Summary
Vitamin D (VD3) has been linked to immunological processes, and its supplementation may have a role in treatment or prevention of diseases with underlying autoimmune or pro‐inflammatory states. As initiators of the immune responses, dendritic cells (DC) are a potential target of VD3 to dampen autoimmunity and inflammation, but the role of DC in VD3‐mediated immunomodulation in vivo is not understood. In addition to being targets of VD3, DC can provide a local source of bioactive VD3 for regulation of T‐cell responses. Here we review existing studies that describe the tolerogenic potential of VD3 on DC, and discuss them in the context of current understanding of DC development and function. We speculate on mechanisms that might account for the potent but poorly understood tolerogenic activities of VD3 and the role of DC as both targets and sources of this hormone.
Keywords: dendritic cells, inflammation, vitamin D
Abbreviations
- 1,25‐OH‐VD3
calcitriol
- cDC1
classical DC 1
- cDC2
classical DC2
- DC
dendritic cell
- GM‐CSF
granulocyte–macrophage colony‐stimulating factor
- GM‐DC
subpopulation of GM‐CSF‐DC with DC features
- GM‐Mac
subpopulation of GM‐CSF‐DC with macrophage features
- GMP
granulocyte–monocyte precursors
- IL‐12
interleukin‐12
- MHCII
MHC class II
- moDC
monocyte‐derived DC
- pre‐cDC
classical DC precursor
- pre‐DC
DC precursor
- pre‐μDC
mucosal DC precursor
- RA
retinoic acid
- RAR
retinoic acid receptor
- Treg
regulatory T
- VD3
vitamin D (as cholecalciferol)
- VDR
vitamin D receptor
- VitA
vitamin A
Dendritic cells (DC) orchestrate the immune response by integrating and balancing environmental signals. They assess imminent danger to the host, and then initiate, direct, amplify or limit subsequent adaptive immune reactions.1 Historically, DC have been described as motile cells within tissues that present antigen to T cells. To detect invading microorganisms or viral infections, DC sense molecular patterns that are associated with pathogens or danger, such as bacterial cell‐wall‐derived lipopolysaccharides, necrotic cell particles or extracellular nucleotides.2 Significantly, DC can also monitor host or commensal metabolism and dietary components, including short‐chain fatty acids and vitamin derivatives.3
Vitamins are organic compounds that are usually obtained from the diet and are crucial to physiological processes.4 The steroid vitamins, vitamin A (VitA) and vitamin D3 (cholecalciferol, here abbreviated as VD3) are precursors to host nuclear hormone receptor ligands that regulate transcription. Both VitA and VD3 and their deficiencies have a tremendous impact on the immune system.5 Vitamin A is solely derived from nutritional sources, recirculates in the intestinal environment via bile and significantly shapes intestinal mucosal immune and barrier functions.5, 6, 7 The source, distribution and specific immunomodulating effects of VD3 are less well understood. In addition to nutritional uptake of cholecalciferol, precursors can also be formed in superficial skin layers when UVB irradiation and spontaneous isomerization non‐enzymatically convert 7‐dehydro‐cholesterol to VD3.8 However, VD3 does not efficiently bind the intracellular receptor vitamin D receptor (VDR). Rather, cytochrome P oxidase Cyp27a1 converts it to 25‐OH‐VD3 which is then further metabolized by Cyp27b1 to 1,25‐OH‐VD3 (calcitriol), the VD3 derivative with the highest ability to activate transcriptional activity through the canonical VDR. Classical studies have shown that Cyp27a1 is highly expressed in liver, where much of 25‐OH‐VD3, the primary circulating form of the vitamin, is generated. Proximal renal tubular cells are a major site of Cyp27b1, generating the active metabolite. However, recent studies show that these enzymes are also present in various other tissues and cell types, including DC (discussed below), allowing local environmental regulation of 1,25‐OH‐VD3 levels and responses in the immune system. In spite of the generation of VD3 from sun exposed skin, VD3 bioavailability may be insufficient as the result of habits of sun avoidance or protection and concomitantly insufficient nutritional uptake. An estimated 40–100% of US or European elderly and 1 billion people worldwide do not have sufficient VD3 levels.8 Although severe complications of childhood VD3 deficiency, most notably rickets, are rarely seen nowadays in countries with western lifestyle, the epidemiological associations of autoimmune diseases with VD3 insufficiency point to the importance of a mechanistic understanding of the interactions of the immune system and VD3.8, 9 Clinical trials are currently ongoing, designed to critically evaluate the efficacy of VD3 supplementation in multiple sclerosis (NCT01490502) and Crohn's disease (NCT02208310).
The primary function of DC, to initiate and refine adaptive immune responses, positions them as potential therapeutic tools in diseases with skewed or missing T‐cell and B‐cell responses. As such, DC therapy has successfully entered the clinic for cancers,10 where cytotoxic T cells are primed by DC differentiated from easily available circulating CD14+ blood monocytes by ex vivo incubation with the tumour antigen PAP fused to granulocyte–macrophage colony‐stimulating factor (GM‐CSF). Similarly, the feasibility of DC‐based immunomodulation is being explored in the setting of autoimmune diseases, where detrimental immune reactions damage host tissue.11 Clearly, a tolerogenic approach that limited or aborted T‐cell or B‐cell responses to self antigens would represent a significant advance in the treatment or prevention of autoimmune disease. Here we consider the physiology and potential therapeutic application of VD3 in the context of the tolerogenic programmes and functions of DC.
