Abstract
The thalamocortical projection to rodent barrel cortex consists of inputs from the ventral posterior medial (VPM) and posterior medial (POm) nuclei that terminate in largely non-overlapping territories in and outside of layer IV. This projection in both rats and mice has been used extensively to study development and plasticity of highly-organized synaptic circuits. Whereas the VPM pathway has been well characterized in both rats and mice, organization of the POm pathway has only been described in rats, and no studies have focused exclusively on the development of the POm projection. Here, using transport of PHA-L or carbocyanine dyes, we characterize the POm thalamocortical innervation of adult mouse barrel cortex and describe its early postnatal development in both mice and rats. In adult mice, POm inputs form a dense plexus in layer Va that extends uniformly underneath layer IV barrels and septa. Innervation of layer IV is very sparse; a clear septal innervation pattern is evident only at the layer IV/Va border. This pattern differs subtly from that described previously in rats. Developmentally, in both species, POm axons are present in barrel cortex at birth, where in mice, they occupy layer IV as it differentiates. In contrast, in rats, POm axons do not enter layer IV until 1–2 days after its emergence from the cortical plate. In both species, arbors undergo progressive and directed growth. However, no layer IV septal innervation pattern emerges until several days after the cytoarchitectonic appearance of barrels and well after the emergence of whisker-related clusters of VPM thalamocortical axons. The mature pattern resolves earlier in rats than in mice. Taken together, these data reveal anatomical differences between mice and rats in development and organization of POm inputs to barrel cortex, with implications for species differences in the nature and plasticity of lemniscal and paralemniscal information processing.
Indexing terms: ventrobasal nuclear complex, synaptic plasticity, DiI, PHA-L, somatosensory cortex, carbocyanine dyes
The rodent somatosensory barrel cortex has been an archetypal model for studying precisely patterned synaptic connectivity for almost forty years (Woolsey and Van der Loos, 1970; Welker and Woolsey, 1974). One principal hallmark of the barrel cortex is the cellular organization of layer IV and the patterning of thalamocortical inputs in relationship to such organization. Granule cells in layer IV, particularly in the posteromedial barrel subfield (PMBSF), where the large facial whiskers are represented, form a series of rosettes or “barrels” that correspond precisely to the number and topographic distribution of facial whiskers on the contralateral face (Van der Loos and Woolsey, 1973). In both rats and mice, afferents from the ventral posterior medial (VPM) nucleus form dense, whisker-related clusters of terminations that target the barrels. This pathway—the so-called lemniscal pathway—conveys signals related to both vibrissal movement and touch (Yu et al., 2006). Developmentally, the temporal and spatial progression of VPM thalamocortical axon (TCA) ingrowth into barrel cortex, colonization of layer IV, and formation of whisker-related clusters that match emergence of layer IV barrels have been well-described during the early postnatal period in both rats and mice (Wise and Jones, 1978; Erzurumlu and Jhaveri, 1990; Catalano et al., 1991; Senft and Woolsey, 1991; Agmon et al., 1993; Schlaggar and O’Leary, 1994; Rebsam et al., 2002).
The cell-dense barrels in layer IV are separated from each other by cell-sparse regions called the septa. In contrast to the wealth of information from both rats and mice about the development and connectivity of the VPM nucleus-to-barrel pathway, the thalamic connectivity of the septa has only been investigated anatomically in rats. In this species, afferents from the medial division of the posterior medial nucleus (POm) terminate in the septa, and extend, as in a column, superficially into layer III and deeper into layer Va (Koralek et al., 1988; Chmielowska et al., 1989; Lu and Lin, 1993; Deschenes et al., 1998). Such POm TCAs also terminate in the dysgranular cortex that separates major body parts within the somatosensory cortex. This pathway—the so-called paralemniscal pathway—conveys signals related to vibrissal movement (Ahissar et al., 2000; Yu et al., 2006). Thus, in rats the two thalamic streams form largely non-overlapping, complementary patterns of terminations in and outside of layer IV, thereby establishing semi-independent circuits for tactile information processing (Koralek et al., 1988; Chmielowska et al., 1989; Lu and Lin, 1993; Kim and Ebner, 1999; Ahissar et al., 2001; Bureau et al., 2006).
