Abstract
Acute kidney injury (AKI) is the rapid onset of decreased kidney function that ultimately increases mortality and morbidity. Stem cell research is a promising avenue for curative and preventative therapies of kidney injury, however, there are many types of stem cells under investigation. Currently there is no research to compare the value of one stem cell method over another. Induced pluripotent stem cells (iPSCs) and spermatogonial stem cells (SSCs) have been shown to differentiate into renal cells, though further clinical research is needed to fully explore potential therapeutic strategies. Mesenchymal stem cells (MSCs) have long been investigated in the preclinical setting and have recently been successful in Phase I clinical trials. MSCs may represent a promising new therapeutic approach to treat AKI as they demonstrate renoprotective effects post-injury via the secretion of promitotic, anti-apoptotic, anti-inflammatory, and immunomodulatory factors. Given the most current research, MSCs appear to offer a promising course of treatment for AKI.
Keywords: Induced pluripotent stem cells, spermatogonial stem cells, mesenchymal stem cells, acute kidney injury, AKI, treatment
Introduction
Acute kidney injury (AKI), previously known as acute renal failure (ARF), is defined by the rapid onset of decreased excretory function [1]. The stage of AKI is based on increased serum creatinine levels concurrent with decreased urine output [2]. Patients that have suffered from AKI have long struggled with diminishing health and increased mortality upon diagnosis. Risk of AKI increases with age and uncontrolled diabetes mellitus and often develops without pre-existing kidney issues. Increases in severity of AKI and number of episodes are associated with an increased risk of Chronic Kidney Disease (CKD). Drug therapies have had limited success and sustainability in the clinical field, which highlights a dire need for curative treatment options, such as stem cell based kidney repair [3].
Stem cells are described by their self-renewal abilities and the capability to develop into various functional cells. There are four classes of developmental potential among stem cells. Totipotent cells are the most versatile, as they can develop into any cell of an organism, including extraembryonic tissues. Pluripotent cells, such as embryonic stem cells, can develop into all cell types in the body of an organism but not into extraembryonic tissues, such as the placenta. Multipotent cells give rise to cells of a specific lineage, for example mesenchymal stem cells give rise to skeletal tissues. Adult stem cells, umbilical cord stem cells, and mesenchymal stem cells are all examples of multipotent cell types. Unipotent stem cells are the most restricted in their potency and can only form one cell type [4].
Induced pluripotent stem cells (iPSCs) are derived from a patient’s tissue and induced into pluripotency. The most common and successful method to inducing pluripotency is through viral vectors, which raises questions as to the dangers associated with using the cells in clinical treatments [5]. Spermatogonial stem cells (SSCs), found in the male testis, are unipotent stem cell lines. When cultured in vitro, embryonic like stem cells can be isolated from SSC cultures [6]. Experiments on SSCs regarding AKI therapy are in the beginning stages and as such there is limited research into the efficacy of the approach.
The majority of research on the therapeutic advantages of stem cell therapies surround mesenchymal stem cells (MSCs). MSCs are derived from nonhematopoietic precursors and have the capacity to differentiate into mesenchymal lineages in vitro. MSC therapy has already proven effective in reducing AKI in experimental models. Clinical trials are indicative that MSC infusion provides no serious threat to the patient and more importantly that it prevented postoperative renal failure [7].
Here, we compare the three different stem cell therapeutic models to establish which venue provides the most promising therapeutic benefits for AKI. The success with MSC therapy, in both preclinical and clinical studies, suggests it will be a viable treatment option in the near future. SSC and iPSC therapies are in the early pre-clinical stages of research but the recent data suggests that continued investigation will unveil SSCs and iPSCs as alternative therapeutic agents.
Induced pluripotent stem cells
Among the different types of stem cells currently under investigation for therapeutic strategies, induced pluripotent stem cells (iPSCs) possess great potential for application in organ regeneration. Organs such as the kidney have long been thought to lack regenerative properties and have thus proved extremely difficult to repair once permanent damage, such as from AKI, has been inflicted. Embryonic as well as adult stem cells have pluripotent properties; however, embryonic stem cell research is often met with ethical dilemmas and adult stem cells are not sufficient to repair acute renal injury. iPSCs are unique in that differentiated adult cells are induced into a pluripotent state through exposure to certain reprogramming factors. If iPSCs can be used for organ regeneration, the need for organ transplantation as well as the use of immunosuppressant drugs can be avoided because new organs could be generated from a patient’s own cells [5].
