Abstract
Background:
There are little data on patient factors that impact diagnosis rates of celiac disease. This study aims to evaluate the association between patient socioeconomic status and the symptoms at diagnosis of celiac disease.
Methods:
A total of 872 patients with biopsy-proven celiac disease were categorized based on the presence or absence of (1) diarrhea and (2) any gastrointestinal symptoms at diagnosis. Univariate and multivariate analyses were used to assess the association between socioeconomic status and symptoms.
Results:
Patients without diarrhea at presentation had a higher mean per capita income (US$34,469 versus US$32,237, p = 0.02), and patients without any gastrointestinal symptoms had a higher mean per capita income (US$36,738 versus US$31,758, p < 0.01) compared with patients having such symptoms. On multivariable analysis adjusting for sex, age, autoimmune or psychiatric comorbidities, and income, per capita income remained a significant predictor of diagnosis without gastrointestinal symptoms (odds ratio: 1.71, 95% confidence interval: 1.17–2.50, p < 0.01), and it showed a trend towards significance in diagnosis without diarrhea (odds ratio: 1.40, 95% confidence interval: 0.98–2.02, p = 0.06).
Conclusions:
Patients with nonclassical symptoms of celiac disease are less likely to be diagnosed if they are of lower socioeconomic status. Celiac disease may be under-recognized in this population due to socioeconomic factors that possibly include lower rates of health-seeking behavior and access to healthcare.
Keywords: diarrhea, gluten-free diet, healthcare disparity, income
Introduction
Celiac disease (CD) is a chronic, immune-mediated disorder triggered in genetically susceptible individuals by the ingestion of gluten, the main storage protein of wheat and related grains. Although it was originally thought to be a rare malabsorption syndrome of childhood, CD is now known as a condition that can affect multiple organ systems and can be diagnosed at any age [Green and Cellier, 2007; Catassi et al. 2007; Fasano et al. 2003; Gomez et al. 2001; Katz et al. 2011; Palabykoglu et al. 2008]. Several studies from both Europe and North America, however, show that despite a rising prevalence of CD over the past several decades, a majority of cases still go undiagnosed [Catassi et al. 2007; Fasano et al. 2003; Gomez et al. 2001; Katz et al. 2011; West et al. 2003]. It is considered that less than 20% of patients with CD are diagnosed, and the mean duration of symptoms prior to diagnosis is 10 years [Rubio-Tapia et al. 2012; Green et al. 2001; Murray et al. 2003].
There are many potential reasons for the low diagnostic rate of CD in the US. Physician factors that may contribute to under-diagnosis include lack of physician recognition of the clinical spectrum of CD and underuse of diagnostic tests when presented with such patients [Catassi et al. 2007; Zipser et al. 2005; Lebwohl et al. 2011]. While the ‘classical’ presentation of adult CD is diarrhea, studies have found that diarrhea has been the main presenting symptom in less than 50% of cases over the past decade [Rampertab et al. 2006]. In fact, it is increasingly common for adults with CD to present with nongastrointestinal symptoms or even ‘silent’ disease [Murray et al. 2003; Rampertab et al. 2006; Lo et al. 2003; Telega et al. 2008]. Though the ease and accuracy of modern serologic testing has increased the detection of CD across all clinical spectra [van der Windt et al. 2010], diagnosing patients with nonclassic manifestations remains a challenge.
In addition to physician factors that delay the diagnosis of CD, patient demographics, awareness, and motivation may also play a role. Research in various medical fields has demonstrated a strong relationship between socioeconomic status and the likelihood of being affected by health disparities, and socioeconomic position is a known risk factor for several disease processes: ranging from asthma to malignancy [Lawlor et al. 2006; Pappas et al. 1993; Shack et al. 2008; Braback et al. 2005]. In CD, the data are conflicting with regards to the impact of socioeconomic status on risk estimates of disease. While some small studies have shown no relationship between socioeconomics and CD diagnosis [Snook et al. 1996; West et al. 2003; Ludvigsson 2005], a recent population-based study from Sweden evaluated more than 29,000 patients and showed that diagnosed CD was less common in individuals with low socioeconomic position [Olen et al. 2012]. The authors suggested that CD might therefore be under-recognized in this population.
The aim of this study was to evaluate the association between patient socioeconomic status and the symptoms at diagnosis of CD. Given that both lower socioeconomic status and nonclassic presentations may be associated with lower rates of CD diagnosis, we hypothesized that nondiarrheal presentations will be less common among CD patients in lower socioeconomic groups.
