Abstract
During human brain development, cortical gyrification, which is believed to facilitate compact wiring of neural circuits, has been shown to follow an inverted U-shaped curve, coinciding with the two-stage neurodevelopmental process of initial synaptic overproduction with subsequent pruning. This trajectory allows postnatal experiences to refine the wiring, which may manifest as endophenotypic changes in cortical gyrification. Diving experts, typical elite athletes who commence intensive motor training at a very young age in their early childhood, serve ideal models for examining the gyrification changes related to long-term intensive diving training. Using local gyrification index (LGI), we compared the cortical gyrification between 12 diving experts and 12 controls. Compared with controls, diving experts showed widespread LGI reductions in regions relevant to diving performance. Negative correlations between LGIs and years of diving training were also observed in diving experts. Further exploratory network efficiency analysis of structural cortical networks, inferred from interregional correlation of LGIs, revealed comparable global and local efficiency in diving experts relative to controls. These findings suggest that gyrification reductions in diving experts may be the result of long-term diving training which could refine the neural circuitry (via synaptic pruning) and might be the anatomical substrate underlying their extraordinary diving performance.
The human cerebral cortex is a highly complex structure with rich gyri and sulci. Gyrification, the process of gyrus and sulcus formation, allows a larger cortical surface area (therefore a greater number of neurons) to fit in the skull, facilitating the development of compact wiring for efficient information processing1. The exact mechanisms underlying the developmental process of cortical gyrification remain unclear, although a number of theories have surfaced. Among these theories, the most attractive model for the development of cortical gyrification is the tension-based hypothesis proposing that cortical gyrification is mainly driven by the tension of the underlying white matter fiber tracts2. Other hypotheses emphasize the developmental mechanisms of the cerebral cortex3 or differential expansion of cortical layers4 as the major causes for cortical gyrification (the gray matter hypothesis). Recent studies supported the opinion that the tension-based hypothesis and the gray matter hypothesis should be considered to be complementary or coexistent in the causal mechanisms of cortical gyrification, rather than as alternatives5.
Cortical gyrification of the human brain begins between 10 and 15 weeks of fetal life6,7. During the first trimester of fetal life, cortical gyrification increases dramatically6, which transforms the cerebral cortical landscape from a relatively smooth, lissencephalic structure into a highly convoluted cortex, resembling the morphology of the adult brain. Existing morphometric studies on the developmental trajectory of human cortical gyrification were mainly conducted using gyrification index (GI) or local gyrification index (LGI), with a higher GI or LGI value indicating a more complex cortical sheet8,9. The findings of these studies suggest that the GI or LGI continues to increase after birth until it reaches its peak somewhere between 2 and 6 postnatal years10,11,12, and is then followed by a protracted declining period12,13,14. These changes likely reflect the two-stage neurodevelopmental process of initial synaptic overproduction followed by experience-dependent synaptic elimination which represents a fine-tuning process of neural circuitry and plays important roles in neural circuit maturation1,14,15. Morphological studies on twins demonstrated that the cerebral volume is highly determined by genetic variations whereas the cortical gyrification is primarily determined by lifelong experience- or environment-related factors16,17,18. Indeed, recent studies of brain morphometry in musicians showed altered morphometric characteristics in the central sulcus in musicians19. Another study documented both increased and decreased cortical gyrification in meditation practitioners compared with controls20. The effect of long-term motor training on the morphology of cortical gyrification, however, remains unknown.
Diving is a competitive sport that greatly exceeds those of simple physical movement or locomotion and demands precise motor control, timing, coordination and excellent proprioceptive sensation during the execution of the motions. Diving experts are typical elite athletes that usually commence intensive motor training at a very young age in their early childhood, and therefore serve ideal models for examining the cerebral neuroplastic changes related to long-term intensive diving training. Of note, our previous studies found that diving experts had significant changes in gray matter volume and cortical thickness in multiple brain areas21,22 as well as regional inflation in the thalamus and pallidum23, suggesting possible changes in the intracortical organization and thalamocortical connections18. According to the aforementioned tension-based hypothesis and gray matter hypothesis of cortical gyrification2,4,5, alterations in cortical gyrification are also expected in the diving experts.
Using surface-based LGI, the present study aims to investigate cortical gyrification changes in diving experts compared with matched controls. Specifically, we fitted vertex-wise linear model to compare the LGI maps between 12 professional diving experts and 12 controls. We hypothesized that diving experts would show LGI changes in multiple brain regions in response to long-term intensive diving training, including some motor cortices and the parahippocampal cortex. Given structural covariance of LGI has been used to construct structural brain networks24, we further performed exploratory network efficiency analyses of the structural cortical networks inferred from interregional correlation of LGIs to examine whether diving experts are more efficient in information processing.