Insights from culture models of DC generation from monocytes
Vitamin D3 has long been proposed as programming DC for tolerance, dampening their ability to activate effector T‐cell generation, while enhancing their potential to induce anti‐inflammatory regulatory T (Treg) cells. This concept, extensively reviewed,3, 5, 12, 13, 14, 15, 16, 17, 18 arose from studies of GM‐CSF‐driven differentiation of human blood CD14+ monocytes into motile cells with dendrites (moDC), capable of presenting antigen on MHC class II (MHCII).19 The properties of these in‐vitro‐generated moDC were substantially altered when 1,25‐OH‐VD3 was included during their differentiation: VD3‐‘tolerized’ moDC were less effective in their induction of T‐cell proliferation,20 but rather induced Treg cells that promoted transplant tolerance.21, 22 MoDC generated in the presence of 1,25‐OH‐VD3 were less capable of producing interleukin‐12 (IL‐12) p70,23 but rather secreted IL‐10.14, 24 These cells also possess decreased densities of the co‐stimulatory molecules CD80 and CD86 and of the antigen‐presenting MHCII complex.20, 23 Hence, with all three pillars of T‐cell activation being hampered by 1,25‐OH‐VD3 modulation of moDC development, its potential as a tolerance‐inducing agent for DC therapy, and as a therapeutic immune modulator against diseases with underlying inappropriate or overwhelming inflammation, was recognized.11, 18
VD3 in metabolic imprinting of DC
In addition to its effects on cytokine and co‐stimulatory molecule expression, VD3 alters the metabolic profile of developing moDC. Maturation and activation of DC is a thermodynamically challenging process, where fatty acid synthesis provides crucial components of endoplasmic reticulum and Golgi organelles, and increased energy is necessary for migration and plasticity.25 To meet these needs, glycolysis breaks down glucose for ATP generation. The resulting pyruvate can be metabolized to acetyl‐CoA and subsequently used for fatty acid genesis, or it can fuel the tricarboxylic acid cycle to create CO2 and proton donors. The latter then drive the respiratory chain to create ATP, a process called oxidative phosphorylation of glucose. However, energy can also be generated without an obligatory need for oxygen through anaerobic glycolysis and consequential excretion of glucose‐derived lactate, useful particularly in relatively anaerobic (hypoxic) environments, as in sites of tissue damage. Rapidly growing tumour cells also show this method of glucose breakdown, but independently of the presence or absence oxygen – then termed aerobic glycolysis, or the Warburg effect.26 The decisive triggers to induce oxidation‐independent Warburg metabolism, and its benefits for a single cell or the organism are still not completely understood.26 Generally, the anabolic needs to synthesize biomolecules during activation, cell division or expansion are supported by Warburg metabolism, converting glucose into carbon donors for fatty acid synthesis or pentose for nucleotide synthesis. At the same time, anabolic use of carbon or excretion of lactate rather than oxidative phosphorylation helps to avoid a build up of glycolysis metabolites. This Warburg metabolic pathway has therefore been proposed as a general anabolic principle of activated and proliferating cells, while glucose breakdown through oxidative phosphorylation is a characteristic of differentiated or resting cells – not only for tumours, but also in normal tissue and the immune system.26, 27 Indeed, in murine GM‐CSF‐DC (see below), Toll‐like receptor stimulation promotes increased anabolic glycolysis rather than oxidative phosphorylation, which is crucial for DC activation, survival and function in the face of increased membrane‐forming demands due to secretory processes or migration.28, 29, 30, 31 However, when 1,25‐OH‐VD3 is given to human monocytes undergoing GM‐CSF differentiation to create tolerogenic DC (as outlined above), an early transcriptional programme is started that engages oxidative phosphorylation.32, 33, 34 By sustaining oxidative phosphorylation as a mode of glucose breakdown, the metabolic pattern used by quiescent cells, VD3 may support or favour immune quiescence and tolerance. This metabolic effect of VD3 could help to explain the association of VD3 deficiency with autoimmune syndromes, and the potentially beneficial effects of VD3 supplementation. Additional studies will be required to further elucidate the specific mechanisms and consequences of metabolism control by VD3.
Potential relevance of culture models to inflammatory (monocyte‐derived) DC in vivo
A potential problem with these seminal studies is that moDC may not be representative of DC in vivo. The artificial culture conditions used to generate them from blood‐derived CD14+ monocytes clearly lack many or most factors (cellular and soluble) that their precursors would experience in vivo. The moDC are perhaps most similar to human CD14+or mouse CX3CR1intermediate Ly6Chigh monocyte‐derived inflammatory DC35 that differentiate from blood‐circulating monocytes in target tissues.36 Although these represent a minor subset of DC in vivo in most settings, they may be important targets of VD3 immunoregulation. Notably, steady‐state intestinal lamina propria leucocytes also contain a population of CD11b+/Sirpa+ CD103– MHCII+ ‘DC’ that originate from monocytes,37 possibly differentiated in the low‐grade and restricted inflammatory setting of the lamina propria (induced by the constant sensing of luminal bacteria). CD11b+/Sirpa+ CD103– DC were increased in frequency in human small intestinal lamina propria with gross findings of inflammation, and these DC showed transcriptional signatures consistant with monocyte derivation as well.38 In experimental autoimmune encephalomyelitis (a murine multiple sclerosis model), central nervous system‐infiltrating CCR2+ monocytes differentiate into pathogenic moDC under the influence of endogenous GM‐CSF and produce IL‐1β to recruit further effector cells initiating and fuelling T‐cell‐mediated pathology. Specific deletion of the GM‐CSF receptor on these monocytes, but not on DC, diminished disease severity.39 Multiple sclerosis is associated with VD3 deficiency; its murine model experimental autoimmune encephalomyelitis, and possibly also multiple sclerosis itself, is ameliorated by VD3 supplementation.40, 41 Hence, we speculate that, paralleling the in vitro studies of moDC outlined above, high VD3 conditions in vivo may redirect monocyte differentiation into tolerogenic versus inflammatory DC and contribute to the potential therapeutic effects of such supplementation. It will be important to characterize moDC, generated under VD3‐deficient versus VD3‐sufficient settings in vivo, to validate this hypothesis.
