peroxide to the olefin. Instead, the authors propose hydrogen-bond mediated association of H2O2 to the fluoroarene ligand, followed by reversible alkene association, and slow intramolecular oxo transfer. The molecular geometry involved in such a transformation is difficult to predict, but it was suggested that the C6F5 group may enhance steric interactions between the substrate and the rigid chiral ligand.36
Scheme 13.
Pt-Catalyzed asymmetric epoxidation of alkenes developed by Strukul.
Prior to Strukul's report, Katsuki and co-workers realized some success with di-μ-oxo titanium(salen) complex 7 and hydrogen peroxide as the oxidant.37 While initial studies only surveyed a limited range of substrates, further examination found that a broad array of terminal olefins underwent asymmetric epoxidation with good to excellent enantioselectivity (Scheme 14).38 Notably, α-branched terminal olefins, a substrate class that was not examined by Strukul, provided the highest levels of enantioenrichment. Lastly, use of competition experiments that employ 1,6-dienes showed that the terminal alkene is more reactive and generated the terminal epoxide in moderate to high regioselectivity.
Scheme 14.
Katsuki's Ti(salen)-catalyzed asymmetric epoxidation of terminal alkenes.
Inspired by the Katsuki studies on epoxidation, Berkessel recently examined simplified ligands that might also offer effective catalysts for Ti-catalyzed alkene epoxidation with H2O2.39 In an important advance, it was found that non-symmetric ligands derived from meso-1,2-diaminocyclohexane, in particular 8 (Scheme 15), offered outstanding reactivity and selectivity in the reaction. Interestingly, ligand 9, a hybrid of ligands 8 and 7, allowed the reaction to be conducted with very low catalyst loadings (0.1 mol %) while still maintaining outstanding selectivit X-ray crystallographic analysis of complexes derived from ligand frameworks 8 and 9, suggests that the configuration of the amine-bearing carbon causes the complexes to provide chiral-at-metal Ti complexes with Δ-chirality although the precise details of the mechanism for oxygen atom transfer remain to be determined.
Scheme 15.

Berkessel's Ti(salen)-catalyzed asymmetric epoxidation of terminal alkenes.
8. Chlorohydroxylation (Section Removed After Publisher Editing)
Note: The peer-reviewed version of this manuscript included the following discussion of asymmetric chlorohydroxylation and dibromination of 1-alkenes reported by Henry. However, during typesetting of this review, a report by Denmark appeared that determined that the Henry results are incorrect with all reactions affording racemic products.[74] Accordingly, this section of the review was removed.
The enantioselective synthesis of chlorohydrins from terminal olefins represents a rare and powerful example of a reaction in which both olefinic carbons can be differentially functionalized in a single step. Early findings by Bäckvall noted that the key to the catalytic synthesis of chlorohydrin from ethylene with Pd(II) salts was the use of an aqueous media containing high concentrations of Cl− and CuCl2 (>2.5 M and >3 M, respectively).40 At low concentrations of Cl− and CuCl2 (<1.0 M for both), the Wacker reaction product (i.e acetaldehyde) was observed. However, later studies by Henry suggested that employment of an appropriate Pd(II) catalyst [i.e. PdCl3(pyridine)−] would allow for measurable chlorohydrin formation even at Cl− concentrations as low as 0.2M.41 Thus, in 1998, Henry and co-workers documented a Pd(II)-catalyzed asymmetric chlorohydrin synthesis with chiral bimetallic Pd-species 10 (Scheme 16).42 With this complex, unfunctionalized terminal olefins were converted to the derived chlorohydrin products with high selectivity, albeit as a mixture of regioisomers. Comparatively, allyl ethers provided the product in much higher regioisomeric selectivity for the terminal chloride product, and in similar levels of enantioselectivity. Notably, only 5–20% of the total product consisted of aldehyde and ketone by products. A significant amount of effort has been spent on determining the mechanism of oxypalladation in Wacker-type processes.43 Through detailed mechanistic studies performed by Henry and others, it is generally accepted that under conditions with high Cl− concentrations, trans-hyxdroxypalladation via an outer-sphere nucleophilic attack is the operative pathway. Alternatively, inner-sphere cis addition is favored with low Cl− concentrations.44 With the stereochemical outcome of the Wacker process being sensitive to catalyst structure and reaction conditions, a complete understanding of the stereochemical features of the Henry process is not currently established.45
Scheme 16.

Pd(binap)-catalyzed chlorohydrin synthesis from 1-alkenes as documented by Henry.
In an important advance, Henry found that by simply varying conditions from chloride- to a bromide-containing medium, enantiomerically enriched vicinal dibromides could be accessed rather than the expected bromohydrin products (results not shown).46 Only allyl ethers and α,β-unsaturated esters were investigated making it unclear whether this alternate mode of reactivity arises from electronic factors, or whether this process would also apply to unfunctionalized terminal alkenes.
9. Aziridination
The asymmetric aziridination of unactivated olefins is particularly difficult not only because of low reactivity of the substrate, but also due to the difficulty of finding highly reactive nitrene sources that are both accessible and easily deprotected.47 Iminoiodane derivates such as PhI=NTs have proven useful for Cu-catalyzed asymmetric aziridinations of electronically activated olefins or those bearing proximal polar functionality.48 However, low reactivity with unfunctionalized terminal olefins suggests that a secondary binding interaction might be necessary to enhance reaction rates via induced intramolecularity.49 Atom-economic azide derivatives were found by many to provide an alternate efficient route to metal nitrene intermediates.50 Still, harsh conditions are often needed for both azide decomposition and N-deprotection, hindering development of asymmetric variants.51
In 2009, Zhang et. al. developed Co(II) porphyrin-based catalyst 11 that performed asymmetric aziridination of unactivated terminal alkenes under mild temperatures (Scheme 17).52 Two keys to the success of this system are notable: first, was use of trichloroethoxysulfonyl azide (TcesN3) as a highly reactive and easily accessible nitrene source; second is the use of amide-containing Co-porphyrin complex 11 where an internal hydrogen bond with the S=O (see inset, Scheme 17) provides organization and enhances both selectivity and reactivity. Zhang's report represents the first highly asymmetric aziridination of terminal alkenes.
