Abstract
Background:
We have investigated the effects of a multispecies probiotic preparation containing a combination of probiotic bacterial genera that included Bifidobacteria, Lactobacilli and a Streptococcus in a mouse model of high-fat diet or obesity-induced liver steatosis.
Methods:
Three groups of C57B1/6J mice were fed either a standard chow or a high-fat diet for 20 weeks, while a third group was fed a high-fat diet for 10 weeks and then concomitantly administered probiotics for a further 10 weeks. Serum, liver and large bowel samples were collected for analysis.
Results:
The expression of the tight-junction proteins ZO-1 and ZO-2 was reduced (p < 0.05) in high-fat diet-fed mice compared to chow-fed mice. Probiotic supplementation helped to maintain tight ZO-1 and ZO-2 expression compared with the high-fat diet group (p < 0.05), but did not restore ZO-1 or ZO-2 expression compared with chow-fed mice. Mice fed a high-fat diet ± probiotics had significant steatosis development compared with chow-fed mice (p < 0.05); steatosis was less severe in the probiotics group compared with the high-fat diet group. Hepatic triglyceride concentration was higher in mice fed a high-fat diet ± probiotics compared with the chow group (p < 0.05), and was lower in the probiotics group compared with the high-fat diet group (p < 0.05). Compared with chow-fed mice, serum glucose, cholesterol concentration and the activity of alanine transaminase were higher (p < 0.05), whereas serum triglyceride concentration was lower (p < 0.05) in mice fed a high-fat diet ± probiotics.
Conclusions:
Supplementation with a multispecies probiotic formulation helped to maintain tight-junction proteins ZO-1 and ZO-2, and reduced hepatic triglyceride concentration compared with a high-fat diet alone.
Keywords: dysbiosis, nonalcoholic fatty liver disease, probiotics, steatosis, tight-junction proteins
Introduction
Nonalcoholic fatty-liver disease (NAFLD) has been estimated to affect as much as 34% of the developed world’s population [Browning et al. 2004; Bedogni et al. 2005]. A leading cause of NAFLD development is the overconsumption of calories [Marchesini et al. 2008]. Dividing the population into lean and obese groups highlights the correlation between NAFLD and obesity. NAFLD has been found in 14% of lean individuals, whereas it afflicts 80-90% of obese individuals [Bellentani et al. 2010; Niaz et al. 2011]. With childhood obesity rates increasing, the prevalence of NAFLD is expected to rise, increasing the burden of fatty liver disease.
Recently, probiotics have been touted as a possible treatment option for NAFLD. Although traditionally recommended for individuals with intestinal disorders, it is emerging that probiotics may have an overarching role in treating diseases of the gastrointestinal (GI) tract. As such, probiotic bacteria have been proposed to prevent or treat NAFLD by providing signals and immune surveillance that regulates the microbiota through cytokine production [Tilg et al. 2000; Hoek and Pastorino, 2004; Mykhal’chyshyn et al. 2013; Alisi et al. 2014; Vitetta et al. 2014; Sepideh et al. 2015]. A proposed mechanistic hypothesis is that probiotics may reduce inflammation by reducing intestinal permeability, preventing the passage of pathogens (or bacterial byproducts such as lipopolysaccharides) [Shing et al. 2014] from passing across the GI tract epithelial barrier.
It has been posited that the attenuation of intestinal permeability by probiotics is due to maintaining or increasing the concentration of tight-junction proteins [(TJPs): ZO-1, ZO-2, PKC-ζ] at the intestinal cell membrane [Parassol et al. 2005; Zyrek et al. 2007]. In addition to the intestine, probiotics may also help to maintain liver function by reducing inflammation through reregulating cytokine production, particularly proinflammatory cytokines that affect the liver (i.e. TNF–α, IL–6 and TGF–β) [Tilg et al. 2000; Hoek and Pastorino, 2004; Hegazy and El-Bedewy, 2010; Rodes et al. 2013]. TGF–β in particular, may activate hepatic stellate cells, leading to fibrogenesis [Khimji et al. 2008]. Probiotics may therefore influence liver physiology by downregulating gut pathobiont species, with shifts that encourage gut microbiome homeostasis, maintain the epithelial physical barrier and reduce proinflammatory activity.
Despite the listed benefits of probiotics (i.e. influencing gut microbial species, intestinal permeability and inflammation) and the known mechanisms for the development of NAFLD (e.g. overconsumption of calories), there have been few studies investigating the effects of probiotics on NAFLD [Aller et al. 2011; Mykhal’chyshyn et al. 2013; Wong et al. 2013; Alisi et al. 2014; Nabavi et al. 2014; Sepideh et al. 2015]. However, these studies incorporated a range of designs (duration, dose and species used), typically include a single probiotic species, and lack investigation into effects on GI permeability. Therefore, there is still a need to further investigate the effects of probiotics. To investigate the effects of probiotics on NAFLD, we used an established model and high-fat diet that has been previously shown to induce NAFLD [Tan et al. 2011; Waller-Evans et al. 2013]. We selected the blend of probiotics based on the synergistic effects of these bacteria. Probiotics administered in combination have greater therapeutic efficacy (e.g. reducing permeability) than species administered individually [Resta-Lenert and Barrett, 2003; Otte et al. 2009].