Methods
We performed a retrospective cohort study utilizing a database that comprised of patients seen at The Celiac Center at Beth Israel Deaconess Medical Center (BIDMC, Boston, Massachusetts, US). Cases were defined as patients with biopsy-proven CD (Marsh classification type 2 or greater).
A total of 1010 patients with biopsy-proven CD were reviewed. CD-presenting symptoms were recorded and separated into the following categories: (1) with or without diarrhea, and (2) with or without any gastrointestinal symptoms (diarrhea, bloating, abdominal pain, weight loss, nausea and vomiting). Presentations without diarrhea included anemia, osteoporosis and screening for at-risk status as well as atypical symptoms.
Socioeconomic and demographic data were obtained from GeoLytics, Inc. (East Brunswick, NJ, US). GeoLytics develops socioeconomic estimates based on an individual’s place of residence, US Census Bureau reports, and limited population estimates. Variables used in this study were mean per capita income and mean household income. The estimates were obtained using the patients’ active US home address at the time of the study. Patients without a listed home address or only a post office box number were excluded, leaving a total of 872 patients.
Univariate analyses (chi-square tests, ANOVA, and Fisher’s exact tests, as appropriate) were used to compare demographic factors, diagnostic characteristics, and mean incomes for those with or without diarrhea symptoms at diagnosis and those with or without gastrointestinal symptoms at diagnosis. Multivariate logistic regression models were constructed to test the association between demographic or diagnostic factors (sex, age, comorbid psychiatric and autoimmune conditions, and per capita income) and diarrhea symptoms at diagnosis. Analyses were performed only for patients with a biopsy-proven diagnosis of CD. Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC). The study was approved by the BIDMC Institutional Review Board (protocol 2010-P-000164/7).
Results
A total of 1010 patients with biopsy-proven CD were reviewed. Full demographic and diagnostic characteristics, as well as symptoms at presentation, are listed for this overall population in Table 1.
Table 1.
Overall study population characteristics.
| Overall study population (n = 1010) | |
|---|---|
| Demographic characteristic | |
| Age at diagnosis (y), mean ± standard deviation | 43.6 ± 16.4 |
| Female, n (%) | 758 (75) |
| Per capita income (US$), mean ± standard deviation | 33,506 ± 14,487 |
| Household income (US$), mean ± standard deviation | 66,676 ± 31,140 |
| Diagnostic characteristics and comorbidities | |
| TTG* level (U/ml), mean ± standard deviation | 98.7 ± 76.3 |
| Hypothyroidism, n (%) | 118 (12) |
| Type 1 diabetes, n (%) | 61 (6) |
| Depression, n (%) | 147 (15) |
| Anxiety, n (%) | 68 (7) |
| Bipolar disorder, n (%) | 8 (1) |
| Symptoms at presentation | |
| Diarrhea, n (%) | 420 (42) |
| Weight loss, n (%) | 194 (19) |
| Anemia, n (%) | 224 (22) |
| Fatigue, n (%) | 97 (10) |
| Abdominal pain, n (%) | 336 (33) |
Immunoglobulin A tissue transglutaminase, reference range <19 U/ml.
A total of 872 patients with CD and an active US postal address were included in the main analysis. The mean age at diagnosis was 43.4 years (±16.2, range 1–89), and 75% were female. In this group, where patients had an active US postal address, the mean per capita income was calculated at US$33,506 (±14,487, range US$5230–116,874), and the mean household income was US$66,676 (±31,140, range US$9625–200,001). The mean immunoglobulin A (IgA) tissue-transglutaminase (TTG) level at diagnosis was 100.5 U/ml (±78.2, range 0–640; reference range for normal <19 U/ml). A minority of patients had a comorbid autoimmune (24%) or psychiatric diagnosis (21%) at the time of CD presentation, and the most common symptom at diagnosis was diarrhea (43%). Compared with the 138 patients without an active US postal address (who were ultimately excluded from further analysis), the 872 study patients had no statistical differences in demographic or diagnostic characteristics, with the exception of diarrhea at presentation: diarrhea was more common in the patients with an active US postal address (43% versus 32%, p = 0.01). Supplementary Table 1 lists a full comparison of patient characteristics between those with and without an active US postal address.