One method used to generate iPSCs is through non-integrative viral vectors. Previous studies have experienced difficulties in controlling copy number and complete incorporation when the vector integrated into the genome. Alternatively, adult cells can be induced to a pluripotent state using non-integrative viral vectors that transiently express the reprogramming factors. Xia et al. used this method with human fibroblasts and successfully generated cells with pluripotency markers. The iPSCs were primed for a ureteric bud progenitor-like fate with a 4-day protocol during which cells were directed toward a mesodermal fate with bone morphogenic protein 4 (BMP-4) and basic fibroblast growth factor (FGF-2). A combination of retinoic acid, activin A, and BMP-2 further specified the cells to intermediate mesoderm and renal-like lineages. Xia and colleagues used the expression of specific cell markers to positively identify the cells as renal-like populations. In order to identify the precise renal lineage to which the cells were directed, the mRNA expression of the induced cells was examined and found to closely resemble the expression profile of ureteric bud progenitor cells. Following the initial differentiation, murine embryonic metanephric mesenchyme cells provided the developmental cues that facilitated the formation of the iPSC-derived ureteric bud progenitor cells into actual ureteric bud structures. Furthermore, Xia et al. generated iPSCs from a patient with polycystic kidney disease (PKD). Using a combination of a three-dimensional culture system and mouse mesenchyme cells, this team successfully created patient-derived ureteric bud structures. Although this study used PKD-iPSCs, this protocol for renal differentiation can be applied to the study of other kidney-related disorders, including AKI [8].
AKI is frequently characterized by damage and the subsequent loss of glomerular podocytes. These complex and specialized cells play an important role in glomerular filtration so the destruction of such cells quickly results in impaired renal function. Unlike the ureteric bud progenitors previously discussed, podocytes are derived from metanephric mesenchyme, and therefore require different factors to drive correct specification. iPSCs can be directed to a podocyte progenitor fate with activin A, BMP-7, and retinoic acid in a suspension culture. After a 10-day incubation, the iPSCs successfully differentiated into podocyte progenitors with cell morphology of primary human podocytes but also a capacity for proliferation. The podocytes derived from iPSCs exhibit the complex cell morphology of adult podocytes, including not only the primary pedicels but secondary and tertiary foot projections as well. Beyond the histological appearance of the iPSC-derived podocytes, the cells generated by Song et al. also expressed podocyte-specific cell markers such as podocin and synapodin. In contrast to primary podocytes that are quiescent in culture, iPSC-derived podocytes maintained proliferation for 3 months, a promising finding for potential organ regeneration. The podocytes generated from iPSCs also exhibited a contractile response to angiotensin II and albumin uptake, similar to normally functioning human podocytes. Using murine embryonic kidneys, iPSC podocytes incorporated into the developing kidney particularly in regions of nephrogenesis . The results of this study show the potential for iPSC-derived kidney cells to both attain the same functionality as primary renal cells as well as integrate into previously existing renal tissue [9].
Thatava et al. [10] furthered the research in iPSC therapy for renal diseases by investigating the generation of iPSCs from patients with polycystic kidney disease, Wilm’s tumor, and systemic lupus erythematosus. Skin biopsies provided keratinocytes that were subsequently induced to a pluripotent state with lentiviral vectors expressing reprogramming factors OCT4, SOX2, KLF4, and c-MYC. The iPSC populations grown in culture expressed cell markers similar to those of embryonic stem cells and were able to differentiate into each of the three germ layers confirming the induction of pluripotency. The proliferative abilities of the cells were further verified in vivo after injection into SCID-Beige mice produced teratomas within 3 to 5 months. The successful production of disease and patient specific iPSC lines provides future opportunities for both disease pathogenesis studies at the cellular level in addition to kidney replacement therapy without the use of a transplant donor or immunosuppressants [10]. There is still much research to be done, however, before iPSCs can effectively and efficiently be used for autologous cell or organ replacement.
