Summary
Diversity and plasticity are hallmarks of macrophages. Classically activated macrophages are considered to promote T helper type 1 responses and have strong microbicidal, pro‐inflammatory activity, whereas alternatively activated macrophages are supposed to be associated with promotion of tissue remodelling and responses to anti‐inflammatory reactions. Transformation of different macrophage phenotypes is reflected in their different, sometimes even opposite, roles in various diseases or inflammatory conditions. MicroRNAs (miRNAs) have emerged as critical regulators of macrophage polarization (MP). Several miRNAs are induced by Toll‐like receptors signalling in macrophages and target the 3′‐untranslated regions of mRNAs encoding key molecules involved in MP. Therefore, identification of miRNAs related to the dynamic changes of MP and understanding their functions in regulating this process are important for discussing the molecular basis of disease progression and developing novel miRNA‐targeted therapeutic strategies. Here, we review the current knowledge of the role of miRNAs in MP with relevance to immune response and inflammation.
Keywords: immune response, inflammation, macrophage polarization, microRNAs, Toll‐like receptors
Abbreviations
- aaMφ
Alternatively activated macrophages
- ABCA1
ATP‐binding cassette transporter A1
- AMPK
AMP‐activated protein kinase
- BCL6
B‐cell lymphoma‐6 protein
- BMDMs
bone‐marrow‐derived macrophage
- C/EBP
CCAAT/enhancer‐binding protein
- caMφ
classically activated macrophages
- CNS
central nervous system
- CSF‐1R
colony‐stimulating factor 1 receptor
- IFN
interferon
- IKKε
IκB kinase ε
- IL‐4
interleukin‐4
- IRAK1
IL‐1 receptor‐associated kinase 1
- IRF
IFN regulatory factor
- JAK 1/2
Janus kinase 1/2
- KLF13
Krüppel‐Like Factor 13
- LPS
lipopolysaccharide
- miRNAs
microRNAs
- MP
macrophage polarization
- MyD88
myeloid differentiation protein 88
- NF‐κB
nuclear factor‐κB
- NOS2
nitric oxide synthase 2
- PDCD4
programmed cell death protein 4
- PGE2
prostaglandin E2
- Ripk1
receptor‐interacting serine‐threonine kinase 1
- SHIP1
Src homology‐2 domain‐containing inositol 5‐phosphatase 1
- SOCS
suppressor of cytokine signalling
- STAT
signal transducer and activator of transcription
- TAMs
tumour‐associated macrophages
- Th1
T helper type 1
- TLRs
Toll‐like receptors
- TNF‐α
tumour necrosis factor‐α
- TRAF
TNF receptor‐associated factor
- TRIF
TIR‐domain containing adapter‐inducing IFN‐β
- UTRs
untranslated regions
- WT
wild‐type
Introduction
Macrophage polarization (MP) is a process by which macrophages express different functional programmes in response to microenvironmental signals.1 Mirroring T helper type 1 (Th1) and Th2 polarization of lymphocytes, macrophages may undergo classical M1 macrophage activation or alternative M2 activation. Although such dichotomy is useful, the M1‐M2 phenotypes of macrophages are oversimplified, attributing to the substantial plasticity of macrophages in vivo.2 Classically activated macrophages (caMφ), stimulated by Toll‐like receptor (TLR) ligands and/or interferon‐γ (IFN‐γ), have been referred to as M[IFN‐γ/lipopolysaccharide (LPS)]. These macrophages are characterized by high expression of pro‐inflammatory cytokines [e.g. interleukin‐1 β (IL‐1β), IL‐6, IL‐12, IL‐23 and tumour necrosis factor‐α (TNF‐α)] and chemokines (e.g. CXCL1‐3, CXCL‐5 and CXCL8‐10), high production of reactive nitrogen intermediates (RNI) and reactive oxygen intermediates (ROI). In contrast, alternatively activated macrophages (aaMφ), stimulated by IL‐4 or IL‐13, have been referred to as M(IL‐4) or M(IL‐13). These macrophages are characterized by high levels of scavenging molecules, expression of mannose and galactose receptors, up‐regulation of CD163, CXCR1, CXCR2 and CCR2, efficient phagocytic activity, production of ornithine and polyamines via the arginase pathway, high levels of anti‐inflammatory cytokines (e.g. IL‐10) and low levels of pro‐inflammatory cytokines (e.g. IL‐12). The caMφ are considered to promote Th1 responses and to have strong microbicidal, pro‐inflammatory and tumoricidal activity, whereas aaMφ are supposed to be associated with promotion of tissue remodelling and tumour progression, and have immunoregulatory effects.3, 4, 5, 6 The considerable diversity and plasticity of macrophages, which switch their phenotype and physiology in response to various environmental cues or under diverse pathophysiological conditions, have been clearly demonstrated.7 The imbalance of MP is reflected in their differential, sometimes even opposing, roles in immune responses and inflammation, subsequently influencing various diseases (Fig. 1).8, 9, 10, 11 Hence, it is necessary to understand the regulatory mechanism of MP.
Figure 1.
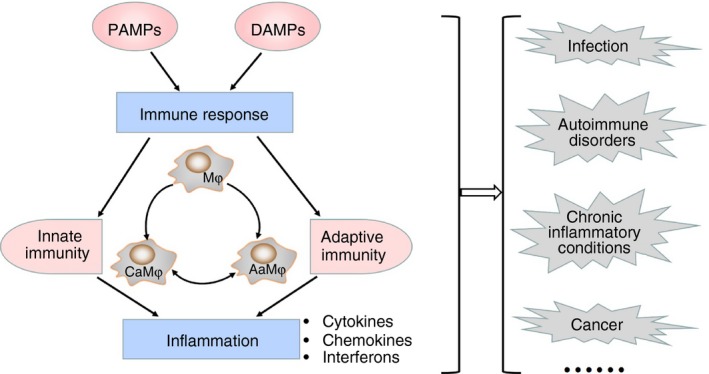
Role of macrophage polarization (MP) in immune response and inflammation. The imbalance of MP is reflected in their differential, sometimes even opposing, roles in immune response and inflammation, subsequently influencing various diseases including infection, autoimmune disorders, chronic inflammatory conditions and cancer.
A coordinated action of various inflammatory modulators, signalling pathway molecules and transcription factors is involved in regulating MP.12, 13, 14 For example, the canonical IFN‐recognition factor (IRF)/signal transducer and activator of transcription (STAT) signalling pathway is crucial in regulating MP. Activation of the IRF/STAT pathway (via STAT1) by IFNs will skew macrophages toward a caMφ phenotype. Interferon‐γ ligand binding to its receptor triggers Janus kinase 1/2 (JAK1/2)‐mediated tyrosine phosphorylation and dimerization of STAT1. Subsequently, the STAT1 homodimer engages cis‐elements known as IFN‐γ‐activated sites in the promoter of target inflammatory genes, such as IL‐12 and inducible nitric oxide synthase. Furthermore, STATs are involved in LPS‐mediated caMφ polarization through activation of TLR4. In response to LPS, autocrine production of IFN‐β binds the receptor of type‐1 IFNs (IFN‐α and IFN‐β) triggering STAT1 and STAT2 phosphorylation. Subsequently, the STAT1‐STAT2 heterodimer recruits IRF9 to bind cis‐elements known as IFN‐activated site and promotes its dependent gene expression. Actually, TLR4 mainly promotes nuclear factor‐κB (NF‐κB)‐mediated transcription of target genes. Under basal conditions, NF‐κB dimers are retained in the cytoplasm in an inactive form bound to IκB. In response to various physiological and environmental stimuli, IκB phosphorylation liberates NF‐κB which translocates to the nucleus to control expression of a subset of target genes. Lipopolysaccharide, as a stimulus of NF‐κB signalling, can bind LPS‐binding protein and deliver the LPS ligand to the receptor, CD14. Subsequently, the CD14–LPS complex interacts with TLR4 in conjunction with extracellular protein MD2 to activate intracellular signalling through NF‐κB pathway. TLR4 signalling proceeds via myeloid differentiation primary response gene 88 (MyD88)‐dependent and/or MyD88‐independent [TIR‐domain containing adapter‐inducing IFN‐β (TRIF) ‐dependent] pathways. The MyD88‐dependent pathway is found to mediate the expression of pro‐inflammatory cytokines, whereas the MyD88‐independent pathway is involved in the induction of IFN‐α and IFN‐β and IFN‐stimulated genes. Both of them can activate IκB kinase (IKK) to phosphorylate IκB and then liberate NF‐κB. In addition, LPS promotes the feed‐forward signalling of NF‐κB by inducing autocrine cytokines, IL‐1β and TNF‐α, to sustain NF‐κB activity. Besides, STAT6 is the pivotal transcription factor in IL‐4‐ or IL‐13‐mediated aaMφ polarization. The IL‐4 and IL‐13 receptors share IL‐4 receptor‐α (IL‐4Rα), an important signal transducer. Binding of IL‐4 or IL‐13 to IL‐4Rα triggers tyrosine phosphorylation on the IL‐4Rα cytoplasmic tail to facilitate recruitment and then tyrosine phosphorylation of STAT6 by JAK1/JAK3 or JAK1/Tyk2, respectively. Subsequent STAT6 homodimerization leads to recruitment of IRF4 and transcription activation of target genes, including Arg1, CD206, Fizz1 and Ym1.4, 15, 16 In recent decades, epigenetic regulation such as that by miRNAs has been identified as critical modulators in this process.17, 18, 19, 20 This review focuses on current understanding of miRNAs manipulation of MP, especially where relevant to immune response and inflammation.