When comparing patients with (n = 376) and without diarrhea (n = 496) at the time of CD diagnosis, demographic and diagnostic characteristics including age, sex, TTG level, and prevalence of comorbid autoimmune conditions (hypothyroidism and type 1 diabetes) were similar (Table 2). The prevalence of certain psychiatric conditions was higher in patients with diarrhea at presentation (depression: 18% versus 13%, p = 0.03; anxiety: 9% versus 6%, p = 0.04), and those with diarrhea had overall higher rates of other symptoms as well, including weight loss (31% versus 10%, p < 0.01), fatigue (12% versus 7%, p < 0.01), and abdominal pain (44% versus 27%, p < 0.01). Based on the socioeconomic analysis performed using the active US postal address, patients whose presentation was without diarrhea had a higher mean per capita income (US$34,469 versus US$32,237, p = 0.02) and a trend towards a higher mean household income (US$70,332 versus US$66,318, p = 0.056).
Table 2.
Univariate analysis: comparison of patients with and without diarrhea at presentation.
| Diarrhea at presentation (n = 376) | No diarrhea at presentation (n = 496) | p value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age at diagnosis (y), mean ± standard deviation | 41.9 ± 15.9 | 44.5 ± 16.2 | 0.08 |
| Female, n (%) | 286 (76) | 369 (74) | 0.57 |
| Per capita income | 32,237 ± 14,454 | 34,469 ± 14,597 | 0.02 |
| Household income | 66,318 ± 28,062 | 70,332 ± 30,483 | 0.056 |
| Diagnostic characteristics and comorbidities | |||
| TTG* level (U/ml) | 97.5 ± 83.1 | 102.9 ± 73.9 | 0.45 |
| Hypothyroidism | 43 (11) | 60 (12) | 0.76 |
| Type 1 diabetes | 19 (5) | 31 (6) | 0.45 |
| Depression | 69 (18) | 65 (13) | 0.03 |
| Anxiety | 35 (9) | 28 (6) | 0.04 |
| Bipolar disorder | 3 (1) | 4 (1) | 0.99 |
| Additional symptoms at presentation | |||
| Weight loss | 116 (31) | 52 (10) | <0.01 |
| Fatigue | 46 (12) | 37 (7) | 0.02 |
| Abdominal pain | 164 (44) | 136 (27) | <0.01 |
IgA tissue transglutaminase, reference range <19 U/ml.
Similar socioeconomic trends were seen when comparing patients with (n = 566) and without (n = 306) any gastrointestinal symptoms at presentation. For patients with any gastrointestinal symptoms, the mean per capita income was US$31,758 (± 13,303), and the mean household income was US$65,304 (± 26,850). By comparison, patients without gastrointestinal symptoms at presentation had a significantly higher mean per capita income of US$36,738 ± 15,980 (p < 0.01) and a significantly higher mean household income of US$76,678 ± 33,970 (p < 0.01).
Analysis was also performed based on per capita income quartiles. The 872 patients for whom income data were available were divided into quartiles of 218 patients, ranging from quartile 1 (income US$5230–23,577) to quartile 4 (income > US$40,432). Patients in each quartile were similar with regard to age at diagnosis, sex, TTG level, and frequency of comorbid autoimmune and psychiatric disorders. Supplementary Table 2 lists the full patient characteristics by per capita income quartile.
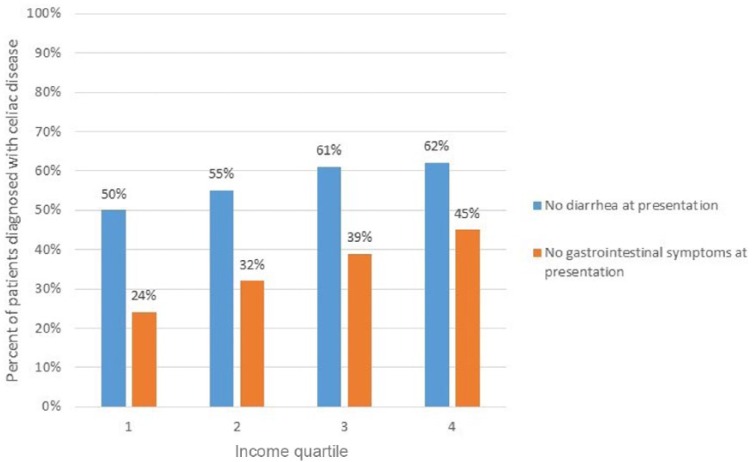
As per capita income quartile increased, the percentage of patients diagnosed without diarrhea or without any gastrointestinal symptoms progressively increased (Figure 1). In the lowest income quartile, 50% of patients were diagnosed based on nondiarrheal presenting symptoms. For the second, third, and fourth quartiles, the percent of patients diagnosed without diarrhea were 55%, 61%, and 62%, respectively (p = 0.01). Similarly, the percentage of patients diagnosed without any gastrointestinal symptoms were 24%, 32%, 39%, and 45% in ascending per capita income quartiles (p < 0.01).