As seen in several of the previously discussed studies, one of the reprogramming factors frequently used in the generation of iPSCs is c-Myc, a gene that plays an important role in cell proliferation and apoptosis. Because c-Myc is a known oncogene that may contribute to tumor development, c-Myc expression is a concerning factor when iPSCs are explored as therapeutic agents for renal injury. Lee et al. investigated the efficacy of iPSCs without exogenous c-Myc as a mechanism for repair and regeneration of renal tissue in AKI. Ischemia and inflammation are two of the most common mechanisms that lead to AKI. iPSCs may be a safe and effective means to repair renal tissue damaged in such a way because iPSCs have been found to suppress intracellular reactive oxygen species and reduce inflammatory cytokines. Lee et al. [3] found that iPSCs created without c-Myc both improved renal function and renal tubular injury as well as eliminated post-transplantation tumorigenesis. Similar to the previous studies discussed, the success of iPSC generation was confirmed by the expression of pluripotency cell markers and the cells’ ability to differentiate into endodermal, mesodermal, and ectodermal lineages. The ability of the iPSCs to attenuate the damaging effects of ischemia-induced AKI was tested by measuring the blood urea nitrogen (BUN) and creatinine levels of rats with AKI following intrarterial, intraperitoneal, and intravenous administration of the iPSCs [3]. Furthermore, after iPSC transplantation, there was a reduction in macrophage proliferation and oxidative stress in the renal tissue. Overall, the survival rates in rats with AKI was significantly increased and renal function improved, indicating iPSC transplantation as a potential therapeutic agent for AKI [3].
Spermatogonial stem cells
Spermatogonial stem cells (SSC) differentiate to produce sperm throughout the life of a male [11]. These cells are considered unipotent in vivo but recent studies have established the ability to dedifferentiate into pluripotent cell lines. SSCs can be cultured long term while maintaining their integrity and under specific conditions can spontaneously convert into embryonic like stem cells (GPSCs). Unlike iPSCs, no reprogramming factors are necessary which reduces the limitations on therapeutic benefits [12]. When cultured with leukemia inhibitory factor or when grown on mitomycin-C treated mouse embryonic fibroblasts, embryonic stem cell like colonies grew from spermatogonial stem cells isolated from mouse adult male testis. Isolation and expansion of these colonies were maintained for over 30 generations. The GPSCs differentiated into all three germ layers from an embryoid body. The pluripotency was further confirmed by formation of teratomas in live mice [13].
The induced pluripotent ability of SSCs offer therapeutic potential and the ability of SSCs to differentiate into renal parenchymal cells has been established. Mouse spermatogonial stem cells transplanted into female mice kidneys developed into mature renal cells. The maturation into renal tubule epithelial cells and glomerular podocytes was shown by the expression of Y chromosome. Though the exact mechanism remains unknown, the ability of spermatogonial stem cells to transform into renal parenchyma provides promising therapeutic potential in the treatment of AKI [14].
A second study was recently conducted in which GPSCs differentiated into functional renal tubular like cells (GTC). During differentiation, GPSCs underwent tubulogenesis, stimulated by type IV collagen and epidermal growth factor. The presence of tight junctions established functionality of the GTCs and the lack of teratoma formation after GTC injection into mouse kidneys exhibited full differentiation. Induced renal injury (IRI) models were used based on a unilateral nephrectomy and subsequent kidney ischemia to assess GTC protective abilities against AKI. Mice that received GTC injections presented with marked reductions in serum creatinine levels compared to mice receiving a saline injection. Since creatinine levels are a marker for kidney function, the decreased levels indicate an improvement in kidney function. Histologic analysis of the ischemic kidney showed a lower number of necrotic tubules and therefore a reduction in tissue damage. There was even an elevation in protective enzyme levels in the GTC treated mice. These experiments established the ability of cells ultimately derived from spermatogonial stem cells to terminally differentiate into functional renal tubular like cells and restore kidney function following ischemic insult [12].
Studies have also confirmed the ability of human SSC to spontaneously differentiate into human embryonic like cells under specific culture conditions. The human embryonic like cells expressed high levels of human ESC markers. The human embryonic like cells formed embryoid bodies in culture which were further successful in differentiating into each of the three primary germ layers [15,16]. In two separate studies, large teratomas were not formed from injection of human embryonic stem like cells into mice [15,16]. This differs from the inclination of human ES cells and iPS cells to form teratomas.
SSCs provide a promising venue for the future. The ability to spontaneously convert into embryonic like cells without genetic modification suggests they may be a better venue than iPSCs. The lack of large teratoma formation with an ability to ultimately differentiate into all three primary germ layers also presents therapeutic benefits. More research is needed to confirm the abilities of SSCs via GTCs to restore kidney function after insult, but preliminary research in mouse models, in addition to the established success of SSC to differentiate into renal parenchymal cells is a step toward developing an efficient therapeutic approach for AKI.