Materials and Methods
Subjects
All subjects of this study participated in our previous studies22,23. The twelve professional diving experts are described in detail in Table 1. In brief, all the twelve diving experts (6 females and 6 males) are national-level masters (mean age, 14.58; SD, 1.68) with top-level diving skills (In China, professional athletes are classified into A, B, C, and D categories to distinguish their competence level, which respectively corresponds to international-level masters, national-level masters, the first-class level and the second-class level). The control group (6 females and 6 males) was matched for age, gender and educational level, and included twelve healthy subjects (mean age, 14.92; SD, 1.38) who were not involved in any extensive physical training or professional experience. All of the subjects were right-handed and were medically and neurologically stable. No subjects had any lifetime histories of substance dependence. Written informed consent from their parents was obtained, and the study was approved by the Institutional Review Board of Beijing MRI Center for Brain Research. Experiments were carried out in accordance with the approved guidelines.
Table 1. Demographic data of the participants.
| Diving experts (n = 12) | Controls (n = 12) | |
|---|---|---|
| Age (year)★ | 14.58 ± 1.68 | 14.92 ± 1.38 |
| Education (year)★ | 7.75 ± 1.82 | 7.92 ± 1.38 |
| Average practice time per day (hour) | 6.54 ± 0.38 | N/A |
| Duration of practice (year) | 10.12 ± 0.86 | N/A |
| Age of commencement (year) | 5.33 ± 0.98 | N/A |
Note: ★Indicates no significant between-group difference using two-sample t-test (p > 0.05).
N/A = not available.
MRI Data Acquisition
High-resolution anatomical images of the whole brain were acquired on a 3-tesla Trio system (Siemens, Erlangen, Germany) with 12-channel head matrix coil using a magnetisation-prepared rapid-acquisition gradient echo sequence. The following parameters were used for the volumetric acquisition: repetition time = 2530 ms, echo time = 3.37 ms, flip angle = 7 degrees, slice thickness = 1.33 mm, FOV = 256 mm, 512 × 512- pixel matrix. The voxel size was 0.5 × 0.5 × 1.33 mm3. The scan time for the T1-weighted sequence was 486 s. During the scanning, each subject reclined in a supine position on the bed of the scanner and was asked to lie still during the imaging time. A foam head holder and padding were placed around the subject’s head. In addition, headphones were provided to block background noise.
Local Gyrification Index
Each scan was processed using FreeSurfer ( http://surfer.nmr.mgh.harvard.edu/) to obtain the LGI. Briefly, the LGI map can be obtained in four steps8. First, the pial surface is reconstructed in three-dimensional space. Second, an outer surface can be obtained from the outer hull which tightly wraps the pial surface. Third, the LGI was calculated for each vertex on the outer surface, as a ratio of areas of circular region centered on this vertex and the area of the corresponding region on the pial surface. Hence, LGI is able to quantify the amount of cortical surface invaginated in the sulci and measure the spatial frequency of cortical gyrification and the depth of the sulci. Fourth, the LGI map is obtained by propagating LGI values from the outer surface to the pial surface. For comparison, all of the individual reconstructed cortical surfaces were aligned to an average template by using a surface-based registration algorithm25. Then the LGI maps were resampled and smoothed with a heat kernel of 10 mm width.
Construction of Gyrification-based Networks
Firstly, each individual LGI map was parcellated using the built-in Desikan atlas of FreeSurfer, which includes 68 anatomical regions (34 in each hemisphere while excluding the corpus callosum). The LGI for each cortical region was calculated as the average LGI of all vertices in that region. Secondly, the interregional correlation matrix C = [cij] (i, j = 1, 2,… N, here N = 68) of each group was obtained by calculating the Pearson correlation coefficients across individuals between the LGIs of every pair of regions24. Prior to the correlation analysis, a linear regression was performed at every cortical region to remove the effects of age, gender and intracranial volume (ICV). Finally, the correlation matrix of each group was thresholded into a binarized matrix B = [bij], where bij is 1 if the absolute value of the correlation coefficient cij between regions i and j is larger than a given correlation threshold, and 0 otherwise.
Network Efficiency Measurements
The undirected network (graph) G was represented by a binarized matrix B with N nodes and K edges, where nodes are cortical regions and edges indicate undirected links corresponding to its nonzero elements. To ensure the networks of two groups have the same number of edges and are comparable, the correlation matrix C of each group was thresholded into a binarized matrix with a fixed sparsity S26, which is defined as the number of edges K in a graph divided by the maximum possible number of edges N(N − 1)/2. Here, a wide range of sparsity thresholds 10% ≤ S ≤ 40% were used.