Figure 1.
Frequency of celiac disease diagnosis based on symptoms by income quartiles.
On multivariable analysis adjusting for sex, age, autoimmune comorbidities, and per capita income, the presence of psychiatric disease remained inversely associated with a nondiarrheal presentation (OR: 0.62, 95% CI: 0.41–0.95, p = 0.03). However, per capita income in the top two quartiles showed a trend towards predicting diagnosis without diarrhea (OR: 1.40, 95% CI: 0.98–2.02, p = 0.06). Table 3 lists the full results of the multivariable regression. When assessing nongastrointestinal symptoms at CD diagnosis, multivariable analysis adjusting for sex, age, and autoimmune or psychiatric comorbidities showed that per capita income in the top two quartiles remained the most significant predictor of diagnosis without gastrointestinal symptoms (OR: 1.71, 95% CI: 1.17–2.50, p < 0.01). Supplementary Table 3 lists the full results of the multivariable regression.
Table 3.
Multivariate analysis: Predictors of celiac disease diagnosis without diarrhea symptoms.
| Variables | OR | 95% CI | p value |
|---|---|---|---|
| Sex | |||
| Male | 1 | ||
| Female | 0.79 | 0.52–1.19 | 0.25 |
| Age (y) | |||
| 1–32 | 1 | ||
| 32–41 | 1.1 | 0.66–1.82 | 0.72 |
| 42–55 | 1.65 | 0.98–2.76 | 0.06 |
| 56+ | 1.29 | 0.78–2.15 | 0.32 |
| Comorbid autoimmune disease | |||
| No | 1 | ||
| Yes | 1.51 | 0.92–2.45 | 0.1 |
| Comorbid psychiatric disease | |||
| No | 1 | ||
| Yes | 0.62 | 0.41–0.95 | 0.03 |
| Per capita income quartile | |||
| Quartiles 1–2 | 1 | ||
| Quartiles 3–4 | 1.40 | 0.98–2.02 | 0.06 |
OR, odds ratio; CI, confidence interval.
Discussion
The underdiagnosis of CD in the US and many other countries is a significant problem, with delayed diagnosis leading not only to decreased quality of life, but also significant morbidity [Choi et al. 2011; Freeman et al. 2009; Soni and Badawy, 2010; Zampieron et al. 2011]. With the exception of a few countries where primary care physician education has led to increases in CD diagnosis [Collin et al. 2007; Dickey et al. 1998], there has been no clear evidence of substantive improvements in diagnosis, and a better understanding of the factors that contribute to underdiagnosis of CD are needed.
To our knowledge, this is the first study specifically evaluating the association between socioeconomic status and symptoms at diagnosis of CD in the US. In this study, we used demographic data from a large population with CD in conjunction with US census data to assess the impact of income on the diagnosis of CD. Our data provide strong evidence for a correlation between higher socioeconomic status, measured by per capita income, and the greater likelihood of being diagnosed with CD in the absence of diarrhea or any gastrointestinal symptoms. Our findings suggest that patients with nonclassic symptoms of CD are less frequently diagnosed when they are of a lower socioeconomic status.
Although we cannot define the exact mechanism by which income leads to differential CD diagnostic patterns, it is known that income modulates health-seeking behavior and access to healthcare, both of which contribute to healthcare disparities [Adler and Newman, 2002]. Previous studies employing county-level data in the US have shown that an income difference of less than US$9000 can lead to significant changes in health status – including a 13% difference in premature mortality [Cheng and Kindig, 2012]. In our study, the difference of the means between consecutive per capita income quartiles ranged from US$7250 to more than US$19,000, and our findings of an inconsistency in CD diagnosis based on symptoms seem consistent with previous studies implicating socioeconomic factors as a determinant of healthcare disparities.