VD3 and DC precursor (pre‐DC) derived DC in the mouse
Recent studies refined our understanding about DC biology, in particular by dissecting the exact ontogeny of subsets that derive from specialized classical DC precursors (pre‐cDC), such as cross‐presenting classical DC 1 (cDC1) or CD4 T‐cell‐stimulating cDC2. Such DC are phenotypically and ontogenically distinct from monocyte‐derived, redifferentiated in vivo moDC that originate from the committed monocyte progenitor36, 42, 43 (Fig. 1). The great majority of ‘professional’ DC in vivo derive from bone marrow pre‐DC.1 In this regard, studies addressing the effects of VD3 on murine DC have mostly cultured whole bone marrow with GM‐CSF or GM‐SCF + IL‐4 to generate CD11c+ cells (GM‐CSF‐DC). In such systems, CD11c+ MHCII+ progeny (fulfilling classical DC definitions) comprise a significant portion of MHCIIintermediate cells having a phenotypic, developmental and transcriptional profile reminiscent of macrophages (‘GM‐Mac’). The MHC‐IIhigh ‘GM‐DC’ are indeed bona fide DC, depend on interferon regulatory factor 4, are probably derived from pre‐cDC and efficiently present antigen to CD4 T cells.44, 45, 46 Using this differentiation protocol, a framework similar to that for human moDC was developed where murine DC are rendered tolerogenic by 1,25‐OH‐VD3 during differentiation. These cells secrete fewer pro‐inflammatory cytokines, express lower levels of co‐stimulatory molecules, and propagate Treg cell conversion and effector T‐cell hyporesponsiveness.47 According to microarray analyses, the above‐mentioned MHC‐IIhigh ‘GM‐DC’ but not MHCIIintermediate ‘GM‐Mac’ express VDR,44 so they are probably the subpopulation described as 1,25‐OH‐D3‐responsive. However, as for human moDC, murine GM‐DC have no exact in vivo counterpart. They show transcriptional hallmarks only partly overlapping with in vivo migratory DC, and are clearly distinct from in vivo lymph‐node‐resident or splenic DC.46
Figure 1.
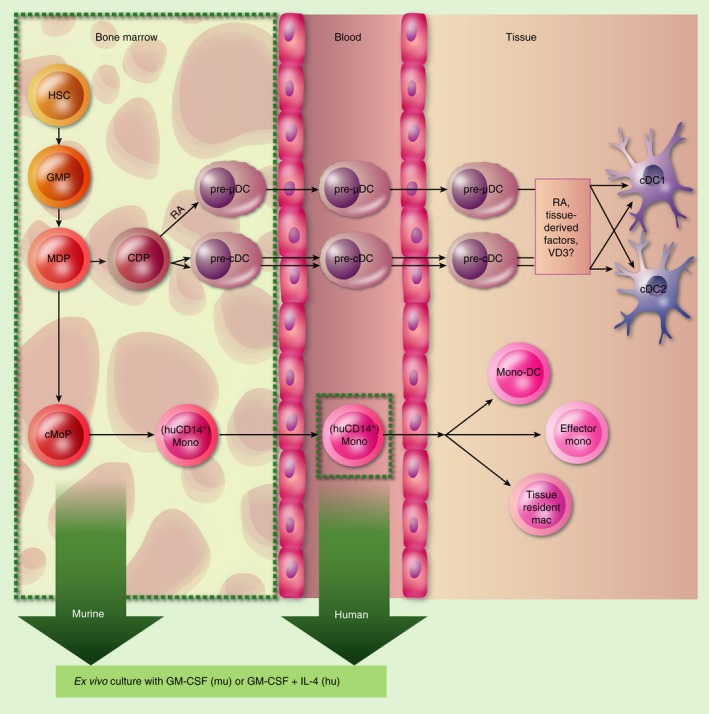
Overview of monocyte‐ and dendritic cell (DC) precursor‐dendritic DC differentiation in vitro and in vivo. Haematopoeitic stem cell‐derived granulocyte–monocyte precursors (GMP) give rise to monocyte‐and‐DC precursors (MDP), after which developmental pathways segregate into a DC lineage with common DC precursors (CDP) and into a monocytic lineage with common monocyte precursors (cMoP). CDP‐derived precursors exhibit early specialization to gut‐tropic mucosal DC precursors (pre‐μDC), regulated by retinoic acid (RA)6, 49 and classical DC precursors (pre‐cDC), and within the latter into cDC1 or cDC2 lineages in the bone marrow.80 Pre‐cDC and pre‐μDC then circulate through blood and home to tissues where they complete their differentiation under tissue‐specific influences.1, 6, 38, 49 The cMoP differentiate to human CD14+/mouse Ly6C+ monocytes and circulate through blood. Upon migration to tissues, they can fulfil various microenvironmentally specific functions.36 Green boxes delineate populations used for GM‐colony‐stimulating factor (GM‐CSF)‐dependent differentiation protocols in most studies addressing DC–vitamin D3 (VD3) interactions.
Hence, the recently improved understanding of DC biology raises questions of whether or not the effects seen in human moDC and in murine in‐vitro‐differentiated GM‐CSF‐DC truly reflect the biology of tissue‐resident DC. The latter still receive multiple input signals from their microenvironment (such as Wnt proteins48) that can shape their capacity to stimulate T cells, and which are often absent or present at non‐physiological levels in in vitro settings. Indeed, the VitA derivative retinoic acid (RA) has a crucial role in DC differentiation: not only does it induce the formation of intestine‐homing pre‐mucosal DC (pre‐μDC) in the bone marrow,49 but it also shapes further differentiation and function in target tissues through its receptor RAR.6, 50, 51, 52, 53 In contrast, a similar role of VD3 and VDR in this context is not known. VD3, Cyp27b1 or VDR deficiencies have a myriad of effects on immune cells and other compartments, such as an impaired intestinal epithelial barrier,54 altered calcium homeostasis55 and constant low‐grade inflammation with elevated IL‐6,56 which makes studies of DC biology in these mice problematic due to secondary effects. Future studies with novel genetic tools, single‐cell‐based, or single‐population‐based analyses and corresponding bioinformatics should help to clarify the cell‐specific influence of VD3 on DC in vivo, with possible implications for patients. Specifically, they will have to determine: which DC in vivo are targeted by 1,25‐OH‐VD3, and at which developmental state; what their ensuing immune functions are; and how dietary supplemented or sun‐induced VD3 participates.
VD3‐programmed DC for immune therapy
Although in vivo counterparts that mirror the VD3‐dependent effects observed in cultured DC remain to be described, in‐vitro‐generated moDC are clearly capable of manipulating adaptive immune responses and are being used in the clinic.10, 57 Hence, the transfer of in‐vitro‐differentiated, VD3‐primed DC bears potential to alter autoinflammatory diseases.58, 59 Protocols to differentiate moDC use purification of CD14+ blood cells, and most likely omit dedicated DC precursors, which circulate in human peripheral blood in low numbers. These blood‐borne pre‐DC can be induced to differentiate into bona fide DC ex vivo.60, 61 For future DC‐based immunotherapy, it will be of interest to compare the tolerogenic potential of preDC‐derived DC and monocyte‐derived DC generated ex vivo with or without VD3. Still, general mechanisms of adoptive DC therapy need to be addressed further, as tolerogenic DC may be harmful when broadly impairing immunosurveillance during malignancies or infection,62 or when presenting loaded antigens in a pro‐inflammatory setting.