MicroRNAs involved in the regulation of MP
MicroRNAs are small, 20–24 nucleotide‐long endogenous non‐coding RNAs that control gene expression by targeting 3′ untranslated regions (UTRs) of mRNAs.21 Several studies have persuasively demonstrated that the excessive or impaired inflammatory responses of macrophages are associated with the deregulation of miRNAs.22 Understanding the expression profiles of many miRNAs in polarized macrophages is the essential step towards unravelling the function of miRNAs in MP. A recent study showed that miR‐127‐3p, miR‐155‐5p, miR‐181a, miR‐204‐5p and miR‐451 were significantly up‐regulated in murine bone marrow‐derived macrophage (BMDMs) stimulated with LPS plus IFN‐γ for 18 hr, whereas miR‐125‐5p, miR‐143‐3p, miR‐145‐5p and miR‐146a‐3p were expressed at high levels in murine BMDMs treated with IL‐4 for 18 hr.23 Moreover, in the analysis of human BMDMs and PMA‐differentiated THP‐1 cells after incubation in polarizing treatments for 8 hr, several miRNAs including miR‐27a, miR‐29b, miR‐125a, miR‐146a and miR‐155 were remarkably up‐regulated in M(IFN‐γ/LPS), whereas expressions of miR‐26a and miR‐193b were increased in M(IL‐4).24 Although species differences or different protocols of MP may contribute to these apparent differences in the expression profiles of miRNAs in macrophages. These findings indicate that miRNAs may play essential roles in the regulation of MP. In particular, TLR signalling triggering has been tightly linked to the control of a different set of miRNAs, which manipulate the molecular mechanism regulating key molecules involved in MP and affect the balance of pro‐inflammatory and anti‐inflammatory responses. Next, we discuss these miRNAs that play vital roles in these processes (Table 1).
Table 1.
Relevance of microRNAs to macrophage polarization
| miRNA | Expression | Factors that regulate its expression | Target genes | Contribution to MP | References | ||
|---|---|---|---|---|---|---|---|
| M1 | M2 | M1 | M2 | ||||
| miR‐155 | ↑ | IFN‐β, TNF‐α, IL‐1 and TLRs | SOCS1, SHIP1, BCL6 C/EBPβ, IL‐13Rα1 | → | ⊣ | 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 | |
| miR‐125a‐5p | ↑(①THP‐1) | ↑(②BMDM) | LPS |
①A20 ②KLF13 |
①→ ②→ |
⊣ | 24, 38 |
| miR‐125b‐5p | ↓ | LPS | TNF‐α, IRF4, A20 | ⊣ | 27, 33, 39, 40, 41, 42, 43 | ||
| miR‐146a | ↑(alveolar macrophages) | acid aspiration | TRAF6, IRF5, IRAK1/2 | ⊣ | → | 44, 45, 46 | |
| miR‐146b | ↑ | LPS | TLR4,MyD88, IRAK1, TRAF6 | ⊣ | 47 | ||
| miR‐124 | ↑(microglia) | IL‐4, IL‐13 | C/EBPα | ⊣ | → | 48, 49, 50, 51 | |
| let‐7c | ↑ | M‐CSF | C/EBPδ, PAK1 | ⊣ | → | 55, 56 | |
| Let‐7f | ↓ | LPS | A20 | ⊣ | 61, 62, 63 | ||
| miR‐21 | ↑ | LPS | ① PDCD4, IL‐12p35, pTEN… ②Stat3 |
①⊣ ②→ |
→ ⊣ |
64 | |
| miR‐511‐3p | ↑ | IL‐4, IL‐13 | Rock2 | → | 66, 71 | ||
| miR‐378‐3p | ↑ | IL‐4 | IL‐4Rα/PI3K/AKT | → | 73, 74 | ||
| miR‐223 | ↑ | IL‐4 | Pknox1 | ⊣ | → | 75 | |
| miR‐342‐5p | ↑ | LPS/IFN‐γ | Bmpr2, Akt1 | → | 77 | ||
| miR‐33 | ↑ | LPS/IFN‐γ | AMPK | → | ⊣ | 79 | |
| miR‐101 | ↑ | LPS | ABCA1, MKP1 | → | 80, 81, 82 | ||
BMDM, bone marrow‐derived macrophage; IFN‐γ, interferon‐γ; IL, interleukin; LPS, lipopolysaccharide; M‐CSF, macrophage colony‐stimulating factor; miRNA, microRNA; MP, macrophage polarization; TLR, Toll‐like receptor; TNF‐α, tumour necrosis factor‐α.
‘↑’, up‐regulation; ‘↓’, down‐regulation.
‘→’, promote; ‘⊣’, inhibit.