The aim of this study was to examine the therapeutic effects of a multispecies probiotic preparation on the integrity of the intestinal epithelial physical barrier, lipid metabolism, liver function and steatosis in mice fed a high-fat diet. We hypothesized that a probiotic supplementation consisting of a mixture of Bifidobacteria, Lactobacilli and a Streptococcus species would mitigate the effects of a high-fat diet by preserving the integrity of the intestinal epithelial barrier, reducing liver steatosis and restoring normal liver function.
Materials and methods
Study design
Some 8-week-old male wild-type mice on a C57B1/6J background were randomly divided into one of three groups: (1) a control group, receiving a standard laboratory chow control diet (chow, n = 9); (2) an HFD group (n = 9); and (3) an HFD group concomitantly supplemented with probiotics from week 10 [n = 10, 1 × 108–9 colony-forming units (CFU)/ml]. One animal was excluded from both the chow and HFD group due to a leaking water bottle contaminating the bedding, causing the animal to get sick, resulting in weight loss. All animals were housed in a specific pathogen free (SPF) facility maintained at 20°C on a 12-hour light/dark cycle with access to clean water and food. At the end of week 20, all mice were euthanized, dissected and samples were stored for later analysis.
Ethics statement
All procedures were carried out in accordance and with approval from The University of Queensland (Permit number: QIMR/313/11/Various Trust Funds) and Queensland Institute of Medical Research Berghofer Medical Research Institute (Permit number: QIMR P829) ethics committees.
Probiotics supplement
Nine species of probiotic bacteria from three genera (represented as percentages of total CFU/ml) were used as a multispecies probiotic blend (Lactobacillus rhamnosus/Lactobacillus casei/Lactobacillus acidophilus/Lactobacillus plantarum/Lactobacillus fermentum comprised 82% of the total CFU; Bifidobacterium lactis/Bifidobacterium breve/Bifidobacterium bifidum comprised 13% of the total CFU; and Streptococcus thermophilus comprised 5% of the total CFU). All species were individually lyophilized and added to a known volume of drinking water (200–300 ml) as a combination probiotic supplement. The water was kept at room temperature (19–23°C) and changed every 48 hours. The total bacterial content of the probiotic blend added to the drinking water was estimated at 1 × 107–8 CFU/ml. This dosage was chosen as it represents a dose per kilogram of body mass equivalent for a human (approximately 1–2 x 109 CFU/kg) based on a mouse consuming 1.5 ml water per 10 g body mass per day.
Diet
The chow diet and HFD were purchased from Specialty Feeds (WA, Australia; HFD product no. SF03–020). The chow diet contained 4.8% total fat (monounsaturated fat 2.0%, polyunsaturated fat, 1.8% and saturated fat 0.7%) and provided 14 MJ/kg of energy. The HFD contained 23.0% total fat (monounsaturated fat 7.6%, polyunsaturated fat 2.0% and saturated fat 12.6%), and provided 20 MJ/kg of energy.
Dissection
At the end of week 20, mice were anaesthetized using an intraperitoneal injection of pentobarbital and xylazine. Once mice were anaesthetized, blood was collected by cardiac puncture and was left to clot at room temperature before serum was removed and stored. Tissue was collected by first removing the liver, followed by the large intestine. The mass of the liver was recorded. Liver and gut tissue samples were placed in formalin and transferred to a 70% ethanol solution after 24 hours for histology.
Large intestine or Swiss roll
The large intestine was cut longitudinally from the caecum to the rectum and opened. The intestinal tract was cleared of faecal matter using a cotton bud and carefully rolled on a wooden toothpick starting from the colon end, with the mucosa on the outside of the roll. The resulting roll was then carefully placed in formalin for histology.
Blood biochemistry
Serum was analyzed spectrophotometrically to determine the activity of alanine transaminase (ALT) and aspartate transaminase (AST) enzymes, and the concentrations of albumin, glucose, cholesterol and triglycerides. Analysis was performed using a Cobas Integra 400 auto-analyzer, with reagents and calibrators supplied by Roche Diagnostics (NSW, Australia).
