Abstract
Background:
Whether low-dose azathioprine (AZA) is effective in maintaining remission in patients with steroid-dependent ulcerative colitis (UC) remains unclear. We assessed the efficacy and safety of low-dose AZA in a Chinese population with UC.
Methods:
We identified steroid-dependent UC patients in clinical remission on AZA maintenance therapy from a territory-wide IBD Registry. Standard- and low-dose AZA were defined as at least 2 mg/kg/day and less than 2 mg/kg/day, respectively. Relapse rates were analyzed by Kaplan–Meier analysis and compared using log-rank test.
Results:
Among 1226 UC patients, 128 (53% male, median duration on AZA 44 months) were included. Median maintenance AZA dose was 1.3 mg/kg/day. 97.7% of the patients were on concomitant oral 5-aminosalicylic acid. Cumulative relapse-free rates in patients on standard-dose and low-dose AZA were 71.2%, 52.8% and 45.2%, and 71.8%, 55.3% and 46.2% at 12, 24 and 36 months, respectively (p = 0.871). Relapse rate within 12 months was higher in patients who withdrew compared with those who maintained on AZA (52.6% versus 29.4%; p = 0.045). Mean corpuscular volume increased after AZA therapy in both of the low-dose [median (interquartile range, IQR): 88.2 (81.4–92.2) versus 95.1 (90.1–100.9) fl, p < 0.001] and standard-dose subgroups [median (IQR) 86.8 (76.9–89.9) versus 94.7 (85.9–99.7) fl, p < 0.001]. Leukopenia occurred in 21.1% of the patients. Patients on standard dose had a higher risk for leukopenia than those on low-dose AZA [odds ratio (OR) 3.9, 95% CI 1.9–8.2, p < 0.001].
Conclusions:
In the Chinese population, low-dose AZA is effective for maintaining remission in steroid-dependent UC patients. Standard-dose AZA was associated with more than threefold increased risk of leukopenia.
Keywords: azathioprine, low dose, steroid-dependent ulcerative colitis
Introduction
The immunomodulating drug, azathioprine (AZA) is effective for maintenance of remission [Timmer et al. 2012] and recommended in most guidelines as a first-line therapy for steroid-dependent ulcerative colitis (UC) [Mowat et al. 2011; Dignass et al. 2012b]. The recommended maintenance dose of AZA is 2.0 to 2.5 mg/kg/day [Mowat et al. 2011; Dignass et al. 2012b], as supported by several small-scale randomized controlled trials showing that maintaining on such dose of AZA is effective in preventing disease relapse [Jewell and Truelove, 1974; Sood et al. 2002]. Dose-response effect of AZA remains unclear. A recent meta-analysis investigating AZA for maintenance of remission in Crohn’s disease [Chande et al. 2015] showed a significant difference favoring 2 mg/kg/day AZA over placebo, whereas no statistically significant differences were found between AZA and placebo for the 2.5 mg/kg/day or 1 mg/kg/day subgroups. However, significantly more serious adverse events were observed in patients on higher doses of AZA.
Studies from Asia have shown that myelotoxicity occurred in approximately 20–40% of patients receiving AZA for treatment of inflammatory bowel disease (IBD) [Hibi et al. 2003; Huang, 2009; Kim et al. 2009; Gao et al. 2012; Ran et al. 2012]. This figure is much higher than that (around 7%) reported in studies from western countries [Gisbert and Gomollon, 2008]. Therefore, most physicians in Asia tend to start AZA at a lower dose and gradually titrate in order to avoid side effects [Huang, 2009; Kim et al. 2009]. Several studies from Asian countries showed that low-dose AZA is effective for maintaining remission in UC patients [Hibi et al. 2003; Kim et al. 2009; Gao et al. 2012; Ran et al. 2012]. However, the efficacy of low-dose AZA in maintaining remission in steroid-dependent UC patients remains unclear due to limited number of small-scale studies, heterogeneous population and short-term follow up.
In this multicenter long-term observational study, we aimed to investigate the efficacy and safety of low-dose AZA in maintaining remission in Chinese patients with steroid-dependent UC, based on data from a territory-wide IBD registry.