miR‐155
miR‐155 is among the first miRNAs to be identified to play diverse roles in immunity and inflammation. It is generally believed that miR‐155 is a multifunctional miRNA.25 Regarding innate immunity and inflammatory response, miR‐155 expression is up‐regulated by various stimulations from cytokines, such as IFN‐β, TNF‐α and IL‐1, or TLR signals including TLR2, TLR3, TLR4 and TLR9 and specific antigens, which suggests the participation of miR‐155 in these immune responses.26, 27
Some studies have shown that miR‐155 enhances classical macrophage activation by repression of negative regulators of pro‐inflammatory responses, including suppressor of cytokine signalling 1 (SOCS1), Src homology‐2 domain‐containing inositol 5‐phosphatase 1 (SHIP1) and B‐cell lymphoma‐6 protein (BCL6). Xu et al. revealed a vital role of the miR‐155/SOCS1 axis in defining PI3K/Akt1‐mediated caMφ skewing in a mouse model of Staphylococcus aureus pulmonary infection.28 Furthermore, Moore et al. reported that miR‐155 was significantly increased in both M(granulocyte–macrophage colony‐stimulating factor/IFN‐γ/LPS)‐polarized primary human BMDMs and microglia. Manipulation of miR‐155 expression in both cell types could influence pro‐inflammatory cytokine secretion and co‐stimulatory surface marker expression by down‐regulating SOCS1 expression.29 These observations suggest that the role of miR‐155 in MP by targeting SOCS1 is important for dictating host defence against bacterial pathogens and adaptive immune responses in the central nervous system (CNS). Another interesting study by O'Connell et al. showed that SHIP1, a negative regulator of TLR pathways, was also repressed by regulating miR‐155 through direct 3′ UTR interactions in LPS‐stimulated wild‐type (WT) versus miR‐155−/− primary macrophages.30 Additionally, in pro‐inflammatory macrophages, miR‐155 directly suppressed the expression of BCL6, a negative transcription factor of the pro‐inflammatory NF‐κB pathway.31
The effect of miR‐155 on MP may not only be in its ability to increase caMφ‐associated gene expression, but also to suppress the aaMφ hallmarks. Previous study showed that miR‐155 inhibited protein expression by targeting 3′ UTR of CCAAT/enhancer‐binding protein (C/EBPβ), a hallmark of aaMφ and a key regulator of Arg1 expression. Further analysis showed that introduction of miR‐155 decreased cytokine production in tumour‐associated macrophages, an aaMφ‐like macrophage.32 In addition, in a study of Akt isoform‐dependent MP, Arranz et al. found that Akt protein kinases differentially contributed to primary murine peritoneal MP, with Akt1 deletion resulting in a caMφ phenotype and Akt2 ablation giving rise to an aaMφ phenotype. This process was mainly mediated by the interaction of miR‐155 with C/EBPβ.33, 34 Besides, Martinez‐Nunez et al. found that miR‐155 directly targeted IL‐13Rα1 and then decreased its protein levels, resulting in diminished activation of STAT6 in human primary macrophages.35
Although miR‐155 appears primarily to promote the pro‐inflammatory activation of multiple macrophages, accumulating evidence suggests that it acts as a negative regulator of overwhelming inflammatory responses by targeting various key molecules involved in pro‐inflammatory signal transduction. This gained support from the work of Tili et al., who showed that while miR‐155 induced TNF‐α production, it could directly target transcript coding for several pro‐apoptotic and anti‐inflammatory proteins involved in LPS signalling, such as Fas‐associated death domain protein, IKKε and receptor‐interacting serine‐threonine kinase 1. It suggests that miR‐155 may exert both positive and negative effects on LPS signalling.27 In further support of this work, Tang and his colleagues identifiedMyD88 as a target gene of miR‐155, which was involved in the negative regulation of Helicobacter pylori‐induced inflammation.36
Altogether, these data uncover pleiotropic roles of miR‐155 in modulation, with possible implications in several human diseases where homeostasis of the immune system might be altered. Initially miR‐155 was assumed to be a pro‐inflammatory miRNA that contributes to macrophage activation. However, the anti‐inflammatory effect of miR‐155 in some cases is not to be ignored. Therefore, further studies focusing on the effect of miR‐155 on MP in reference to inflammation and immune response are clearly required before further considering miR‐155 as a therapeutic target.
miR‐125 family
The ubiquitin‐editing enzyme A20 (TNFAIP3) is thought to be a negative regulator of NF‐κB signalling in many cell types of the immune system.37 Graff et al. showed that miR‐125a‐5p was up‐regulated in M(LPS). Transfection with M(LPS) of miR‐125a‐5p mimics increased the expression of caMφ‐associated transcripts, which might be at least partially explained by repression of A20.24 In addition, in a recent study, Banerjee et al. found that miR‐125a‐5p was expressed at a higher level in M‐BMDMs (aaMφ) than in GM‐BMDMs (caMφ). Importantly, miR‐125a‐5p suppressed classical activation and diminished the bactericidal activity of macrophages, but enhanced alternative macrophage activation and phagocytic activity to ingest apoptotic cells. Furthermore, they revealed that this effect of miR‐125a‐5p on macrophage activation was mediated by KLF13, known to regulate T‐cell activation and promote T‐cell‐associated inflammation.38
In contrast to miR‐125a‐5p, miR‐125b‐5p has the same ‘seed’ sequence as miR‐125a‐5p does, which was found to be inhibited upon inflammatory stimulation, such as LPS.33, 39 Overall, it has been implied that miR‐125b can target 3′ UTR of TNF‐α and substantially decrease the stability of TNF‐α transcript, negatively regulating inflammatory responses.27, 40, 41 However, miR‐125b potentiated pro‐inflammatory macrophage activation via targeting IRF4 and A20 as well.42, 43 Moreover, over‐expression of miR‐125b induced the expression of co‐stimulatory factor and enhanced responsiveness to IFN‐γ and elicited activated macrophages.42
Collectively, these observations suggest that the miR‐125 family may have a direct or indirect effect on MP. Different cell systems and models such as primary macrophages or culture cells, may account for the discrepancy between these studies. Hence, further experimental studies need to be performed before miR‐125 can be viewed as a therapy target for prevention of inflammation in various diseases.
miR‐146 family
The miR‐146 family is composed of two evolutionarily conserved miRNAs: miR‐146a and miR‐146b. miR‐146a was first identified as a negative feedback regulator in macrophage activation by directly targeting the downstream signalling molecules of TLRs signalling, such as IL‐1 receptor‐associated kinase 1 (IRAK1) and TNF receptor‐associated factor 6 (TRAF6).44 Furthermore, miR‐146a also acted as a negative regulator of the RIG‐I‐dependent pathway and inhibited type I IFN production in macrophages by targeting IRAK2.45 In a recent study, Vergadi et al. showed that miR‐146a expression was increased in primary alveolar macrophages at 24 hr after acid aspiration, which acquired a classical activation phenotype. Interestingly, alveolar macrophages from acid‐injured Akt2−/− mice exhibited an alternatively activated phenotype. Expression of miR‐146a was higher in Akt2−/− macrophages compared with WT macrophages before acid aspiration. Moreover, transfection of WT macrophages with miR‐146a inhibited LPS‐induced nitric oxide synthase 2 (NOS2) expression and alternative macrophage activation, while inhibition of miR‐146a in Akt2−/− macrophages restored NOS2 induction. Furthermore, over‐expression of miR‐146a in WT macrophages led to inhibition of TRAF6 and IRF5, and this effect of miR‐146a in Akt2−/− macrophages was less prominent compared with WT macrophages.46
Besides miR‐146a, Curtale et al. recently identified miR‐146b as an IL‐10‐dependent miRNA in the human and murine systems.47 They provided further evidence that the LPS‐dependent induction of miR‐146b in murine BMDMs was remarkably suppressed when macrophages were obtained from IL‐10−/− mice.47 Importantly, over‐expression of miR‐146b in THP‐1 cells resulted in a significant decrease in the LPS‐medicated production of several pro‐inflammatory cytokines (TNF‐α, IL‐6 and IL‐8) and chemokines (CCL2, CCL3, CCL7 and CXCL10). Further work by these authors showed that miR‐146b has this anti‐inflammatory activity by directly targeting multiple elements, including TLR4, MyD88, IRAK‐1 and TRAF6.47
Based on the above findings, miR‐146a may be of critical importance in promoting macrophages towards an alternatively activated phenotype, which seems to be a potential and promising target for aseptic acute respiratory distress syndrome. Nevertheless, the impaired bacterial clearance as a consequence of aaMφ induction greatly hampers its clinical use in septic acute respiratory distress syndrome. In addition, although miR‐146b has generally been shown to negatively regulate inflammation, the effect of miR‐146b on MP is not fully understood.
miR‐124
Tissue‐resident macrophages often possess an aaMφ‐like phenotype under normal physiological conditions. For instance, Ponomarev et al. showed that microglia, the CNS‐resident macrophages, expressed a number of aaMφ‐associated genes, including Fizz1, Ym1, IL‐4 and IL‐10.48 Intriguingly, miR‐124 has been identified as a crucial regulator of microglial differentiation in the CNS microenvironment. In further support of this work, Ponomarev et al. demonstrated that brain‐specific miR‐124 was expressed in microglia but not in peripheral macrophages. Moreover, transfection of BMDMs with miR‐124 led to the attenuated expression of several caMφ markers, such as MHC class II, CD86, TNF‐α and NOS2, whereas the up‐regulation of the aaMφ markers, including transforming growth factor‐β, Arg1 and Fizz1. Further analysis showed that miR‐124 controlled these numerous markers of macrophage activation by directly inhibiting C/EBPα and its downstream target PU.1.49 In addition, their study in experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis, revealed that treatment of mice with miR‐124 could decrease the absolute numbers of macrophages and activate CD45hi microglia, and ameliorate both clinical symptoms and inflammation as well as enhance recovery in the CNS.49 Briefly, their findings suggest that miR‐124 is involved in microglial differentiation during CNS development and acts as a new inhibitor of macrophage activation in the periphery during experimental autoimmune encephalomyelitis.50
Besides the CNS, aaMφ also play a critical role in the regulation of the allergic immune responses. Researchers from the same laboratory further studied whether aaMφ induced miR‐124 expression.51 They found that exposure of cells to IL‐4 and IL‐13 resulted in the up‐regulation of miR‐124 in BMDMs as well as in RAW 264.7 cells. More importantly, IL‐4 could increase the expression of three miR‐124 precursor transcripts with prominent expression of pri‐miR‐124.3, indicating up‐regulation of miR‐124 by IL‐4 on a transcriptional level. Further study showed that miR‐124 inhibitor abrogated the up‐regulation of CD206, Ym1 as well as the down‐regulation of CD86, NOS2, TNF‐α in M(IL‐4). In keeping with these results, their study in patients with allergies and bronchial asthma revealed that the numbers of human CD14+ CD16+ intermediate monocytes were remarkably increased, and these cells expressed miR‐124 at high levels and exhibited other properties of aaMφ‐like cells.51
In summary, these observations suggest that miR‐124 may act as a universal regulator of MP in various subsets of monocytic cells and tissue‐resident macrophages including microglia and lung macrophages.