DC as a source of 1,25‐OH‐VD3 for T cell programming
In addition to its potential to skew DC development, VD3 directly modulates T‐cell responses. The presence of 1,25‐OH‐VD3 during T‐cell activation inhibits their proliferation, favours Treg cell development, and alters trafficking receptor expression.63, 64, 65 However, circulating levels of 1,25‐OH‐VD3 in vivo are too low to mediate these effects,8, 66 and 25‐OH‐VD3, the major circulating form of the vitamin, does not itself activate VDR‐dependent transcription. Hence, 1,25‐OH‐VD3 must be generated locally to effect T‐cell programming, and DC can fulfil this function: both in‐vitro‐derived human moDC and subsets of in vivo DC can generate and present the hormone to T cells.67 At the level of gene expression, all physiological in vivo subsets of mouse DC, including plasmacytoid DC, and both cDC1 and cDC2 express the 25‐hydroxylase Cyp27a1 (Immgen database, http://www.immgen.org/). In contrast, none of the analysed mouse DC subsets expressed Cyp27b1 above a threshold level of detection (Immgen). However, other studies have reported the expression and metabolic activity of Cyp27b1 in human moDC that subsequently influences T‐cell activity, although the degree to which DC can produce 1,25‐OH‐VD3 in vitro varies with their activation and differentiation status.23, 67, 68, 69 Similarly to macrophages, direct stimulation of human and mouse GM‐CSF‐DC with pathogen‐associated molecular pattern or pro‐inflammatory cytokines triggers Cyp27b1 expression, as does T‐cell contact, especially in a pro‐inflammatory cytokine milieu.23, 69, 70, 71 Moreover, DC isolated from skin‐draining afferent lymph of sheep have also been shown to metabolize VD3 to 1,25‐OH‐VD3.67 Interestingly, human T cells also express Cyp27b1 upon activation and so can carry out the final VD3 conversion step on their own.67 Hence, when Cyp27b1 is not expressed by DC, activated T cells and DC together can still convert VD3 to 1,25‐OH‐VD3 through cross‐cellular metabolism, a process that has been demonstrated experimentally in IL‐12‐stimulated co‐cultures of human moDC and naive peripheral blood T cells.67
In differentiating moDC, 25‐OH‐VD3 at concentrations similar to those in serum (10–100 nm) is sufficient for DC generation of 1,25‐OH‐VD3 and autocrine imprinting of a tolerogenic phenotype (i.e. T‐cell hyporesponsiveness induction or metabolic switches),23, 32 but these serum 25‐OH‐VD3 levels are too low for the autocrine/paracrine induction of CCR10 on T cells in moDC : T‐cell co‐cultures67 (Fig. 2). We speculate that local concentrations above those present in circulation are induced in skin and draining lymph by UVB irradiation and confine epidermotropism in T cells through expression of CCR10, a chemoattractant receptor for the keratinocyte‐expressed chemokine CCL28 while suppressing gut‐homing properties (integrin α 4 β 7 and CCR9 expression).67 In contrast, tolerogenic imprinting of DC might be a broader, not skin‐specific mechanism of immunomodulation. If the hormone were presented in a targeted fashion at the immunological synapse, its action could be limited to responding T cells. Local diffusion could affect nearby bystander cells such as stromal fibroblasts or endothelial cells. Such paracrine secretion would therefore influence the tissue‐specific properties of immune instruction. Perhaps skin‐draining DC, which can synthesize 1,25‐OH‐VD3 from D3,67 also transport VD3 after sun exposure, creating a characteristic environment in skin‐draining lymph nodes. This may provide a mechanism to direct skin‐homing T cells to the epidermis in response to sun‐induced damage, where VD3 is high due to UVB‐induced conversion of 7‐dehydro‐cholesterol and subsequent isomerization. Indeed, in the parallel VitA/RA system, DC draining the intestine are thought not only to express RA synthetic capability, but also to transport RA or its precursors for processing and presentation in the draining mesenteric lymph node.5, 66, 72
Figure 2.
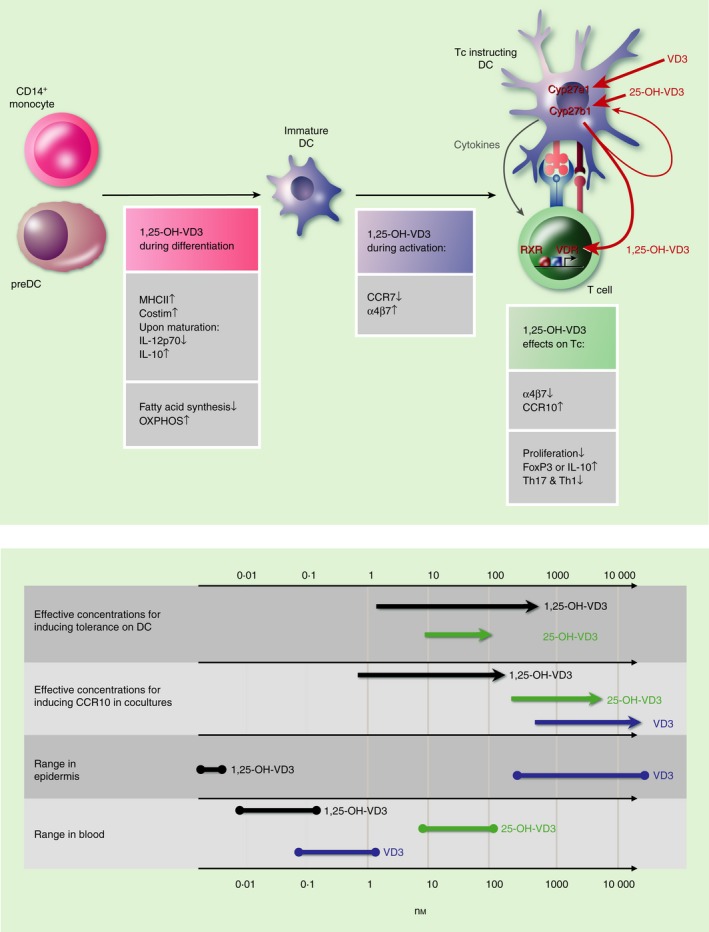
Calcitriol (1,25‐OH‐VD3) and dendritic cell (DC) function during different stages of immune activation. Upper panel, left: 1,25‐OH‐VD3 acting on granulocyte–macrophage colony‐stimulating factor (GM‐CSF) ‐differentiating precursors renders DC ‘tolerogenic’ by inhibiting T‐cell stimulation pillars, especially after DC maturation (e.g. performed with tumour necrosis factor‐α or lipopolysaccharide). Also, profound metabolic patterns are induced to promote oxidative phosphorylation and reduce fatty acid synthesis. Middle: 1,25‐OH‐VD3 during DC maturation alters trafficking receptor profiles to redirect migration (see text). Right: Maturation induces DC‐intrinsic up‐regulation of Cyp27b1. Paracrine DC‐derived 1,25‐OH‐VD3 serves as a fourth pillar of DC : T‐cell interaction and promotes regulatory T‐cell generation, inhibits effector T‐cell proliferation and imprints trafficking patterns in instructed T cells. Autocrine 1,25‐OH‐VD3 possibly has effects on DC themselves as well. Lower panel: range of concentrations of VD3 and metabolites in blood and epidermis in vivo and required for immune effects in vitro.