In this study, we employed the network efficiency measurements to examine the global topological properties of the structural cortical networks obtained for the two groups. For a graph G with N nodes and K edges, the global efficiency Eglob (G) is defined as27
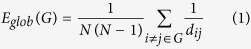 |
where dij is the shortest path length between node i and node j in G. The local efficiency Eloc(G) is measured as27
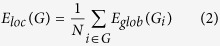 |
where Gi denotes the subgraph composed of the nearest neighbors of node i.
Statistical Analysis
Vertex-by-vertex contrasts of LGI were performed between diving experts and controls. Specifically, each contrast was entered into a vertex-by-vertex GLM including diagnosis, sex, exact age and ICV as covariates. Subsequently, random field theory (RFT) was used to assess the significance of the statistical maps and to correct for multiple comparisons28. Clusters were first reported reaching a significance level of P < 0.05 RFT corrected. Those reaching a looser significance level of P < 0.005 uncorrected were also indicated.
Within the diving experts, vertex-wise correlation analyses were performed to determine the associations between LGI and years of diving training, while removing the partial effect of age, sex and ICV. Then, the same procedures as the above between-group analysis on LGI were repeated to correct for multiple comparisons and to report the results of the correlation analysis.
For group comparison of the network efficiency parameters, non-parametric permutation test with 1000 repetitions was adopted. For each iteration, we reassigned the LGIs of each participant randomly to one of two new groups with the sample size identical as the two groups. For each randomized group, we calculated the global efficiency and local efficiency as well as their normalized integrals (area under the curve divided by the length of integration interval) across a range of sparsity thresholds (10% ≤ S ≤ 40%). Differences of the normalized integrals of each efficiency parameter were then calculated from two randomized groups to obtain a null distribution of differences, against which we can compute the P values of the actual difference of normalized integrals of the efficiency parameters between diving experts and controls.
Results
Vertex-wise LGI Analysis
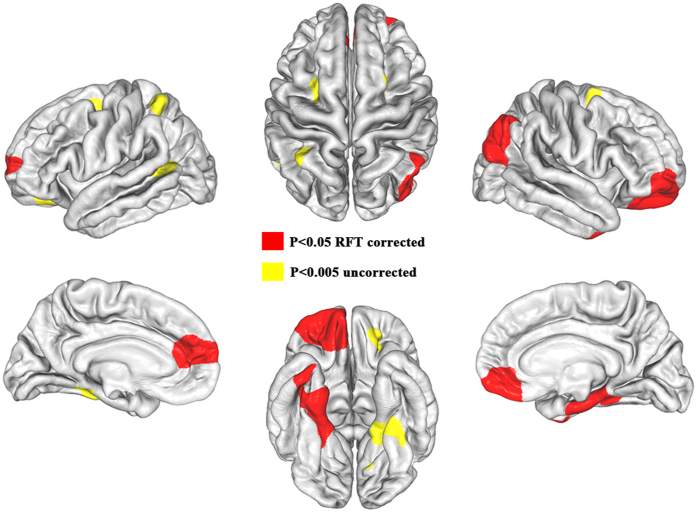
Compared with controls, we found four clusters where diving experts had significant LGI reductions (P < 0.05 RFT corrected, red clusters in Fig. 1). These clusters involve the bilateral anterior cingulate gyrus, the right superior frontal gyrus and right orbitofrontal cortex, inferior and superior parietal cortex, parahippocampal gyrus, lingual and fusiform gyrus, inferior temporal gyrus and entorhinal cortex. In addition, we found six more clusters showing LGI reductions under a looser threshold of P < 0.005 uncorrected (yellow clusters in Fig. 1), in bilateral caudal middle frontal regions (dorsal premotor cortices), left precentral gyrus, superior parietal cortex, superior temporal sulcus, parahippocampal gyrus, lingual and fusiform gyrus, and orbitofrontal cortex in diving experts. For visualization, regions of difference were projected onto the pial surface of the average template.
Figure 1. Cortical areas with LGI reductions in diving experts compared with controls.
Red clusters are those where significance reached P < 0.05 RFT corrected. Yellow clusters are those where significance reached a looser threshold of P < 0.005 uncorrected.
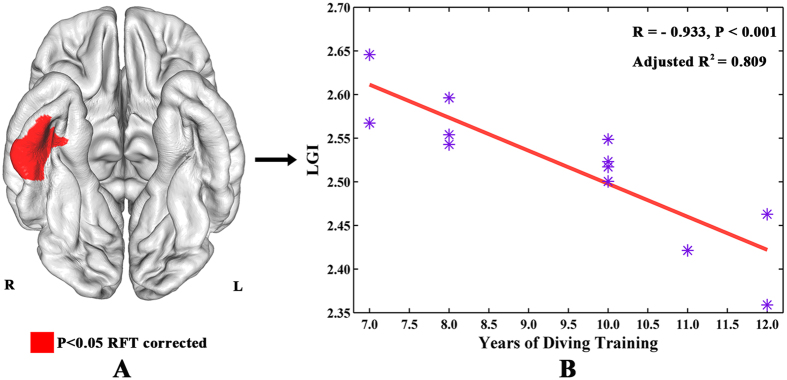
Within the diving experts, we found a cluster in the medial temporal lobe where LGIs were negatively correlated with years of diving training (P < 0.05 RFT corrected). This cluster involves the right inferior temporal gyrus, fusiform gyrus, parahippocampal gyrus and entorhinal cortex (Fig. 2). Under a looser threshold of P < 0.005 uncorrected, we found one more cluster in the right middle frontal gyrus (premotor cortex) where LGIs were negatively correlated with years of diving training.