Let‐7 family
Let‐7, one of the first tumour‐suppressive miRNAs identified, was initially demonstrated to manipulate developmental timing in Caenorhabditis elegans.52, 53 Let‐7 belongs to a highly conserved miRNA family comprising 12 genes, which encode for nine different miRNAs (let‐7a to let‐7i, miR‐98).54 Members of the let‐7 family including let‐7c, let‐7e and let‐7i have been found to play pleiotropic roles in inflammatory responses to various stimuli. Among them, the role of let‐7 family in the regulation of MP has also been addressed. For example, a study performed by Banerjee et al. showed that let‐7c levels were markedly higher in M‐BMDM (aaMφ) than in GM‐BMDM (caMφ) and were also elevated in alveolar macrophages, which are known to exhibit an aaMφ phenotype.55 They further found that transfection of mimics for let‐7c in GM‐BMDM significantly decreased the caMφ hallmarks and diminished the bactericidal activity of GM‐BMDM while facilitating macrophage polarization to an alternatively activated phenotype and enhancing their ability to engulf apoptotic cells. Conversely, let‐7c siRNA in M‐BMDM could promote classical macrophage activation and down‐regulate the aaMφ markers. Further study showed that let‐7c directly targeted C/EBPδ.55 Their findings strongly suggest that let‐7c plays a critical role in the regulation of MP. Additionally, Zhang et al. provided further evidence for the role of let‐7c in classical MP. They showed that epigenetic loss of let‐7c owing to up‐regulation of EZH2 induced PAK1 expression and pro‐inflammatory phenotype in M(IFN‐γ/LPS), which was mediated by the NIK–IKK–NF‐κB pathway. Furthermore, pharmacological and genetic ablation with EZH2/let‐7c/PAK1 signalling damped classical macrophage activation.56
A recent study reported that let‐7e, one member of let‐7 family, was rapidly up‐regulated in M(LPS). Moreover, let‐7e over‐expression in Akt1−/− macrophages could restore sensitivity and tolerance to LPS in culture and in vivo by targeting TLR4.33 Another interesting study performed by Kumar and his colleagues showed that down‐regulation of let‐7f blunted the inflammatory response and the production of iNOS by directly targeting A20 in macrophages during Mycobacterium tuberculosis infection. Intriguingly, further study showed that over‐expression of let‐7f elevated the production of cytokines, such as TNF‐α and IL‐1β.57 In addition, down‐regulation of miR‐98 upon LPS treatment augmented TLR4‐triggered IL‐10 production and miR‐98 over‐expression could inhibit this effect, which might be a crucial regulatory mechanism for controlling pro‐inflammatory immune responses.58
Taken together, these observations indicate that let‐7e, let‐7f and miR‐98 may have an indirect impact on MP. In further research, the direct effect of the let‐7 family on MP needs to be determined.
miR‐21
miR‐21 is identified as a negative regulator of tumour‐suppressor PDCD4 and is up‐regulated in various cancers and therefore has been called an ‘oncomiR’.59 PDCD4 is also a pro‐inflammatory protein, which promotes NF‐κB activation and inhibits IL‐10 expression.60 A previous study by Sheedy et al. showed that miR‐21 negatively regulated PDCD4 expression in a MyD88‐dependent or TRIF‐dependent manner after LPS stimulation. They transfected RAW 264.7 cells with an miR‐21 precursor and found that miR‐21 remarkably blocked NF‐κB signalling and increased IL‐10 expression in response to LPS. In contrast, transfection with anti‐miR‐21 or specifically targeted protection of the miR‐21 site in PDCD4 mRNA exhibited the opposite effect.61 Subsequently, accumulating evidence has demonstrated that miR‐21 plays a vital role in inflammatory responses, especially in the regulation of MP. First, a study by Lu et al. found that miR‐21 negatively regulated the expression of the pro‐inflammatory cytokine IL‐12p35, a caMφ‐associated gene.62 Consistent with an anti‐inflammatory role of miRNA‐21, recent evidence supported the idea that miR‐21 acted as a novel molecular hub that integrated signals from the CSF‐1R pTyr‐721/PI3K axis to suppress the pro‐inflammatory MP and enhance the anti‐inflammatory macrophage phenotype.63
However, Wang et al. demonstrated that the interaction between miR‐21 and prostaglandin E2 (PGE2) was a determining factor in driving classical macrophage activation and inhibiting alternative macrophage activation. They showed that caMφ markers (TNF‐α, IL‐1β, IL‐6 and IL‐12p40) were either not expressed or were expressed at lower levels, whereas the levels of aaMφ markers (IL‐10, Arg‐1, RETNLα, and Chi3 l3) were higher in miR‐21−/− peritoneal macrophages than those observed in WT macrophages. Conversely, transfection of macrophages with an miR‐21 mimic observably suppressed the expression of genes enriched in aaMφ and increased the expression of genes present in caMφ.64 Furthermore, the lipid mediator PGE2, which has anti‐inflammatory effects in macrophages by inhibiting TNF‐α levels and enhancing IL‐10 expression,65 significantly inhibited miR‐21 expression.64 They also found that miR‐21 was an endogenous brake in PGE2‐mediated generation of alternative macrophage activation in a STAT3‐dependent manner.64
In short, these findings suggest that miR‐21 is a homeostatic modulator of MP even without conventional caMφ and aaMφ stimuli. Nevertheless, miR‐21 may exert an opposite effect on MP depending on cell type and in vivo microenvironment.
miR‐511‐3p
miR‐511‐3p is embedded within the fifth intron of both human and mouse MRC1 genes, which encode MRC1 (also called as CD206). Squadrito et al. showed that robust expression and bioactivity of miR‐511‐3p were found in aaMφ. Intriguingly, miR‐511‐3p over‐expression could broadly and specifically tune down the expression of aaMφ genes; however, it did not alter gene signature of pro‐inflammatory CD11c+ macrophages.66 Moreover, miR‐511‐3p directly and selectively targeted Rock2,66 which is a serine‐threonine kinase67 and phosphorylates IRF4,68 a transcription factor that induces alternative macrophage activation. Tumour‐associated macrophages (TAMs) have been demonstrated to exhibit an aaMφ‐like phenotype, which is associated with tumour progression.69, 70 Unexpectedly, enforced miR‐511‐3p activity in TAMs attenuated the protumoral genetic programmes of MRC1+ TAMs and inhibited blood vessel formation as well as tumour growth.66 These interesting findings suggest that although miR‐511‐3p is up‐regulated in aaMφ, it reprogrammes TAMs from a protumoral to an antitumoral phenotype.