1,25‐OH‐VD3 can be locally inactivated by Cyp24a1‐mediated hydroxylation to a metabolite without VDR‐dependent transcriptional activity. In this way, 1,25‐OH‐VD3‐induced autocrine Cyp24a1 induction can form a negative feedback loop of local VD3 activity in vivo. Although differential Cyp24a1 levels have been reported for in‐vitro‐differentiated moDC versus macrophages,68 the relevance of this loop is unclear for the control of immune cell trafficking. Future studies will be required to elucidate potential region‐specific T‐cell trafficking patterns induced by VD3 generation and degradation, similar to those outlined for VitA/RA and gut trafficking in recent years.
VD3 influences the migratory patterns of DC
Vaccination has proven to be the most successful disease‐preventing medical intervention. Although discovered centuries ago, the exact mechanisms responsible for an induction of competent T‐cell and B‐cell responses have been subject to continuous debate. Generally, a major pitfall is the poor induction of cross‐compartment specific immune responses, specifically the protection of mucosal surfaces (a main entry site for many pathogens) after subcutaneous or intramuscular vaccinations. In contrast, oral or intranasal application together with cholera toxin induces potent mucosal protection, but toxicity limits its clinical use. 1,25‐OH‐VD3, when co‐administered subcutaneously with the adjuvant alum has been shown to overcome this limitation in a murine model, initiating an intestinal and pulmonary IgA response after subcutaneous vaccination.73 One hypothesis for these observations is an altered migratory pattern of DC originating at the site of vaccination. Bone‐marrow‐derived GM‐CSF‐DC that were matured (after GM‐CSF differentiation) with lipopolysaccharide and concomitant 1,25‐OH‐VD3 showed an altered migratory pattern when injected subcutaneously.74 Whereas control DC readily migrated to the draining lymph node, their CCR7 expression sequesters them at sites of CCL19/21 production in the first lymph node they encounter, where they then fulfil their bona fide role of presenting antigen. In contrast, maturation in the presence of 1,25‐OH‐VD3 rapidly but transiently decreased the surface CCR7 expression of GM‐CSF‐DC, allowing them to migrate beyond this initial encounter to non‐draining lymph nodes and Peyer's patches through a transient up‐regulation of intestinal‐targeting integrin α 4 β 7 and associated with expression of the intestinal cDC2 markers CD103 and DCIR2/Clec4a4.74, 75 Notably, such altered trafficking patterns were also observed for endogenous tissue‐resident DC following subcutaneous injection of fluorescent microspheres and concomitant 1,25‐OH‐VD3 injection.74 These migrated DC then fulfilled their T‐cell instructing role in the respective sites: while activated T cells specific for the vaccinating antigen were only found in draining lymph nodes in the absence of 1,25‐OH‐VD3 co‐vaccination, 1,25‐OH‐VD3 co‐vaccination resulted in DC‐instructed antigen‐specific T cells in both draining and non‐draining lymph nodes as well as in Peyer's patches.74 In related studies of a human skin explant model, intradermally injected 1,25‐OH‐VD3 increased the migration of dermal CD14+ DC, a monocyte‐derived DC population,76 while repressing the T‐cell stimulatory capacities of total migrating DC.77 Hence, the presence of 1,25‐OH‐VD3 during DC activation shapes their migratory capacity upon antigen uptake, and potentially modulates the scope of their induced T‐cell responses not with respect to antigen specificity, but rather to T‐cell skewing and trafficking potential. 1,25‐OH‐VD3 regulation of DC migratory properties during antigen responses may therefore represent an additional mechanism for regulation of immune responses by local VD3 metabolism. Similarly to UVB‐induced VD3 conversion in skin, intestinal infection, epithelial barrier breach and inflammation might induce local Cyp27b1 in lamina propria DC or macrophages, which could result in conversion of dietary or systemic VD3 metabolites and act on intestinal DC during their activation. In this scenario, VDR signalling in DC could compete with VitA effects. Indeed, the canonical nuclear receptors for VD3 and RA, VDR and RARα, often counteract each other's signalling, potentially through competition for their common heterodimeric partner, RXR. Such competition has been documented for T cells: RA induction of integrin α 4 β 7‐mediated and CCR9‐mediated intestinal T‐cell homing is antagonized by 1,25‐OH‐VD3 in vitro.67 However, VD3 can also cooperate with RA to induce VitA metabolizing enzymes in human (but not mouse) intestine‐derived or blood‐derived DC subsets,78 whereas it suppresses them in mouse GM‐CSF‐DC.79 Hence, the interplay of vitamins A and D and their role in shaping immune responses still requires further investigation; it is likely to be context‐, cell‐type‐ and species‐dependent.