Hepatic triglycerides assay
Liver tissue was homogenized (Polytron PT1200, Kinematics, Switzerland) in a 1.5% potassium chloride (KCl) solution (2.3 g KCl in 200 ml water). 500 µl of homogenate was extracted using a 2:1 chloroform/methanol mixture. Extracts were dried under nitrogen and stored at −80°C until analysis. For analysis, samples were reconstituted using 2% triton-x with the aid of sonication. Samples were further diluted with 2% triton-x, for a final ratio of 1:6, ready for analysis. Triglyceride concentration was measured using a spectrophotometer (Cobas Mira, Roche Diagnostics, Australia) and a kit and calibrators were supplied by Novachem (Victoria, Australia).
Protein determinations
Total liver protein was measured as per the manufacturer’s direction using a Pierce BCA protein assay kit supplied by Thermo Scientific (Victoria, Australia).
Tight-junction proteins ZO-1, ZO-2
Tight-junction proteins ZO-1 and ZO-2 were detected on large intestine Swiss roll histology slides by standard immunohistochemistry using the DAKO Envision System (Dako, Vic, Australia). Tissue sections were stained with rabbit antihuman polyclonal antibodies against ZO-1 (2.5 μg/ml; 1:100 dilution of the stock; catalogue no. 450-02129) and ZO-2 (10 μg/ml; 1:25 dilution of the stock; catalogue no. 000-05499) (Lifespan Biosciences, WA, USA).
Histological scoring
Histology was blindly scored on large intestinal Swiss roll sections with ZO-1 and ZO-2 staining and liver sections with haematoxylin and eosin, and oil red O staining. Digital images of the Swiss roll section were captured using the Aperio ScanScope XT Slide Scanner (Aperio Technologies, Vista, CA, USA) under 20 × objective magnification. Ten images were captured and scored for each sample. The quantitative scoring of ZO-1 and ZO-2 expression of each tissue section was then analyzed using the positive pixel algorithm of Aperio Imagescope by an experienced molecular biologist. Liver sections (one section per mouse liver) were blindly scored by an experienced pathologist for diagnosis, NAFLD activity score, steatosis grade and percentage, portal inflammation, lobular inflammation and ballooning, Mallory’s hyaline, fibrosis stage, portal score and centrilobular score using a previously published method [Kleiner et al. 2005].
Statistical analysis
All data are presented as mean ± standard deviation, unless otherwise stated. Data were tested for normality of the distribution, and statistical analysis was performed with the statistical software GraphPad Prism 6 (California, USA) and Stata (v14; Texas, USA). Comparison between groups for continuous data (body mass, TJPs ZO-1 and ZO-2, serum ALT, AST, albumin, glucose, cholesterol and triglycerides, hepatic triglycerides and steatosis %) was carried out using a one-way analysis of variance (ANOVA) with a Tukey’s multiple comparison post hoc test, or a Kruskal-Wallis test for nonparametric data with a Dunn’s multiple comparisons post hoc test of significance between individual groups (GraphPad). Comparison between groups for ordinal data (lobular inflammation, ballooning and portal inflammation) was carried out using a Kruskal Wallis test (Stata). Differences were considered significant when p was less than 0.05 (*p < 0.05 compared with the chow group; #p < 0.05 compared with the HFD group). Correlation calculations were carried out using a Pearson correlation coefficient using GraphPad Prism 6.
