Abstract
Inflammatory bowel disease (IBD) is a chronic, often relapsing, condition that deeply impacts the quality of life for many patients. Although there have been significant advances in medical treatments, a large proportion of patients become refractory to available therapeutic options. Stem-cell therapy through hematopoietic stem cells (HSCs) or mesenchymal stem (stromal) cells (MSCs) is a promising therapeutic option for severe refractory cases especially when surgery is not feasible. In HSC transplantation, the objective is to destroy the ‘autoreactive’ immune cells responsible for disease chronicity, and to re-establish gut tolerance to gut microbes. In perianal Crohn’s disease (CD), the objective is to deposit MSCs locally in fistulizing tracts to down-regulate the local immune response and induce wound healing. Results from upcoming and ongoing clinical trials will set the path of these novel therapeutic options that have the capability to successfully treat severe refractory Crohn’s patients.
Keywords: Crohn’s disease, hematopoietic stem cells, inflammatory bowel disease, mesenchymal stem cells, stem-cell therapy
Introduction
Inflammatory bowel diseases (IBDs) can be categorized as ulcerative colitis (UC) or Crohn’s disease (CD) and may affect any location throughout the gastrointestinal tract. Unfortunately, incidence and prevalence of IBD is ever-increasing for both pediatric and adult populations, signaling its emergence as a global disease especially in Western countries [Gazouli et al. 2014]. Every 1 in 200 Americans bears a diagnosis [Molodecky et al. 2012]. Symptoms of active disease include abdominal pain, diarrhea, unintended weight loss, and most commonly fatigue [Hendrickson et al. 2002; Bielefeldt et al. 2009]. Quality of life is impacted and patients become more susceptible to develop colorectal cancer [Beaugerie and Itzkowitz, 2015]. Seen also are high rates of surgeries, hospitalizations, and drug adverse side-effects [Ramirez and Fleshner, 2006]. Resection rate for CD is at 29% and colectomy rate in UC is lower at 12%; both rates measured within 7 years of initial diagnosis [Vester-Andersen et al. 2014].
IBD is a destructive and debilitating lifetime condition that has a significant impact on quality of life [Mitchell et al. 1988]. Disease relapse affects many aspects of patients’ lives. Homeostatic mental wellbeing and physical health are at risk, not to mention the profound impact IBD has on personal relationships and work productivity [Zand et al. 2015]. In a patient survey involving over 5600 responses, three quarters of the members reported that IBD symptoms affect their ability to enjoy leisure activities, and around 69% of the same members report symptoms that affect their ability to be productive at work [Ghosh and Mitchell, 2007; Zand et al. 2015]. The ultimate goal in treating IBD is to achieve deep remission (symptom control and endoscopic healing of mucosal lesions [Rogler et al. 2013]), and reduce long-term disability while maintaining a normal quality of life [Hommes et al. 2012].
The current treatment for IBD is centered upon symptom control in a stepwise approach. This method begins with medications: 5-aminosalicylic acid (5-ASA) agents and antibiotics. Subsequently followed by corticosteroids, immunomodulators, and biologics. When all else fails, the final option tends to be surgery [Thomas and Lodhia, 2014]. Half of CD patients require surgical resection at some point during the disease course [Peyrin-Biroulet and Lemann, 2011]. However, some CD patients refuse surgery or are not eligible candidates given the large extent of small bowel disease. The high risk of developing short-bowel syndrome is a factor to consider. A particular subset of patients exists who are refractory to all current medical therapies and cannot undergo surgery [Hwang and Varma, 2008]. Stem-cell therapy is a promising alternative to treat ongoing tissue damage by resetting the underlying disease process, through alteration of the mucosal immune response [Heslop et al. 2015]. Existing and ongoing studies show promising yet inconclusive results. The outcomes obtained from past and current clinical trials have potential to add a new branch of disease management for patients with IBD, significantly improving the quality of life for those who need it the most.
Etiopathogenesis of IBD
While there have been significant advances into the pathogenetic insight of IBD, the exact etiology is still unknown. Genome-wide association studies (GWASs) helped to identify genetic risk loci, where 28 markers are shared between UC and CD [Franke et al. 2010; Anderson et al. 2011]. The anti-inflammatory cytokine interleukin (IL)-10 locus was initially associated with UC [Franke et al. 2008] later to be associated also in CD [Franke et al. 2010]. Deficiency in IL-10 and its receptor leads to severe early-onset colitis [Shah et al. 2012]. The manifestation of IBD often involves genetic and nongenetic cues; orchestration of complex genetic [Franke et al. 2010; Fiocchi, 2012], environmental [Cosnes et al. 2011], and microbial [Chassaing and Darfeuille-Michaud, 2011] factors. A genetically susceptible host can develop a dysregulated immune response to commensal bacteria and luminal antigens [Lanzoni et al. 2008]. Environmental stimuli can also trigger a change in the innate and adaptive immune function in epithelial barrier function and microbiome composition leading to an active disease state [Baumgart and Sandborn, 2012; Ordas et al. 2012; Scharl and Rogler, 2012].
Although the etiology is not fully elucidated, IBD is nonetheless classified as an autoimmune disease. Autoimmune activation is seen with circulating antibodies against epithelial barrier function and commensal enteric bacterial population involvement [Broberger and Perlmann, 1959; Wen and Fiocchi, 2004]. In both UC and CD, antibodies are present against a range of autoantigens including lymphocyte antigens [Korsmeyer et al. 1975]. Two commonly studied autoantibodies in autoimmune diseases are: atypical perinuclear antineutrophil cytoplasmic antibodies (P-ANCAs) and anti-Saccharomyces cerevisiae antibodies (ASCAs) [Das and Biancone, 2008]. A study by Duerr and colleagues showed P-ANCAs are present in 60–75% of UC patients with primary sclerosing cholangitis [Duerr et al. 1991]. More recent studies show autoimmunity against human tropomyosin isoform 5 (hTM5) as a critical epithelial autoantigen in UC, which can induce both cellular and humoral immune responses [Geng et al. 2001; Das et al. 2008; Ebert et al. 2009]. These hTM5-specific immunoglobulin G (IgG) autoantibodies show a direct pathogenic effect on the destruction of colonic epithelial cells.
Although current research has reached significant advancements in understanding the underlying course of action in the development of IBD, the exact mechanisms are still vague and unclear. This results in a difficult treatment course for IBD patients. Until the exact pathways and mechanisms are understood, current medical treatment for IBD is aimed at symptom control, often by the way of immunosuppression.
Stem-cell therapy
Stem cells are defined as undifferentiated cells having the capacity to differentiate into tissue or organ-specific cells, and are characterized as either embryonic or somatic (adult) stem cells. Stem cells differ depending on origin and function [Smith et al. 2006]. Stem-cell therapy can either be autologous (isolated from the same individual) or allogeneic (isolated from a donor, ideally human leukocyte antigen [HLA]-matched). There has been inconsistent yet promising results from clinical trials in the past few decades, using stem-cell therapy through hematopoietic stem cells or mesenchymal stem cells [Reya et al. 2001]. Transplant data from 2013 show that over 320,000 patients have received either autologous or allogeneic stem-cell transplants for various indications [Pasquini and Zhu, 2015].
Hematopoietic stem-cell therapy
Hematopoietic stem cells are multipotent cells that have a self-renewal property, and are capable of differentiating into blood and immune cells. These stem cells can be isolated from the bone marrow (BM), umbilical cord blood, or more commonly peripheral blood [Da Silva Meirelles et al. 2006]. They are progenitors of both myeloid (monocytes, erythrocytes macrophages, neutrophils, and dendritic cells) and lymphoid (T cells, B cells, and natural killer cells) lineages [Pittenger et al. 1999]. HSCs have the capability to directly migrate to a damaged tissue or differentiate to epithelial or immunomodulatory cells in order to restore normal mucosal tissue [Kavanagh and Kalia, 2011].