Figure 2. Relationship between LGIs and years of diving training in diving experts.
(A) Cortical areas showing significant negative correlations between LGIs and years of diving training in diving experts (P < 0.05 RFT corrected). (B) Scatter plot showing the negative correlation between years of diving training and the mean LGIs of the significant cluster in vertex-wise correlation analyses.
Network Efficiency Analysis
Non-parametric permutation test revealed no significant difference of the normalized integrals of the global efficiency (P = 0.37) and local efficiency (P = 0.48) between diving experts and controls.
Discussion
Using a surface-based LGI, the present study investigated the cortical gyrification changes in diving experts compared with matched controls. We also performed network efficiency analyses of structural cortical networks inferred from interregional correlation of LGIs to explore whether diving experts are more efficient in information processing. The first major finding was the widespread LGI reduction in diving experts. The cortical areas with LGI reductions were almost symmetrically distributed over both hemispheres, involving the bilateral premotor cortex, bilateral medial temporal lobe, bilateral prefrontal cortex, left precentral gyrus, superior parietal cortex, superior temporal sulcus, and the right inferior parietal cortex. Within the diving experts, vertex-wise correlation analyses revealed two clusters involving the right inferior temporal gyrus, fusiform gyrus, entorhinal cortex and premotor cortex where LGIs were negatively correlated with years of diving training. Next, we documented comparable global and local efficiency in diving experts relative to controls. Taken together, these findings indicate that cortical gyrification changes in diving experts may be the result of long-term diving training which could refine the neural circuitry and might be the anatomical substrate underlying their extraordinary diving performance.
As demonstrated by many previous studies, long-term intensive training could influence brain structure and function along with the underlying connectivity. In the present study, we investigated the specific effect of long-term diving training on cortical gyrification by examining diving experts vs matched controls. Compared with controls, widespread LGI reductions were observed in diving experts. Among the regions showing significant LGI reductions, the differences seen in the posterior parietal cortex, medial temporal lobe and premotor cortex were of particular interest. These regions were shown to be directly or indirectly connected with the vestibular nuclei29,30, and were considered to be key nodes of the vestibular system, which plays important roles in postural and oculomotor control, bodily perception, spatial cognition31, as well as in spatial memory, spatial learning and spatial navigation32. The vestibular system codes three dimensional head movements in space by sensing the angular and linear accelerations31,32. For diving experts, the objective of diving practice is to produce the intended body parabolic trajectory with multiple twists and somersaults, which highlights the importance of postural and oculomotor control as well as a good sense of proprioception and spatial perception33. As a closed loop control movements with much complexity, spatial navigation on movements is highly required to achieve the best possible diving. The cluster observed in the medial temporal lobe also involved some visual cortex areas, such as the lingual and fusiform gyrus, which have been found to project to the hippocampal formation and to be very important for visually guided spatial memory and navigation34. This finding is in line with the fact that visual spotting is a commonly used technique to assist in spatial orientation and knowing when to come out of the somersaulting dives33. In diving experts, the LGIs of this cluster were shown to be negatively correlated with years of diving training, which might reflect the involvement of this region in the storage and retrieval of spatial memories35,36. On the other hand, the cortical gyrification changes observed in the caudal middle frontal gyrus (dorsal premotor cortex), precentral gyrus, and posterior parietal cortex may be associated with motor functioning given that these regions have the anatomical substrate to influence motor output through direct or indirect connections with the primary motor cortex and the spinal cord37,38. In fact, the dorsal premotor cortex plays a dominant role in movement selection and planning39,40 and is considered to be a structure of key importance in motor learning41. The posterior parietal cortex, including the inferior parietal lobule and superior parietal lobule, has been involved in intention to perform specific motor acts42, preparation and redirection of movements39,40. The posterior parietal cortex has also been considered to be an important hub where several different types of information are integrated43,44. The cortical gyrification changes observed in these regions may indicate that diving experts might be more efficient in integrating multi-modal information in this fronto-parietal network to produce an output that reflects the selection, preparation, and execution of movements45.