Because of the similarity between rats and mice in the VPM projection to barrels, it is often assumed that the POm innervation of barrel cortex in mice would also be similar, if not identical, to that in rats. However, several lines of evidence suggest that this may not necessarily be so. First, the cellular organization of layer IV differs between the two rodent species (Welker and Woolsey, 1974). In rats, the barrel walls and centers are relatively uniform in cell density, and the septa that separate them are wide. In contrast, in mice, barrels are more easily discernible cytoarchitecturally than in rats because the barrel hollows are less cell-dense than the barrel walls. However, septa are much narrower than in rats, so much so that barrels can often appear fused, suggesting that connectivity of the septa in mice may differ from that in rats. Second, electrophysiological studies of intrinsic barrel cortex circuitry subserving lemniscal and paralemniscal processing indicate that interlaminar pathways connecting layers IV and Va with supragranular layers in mice differ in comparison with those in rats (Shepherd and Svoboda, 2005; Bureau et al., 2006). Third, differences between these rodent species in other types of barrel cortex input/output circuitry have been described. For example, a recent study of mouse sensory-motor cortex has shown that large numbers of callosal neurons that establish a dual projection to ipsilateral frontal cortex during early development persist into adulthood (Mitchell and Macklis, 2005), whereas in rats, such dual projection neurons are transient, undergoing a developmentally regulated pruning of one or the other projection (Ivy and Killackey, 1981; O’Leary et al., 1981). Given the widespread reliance on genetically manipulated mice as tools for understanding the molecular algorithms that drive thalamocortical innervation of barrel cortex and the emergence of barrel architecture (e.g. Abdel-Majid et al., 1998; Maier et al., 1999; Rebsam et al., 2002; Dufour et al., 2003; Lee et al., 2005; Shimogori and Grove, 2005; Inan et al., 2006; Ince-Dunn et al., 2006), a complete understanding of the development and connectivity of the POm nucleus-to-barrel cortex pathway in mice is particularly important for extrapolating results from genetically manipulated mice to rats, which, because of their larger brain size, more often serve as subjects in acute experiments. Thus, the purpose of the present study was two-fold. Our first goal was to characterize the POm innervation of adult mouse barrel cortex, with emphasis on comparing such patterns with those that have been described previously in rats. Our second goal was then to describe the early postnatal development of POm innervation in both rats and mice. A recent study has described the development of POm TCAs in rats in the context of projections to layer I (Galazo et al., 2007); however, no studies have investigated the development of this circuitry in rats or mice in relationship to the development of the VPM projection to barrels.
MATERIALS AND METHODS
Animals
This study was conducted on the postnatal brains of 241 CFW mice and 175 Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), ranging in age from postnatal (P) day 0 (the first 24 hours after birth) through P10, and adult mice (>P45). Both male and female animals were used. The treatment of all animals was in strict accordance with procedures mandated by Mount Sinai’s Institutional Animal Care and Use Committee and with the guidelines established by the National Institutes of Health.
PHA-L labeling of TCAs in adult mice and rat pups
Adult mice were anesthetized with an intraperitoneal injection of a combination of ketamine (100 mg/kg) and xylazine (20 mg/kg) and placed into a stereotaxic instrument. A dental drill was used to remove a small portion of the skull overlying the position of the thalamus. A glass micropipette was lowered into the POm nucleus (1.7 mm posterior and 1.2 mm lateral to bregma, and 3.3 mm ventral to the cortical surface) and 2.5% Phaseolus vulgaris leucoagglutinin (PHA-L; diluted in 0.01 M phosphate buffer, pH 8) was deposited iontophoretically (5 μA positive current, with a 7 seconds on/off duty cycle for 5–10 minutes). Animals survived for 5–7 days, then were anesthetized as above and perfused transcardially first with 1% paraformaldehyde followed by 4% paraformaldehyde, both in 0.1 M phosphate-buffered saline (PBS). Brains were postfixed for six hours and cryoprotected. Rat pups (aged P6) were immobilized on ice and placed in a modified stereotaxic frame. A glass micropipette was lowered into the POm nucleus (2.2 mm posterior and 1.8 – 2.0 mm lateral to bregma, and 2.5 mm ventral to the skull surface), and PHA-L was ejected as described above, with the exception that iontophoresis lasted 2–5 min. Animals survived for 48 hrs, then were perfused with normal saline followed by fixative as described above. Brains were postfixed for 