The HSC multistep procedure begins with a pretransplant screening process that involves (but is not limited to) history and examination, blood tests, serologies, colonoscopy, small bowel magnetic resonance imaging (MRI), or pelvic MRI (only if perianal disease), and BM aspiration. The subsequent transplant procedure involves three main steps:
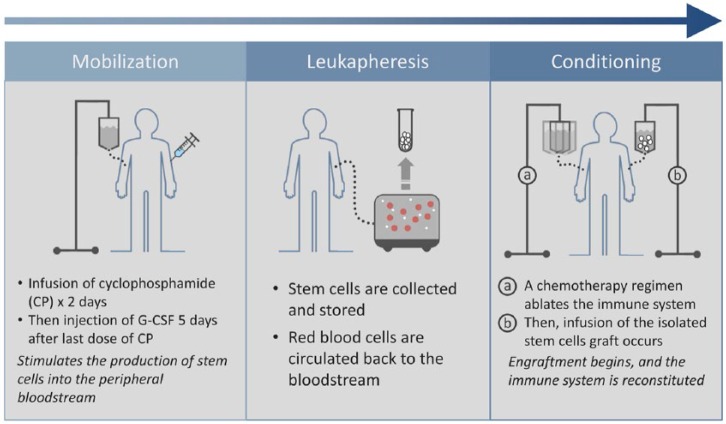
Hematopoietic stem cell transplantation (HSCT) begins with mobilization of stem cells from the patient (autologous [Figure 1]) or an HLA-matched donor (allogeneic) which begins with infusion of cyclophosphamide (CP) for 2 consecutive days to remove lymphocyte cells. Then 5 days following the last administration of CP, involves subcutaneous injection of granulocyte colony-stimulating factor (G-CSF) to stimulate BM to produce stem cells that are subsequently released to the bloodstream [Sallerfors and Olofsson, 1992]. Administration of G-CSF continues for 4–5 days and ends the day before leukapheresis. Prophylactic antibiotics are recommended.
The second step, leukapheresis, aims to collect the CD34+ cells from the peripheral blood or BM up to a target number of 3–8 × 106. Collected cells are then cryopreserved until infusion.
The final transplant step is conditioning with transplantation. This step involves total body immune ablation through chemotherapy to eliminate autoreactive cells. Intravenous CP is administered for 4 consecutive days; with infusion of rabbit antithymocyte globulin (rbATG) 2 days after the first dose of CP. Methylpred-nisolone is also administered to improve tolerability. Transplantation of the isolated cells begins 5 days after the first administration of CP and 24 hours following the end of rbATG and methylprednisolone. The stem cells are then transplanted through a central venous catheter to the patient in varying dosages. Careful isolation is essential to reduce the risk of transplant morbidities and mortality. Engraftment and reconstitution of the immune system last about 2–4 weeks. Postengraphment is the final phase of HSCT, where follow up is essential, especially in allogenic HSCT, when risk of chronic graft-versus-host disease (GvHD) can occur. In GvHD the donor effector cells are activated and attack the recipient’s immune system [Lee et al. 2003]. Mild forms of chronic GvHD can be successfully treated with steroids or immunosuppressive medications (e.g. azathioprine) [Iwasaki, 2004]. Patients follow up with their physicians 1, 2, 4, 6, 12, and 24 months after discharge.
Figure 1.
Autologous stem-cell transplantation.
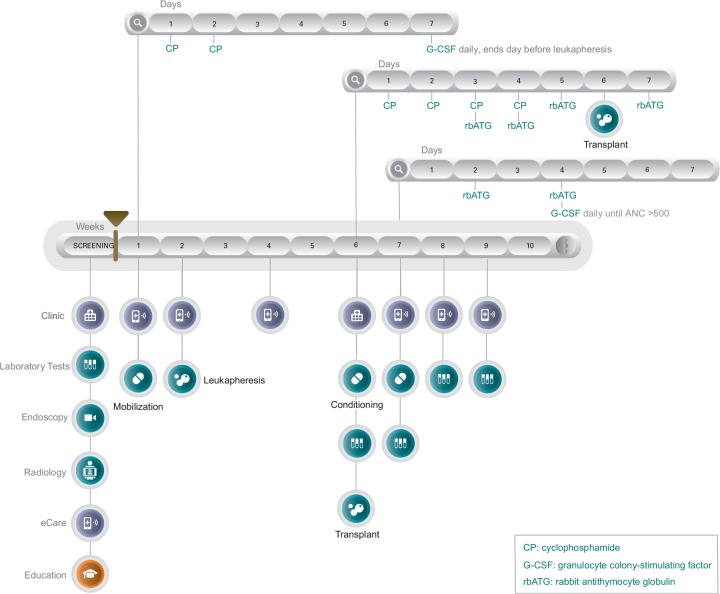
The protocol for stem-cell transplantation is very precise and can be confusing for the patient to follow and understand without a guideline and proper education. Implemented and designed by UCLA Center for IBD, Los Angeles, USA, is an individualized scenario where each step of the transplant process is laid out in a detailed care pathway accessible to the patient and provider. The following HSCT Care Pathway [Figure 2] begins with a screening period. During this time, indication for transplant is established and a multidisciplinary care team is coordinated including GI, hematology, and oncology. When not hospitalized, patients capture their symptoms through a mobile application (eCare). All screening examinations and tests are performed prior to mobilization at week 1. Mobilization (week 1) and leukapheresis (week 2) can be carried out in an outpatient setting. Hospitalization occurs at week 6, initiating the conditioning phase, and infusion of CD34+ stem cells. The patient is followed closely for about 4–5 weeks post-infusion to monitor for side-effects, toxicity, and infections. When absolute neutrophil count (ANC) reaches >500 the patient is discharged and then is scheduled for follow-up visits. The HSCT Care Pathway is customizable beyond standard protocol procedures, and can be reset or extended.
Figure 2.
HSCT Care PathwayTM.
ANC, absolute neutrophil count.
Modeling of an individualized and customizable scenario is clearly laid out in a visual timeline that is available to both the patient and provider on the web or a mobile platform. Each circle is an active icon where the patient can click through to pull information to see results of lab tests, schedule a clinic visit, or capture their symptoms.
Historically, animal models provided first insight into HSCT showing it could be an efficacious mode of treatment for autoimmune diseases [Bekkum, 2000; Van Bekkum, 2000; Ikehara, 2001]. Initially, HSCT was used to treat malignant hematological indications in humans [Yin and Jowitt, 1992; Bierman et al. 2003]. In clinical practice, several case studies reported improvement of autoimmune disease activity following HSCT for concomitant aplastic anemia or malignancy [Van Deen, 2012]. In a case where two patients with severe aplastic anemia underwent allogeneic BM transplant (BMT), following transplantation their case of severe rheumatoid arthritis had become quiescent in junction with resolution of the aplastic anemia indication. Reported remission lasted for 13 years following transplantation [Lowenthal et al. 1993]. Expanding further, experimental results and phase I–II trials show that high-dose chemotherapy with autologous HSCT can successfully treat severe cases of various autoimmune diseases [Gratwohl et al. 2005]. Historically, autoimmune diseases treated with HSCT include but are not limited to multiple sclerosis [Burt et al. 1995], lupus, and rheumatoid arthritis [Jacobs et al. 1986]. The central aim of HSCT effectiveness is to ‘reset’ the immune system by eliminating self-reactive T-lymphocytes and memory cells that contribute as the effectors of a dysregulated immune system [Martinez-Montiel Mdel et al. 2014]. Retrospective analyses and prospective studies show feasibility and safety of auto- and allo-HSCT for autoimmune diseases [Daikeler et al. 2009; Farge et al. 2010].