Scheme 17.
X. Peter Zhang's asymmetric aziridination of 1-alkenes with TcesN3.
Building on Zhang's precedent, the Katsuki group also reported a mild Ru(CO)(salen)-catalyzed asymmetric aziridination of unactivated terminal olefins utilizing 2-(trimethylsilyl)ethanesulfonyl azide (SESN3) as a nitrene source (Scheme 18).53 Employing 3 mol % of complex 12 and equimolar amounts of azide and alkene provided high yields and excellent enantioselectivities for a variety of terminal alkenes. Importantly, Katsuki's report provided an expanded substrate scope that included non-conjugated dienes, bromide- and ether-containing alkenes, as well as α-branched olefins. Of note, the aziridine protecting group was easily removed through use of tris(dimethylamino)sulfonium difluorotrimethylsilicate (TASF). Similar to Zhang's system in Scheme 17, it is proposed that catalyst 12 provides internal activation of the nitrene, in this case by overlap between the sulfonyl oxygen atom and a low-lying σ* C-F orbital (see inset, Scheme 18). Thus, in both the Zhang and Katuski systems, the catalysts likely benefit from organization that limits the number of competing transition states thereby offering excellent selectivity.
Scheme 18.
Katsuki's Ru(salalen)-catalyzed aziridination of terminal alkenes.
10. Cyclopropanation
The catalytic asymmetric cyclopropanation of alkenes represents one of the most general methods of accessing chiral cyclopropane units, a motif which is present in a number of natural or synthetic pharmaceutically relevant compounds.54 Since the seminal report by Nozaki and Noyori,55 many reliable asymmetric transition metal-catalyzed processes have been developed for cyclopropane synthesis.56 Impressively, unactivated terminal alkenes have been found to undergo selective transition metal-catalyzed cyclopropanation with many stabilized diazo compounds. Effective catalysts have been developed that are based on Co(II),57 Cu(I),58 Ru(II),59 Rh(II),60 and Ir(III)61 complexes (major contributions are summarized in Scheme 19). The body of work in this field is large compared to that of other asymmetric transformations of terminal alkenes, and thus only a few key examples will be discussed in detail.
Scheme 19.
Selected examples of asymmetric transition-metal-catalzed cyclopropanation of 1-hexene.
While many methods are available for the generation of thermodynamically stable trans-disubstituted58 and trisubstituted cyclopropanes,57,60 synthesis of cis-disubstituted cyclopropane derivatives in high diastereo- and enantioselectivity is still challenging. In 2008, Katsuki and co-workers reported an asymmetric iridium-catalyzed cis-selective cyclopropanation of nonconjugated olefins (Scheme 20).61 Employing 1 mol% of aryliridium-salen complex 13 in the presence of ethyl α-diazoacetate and a large excess of olefin furnished cis-disubstituted cyclopropanes in high yields and excellent enantioselectivities. Of note, subsequent manipulation of the pendant ester moiety provides a straightforward method for chain extension or functional group installation.
Scheme 20.
Katsuki's Ir-catalyzed asymmetric cyclopropanation of alkenes.
Although the authors state that a detailed mechanism of the stereochemical control is unclear, they do provide a stereochemical model based on crystal structures of the catalyst and with the help of density functional theory calculations. It is suggested that the diazo precursor replaces a weakly bound methanol at the apical position to provide the corresponding Ir-carbenoid intermediate displayed in Figure 4. With the Ccarbene-Cester bond bisecting two Ir-N vectors, an incoming olefin is favored to attack the carbenoid carbon from the least hindered side over one of the naphthalene rings via a perpendicular, side-on approach. Subsequently, a counter-clockwise rotation of the olefin positions the substitutent away from the ester moiety of the carbenoid, leading to formation of the major isomer. Note that a clockwise rotation is unfavored due to development of steric repulsion between the olefin substituent and the basal salen ligand.
Figure 4.
Stereochemical model for the Ir-salen catalyzed cyclopropanation of terminal alkenes.
In 2010, the Zhang group reported on a highly versatile chiral cobalt(II) porphyrin complex 14 that was found to catalyze the asymmetric cyclopropanation of a variety of activated and unactivated alkenes with tert-butyl α-cyanodiazoacetate as the carbene source (Scheme 21).57 High enantioinduction and E-selectivity was achieved for all substrates.62 Unlike most metal-catalyzed carbene transfer methods, the reaction proceeded efficiently with olefin as the limiting reagent. Furthermore, the reaction is performed under solvent-free conditions and does not require slow addition of the diazoacetate.63 Through appropriate reagent choice, either the ester group or the cyano group could be reduced chemoselectively with complete preservation of stereochemistry.
Scheme 21.
Zhang's Co-catalyzed asymmetric terminal alkene cyclopropanation followed by chemoselective reduction.