24 hours, then cryoprotected. Blocks containing the thalamus and the posteromedial barrel subfield (PMBSF) from all animals were cut into serial 50 μm-thick sections on a freezing microtome in the coronal plane or, for some blocks containing the PMBSF, in an oblique tangential plane parallel to the pial surface. One series was processed histochemically for cytochrome oxidase (CO) activity (Wong-Riley, 1979), which was used to reveal nuclear, laminar and compartmental (layer IV barrel/septal) boundaries. The adjacent series was processed immunocytochemically for the detection of PHA-L by blocking sections overnight in 2% normal goat serum (NGS) and 0.03% Triton-X 100 in potassium phosphate buffered saline (KPBS), followed by incubation for 48 hours in anti-PHA-L (E + L) primary antisera (1:2000; affinity-purified rabbit polyclonal antisera, catalog number AS-2300, lot number T0104; Vector Laboratories, Burlingame, VT) containing 2% NGS in KPBS. Sections were then briefly washed, incubated in biotinylated goat anti-rabbit IgG (1:400, Vector) in PBS, then incubated in an avidin-biotin-peroxidase solution (Vectastain Elite kit, Vector). Antibody binding was visualized by a 5-minute incubation in 0.022% 3,3′-diaminobenzidine tetrachloride (Sigma-Aldrich, St. Louis, MO) to which 0.003% hydrogen peroxide was added. After washing, sections were mounted on gelatin-subbed slides, dehydrated, cleared in xylene and coverslipped. To verify the specificity of the anti-PHA-L antisera, control sections through the PMBSF from brains that had not received injection of PHA-L were processed immunocytochemically as described above. In these control sections, no immunolabeling was observed.
Carbocyanine dye labeling in rat and mouse pups
Mice and rats aged P0–P10 were anesthetized (mice: Avertin, 350 mg/kg; rats: a mixture of ketamine, 100 mg/kg and xylazine, 20 mg/kg) and perfused transcardially briefly with 0.9% saline followed by 4% paraformaldehyde in 0.1 M PBS. Following 24–36 hours of postfixation, brains were hemisected through the midline in order to visualize the thalamus on the medial surface. A second, coronal cut was then made through the diencephalon approximately at the level of the medial habenular nucleus, thereby exposing the caudal end of the thalamus. A dissecting microscope was then used to visualize on the caudal face of the block both VPM and POm, and to visually guide the accurate placement of glass micropipettes containing solutions or crystals of carbocyanine dye into the POm nucleus alone, or in some cases, into VPM and POm simultaneously. Both DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) and DiD (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate, both from Molecular Probes, Eugene, OR) were used for simultaneous injections of VPM and POm. Approximately one-half of the brains received DiI into POm and DiD into VPM; the order was reversed for the other one-half. For single-labeling of POm, some brains received DiI, others received DiD. We found no evidence that the innervation patterns observed arising from either nucleus differed depending on the dye used. For injections, the carbocyanine dyes were diluted in dimethylformamide to a final concentration of 2.5%; a small amount of the dye was drawn through the tip of a pipette into the tissue by capillary action at single or multiple spots within the target nucleus. Only DiI was alternatively delivered in some brains by embedding a small crystal into the target nucleus. Brains were then incubated at 37°C in 4% paraformaldehyde for 1–4 weeks to allow for diffusion along the lipid bilayer of the thalamocortical axons, after which they were sectioned at a setting of 100 μm on a vibratome in either a coronal plane or in an oblique tangential plane through the PMBSF. Selected sections were counterstained with a fluorescent Nissl stain (Neurotrace 500/525, Molecular Probes; diluted 1:300 in 0.1 M PBS for 20 minutes). Sections were then washed, wet-mounted and examined immediately by confocal microscopy. Only sections with low background fluorescence, minimal retrograde labeling, and little or no transcellular labeling of radial glia (Godement et al., 1987) were included in the study.
Microscopy and Analysis
Sections from adult mouse brains containing immunolocalized PHA-L or reacted for CO activity were examined under darkfield and brightfield optics with a Zeiss Axiophot photomicroscope (Carl Zeiss, Thornwood, NY). Digital images of PHA-L immunolabeling or CO activity were acquired with a Spot camera (Diagnostic Instruments, Sterling Heights, MI) and imported to Adobe Photoshop CS (Adobe Systems, Mountain View, CA), where individual images were assembled into photomontages and subjected to minimal adjustments in contrast and brightness. Laminar and compartmental boundaries were then determined by aligning immunolabeled sections with their adjacent CO-reactive sections using blood vessels and other fiducials as landmarks.