To further ascertain the role of miR‐511 in aaMφ, Karo‐Atar et al. demonstrated that miR‐511 expression was elevated by IL‐4 and IL‐13 stimulation in vitro and in vivo, and in a mouse model of allergic airway disease, which is associated with induced IL‐4 and IL‐13 production. Moreover, global transcriptome analyses following miR‐511 over‐expression suggested that miR‐511 could modulate diverse activities of aaMφ, such as cellular proliferation, metabolism and immune responses. However, they also found that transfection of miR‐511 mimic alone in BMDMs was not sufficient to affect the polarized phenotype of macrophages. Furthermore, over‐expression of miR‐511 following IFN‐γ/Escherichia coli or IL‐4‐activation was still not sufficient to skew macrophages towards an alternatively activated phenotype. Collectively, these findings identify miR‐511 as a bona fide aaMφ‐associated miRNA possibly by governing molecular checkpoints linked to IL‐4/IL‐13‐induced macrophage activities, not MP.71
miR‐378‐3p
miR‐378‐3p is located in an intronic region of PPARGC1b that encodes peroxisome proliferator‐activated receptor γ (PPARγ)‐coactivator‐1β,72 a protein related to the alternative activation of macrophages.73 This suggests that miR‐378‐3p may be tightly involved in the regulation of aaMφ. In a recent study in a mouse model of helminth‐induced alternative activation, Rückerl et al. found that miR‐378‐3p was significantly up‐regulated in vivo. Consistent with this observation, in vitro experiments confirmed that miR‐378‐3p was specifically induced upon stimulation with IL‐4. Additionally, in silico analysis and miRNA manipulation studies identified potential miR‐378‐3p targets in the IL‐4‐Rα/PI3K/Akt‐signalling cascade, which is an important component of aaMφ.74 Altogether, miR‐378‐3p may manipulate in a negative feedback loop for limiting and controlling MP in the alternative activation setting.
miR‐223
Macrophage polarization plays an important role in the regulation of adipose tissue inflammation. miR‐223 was greatly enriched in bone marrow and macrophages isolated from adipose tissue. Moreover, dramatically elevated miR‐223 levels were observed in IL‐4‐treated BMDMs, whereas LPS stimulation slightly decreased miR‐223 expression in cultured BMDMs. Furthermore, miR‐223−/− macrophages generated an enhanced response to LPS in the induction of pro‐inflammatory cytokines IL‐1β, IL‐6 and TNF‐α, whereas the expression of PPARγ and Arg‐1 was remarkably reduced in the absence of miR‐223. In addition, Pknox1 was a genuine target of miR‐223 and played a vital role in the control of MP. Collectively, miR‐223 deficiency promoted inflammatory responses (e.g. macrophage infiltration, pro‐inflammatory cytokine expression, and p65 phosphorylation) and down‐regulated insulin signalling of adipose tissue from high‐fat‐diet‐fed mice, accompanied by inappropriate adipokine expression.75 Therefore, miR‐223‐controlled MP was crucial for adipose tissue function.
miR‐187
Described as the first IL‐10‐dependent miRNA, miR‐187 directly targets the stability and translation of TNF‐α mRNA, and indirectly reduces the production of IL‐6 and IL‐12p40 by down‐regulation of IκBζ, a positive transcriptional regulator of the latter two cytokines.76 This study demonstrates that miR‐187 plays an essential role in regulating IL‐10‐driven anti‐inflammatory responses.
miR‐342‐5p
miR‐342‐5p was shown to be the most prominently up‐regulated miRNA and was expressed in lesional macrophages.77 Furthermore, miR‐342‐5p directly targeted Akt1 through its 3′ UTR. On the one hand, up‐regulation of miR‐342‐5p triggered down‐regulation of Akt1 in M(IFN‐γ/LPS), which in turn resulted in a loss of the intrinsic anti‐inflammatory functions of Akt1. On the other hand, Akt1 suppression by miR‐342‐5p in macrophages could induce pro‐inflammatory macrophage markers (NOS2 and IL‐6) by up‐regulating miR‐155. In contrast, miR‐342‐5p inhibitor could reduce atherosclerotic lesion formation and substantially suppress Akt1‐dependent NOS2 expression in lesional macrophages.77 Therefore, given the controversy regarding the function of miR‐155 in MP, the use of miR‐342‐5p as a promising therapeutic target may currently be limited and the potential mechanism of miR‐342‐5p needs to be further addressed.
miR‐33 and miR‐101
Senescent macrophages are relevant to an anti‐inflammatory profile, which is clearly reflected in the gene expression signature. The expression of caMφ markers including IL‐6, IL‐1β and prostaglandin‐endoperoxide synthase 2 was significantly decreased in aged macrophages compared with young macrophages, whereas the aaMφ hallmarks, such as IL‐10 and CD163, were remarkably up‐regulated.78 Sene et al. showed that miR‐33 expression was significantly increased in aged macrophages compared with young macrophages, consistent with decreased expression of ATP‐binding cassette transporter A1 (ABCA1), which was observed in old macrophages and reversed the alternative pro‐angiogenic polarization of macrophages towards an aaMφ‐like disease‐promoting phenotype. Moreover, miR‐33 inhibition markedly increased the mRNA and protein expression of ABCA1 in young and old macrophages. Importantly, antagonism of endogenous miR‐33 could promote MP towards a classically activated phenotype.78 However, in a recent study, Ouimet et al. found that the expression of miR‐33 was higher in in BMDMs(IFN‐γ/LPS) compared with BMDMs(IL‐4) for 24 hr, pointing to a potential role of miR‐33 in MP.79 Further study showed that transfection with miR‐33 mimic in mouse peritoneal macrophages significantly increased the mRNA expression of M(IFN‐γ/LPS) markers including IL‐1β, IL‐6 and NOS2 and suppressed M(IL‐4) hallmarks CD206 and Fizz1. Conversely, inhibition of miR‐33 showed a remarkable increase in expression of M(IL‐4) markers and decreased expression of M(IFN‐γ/LPS) markers compared with macrophages transfected with control oligonucleotides. Mechanically, miR‐33 promotes a pro‐inflammatory macrophage state by targeting the AMP‐activated protein kinase, thereby reducing fatty acid oxidation and increasing glycolysis, consequently affecting both innate and adaptive immune responses.79
Another miRNA, which also could negatively regulate the expression of ABCA1 under inflammatory conditions such as IL‐6 treatment, was pro‐inflammatory miR‐101.80 Besides, miR‐101 was induced by caMφ stimuli such as TLR agonists and directly targeted MKP‐1, which deactivates mitogen‐activated protein kinase signalling. Over‐expression of miR‐101 in macrophages decreased the expression of MKP‐1 by LPS and prolonged activation of p38 and JNK, and increased the secretion of caMφ‐associated cytokines such as IL‐1β, IL‐6 and TNF‐α. Conversely, inhibition of miR‐101 increased induction of MKP‐1 and shortened activation of p38 and JNK as well as the caMφ hallmarks.81 These findings indicated that miR‐101 plays a critical role in MP of innate immune response and may be a potential anti‐inflammatory target. This notion was later confirmed in a recent study. Gao et al. demonstrated that genkwanin exerted anti‐inflammatory effect mainly through the regulation of the miR‐101/MKP‐1/mitogen‐activated protein kinase pathway in LPS‐stimulated RAW 264.7 cells.82
miR‐9, miR‐147, miR‐203 and miR‐27
The TLRs are predominant receptors that enable macrophages to recognize numerous invading microbial pathogens. Hitherto, 13 TLRs have been identified, each of which recognizes a unique pathogen‐associated molecular pattern. For instance, TLR2 recognizes lipopeptides whereas TLR4 interplays with LPS. As for TLR3, ‐7, ‐8 and ‐9, all of them recognize microbial nucleic acids. Subsequently, binding of ligands to TLRs can activate NF‐κB signalling and IRF3/7 in a MyD88‐dependent or TRIF‐dependent manner.83, 84 A previous study by Bazzoni et al. demonstrated that miR‐9 induced by TLR2/4 activation repressed the expression of NF‐κB p50 and then inhibited NF‐κB activation in LPS‐stimulated human monocytes.85 Another miRNA, miR‐147, was found to act as a negative regulator of TLR‐induced signalling in murine macrophages. In support of this work, Liu et al. showed that miR‐147 was significantly up‐regulated in LPS‐stimulated murine macrophages and in the lungs of LPS‐treated mice in vivo. Moreover, TLR4‐induced miR‐147 expression required activation of both NF‐κB and IRF3 in an MyD88‐dependent and TRIF‐dependent manner. In subsequent experiments, they found that miR‐147 over‐expression decreased while miR‐147 silencing increased the expression of inflammatory cytokines in macrophages treated with ligands to TLR2, TLR3 and TLR4.86 In addition, miR‐203 was shown to play a negative role of feed‐forward loop in regulating the immune response against LPS or bacillus Calmette–Guérin infection, which was at least partially by targeting the 3′ UTR of MyD88 and post‐transcriptionally down‐regulating the expression of MyD88, subsequently reducing the expression of inflammatory mediators (e.g. NF‐κB, TNF‐α and IL‐6) in RAW264.7 cells.87 Collectively, miR‐9, miR‐21 and miR‐203 can be induced by several TLR agonists in a MyD88‐dependent or TRIF‐dependent manner and are supposed to restrain inflammatory responses by inhibiting NF‐κB signalling activation in macrophages. Although miR‐9, miR‐21 and miR‐203 have limited direct effect on MP of caMφ, these studies still indicate that they may play an indispensable role in suppressing macrophage activation.