Mesenchymal stem cells (MSC)
Mesenchymal stem cells (MSCs) are adult, nonhematopoietic precursor cells derived most commonly from bone marrow, but can also be derived from a variety of tissues such as umbilical cord blood, fetal membrane, and adipose tissue. MSCs are multipotent, meaning they can differentiate into more than one cell type, but not all cell types. MSCs are derived from the mesodermal germ layer and differentiate into mesenchymal (osteoblasts, adipocytes, and chondroblasts) as well as non mesenchymal lineages [7]. Differentiation of MSCs into specific cell types can be directed under certain culture conditions. MSCs are isolated from a small sample of bone marrow and easily expanded in vitro. Unlike embryonic stem cells and iPSCs, MSCs are easily cultured and expanded. Thus, MSCs are a practical option for clinical therapies due to ease of preparation. MSCs remain among the top choices for cellular based therapies due to their immunomodulatory properties which reduce inflammation and the potential to ameliorate autoimmunity. The multitude of therapeutic factors released by MSCs and their beneficial effects are demonstrated in Figure 1 [17]. Given the benefits of MSCs, they are currently undergoing clinical trials for a multitude of human diseases, including AKI.
Figure 1.
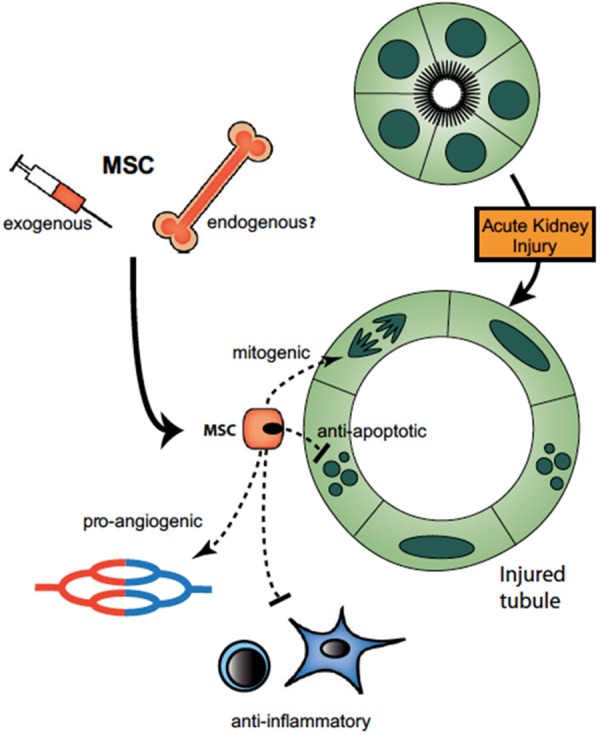
Model for paracrine actions of MSCs on the injured tubule. After an acute kidney injury, injected MSCs home to injury sites and may be recruited from endogenous niches (bone marrow or kidney) as well. MSCs bind to glomerular and/or peritubular capillary endothelium and both protect the kidney from further injury and accelerate repair. Paracrine mediators play important roles in repair, including VEGF, IGF, HGF, PGE2, and other soluble factors that exert mitogenic, antiapoptotic, proangiogenic and anti-inflammatory effects [17].
Paracrine and endocrine effects of MSC
It was originally believed that MSCs provided a therapeutic basis for regeneration by engrafting at the site of injury via transdifferentiation-the ability of adult stem cells of one lineage to differentiate into a different cell type, in this case renal parenchymal cells. However, further testing revealed that engraftment of MSCs to the site of injury was very rare and therefore was not likely to be the sole cause of regeneration of after AKI [18]. This discovery led to the idea that paracrine factors exerted by stem cells, acting in conjunction with endocrine signals, were possible mechanisms of repair post-injury [18]. MSCs secrete a wide variety of growth factors, cytokines, and polypeptides that provide therapeutic effects. Polypeptides secreted by MSCs have been shown to enhance epithelial proliferation, modulate inflammation, and promote angiogenesis, thus making MSCs good therapeutic candidates for renal injury. IGF-1 and HGF are two MSC-derived factors that promote renal blood flow and protect against ischemia [19]. Through the secretion of growth factors and cytokines, MSCs are able to exert complex paracrine and endocrine signaling cascades that aid in repair during AKI.