In the largest cohort study, the outcomes of 900 patients from 1996–2007 were measured for autologous HSCT transplants of various indications [Farge et al. 2010]. The survival rate of autoimmune HSCT transplants was 85% at 5 years with 43% progression-free survival. Around 30% of patients showed a complete response, despite full immune reconstitution. Analysis showed the type of autoimmune disease was most relevant to the outcome, rather than the transplant technique. Around half of the deaths (often due to infection) were treatment related and appeared to be strongly correlated with the transplant center and not the conditioning regimen [Farge et al. 2010]. The following prospective study involved a high rate of complications. It involved 26 refractory CD patients who underwent an auto-HSCT. During the mobilization phase, 16 cases of febrile neutropenia, 1 case of bacteremia, and 2 cases related to septic shock were seen. A total of 21 patients then entered the conditioning phase. Infectious complications continued, and noninfectious complications occurred in 6 patients: 1 who suffered an rbATG drug reaction, 12 mucositis events, and 2 hemorrhagic complications. In addition, 1 patient suffered from a cytomegalovirus leading to death by multiorgan failure [Jauregui-Amezaga et al. 2014]. Initially, IBD patients underwent HSCT for other indications (such as leukemia and non-Hodgkin’s lymphoma [NHL]). The first case of a CD patient undergoing an autologous BMT for NHL occurred in 1993, resulting in a 6-month remissive state [Drakos et al. 1993]. Other study results varied but were promising, showing a remissive state for the primary indication along with clinical and endoscopic improvement for UC or CD [Yin et al. 1992; Drakos et al. 1993; Castro et al. 1996; Kashyap, 1998; Lopez-Cubero et al. 1998]. By complete immune ablation and reconstitution with hematopoietic stem cells, the body is able to generate naïve lymphoid and myeloid cells [Swenson and Theise, 2010], thus reducing T-cell activity against mucosal self-antigens and inflammation. The inflammatory process is controlled, but the underlying genetic predispositions are not modified with an autologous transplant. Small studies have been performed showing auto-HSCT’s ability to induce a remissive state. A 2010 study by Burt and colleagues included 24 CD patients where remission rates (defined as supplementary medication was not initiated) were at 91%, 57%, and 19% at years 1, 3, and 5 respectively [Burt et al. 2010]. Another study showed all patients entered clinical remission at a 3-month follow up following transplantation; then at a 16.5-month follow up, 75% of patients remained in clinical and endoscopic remission. However, it is important to note the small sample size only included four CD patients [Cassinotti et al. 2008].
Although data are limited, there are a few case reports of UC patients undergoing stem-cell transplantation for other indications. One patient was showed to have prolonged clinical remission after high-dose chemotherapy and peripheral-blood stem-cell (PBSC) transplantation for high-risk breast cancer. Following transplant, the patient reported no symptoms and was off IBD-related medication for 2 years [Castro et al. 1996]. Another case of a 57-year-old woman with distal UC undergoing auto-HSCT for breast cancer was asymptomatic and discontinued IBD maintenance therapy 6 months after transplant. However, it is important to note that the patient did not have any IBD-related symptoms before the transplant and there was no colonoscopy to confirm disease remission at 6 months. At a 25 month follow up, the patient developed rectal bleeding and became asymptomatic only after restarting maintenance mesalamine therapy [Marti et al. 2001]. Stem-cell transplants in UC show a favorable but short-lived response. There is a randomized, double-blind, placebo-controlled phase II study of MultiStem® Cell Therapy (Athersys Inc., USA) for moderate-to-severe UC. The study showed good tolerability but failed to show efficacy over 8 weeks [Lehmann, 2014]. MultiStem is a manufactured biologic product isolated from human BM. The single intravenous (IV) administration did not show statistically significant improvement.
Methods in HSCT for IBD still vary, and the protocols are ever changing. For the most part, studies select CD34+ PBSCs to use for transplant. The reason is that CD34+ is a hematopoietic progenitor cell antigen that functions as a cell–cell adhesion factor [Satterthwaite et al. 1992; Simmons et al. 1992]. When attempting to isolate HSCs, effector cells are present in the blood sample. Theoretically when selecting for CD34+ in the sample, the stem cells are selected and not the effector cells [Larocca et al. 2006]. Two studies carried out by Cassinotti and colleagues evaluated the safety and efficacy of unselected CD34+ PBSCs for auto-HSCT [Cassinotti et al. 2008; Clerici et al. 2011; Cassinotti et al. 2012]. For both studies, no improvement was seen following mobilization but the procedure was well tolerated. In the 2007 study with four refractory CD patients, preliminary results showed 75% of patients remained in clinical and endoscopic remission at a 16.5-month follow up, with full fistula closure observed for all patients. In a study 4 years later, following transplantation all patients (n = 6) remained in remission for 1–3 months without any immunosuppressive treatment. Late outcomes at 1-year follow up showed five out of six of the patients displayed full response and maintained both clinical and endoscopic remission without further treatment [Clerici et al. 2011]. The protocol to extract unselected CD34+ PBSCs is less technical and expensive to perform. This offers a potential opportunity for midsized transplant centers to provide HSCT for their refractory patients. However, the studies mentioned above involved a limited sample size. There is a need for long-term follow up and randomized control studies with a larger cohort of patients.
A study carried out by Oyama and colleagues showed improvement with hematopoietic autologous peripheral blood (PB) HSCT with low-dose CP and G-CSF [Oyama et al. 2005]. At an 18.5 month follow up, 11 out of 12 patients were off all immunosuppressive drugs and had achieved clinical remission. It is important to note this study measured clinical outcomes and did not report on any endoscopic findings. A monocenter phase I and II trial carried out by Hasselblatt and colleagues measured the outcomes of 12 patients with refractory CD undergoing autologous PB HSCT with immunoablation conditioning [Hasselblatt et al. 2012]. Mobilization was carried out with CP and G-CSF, harvesting CD30+-selected PBSCs. Around 56% of the patients showed clinical and endoscopic improvement in response to the mobilization procedure. Continuing with nine patients entering the conditioning phase, five patients remained in a remissive state at a 6-month follow up. One of these patients showed complete remission of pyoderma gangrenosum extraintestinal manifestation, and another patient had a complete fistula closure. However, seven of the nine patients had a relapse of disease at a 3-year follow-up. It is interesting to note that mucosal healing achieved in 56% of patients in this study is noticeably higher that previous results of phase II trials involving TNF-α inhibitors as the mode of therapy with no transplant [Hanauer et al. 2002].
The ASTIC study, currently the only multicenter randomized phase III interventional study, carried out by the European Group for Blood and Marrow Transplantation (EBMT) aimed to test the potential clinical benefits of high-dose immunoablation of early versus delayed (59 weeks) auto-HSCT. All 45 patients were mobilized prior to randomization. The early group received immediate HSCT 4 weeks post-leukapheresis, while the control delay group waited a year for transplantation and continued conventional therapy. A stringent primary endpoint for full clinical remission was defined by a Crohn’s Disease Activity Index (CDAI) score <150, no use of corticosteroid, immunosuppressive, or biologic drugs for the last 3 months. Lastly, no endoscopic or radiological evidence of active disease along any part of the GI. Secondary endpoints were the individual components of the primary composite outcome along with labs, quality of life (QoL), and imaging. Results from this long-term study have just been released, where only 2 out of 23 patients from the early treatment group achieved full clinical and endoscopic remission compared to 1 out of 22 from the controlled delay group [Hawkey et al. 2015]. Showing there is no statistical difference in sustained disease remission between the HSCT-treated group versus conventional therapy controls. The trial also stated all patients experienced a non-serious adverse complication, with infections as the most common. One patient in the early treatment group died 20 days after the conditioning phase, due to sinusoidal obstructive syndrome.