In conclusion, seminal studies show that DC can metabolize VD3 for programming of T cells, and suggest that 1,25‐OH‐VD3 also interacts directly with DC to influence their migration and their capacity to instruct T cells and hence to initiate, fine tune or dampen immune reactions. However, our understanding of the complexity of both VD3 and DC biology has grown considerably in recent years, and additional studies are required to address the role of DC–VD3 interactions in the potentially beneficial effects of VD3 supplementation reported in some autoimmune and inflammatory diseases. Understanding the diverse mechanisms of VD3 action will be crucial for the appropriate application of VD3 supplementation for therapy or prophylaxis that might evolve from currently ongoing clinical trials in autoimmune disease.
Disclosures
The authors disclose no conflicts.
References
- 1. Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 2013; 31:563–604. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3853342&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol 2015; 16:343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiss M, Czimmerer Z, Nagy L. The role of lipid‐activated nuclear receptors in shaping macrophage and dendritic cell function: from physiology to pathology. J Allergy Clin Immunol 2013; 132:264–86. URL http://dx.doi.org/10.1016/j.jaci.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 4. Veldhoen M, Brucklacher‐Waldert V. Dietary influences on intestinal immunity. Nat Rev Immunol 2012; 12:696–708. URL http://www.ncbi.nlm.nih.gov/pubmed/23007570. [DOI] [PubMed] [Google Scholar]
- 5. Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 2008; 8:685–98. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2906676&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zeng R, Bscheider M, Lahl K, Lee M, Butcher EC. Generation and transcriptional programming of intestinal dendritic cells: essential role of retinoic acid. Mucosal Immunol 2015; 9:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spencer SP, Wilhelm C, Yang Q, Hall J a, Bouladoux N, Boyd a et al Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 2014; 343:432–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24458645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holick MF. Vitamin D Deficiency. N Engl J Med 2007; 357:266–81. [DOI] [PubMed] [Google Scholar]
- 9. Yang C‐Y, Leung PSC, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol 2013; 45:217–26. URL http://www.ncbi.nlm.nih.gov/pubmed/23359064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF et al Sipuleucel‐T immunotherapy for castration‐resistant prostate cancer. N Engl J Med 2010;2373–83. [DOI] [PubMed] [Google Scholar]
- 11. van Brussel I, Lee WP, Rombouts M, Nuyts AH, Heylen M, de Winter BY et al Tolerogenic dendritic cell vaccines to treat autoimmune diseases: can the unattainable dream turn into reality? Autoimmun Rev 2014; 13:138–50. URL http://dx.doi.org/10.1016/j.autrev.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 12. Adams JS, Hewison M. Extrarenal expression of the 25‐hydroxyvitamin D‐1‐hydroxylase. Arch Biochem Biophys 2012; 523:95–102. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3361592&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hart PH, Gorman S, Finlay‐Jones JJ. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol 2011; 11:584–96. URL http://dx.doi.org/10.1038/nri3045. [DOI] [PubMed] [Google Scholar]
- 14. Nikolic T, Roep BO. Regulatory multitasking of tolerogenic dendritic cells – lessons taken from vitamin d3‐treated tolerogenic dendritic cells. Front Immunol 2013; 4:113. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3653108&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hewison M, Adams JS. Extrarenal 1a‐hydroxylase. Vitam D 2011;777–804..URL http://www.crossref.org/deleted_DOI.html
- 16. Adorini L. Control of adaptive immunity by vitamin D receptor agonists [Internet]. 3rd edn. Vitamin D. Elsevier; 2011. 1789–809pp. URL http://dx.doi.org/10.1016/B978-0-12-381978-9.10092-7. [Google Scholar]
- 17. Barragan M, Good M, Kolls J. Regulation of dendritic cell function by vitamin D. Nutrients 2015; 7:8127–51. URL http://www.mdpi.com/2072-6643/7/9/5383/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adorini L, Penna G. Induction of tolerogenic dendritic cells by vitamin D receptor agonists. Handb Exp Pharmacol 2009; 188:251–73. [DOI] [PubMed] [Google Scholar]
- 19. Romani N, Gruner S, Brang D, Eckhart K, Lenz A, Trockenbacher B et al Proliferating dendritic cell progenitors in human blood. J Exp Med 1994; 180:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Penna G, Adorini L. 1,25‐Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 2000; 164:2405–11. URL http://www.jimmunol.org/cgi/doi/10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 21. Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli A M, Adorini L. Regulatory T cells induced by 1,25‐dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol 2001; 167:1945–53. URL http://www.jimmunol.org/cgi/doi/10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 22. Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S et al 1,25‐Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol 2006; 178:145–53. URL http://www.jimmunol.org/cgi/doi/10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 23. Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG et al Differential regulation of vitamin d receptor and its ligand in human monocyte‐derived dendritic cells. J Immunol 2003; 170:5382–90. URL http://www.jimmunol.org/cgi/doi/10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 24. Van Halteren AGS, Van Etten E, De Jong EC, Bouillon R, Roep BO, Mathieu C. Redirection of human autoreactive T‐cells upon interaction with dendritic cells modulated by TX527, an analog of 1,25 dihydroxyvitamin D3. Diabetes 2002; 51:2119–25. [DOI] [PubMed] [Google Scholar]