Role of MSC-derived microvesicles in renal repair after injury
Interestingly, MSCs have recently been found to secrete another substance, microvesicles, which were shown to exert anti-apoptotic effects, thus aiding in cell survival after injury. Microvesicles are small particles released from MSC in a paracrine fashion that deliver messenger RNA (mRNA), micro RNA, or proteins that may function in reprogramming injured cells or promote secretion of cytoprotective factors [20]. Each of these effects has been demonstrated in injury models of various organ systems, such as acute lung injury, acute kidney injury (AKI), and acute brain injury. Bruno et al. investigated the therapeutic effects of microvesicles derived from MSCs in SCID mice upon injection of cisplatin, a chemotherapy drug that is toxic to the kidney and thus induces AKI. Cisplatin-injected SCID mice received a single injection of microvesicles (siMV), multiple injections of mi-crovesicles (miMV), and a control group with no MSC-derived microvesicles injected. Microvesicles derived from MSCs reduced mortality of cisplatin-injected mice. By day 21, the survival rate in cisplatin-injected mice with siMV was 40% and from miMV was 80% with respect to the control group. Treatment with miMV was significantly more effective than siMVs by day 21, indicating that microvesicles derived from MSC aid in the AKI repair process, perhaps in a dose-dependent manner.
Immunomodulation
Acute kidney injury can be caused by ischemia-reperfusion, a process that begins with lack of oxygen and nutrients traveling to tissue. When blood flow is restored, oxidative stress and an inflammatory response are triggered, leading to damage and acute injury [21]. Upon insult, macrophages and neutrophils migrate to damaged tissue and release pro-inflammatory cytokines, chemokines, and reactive oxygen species. In addition, cells of the adaptive immune response, natural killer cells, B and T lymphocytes, and mast cells are also present and take part in the inflammatory response. This process of ischemia-reperfusion can be simulated in animal models to induce acute kidney injury for further study.
Mesenchymal stem cells (MSCs) have the ability to travel to these inflammatory sites, making stem cell therapy very promising for repair of acute kidney injury. Under normal physiological circumstances, MSCs given intravenously will often migrate to the bone marrow [22]. However, upon injury MSCs will migrate to the damaged site. This homing capability is driven by chemokines released from the damaged site as well as the MSCs themselves. Pathways include stromal cell-derived factor-1/CXCR4 and CD44 which are both up-regulated following ischemic injury [23,24]. Once at the site of injury, MSCs can reprogram monocytes and macrophages to shift from a pro-inflammatory to anti-inflammatory state. This shift is associated with decreased levels of TNF-α, IL-1beta, and interferon-γ which in turn helps to reduce the inflammatory response [25]. MSCs also release a number of soluble factors to increase anti-inflammatory activity including interleukin 1, interleukin 10, and prostaglandin E2.
Previous studies have reported on the anti-inflammatory nature of PGE2 and its effects on cytokines, such as IL-10. The immunosuppressive properties of IL-10 dampen the effect of immune cells, neutrophils and monocytes, to reduce chemokine and cytokine production and therefore, the inflammatory response. Bone marrow MSC (BM-MSC) are capable of triggering IL-10 production by reprogramming macrophages, leading to the aforementioned anti-inflammatory response [26].
Tsuda et al. has verified this pathway by introducing an anti-IL-10 antibody to MSC transplanted rats, which lessened the therapeutic ability of MSC following ischemia-reperfusion related injury. Other factors of the inflammatory response at play in ischemia-reperfusion conditions include monocyte chemotactic protein (MCP-1 or CCL2) and IL-6. When treated with fetal membrane MSC (FM-MSC), macrophage infiltration has been shown to decrease, and this may be due to FM-MSC ability to decrease MCP-1 and IL-6 activity. FM-MSC treatment in animal models with myocarditis has also been shown to involve the adaptive immune response, by reducing activation, proliferation, and infiltration of T cells upon injury [27]. This decline in T cell involvement provides further evidence of MSC immunomodulatory capability extending beyond the anti-inflammatory response. This ability may transfer well from the myocarditis model into therapeutic options for AKI with further research.
Anti-apoptotic effects of MSC
Ischemia-reperfusion injuries leading to AKI have been associated with accumulation of reactive oxygen species (ROS) and apoptosis. The process of ROS accumulation is referred to as oxidative stress, which results in elevated levels of free radicals that can be harmful to tissues. Ischemia-reperfusion injury has been shown to increase ROS levels, leading to increased tissue damage. This process, coupled with elevated apoptotic proteins, plays a role in destroying renal tissue during AKI. MSC treatment has been found to reduce oxidative stress and apoptosis in animal models following ischemia-reperfusion injury [21].