Past research has shown that a lack of physician awareness hinders diagnosis of CD based on more subtle or atypical symptoms [Catassi et al. 2007], a fact that is consistent with our findings. However, our work shows that patient factors are also likely to be relevant. While there are some published data evaluating socioeconomic characteristics in those diagnosed with CD in Europe [Snook et al. 1996; West et al. 2003; Ludvigsson 2005; Olen et al. 2012], there is a paucity of such data in the US population with CD. One of the inherent difficulties in obtaining socioeconomic estimates is the lack of data available at the individual subject level. We acknowledge that our method of determining socioeconomic position, using estimates based on patients’ place of residence, has pitfalls, including the possibility that patients may have moved residences after their initial diagnosis and the inaccuracies of applying population characteristics to the individual. However, population estimates are commonly employed when assessing socioeconomic characteristics for governmental research and public planning purposes [Easa and Chan, 2000; Scotch et al. 2006], and our source of socioeconomic and demographic data, GeoLytics, Inc., has been used in the study of other disease states. One such study from a large, tertiary-care medical center in California used census data to estimate the effect of income on hemoglobin A1c (HbA1c) levels in diabetic patients [Geraghty et al. 2010]. This study found that patients with higher HbA1c levels tended to have lower incomes, and patients with health insurance tended to have lower HbA1c levels than those without health insurance. Similar US studies have used various means of measuring socioeconomic position to demonstrate that lower socioeconomic class is associated with delayed diagnosis and increased morbidity and mortality from diseases including melanoma, prostate cancer, and systemic lupus erythematous [Mandala et al. 2011; Rapiti et al. 2009; Cooper et al. 2007]. Our study suggests that similar to these other disease states, CD patients with lower incomes may be less likely to seek prompt medical care for health complaints; ultimately preventing a timely diagnosis.
We acknowledge several limitations of our study. First, all patients in the study were seen at a single referral center in Boston. Though the catchment area of The Celiac Center at BIDMC is across the New England region (including patients residing in Massachusetts, New Hampshire, and Maine), the generalizability of our findings to other regions of the US, and certainly abroad, may be limited. Notably, 94.5% of the patients seen at our Celiac Center are white by self identification. Given the homogeneity of the study population, we did not include race or ethnicity in our analysis, but this critical confounder should be assessed in future studies. Second, there are several limitations of ‘Geocoding,’ which were enumerated above. Third, our limited sample size (consisting of patients from only one medical center) likely affected the outcomes of our multivariate analysis. Although per capita income remained a predictor of nongastrointestinal symptoms at the time of CD diagnosis after adjusting for various patient characteristics, per capita income was marginally below the level of significance when predicting nondiarrheal symptoms. It is possible that a larger sample size would have yielded significant results with more narrow confidence intervals. Another finding that was surprising in our multivariate analysis was the fact that comorbid psychiatric conditions (depression, anxiety, and bipolar disorder) were predictive of diarrhea symptoms at CD presentation. Although we cannot definitively investigate or explain this finding as part of our current study, we believe that the increased prevalence of irritable bowel syndrome in both CD and psychiatric disorders may be driving this association [Chey et al. 2015]. Fourth, the current study measured the mode of clinical presentation of patients with confirmed CD and correlated this to their socioeconomic status. The study did not measure rates of diagnosis in patients of differing socioeconomic classes, which would have been a more direct measure of the impact of income on CD presentation and diagnosis, but would require screening individuals who have not sought care at a CD center. This is an area that should be specifically addressed in future studies.
In conclusion, this study demonstrates that patients with CD of a lower socioeconomic status are more likely to have a diarrheal or gastrointestinal symptomatic presentation. This raises the concern that nonclassical CD may be missed among those of a lower socioeconomic status. We believe that factors such as decreased access to care and differences in health-seeking behavior may contribute to the underdiagnosis of CD in groups of lower socioeconomic status. As CD may be under-recognized in this population, we encourage physicians to more strongly consider CD in patients of lower income and socioeconomic status, even if they lack diarrhea.
Supplementary Material
Footnotes
Funding: Daniel Leffler was supported by the Celiac Center Research Fund (BIDMC, Boston) and the NIH (grant number DK1042103881).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Abhik Roy, Department of Gastroenterology, Columbia University, 622 West 168th Street, PH7 West (Room 318), New York, NY 10032, USA.