- 25. Pearce EJ, Everts B.Dendritic cell metabolism. Nat Publ Gr [Internet]. Nature Publishing Group 2015;15:18–29. URL http://dx.doi.org/10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heiden MG Vander, Cantley LC, Thompson CB, Mammalian P, Exhibit C, Metabolism A. Understanding the Warburg Effect: cell proliferation. Science 2009; 324:1029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pearce E, Pearce E. Metabolic pathways in immune cell activation and quiescence. Immunity 2013; 38:633–43. URL http://dx.doi.org/10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, Deberardinis RJ et al Toll‐like receptor‐induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 2010; 115:4742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Everts B, Amiel E, Van Der Windt GJW, Freitas TC, Chott R, Yarasheski KE et al Commitment to glycolysis sustains survival of NO‐producing inflammatory dendritic cells. Blood 2012; 120:1422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Everts B, Amiel E, Huang SC‐C, Smith AM, Chang C‐H, Lam WY et al TLR‐driven early glycolytic reprogramming via the kinases TBK1‐IKKɛ supports the anabolic demands of dendritic cell activation. Nat Immunol 2014; 15:323–32. URL http://www.ncbi.nlm.nih.gov/pubmed/24562310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou XY, Tomatsu S, Fleming RE, Parkkila S, Waheed a, Jiang J et al HFE gene knockout produces mouse model of hereditary hemochromatosis. Proc Natl Acad Sci U S A 1998; 95:2492–7. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=19387&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferreira GB, Vanherwegen A‐S, Eelen G, Gutiérrez ACF, Van Lommel L, Marchal K et al Vitamin D3 induces tolerance in human dendritic cells by activation of intracellular metabolic pathways. Cell Rep [Internet]. 2015;10:711–25. URL http://linkinghub.elsevier.com/retrieve/pii/S2211124715000261. [DOI] [PubMed] [Google Scholar]
- 33. Ferreira GB, Overbergh L, Verstuyf A, Mathieu C. 1α,25‐Dihydroxyvitamin D3 and its analogs as modulators of human dendritic cells: a comparison dose‐titration study. J Steroid Biochem Mol Biol 2013; 136:160–5.. URL http://www.ncbi.nlm.nih.gov/pubmed/23098690. [DOI] [PubMed] [Google Scholar]
- 34. Ferreira GB, Van Etten E, Lage K, Hansen DA, Moreau Y, Workman CT, et al Proteome analysis demonstrates profound alterations in human dendritic cell nature by TX527, an analogue of vitamin D. Proteomics 2009; 9:3752–64. [DOI] [PubMed] [Google Scholar]
- 35. Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B et al Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010; 33:375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization [Internet]. Annual review of immunology. 2015. 643–75 pp. URL http://www.annualreviews.org/doi/abs/10.1146/annurev-immunol-032414-112220. [DOI] [PubMed]
- 37. Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M et al Origin of the lamina propria dendritic cell network. Immunity [Internet]. Elsevier Ltd 2009. 31:513–25. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2778256&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Watchmaker PB, Lahl K, Lee M, Baumjohann D, Morton J, Kim SJ et al Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nat Immunol 2014; 15:98–108. URL http://www.ncbi.nlm.nih.gov/pubmed/24292363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Croxford AL, Lanzinger M, Hartmann FJ, Schreiner B, Mair F, Pelczar P et al The cytokine GM‐CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity 2015; 43:1–13. URL http://linkinghub.elsevier.com/retrieve/pii/S1074761315003222. [DOI] [PubMed] [Google Scholar]
- 40. Smolders J, Damoiseaux J, Menheere P, Hupperts R. Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol 2008; 194:7–17. [DOI] [PubMed] [Google Scholar]
- 41. Soilu‐Hanninen M, Aivo J, Lindstrom B‐M, Elovaara I, Sumelahti M‐L, Farkkila M et al A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon‐1β in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2012; 83:565–71. URL http://jnnp.bmj.com/cgi/doi/10.1136/jnnp-2011-301876. [DOI] [PubMed] [Google Scholar]
- 42. Hettinger J, Richards DM, Hansson J, Barra MM, Joschko A‐C, Krijgsveld J et al Origin of monocytes and macrophages in a committed progenitor. Nat Immunol 2013; 14:821–30. URL http://www.ncbi.nlm.nih.gov/pubmed/23812096. [DOI] [PubMed] [Google Scholar]
- 43. Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU et al Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 2014; 14:571–8. URL http://www.ncbi.nlm.nih.gov/pubmed/25033907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Helft J, Böttcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU et al GM‐CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c+MHCII+ macrophages and dendritic cells. Immunity 2015; 42:1197–211. URL http://linkinghub.elsevier.com/retrieve/pii/S1074761315002162. [DOI] [PubMed] [Google Scholar]
- 45. Gao Y, Nish S, Jiang R, Hou L, Licona‐Limón P, Weinstein J et al Control of T helper 2 responses by transcription factor IRF4‐dependent dendritic cells. Immunity 2013; 39:722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vander Lugt B, Khan A a, Hackney J a, Agrawal S, Lesch J, Zhou M et al Transcriptional programming of dendritic cells for enhanced MHC class II antigen presentation. Nat Immunol 2014; 15:161–7 URL http://www.ncbi.nlm.nih.gov/pubmed/24362890. [DOI] [PubMed] [Google Scholar]
- 47. Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1α,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor‐dependent pathway that promotes a persistent state of immaturity in vitro and in vivo . Proc Natl Acad Sci U S A 2001; 98:6800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oderup C, LaJevic M, Butcher EC. Canonical and noncanonical Wnt proteins program dendritic cell responses for tolerance. J Immunol 2013; 190:6126–34. URL http://www.ncbi.nlm.nih.gov/pubmed/23677472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zeng R, Oderup C, Yuan R, Lee M, Habtezion a, Hadeiba H et al Retinoic acid regulates the development of a gut‐homing precursor for intestinal dendritic cells. Mucosal Immunol 2013; 6:847–56. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3612556&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klebanoff C a, Spencer SP, Torabi‐Parizi P, Grainger JR, Roychoudhuri R, Ji Y et al Retinoic acid controls the homeostasis of pre‐cDC‐derived splenic and intestinal dendritic cells. J Exp Med 2013; 210:1961–76. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3782040&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis‐protective regulatory T‐cell differentiation through dendritic cell conditioning. Mucosal Immunol 2009; 2:340–50. URL http://dx.doi.org/10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 52. Molenaar R, Knippenberg M, Goverse G, Olivier BJ, de Vos AF, O'Toole T et al Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut‐draining lymph node stromal cells is controlled by dietary vitamin A. J Immunol 2011; 186:1934–42. URL http://www.ncbi.nlm.nih.gov/pubmed/21220692. [DOI] [PubMed] [Google Scholar]