11. Carboalumination
In 1995, Negishi and co-workers described a zirconium-catalyzed asymmetric carboalumination (ZACA) of monosubstituted alkenes, adding to the list of stereoselective C-C bond-forming reactions available for this challenging substrate class.64 Subsequent to treatment of the olefin starting material with 8 mol % of (−)- (NMI)2ZrCl2 and equimolar amounts of trimethylaluminum, the carboaluminated products could be oxidized in situ to provide β-methyl primary alcohols in good yield and moderate enantioselectivity. Initially, extending the methodology to include other trialkylaluminum reagents resulted in low enantioselectivity. However, upon further evaluation, a dramatic solvent effect was observed and high enantioinduction was eventually obtained when using 1,1-dichloroethane in place of 1,2-dichloroethane for other trialkylaluminum reagents (Scheme 22).65 While the authors do not offer a mechanistic rationale for this useful result, they have noted that use of more polar solvents suppresses carbometallation pathways involving metallacycles that are observed with reactions performed in hexanes. Through the principle of statistical enantiomeric amplification,66 asymmetric carboalumination has been utilized for the syntheses of highly enantioenriched deoxypolypropionates via a one-pot tandem ZACA/Pd-catalyzed vinylation process.67
Scheme 22.
Negishi's Zr-catalyzed carboalumination (ZACA) of terminal alkenes.
Negishi and co-workers have proposed a stereochemical model for the asymmetric methylalumination of alkenes (Figure 5).68 It has been suggested that a stereoselective methylzirconation is operative, in which C2 symmetric cationic Zr-intermediate 15 interacts with the re-face of the olefin. Alternatively, an associated complex, in which Zr-intermediate 16 is the active complex, is also plausible. In both scenarios, the alkyl substituent of the alkene occupies the least hindered quadrant of the metal, with the neomenthyl unit controlling the orientation of the alkene.
Figure 5.
Proposed stereochemical model for the Zr-catalyzed methylalumination of 1-alkenes.
12. Diboration
Organoboronates are useful reagents in organic synthesis due to their high chemical stability and accessibility. Also of note, a wide array of stereospecific transformations can be performed on organoboronates, making them versatile intermediates for transformation into other useful products. Thus, the asymmetric construction of adjacent borylated centers by diboration of an alkene represents a versatile strategy for alkene difunctionalization, thereby connecting simple alkene starting materials to a broad array of chiral building blocks.
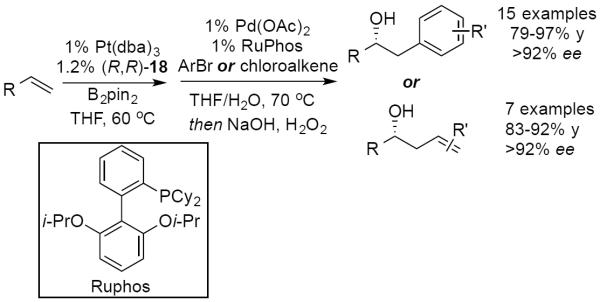
In 2009, our lab reported the first highly enantioselective diboration of simple terminal alkenes.69 Utilizing 3 mol % Pt(dba)3 and 6 mol % of TADDOL-derived phosphonite (R,R)-17 with bis(pinacolato)diboron (B2pin2), terminal 1,2-diols could be obtained in high yields and excellent enantioselectivity (Scheme 23) following an oxidative work-up. Furthermore, it was found that a single-flask diboration-homologation-oxidation sequence could generate 1,4-diols without loss of stereoselection. Later, additional optimization of catalyst structure, ligand:catalyst ratio, catalyst loading, and an expansion of the substrate scope was performed (Scheme 24).70 With commercially available TADDOL-derived phosphonite (R,R)-18, catalyst loading could be reduced to 0.5 mol % Pt, or even as low as 0.2 mol % Pt without diminishing enantiomeric ratios. Of note, di- or trisubstituted alkenes remained completely unreactive under the reaction conditions, and thus diboration provides a regiocomplementary strategy for vicinal diol construction relative to Sharpless asymmetric dihydroxylation (Scheme 24, eq. 1 and 2).71
Scheme 23.
Pt-Catalyzed enantioselective diboration of terminal alkenes reported in 2009.
Scheme 24.
Optimized Pt-catalyzed asymemtric alkene diboration.
Subsequent to optimization of diboration conditions, a one-pot diboration/cross-coupling (DCC) cascade sequence was developed by our group (Scheme 25).72 In this strategy, subsequent to diboration, addition of 1 mol % Pd(OAc)2 and 1 mol % RuPhos allowed efficient regioselective cross-coupling of the terminal boronate with both aryl and vinyl electrophiles. Notably, the internal boronate of the diboration product was not reactive in cross-coupling; thus, the DCC sequence provides a method of differentiating the two boronates in complexity-generating reaction sequences.
Scheme 25.
Single-flask asymmetric diboration/cross-coupling (DCC) applied to terminal alkenes.
Extensive experimental and computational studies have been performed on the Pt-catalyzed asymmetric diboration reaction. Through kinetic analysis and natural abundance 12C/13C kinetic isotope effect experiments, it was found that addition of a Pt-B bond across the alkene is irreversible, turnover-limiting, and therefore the stereochemistry-controlling step of the catalytic cycle. Additional study of the insertion reaction with probe substrates suggested that a 2,1-insertion mode was operative, in which an internal C-Pt bond is furnished rather than a terminal C-Pt bond. Thus, it is proposed that the insertion mode positions the prochiral carbon of the olefin in close proximity to the chiral ligand, enhancing stereoinduction. To some extent, this uncommon insertion mode also explains why similar selectivities are observed for aliphatic olefins and for styrenic olefins that benefit from π-benzyl stabilization.