Additional areas of LGI reductions were found in the medial prefrontal cortices (including the anterior cingulate gyrus, medial superior frontal gyrus and medial orbitofrontal gyrus) and lateral orbitofrontal cortex. The medial prefrontal cortex has been shown to play important roles in retrieval of both recent and remote memories46 as well as in decision-making including conflict monitoring47, error detection48 and reward-guided learning49. Several lines of evidence indicate that medial prefrontal cortex likely forms and stores schema which maps context and events onto appropriate actions and could direct the correct motor response to a given set of events in light of past experience50. Hence, the cortical gyrification changes in the medial prefrontal cortex may relate to the formation of the repertoire of diving experiences through long-term intensive diving training, which enables the diving experts to choose the best diving practice50. Additionally, the anterior prefrontal cortex has been involved in the exploration of alternative behavioral options during execution of a prevailing behavioral plan51, the cortical gyrification changes in the orbitofrontal cortex may reflect a built-up and maintenance of an optimal diving strategy as a result of exploring and evaluating potential parameters of the timing, strength and coordination during long-term diving practice51,52,53. This interpretation is supported by a previous study reporting that elite divers have higher levels of thrill and adventure seeking, experience seeking and disinhibition than non-divers54. However, the exact contribution of cortical gyrification changes observed in the prefrontal cortex to the excellent performance of diving experts remains unclear and needs further investigations.
To test whether diving experts are more efficient in information processing, we performed exploratory network efficiency analyses on the structural networks inferred from interregional correlation of LGI. Unexpectedly, we observed no significant difference in the normalized integrals of global efficiency and local efficiency between diving experts and controls. The negative result of the network efficiency analyses could be due to the small sample size of the current study, which resulted in low statistical power.
Over the years, a number of studies have been conducted to investigate the developmental trajectory of cortical gyrification. Specifically, a longitudinal study on the cortical gyrification development of infants in the first 2 postnatal years reported that the cortical LGI has age-related and marked development with 16.1% increase in the first year and 6.6% increase in the second year10. By imaging healthy adolescents twice with a two-year gap, another longitudinal study found that the cortical surface flattens during adolescence13. Meanwhile, studies assessing the age effect on the cortical gyrification development in healthy subjects elder than 6 years reported negative correlations between age and LGI14,55. These studies indicate that the development of the cortical LGI may follow an inverted U-shaped curve12 which reaches its peak somewhere between 2 and 6 postnatal years11. Such an inverted U-shaped curve of cortical gyrification development is consistent with the two-stage neurodevelopmental process of exuberant proliferation of neurons and their synaptic connections followed by elimination of the excess15. The rapid cortical LGI growth of the first two years is likely associated with the increase of dendritic arborization, the growth of the terminal axon arborization, synaptogenesis, and glial proliferation10, whereas the subsequent LGI decline may be due to the synaptic pruning as a fine-tuning process of the neural circuitry in preparation for optimal behaviors1,10. Indeed, age-related changes in synaptic and dendritic arborization may lead to decreasing tensile forces which could result in widening of the sulci and greater curvature of the gyri13,56. In the current study, both diving experts and controls were all elder than 6 years, and therefore had entered the LGI declining stage. The observed LGI reductions in diving experts indicated that diving experts may either have a faster rate or earlier time of LGI declining or both, which presumably result from increased rate or earlier onset time of synaptic pruning in diving experts. Further exploratory correlation analyses between global LGI and age for each group revealed a significant negative correlation in the control group, whereas such an age-related LGI decline was not found in diving experts (Fig. S1 in the Supplementary Materials). This line of evidence supported the speculation that the LGI reductions in diving experts may result from earlier onset time of synaptic pruning due to intensive diving training. However, this interpretation should be considered as preliminary since the age ranges of the two groups are narrowly distributed. Moreover, we cannot rule out the possibility that increased rate of synaptic pruning is implicated in causing the LGI reductions in the diving experts57 given that such cross-sectional correlation analyses cannot reflect the dynamic relationship between LGI and age. Together, the LGI reductions in the diving experts may be related to the fine-tuning process (via synaptic pruning) of neural circuitry.
In addition, the observed cortical gyrification alterations in diving experts can be explained by prevailing theories about the development of cortical gyrification2,4,5. In fact, voxel-based morphometry on the white matter volume of the diving experts revealed significant elevated white matter volume in the thalamus, parahippocampal gyrus, insular cortex and brainstem (Fig. S2 in the Supplementary Materials). The previously reported significant regional inflation in the thalamus and globus pallidus23, which are key nodes of the basal ganglia-thalamo-cortical network and have reciprocal connections with the cerebral cortex58,59, also suggest possible alterations in white matter tract in diving experts. On the other hand, although the present study provided no direct evidence of altered growth rate of different cortical layers, the gray matter density and cortical thickness changes observed in our previous studies are indicative of altered intracortical organization in the diving experts4,21,22. Furthermore, considering the reciprocal thalamocortical connections between the thalamus and different cortical layers58,59, altered cortical afferent due to changes in the thalamus may result in differential plastic changes in different cortical layers of diving experts18,56.