Methods
Steroid-dependent UC patients, defined as patients who were unable to reduce systemic steroids below the equivalent of prednisolone 10 mg/day within 3 months of starting steroids without recurrent active disease, or who had a relapse within 3 months of stopping steroids [Dignass et al. 2012a], diagnosed between 1981 and 2013, were identified from the Hong Kong IBD Registry. This registry represents the first territory-wide IBD registry in the Chinese population. It includes retrospective and prospective data collection of patients with IBD across 13 major public hospitals, and covers over 95% of IBD patients in Hong Kong. The diagnosis of UC was confirmed according to the Lennard-Jones criteria based on clinical symptoms, endoscopy and histology [Lennard-Jones, 1989]. Only patients who were on AZA for at least 4 months and had achieved steroid-free clinical remission for at least 3 months were included. Clinical remission was defined as the 6-point Mayo subscore of 0 (no diarrhea and no rectal bleeding). Clinical relapse was defined as a 6-point Mayo subscore of greater than 0, or increased symptoms that required adjustment or escalation of medication. Severity of disease relapse was assessed according to the Truelove and Witts criteria [Truelove and Witts, 1955]. Leukopenia was defined as a white blood cell count (WBC) less than 3 × 109/l, and severe neutropenia was defined as neutrophil count less than 0.5 × 109/l [Gisbert and Gomollon, 2008]. Recurrent leukopenia was defined as recurrence of leukopenia during AZA therapy (including patients who were rechallenged with AZA after previous discontinuation, and those who were put on the previous dosage at which leukopenia occurred). Hepatotoxicity was defined as an increase in serum alanine transaminase greater than twice the upper normal limit and resolution after withdrawal of treatment or reduction in the dosage [Avallone et al. 2014].
Data collection
Data were extracted retrospectively from patient electronic files into standardized forms specifically designed for the registry. Additional data were obtained from patients’ interviews. Data were entered into the registry database independently by two research staff. Patients’ age, gender, smoking status (current smokers were defined as subjects who still smoked, exsmokers were defined as those who had stopped smoking and nonsmokers were patients who had never smoked at AZA initiation), family history of IBD within first-degree relatives, history of appendectomy, duration of UC at AZA initiation, disease extent (according to the Montreal classification) [Satsangi et al. 2006], duration of AZA maintenance therapy, peak and maintenance dosages of AZA, laboratory markers including complete blood cell count (CBC), erythrocyte sedimentation rate and C-reactive protein, and concomitant aminosalicylic acid (ASA) and allopurinol were recorded.
Statistical analysis
Cumulative relapse-free rates and time to relapse were compared between patients on standard-dose (⩾2 mg/kg/day) and low-dose (<2 mg/kg/day) AZA. Time to relapse was compared between patients who maintained on, and discontinued, AZA. Categorical variables were analyzed using chi-square test or Fisher’s exact test. Continuous variables were analyzed using t-test if they were normally distributed or Mann–Whitney U test if there was a skewed distribution. Cumulative relapse and moderate or severe relapse rates were analyzed by Kaplan–Meier analysis and compared using log-rank test. Two-sided p values of less than 0.05 were considered significant. Statistical analyses were performed with IBM SPSS Statistics 20.0.
Ethical considerations
The study was approved by the ethics committee of each participating center.
Results
Patient characteristics
Among 1226 UC patients within the Hong Kong IBD registry, 278 (22.7%) had steroid-dependent disease. Of 210 patients prescribed with AZA, 49 were not taking AZA for at least 4 months (42 were on low-dose AZA). Of 161 patients on AZA for at least 4 months (median duration on AZA, 44 months), 128 (79.5%) who achieved steroid-free clinical remission for at least 3 months were included. The remaining 33 patients (15 on standard-dose and 18 on low-dose AZA) had not achieved steroid-free clinical remission. The baseline characteristics of included patients are shown in Table 1. Among 35 (27.3%) patients who had reached standard dose, 22 (17.2%) maintained on standard dose. Reasons for the other 13 patients who reached standard dose, but maintained on low dose were leukopenia (n = 7), stable disease (n = 4) and unknown (n = 2).
Table 1.
Baseline characteristics.