Lastly, an impressive advance was recorded by Nishiyama who described a Rh(Phebox) catalyst capable of diborating a variety of activated and unactivated terminal alkenes in good yields and high enantiomeric excess (Scheme 26).73 Use of 1 mol % 19 and 5 mol % NaOtBu efficiently catalyzed the addition of B2pin2 across an olefin, providing enantiomercially-enriched vicinal 1,2-diols upon oxidation with NaBO3. Due to the fact that the reaction is base-promoted, the authors conclude that either σ-bond metathesis or transmetallation is involved in forming a Rh(III)-boryl species from a Rh(III)-OtBu intermediate, followed by Rh-B bond alkene insertion.
Scheme 26.
Nishiyama's Rh(Phebox)-catalyzed diboration/oxidation of terminal alkenes.
7. Summary and Outlook
Simple α-olefins are abundant and accessible from both large-scale industrial and smaller-scale synthetic processes, making them ideal substrates for asymmetric catalysis. Despite recent advances in catalytic olefin functionalization, highly enantioselective transformations of terminal aliphatic olefins are still uncommon. Additional progress in catalyst design and reaction engineering that allows both high facial selectivity and control of regioselection is critical to developing practical catalytic enantioselective processes. Nonetheless, recent approaches have led to the development of unique and highly versatile catalysts that promote selective reactions for both activated and unactivated olefins alike, bringing new hope for the discovery of more strategic and concise methods for chemical synthesis.
Acknowledgements
Our work in the area of terminal alkene functionalization has been supported by the US National Institutes of Health (GM-59417). JRC has been supported by a LaMattina Fellowship.
Biographies

John Ryan Coombs earned his B.Sc. from Fordham University in 2010. Subsequently, he received his Ph.D. at Boston College as a LaMattina fellow under the guidance of Prof. James P. Morken, where he primarily studied the Pt-catalyzed asymmetric diboration of unsaturated hydrocarbons. He is currently a research investigator at Bristol-Myers Squibb in New Brunswick, New Jersey.

James P. Morken received his B. Sc. from the University of California at Santa Barbara, his Ph.D. at Boston College with Prof. Amir H. Hoveyda, and was a postdoctoral fellow with Prof. Stuart L. Schreiber at Harvard University. He began his independent carrer at UNC Chapel Hill in 1997 and moved to Boston College in 2006. He is currently the Louise and Jim Vanderslice and Family Professor of Chemistry. His research focuses on catalytic asymmetric organic synthesis.
References
- [1].Marciniec B, Maciejewski H, Pietraszuk C, Pawluc P. In: Hydrosilylation: A Comprehensive Review on Recent Advances. Marciniec B, editor. Springer; New York: 2008. [Google Scholar]
- [2].a) Chalk AJ, Harrod JF. J. Am. Chem. Soc. 1965;87:16–21. [Google Scholar]; b) Duckett SB, Perutz RN. Organometallics. 1992;11:90–98. [Google Scholar]
- [3].a) Schroeder MA, Wrighton MS. J. Organomet. Chem. 1977;128:345–358. [Google Scholar]; b) Bergens SH, Noheda P, Whelan J, Bosnich B. J. Am. Chem. Soc. 1992;114:2128–2135. [Google Scholar]; c) Brookhart M, Grant BE. J. Am. Chem. Soc. 1993;115:2151–2156. [Google Scholar]
- [4].Uozumi Y, Hayashi T. J. Am. Chem. Soc. 1991;113:9887–9888. [Google Scholar]
- [5].For a review on C-Si bond oxidation, see: Jones GR, Landais Y. Tetrahedron. 1996;52:7599–7662.
- [6].Uozumi Y, Kenji K, Hayashi T, Yanagi K, Fukuyo E. Bull. Chem. Soc. Jpn. 1995;68:713–722. [Google Scholar]
- [7].a) Claver C, van Leeuwen PWNM, editors. Rhodium Catalyzed Hydroformylation. Kluwer Academic Publishers; Doordrecht, Netherlands: 2000. [Google Scholar]; b) Franke R, Selent D, Börner A. Chem. Rev. 2012;112:5675–5732. doi: 10.1021/cr3001803. [DOI] [PubMed] [Google Scholar]
- [8].Besides Rh, Pt(II)-catalyzed asymmetric hydroformylation of activated alkenes is particularly successful. For a review, see: Pospech J, Fleischer I, Franke R, Buchholz S, Beller M. Angew. Chem. Int. Ed. 2013;52:2852–2872. doi: 10.1002/anie.201208330.
- [9].For reviews on Rh-catalyzed asymmetric hydroformylations, see: Klosin J, Landis CR. Acc. Chem. Res. 2007;40:1251–1259. doi: 10.1021/ar7001039.; van Leeuwen PWNM, Kamer PCJ, Claver C, Pámies O, Diéguez M. Chem. Rev. 2011;111:2077–2118. doi: 10.1021/cr1002497.; Fernández-Pérez H, Etayo P, Panossian A, Vidal-Ferran A. Chem. Rev. 2011;111:2119–2176. doi: 10.1021/cr100244e.; For selected recent examples of Rh-catalyzed AHF of functionalized terminal alkenes, see: Zhang XW, Coo BN, Yu SC, Zhang XM. Angew. Chem. Int. Ed. 2010;49:4047–4050. doi: 10.1002/anie.201000955.. Worthy AD, Joe CL, Lightburn TE, Tan KL. J. Am. Chem. Soc. 2010;132:14757–14759. doi: 10.1021/ja107433h.; McDonald RI, Wong GW, Neupane RP, Stahl SS, Landis CR. J. Am. Chem. Soc. 2010;132:14027–14029. doi: 10.1021/ja106674n.; Allmendinger S, Kinuta H, Breit B. Adv. Synth. Catal. 2015;357:41–45.