This study had some limitations that should be addressed. First, due to the cross-sectional design of the current study, it is indefinite that the cortical gyrification reductions we found were directly caused by diving training. Hence, the major future challenge is to figure out whether the cortical gyrification changes in diving experts were actually induced by diving training, or whether they are inherent prerequisites for the beginning and continuation of diving. Second, the sample size of the current study is relatively small, making the findings of network analysis preliminary in nature. Moreover, the cortical networks were constructed by assessing the interregional structural covariance of cortical gyrification, which only provided an indirect measure of the anatomical networks. Therefore, more comprehensive network analyses with diffusion tensor imaging and also a larger sample size are warranted.
Using a surface-based LGI, we found widespread cortical gyrification reductions in diving experts compared with controls. Within the diving experts, vertex-wise correlation analyses showed significant negative correlations between years of diving training and LGIs of a cluster in the medial temporal lobe. Meanwhile, graph-theoretic network analysis showed comparable global efficiency and local efficiency in diving experts relative to controls. These findings suggests that cortical gyrification reductions in diving experts may be the result of long-term diving training which could refine the neural circuitry (via synaptic pruning) and might be the anatomical substrate underlying their extraordinary diving performance.
Additional Information
How to cite this article: Zhang, Y. et al. Effects of Long-term Diving Training on Cortical Gyrification. Sci. Rep. 6, 28243; doi: 10.1038/srep28243 (2016).
Supplementary Material
Acknowledgments
The authors would like to thank Seun Jeon for his helpful suggestions in improving the manuscript. The study was supported by grants from the National Natural Science Foundation of China (31200794 and 81503670), the Beijing Municipal Science & Technology Commission (Grant No. D151100002315003), the open research fund of Key lab of Behavioral Science from the Institute of Psychology, Chinese Academy of Sciences and China Postdoctoral Science Foundation (No. 20060390540).
Footnotes
Author Contributions Y.Z., L.Z., W.B., G.W., A.E. and T.J. were involved in the design of this study and contributed to the writing of the manuscript. Y.Z. and L.Z. performed the analyses. G.W. and Y.W. were involved in data collection. Y.Z., L.Z. and Y.W. wrote the first draft of the manuscript.
References
- White T., Su S., Schmidt M., Kao C. Y. & Sapiro G. The development of gyrification in childhood and adolescence. Brain Cogn. 72, 36–45, 10.1016/j.bandc.2009.10.009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D. C. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385, 313–318, 10.1038/385313a0 (1997). [DOI] [PubMed] [Google Scholar]
- Kriegstein A., Noctor S. & Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat. Rev. Neurosci. 7, 883–890, 10.1038/nrn2008 (2006). [DOI] [PubMed] [Google Scholar]
- Caviness V. S. Jr. Mechanical model of brain convolutional development. Science 189, 18–21 (1975). [DOI] [PubMed] [Google Scholar]
- Zilles K., Palomero-Gallagher N. & Amunts K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 36, 275–284, 10.1016/j.tins.2013.01.006 (2013). [DOI] [PubMed] [Google Scholar]
- Chi J. G., Dooling E. C. & Gilles F. H. Gyral development of the human brain. Ann. Neurol. 1, 86–93, 10.1002/ana.410010109 (1977). [DOI] [PubMed] [Google Scholar]
- Zilles K. et al. Quantitative analysis of sulci in the human cerebral cortex: development, regional heterogeneity, gender difference, asymmetry, intersubject variability and cortical architecture. Hum. Brain Mapp. 5, 218–221, (1997). [DOI] [PubMed] [Google Scholar]
- Schaer M. et al. A surface-based approach to quantify local cortical gyrification. IEEE Trans. Med. Imaging 27, 161–170, 10.1109/TMI.2007.903576 (2008). [DOI] [PubMed] [Google Scholar]
- Zilles K., Armstrong E., Schleicher A. & Kretschmann H. J. The human pattern of gyrification in the cerebral cortex. Anat. Embryol. (Berl.) 179, 173–179 (1988). [DOI] [PubMed] [Google Scholar]