Although miRNAs have been demonstrated to be major regulators in the innate immune response, only a small number of miRNAs have been involved in negative feedback loops, which ameliorate inflammation. Xie et al. identified miR‐27a down‐regulation in BMDM(LPS). Over‐expression of miR‐27a in BMDMs could elevate the levels of pro‐inflammatory cytokines, whereas miR‐27a knockdown diminished the expression of these cytokines. Further study showed that miR‐27a could prevent overly exuberant TLR2/4‐driven inflammatory response of macrophages by targeting IL‐10 and reducing IL‐10 secretion.88
Conclusion
Although lots of miRNAs can regulate the inflammatory response in different macrophages, only a few miRNAs are known to be involved in MP, which reflects the fact that only a limited number of protein regulators and transcriptional factors are shown to participate in both processes. This review summarizes the information on miRNA functions directly or indirectly related to MP (Fig. 2). Accumulating evidence has shown that the miRNA profiles are differentially expressed in MP. The fact that miRNAs are very specific for different tissue and cell types, many studies profiled the miRNA patterns in various cell types, which put forward the potential role of miRNAs in MP. Deregulation of miRNA in MP was a result of impaired miRNA‐biogenesis, genomic or epigenetic alterations, leading to the effect of miRNA on MP of caMφ and aaMφ. Delineation of the role of miRNAs in MP provides insights into their regulation of immune response and inflammation, subsequently affecting various diseases. So far, specific miRNA mimics or antagomirs are found to control immune response and inflammation, and the use of those miRNAs to treat inflammatory diseases represents a potent therapeutic approach.89, 90, 91 For example, miR‐21 antagomirs might be a good therapeutic agent in acute inflammation that not only elevates the numbers of aaMφ but also inhibits the levels of various inflammatory cytokines including IL‐1β and TNF‐α. Alternatively, miR‐21 mimics suppressed chronic inflammatory responses by preventing alternative macrophage activation. Besides acute and chronic inflammation, additional studies investigating the role of this miRNA in different diseases including tumours are warranted.64, 92 Ideally, miRNAs could be a better therapeutic approach over single gene therapy because each miRNA can target multiple genes involved in MP. Nevertheless, this implies that it still remains a major challenge to identify the specific targets of any single miRNA. To overcome this limitation, several advanced techniques, including RNA immunoprecipitation (RIP)‐microarray, RIP sequencing, photoactivatable‐ribonucleoside‐enhanced cross‐linking and immunoprecipitation (PAR‐CLIP), have been developed to define bona fide miRNA targets;93 in contrast, each target gene in MP can also cooperatively bind to various miRNAs, especially in the tumour microenvironment. A better understanding of how multiple target miRNAs can cooperate to properly balance MP in various diseases, especially cancers, is needed. Besides, miRNA‐based therapeutic approach becomes more complex due to interactions between different miRNAs, which limits our understanding of how miRNA networks can work together to regulate MP. Recently, Ding et al. found a three‐nucleotide periodic repeat pattern in the structure of coding regions and a less structured region immediately upstream of the start codon, more importantly, these features were strongly correlated with translation efficiency. They also found patterns of strong and weak secondary structure at sites of alternative polyadenylation and 5′ splice sites that were associated with unspliced events, which suggests that the nature of these interactions depends on RNA secondary structures, resulting in a defined set of miRNA targets that may be physiologically related in different cell types.94 Therefore, there is still a long way to go before miRNA‐based therapy due to the above limitations. Much more work needs to be done to determine the precise functions and roles of miRNAs, particularly with reference to regulation of MP before using them as biomarkers and therapies.
Figure 2.
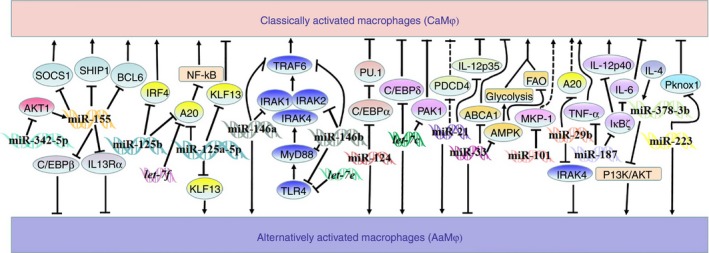
Role of microRNAs (miRNAs) in the regulation of macrophage activation and polarization. Names in double‐strand refer to microRNAs; arrows depicting activation or inhibition between the indicators mean that the upper indicator normally activates or inhibits the one below; solid line implies that the relationship between two indicators is direct, and dotted line implies the relationship between two indicators is indirect in the regulation of macrophage polarization.
Disclosures
The authors declare no financial or commercial conflict of interest.
Acknowledgements
This work was partially supported by National Natural Science Foundation of China (81273526, 81473268, 81500344 and 81500473), the Natural Science Foundation of Anhui Province (1308085MH145), and the Anhui Provincial Key Scientific and Technological Project (1301042212).