- [10].a) Torrent M, Solá M, Frenking G, G. Chem. Rev. 2010;100:439–494. doi: 10.1021/cr980452i. [DOI] [PubMed] [Google Scholar]; (b) Nozaki K, Ojima I. In: Catalytic Asymmetric Synthesis. 2nd ed. Ojima I, editor. Wiley-VCH; New York: 2000. [Google Scholar]
- [11].a) Sakai N, Mano S, Nozaki K, Takaya H. J. Am. Chem. Soc. 1993;115:7033–7034. [Google Scholar]; b) Nozaki K, Sakai N, Nanno T, Higashijima T, Mano S, Horiuchi T, Takaya H. J. Am. Chem. Soc. 119:4413–4423. 997. [Google Scholar]
- [12].Aguado-Ullate S, Saureu S, Guasch L, Carbó JJ. Chem. Eur. J. 2012;18:995–1005. doi: 10.1002/chem.201101230. [DOI] [PubMed] [Google Scholar]
- [13].Note that calculations were performed with styrene and (E)-2-butene: Gleich D, Schmid R, Herrmann WA. Organometallics. 1998;17:2141–2143.
- [14].Noonan GM, Fuentes JA, Cobley CJ, Clarke ML. Angew. Chem. Int. Ed. 2012;51:2477–2480. doi: 10.1002/anie.201108203. [DOI] [PubMed] [Google Scholar]
- [15].For general review on catalytic hydroamination reactions, see: Brunet JJ, Neilbecker D. In: Catalytic Heterofunctionalization. Togni A, Grützmacher H, editors. Wiley-VCH; Weinheim, Germany: 2001. p. 91.; Beller M, Seayad J, Tillack A, Jiao H. Angew. Chem. Int. Ed. 2004;43:3368–3398. doi: 10.1002/anie.200300616.; Müller TE, Hultzsch KC, Yus M, Foubelo F, Tada M. Chem. Rev. 2008;108:3795–3892. doi: 10.1021/cr0306788.. Huang L, Arndt M, Gooßen K, Heydt H, Gooßen LJ. Chem. Rev. 2015;115:2596–2697. doi: 10.1021/cr300389u.
- [16].a) Sevov CS, Jianrong Z, Hartwig JF. J. Am. Chem. Soc. 2014;136:3200–3207. doi: 10.1021/ja412116d. [DOI] [PubMed] [Google Scholar]; b) Sevov CS, Zhou J, Hartwig JF. J. Am. Chem. Soc. 2014;134:11960–11963. doi: 10.1021/ja3052848. [DOI] [PubMed] [Google Scholar]; c) Pan S, Endo K, Shibata T. Org. Lett. 2012;14:780–783. doi: 10.1021/ol203318z. [DOI] [PubMed] [Google Scholar]
- [17].For early examples, see: Zhang J, Yang C-G, He C. J. Am. Chem. Soc. 2006;128:1798–1799. doi: 10.1021/ja053864z.; Liu X-Y, Li C-H, Che C-M. Org. Lett. 2006;8:2707–2710. doi: 10.1021/ol060719x.; Giner X, Nájera C. Org. Lett. 2008;10:2919–2922. doi: 10.1021/ol801104w.; Corma A, Leyva-Pérez A, Sabater MJ. Chem. Rev. 2011;111:1657–1712. doi: 10.1021/cr100414u. For a review, see:
- [18].For an early example, see: Karshtedt D, Bell AT, Tilley TD. J. Am. Chem. Soc. 2005;127:12640–12646. doi: 10.1021/ja052836d.
- [19].a) Reznichenko AL, Nguyen HN, Hultzsch KC. Angew. Chem. Int. Ed. 2010;49:8984–8987. doi: 10.1002/anie.201004570. [DOI] [PubMed] [Google Scholar]; b) Reznichenko AL, Hultzsch KC. Organometallics. 2013;32:1397–1408. [Google Scholar]
- [20].Zhang Z, Lee SD, Widenhoefer RA. J. Am. Chem. Soc. 2009;131:5372–5373. doi: 10.1021/ja9001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].For comprehensive reviews of C-C bond functionalization with chiral bis(gold)phosphine complexes, see: Widenhoefer RA. Chem. Eur. J. 2008;14:5382–5391. doi: 10.1002/chem.200800219.; Bongers N, Krause N. Angew. Chem. Int. Ed. 2008;47:2178–2181. doi: 10.1002/anie.200704729.
- [22].a) Michael JP. Nat. Prod. Rep. 2005;22:627–646. doi: 10.1039/b413750g. [DOI] [PubMed] [Google Scholar]; b) Arena CG, Aricò G. Curr. Org. Chem. 2010;14:546–580. [Google Scholar]; c) Bull JA, Mousseau JJ, Pelletier G, Charette AB. Chem. Rev. 2012;112:2642. doi: 10.1021/cr200251d. [DOI] [PubMed] [Google Scholar]
- [23].For asymmetric C-H alkylation of arenes and heteroarenes with activated olefins, see: Thalji RK, Ellman JA, Bergman RG. J. Am. Chem. Soc. 2004;126:7192–7193. doi: 10.1021/ja0394986.; Wilson RM, Thalji RK, Bergman RG, Ellman JA. Org. Lett. 2006;8:1745–1747. doi: 10.1021/ol060485h.; Zheng J, You S-L. Angew. Chem. 2014;126:13460–13463.; Lee P-S, Yoshikai N. Org. Lett. 2015;17:22–25. doi: 10.1021/ol503119z.; Filloux CM, Rovis T. J. Am. Chem. Soc. 2015;137:508–517. doi: 10.1021/ja511445x.