Confocal images of fluorescent dye labeling were acquired with a Zeiss 510 Meta laser-scanning confocal microscope using10× (0.32 N.A.) or 20× (0.8 N.A.) objectives. Images of DiI labeling were acquired with a HeNe-543 laser and a λ560–615 filter; images of DiD were acquired with a HeNe-633 laser and a λ650–745 filter; images of Nissl staining were obtained by using an Argon/2–488 laser and a λ505–550 filter. Optical sections 1–4 μm-thick were acquired through the full depth of each section. Stacks of optical sections spanning the full tissue section thickness, or from a selected subset, were then digitally superimposed and imported to Adobe Photoshop CS, where minimal adjustments in contrast and brightness were made and channels (carbocyanine dyes and Nissl stain) were separated. To display single-dye labeling of POm TCAs, images were inverted to present carbocyanine dye labeling as a black signal on a white/grey background. For consistency in displaying the simultaneous labeling of VPM and POm TCAs using two different carbocyanine dyes, we pseudocolored labeling from the POm nucleus red and that from the VPM nucleus blue in all figures, regardless of which dye (DiI or DiD) was actually used.
For analysis of single POm TCA morphology in rats and mice, we used material cut in the coronal plane in which the intracortical arbors of single POm TCAs could be clearly isolated. Additionally, axons were selected for analysis if: 1) they came from brains in which thalamic labeling was restricted to the POm nucleus; 2) their parent intracortical branch could be followed to the white matter; and 3) their terminal arbor was contained within the PMBSF or, in the youngest brains prior to the cytoarchitectonic appearance of barrels, within the presumptive position of the PMBSF using established landmarks (Senft and Woolsey, 1991). For both rats and mice, three age-groups of animals were analyzed: P0, P4, and P8 (n = 6–9 animals/species/age-group). For each species, 35–58 single axons were traced per age-group, with 2–12 axons traced from 1–5 sections per animal. Optical confocal sections through the full-depth of the tissue slice were acquired and digitally superimposed as described above; laminar boundaries were then assigned directly from the same, Nissl-counterstained sections. The composite images were then exported to Neurolucida (MBF Bioscience, Williston, VT) where they were traced. To determine the maximal tangential (mediolateral) spread of single arbors within the coronal plane of section, the two most widely-separated axon endpoints, regardless of which layer they occupied, were projected onto a line drawn parallel to the pial surface and the horizontal distance between them was measured. Mean values of such distances were determined for each animal, and then used to calculate the mean and standard deviation per age-group. To estimate the progression of innervation by layer at P0 and P4 (i.e., Fig. 10), the laminar position of the endpoint of the most superficially extending branch was noted. Calculations were done separately for each species. The number of superficial-most endpoints was tallied for each layer and expressed as a percentage of the total number of endpoints for all animals examined at that age. Since all endpoints for each layer were pooled across animals, there are no error bars attached to the graphs of Figure 10. Statistical significance between species at each of the two developmental ages was calculated using a Fisher exact probability test, where p < 0.05 was considered significant. Final figure layout and graphics were completed using Adobe InDesign.
RESULTS
Carbocyanine dyes were used to label POm TCAs in early postnatal brains, but such dyes transport poorly along myelinated axons of adult brains (Vercelli et al., 2000). Thus, we used the anterograde tracer PHA-L to label TCAs in adult mice. In all cases, our analysis was restricted to the intracortical trajectories of labeled TCAs, focusing on laminar and compartmental (barrel/septa) termination patterns in the PMBSF and surrounding dysgranular cortex. No attempt was made to analyze the subcortical trajectories of labeled TCAs. We describe first the adult pattern of POm TCA innervation in mice as this has never been reported previously, followed by a description of the developmental sequences of each species. Here, our focus was on the first ten postnatal days, previous studies having established this as the major period over which TCAs from VPM and other sensory nuclei innervate the cortex, colonize layer IV, and adult-like patterns of distribution resolve (Lund and Mustari, 1977; Wise and Jones, 1978; Senft and Woolsey, 1991; Agmon et al., 1993; Kageyama and Robertson, 1993; Catalano et al., 1996).