Results
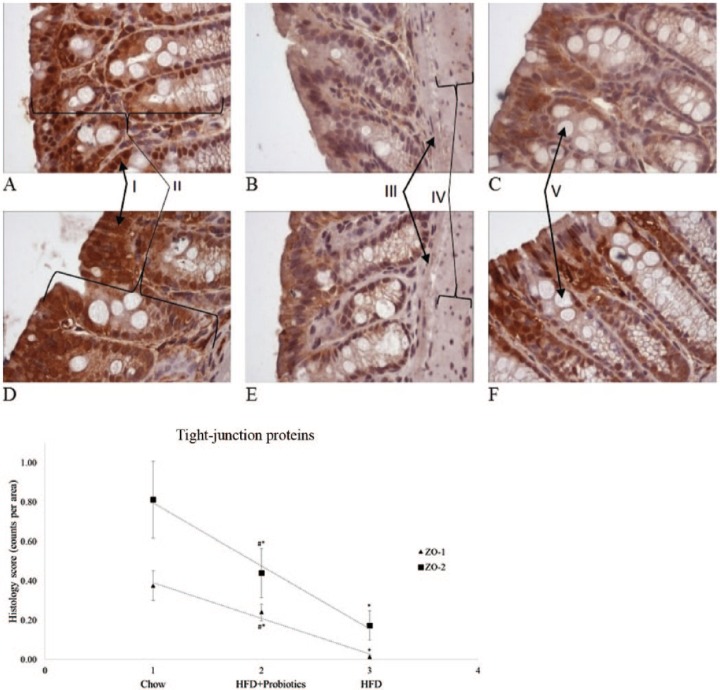
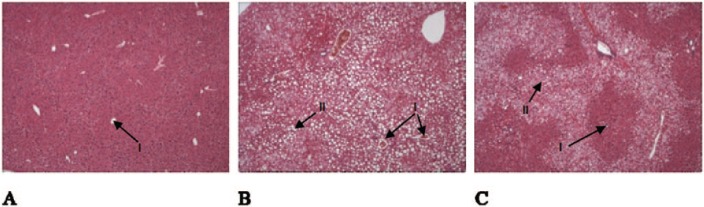
There was no difference (p = 0.18) in initial body mass of the chow, HFD and HFD + probiotics groups. The gain in body mass was similar between the chow and HFD groups for the first 7 weeks. At week 8, compared with chow-fed mice, body mass was significantly greater for the HFD group (4.1%) and HFD + probiotics group [(7.7%), p < 0.05] (Table 1). By the end of the 20th week, compared with chow-fed mice, body mass remained significantly greater for the HFD group (27.7%) and HFD + probiotics group [(26.2%), p < 0.05)]. Liver mass was significantly greater in both HFD-fed groups compared with chow-fed mice (p < 0.05) (Table 1).Semiquantitative analysis of the large intestine Swiss rolls showed that expression of the TJPs ZO-1 and ZO-2 was significantly lower in both HFD groups compared with the chow group (p < 0.05) (Figure 1). The HFD + probiotics group showed significantly greater expression of ZO-1 and ZO-2 compared with HFD mice (p < 0.05) (Figure 1). Histological examination of livers from chow-fed mice showed no steatosis development (Table 2) or fat-droplet accumulation. By contrast, HFD mice demonstrated development of steatosis, with large fat droplets, lobular inflammation and ballooning present. Compared with the HFD group, the HFD + probiotics group showed a nonsignificant reduction (p = 0.36) in steatosis percentage (Table 2) and visible reductions in fat droplets (Figure 2 A–C).
Table 1.
Body mass and liver mass.
| Chow (n = 9) | HFD (n = 9) | HFD + probiotics (n = 10) | |
|---|---|---|---|
| Body mass 0 weeks (g) | 23.9 ± 1.0 | 24.8 ± 0.7 | 24.5 ± 0.8 |
| Body mass week 8 (g) | 31.3 ± 1.2 | 32.6 ± 2.6* | 33.7 ± 3.5* |
| Body mass week 20 (g) | 33.6 ± 1.9 | 46.5 ± 4.1* | 45.5 ± 4.8* |
| Liver mass week 20 (g) | 1.7 ± 0.3 | 2.4 ± 0.7* | 2.7 ± 1.2* |
HFD, high-fat diet; *p < 0.05.
Figure 1.
Histological examination of the large intestine Swiss rolls for the tight-junction proteins ZO-1 and ZO-2. ZO-1: (A) chow-fed mouse; (B) HFD-fed mouse; (C) mouse fed HFD and supplemented with probiotics; for ZO-2: (D) chow-fed mouse; (E) HFD-fed mouse; (F) mouse fed HFD and supplemented with probiotics. All figures represent the median for that group. (I) Staining (brown) of tight-junction proteins; (II) mucosa; (III) muscularis mucosae; (IV) submucosa; (V) goblet cell. 400 × magnification; HFD, high-fat diet; *p < 0.05 compared with chow-fed mice; #p < 0.05 compared with HFD-fed mice.
Table 2.
Liver histology grading.
| Chow (n = 9) | HFD (n = 9) | HFD + probiotics (n = 10) | |
|---|---|---|---|
| Steatosis grade | 0.0 ± 0.0 | 2.4 ± 0.7* | 2.0 ± 1.3* |
| Steatosis % | 0.1 ± 0.3 | 72.8 ± 27.2* | 59.2 ± 38.7* |
| Portal inflammation | 0.0 ± 0.0 | 0.2 ± 0.4 | 0.0 ± 0.0 |
| Lobular inflammation | 0.6 ± 0.7 | 1.9 ± 0.9* | 1.7 ± 1.1 |
| Ballooning | 0.2 ± 0.4 | 1.3 ± 0.7* | 1.1 ± 0.6* |
HFD, high-fat diet; *p < 0.05.
Figure 2.
Liver histology for lipid deposits. (A) Mouse fed a standard chow diet with 0% steatosis; (B) mouse fed HFD with 85% steatosis; (C) mouse fed HFD with probiotics supplementation with 60% steatosis. All figures represent the median for that group. (I) Central vein; (II) fat deposits. 20 × magnification; HFD, high-fat diet.