- [24].Rodewald S, Jordan RF. J. Am. Chem. Soc. 1994;116:4491–4492. [Google Scholar]
- [25].Song G, O WWN, Hou Z. J. Am. Chem. Soc. 2014;136:12209–12212. doi: 10.1021/ja504995f. [DOI] [PubMed] [Google Scholar]
- [26].Hentges SG, Sharpless KB. J. Am. Chem. Soc. 1980;102:4263–4265.; Kolb HC, Van Nieuwenhze MS, Sharpless KB. Chem. Rev. 1994;94:2483–2547. For a review, see:
- [27].Previous to an asymmetric variant, Criegee discovered that pyridine can significantly accelerate reaction rates of OsO4 with olefins. However, a seminal report by Sharpless suggested that chiral pyridine derivatives provided little to no enantioinduction due to low binding affinity of pyridine for OsO4: Criegee R. Justus Liebigs Ann. Chem. 1936;522:75–96.; Criegee R. Angew. Chem. 1938;51:519–520.
- [28].Oishi T, Hirama M. J. Org. Chem. 1989;54:5834–5835. [Google Scholar]
- [29].Jacobsen EN, Markó I, Mungall WS, Schröder G, Sharpless KB. J. Am. Chem. Soc. 1988;110:1968–1970. [Google Scholar]
- [30].Wai JSM, Markó I, Svendsen JS, Finn MG, Jacobsen EN, Sharpless KB. J. Am. Chem. Soc. 1989;111:1123–1125. [Google Scholar]
- [31].Kwong H-L, Sorato C, Ogini Y, Chen H, Sharpless KB, K. B. Tetrahedron Lett. 1990;31:2999–3002. [Google Scholar]
- [32].Becker H, Sharpless KB. Angew. Chem. Int. Ed. Engl. 1996;35:448–451. [Google Scholar]
- [33].For helpful reviews, see: Xia Q-H, Ge H-Q, Ye C-P, Liu Z-M, Su K-X. Chem. Rev. 2005;105:1603–1662. doi: 10.1021/cr0406458.; De Faveri G, Ilyashenko G, Watkinson M. Chem. Soc. Rev. 2011;40:1722–1760. doi: 10.1039/c0cs00077a.; Zhu Y, Wang Q, Cornwall RG, Shi Y. Chem. Rev. 2014;114:8199–8256. doi: 10.1021/cr500064w.
- [34].a) Tokunaga M, Larrow JF, Kakiuchi F, Jacobsen EN. Science. 1997;277:936–938. doi: 10.1126/science.277.5328.936. [DOI] [PubMed] [Google Scholar]; b) Robinson DEJE, Bull SD. Tetrahedron Asymmetry. 2003;14:1407–1446. [Google Scholar]
- [35].Colladon M, Scarso A, Sgarbossa P, Michelin RA, Strukul G. J. Am. Chem. Soc. 2006;128:14006–14007. doi: 10.1021/ja0647607.; Colladon M, Scarso A, Sgarbossa P, Michelin RA, Strukul G. J. Am. Chem. Soc. 2007;129:7680–4689. doi: 10.1021/ja071142x. For related work, see:
- [36].Pizzo E, Sgarbossa P, Scarso A, Michelin RA, Strukul G. Organometallics. 2006;25:3056–3062. [Google Scholar]
- [37].The enantioselective epoxidation of 1-octene was reported utilizing 1 mol% 7 (70% y, 82% ee). However, no other unactivated terminal alkenes were investigated: Matsumoto K, Sawada Y, Saito B, Sakai K, Katsuki T. Angew. Chem. Int. Ed. 2005;44:4935–4939. doi: 10.1002/anie.200501318.
- [38].Sawada Y, Matsumoto K, Katsuki T. Angew. Chem. Int. Ed. 2007;46:4559–4561. doi: 10.1002/anie.200700949. [DOI] [PubMed] [Google Scholar]
- [39].a) Berkessel A, Günther T, Wang Q, Neudörfl J-M. Angew. Chem. Int. Ed. 2013;52:8467–8471. doi: 10.1002/anie.201210198. [DOI] [PubMed] [Google Scholar]; b) Wang Q, Neudörfl J-M, Berkessel A. Chem. Eur. J. 2015;21:247–254. doi: 10.1002/chem.201404639. [DOI] [PubMed] [Google Scholar]
- [40].Bäckvall JE, Åkermark B, Ljunggren SO. J. Am. Chem. Soc. 1979;101:2411–2416. [Google Scholar]
- [41].a) Francis JW, Henry PM. J. Mol. Catal. A: Chem. 1995;99:77–86. [Google Scholar]; b) Lai J-Y, Wang F-S, Guo G-Z, Dai L-X. J. Org. Chem. 1993;58:6944–6946. [Google Scholar]
- [42].El-Qisairi A, Hamed O, Henry PM. J. Org. Chem. 1998;63:2790–2791. doi: 10.1021/jo005627e. [DOI] [PubMed] [Google Scholar]
- [43].For comprehensive reviews, see: Keith JA, Henry PM. Angew. Chem. Int. Ed. 2009;48:9038–9049. doi: 10.1002/anie.200902194. McDonald RI, Liu G, Stahl SS. Chem. Rev. 2011;111:2981–3091. doi: 10.1021/cr100371y.