The authors concluded the study against widespread use of HSCT for patients with refractory CD; A stark contrast from the preliminary results, showing promising durable benefit 1 year after transplant [Hawkey, 2014]. The authors did address the stringent endpoint to justify treatment toxicity. However, before dismissing the widespread use of HSCT for refractory CD patients it is important to dissect the suboptimal end points used in the study design [Hommes and Lacey, 2016]. If a patient did not meet any composite criteria, the transplant was classified as a failed treatment, including use of corticosteroid or immunosuppressive drugs. A maintenance immunosuppressive regimen could have had a positive impact on the rates of remission. Studies have shown induction should include maintenance therapy in order to prevent relapse of the disease [Burt et al. 2010]. A different conclusion can be reached if the endpoints were less demanding in addition to including maintenance therapy after transplant for this difficult-to-treat refractory patient population. Allogeneic HSCT has the ability to genetically correct the underlying disease with a healthy donor [Lopez-Cubero et al. 1998]. However, it is associated with a higher risk of complication and mortality rate when compared to auto-HSCT. There is also a high risk of a noninfectious adoptive autoimmunity transfer of IBD, leading to a phenotypic manifestation in patients receiving allogeneic BMT. A newly diagnosed fulminant Crohn’s colitis occurred in a patient with NHL following nonmyeloablative stem-cell transplantation [Sonwalkar et al. 2003]. There are also two cases of patients developing UC after BMT [Spiers, 1984; Baron et al. 1998]. Learning from these unfortunate cases, it is important to fully screen donors for known genetic risk factors and avoid mismatches for HLA class genes.
Although the limited number of phase I–III trials show promising results, the occurrence of relapse makes it difficult to categorize HSCT as a complete curative treatment. When remission rates drop from 91% at 1 year to 19% at 5 years, long-term outcome measurements are necessary [Burt et al. 2010]. It is also interesting to note that most studies did not initiate any form of combination therapy post-transplant. Future studies could look into the safety and efficacy of combination therapy following HSCT. Expert transplant centers should collaborate to continue biobanking and grow the registry of both successful and unsuccessful HSCT cases. Having more information about individual cases will provide new insights to treatment mechanisms leading to improvements in protocol. A risk–benefit analysis must be fully discussed with the patient before initiating treatment.
Mesenchymal cell transplants
Mesenchymal stem cells (MSCs) are multipotent cells capable of differentiating into several types of cells and are present in all tissues of the body [Da Silva Meirelles et al. 2006]. Friedenstein and colleagues first described the mesenchymal stem- cell concept in 1974 when it was isolated from BM [Friedenstein et al. 1974]. The cells can also be successfully isolated from umbilical cord blood or adipose tissue for clinical application [Zuk et al. 2002; Sensebe et al. 2010]. Adipose-derived stem cells are easily extracted in large quantities with reduced morbidity and discomfort for the patient.
A study by Melief and colleagues confirmed that BM and adipose-derived MSCs had similar immune-modulating functions for multilineage differentiation, while adipose tissue had more potent modulating property over BM due to the increased level of cytokine secretion [Melief et al. 2013]. The International Society for Cellular Therapy has set specific defining characteristics for a stem cell to be labeled as mesenchymal: (1) it must have the ability to adhere to plastic under standard culture conditions; (2) it must express CD105, CD73, and CD90 (but not express CD45, CD34, CD14, CD11b, CD79α, CD19, or HLA-class II molecules); (3) cells must have multipotent in vitro differentiation potential to osteoblasts, adipocytes and chondroblasts [Dominici et al. 2006].
In addition to differentiation, and more relevant to clinical practice, MSCs have immunomodulating capabilities to downregulate mucosal immune reactivity and promote tissue healing [Bartholomew et al. 2002; Krampera et al. 2003; Stappenbeck and Miyoshi, 2009]. Additional studies show that MSCs have the ability to inhibit T-cell proliferation in vitro [Bartholomew et al. 2002; Le Blanc et al. 2003a], and inhibit lymphocyte proliferation by activating a programmed cell death pathway [Augello et al. 2005]. The specific immunomodulating mechanisms of MSCs are still unclear, but evidence shows that the influence of the target cell occurs within close proximity and is not solely dependent on cell-to-cell contact [Di Nicola et al. 2002; Ankrum et al. 2014]. Promising results demonstrate the functional ability of the potential of MSCs to influence the immune response and dampen inflammation, affirming their clinical practicality for therapeutic transplantation in autoimmune diseases.
Administration of MSCs over HSCT is advantageous in regards to their low immunogenicity property, eliminating the need for chemotherapy, which is necessary in HSCT. The thought of low immunogenicity of MSCs stem from the fact that they express low levels of major histocompatability complex (MHC) class I, no MHC class II, and no costimulatory molecules that would activate T cells [Le Blanc et al. 2003b; Ankrum et al. 2014]. MHC molecules are found at the cell surface and function to display intracellular proteins to cytotoxic T cells, thus triggering an immune response against the nonself antigen [Janeway et al. 2001]. MHCs are also synonymous with HLAs, which are alloantigens that are surface proteins responsible for immune system regulation [Bjorkman et al. 1987]. Low immunogenicity would give allogeneic MSCs the ability to evade the immune system and allow their usage across MHC barriers [Schu et al. 2012]. Due to the nonimmunogenic properties and ease of expansion, unmatched allogeneic MSCs are advantageous in that they can be mass manufactured making it easier for midsized transplant centers to provide their patient population with the possibility of transplantation. However, in vitro work by Nauta and colleagues explains that MCSs are not intrinsically immunoprivileged. Under varying conditions, allogeneic MSCs are capable of stimulating a memory T-cell response that can result in graft rejection [Nauta et al. 2006].
Today, MSCs have been used to treat CD in systemic (intra-arterial or intravenous) transplantation for active luminal disease or local administration for fistulizing disease. There are only a few studies reporting mesenchymal stem cell transplantation (MSCT) for CD and UC [Lazebnik et al. 2012a; Lazebnik et al. 2012b; Liang et al. 2012]. At least 25% of CD patients develop debilitating perianal fistulas within 20 years of diagnosis [Hellers et al. 1980; Schwartz et al. 2002], with complex fistulas present 78% of the time, and are discernibly more difficult to treat over simple fistulas [Molendijk et al. 2014]. Treatment for fistulas has shifted from a surgically-based approach to a more immunosuppressant and biologic step-wise approach. Unfortunately despite the best medical advances, durable fistula closure rates remain low at 37% [Molendijk et al. 2014].
In regards to local administration, currently accepted forms of treatment for MSCs in IBD are indicated for the fistulizing phenotype in CD. Two studies in 2013 aimed to heal fistulizing CD, one study with autologous MSCs and the other with allogeneic adipose MSCs. For autologous treatment, results showed complete fistula healing in 82% (27 out of 33) patients 8 weeks following final injection [Lee et al. 2013]. The allogeneic treatment demonstrated full closure in 30% of the patients, with the remaining patients showing a reduction of draining from fistula tracts [De La Portilla et al. 2013]. Local administration of autologous MSCs was shown to be well tolerated and feasible in a study that included 10 CD patients with actively-draining complex perianal and enterocutaneous fistulas. Following ex vivo isolation and expansion, all patients showed either complete or partial fistula closure without any adverse effects. All patients showed a reduction in disease activity, most often after the second administration [Ciccocioppo et al. 2011]. However, drawbacks of auto-MSCT are seen with the efficacy of harvesting time, which requires several weeks for ex vivo expansion [Brandenberger, 2011]. In comparison, allogeneic transplants seem to be more convenient in terms of time and feasible due to MSCs low immunogenicity characteristic from healthy donors. In the most recent study carried out by Molendijk and colleagues involved the first placebo controlled, double-blinded, randomized, dose-escalating clinical trial to date [Molendijk et al. 2015]. This study included 21 CD patients undergoing local administration of allogeneic BM MSCs for perianal fistulas. Throughout the 24-week trial, no serious adverse events occurred. Also, the dosage of 3 × 107 MSCs showed most efficacious (when compared to 1 × 107 and 9 × 107 MSCs) with a 85.7% successful fistula healing rate at week 12; only 33% showed healing for the placebo group.