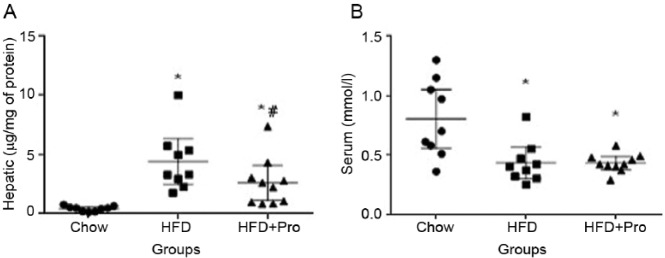
Compared with chow-fed mice, hepatic triglyceride concentration was significantly higher in both HFD groups (p < 0.01) (Figure 3A). In partial support of the liver histology scores and observation, hepatic triglyceride concentration was significantly lower in the HFD + probiotics group compared with the HFD group (p < 0.05) (Figure 3A). Serum triglyceride concentration was significantly lower in both HFD groups compared with chow-fed mice (p < 0.05) (Figure 3B), whereas it was not significantly different between the HFD and HFD + probiotics groups. In the HFD group, there was a trend towards a negative correlation between serum and hepatic triglyceride concentrations (R2 = 0.391; p = 0.07) (Figure 4). This relationship was weaker in the HFD + probiotics group (R2 = 0.293; p = 0.10), and no such relationship was evident in chow-fed mice (R2 = 0.082; p = 0.45). The activity of serum ALT and the concentrations of glucose and cholesterol were significantly higher in HFD-fed mice compared with chow-fed mice (Figure 5A–C), but they were not significantly different between the HFD and HFD + probiotics groups. There was no difference in serum AST activity or albumin concentration between any of the groups (Figure 5D–E).
Figure 3.
Hepatic and serum triglyceride concentrations. (A) Hepatic triglyceride concentrations (B) Serum triglyceride concentration. HFD, high-fat diet; *p < 0.05 compared with chow-fed mice; #p < 0.05 compared with HFD-fed mice.
Figure 4.
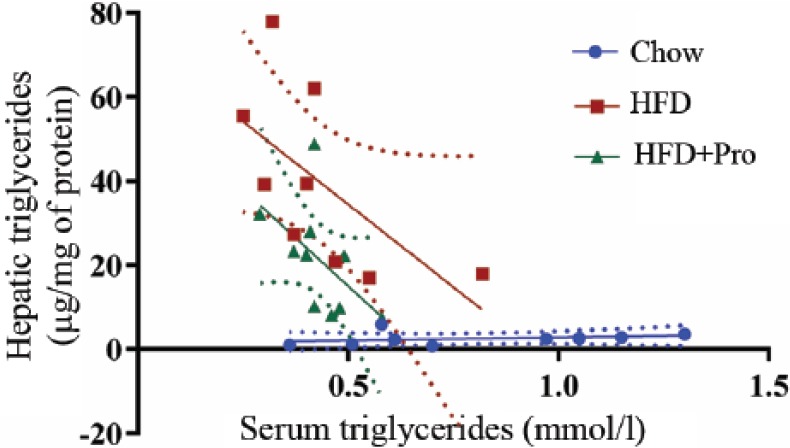
Serum and hepatic triglyceride mobilization. An inverse relationship between serum and hepatic triglycerides when an HFD was consumed. Chow-fed mice, R2 = 0.082, p = 0.45; HFD-fed mice, R2 = 0.391, p = 0.07; HFD-fed mice supplemented with probiotics, R2 = 0.293, p = 0.10. HFD, high-fat diet.
Figure 5.
Serum biochemical data. (A) ALT, (B) glucose, (C) cholesterol, (D) AST, (E) albumin. HFD, high-fat diet; ALT, alanine transaminase; AST, aspartate aminotransferase; *p < 0.05 compared with chow-fed mice.
Discussion
The aim of this study was to examine the therapeutic effects of a multispecies probiotic preparation on the integrity of the intestinal epithelial barrier, lipid metabolism, liver function and steatosis in mice fed a high-fat diet. The main findings were that the supplementation with a multispecies probiotic formulation helped to maintain TJPs ZO-1 and ZO-2, and reduced hepatic triglyceride concentrations compared with an HFD alone. These findings have implications for advancing probiotics and the GI tract microbiome as a potential point of treatment for NAFLD.
In this study we examined the effects of probiotics on the development of NAFLD, and hypothesized that probiotics supplementation would mitigate the effects of a high-fat diet in part by preserving the integrity of the intestinal epithelial barrier. Our hypothesis was derived from current literature showing that probiotics maintain transepithelial resistance (permeability) when cells are exposed to an insult [Parassol et al. 2005; Zyrek et al. 2007]. We have also previously shown that probiotics reduce intestinal permeability (indicated by lower serum lipopolysaccharide concentration) in athletes who are under physiological stress [Shing et al. 2014].