| All patients (n = 128) | Maintained on low-dose AZA (n = 106) | Maintained on standard-dose AZA (n = 22) | p value* | |
|---|---|---|---|---|
| Age at diagnosis, median (IQR) (years) | 40 (29–55) | 43 (29–54) | 32 (25–55) | 0.326 |
| Male, n (%) | 68 (53.1) | 59 (55.7) | 9 (40.9) | 0.207 |
| Age at AZA commencement, median (IQR) (years) | 46 (36–59) | 48 (37–59) | 44 (32–57) | 0.505 |
| Duration of UC at AZA initiation, median (IQR) (years) | 3.0 (1.0–8.8) | 3.0 (1.0–8.0) | 3.5 (1.0–9.2) | 0.814 |
| Duration on AZA maintenance therapy, median (IQR) (months) | 44 (21–72) | 46 (22–77) | 33 (14–58) | 0.093 |
| Smoking status at AZA commencement | 0.612 | |||
| Current smoker, n (%) | 3 (2.7) | 3 (3.3) | 0 | |
| Exsmoker, n (%) | 17 (15.5) | 15 (16.3) | 2 (11.1) | |
| Nonsmoker, n (%) | 90 (81.8) | 74 (80.4) | 16 (88.9) | |
| FDR with IBD, n (%) | 4 (3.3) | 3 (3.0) | 1 (4.8) | 0.538 |
| History of appendectomy, n (%) | 3 (2.3) | 0 | 3 (13.6) | 0.005 |
| UC extent | 0.809 | |||
| Proctitis, n (%) | 2 (1.6) | 2 (1.9) | 0 | |
| Left sided colitis, n (%) | 29 (22.7) | 24 (22.6) | 5 (22.7) | |
| Extensive colitis, n (%) | 97 (75.8) | 80 (75.5) | 17 (77.3) | |
| Concomitant ASA, n (%) | 125 (97.7) | 104 (98.1) | 21 (95.5) | 0.435 |
| Concomitant allopurinol, n (%) | 0 | 0 | 0 | – |
| Maintenance dose of AZA, median (IQR) (mg/kg/day) | 1.3 (0.9–1.7) | 1.2 (0.8–1.5) | 2.1 (2.0–2.2) | – |
| Peak dose of AZA, median (IQR) (mg/kg/day) | 1.6 (1.2–2.0) | 1.4 (1.2–1.8) | 2.1 (2.0–2.4) | – |
Patients maintained on low-dose AZA versus standard-dose AZA.
AZA, azathioprine; IQR, interquartile range; UC, ulcerative colitis; FDR, first-degree relative; IBD, inflammatory bowel disease; ASA, aminosalicylic acid.
Efficacy of azathioprine
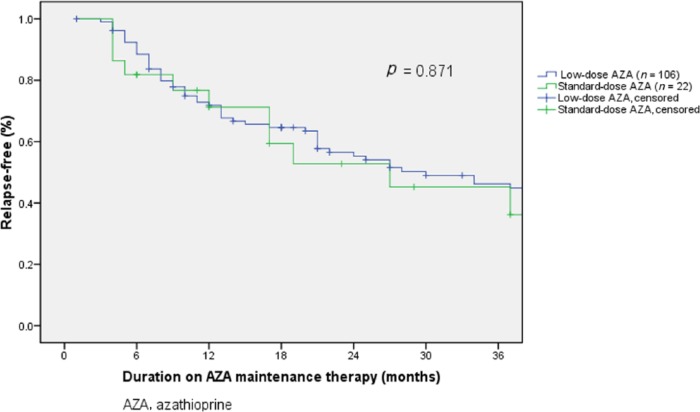
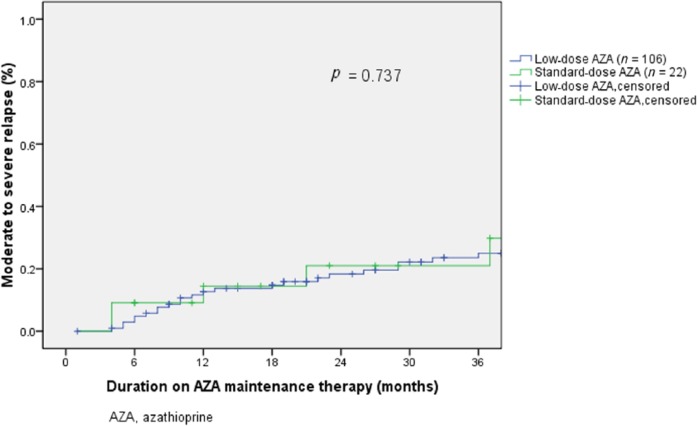
Cumulative relapse-free rates were similar between patients on standard-dose and low-dose AZA (71.2%, 52.8% and 45.2% versus 71.8%, 55.3% and 46.2% during 12, 24 and 36 months, respectively, p = 0.871, Figure 1). Median time to relapse was 17 (IQR 6–31) months in patients on standard-dose, and 21 (IQR 9–44) months among patients on low-dose AZA (p = 0.21). Rates of moderate or severe relapse were not significantly different between standard-dose group and low-dose group: 14.4%, 21.0% and 21.0% versus 12.7%, 18.3% and 25.0% during 12, 24 and 36 months, respectively (p = 0.737, Figure 2).