References
- 1. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003; 3:23–35. [DOI] [PubMed] [Google Scholar]
- 3. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010; 32:593–604. [DOI] [PubMed] [Google Scholar]
- 4. Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol 2013; 120:163–84. [DOI] [PubMed] [Google Scholar]
- 5. Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci 2008; 13:453–61. [DOI] [PubMed] [Google Scholar]
- 6. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S et al Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010; 11:889–96. [DOI] [PubMed] [Google Scholar]
- 8. Heymann F, Peusquens J, Ludwig‐Portugall I, Kohlhepp M, Ergen C, Niemietz P et al Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 2015; 62:279–91. [DOI] [PubMed] [Google Scholar]
- 9. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11:723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013; 496:445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu XQ, Yang Y, Li WX, Cheng YH, Li XF, Huang C et al Telomerase reverse transcriptase acts in a feedback loop with NF‐κB pathway to regulate macrophage polarization in alcoholic liver disease. Sci Rep 2016; 6:18685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C et al Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal 2014; 26:192–7. [DOI] [PubMed] [Google Scholar]
- 13. Motwani MP, Gilroy DW. Macrophage development and polarization in chronic inflammation. Semin Immunol 2015; 27:257–66. [DOI] [PubMed] [Google Scholar]
- 14. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front Immunol 2014; 5:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tugal D, Liao X, Jain MK. Transcriptional control of macrophage polarization. Arterioscler Thromb Vasc Biol 2013; 33:1135–44. [DOI] [PubMed] [Google Scholar]
- 16. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas . J Clin Invest 2012; 122:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Y, Zhao L, Shi B, Ma S, Xu Z, Ge Y et al Functions of miR‐146a and miR‐222 in tumor‐associated macrophages in breast cancer. Sci Rep 2015; 5:18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma S, Liu M, Xu Z, Li Y, Guo H, Ge Y et al A double feedback loop mediated by microRNA‐23a/27a/24‐2 regulates M1 versus M2 macrophage polarization and thus regulates cancer progression. Oncotarget 2015; 7:13502–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saha B, Momen‐Heravi F, Kodys K, Szabo G. MicroRNA Cargo of extracellular vesicles from alcohol‐exposed monocytes signals naive monocytes to differentiate into M2 macrophages. J Biol Chem 2016; 291:149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Talari M, Kapadia B, Kain V, Seshadri S, Prajapati B, Rajput P et al MicroRNA‐16 modulates macrophage polarization leading to improved insulin sensitivity in myoblasts. Biochimie 2015; 119:16–26. [DOI] [PubMed] [Google Scholar]
- 21. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136:215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baltimore D, Boldin MP, O'Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol 2008; 9:839–45. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Zhang M, Zhong M, Suo Q, Lv K. Expression profiles of miRNAs in polarized macrophages. Int J Mol Med 2013; 31:797–802. [DOI] [PubMed] [Google Scholar]
- 24. Graff JW, Dickson AM, Clay G, McCaffrey AP, Wilson ME. Identifying functional microRNAs in macrophages with polarized phenotypes. J Biol Chem 2012; 287:21816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR‐155 gene: a typical multifunctional microRNA. Biochim Biophys Acta 2009; 1792:497–505. [DOI] [PubMed] [Google Scholar]
- 26. O'Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA‐155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A 2007; 104:1604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B et al Modulation of miR‐155 and miR‐125b levels following lipopolysaccharide/TNF‐α stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol 2007; 179:5082–9. [DOI] [PubMed] [Google Scholar]
- 28. Xu F, Kang Y, Zhang H, Piao Z, Yin H, Diao R et al Akt1‐mediated regulation of macrophage polarization in a murine model of Staphylococcus aureus pulmonary infection. J Infect Dis 2013; 208:528–38. [DOI] [PubMed] [Google Scholar]
- 29. Moore CS, Rao VT, Durafourt BA, Bedell BJ, Ludwin SK, Bar‐Or A et al miR‐155 as a multiple sclerosis‐relevant regulator of myeloid cell polarization. Ann Neurol 2013; 74:709–20. [DOI] [PubMed] [Google Scholar]
- 30. O'Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR‐155. Proc Natl Acad Sci U S A 2009; 106:7113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nazari‐Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR et al MicroRNA‐155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest 2012; 122:4190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He M, Xu Z, Ding T, Kuang DM, Zheng L. MicroRNA‐155 regulates inflammatory cytokine production in tumor‐associated macrophages via targeting C/EBPbeta. Cell Mol Immunol 2009; 6:343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V et al The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity 2009; 31:220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED et al Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci U S A 2012; 109:9517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinez‐Nunez RT, Louafi F, Sanchez‐Elsner T. The interleukin 13 (IL‐13) pathway in human macrophages is modulated by microRNA‐155 via direct targeting of interleukin 13 receptor α1 (IL13Rα1). J Biol Chem 2011; 286:1786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tang B, Xiao B, Liu Z, Li N, Zhu ED, Li BS et al Identification of MyD88 as a novel target of miR‐155, involved in negative regulation of Helicobacter pylori‐induced inflammation. FEBS Lett 2010; 584:1481–6. [DOI] [PubMed] [Google Scholar]
- 37. Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S et al De‐ubiquitination and ubiquitin ligase domains of A20 downregulate NF‐κB signalling. Nature 2004; 430:694–9. [DOI] [PubMed] [Google Scholar]
- 38. Banerjee S, Cui H, Xie N, Tan Z, Yang S, Icyuz M et al miR‐125a‐5p regulates differential activation of macrophages and inflammation. J Biol Chem 2013; 288:35428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murphy AJ, Guyre PM, Pioli PA. Estradiol suppresses NF‐κB activation through coordinated regulation of let‐7a and miR‐125b in primary human macrophages. J Immunol 2010; 184:5029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang HC, Yu HR, Huang LT, Chen RF, Lin IC, Ou CY et al miRNA‐125b regulates TNF‐α production in CD14 + neonatal monocytes via post‐transcriptional regulation. J Leukoc Biol 2012; 92:171–82. [DOI] [PubMed] [Google Scholar]
- 41. Rajaram MV, Ni B, Morris JD, Brooks MN, Carlson TK, Bakthavachalu B et al Mycobacterium tuberculosis lipomannan blocks TNF biosynthesis by regulating macrophage MAPK‐activated protein kinase 2 (MK2) and microRNA miR‐125b. Proc Natl Acad Sci U S A 2011; 108:17408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chaudhuri AA, So AY, Sinha N, Gibson WS, Taganov KD, O'Connell RM et al MicroRNA‐125b potentiates macrophage activation. J Immunol 2011; 187:5062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim SW, Ramasamy K, Bouamar H, Lin AP, Jiang D, Aguiar RC. MicroRNAs miR‐125a and miR‐125b constitutively activate the NF‐κB pathway by targeting the tumor necrosis factor α‐induced protein 3 (TNFAIP3, A20). Proc Natl Acad Sci U S A 2012; 109:7865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF‐κB‐dependent induction of microRNA miR‐146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 2006; 103:12481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hou J, Wang P, Lin L, Liu X, Ma F, An H et al MicroRNA‐146a feedback inhibits RIG‐I‐dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol 2009; 183:2150–8. [DOI] [PubMed] [Google Scholar]
- 46. Vergadi E, Vaporidi K, Theodorakis EE, Doxaki C, Lagoudaki E, Ieronymaki E et al Akt2 deficiency protects from acute lung injury via alternative macrophage activation and miR‐146a induction in mice. J Immunol 2014; 192:394–406. [DOI] [PubMed] [Google Scholar]
- 47. Curtale G, Mirolo M, Renzi TA, Rossato M, Bazzoni F, Locati M. Negative regulation of Toll‐like receptor 4 signaling by IL‐10‐dependent microRNA‐146b. Proc Natl Acad Sci U S A 2013; 110:11499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ponomarev ED, Maresz K, Tan Y, Dittel BN. CNS‐derived interleukin‐4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J Neurosci 2007; 27:10714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA‐124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP‐α‐PU.1 pathway. Nat Med 2011; 17:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ponomarev ED, Veremeyko T, Weiner HL. MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia 2013; 61:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Veremeyko T, Siddiqui S, Sotnikov I, Yung A, Ponomarev ED. IL‐4/IL‐13‐dependent and independent expression of miR‐124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PLoS ONE 2013; 8:e81774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grishok A, Pasquinelli AE, Conte D, Li N, Parrish S, Ha I et al Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 2001; 106:23–34. [DOI] [PubMed] [Google Scholar]