Histological analysis of the large intestine showed that an HFD reduced the expression of ZO-1 and ZO-2 compared with chow-fed mice, whereas the administration of probiotics partly attenuated this effect. The magnitude of the probiotics effect is consistent with probiotics preventing the degradation of ZO-1 and ZO-2. That is, if we assume the chow group represents 100% TJP expression (maximum expression) and the HFD group represents 0% TJP expression (minimum expression), we can establish an approximate rate of TJP degradation over the 20 weeks. The HFD + probiotic group therefore represents approximately 50% TJP expression (Figure 1) and equates to the expression expected in the HFD group at week 10, assuming TJP degradation is linear. This is of note, as this is when we introduced probiotics.
We cannot establish a definitive association, but maintaining the integrity of the gut physical barrier through the administration of probiotics, may have helped to attenuate the progression of NAFLD. This is supported by the reduction in hepatic triglyceride concentrations seen in the mice fed an HFD and supplemented with probiotics. We speculate that this may be in part the result of reducing the translocation of pathogens and byproducts across the intestinal epithelial barrier in response to an HFD-triggered dysbiosis. This is very much likely to matter given that the gut physical barrier, formed by intestinal epithelial cells, maintains homeostasis in the intestine in a continuous co-operative process with the innate immune system, importantly linking it to intestine-resident macrophages and to dendritic cells [Peterson and Artis, 2014].
Regulating intestinal epithelial permeability is one of the most important physiological defences against pathogenic bacteria and exogenous pathogens. In a recent report, Spadoni and colleagues [Spadoni et al. 2015] demonstrated this in both mice and humans. Specifically, they identified a gut–vascular barrier (GVB) that regulates the translocation of antigens into the bloodstream, and prevents the entry of large particles (i.e. intestinal bacteria and antigens). However, in certain circumstances (obesity, diet, inflammation, dysbiosis, etc.), the GVB can be compromised, allowing larger particles to pass through to the bloodstream where they can reach and affect the organs. Spadoni and colleagues also demonstrated that GVB impairment occurs independently to liver damage. These data are complementary to our present findings that an HFD alters TJP expression, and offer supporting evidence that intestinal permeability resulting from the overconsumption of calories can cause (and potentially rescue) liver damage.
We also observed reduced lipid accumulation from the liver in mice fed an HFD and supplemented with probiotics. We did not determine the cellular mechanism(s) responsible for this observation. Nevertheless, we did identify some physiological responses that may point to the mechanism(s) involved in the increased deposition of hepatic lipids in mice fed an HFD. In both HFD-fed groups, the observed increase in liver triglycerides was accompanied by decreased serum triglycerides (Figure 4). Experimental studies have reported that under regulated conditions, as liver triglyceride concentrations increase, there is an adaptation response that may increase or decrease cell-signalling (reactive oxygen species dependent) mechanisms in order to increase the mobilization of fat to clear it from the liver [Nussbaum et al. 2013].
Despite the beneficial effects of probiotics, these bacteria cannot be deemed a panacea to a high calorie diet. In this study, 30% of the mice (n = 3) showed no effect from the administration of probiotics; consequently additional factors that influence the GI microbiome could also be important considerations. Colonization of the GI tract is not a uniform event, and begins post birth as the newborn is exposed to maternal and environmental microbes [Tapiainen et al. 2006]. Animal housing differences, variable bacterial colonization of the GI tract and disease development may have contributed to the observed variation in probiotic effects. A weakness of this study in this regard was the lack of GI tract microbiome analysis.
This study proposes that probiotics are not a panacea for an overconsumption of calorie-dense foods through a high-saturated-fat diet in the hope of downregulating liver fatty acid metabolism. Rather, probiotics may help reduce the severity and possibly the rate of progression of NAFLD to cirrhosis or hepatocellular carcinoma, resulting from the consumption of a high-caloric or high-fat diet, by modulating intestinal epithelial permeability, attenuating inflammation and lipid metabolism in the liver.
Future research directions
It was beyond the scope of the current study to test the efficacy of the individual bacterial species in the probiotics supplement. Furthermore, the effects of individual species may not translate to the effects of a combination probiotic formulation, that is, individual bacteria work differently in the presence of other species. However, investigating the effects of individual bacterial strains may help develop better probiotic combinations and is something for future studies. Studies that investigate the role of multispecies probiotics and their effect on the gut–liver axis should also employ research designs that can determine whether improvement in gut dysbiosis is accompanied by a beneficial shift in the GI tract microbiome that then pre-empts improvement in liver steatosis. To date, only a few studies have investigated the microbiome associated with NAFLD and nonalcoholic steatohepatitis conditions [Mouzaki et al. 2013; Zhu et al. 2013; Jiang et al. 2015]. These studies reported a difference in the microbiota population between patients with liver disease and healthy individuals. An extension to the present study would involve analyzing changes in the microbiota associated with an HFD and probiotics supplementation. This analysis would provide valuable information on the effects of probiotics on a dysbiotic microbiome, and provide a potential answer to why some subjects respond to probiotics therapy and some do not.