Figure 1.
Relapse-free rate and azathioprine dose.
Figure 2.
Moderate to severe relapse and azathioprine dose.
Regarding the laboratory markers, mean corpuscular volume (MCV) increased significantly after AZA treatment in both of the low-dose [median (IQR): 88.2 (81.4–92.2) versus 95.1 (90.1–100.9) fl, p < 0.001] and standard-dose subgroups [median (IQR): 86.8 (76.9–89.9) versus 94.7 (85.9–99.7) fl, p < 0.001]. Median lymphocyte counts were 1.3 (IQR 0.9–1.8) × 109/l and 1.3 (IQR 1.0–1.6) × 109/l after low-dose and standard-dose AZA therapy, respectively (p = 0.649).
There were 24 patients who discontinued AZA during clinical remission. The median maintenance dose of AZA was 1.2 (IQR 0.9–1.5) mg/kg/day and the median duration on AZA was 50 (IQR 26–84) months. Relapse rate at 12 months was significantly higher among patients who discontinued AZA than those who maintained on AZA (52.6% versus 29.4%, p = 0.045). Median time to relapse was significantly shorter in patients who discontinued AZA than those who maintained on treatment [10 (IQR 2–15) months versus 15 (IQR 7–36) months, p = 0.012].
During AZA maintenance therapy, one patient on standard-dose AZA (4.5%) and five patients on low-dose AZA (4.7%) used biologics to control active disease (p = 1.000). Among the five patients on low-dose AZA, three patients had myelotoxicity that prevented escalation to standard dose. One patient on standard-dose (5.3%) and five patients on low-dose AZA (4.7%) had colectomy for acute severe UC (p = 1.000).
Side effects
The most frequently observed side effects were leukopenia (n = 27), hepatotoxicity (n = 5), and nausea (n = 5). With a total follow-up duration of 562 person-years, leukopenia occurred in 21.1% of the patients. The incidence rate of leukopenia was 48 per 1000 person-years and the incidence rate of severe neutropenia was 2 per 1000 person-year. The median dosage of AZA at which leukopenia was observed was 1.5 (IQR 1.2–1.8) mg/kg/day. Patients on standard-dose AZA were significantly more likely to have leukopenia (49.1%) than those on low-dose AZA (19.8%) (OR 3.9, 95% CI 1.9–8.2, p < 0.001).
Leukopenia caused AZA discontinuation in 13 (10.2%) patients (temporal withdrawal: n = 11; permanent withdrawal: n = 2). Among the 11 patients who resumed AZA, six had recurrent leukopenia. Of the 14 patients who had leukopenia but did not discontinue AZA, one maintained the AZA dosage and 13 reduced dosage; five patients had recurrent leukopenia. Overall, recurrent leukopenia occurred in 11 out of 25 (44%) patients.
Discussion
This long-term multicenter observational study using data from a territory-wide IBD registry showed that a substantial proportion of steroid-dependent patients with UC (82.8%) were maintained on low-dose AZA. Low-dose AZA was effective in maintaining remission in the Chinese population. Leukopenia is the main side effect, and the rate of recurrent leukopenia is high. The risk for leukopenia increased significantly with higher AZA dose.
Low-dose AZA was effective in our population. Around 70%, 55% and 45% maintained clinical remission at 12, 24 and 36 months, respectively. Although using a strict definition for remission, the relapse rate in our cohort was comparable with that reported in previous studies that investigated the efficacy of standard-dose AZA in maintaining remission (sustained remission rate: 40–76% at 12 months; 45–63% during 3 years) [Jewell and Truelove, 1974; Sood et al. 2000, 2002, 2003; Chebli et al. 2010]. Being consistent with the results of a recent systematic review [Torres et al. 2015], relapse rate in our cohort increased significantly after AZA withdrawal. The proportion of patients requiring escalation to biologics or surgery was around 5%, lower than that reported by studies in which patients maintained on standard-dose AZA [Jharap et al. 2010; Sood et al. 2015].
Leukopenia occurred in 21% of the patients with an incidence rate of 48 per 1000 person-years, higher than 7% and 30 per 1000 person-years, as reported by most studies from western countries [Gisbert and Gomollon, 2008]. Our results were comparable with those from eastern countries [Hibi et al. 2003; Huang, 2009; Kim et al. 2009; Gao et al. 2012; Ran et al. 2012]. The incidence rate of severe neutropenia was 2 per 1000 person-years in our cohort, lower than 9 per 1000 person-years in literature [Gisbert and Gomollon, 2008]. This may be attributed to the common clinical practice of gradual-dose titration and closely monitoring of CBC, through which the dose could be timely adjusted once leukopenia occurred.