- [44].For selected examples, see: Gregor N, Zaw K, Henry PM. Organometallics. 1984;3:1251–1256. Francis JW, Henry PM. Organometallics. 1991;10:3498–3503. Hamed O, Thompson C, Henry PM. J. Org. Chem. 1997;62:7082–7083. doi: 10.1021/jo971051q. Keith JA, Nielsen RJ, Oxgaard J, Goddard WA. J. Am. Chem. Soc. 2007;129:12342–12343. doi: 10.1021/ja072400t.
- [45].a) Comas-Vives A, Stirling A, Lledós A, Ujaque G. Chem. Eur. J. 2010;16:8738–8747. doi: 10.1002/chem.200903522. [DOI] [PubMed] [Google Scholar]; b) Angerson BJ, Keith JA, Sigman MS. J. Am. Chem. Soc. 2010;132:11872–11874. doi: 10.1021/ja1057218. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kóvacs G, Stirling A, Lledós A, Ujaque G. Chem. Eur. J. 2012;18:5612–5619. doi: 10.1002/chem.201102138. [DOI] [PubMed] [Google Scholar]; d) Stirling A, Nair NN, Lledós A, Ujaque G. Chem. Soc. Rev. 2014;43:4940–4952. doi: 10.1039/c3cs60469a. [DOI] [PubMed] [Google Scholar]; e) Kočovský P, Bäckvall J-E. Chem. Eur. J. 2015;21:36–56. doi: 10.1002/chem.201404070. For an extensive review, see: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].El-Qisairi AK, Qaseer HA, Katsigras G, Lorenzi P, Trivedi U, Tracz S, Hartman A, Miller JA, Henry PM. Org. Lett. 2003;5:439–441. doi: 10.1021/ol0273093. [DOI] [PubMed] [Google Scholar]
- [47].Degennaro L, Trinchera P, Luisi R. Chem. Rev. 2014;114:7881–7929. doi: 10.1021/cr400553c. [DOI] [PubMed] [Google Scholar]
- [48].For early examples, see: Li Z, Conser KR, Jacobsen EN. J. Am. Chem. Soc. 1993;115:5326–5327. Evans DA, Faul MM, Bilodeau MT, Anderson BA, Barnes DM. J. Am. Chem. Soc. 1993;115:5328–5329. Sanders CJ, Gillespie KM, Bell D, Scott P. J. Am. Chem. Soc. 2000;122:7132–7133.
- [49].a) Fruit C, Müller P. Tetrahedron: Asymmetry. 2004;15:1019–1026. [Google Scholar]; b) Wang X, Ding K. Chem. Eur. J. 2006;12:4568–4575. doi: 10.1002/chem.200501109. [DOI] [PubMed] [Google Scholar]; c) Anada M, Tanaka M, Washio T, Yamawaki M, Abe T, Hashimoto S. Org. Lett. 2007;9:4559–4562. doi: 10.1021/ol702019b. [DOI] [PubMed] [Google Scholar]
- [50].For a review, see: Driver TG. Org. Biomol. Chem. 2010;8:3831–3846. doi: 10.1039/c005219c.
- [51].a) Li Z, Quan RW, Jacobsen EN. J. Am. Chem. Soc. 1995;117:5889–5890. [Google Scholar]; b) Bergmeier SC, Seth PP. Tetrahedron Lett. 1999;40:6181–6184. [Google Scholar]; c) Katsuki T. Chem. Lett. 2005:1304–1309. [Google Scholar]
- [52].a) Subbarayan V, Ruppel JV, Zhu S, Perman JA, Zhang XP. Chem. Commun. 2009:4266–4268. doi: 10.1039/b905727g. [DOI] [PubMed] [Google Scholar]; b) Ruppel JV, Jones JE, Huff CA, Kamble RM, Chen Y, Zhang XP. Org. Lett. 2008;10:1995–1998. doi: 10.1021/ol800588p. [DOI] [PubMed] [Google Scholar]; c) Suarez AIO, Jiang H, Zhang XP, de Bruin B. Dalton Trans. 2011;50:5697–5705. doi: 10.1039/c1dt10027k. For mechanistic analysis, see: [DOI] [PubMed] [Google Scholar]
- [53].Kim C, Uchida T, Katsuki T. Chem. Commun. 2012;48:7188–7190. doi: 10.1039/c2cc32997b. [DOI] [PubMed] [Google Scholar]
- [54].a) Carson CA, Kerr MA. Chem. Soc. Rev. 2009;38:3051–3060. doi: 10.1039/b901245c. [DOI] [PubMed] [Google Scholar]; b) Reissig H-U, Zimmer R. Chem. Rev. 2003;103:1151–1196. doi: 10.1021/cr010016n. [DOI] [PubMed] [Google Scholar]
- [55].a) Nozaki H, Takaya H, Noyori R. Tetrahedron Lett. 1965;6:2563–2567. [Google Scholar]; b) Nozaki H, Takaya H, Moriuti S, Noyori R. Tetrahedron. 1968;24:3655–3669. [Google Scholar]
- [56].For a recent review, see: Bartoli G, Bencivenni G, Dalpozzo R. Synthesis. 2014;46:979–1029.