Anti-apoptotic pathways of MSCs have been further elucidated in animal models undergoing cisplatin-induced acute kidney injury. Introduction of cisplatin into animal models can cause AKI by triggering apoptotic pathways that destroy tubular cells. Cisplatin injection decreases the levels of anti-apoptotic proteins like Bcl-2 and increases pro-apoptotic proteins like Bax. MSCs up-regulate Bcl-2 and down-regulates Bax, leading to an anti-apoptotic response [28].
Clinical trials
Clinical data on MSC capacity to treat AKI demonstrated very positive results, with patients requiring a shorter hospital stay, decreased chance of readmission, and prevention of further renal damage. MSC treated cells demonstrated a rise in proliferating cell nuclear antigen (PCNA), a marker for regeneration, when compared to control cells, as seen in Table 1 [29]. MSC treated cells also displayed a significantly lower apoptotic index, as determined by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, which measures the level of apoptotic proteins interleukin-1β, tumor necrosis factor-α, interferon-γ. The increase in proliferative cells and decrease in apoptotic proteins led to an overall lower kidney injury score compared to control cells [30].
Table 1.
Survival rates for saline (control), bone marrow-MSC, and cord blood-MSC following cisplatin injection and subsequent damage. Saline treated cells had a 0 survival rate 7 days after cisplatin injection. Bone marrow-MSC treated cells had a 50 survival rate, and cord blood-MSC had an 86 survival rate. Table is adapted from Morigi 2013 [29]
| Mesenchymal Stem Cells and Kidney Repair | |||
|---|---|---|---|
| Comparative effect of stem cells of different origin in experimental ARF | |||
|
| |||
| Treatment | Blood Urea Nitrogen (mg/dL) | Renal Histologya | Survivalb |
| Saline | >140 | Damaged | 0 |
| Bone marrow-MSC | 63 ± 5 | Preserved | 50 |
| Cord blood-MSC | 58 ± 7 | Preserved | 86 |
On day 4 from cisplatin injection.
On day 7 from cisplatin injection.
Alternatives to bone marrow-derived MSC
BM-MSC has shown promising results in both preclinical and clinical studies with regards to AKI treatment. However, BM-MSC collection is relatively invasive and does not always provide a large source of MSC, which poses potential limitations on its usage. This has opened the door to other potential stem cell options. Fetal membrane MSC (FM-MSC), collected in a non-invasive manner and in large quantities, have been shown to provide promising protection and treatment possibilities for AKI [27]. Another alternative to BM-MSCs is cord blood-MSCs (CB-MSC), which have shown greater survival rates when compared to BM-MSCs [29]. CB-MSC, BM-MSC, and a saline control were injected with cisplatin and survival of rats was measured 7 days following injection. The survival of rats treated with CB-MSC was 86, BM-MSC was 50, and saline control was 0 [25]. CB-MSC recorded increased gene expression for multiple factors of angiogenesis [31].
Conclusions
In recent years, there has been a multitude of research regarding the application of stem cell therapies for the treatment of acute kidney injury. This paper addresses three different types of stem cells (MSCs, iPSCs, and SSCs) that have shown to be most promising for the development of reparative and regenerative treatment for AKI. The goal was to provide a summary of the latest research regarding stem cell therapy as supported by the current body of scientific evidence. A concise summary of these findings can be found in Table 2.
Table 2.