There are several reasons for the effectiveness of low-dose AZA in our cohort. First of all, different thiopurine metabolic features between Asians and Caucasians may account for such differences. 6-thioguanine nucleotide (6-TGN) is considered to be the most therapeutic metabolite [Amin et al. 2015]. Higher 6-TGN levels lead to both of the better clinical response and more myelotoxicity [Dubinsky et al. 2000; Osterman et al. 2006; Moreau et al. 2014; Amin et al. 2015]. Roblin and colleagues found a 6-TGN level greater than 250 pmol/8 × 108 red blood cells as the only predictor for favorable clinical remission [Roblin et al. 2005]. A previous study has found that the 6-TGN concentrations in Japanese IBD patients on low-dose thiopurine were comparable with those reported from western countries [Andoh et al. 2008]. Although we were not able to test thiopurine metabolites in this study, we used surrogate markers. Elevated MCV level was reported as a predictor for 6-TGN levels [Kneebone et al. 2014; Kopylov et al. 2015], and lower lymphocyte count was independently associated with therapeutic level of 6-TGN [Kopylov et al. 2015]. The significant rise in MCV and lower lymphocyte count after therapy in our study attested to the probable attainment of high 6-TGN concentrations on low-dose AZA in our cohort. We further analyzed MCV levels among patients after AZA therapy and found that more patients with MCV greater than 90 fl after treatment achieved remission (88.1% versus 70.7% of patients with MCV < 90 fl, p = 0.011). It indicated that higher MCV levels after treatment could predict AZA response. Additionally, over 90% of UC patients in Hong Kong are prescribed ASA (unpublished data from the Hong Kong IBD Registry). For steroid-dependent patients, AZA is added on as an escalation of therapy. The high concomitant use of ASA may contribute to the satisfactory efficacy of low-dose AZA. A multicenter study from Italy found that concomitant ASA use predicted sustained remission during AZA therapy [Cassinotti et al. 2009]. Studies from Japan showed that low-dose thiopurine was effective for maintenance of remission, and all of the patients included were on concomitant ASA [Hibi et al. 2003; Andoh et al. 2008]. Although the anti-inflammatory effect of ASA may account for a part, previous studies have shown that adding ASA to thiopurine led to increased level of 6-TGN [De Boer et al. 2007; De Graaf et al. 2010; Gao et al. 2012].
Our study has several strengths. To the best of the authors’ knowledge, this is the first long-term observational multicenter study investigating the efficacy of low-dose AZA in maintaining remission in steroid-dependent UC patients. Due to the high myelotoxicity rate caused by AZA therapy among Asians, most practitioners tend to start AZA at a low dose and gradually titrate to a minimal effective dose in order to avoid side effects. We have reinforced the findings of previous studies from our Asian partners that were based on a small sample size from a single hospital [Hibi et al. 2003; Ran et al. 2012], or did not separate UC from Crohn’s disease [Kim et al. 2009]. Our findings support the ‘titration’ strategy during AZA therapy and the probable achievement of high 6-TGN levels on low-dose AZA in our population.
This study also has some limitations. Firstly, no thiopurine metabolic levels were measured, such as thiopurine methyltransferase (TPMT) genotype or phenotype and metabolites, as they are so far not routinely available in Hong Kong. TPMT is a principal enzyme in thiopurine metabolism [Amin et al. 2015]. Although TPMT genetic polymorphisms result in different enzyme activity, TPMT genotyping before initiating thiopurine therapy is of limited use in the Asian population [Takatsu et al. 2009; Yang et al. 2014]. A recent Korean study has discovered that nucleoside diphosphate-linked moiety X-type motif 15 was strongly associated with thiopurine-induced leukopenia in Asians [Yang et al. 2014]. Unfortunately, as these tests are not available in most places in Asia, including Hong Kong, we could not provide these data. Instead of directly measuring 6-TGN, the significant increase of MCV and low lymphocyte-count level in our study indicated the probable high 6-TGN concentrations on low-dose AZA therapy. Our results, which were based on a territory-wide population, reflect the real-life settings of AZA use in clinical practice. As it is shown in a large worldwide survey of IBD experts [Roblin et al. 2011] that limited availability or reimbursement of thiopurine metabolism tests results in relative underutilization of these tests, access to and reimbursement of TPMT phenotype and thiopurine-metabolite monitoring becomes urgent in our country. Routine performance of these tests in our clinical practice should optimize therapeutic strategy in the future. Secondly, most data were retrospectively retrieved; missing data were inevitable. Although patients were followed up with clinical and laboratory assessment regularly (every 3–6 months), we were unable to evaluate drug compliance. It is also possible that several changes in management of AZA therapy have occurred during the wide period of observation (from 1981 to 2013) that may lead to bias. Thirdly, the sample size of patients on standard-dose AZA was small, which made it underpowered to perform statistical analysis for some variables and subgroup analyses. However, the overlapping curves between standard- and low-dose groups suggested that the insignificant difference was unlikely caused by underpower. In addition, our results were derived from a Chinese population with a high rate of concomitant 5-ASA use (97.7%). These results might not be extrapolated to other populations. There may also be some bias with data derived from a registry.