- [57].Zhu S, Xu X, Perman JA, Zhang P. J. Am. Chem. Soc. 2010;132:12796–12799. doi: 10.1021/ja1056246. [DOI] [PubMed] [Google Scholar]
- [58].a) Lo MM-C, Fu GC. J. Am. Chem. Soc. 1998;120:10270–10271. [Google Scholar]; b) Minuth T, Boysen MMK. Synthesis. 2010:2799. [Google Scholar]
- [59].Hoang VDM, Reddy PAN, Kim T-J. Tetrahedron Lett. 2007;48:8014–8017. [Google Scholar]
- [60].a) Davies HML, Bruzinski PR, Lake DH, Kong N, Fall MJ. J. Am. Chem. Soc. 1996;118:6897–6907. [Google Scholar]; b) Lindsay VNG, Lin W, Charette AB. J. Am. Chem. Soc. 2009;131:16383–16385. doi: 10.1021/ja9044955. [DOI] [PubMed] [Google Scholar]; c) Chuprakov S, Kwok SW, Zhang L, Lercher L, Fokin VV. J. Am. Chem. Soc. 2009;131:18034–18035. doi: 10.1021/ja908075u. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lindsay VNG, Nicolas C, Charette AB. J. Am. Chem. Soc. 2011;133:8972–8981. doi: 10.1021/ja201237j. [DOI] [PubMed] [Google Scholar]; e) Marcoux D, Azzi S, Charette AB. J. Am. Chem. Soc. 2009;131:6970–6972. doi: 10.1021/ja902205f. [DOI] [PubMed] [Google Scholar]; f) Grimster N, Zhang L, Fokin VV. J. Am. Chem. Soc. 2010;132:2510–2511. doi: 10.1021/ja910187s. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Qin C, Boyarskikh V, Hansen JH, Hardcastle KI, Musaev DG, Davies HML. J. Am. Chem. Soc. 2011;133:19198–19204. doi: 10.1021/ja2074104. [DOI] [PubMed] [Google Scholar]; (h) Lindsay VNG, Fiset D, Gritsch PJ, Azzi S, Charette AB. J. Am. Chem. Soc. 2013;135:1463–1470. doi: 10.1021/ja3099728. [DOI] [PubMed] [Google Scholar]
- [61].Suematsu H, Kanchiku S, Uchida T, Katsuki T, T. J. Am. Chem. Soc. 2008;130:10327–10337. doi: 10.1021/ja802561t. [DOI] [PubMed] [Google Scholar]
- [62].For a detailed mechanistic study pertaining to the Co(II) porphyrin-catalyzed cyclopropanation, see: Dzik WI, Xue X, Zhang XP, Reek JNH, de Bruin B. J. Am. Chem. Soc. 2010;132:10891–10892. doi: 10.1021/ja103768r.
- [63].Slow diazoalkane addition is often required in order prevent formation of carbene dimerization byproducts typically observed at high concentrations of diazoalkane: Dörwald FZ. Metal Carbenes in Organic Synthesis. Wiley-VCH; Weinheim, Germany: 1999. p. 116.
- [64].Kondakov DY, Negishi E. J. Am. Chem. Soc. 1995;117:10771–10772. [Google Scholar]
- [65].Kondakov DY, Negishi E. J. Am. Chem. Soc. 1996;118:1577–1578. [Google Scholar]
- [66].Statistical enantiomeric amplification is a principle which predicts, through the mass action law, that a combination of two compounds with low enantiomeric excess can generate a new compound containing two chiral centers in much greater enantiomeric excess (i.e. two chiral species of 80% ee can theoretically generate a new compound in 97.6% ee at the expense of lower yields). See: Negishi E. Dalton Trans. 2005:827–848. doi: 10.1039/b417134a.
- [67].a) Huo S, Negishi E. Org. Lett. 2001;3:3253–3256. doi: 10.1021/ol010142d. [DOI] [PubMed] [Google Scholar]; b) Negishi E, Tan Z, Liang B, Novak T. Proc. Natl. Acad. Sci. 2004;101:5782–5787. doi: 10.1073/pnas.0307514101. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Magnin-Lachaux M, Tan Z, Liang B, Negishi E. Org. Lett. 2004;6:1425–1427. doi: 10.1021/ol0497483. [DOI] [PubMed] [Google Scholar]; d) Novak T, Tan Z, Liang B, Negishi E. J. Am. Chem. Soc. 2005;127:2838–2839. doi: 10.1021/ja043534z. [DOI] [PubMed] [Google Scholar]; e) Liang B, Novak T, Tan Z, Negishi E. J. Am. Chem. Soc. 2006;128:2770–2771. doi: 10.1021/ja0530974. [DOI] [PubMed] [Google Scholar]; f) Xu S, Lee C-T, Wang G, Negishi E. Chem. Asian, J. 2013;8:1829–1835. doi: 10.1002/asia.201300311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].a) Kondakov DY, Negishi E. J. Am. Chem. Soc. 1995;117:10771–10772. [Google Scholar]; b) Negishi E. Chem. Eur. J. 1999;5:411–420. [Google Scholar]
- [69].Kliman LT, Mlynarski SN, Morken JP. J. Am. Chem. Soc. 2009;131:13210–13211. doi: 10.1021/ja9047762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Coombs JR, Haeffner F, Kliman LT, Morken JP. J. Am. Chem. Soc. 2013;135:11222–11231. doi: 10.1021/ja4041016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Xu D, Crispino GA, Sharpless KB. J. Am. Chem. Soc. 1992;114:7570–7571. [Google Scholar]
- [72].Mlynarski SN, Schuster CH, Morken JP. Nature. 2013;505:386–390. doi: 10.1038/nature12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Toribatake K, Nishiyama H. Angew. Chem. Int. Ed. 2013;52:11011–11015. doi: 10.1002/anie.201305181. [DOI] [PubMed] [Google Scholar]
- [74].Denmark SE, Carson N. Org. Lett. 2015;17:5728–5731. doi: 10.1021/acs.orglett.5b02650. [DOI] [PMC free article] [PubMed] [Google Scholar]