In addition to local injections of MSCs, there have been significant advances in systemic infusions of MSCs for luminal CD. Unique to MSCs, an ‘off-the-shelf’ product (Prochymal) has been developed by a third-party laboratory Osiris Therapeutics Inc. (USA) These cells are isolated from the BM of healthy donors and expanded ex vivo. The MSCs purified by Osiris are cultured and packaged. Remarkably, up to 10,000 dosages can be obtained from a single donor [Rattue, 2012]. Several phase I–II studies included the Prochymal MSC product for transplant. The first human trial of systemic MSCs was carried out by Onken and colleagues and evaluated nine patients with active CD, who previously failed immunosuppressants and a course of steroids [Onken et al. 2006]. Patients were randomized to intravenously receive Prochymal allogeneic MSCs in varying doses. A total of 3 of 10 patients achieved clinical remission by day 14 following transplantation. No serious adverse events occurred during treatment in all groups, and infusions were well tolerated. Duijvestein and colleagues continued to show that autologous BM MSCT appeared to be well tolerated and feasible in the treatment of refractory luminal CD [Duijvestein et al. 2010]. Additionally, no serious adverse events occurred during harvesting and IV administration.
A phase II study, carried out by Forbes and colleagues measured clinical response at 42 days after a 4-week course of allogeneic infusions of MSCs [Forbes et al. 2014]. Among the 15 patients, a reduction in the CDAI score was seen along with 8 patients entering clinical remission. Currently, a large (330 enrolled patients) multicenter, randomized, double-blind, phase III trial is underway with an expected completion in late 2018 [Raina, 2007]. The purpose of the study is to evaluate the safety and efficacy of Prochymal MSCs at different IV doses for moderate-to-severe CD. Two preliminary analyses have been performed thus far. Of the 207 patients, 148 have successfully reached the 28-day primary endpoint assessment of an absolute CDAI score <150, signifying a clinical remissive state [Meldrum, 2014].
Notwithstanding the promising improvement of clinical outcomes, it is necessary to consider the current hazard and safety before enrolling a patient for MSCT. A few studies isolated various modes of MHCs to verify there is no risk for chromosomal aberration development after long-term culturing or induction of tumors during in vitro and in vivo experiments [Bernardo et al. 2007; Macias et al. 2010; Tarte et al. 2010]. Culture standards need to be perfected, and all possible hazards in the cell sample need to be eliminated before administration. Several phase I–III trials were carried out with historical or concomitant immunomodulating therapy. A study by Duijvestein and colleagues investigated the effect of immunosuppressive drugs (i.e., azathioprine, methotrexate, 6-MP, and anti-TNFα) on the MSC phenotype, survival, and differentiation or immunosuppressive capacity. Their results confirmed mesenchymal stromal cell function is not affected by common drugs used to treat refractory IBD patients [Duijvestein et al. 2011]. Moreover, 6-MP and anti-TNFα antibodies enhanced the inhibitory effect, suggesting clinical safety and usefulness of combination therapy.
As discussed in existing literature, there is a complex variety of pathways and mechanisms through which MSCs respond to damaged inflamed tissue in the gut. Future studies should focus on the exact mechanism in which MSCs operate, providing vital information to improve current therapeutic strategies. It is also valuable to look into initiating combination therapy following transplantation to improve remission rates [Duijvestein et al. 2011]. Continuing exploration of other stem-cell sources in preclinical animal models is essential. In addition to an understanding of basic science, the protocols for isolation and administration should improve as we move forward towards stem-cell therapies for refractory CD patients and other autoimmune diseases.
Induced pluripotent stem cells
It is possible that mature specialized cells can be reprogrammed to become pluripotent [Gurdon, 1962]. Induced pluripotent stem cells (iPSCs) have this capability and hold great potential for regenerative medicine. Induction to an embryonic-like cell stage is initiated by the introduction of specific transcription factors (Oct4, Sox2, Klf4, and c-Myc) [Takahashi and Yamanaka, 2006]. The vast capacity to differentiate and infinite ability to propagate are ideal for replacing damaged and diseased tissue. An innovative protocol has been published for the direct generation of intestinal tissue from human embryonic stem cells (ESCs) and iPSCs in vitro, by manipulating growth factors [Spence et al. 2011]. Also, use of iPSCs eliminates the ethical issue that exists with the use of ESCs .
It is known that regulatory T cells (Tregs) can be used to affect autoimmunity and organ rejection; however, human therapeutic options for Tregs are limited due to the challenging nature of isolating an ideal amount of functional Tregs. A study by Haque and colleagues provided an approach to generate functional Tregs, induced by iPSCs [Haque et al. 2012]. Results from this study provide a novel therapeutic approach to treat autoimmune diseases from Tregs, maintaining immunological tolerance and suppressing autoreactive cells. Adoptive cell transfer (ACT) of Tregs showed encouraging results in experimental settings for autoimmune diseases such as rheumatoid arthritis [Wright et al. 2009] and systemic lupus [Scalapino and Daikh, 2009]. Conversely, due to its similarity with ESCs there is still a significant risk of teratoma formation [Knoepfler, 2009].
Animal and human models exploring iPSCs in IBD have not yet been explored, and there is limited literature on case studies for iPSCs used to treat autoimmune diseases. Further development of novel therapeutic strategies should involve extensive research and assessment of safety and should eliminate risk factors associated with the transplant before it is introduced in the clinical environment.
Conclusion
The goal in treating IBD remains the same: to achieve deep remission and halt any ongoing disease progression. Hematopoietic and mesenchymal stem cells have been shown to be a potential alternative therapy for disease control in refractory CD. Continued in-depth investigation is warranted to fully understand the complex cellular mechanisms. The information from current ongoing phase III clinical trials will provide a valuable roadmap for the future of stem-cell therapy. Cellular therapy must not be limited to HSCs or MSCs, other cellular therapies should continue to be explored in a preclinical setting. Future investigation must focus on perfecting safety and feasibility, with the goal of improving quality of life for the patient.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Natalie E. Duran, Center for Inflammatory Bowel Diseases, Melvin and Bren Simon Digestive Diseases Center, David Geffen School of Medicine, University of California at Los Angeles, 10945 Le Conte Avenue #2338D, Los Angeles, CA 90095, USA.