In conclusion, low-dose AZA is effective for maintaining remission in steroid-dependent UC patients in the Chinese population. Leukopenia is common and standard-dose AZA was associated with a more than threefold increased risk of leukopenia. Randomized controlled trials with metabolite monitoring in Asians are needed to further investigate thiopurine metabolism and its optimal use in the Asian population.
Acknowledgments
We acknowledge the help of Tiffany M. L. Chung and Catherine Y. Y. Iu, Mandy Lee and Whitney Tang in coordinating the data collection. We are grateful to Dr. Sai Ho Wong, Dr. Raymond W. H. Tong and all other gastroenterologists and staff at every hospital who have assisted in identification and recruitment of patients. We acknowledge the Jessie and Thomas Tam Foundation and AbbVie Hong Kong for supporting the Hong Kong IBD Registry.
Footnotes
Funding: This work was supported by Jessie and Thomas Tam Foundation and unrestricted educational grant from Abbvie pharmaceuticals, Hong Kong. The study sponsors have no contribution in the study design, analysis, interpretation of data and publication.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Hai Yun Shi, Department of Medicine and Therapeutics, Institute of Digestive Disease, State Key Laboratory of Digestive Disease, LKS Institute of Health Sciences, The Chinese University of Hong Kong, Hong Kong, China Department of Gastroenterology & Hepatology, Beijing Friendship Hospital, Capital Medical University, Beijing Digestive Disease Center, National Clinical Research Center for Digestive Diseases, China.
Francis K. L. Chan, Department of Medicine and Therapeutics, Institute of Digestive Disease, State Key Laboratory of Digestive Disease, LKS Institute of Health Sciences, The Chinese University of Hong Kong, Hong Kong, China
Wai Keung Leung, Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong, China.
Michael K. K. Li, Department of Medicine and Geriatrics, Tuen Mun Hospital, Hong Kong, China
Chi Man Leung, Department of Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong, China.
Shun Fung Sze, Department of Medicine, Queen Elizabeth Hospital, Hong Kong, China.
Jessica Y. L. Ching, Department of Medicine and Therapeutics, Institute of Digestive Disease, State Key Laboratory of Digestive Disease, LKS Institute of Health Sciences, The Chinese University of Hong Kong, Hong Kong, China
Fu Hang Lo, Department of Medicine and Geriatrics, United Christian Hospital, Hong Kong, China.
Steven W. C. Tsang, Department of Medicine, Tseung Kwan O Hospital, Hong Kong, China
Edwin H. S. Shan, Department of Medicine and Geriatrics, Caritas Medical Center, Hong Kong, China
Lai Yee Mak, Department of Medicine, North District Hospital, Hong Kong, China.
Belsy C. Y. Lam, Department of Medicine and Geriatrics, Kwong Wah Hospital, Hong Kong, China
Aric J. Hui, Department of Medicine, Alice Ho Miu Ling Nethersole Hospital, Hong Kong, China
Wai Hung Chow, Department of Medicine, Yan Chai Hospital, Hong Kong, China.
Marc T. L. Wong, Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong, China
Ivan F. N. Hung, Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong, China
Yee Tak Hui, Department of Medicine, Queen Elizabeth Hospital, Hong Kong, China.
Yiu Kay Chan, Department of Medicine and Geriatrics, Caritas Medical Center, Hong Kong, China.
Kam Hon Chan, Department of Medicine, North District Hospital, Hong Kong, China.
Ching Kong Loo, Department of Medicine and Geriatrics, Kwong Wah Hospital, Hong Kong, China.
Carmen K. M. Ng, Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong, China
Wai Cheung Lao, Department of Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong, China.
Marcus Harbord, Department of Gastroenterology, Chelsea and Westminster Hospital, London, UK.