Shilpa Mehra, Division of Gastroenterology and Hepatology, Montefiore Medical Center, Bronx, NY, USA.
Ciarán P. Kelly, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA, USA
Sohaib Tariq, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Kumar Pallav, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Melinda Dennis, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Ann Peer, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Benjamin Lebwohl, Department of Gastroenterology, Columbia University, New York, NY, USA.
Peter H. R. Green, Department of Gastroenterology, Columbia University, New York, NY, USA
Daniel A. Leffler, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA, USA
References
- Adler N., Newman K. (2002) Socioeconomic disparities in health: pathways and policies. Health Affairs 21: 60–76. [DOI] [PubMed] [Google Scholar]
- Braback L., Hjern A., Rasmussen F. (2005) Social class in asthma and allergic rhinitis: a national cohort study over three decades. Eur Respir J 26: 1064–1068. [DOI] [PubMed] [Google Scholar]
- Catassi C., Kryszak D., Louis-Jacques O., Duerksen D., Hill I., Crowe S., et al. (2007) Detection of celiac disease in primary care: a multicenter case-finding study in North America. Am J Gastroenterol 102: 1454–1460. [DOI] [PubMed] [Google Scholar]
- Cheng E., Kindig D. (2012) Dispartities in premature mortality between high and low income US counties. Preventing Chronic Disease 9: 1–11. [PMC free article] [PubMed] [Google Scholar]
- Chey W., Kurlander J., Eswaran S. (2015) Irritable bowel syndrome: a clinical review. JAMA 313: 949–958. [DOI] [PubMed] [Google Scholar]
- Choi J., Lebwohl B., Wang J., Lee S., Murray J., Sauer M., et al. (2011) Increased prevalence of celiac disease in patients with unexplained infertility in the United States. J Reprod Med 56: 199–203. [PMC free article] [PubMed] [Google Scholar]
- Collin P., Huhtala H., Virta L., Kekkonen L., Reunala T. (2007) Diagnosis of celiac disease in clinical practice: physician’s alertness to the condition essential. J Clin Gastroenterol 41: 152–156. [DOI] [PubMed] [Google Scholar]
- Cooper G., Treadwell E., St Clair E., Gilkeson G., Dooley M. (2007) Sociodemographic associations with early disease damage in patients with systemic lupus erythematosus. Arthritis Rheum 57: 993–999. [DOI] [PubMed] [Google Scholar]
- Dickey W., McMillan S., Hughes D. (1998) Identification of coeliac disease in primary care. Scand J Gastroenterol 33: 491–493. [DOI] [PubMed] [Google Scholar]
- Easa S., Chan Y. (2000) American Society of Civil Engineers, Geographic Information Systems Committee. Urban planning and development applications of GIS, American Society of Civil Engineers: Reston. [Google Scholar]
- Fasano A., Berti I., Gerarduzzi T., Not T., Colletti R., Drago S., et al. (2003) Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med 163: 286–292. [DOI] [PubMed] [Google Scholar]
- Freeman H. (2009) Adult celiac disease and its malignant complications. Gut Liver 3: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty E., Balsbaugh T., Nuovo J., Tandon S. (2010) Using Geographic Information Systems (GIS) to assess outcome disparities in patients with type 2 diabetes and hyperlipidemia. J Am Board Fam Med 23: 88–96. [DOI] [PubMed] [Google Scholar]
- Gomez J., Selvaggio G., Viola M., Pizarro B., la Motta G., de Barrio S., et al. (2001) Prevalence of celiac disease in Argentina: screening of an adult population in the La Plata area. Am J Gastroenterol 96: 2700–2704. [DOI] [PubMed] [Google Scholar]
- Green P., Cellier C. (2007) Celiac disease. N Engl J Med 357: 1731–1743. [DOI] [PubMed] [Google Scholar]
- Green P., Stavropoulos S., Panagi S., Goldstein S., Mcmahon D., Absan H., et al. (2001) Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol 96: 126–131. [DOI] [PubMed] [Google Scholar]
- Katz K., Rashtak S., Lahr B., Melton L., Krause P., Maggi K., et al. (2011) Screening for celiac disease in a North American population: sequential serology and gastrointestinal symptoms. Am J Gastroenterol 106: 1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D., Sterne J., Tynelius P., Davey Smith G., Rasmussen F. (2006) Association of childhood socioeconomic position with cause-specific mortality in a prospective record linkage study of 1,839,384 individuals. Am J Epidemiol 164: 907–915. [DOI] [PubMed] [Google Scholar]