Daniel W. Hommes, Department of Digestive Diseases, David Geffen School of Medicine, UCLA, Los Angeles, CA, USA
References
- Anderson C., Boucher G, Lees C., Franke A., D’Amato M., Taylor K., et al. (2011) Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 43: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankrum J., Ong J., Karp J. (2014) Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotech 32: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augello A., Tasso R., Negrini S., Amateis A., Indiveri F., Cancedda R., et al. (2005) Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol 35: 1482–1490. [DOI] [PubMed] [Google Scholar]
- Baron F., Hermanne J., Dowlati A., Weber T., Thiry A., Fassotte M., et al. (1998) Bronchiolitis obliterans organizing pneumonia and ulcerative colitis after allogeneic bone marrow transplantation. Bone Marrow Transpl 21: 951–954. [DOI] [PubMed] [Google Scholar]
- Bartholomew A., Sturgeon C., Siatskas M., Ferrer K., Mcintosh K., Patil S., et al. (2002) Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30: 42–48. [DOI] [PubMed] [Google Scholar]
- Baumgart D., Sandborn W. (2012) Crohn’s disease. Lancet 380: 1590–1605. [DOI] [PubMed] [Google Scholar]
- Beaugerie L., Itzkowitz S.H. (2015) Cancers complicating inflammatory bowel disease. New England J Med 372: 1441–1452. [DOI] [PubMed] [Google Scholar]
- Bekkum D. (2000) Immune ablation and stem-cell therapy in autoimmune disease. experimental basis for autologous stem-cell transplantation. Arthritis Res 2: 281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo M., Zaffaroni N., Novara F., Cometa A., Avanzini M., Moretta A., et al. (2007) Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res 67: 9142–9149. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K., Davis B., Binion D. (2009) Pain and inflammatory bowel disease. Inflamm Bowel Dis 15: 778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman P., Sweetenham J., Loberiza F., Taghipour G., Lazarus H., Rizzo J., et al. (2003) Syngeneic hematopoietic stem-cell transplantation for non-Hodgkin’s lymphoma: a comparison with allogeneic and autologous transplantation-the lymphoma working committee of the international bone marrow transplant registry and the European group for blood and marrow transplantation. J Clin Oncol 21: 3744–3753. [DOI] [PubMed] [Google Scholar]
- Bjorkman P., Saper M., Samraoui B., Bennett W., Strominger J., Wiley D. (1987) Structure of the human class I histocompatibility antigen, HLA-A2. Nature 329: 506–512. [DOI] [PubMed] [Google Scholar]
- Brandenberger R. (2011) Cell therapy bioprocessing BioProcess Int 30–37. [Google Scholar]
- Broberger O., Perlmann P. (1959) Autoantibodies in human ulcerative colitis. J Exp Med 110: 657–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt R., Burns W., Hess A. (1995) Bone marrow transplantation for multiple sclerosis. Bone Marrow Transpl 16: 1–6. [PubMed] [Google Scholar]
- Burt R., Craig R., Milanetti F., Quigley K., Gozdziak P., Bucha J., et al. (2010) Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn’s disease: long-term follow up. Blood 116: 6123–6132. [DOI] [PubMed] [Google Scholar]
- Cassinotti A., Annaloro C., Ardizzone S., Onida F., Della Volpe A., Clerici M., et al. (2008) Autologous haematopoietic stem cell transplantation without CD34+ cell selection in refractory Crohn’s disease. Gut 57: 211–217. [DOI] [PubMed] [Google Scholar]
- Cassinotti A., Onida F., Annaloro C., Sampietro G., Fociani P., Fichera M., et al. (2012) P362 autologous haematopoietic stem cell transplantation without cd34+ cell selection for refractory Crohn’s disease: the Milan experience after 5 years. J Crohns Colitis 6: S153–S154. [Google Scholar]
- Castro J., Benich H., Smith L., Kalter S., Bachier C., Meneghetti C., et al. (1996) Prolonged clinical remission in patients with inflammatory bowel disease (IBD) after high dose chemotherapy (HDC) and autologous blood stem cell transplantation. Blood 88: 133A. [Google Scholar]
- Chassaing B., Darfeuille-Michaud A. (2011) The Commensal Microbiota and Enteropathogens in the Pathogenesis of Inflammatory Bowel Diseases. Gastroenterology 140: 1720–1728. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R., Bernardo M., Sgarella A., Maccario R., Avanzini M., Ubezio C., et al. (2011) Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut 60: 788–798. [DOI] [PubMed] [Google Scholar]
- Clerici M., Cassinotti A., Onida F., Trabattoni D., Annaloro C., Della Volpe A., et al. (2011) Immunomodulatory effects of unselected haematopoietic stem cells autotransplantation in refractory Crohn’s disease. Dig Liver Dis 43: 946–952. [DOI] [PubMed] [Google Scholar]
- Cosnes J., Gower-Rousseau C., Seksik P., Cortot A. (2011) Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 140: 1785–1794. [DOI] [PubMed] [Google Scholar]
- Da Silva Meirelles L., Chagastelles P., Nardi N. (2006) Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119: 2204–2213. [DOI] [PubMed] [Google Scholar]
- Daikeler T., Hugle T., Farge D., Andolina M., Gualandi F., Baldomero H., et al. (2009) allogeneic hematopoietic sct for patients with autoimmune diseases. Bone Marrow Transpl 44: 27–33. [DOI] [PubMed] [Google Scholar]
- Das K., Biancone L. (2008) Is IBD an autoimmune disorder? Inflamm Bowel Dis 14:S97–S101. [DOI] [PubMed] [Google Scholar]
- De La Portilla F., Alba F., Garcia-Olmo D., Herrerias J., Gonzalez F., Galindo A. (2013) Expanded allogeneic adipose-derived stem cells (eascs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis 28: 313–323. [DOI] [PubMed] [Google Scholar]
- Di Nicola M., Carlo-Stella C., Magni M., Milanesi M., Longoni P., Matteucci P., et al. (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99: 3838–3843. [DOI] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317. [DOI] [PubMed] [Google Scholar]
- Drakos P., Nagler A., Or R. (1993) Case of Crohn’s disease in bone marrow transplantation. Am J Hematol 43: 157–158. [DOI] [PubMed] [Google Scholar]
- Duerr R., Targan S., Landers C., Larusso N., Lindsay K., Wiesner R., et al. (1991) Neutrophil cytoplasmic antibodies: a link between primary sclerosing cholangitis and ulcerative colitis. Gastroenterology 100: 1385–1391. [PubMed] [Google Scholar]
- Duijvestein M., Molendijk I., Roelofs H., Vos A., Verhaar A., Reinders M., et al. (2011) Mesenchymal stromal cell function is not affected by drugs used in the treatment of inflammatory bowel disease. Cytotherapy 13: 1066–1073. [DOI] [PubMed] [Google Scholar]
- Duijvestein M., Vos A., Roelofs H., Wildenberg M., Wendrich B., Verspaget H., et al. (2010) Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut 59: 1662–1669. [DOI] [PubMed] [Google Scholar]
- Ebert E., Geng X., Bajpai M., Pan Z., Tatar E., Das K. (2009) Antibody to tropomyosin isoform 5 and complement induce the lysis of colonocytes in ulcerative colitis. Am J Gastroenterology 104: 2996–3003. [DOI] [PubMed] [Google Scholar]
- Farge D., Labopin M., Tyndall A., Fassas A., Mancardi G., Van Laar J., et al. (2010) Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years’ experience from the European group for blood and marrow transplantation working party on autoimmune diseases. Haematologica 95: 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocchi C. (2012) Genes and ‘in-vironment’: how will our concepts on the pathophysiology of inflammatory bowel disease develop in the future? Dig Dis 30: 2–11. [DOI] [PubMed] [Google Scholar]
- Forbes G., Sturm M., Leong R., Sparrow M., Segarajasingam D., Cummins A., et al. (2014) A phase II study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol 12: 64–71. [DOI] [PubMed] [Google Scholar]
- Franke A., Balschun T., Karlsen T., Sventoraityte J., Nikolaus S., Mayr G., et al. (2008) Sequence variants in il10, arpc2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet 40: 1319–1323. [DOI] [PubMed] [Google Scholar]
- Franke A., McGovern D., Barrett J., Wang K., Radford-Smith G., Ahmad T., et al. (2010) Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet 42: 1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein A., Chailakhyan R., Latsinik N., Panasyuk A., Keiliss-Borok I. (1974) Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 17: 331–340. [DOI] [PubMed] [Google Scholar]
- Gazouli M., Roubelakis M., Theodoropoulos G. (2014) Stem cells as potential targeted therapy for inflammatory bowel disease. Inflamm Bowel Dis 20: 952–955. [DOI] [PubMed] [Google Scholar]