Justin C. Y. Wu, Department of Medicine and Therapeutics, Institute of Digestive Disease, State Key Laboratory of Digestive Disease, LKS Institute of Health Sciences, The Chinese University of Hong Kong, Hong Kong, China
Joseph J. Y. Sung, Department of Medicine and Therapeutics, Institute of Digestive Disease, State Key Laboratory of Digestive Disease, LKS Institute of Health Sciences, The Chinese University of Hong Kong, Hong Kong, China
Siew C. Ng, Department of Medicine and Therapeutics, Institute of Digestive Disease, State Key Laboratory of Digestive Disease, LKS Institute of Health Science, The Chinese University of Hong Kong, Hong Kong, China.
References
- Amin J., Huang B., Yoon J., Shih D. (2015) Update 2014: advances to optimize 6-mercaptopurine and azathioprine to reduce toxicity and improve efficacy in the management of IBD. Inflamm Bowel Dis 21: 445–452. [DOI] [PubMed] [Google Scholar]
- Andoh A., Tsujikawa T., Ban H., Hashimoto T., Bamba S., Ogawa A., et al. (2008) Monitoring 6-thioguanine nucleotide concentrations in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol 23: 1373–1377. [DOI] [PubMed] [Google Scholar]
- Avallone E., Pica R., Cassieri C., Zippi M., Paoluzi P., Vernia P. (2014) Azathioprine treatment in inflammatory bowel disease patients: type and time of onset of side effects. Eur Rev Med Pharmacol Sci 18: 165–170. [PubMed] [Google Scholar]
- Cassinotti A., Actis G., Duca P., Massari A., Colombo E., Gai E., et al. (2009) Maintenance treatment with azathioprine in ulcerative colitis: outcome and predictive factors after drug withdrawal. Am J Gastroenterol 104: 2760–2767. [DOI] [PubMed] [Google Scholar]
- Chande N., Patton P., Tsoulis D., Thomas B., Macdonald J. (2015) Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev: CD000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebli L., Chaves L., Pimentel F., Guerra D., Barros R., Gaburri P., et al. (2010) Azathioprine maintains long-term steroid-free remission through 3 years in patients with steroid-dependent ulcerative colitis. Inflamm Bowel Dis 16: 613–619. [DOI] [PubMed] [Google Scholar]
- De Boer N., Wong D., Jharap B., De Graaf P., Hooymans P., Mulder C., et al. (2007) Dose-dependent influence of 5-aminosalicylates on thiopurine metabolism. Am J Gastroenterol 102: 2747–2753. [DOI] [PubMed] [Google Scholar]
- De Graaf P., De Boer N., Wong D., Karner S., Jharap B., Hooymans P., et al. (2010) Influence of 5-aminosalicylic acid on 6-thioguanosine phosphate metabolite levels: a prospective study in patients under steady thiopurine therapy. Br J Pharmacol 160: 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignass A., Eliakim R., Magro F., Maaser C., Chowers Y., Geboes K., et al. (2012a) Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis 6: 965–990. [DOI] [PubMed] [Google Scholar]
- Dignass A., Lindsay J., Sturm A., Windsor A., Colombel J., Allez M., et al. (2012b) Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 6: 991–1030. [DOI] [PubMed] [Google Scholar]
- Dubinsky M., Lamothe S., Yang H., Targan S., Sinnett D., Théorêt Y., et al. (2000) Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 118: 705–713. [DOI] [PubMed] [Google Scholar]
- Gao X., Zhang F., Ding L., Liu H., Wang X., Chen B., et al. (2012) The potential influence of 5-aminosalicylic acid on the induction of myelotoxicity during thiopurine therapy in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol 24: 958–964. [DOI] [PubMed] [Google Scholar]
- Gisbert J., Gomollon F. (2008) Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol 103: 1783–1800. [DOI] [PubMed] [Google Scholar]
- Hibi T., Naganuma M., Kitahora T., Kinjyo F., Shimoyama T. (2003) Low-dose azathioprine is effective and safe for maintenance of remission in patients with ulcerative colitis. J Gastroenterol 38: 740–746. [DOI] [PubMed] [Google Scholar]
- Huang L. (2009) Current use of immunosuppressive agents in inflammatory bowel disease patients in East China. World Journal of Gastroenterology 15: 3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell D., Truelove S. (1974) Azathioprine in ulcerative colitis: final report on controlled therapeutic trial. Br Med J 4: 627–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jharap B., Seinen M., De Boer N., Van Ginkel J., Linskens R., Kneppelhout J., et al. (2010) Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis 16: 1541–1549. [DOI] [PubMed] [Google Scholar]