- Li G. et al. Mapping longitudinal development of local cortical gyrification in infants from birth to 2 years of age. J. Neurosci. 34, 4228–4238, 10.1523/JNEUROSCI.3976-13.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A. et al. How does your cortex grow? J. Neurosci. 31, 7174–7177, 10.1523/JNEUROSCI.0054-11.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treble A., Juranek J., Stuebing K. K., Dennis M. & Fletcher J. M. Functional significance of atypical cortical organization in spina bifida myelomeningocele: relations of cortical thickness and gyrification with IQ and fine motor dexterity. Cereb. Cortex 23, 2357–2369, 10.1093/cercor/bhs226 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman-Gomez Y. et al. The human cerebral cortex flattens during adolescence. J. Neurosci. 33, 15004–15010, 10.1523/JNEUROSCI.1459-13.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogstrom L. J., Westlye L. T., Walhovd K. B. & Fjell A. M. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cereb. Cortex 23, 2521–2530, 10.1093/cercor/bhs231 (2013). [DOI] [PubMed] [Google Scholar]
- Petanjek Z. et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. USA 108, 13281–13286, 10.1073/pnas.1105108108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley A. J., Jones D. W. & Weinberger D. R. Genetic variability of human brain size and cortical gyral patterns. Brain 120 (Pt 2), 257–269 (1997). [DOI] [PubMed] [Google Scholar]
- Kochunov P. et al. Genetics of primary cerebral gyrification: Heritability of length, depth and area of primary sulci in an extended pedigree of Papio baboons. Neuroimage 53, 1126–1134, 10.1016/j.neuroimage.2009.12.045 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T., Andreasen N. C. & Nopoulos P. Brain volumes and surface morphology in monozygotic twins. Cereb. Cortex 12, 486–493 (2002). [DOI] [PubMed] [Google Scholar]
- Amunts K. et al. Motor cortex and hand motor skills: structural compliance in the human brain. Hum. Brain Mapp. 5, 206–215, (1997). [DOI] [PubMed] [Google Scholar]
- Luders E. et al. The unique brain anatomy of meditation practitioners: alterations in cortical gyrification. Front. Hum. Neurosci. 6, 34, 10.3389/fnhum.2012.00034 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G., Luo J. & Li Y. Brain structure in diving players on MR imaging studied with voxel-based morphometry. Progress in Natural Science 19, 1397–1402 (2009). [Google Scholar]
- Wei G., Zhang Y., Jiang T. & Luo J. Increased cortical thickness in sports experts: a comparison of diving players with the controls. PLoS One 6, e17112, 10.1371/journal.pone.0017112 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. et al. Regional inflation of the thalamus and globus pallidus in diving players. Med. Sci. Sports Exerc. 45, 1077–1082, 10.1249/MSS.0b013e31827f4370 (2013). [DOI] [PubMed] [Google Scholar]
- Palaniyappan L., Park B., Balain V., Dangi R. & Liddle P. Abnormalities in structural covariance of cortical gyrification in schizophrenia. Brain Struct Funct, 10.1007/s00429-014-0772-2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M. I., Tootell R. B. & Dale A. M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 8, 272–284 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S. & Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 3, e17, 10.1371/journal.pcbi.0030017 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora V. & Marchiori M. Efficient behavior of small-world networks. Phys. Rev. Lett. 87, 198701 (2001). [DOI] [PubMed] [Google Scholar]
- Hayasaka S., Phan K. L., Liberzon I., Worsley K. J. & Nichols T. E. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage 22, 676–687, 10.1016/j.neuroimage.2004.01.041 (2004). [DOI] [PubMed] [Google Scholar]
- Mast F. W., Preuss N., Hartmann M. & Grabherr L. Spatial cognition, body representation and affective processes: the role of vestibular information beyond ocular reflexes and control of posture. Front. Integr. Neurosci. 8, 44, 10.3389/fnint.2014.00044 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinder M. E. & Taube J. S. Differentiating ascending vestibular pathways to the cortex involved in spatial cognition. J. Vestib. Res. 20, 3–23, 10.3233/VES-2010-0344 (2010). [DOI] [PubMed] [Google Scholar]
- Lopez C. & Blanke O. The thalamocortical vestibular system in animals and humans. Brain Res Rev 67, 119–146, 10.1016/j.brainresrev.2010.12.002 (2011). [DOI] [PubMed] [Google Scholar]
- Hitier M., Besnard S. & Smith P. F. Vestibular pathways involved in cognition. Front. Integr. Neurosci. 8, 59, 10.3389/fnint.2014.00059 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien R. Springboard and platform diving 2 edn (Human Kinetics, 2002). [Google Scholar]
- Smith C. D. et al. MRI diffusion tensor tracking of a new amygdalo-fusiform and hippocampo-fusiform pathway system in humans. J. Magn. Reson. Imaging 29, 1248–1261, 10.1002/jmri.21692 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom A. D. et al. Cellular networks underlying human spatial navigation. Nature 425, 184–188, 10.1038/nature01964 (2003). [DOI] [PubMed] [Google Scholar]