- 53. Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE et al The 21‐nucleotide let‐7 RNA regulates developmental timing in Caenorhabditis elegans . Nature 2000; 403:901–6. [DOI] [PubMed] [Google Scholar]
- 54. Jerome T, Laurie P, Louis B, Pierre C. Enjoy the Silence: The Story of let‐7 MicroRNA and Cancer. Curr Genomics 2007; 8:229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M et al MicroRNA let‐7c regulates macrophage polarization. J Immunol 2013; 190:6542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang W, Liu H, Liu W, Liu Y, Xu J. Polycomb‐mediated loss of microRNA let‐7c determines inflammatory macrophage polarization via PAK1‐dependent NF‐κB pathway. Cell Death Differ 2015; 22:287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kumar M, Sahu SK, Kumar R, Subuddhi A, Maji RK, Jana K et al MicroRNA let‐7 modulates the immune response to Mycobacterium tuberculosis infection via control of A20, an inhibitor of the NF‐κB pathway. Cell Host Microbe 2015; 17:345–56. [DOI] [PubMed] [Google Scholar]
- 58. Liu Y, Chen Q, Song Y, Lai L, Wang J, Yu H et al MicroRNA‐98 negatively regulates IL‐10 production and endotoxin tolerance in macrophages after LPS stimulation. FEBS Lett 2011; 585:1963–8. [DOI] [PubMed] [Google Scholar]
- 59. Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S et al MicroRNA‐21 (miR‐21) post‐transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 2008; 27:2128–36. [DOI] [PubMed] [Google Scholar]
- 60. Hilliard A, Hilliard B, Zheng SJ, Sun H, Miwa T, Song W et al Translational regulation of autoimmune inflammation and lymphoma genesis by programmed cell death 4. J Immunol 2006; 177:8095–102. [DOI] [PubMed] [Google Scholar]
- 61. Sheedy FJ, Palsson‐McDermott E, Hennessy EJ, Martin C, O'Leary JJ, Ruan Q et al Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR‐21. Nat Immunol 2010; 11:141–7. [DOI] [PubMed] [Google Scholar]
- 62. Lu TX, Munitz A, Rothenberg ME. MicroRNA‐21 is up‐regulated in allergic airway inflammation and regulates IL‐12p35 expression. J Immunol 2009; 182:4994–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Caescu CI, Guo X, Tesfa L, Bhagat TD, Verma A, Zheng D et al Colony stimulating factor‐1 receptor signaling networks inhibit mouse macrophage inflammatory responses by induction of microRNA‐21. Blood 2015; 125:e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang Z, Brandt S, Medeiros A, Wang S, Wu H, Dent A et al MicroRNA 21 is a homeostatic regulator of macrophage polarization and prevents prostaglandin E2‐mediated M2 generation. PLoS ONE 2015; 10:e0115855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Domingo‐Gonzalez R, Katz S, Serezani CH, Moore TA, Levine AM, Moore BB. Prostaglandin E2‐induced changes in alveolar macrophage scavenger receptor profiles differentially alter phagocytosis of Pseudomonas aeruginosa and Staphylococcus aureus post‐bone marrow transplant. J Immunol 2013; 190:5809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Squadrito ML, Pucci F, Magri L, Moi D, Gilfillan GD, Ranghetti A et al miR‐511‐3p modulates genetic programs of tumor‐associated macrophages. Cell Rep 2012; 1:141–54. [DOI] [PubMed] [Google Scholar]
- 67. Knipe RS, Tager AM, Liao JK. The Rho kinases: critical mediators of multiple profibrotic processes and rational targets for new therapies for pulmonary fibrosis. Pharmacol Rev 2015; 67:103–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Biswas PS, Gupta S, Chang E, Song L, Stirzaker RA, Liao JK et al Phosphorylation of IRF4 by ROCK2 regulates IL‐17 and IL‐21 production and the development of autoimmunity in mice. J Clin Invest 2010; 120:3280–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol 2015; 36:229–39. [DOI] [PubMed] [Google Scholar]
- 70. Raggi C, Mousa HS, Correnti M, Sica A, Invernizzi P. Cancer stem cells and tumor‐associated macrophages: a roadmap for multitargeting strategies. Oncogene 2016; 35:671–82. [DOI] [PubMed] [Google Scholar]
- 71. Karo‐Atar D, Itan M, Pasmanik‐Chor M, Munitz A. MicroRNA profiling reveals opposing expression patterns for miR‐511 in alternatively and classically activated macrophages. J Asthma 2015; 52:545–53. [DOI] [PubMed] [Google Scholar]
- 72. Eichner LJ, Perry MC, Dufour CR, Bertos N, Park M, St‐Pierre J et al miR‐378(*) mediates metabolic shift in breast cancer cells via the PGC‐1β/ERRγ transcriptional pathway. Cell Metab 2010; 12:352–61. [DOI] [PubMed] [Google Scholar]
- 73. Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR et al Oxidative metabolism and PGC‐1beta attenuate macrophage‐mediated inflammation. Cell Metab 2006; 4:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ruckerl D, Jenkins SJ, Laqtom NN, Gallagher IJ, Sutherland TE, Duncan S et al Induction of IL‐4Rα‐dependent microRNAs identifies PI3K/Akt signaling as essential for IL‐4‐driven murine macrophage proliferation in vivo . Blood 2012; 120:2307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H et al A novel regulator of macrophage activation: miR‐223 in obesity‐associated adipose tissue inflammation. Circulation 2012; 125:2892–903. [DOI] [PubMed] [Google Scholar]
- 76. Rossato M, Curtale G, Tamassia N, Castellucci M, Mori L, Gasperini S et al IL‐10‐induced microRNA‐187 negatively regulates TNF‐α, IL‐6, and IL‐12p40 production in TLR4‐stimulated monocytes. Proc Natl Acad Sci U S A 2012; 109:E3101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wei Y, Nazari‐Jahantigh M, Chan L, Zhu M, Heyll K, Corbalan‐Campos J et al The microRNA‐342‐5p fosters inflammatory macrophage activation through an Akt1‐ and microRNA‐155‐dependent pathway during atherosclerosis. Circulation 2013; 127:1609–19. [DOI] [PubMed] [Google Scholar]
- 78. Sene A, Khan AA, Cox D, Nakamura RE, Santeford A, Kim BM et al Impaired cholesterol efflux in senescent macrophages promotes age‐related macular degeneration. Cell Metab 2013; 17:549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ouimet M, Ediriweera HN, Gundra UM, Sheedy FJ, Ramkhelawon B, Hutchison SB et al MicroRNA‐33‐dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J Clin Invest 2015; 125:4334–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang N, Lei J, Lei H, Ruan X, Liu Q, Chen Y et al MicroRNA‐101 overexpression by IL‐6 and TNF‐α inhibits cholesterol efflux by suppressing ATP‐binding cassette transporter A1 expression. Exp Cell Res 2015; 336:33–42. [DOI] [PubMed] [Google Scholar]
- 81. Zhu QY, Liu Q, Chen JX, Lan K, Ge BX. MicroRNA‐101 targets MAPK phosphatase‐1 to regulate the activation of MAPKs in macrophages. J Immunol 2010; 185:7435–42. [DOI] [PubMed] [Google Scholar]
- 82. Gao Y, Liu F, Fang L, Cai R, Zong C, Qi Y. Genkwanin inhibits proinflammatory mediators mainly through the regulation of miR‐101/MKP‐1/MAPK pathway in LPS‐activated macrophages. PLoS ONE 2014; 9:e96741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Barton GM, Medzhitov R. Toll‐like receptor signaling pathways. Science 2003; 300:1524–5. [DOI] [PubMed] [Google Scholar]
- 84. Gay NJ, Symmons MF, Gangloff M, Bryant CE. Assembly and localization of Toll‐like receptor signalling complexes. Nat Rev Immunol 2014; 14:546–58. [DOI] [PubMed] [Google Scholar]
- 85. Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, Mori L et al Induction and regulatory function of miR‐9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci U S A 2009; 106:5282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y, Abraham E. miR‐147, a microRNA that is induced upon Toll‐like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci U S A 2009; 106:15819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wei J, Huang X, Zhang Z, Jia W, Zhao Z, Zhang Y et al MyD88 as a target of microRNA‐203 in regulation of lipopolysaccharide or Bacille Calmette–Guérin induced inflammatory response of macrophage RAW264.7 cells. Mol Immunol 2013; 55:303–9. [DOI] [PubMed] [Google Scholar]
- 88. Saha B, Bruneau JC, Kodys K, Szabo G. Alcohol‐induced miR‐27a regulates differentiation and M2 macrophage polarization of normal human monocytes. J Immunol 2015; 194:3079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Alam MM, O'Neill LA. MicroRNAs and the resolution phase of inflammation in macrophages. Eur J Immunol 2011; 41:2482–5. [DOI] [PubMed] [Google Scholar]
- 90. Liu G, Abraham E. MicroRNAs in immune response and macrophage polarization. Arterioscler Thromb Vasc Biol 2013; 33:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bi Y, Liu G, Yang R. MicroRNAs: novel regulators during the immune response. J Cell Physiol 2009; 218:467–72. [DOI] [PubMed] [Google Scholar]
- 92. Wu XQ, Huang C, Liu XH, Li J. MicroRNA let‐7a: a novel therapeutic candidate in prostate cancer. Asian J Androl 2014; 16:327–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Thomas M, Lieberman J, Lal A. Desperately seeking microRNA targets. Nat Struct Mol Biol 2010; 17:1169–74. [DOI] [PubMed] [Google Scholar]
- 94. Ding Y, Tang Y, Kwok CK, Zhang Y, Bevilacqua PC, Assmann SM. In vivo genome‐wide profiling of RNA secondary structure reveals novel regulatory features. Nature 2014; 505:696–700. [DOI] [PubMed] [Google Scholar]