- Kim D., Kim Y., Kim B., Chang D., Son H., Rhee P., et al. (2009) The efficacy of low dose azathioprine/6-mercaptopurine in patients with inflammatory bowel disease. Hepatogastroenterology 56: 1395–1402. [PubMed] [Google Scholar]
- Kneebone A., Poon S., Asher R., Jackson R., Gregg B., Kerr S., et al. (2014) Mean corpuscular volume but not lymphocyte count is a predictor of thiopurine dose adequacy and toxicity. Gut 63: A157–A158. [Google Scholar]
- Kopylov U., Battat R., Benmassaoud A., Paradis-Surprenant L., Seidman E. (2015) Hematologic indices as surrogate markers for monitoring thiopurine therapy in IBD. Dig Dis Sci 60: 478–484. [DOI] [PubMed] [Google Scholar]
- Lennard-Jones J. (1989) Classification of inflammatory bowel disease. Scand J Gastroenterol 170: 2–6. [DOI] [PubMed] [Google Scholar]
- Moreau A., Paul S., Del Tedesco E., Rinaudo-Gaujous M., Boukhadra N., Genin C., et al. (2014) Association between 6-thioguanine nucleotides levels and clinical remission in inflammatory disease: a meta-analysis. Inflamm Bowel Dis 20: 464–471. [DOI] [PubMed] [Google Scholar]
- Mowat C., Cole A., Windsor A., Ahmad T., Arnott I., Driscoll R., et al. (2011) Guidelines for the management of inflammatory bowel disease in adults. Gut 60: 571–607. [DOI] [PubMed] [Google Scholar]
- Osterman M., Kundu R., Lichtenstein G., Lewis J. (2006) Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology 130: 1047–1053. [DOI] [PubMed] [Google Scholar]
- Ran W., Ouyang Q., Dong L., Xue L. (2012) [A retrospective analysis of azathioprine in the treatment of 24 patients with refractory ulcerative colitis]. Zhonghua Nei Ke Za Zhi 51: 613–617. [PubMed] [Google Scholar]
- Roblin X., Oussalah A., Chevaux J., Sparrow M., Peyrin-Biroulet L. (2011) Use of thiopurine testing in the management of inflammatory bowel diseases in clinical practice: a worldwide survey of experts. Inflamm Bowel Dis 17: 2480–2487. [DOI] [PubMed] [Google Scholar]
- Roblin X., Serre-Debeauvais F., Phelip J., Faucheron J., Hardy G., Chartier A., et al. (2005) 6-tioguanine monitoring in steroid-dependent patients with inflammatory bowel diseases receiving azathioprine. Aliment Pharmacol Ther 21: 829–839. [DOI] [PubMed] [Google Scholar]
- Satsangi J., Silverberg M., Vermeire S., Colombel J. (2006) The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 55: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A., Kaushal V., Midha V., Bhatia K., Sood N., Malhotra V. (2002) The beneficial effect of azathioprine on maintenance of remission in severe ulcerative colitis. J Gastroenterol 37: 270–274. [DOI] [PubMed] [Google Scholar]
- Sood A., Midha V., Sood N., Avasthi G. (2003) Azathioprine versus sulfasalazine in maintenance of remission in severe ulcerative colitis. Indian J Gastroenterol 22: 79–81. [PubMed] [Google Scholar]
- Sood A., Midha V., Sood N., Kaushal V. (2000) Role of azathioprine in severe ulcerative colitis: one-year, placebo-controlled, randomized trial. Indian J Gastroenterol 19: 14–16. [PubMed] [Google Scholar]
- Sood R., Ansari S., Clark T., Hamlin P., Ford A. (2015) Long-term efficacy and safety of azathioprine in ulcerative colitis. J Crohns Colitis 9: 191–197. [DOI] [PubMed] [Google Scholar]
- Takatsu N., Matsui T., Murakami Y., Ishihara H., Hisabe T., Nagahama T., et al. (2009) Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol 24: 1258–1264. [DOI] [PubMed] [Google Scholar]
- Timmer A., McDonald J., Tsoulis D., MacDonald J. (2012) Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev: CD000478. [DOI] [PubMed] [Google Scholar]
- Torres J., Boyapati R., Kennedy N., Louis E., Colombel J., Satsangi J. (2015) Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology 149: 1716–1730. [DOI] [PubMed] [Google Scholar]
- Truelove S., Witts L. (1955) Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 2: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Hong M., Baek J., Choi H., Zhao W., Jung Y., et al. (2014) A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet 46: 1017–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]