- 53. Jaensson‐Gyllenbäck E, Kotarsky K, Zapata F, Persson EK, Gundersen TE, Blomhoff R et al Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut‐tropic T cells. Mucosal Immunol 2014; 4:438–47. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3130189&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate‐induced colitis. J Nutr 2013; 143:1679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008; 29:726–76. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2583388&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wu S, Liao AP, Xia Y, Li YC, Li J‐D, Sartor RB et al Vitamin D receptor negatively regulates bacterial‐stimulated NF‐κB activity in intestine. Am J Pathol 2010; 177:686–97. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2913341&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Palucka K, Banchereau J. Dendritic‐cell‐based therapeutic cancer vaccines. Immunity 2013; 39:38–48. URL http://dx.doi.org/10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chu C‐C, Ali N, Karagiannis P, Di Meglio P, Skowera A, Napolitano L et al Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL‐10 and induce regulatory T cells that suppress skin inflammation. J Exp Med 2012; 209:935–45. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3348099&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ferreira GB, Gysemans C a, Demengeot J, da Cunha JPMCM, Vanherwegen A‐S, Overbergh L et al 1,25‐Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J Immunol 2014; 192:4210–20. URL http://www.ncbi.nlm.nih.gov/pubmed/24663679. [DOI] [PubMed] [Google Scholar]
- 60. Lee J, Breton G, Oliveira TYK, Zhou YJ, Aljoufi a., Puhr S et al Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J Exp Med 2015; 212:385–399. URL http://www.jem.org/cgi/doi/10.1084/jem.20141442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Breton G, Lee J, Zhou YJ, Schreiber JJ, Keler T, Puhr S et al Circulating precursors of human CD1c+ and CD141+ dendritic cells. J Exp Med 2015; 212:401–413. URL http://www.jem.org/cgi/doi/10.1084/jem.20141441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Caspi RR. Immunotherapy of autoimmunity and cancer: the penalty for success. Nat Rev Immunol 2008; 8:970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kongsbak M, Levring TB, Geisler C, von Essen MR. The vitamin d receptor and T cell function. Front Immunol 2013; 4:148 URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3684798&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cantorna MT, Waddell A. The vitamin D receptor turns off chronically activated T cells. Ann N Y Acad Sci 2014; 1317:70–5. URL http://www.ncbi.nlm.nih.gov/pubmed/24673331. [DOI] [PubMed] [Google Scholar]
- 65. Palmer MT, Lee YK, Maynard CL, Oliver JR, Bikle DD, Jetten AM et al Lineage‐specific effects of 1,25‐dihydroxyvitamin D3 on the development of effector CD4 T cells. J Biol Chem 2011; 286:997–1004. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3020784&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue‐selective lymphocyte trafficking. Nat Immunol 2008; 9:981–7. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3171274&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D et al DCs metabolize sunlight‐induced vitamin D3 to “program” T cell attraction to the epidermal chemokine CCL27. Nat Immunol 2007; 8:285–93. URL http://www.ncbi.nlm.nih.gov/pubmed/17259988. [DOI] [PubMed] [Google Scholar]
- 68. Kundu R, Chain BM, Coussens AK, Khoo B, Noursadeghi M. Regulation of CYP27B1 and CYP24A1 hydroxylases limits cell‐autonomous activation of vitamin D in dendritic cells. Eur J Immunol 2014; 44:1781–90. URL http://www.ncbi.nlm.nih.gov/pubmed/24643654. [DOI] [PubMed] [Google Scholar]
- 69. Széles L, Keresztes G, Töröcsik D, Balajthy Z, Krenács L, Póliska S et al 1,25‐dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol 2009; 182:2074–83. [DOI] [PubMed] [Google Scholar]
- 70. Jeffery LE, Wood AM, Qureshi OS, Hou TZ, Gardner D, Briggs Z et al Availability of 25‐hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol 2012; 189:5155–64. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3504609&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Enioutina EYY, Bareyan D, Daynes R a. TLR‐induced local metabolism of vitamin D3 plays an important role in the diversification of adaptive immune responses. J Immunol 2009; 182:4296–305. URL http://www.ncbi.nlm.nih.gov/pubmed/19299729. [DOI] [PubMed] [Google Scholar]
- 72. Sun C‐M, Hall J a, Blank RB, Bouladoux N, Oukka M, Mora JR et al Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 2007; 204:1775–85. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2118682&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Daynes RA, Enioutina EY, Butler S, Mu HH, McGee ZA, Araneo BA. Induction of common mucosal immunity by hormonally immunomodulated peripheral immunization. Infect Immun 1996; 64:1100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Enioutina EY, Bareyan D, Daynes RA. Vitamin D3‐mediated alterations to myeloid dendritic cell trafficking in vivo expand the scope of their antigen presenting properties. Vaccine 2007; 25:1236–49. [DOI] [PubMed] [Google Scholar]
- 75. Enioutina EY, Bareyan D, Daynes RA. TLR ligands that stimulate the metabolism of vitamin D3 in activated murine dendritic cells can function as effective mucosal adjuvants to subcutaneously administered vaccines. Vaccine 2008; 26:601–13. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2632860&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McGovern N, Schlitzer A, Gunawan M, Jardine L, Shin A, Poyner E et al Human dermal CD14+ cells are a transient population of monocyte‐derived macrophages. Immunity 2014; 41:465–77. URL http://linkinghub.elsevier.com/retrieve/pii/S1074761314003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bakdash G, Schneider LP, Van Capel TMM, Kapsenberg ML, Teunissen MBM, De Jong EC. Intradermal application of vitamin D3 increases migration of CD14+ dermal dendritic cells and promotes the development of Foxp3+ regulatory T cells. Hum Vaccines Immunother 2015; 9:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sato T, Kitawaki T, Fujita H, Iwata M, Iyoda T, Inaba K et al Human CD1c+ myeloid dendritic cells acquire a high level of retinoic acid‐producing capacity in response to vitamin D₃. J Immunol 2013; 191:3152–60. URL http://www.ncbi.nlm.nih.gov/pubmed/23966631. [DOI] [PubMed] [Google Scholar]
- 79. Chang S‐Y, Cha H‐R, Chang J‐H, Ko H‐J, Yang H, Malissen B et al Lack of retinoic acid leads to increased langerin‐expressing dendritic cells in gut‐associated lymphoid tissues. Gastroenterology 2010; 138:1468–78. URL http://www.ncbi.nlm.nih.gov/pubmed/19914251. [DOI] [PubMed] [Google Scholar]
- 80. Schlitzer A, Sivakamasundari V, Chen J, Sumatoh HRB, Schreuder J, Lum J et al Identification of cDC1‐ and cDC2‐committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat Immunol 2015; 16:718–28. URL http://www.nature.com/doifinder/10.1038/ni.3200. [DOI] [PubMed] [Google Scholar]