Comparison of iPSCs, SSCs, and MSCs for treatment of AKI
| Harvest Method | Reprogramming Factors | Differentiation into Kidney Progenitors | Pre-clinical Benefits | Clinical Trial Results | |
|---|---|---|---|---|---|
| Induced Pluripotent Stem Cells | Skin biopsy (provides keratinocytes and fibroblasts) | OCT-4, SOX-2, KLF-4, and/or c-MYC | Ureteric bud cells and podocyte | ●Reduced severity of AKI in rats | None |
| ●Reduced macrophage proliferation in kidney | |||||
| ●Reduced oxidative stress in kidney | |||||
| Spermatogonial Stem Cells | Testis biopsy | Not Applicable | Epithelial cells of the renal tubule and podocytes (via human embryonic like cells) | ●Differentiate into functional renal tubular like cells | None |
| ●Administration to AKI rats reduced serum creatinine levels and reduced necrotic tubules | |||||
| Mesenchymal Stem Cells | Biopsy from bone marrow, cord blood, or fetal membrane | Not Applicable | Renal parenchymal cells, glomerular and tubular epithelial cells, glomerular and tubular interstitial cells | ●Immunomodulatory and anti-inflammatory effects | ●Reduced hospital stay |
| ●Anti-apoptotic | ●Decreased readmission rates | ||||
| ●Mitogenic | ●Prevention of further renal damage | ||||
| ●Lower kidney injury score compared to control |
A summary of the present research regarding stem cell-based therapies for acute kidney injury (AKI). Although many positive outcomes have been observed in pre-clinical settings for the use of spermatogonial stem cells (SSCs) and induced pluripotent stem cells (iPSCs) in the treatment of AKI, the current body of research on mesenchymal stem cell (MSC) treatments is more robust. MSC-based therapies are the only stem cell therapies currently in Phase I clinical trials for the treatment of AKI.
In regards to iPSCs, many of the recent studies have established quick, safe, and effective protocols for inducing pluripotency in adult cells, directed differentiation toward a renal cell lineage, and used these cells to treat the damaging effects of AKI. In addition, a variety of adult cells types can be used for the generation of iPSCs so harvesting methods are relatively non-invasive and viable options to most individuals. One of the major concerns with iPSC use, however, is that of teratoma formation. One of the reprogramming factors used to induce pluripotency is c-Myc, a known oncogene that can lead to tumorigenesis. Only one study thus far has addressed this issue and generated iPSCs without exogenous c-Myc.
Similarly, SSC-based therapies have been found to have several positives and negatives for the treatment of AKI. SSCs have shown very limited teratoma formation and do not require the reprogramming factors used in iPSCs to reach a pluripotent state, bypassing one of the major concerns associated with iPSC use. These stem cells have also exhibited a relatively long time frame of proliferation, allowing for long-term culturing without deterioration. Researchers have demonstrated the ability of SSCs to differentiate into renal lineages, but there has yet to be studies directly addressing the therapeutic effects of human derived SSCs for AKI. Furthermore, even though they can be used in both genders, SSCs can only be harvested from male patients and require a fairly invasive procedure.
Although both iPSCs and SSCs show great promise as therapeutic agents for AKI, the amount of research currently available is insufficient to make a definitive conclusion in favor of one cell type or the other for treatment of AKI. Additionally, the vast amount of research that has been done on MSCs, up to Phase I clinical trials, has led us to conclude that currently the best course of action for stem cell-based treatment of AKI is MSC-derived therapy. Currently ongoing Phase II clinical trials will likely expand upon the knowledge base of MSC-derived therapies.
MSC therapies have shown multiple beneficial outcomes including anti-inflammatory, mitogenic, anti-apoptotic, and pro-angiogenic effects with no detrimental side effects thus far. Additionally, MSCs can be cultivated from a variety of sources. While bone marrow is the most common source of MSCs, its collection is relatively invasive, which has led to research involving less invasive MSC sources from umbilical cord blood and fetal membranes, both of which show promising pre-clinical data for AKI treatment. While all three forms of MSCs seem to be very promising in treating ischemia injury, further research is needed to determine which type of MSCs are most beneficial for AKI and other forms of acute organ injury. After considering the evidence regarding the three types of stem cells included in this review (iPSCs, SSCs, and MSCs), MSCs may offer the most promising results in treatment of AKI, both preclinically and clinically, and further research is likely to yield exciting and encouraging results in the field of stem cell regeneration.
Acknowledgements
We acknowledge the guidance of Dr. G. Ian Gallicano throughout our research and writing process.
Disclosure of conflict of interest
None.