Footnotes
Funding: Luis Vitetta provided funding through his National Institute of Complementary Medicine and National Health and Medical Research Council of Australia competitive funding and Industry support for research into probiotics. This work was supported in part by a project grant from the National Health and Medical Research Council of Australia (APP1029574) to DC.
Conflict of interest statement: Luis Vitetta has received National Institute of Complementary Medicine and Industry support for research into probiotics. David Briskey received a student scholarship from National Institute of Complementary Medicine to undertake a PhD.
Contributor Information
David Briskey, School of Human Movement and Nutrition Sciences, The University of Queensland, Brisbane, Queensland, Australia.
Mandy Heritage, Gallipoli Medical Research Centre, Greenslopes Hospital, Brisbane, Australia School of Medicine, The University of Queensland, Brisbane, Australia.
Lesley-Anne Jaskowski, Gallipoli Medical Research Centre, Greenslopes Hospital, Brisbane, Australia School of Medicine, The University of Queensland, Brisbane, Australia.
Jonathan Peake, School of Biomedical Sciences and Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, Australia.
Glenda Gobe, Centre for Kidney Disease Research, School of Medicine, The University of Queensland, Translational Research Institute, Queensland, Australia.
V. Nathan Subramaniam, Gallipoli Medical Research Centre, Greenslopes Hospital, Brisbane, Australia Queensland Institute of Medical Research Berghofer Medical Research Institute, Brisbane, Australia Envoi Specialist Pathologists, Brisbane, Australia.
Darrell Crawford, Gallipoli Medical Research Centre, Greenslopes Hospital, Brisbane, Australia School of Medicine, The University of Queensland, Brisbane, Australia.
Catherine Campbell, Envoi Specialist Pathologists, Brisbane, Australia.
Luis Vitetta, Sydney Medical School, University of Sydney, Medlab Clinical Ltd., Sydney, New South Wales, 2006, Australia.
References
- Alisi A., Bedogni G., Baviera G., Giorgio V., Porro E., Paris C., et al. (2014) Randomised clinical trial: the beneficial effects of Vsl#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 39: 1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aller R., De Luis D., Izaola O., Conde R., Gonzalez Sagrado M., Primo D., et al. (2011) Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci 15: 1090–1095. [PubMed] [Google Scholar]
- Bedogni G., Miglioli L., Masutti F., Tiribelli C., Marchesini G., Bellentani S. (2005) Prevalence of and risk factors for nonalcoholic fatty liver disease: the DIONYSOS nutrition and liver study. Hepatology 42: 44–52. [DOI] [PubMed] [Google Scholar]
- Bellentani S., Scaglioni F., Marino M., Bedogni G. (2010) Epidemiology of non-alcoholic fatty liver disease. Dig Dis 28: 155–161. [DOI] [PubMed] [Google Scholar]
- Browning J., Szczepaniak L., Dobbins R., Nuremberg P., Horton J., Cohen J., et al. (2004) Prevalence of hepatic steatosis in an urban population in the united states: impact of ethnicity. Hepatology 40: 1387–1395. [DOI] [PubMed] [Google Scholar]
- Hegazy S., El-Bedewy M. (2010) Effect of probiotics on pro-inflammatory cytokines and NF-kappaB activation in ulcerative colitis. World J Gastroenterol 16: 4145–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek J., Pastorino J. (2004) Cellular signaling mechanisms in alcohol-induced liver damage. Semin Liver Dis 24: 257–272. [DOI] [PubMed] [Google Scholar]
- Jiang W., Wu N., Wang X., Chi Y., Zhang Y., Qiu X., et al. (2015) Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep 5: 8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khimji A., Shao R., Rockey D. (2008) Divergent transforming growth factor-beta signaling in hepatic stellate cells after liver injury: functional effects on ECE-1 regulation. The American journal of pathology 173: 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D., Brunt E., Van Natta M., Behling C., Contos M., Cummings O., et al. (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- Marchesini G., Moscatiello S., Di Domizio S., Forlani G. (2008) Obesity-associated liver disease. J Clin Endocrinol Metab 93: S74–80. [DOI] [PubMed] [Google Scholar]
- Mouzaki M., Comelli E., Arendt B., Bonengel J., Fung S., Fischer S., et al. (2013) Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 58: 120–127. [DOI] [PubMed] [Google Scholar]
- Mykhal’chyshyn H., Bodnar P., Kobyliak N. (2013) Effect of probiotics on proinflammatory cytokines level in patients with type 2 diabetes and nonalcoholic fatty liver disease. Lik Sprava 2: 56–62. [PubMed] [Google Scholar]