- Geng X., Liu J., Lin J., Taniguchi M., Kosa E., Glazier K., et al. (2001) Autoimmunity against human tropomyosin isoform 5 (HTM5) in ulcerative colitis: antigen specific T cell response against the c terminal domain of HTM5. Gastroenterology 120: A520–A521. [Google Scholar]
- Ghosh S., Mitchell R. (2007) Impact of inflammatory bowel disease on quality of life: results of the European federation of Crohn’s and ulcerative colitis associations (EFCCA) patient survey. J Crohns Colitis 1: 10–20. [DOI] [PubMed] [Google Scholar]
- Gratwohl A., Passweg J., Bocelli-Tyndall C., Fassas A., Van Laar J., Farge D., et al. (2005) Autologous hematopoietic stem cell transplantation for autoimmune diseases. Bone Marrow Transpl 35: 869–879. [DOI] [PubMed] [Google Scholar]
- Gurdon J. (1962) The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol 10: 622–640. [PubMed] [Google Scholar]
- Hanauer S., Feagan B., Lichtenstein G., Mayer L., Schreiber S., Colombel J., et al. (2002) Maintenance infliximab for Crohn’s disease: the Accent I randomised trial. Lancet 359: 1541–1549. [DOI] [PubMed] [Google Scholar]
- Haque R., Lei F., Xiong X., Bian Y., Zhao B., Wu Y., et al. (2012) Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity. J Immunol 189: 1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselblatt P., Drognitz K., Potthoff K., Bertz H., Kruis W., Schmidt C., et al. (2012) Remission of refractory Crohn’s disease by high-dose cyclophosphamide and autologous peripheral blood stem cell transplantation. Aliment Pharmacol Ther 36: 725–735. [DOI] [PubMed] [Google Scholar]
- Hawkey C. (2014) OC-007 haemopoetic stem cell transplantation for severe resistant Crohn’s disease: preliminary evidence for durable benefit. Gut 63: A4. [Google Scholar]
- Hawkey C., Allez M., Clark M., Labopin M., Lindsay J., Ricart E., et al. (2015) Autologous hematopoetic stem cell transplantation for refractory crohn disease: a randomized clinical trial. JAMA 314: 2524–2534. [DOI] [PubMed] [Google Scholar]
- Hellers G., Bergstrand O., Ewerth S., Holmstrom B. (1980) Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut 21: 525–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson B., Gokhale R., Cho J. (2002) Clinical aspects and pathophysiology of inflammatory bowel disease. Clinical Microbiology Reviews 15: 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop J., Hammond T., Santeramo I., Tort Piella A., Hopp I., Zhou J., et al. (2015) Concise review: workshop review: understanding and assessing the risks of stem cell-based therapies. Stem Cells Transl Med 4: 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommes D., Colombel J., Emery P., Greco M., Sandborn W. (2012) Changing Crohn’s disease management: need for new goals and indices to prevent disability and improve quality of life. J Crohns Colitis 6: S224–S234. [DOI] [PubMed] [Google Scholar]
- Hommes D., Lacey P. (2016) Stem cells: HSCT for Crohn’s disease: work in progress or a bridge too far? Nat Rev Gastroenterol Hepatol 13: 128–130. [DOI] [PubMed] [Google Scholar]
- Hwang J., Varma M. (2008) Surgery for inflammatory bowel disease. World J Gastroenterol 14: 2678–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S. (2001) Treatment of autoimmune diseases by hematopoietic stem cell transplantation. Exp Hematol 29: 661–669. [DOI] [PubMed] [Google Scholar]
- Iwasaki T. (2004) Recent advances in the treatment of graft-versus-host disease. Clin Med Res 2: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs P., Vincent M., Martell R. (1986) Prolonged remission of severe refractory rheumatoid arthritis following allogeneic bone marrow transplantation for drug-induced aplastic anaemia. Bone Marrow Transpl 1: 237–239. [PubMed] [Google Scholar]
- Janeway C., Travers P., Walport M., Shlomchik M. (2001) Immunobiology: The Immune System In Health And Disease, 5th edition Garland Science: New York. [Google Scholar]
- Jauregui-Amezaga A., Rovira M., Pinó Donnay S., Marín P., Feu F., Elizalde J., et al. (2014) P471 hematopoietic stem cell transplantation in refractory Crohn’s disease: feasibility and toxicity. J Crohns Colitis 8: S263–S263. [Google Scholar]
- Kashyap A. (1998) Autologous bone marrow transplantation for non-hodgkin’s lymphoma resulting in long-term remission of coincidental Crohn’s disease. Brit J Haematol 103: 651–652. [DOI] [PubMed] [Google Scholar]
- Kavanagh D., Kalia N. (2011) Hematopoietic stem cell homing to injured tissues. Stem Cell Rev 7: 672–682. [DOI] [PubMed] [Google Scholar]
- Knoepfler P. (2009) Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells (Dayton, Ohio) 27: 1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer S., Williams R., Wilson I., Strickland R. (1975) Lymphocytotoxic antibody in inflammatory bowel disease: a family study. New England J Med 293: 1117–1120. [DOI] [PubMed] [Google Scholar]
- Krampera M., Glennie S., Dyson J., Scott D., Laylor R., Simpson E., et al. (2003) Bone marrow mesenchymal stem cells inhibit the response of naïve and memory antigen-specific T cells to their cognate peptide. Blood 101: 3722–3729. [DOI] [PubMed] [Google Scholar]
- Lanzoni G., Roda G., Belluzzi A., Roda E., Bagnara G. (2008) Inflammatory bowel disease: moving toward a stem cell-based therapy. World J Gastroenterol 14: 4616–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca A., Piaggio G., Podesta M., Pitto A., Bruno B., Di Grazia C., et al. (2006) Boost of CD34+-selected peripheral blood cells without further conditioning in patients with poor graft function following allogeneic stem cell transplantation. Haematologica 91: 935–940. [PubMed] [Google Scholar]
- Lazebnik L., Knyazev O., Parfenov A., Ruchkina I., Shcherbakov P., Khomeriki S., et al. (2012a) Optimization of cell therapy in patients with inflammatory bowel diseases. Ter Arkh 84: 10–17. [PubMed] [Google Scholar]
- Lazebnik L., Lychkova A., Knyazev O. (2012b) Treatment of experimental ulcerative colitis. Bull Exp Biol Med 153: 889–892. [DOI] [PubMed] [Google Scholar]
- Le Blanc K., Tammik C., Rosendahl K., Zetterberg E., Ringden O. (2003a) HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 31: 890–896. [DOI] [PubMed] [Google Scholar]
- Le Blanc K., Tammik L., Sundberg B., Haynesworth S., Ringden O. (2003b) Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 57: 11–20. [DOI] [PubMed] [Google Scholar]
- Lee S., Vogelsang G., Flowers M. (2003) Chronic graft-versus-host disease. Biol Blood Marrow Transpl 9: 215–233. [DOI] [PubMed] [Google Scholar]
- Lee W., Park K., Cho Y., Yoon S., Song K., Kim Do S., et al. (2013) Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells 31: 2575–2581. [DOI] [PubMed] [Google Scholar]
- Lehmann W. (2014) Athersys announces results from phase II study of multistem(r) cell therapy for ulcerative colitis; multistem demonstrates good tolerability and safety profile but fails to show efficacy over 8 weeks in patients with chronic, advanced ulcerative colitis. Press release Athersys Inc. Available at: Athersys.com.
- Liang J., Zhang H., Wang D., Feng X., Wang H., Hua B., et al. (2012) Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut 61: 468–469. [DOI] [PubMed] [Google Scholar]
- Lopez-Cubero S., Sullivan K., McDonald G. (1998) Course of Crohn’s disease after allogeneic marrow transplantation. Gastroenterology 114: 422–440. [DOI] [PubMed] [Google Scholar]
- Lowenthal R., Cohen M., Atkinson K., Biggs J. (1993) Apparent cure of rheumatoid arthritis by bone marrow transplantation. J Rheumatol 20: 137–140. [PubMed] [Google Scholar]
- Macias M., Grande J., Moreno A., Dominguez I., Bornstein R., Flores A. (2010) Isolation and characterization of true mesenchymal stem cells derived from human term decidua capable of multilineage differentiation into all 3 embryonic layers. Am J Obstet Gynecol 203: 495, e499,, e423. [DOI] [PubMed] [Google Scholar]
- Marti J., Mayordomo J., Isla M., Saenz A., Escudero P., Tres A. (2001) PBSC autotransplant for inflammatory bowel disease (IBD): a case of ulcerative colitis. Bone Marrow Transpl 28: 109–110. [DOI] [PubMed] [Google Scholar]
- Martinez-Montiel Mdel P., Gomez-Gomez G., Flores A. (2014) Therapy with stem cells in inflammatory bowel disease. World J Gastroenterol 20: 1211–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum J. (2014) Mesoblast provides update on clinical programs of prochymal for Crohn’s disease and acute graft versus host disease. Mesoblast Ltd. press release 28 April 2014. Available at: mesoblast.com.