References
- 1.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 2.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee P, Chien Y, Chiou G, Lin C, Chiou C, Tarng D. Induced pluripotent stem cells without c-Myc attenuate acute kidney injury via downregulating the signaling of oxidative stress and inflammation in ischemia-reperfusion rats. Cell Transplant. 2012;21:2569–2585. doi: 10.3727/096368912X636902. [DOI] [PubMed] [Google Scholar]
- 4.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morales EE, Wingert RA. Renal stem cell reprogramming: Prospects in regenerative medicine. World J Stem Cells. 2014;6:458–466. doi: 10.4252/wjsc.v6.i4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M, Niwa O, Oshimura M, Heike T, Nakahata T, Ishino F, Ogura A, Shinohara T. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 8.Xia Y, Nivet E, Sancho-Martinez I, Gallegos T, Suzuki K, Okamura D, Wu MZ, Dubova I, Esteban CR, Montserrat N, Campistol JM, Izpisua Belmonte JC. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol. 2013;15:1507–15. doi: 10.1038/ncb2872. [DOI] [PubMed] [Google Scholar]
- 9.Song B, Smink AM, Jones CV, Callaghan JM, Firth SD, Bernard CA, Laslett AL, Kerr PG, Ricardo SD. The directed differentiation of human iPS cells into kidney podocytes. PLoS One. 2012;7:e46453. doi: 10.1371/journal.pone.0046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thatava T, Armstrong AS, De Lamo JG, Edukulla R, Khan YK, Sakuma T, Ohmine S, Sundsbak JL, Harris PC, Kudva YC, Ikeda Y. Successful disease-specific induced pluripotent stem cell generation from patients with kidney transplantation. Stem Cell Res Ther. 2011;2:48. doi: 10.1186/scrt89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dym M. Spermatogonial stem cells of the testis. Proc Natl Acad Sci U S A. 1994;91:11287–11289. doi: 10.1073/pnas.91.24.11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Chiara L, Fagoonee S, Ranghino A, Bruno S, Camussi G, Tolosano E, Silengo L, Altruda F. Renal cells from spermatogonial germline stem cells protect against kidney injury. J Am Soc Nephrol. 2014;25:316–328. doi: 10.1681/ASN.2013040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 14.Wu D, He D, Li X, Liu Z. Differentiations of transplanted mouse spermatogonial stem cells in the adult mouse renal parenchyma in vivo. Acta Pharmacol Sin. 2008;29:1029–1034. doi: 10.1111/j.1745-7254.2008.00836.x. [DOI] [PubMed] [Google Scholar]
- 15.Golestaneh N, Kokkinaki M, Pant D, Jiang J, DeStefano D, Fernandez-Bueno C, Rone JD, Haddad BR, Gallicano GI, Dym M. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009;18:1115–26. doi: 10.1089/scd.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, Gromoll J, Turek PJ, Reijo-Pera RA. Isolation and Characterization of Pluripotent Human Spermatogonial Stem Cell-Derived Cells. Stem Cells. 2009;27:138–149. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Medicine. 2008;59:311. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 18.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, Abbate M, Zoja C, Remuzzi G. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- 20.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, Shao PL, Chang KC, Leu S, Yip HK. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;9:51. doi: 10.1186/1479-5876-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, Sturgeon C, Hewett T, Chung T, Stock W, Sher D, Weissman S, Ferrer K, Mosca J, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29:244–255. doi: 10.1016/s0301-472x(00)00635-4. [DOI] [PubMed] [Google Scholar]
- 23.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 24.Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 25.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 26.Bouffi C, Bony C, Courties G, Jorgensen C, Noel D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One. 2010;5:e14247. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuda H, Yamahara K, Otani K, Okumi M, Yazawa K, Kaimori JY, Taguchi A, Kangawa K, Ikeda T, Takahara S, Isaka Y. Transplantation of allogenic fetal membrane-derived mesenchymal stem cells protects against ischemia/reperfusion-induced acute kidney injury. Cell Transplant. 2014;23:889–899. doi: 10.3727/096368913X665594. [DOI] [PubMed] [Google Scholar]
- 28.Qi S, Wu D. Bone marrow-derived mesenchymal stem cells protect against cisplatin-induced acute kidney injury in rats by inhibiting cell apoptosis. Int J Mol Med. 2013;32:1262–1272. doi: 10.3892/ijmm.2013.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morigi M, Benigni A. Mesenchymal stem cells and kidney repair. Nephrol Dial Transplant. 2013;28:788–793. doi: 10.1093/ndt/gfs556. [DOI] [PubMed] [Google Scholar]
- 30.Westenfelder C, Togel FE. Protective actions of administered mesenchymal stem cells in acute kidney injury: relevance to clinical trials. Kidney Int Suppl (2011) 2011;1:103–106. doi: 10.1038/kisup.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panepucci RA, Siufi JL, Silva WA Jr, Proto-Siquiera R, Neder L, Orellana M, Rocha V, Covas DT, Zago MA. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004;22:1263–1278. doi: 10.1634/stemcells.2004-0024. [DOI] [PubMed] [Google Scholar]