- Nabavi S., Rafraf M., Somi M., Homayouni-Rad A., Asghari-Jafarabadi M. (2014) Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci 97: 7386–7393. [DOI] [PubMed] [Google Scholar]
- Niaz A., Ali Z., Nayyar S., Fatima N. (2011) Prevalence of NAFLD in healthy and young male individuals. ISRN Gastroenterol 2011: 363546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum J., Liu L., Hasan S., Schaub M., McClendon A., Stainier D., et al. (2013) Homeostatic generation of reactive oxygen species protects the zebrafish liver from steatosis. Hepatology 58: 1326–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte J., Mahjurian-Namari R., Brand S., Werner I., Schmidt W., Schmitz F. (2009) Probiotics regulate the expression of Cox-2 in intestinal epithelial cells. Nutr Cancer 61: 103–113. [DOI] [PubMed] [Google Scholar]
- Parassol N., Freitas M., Thoreux K., Dalmasso G., Bourdet-Sicard R., Rampal P. (2005) Lactobacillus casei DN-114 001 inhibits the increase in paracellular permeability of enteropathogenic Escherichia coli-infected T84 cells. Res Microbiol 156: 256–262. [DOI] [PubMed] [Google Scholar]
- Peterson L., Artis D. (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14: 141–153. [DOI] [PubMed] [Google Scholar]
- Resta-Lenert S., Barrett K. (2003) Live Probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (Eiec). Gut 52: 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodes L., Khan A., Paul A., Coussa-Charley M., Marinescu D., Tomaro-Duchesneau C., et al. (2013) Effect of probiotics Lactobacillus and Bifidobacterium on gut-derived lipopolysaccharides and inflammatory cytokines: an in vitro study using a human colonic microbiota model. J Microbiol Biotechnol 23: 518–526. [DOI] [PubMed] [Google Scholar]
- Sepideh A., Karim P., Hossein A., Leila R., Hamdollah M., Mohammad E., et al. (2015) Effects of multistrain probiotic supplementation on glycemic and inflammatory indices in patients with nonalcoholic fatty liver disease: a double-blind randomized clinical trial. J Am Coll Nutr: 1–6. [DOI] [PubMed] [Google Scholar]
- Shing C., Peake J., Lim C., Briskey D., Walsh N., Fortes M., et al. (2014) Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur J Appl Physiol 114: 93–103. [DOI] [PubMed] [Google Scholar]
- Spadoni I., Zagato E., Bertocchi A., Paolinelli R., Hot E., Di Sabatino A., et al. (2015) A Gut-vascular barrier controls the systemic dissemination of bacteria. Science 350: 830–834. [DOI] [PubMed] [Google Scholar]
- Tan T., Crawford D., Jaskowski L., Murphy T., Heritage M., Subramaniam V., et al. (2011) Altered lipid metabolism in HFE-knockout mice promotes severe NAFLD and early fibrosis. Am J Physiol Gastrointest Liver Physiol 301: G865–876. [DOI] [PubMed] [Google Scholar]
- Tapiainen T., Ylitalo S., Eerola E., Uhari M. (2006) Dynamics of gut colonization and source of intestinal flora in healthy newborn infants.APMIS : acta pathologica, microbiologica, et immunologica Scandinavica 114: 812–817. [DOI] [PubMed] [Google Scholar]
- Tilg H., Diehl A. (2000) Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med 343: 1467–1476. [DOI] [PubMed] [Google Scholar]
- Vitetta L., Manuel R., Zhou J., Linnane A., Hall S., Coulson S. (2014) The overarching influence of the gut microbiome on end-organ function: the role of live probiotic cultures. Pharmaceuticals (Basel) 7: 954–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller-Evans H., Hue C., Fearnside J., Rothwell A., Lockstone H., Calderari S., et al. (2013) Nutrigenomics of high fat diet induced obesity in mice suggests relationships between susceptibility to fatty liver disease and the proteasome. PLoS One 8: e82825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V., Won G., Chim A., Chu W., Yeung D., Li K., et al. (2013) Treatment of nonalcoholic steatohepatitis with probiotics. a proof-of-concept study. Ann Hepatol 12: 256–262. [PubMed] [Google Scholar]
- Zhu L., Baker S., Gill C., Liu W., Alkhouri R., Baker R., et al. (2013) Characterization of gut microbiomes in nonalcoholic steatohepatitis (nash) patients: a connection between endogenous alcohol and nash. Hepatology 57: 601–609. [DOI] [PubMed] [Google Scholar]
- Zyrek A., Cichon C., Helms S., Enders C., Sonnenborn U., Schmidt M. (2007) Molecular mechanisms underlying the probiotic effects of Escherichia coli nissle 1917 involve ZO-2 and pkczeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol 9: 804–816. [DOI] [PubMed] [Google Scholar]