- Melief S., Schrama E., Brugman M., Tiemessen M., Hoogduijn M., Fibbe W., et al. (2013) Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells 31: 1980–1991. [DOI] [PubMed] [Google Scholar]
- Mitchell A., Guyatt G., Singer J., Irvine E., Goodacre R., Tompkins C., et al. (1988) Quality of life in patients with inflammatory bowel disease. J Clin Gastroenterol 10: 306–310. [DOI] [PubMed] [Google Scholar]
- Molendijk I., Bonsing B., Roelofs H., Peeters K., Wasser M., Dijkstra G., et al. (2015) Allogeneic bone marrow-derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn’s disease. Gastroenterology 149: 918–27, e6. [DOI] [PubMed] [Google Scholar]
- Molendijk I., Nuij V., Van Der Meulen-De Jong A., Van Der Woude C. (2014) Disappointing durable remission rates in complex Crohn’s disease fistula. Inflamm Bowel Dis 20: 2022–2028. [DOI] [PubMed] [Google Scholar]
- Molodecky N., Soon I., Rabi D., Ghali W., Ferris M., Chernoff G., et al. (2012) Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142: 46–54 e42, quiz e30. [DOI] [PubMed] [Google Scholar]
- Nauta A., Westerhuis G., Kruisselbrink A., Lurvink E., Willemze R., Fibbe W. (2006) Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 108: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken J., Gallup D., Hanson J., Pandak M., Custer L. (2006) Successful outpatient treatment of refractory Crohn’s disease using adult mesenchymal stem cells. American College of Gastroenterology Conference: Abstract 121. [Google Scholar]
- Ordas I., Eckmann L., Talamini M., Baumgart D., Sandborn W. (2012) Ulcerative colitis. Lancet 380: 1606–1619. [DOI] [PubMed] [Google Scholar]
- Oyama Y., Craig R., Traynor A., Quigley K., Statkute L., Halverson A., et al. (2005) Autologous hematopoietic stem cell transplantation in patients with refractory Crohn’s disease. Gastroenterology 128: 552–563. [DOI] [PubMed] [Google Scholar]
- Pasquini M., Zhu X. (2015) Current use and outcome of hematopoietic stem cell transplantation: 2014 CIBMTR summary slides. Available at: http://www.cibmtr.org.
- Peyrin-Biroulet L., Lemann M. (2011) Review article: remission rates achievable by current therapies for inflammatory bowel disease. Aliment Pharmacol Ther 33: 870–879. [DOI] [PubMed] [Google Scholar]
- Pittenger M., Mackay A., Beck S., Jaiswal R., Douglas R., Mosca J., et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147. [DOI] [PubMed] [Google Scholar]
- Raina P. (2007) Evaluation of Prochymal® adult human stem cells for treatment-resistant moderate-to-severe Crohn’s disease. ClinicalTrials.gov identifier: NCT00482092. [Google Scholar]
- Ramirez R., Fleshner P. (2006) Reoperative inflammatory bowel disease surgery. Clin Colon Rectal Surg 19: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattue P. (2012) Prochymal - first stem cell drug approved. Medical News Today 22 May 2012. [Google Scholar]
- Reya T., Morrison S., Clarke M., Weissman I. (2001) Stem cells, cancer, and cancer stem cells. Nature 414: 105–111. [DOI] [PubMed] [Google Scholar]
- Rogler G., Vavricka S., Schoepfer A., Lakatos P. (2013) Mucosal healing and deep remission: what does it mean? World J Gastroenterol 19: 7552–7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallerfors B., Olofsson T. (1992) Granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) secretion by adherent monocytes measured by quantitative immunoassays. Eur J Haematol 49: 199–207. [DOI] [PubMed] [Google Scholar]
- Satterthwaite A., Burn T., Le Beau M., Tenen D. (1992) Structure of the gene encoding CD34, a human hematopoietic stem cell antigen. Genomics 12: 788–794. [DOI] [PubMed] [Google Scholar]
- Scalapino K., Daikh D. (2009) Suppression of glomerulonephritis in NZB/NZW lupus prone mice by adoptive transfer of ex vivo expanded regulatory T cells. PLoS One 4: e6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharl M., Rogler G. (2012) Inflammatory bowel disease pathogenesis: what is new? Curr Opin Gastroenterology 28: 301–309. [DOI] [PubMed] [Google Scholar]
- Schu S., Nosov M., O’Flynn L., Shaw G., Treacy O., Barry F., et al. (2012) Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med 16: 2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D., Loftus E., Tremaine W., Panaccione R., Harmsen W., Zinsmeister A., et al. (2002) The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology 122: 875–880. [DOI] [PubMed] [Google Scholar]
- Sensebe L., Krampera M., Schrezenmeier H., Bourin P., Giordano R. (2010) Mesenchymal stem cells for clinical application. Vox Sang 98: 93–107. [DOI] [PubMed] [Google Scholar]
- Shah N., Kammermeier J., Elawad M., Glocker E. (2012) Interleukin-10 and interleukin-10-receptor defects in inflammatory bowel disease. Curr Allergy Asthma Rep 12: 373–379. [DOI] [PubMed] [Google Scholar]
- Simmons D., Satterthwaite A., Tenen D., Seed B. (1992) Molecular cloning of a CDNA encoding CD34, a sialomucin of human hematopoietic stem cells. J Immunol 148: 267–271. [PubMed] [Google Scholar]
- Smith B., Tang K., Nutbeam D. (2006) WHO health promotion glossary: new terms. Health Promot Int 21: 340–345. [DOI] [PubMed] [Google Scholar]
- Sonwalkar S., James R., Ahmad T., Zhang L., Verbeke C., Barnard D., et al. (2003) Fulminant Crohn’s colitis after allogeneic stem cell transplantation. Gut 52: 1518–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J., Mayhew C., Rankin S., Kuhar M., Vallance J., Tolle K., et al. (2011) Directed Differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers A. (1984) Ulcerative colitis after bone-marrow transplantation for acute leukemia. N Engl J Med 311: 1259. [DOI] [PubMed] [Google Scholar]
- Stappenbeck T., Miyoshi H. (2009) The role of stromal stem cells in tissue regeneration and wound repair. Science 324: 1666–1669. [DOI] [PubMed] [Google Scholar]
- Swenson E., Theise N. (2010) Stem cell therapeutics: potential in the treatment of inflammatory bowel disease. Clin Exp Gastroenterol 3: 1–10. [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676. [DOI] [PubMed] [Google Scholar]
- Tarte K., Gaillard J., Lataillade J., Fouillard L., Becker M., Mossafa H., et al. (2010) Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 115: 1549–1553. [DOI] [PubMed] [Google Scholar]
- Thomas A., Lodhia N. (2014) Advanced therapy for inflammatory bowel disease: a guide for the primary care physician. J Am Board Fam Med 27: 411–420. [DOI] [PubMed] [Google Scholar]
- Van Bekkum D. (2000) Stem cell transplantation in experimental models of autoimmune disease. J Clin Immunol 20: 10–16. [DOI] [PubMed] [Google Scholar]
- Van Deen W. (2012) Hematopoietic Stem Cells: New Research. Montgomery W., Burton H. (eds). Nova Science Publishers. [Google Scholar]
- Vester-Andersen M., Prosberg M., Jess T., Andersson M., Bengtsson B., Blixt T., et al. (2014) Disease course and surgery rates in inflammatory bowel disease: a population-based, 7-year follow-up study in the era of immunomodulating therapy. Am J Gastroenterol 109: 705–714. [DOI] [PubMed] [Google Scholar]
- Wen Z., Fiocchi C. (2004) Inflammatory bowel disease: autoimmune or immune-mediated pathogenesis? Clin Dev Immunol 11: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G., Notley C., Xue S., Bendle G., Holler A., Schumacher T., et al. (2009) Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Nat Acad Sci USA 106: 19078–19083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Jowitt S. (1992) Resolution of immune-mediated diseases following allogeneic bone marrow transplantation for leukaemia. Bone Marrow Transpl 9: 31–33. [PubMed] [Google Scholar]
- Zand A., Van Deen W., Inserra E., Hall L., Kane E., Centeno A., et al. (2015) Presenteeism in inflammatory bowel diseases: a hidden problem with significant economic impact. Inflamm Bowel Dis 21: 1623–1630. [DOI] [PubMed] [Google Scholar]
- Zuk P., Zhu M., Ashjian P., De Ugarte D., Huang J., Mizuno H., et al. (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13: 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]