- Pilly P. K. & Grossberg S. How do spatial learning and memory occur in the brain? Coordinated learning of entorhinal grid cells and hippocampal place cells. J. Cogn. Neurosci. 24, 1031–1054, 10.1162/jocn_a_00200 (2012). [DOI] [PubMed] [Google Scholar]
- Picard N. & Strick P. L. Imaging the premotor areas. Curr. Opin. Neurobiol. 11, 663–672 (2001). [DOI] [PubMed] [Google Scholar]
- Karabanov A. et al. Timing-dependent modulation of the posterior parietal cortex-primary motor cortex pathway by sensorimotor training. J. Neurophysiol. 107, 3190–3199, 10.1152/jn.01049.2011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiber M. P. et al. Cortical areas and the selection of movement: a study with positron emission tomography. Exp. Brain Res. 84, 393–402 (1991). [DOI] [PubMed] [Google Scholar]
- Rushworth M. F., Johansen-Berg H., Gobel S. M. & Devlin J. T. The left parietal and premotor cortices: motor attention and selection. Neuroimage 20, Suppl 1, S89–100 (2003). [DOI] [PubMed] [Google Scholar]
- Hardwick R. M., Rottschy C., Miall R. C. & Eickhoff S. B. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage 67, 283–297, 10.1016/j.neuroimage.2012.11.020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M. et al. Movement intention after parietal cortex stimulation in humans. Science 324, 811–813, 10.1126/science.1169896 (2009). [DOI] [PubMed] [Google Scholar]
- Singh-Curry V. & Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia 47, 1434–1448, 10.1016/j.neuropsychologia.2008.11.033 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen R. A. Multimodal integration for the representation of space in the posterior parietal cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 352, 1421–1428, 10.1098/rstb.1997.0128 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise S. P., Boussaoud D., Johnson P. B. & Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu. Rev. Neurosci. 20, 25–42, 10.1146/annurev.neuro.20.1.25 (1997). [DOI] [PubMed] [Google Scholar]
- Gonzalez C. et al. Medial prefrontal cortex is a crucial node of a rapid learning system that retrieves recent and remote memories. Neurobiol. Learn. Mem. 103, 19–25, 10.1016/j.nlm.2013.04.006 (2013). [DOI] [PubMed] [Google Scholar]
- Botvinick M. M., Cohen J. D. & Carter C. S. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8, 539–546, 10.1016/j.tics.2004.10.003 (2004). [DOI] [PubMed] [Google Scholar]
- Holroyd C. B., Coles M. G. & Nieuwenhuis S. Medial prefrontal cortex and error potentials. Science 296, 1610–1611 author reply 1610-1611 (2002). [DOI] [PubMed] [Google Scholar]
- Rushworth M. F., Noonan M. P., Boorman E. D., Walton M. E. & Behrens T. E. Frontal cortex and reward-guided learning and decision-making. Neuron 70, 1054–1069, 10.1016/j.neuron.2011.05.014 (2011). [DOI] [PubMed] [Google Scholar]
- Euston D. R., Gruber A. J. & McNaughton B. L. The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070, 10.1016/j.neuron.2012.12.002 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E. & Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science 318, 594–598, 10.1126/science.1142995 (2007). [DOI] [PubMed] [Google Scholar]
- Koechlin E., Basso G., Pietrini P., Panzer S. & Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature 399, 148–151, 10.1038/20178 (1999). [DOI] [PubMed] [Google Scholar]
- Popa T., Bonifazi M., della Volpe R., Rossi A. & Mazzocchio R. Anticipatory control of impending postural perturbation in elite springboard divers. Eur. J. Appl. Physiol. 104, 1007–1011, 10.1007/s00421-008-0856-x (2008). [DOI] [PubMed] [Google Scholar]
- Hinton-Bayre A. D. & Hanrahan S. J. Sensation seeking, physical self-concept and attentional style in elite springboard and platform divers. Journal of Human Movement Studies 37, 183–203 (1999). [Google Scholar]
- Klein D. et al. Adolescent brain maturation and cortical folding: evidence for reductions in gyrification. PLoS One 9, e84914, 10.1371/journal.pone.0084914 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. & Hilgetag C. C. In Handbook of developmental cognitive neuroscience (eds Nelson C. A. & Luciana M. ) (The MIT Press, Cambridge, MA, 2008). [Google Scholar]
- Sun D. et al. Brain surface contraction mapped in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Mol. Psychiatry 14, 976–986, 10.1038/mp.2008.34 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M. & Rubenstein J. L. Patterning and plasticity of the cerebral cortex. Science 310, 805–810, 10.1126/science.1112070 (2005). [DOI] [PubMed] [Google Scholar]
- Wise S. P. & Jones E. G. Developmental studies of thalamocortical and commissural connections in the rat somatic sensory cortex. J. Comp. Neurol. 178, 187–208, 10.1002/cne.901780202 (1978). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.