- Lebwohl B., Kapel R., Neugut A., Green P., Genta R. (2011) Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest Endosc 74: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo W., Sano K., Lebwohl B., Diamond B., Green P. (2003) Changing presentation of adult celiac disease. Dig Dis Sci 48: 395–398. [DOI] [PubMed] [Google Scholar]
- Ludvigsson J. (2005) Socio-economic characteristics in children with coeliac disease. Acta Paediatr 94: 107–113. [DOI] [PubMed] [Google Scholar]
- Mandala M., Imberti G., Piazzalunga D., Belfiglio M., Lucisano G., Labianca R., et al. (2011) Association of socioeconomic status with Breslow thickness and disease-free and overall survival in stage I–II primary cutaneous melanoma. Mayo Clin Proc 86: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J., Van Dyke C., Plevak M., Dierkhising R., Zinsmeister A., Melton L. (2003) Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol 1: 19–27. [DOI] [PubMed] [Google Scholar]
- Olen O., Bihagen E., Rasmussen F., Ludvigsson J. (2012) Socioeconomic position and education in patients with celiac disease. Dig and Liv Dis 44: 471–476. [DOI] [PubMed] [Google Scholar]
- Palabykoglu M., Botoman V., Coban S., Ormeci N., Bonner G., Woodhouse S., et al. (2008) A tale of two cities: typical celiac sprue presenting symptoms are significantly more common in Turkish than in US Patients. J Clin Gastroenterol 42: 62–65. [DOI] [PubMed] [Google Scholar]
- Pappas G., Queen S., Hadden W., Fisher G. (1993) The increasing disparity in mortality between socioeconomic groups in the United States, 1960 and 1986. N Engl J Med 329: 103–109. [DOI] [PubMed] [Google Scholar]
- Rampertab S., Pooran N., Brar P., Singh P., Green P. (2006) Trends in the presentation of celiac disease. Am J Med 119: 355 e9–14. [DOI] [PubMed] [Google Scholar]
- Rapiti E., Fioretta G., Schaffar R., Neyroud-Caspar I., Verkooijen H., Schmidlin F., et al. (2009) Impact of socioeconomic status on prostate cancer diagnosis, treatment, and prognosis. Cancer 115: 5556–5565. [DOI] [PubMed] [Google Scholar]
- Rubio-Tapia A., Ludvigsson J., Brantner T., Murray J., Everhart J. (2012) The prevalence of celiac disease in the United States. Am J Gastroenterol 107: 1538–1544. [DOI] [PubMed] [Google Scholar]
- Scotch M., Parmanto B., Gadd C., Sharma R. (2006) Exploring the role of GIS during community health assessment problem solving: experiences of public health professionals. Int J Health Geogr 5: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shack L., Jordan C., Thomson C., Mak V., Moller H. (2008) Variation in incidence of breast, lung and cervical cancer and malignant melanoma of skin by socioeconomic group in England. BMC Cancer 8: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook J., Dwyer L., Lee-Elliott C., Khan S., Wheeler D., Nicholas D. (1996) Adult coeliac disease and cigarette smoking. Gut 39: 60–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni S., Badawy S. (2010) Celiac disease and its effect on human reproduction: a review. J Reprod Med 55: 3–8. [PubMed] [Google Scholar]
- Telega G., Bennet T., Werlin S. (2008) Emerging new clinical patterns in the presentation of celiac disease. Arch Pediatr Adolesc Med 162: 164–168. [DOI] [PubMed] [Google Scholar]
- Van der Windt D., Jellema P., Mulder C., Kneepkens C., van der Horst H. (2010) Diagnostic testing for celiac disease among patients with abdominal symptoms: a systematic review. JAMA 17: 1738–1746. [DOI] [PubMed] [Google Scholar]
- West J., Logan R., Hill P., Lloyd A., Lewis S., Hubbard R., et al. (2003) Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. Gut 52: 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieron A., Daicampi C., Martin A., Buja A. (2011) Quality of life in adult celiac disease in a mountain area of northeast Italy. Gastroenterol Nurs 34: 313–319. [DOI] [PubMed] [Google Scholar]
- Zipser R., Farid M., Baisch D., Patel B., Patel D. (2005) Physician awareness of celiac disease: a need for further education. J Gen Intern Med 20: 644–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.