Adult termination pattern of POm TCAs in mouse barrel cortex
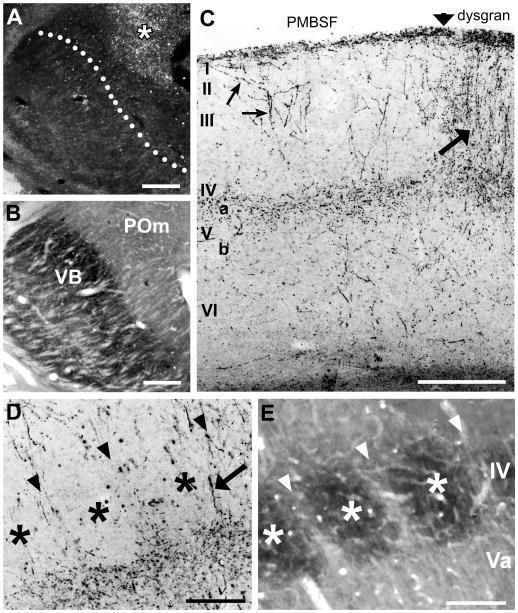
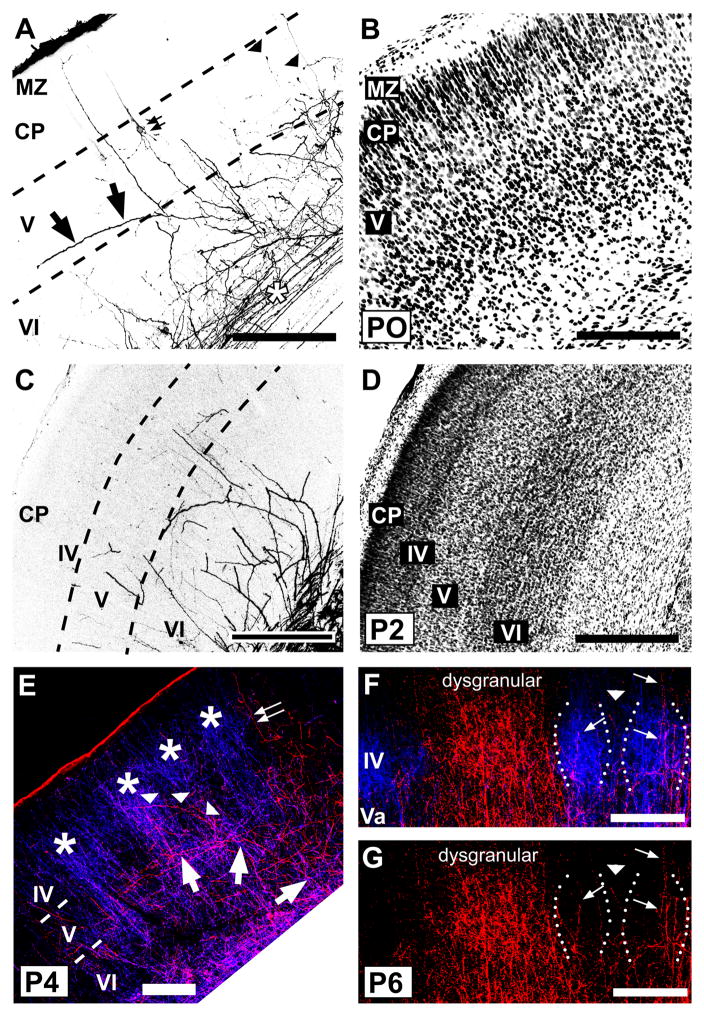
Small, focal injections of PHA-L into POm of adult mice (Fig. 1A, B) produced TCA terminations within several discrete cortical areas, including primary motor and somatosensory cortex (Fig. 1C) as expected (Donoghue and Parham, 1983; Spreafico et al., 1987; Deschenes et al., 1998). Within the PMBSF, when viewed in sections cut in the coronal plane, labeled axons were sparsely distributed in layers VI and Vb, but formed a dense terminal-like plexus in layer Va that appeared uniformly distributed across the mediolateral extent of the field, thereby extending across layer Va territory subjacent to both barrels and septa in layer IV (Fig. 1C,D). However, at the layer IV/Va border, there was a hint of a scalloped appearance to the distribution of fibers, with the labeled fiber plexus extending superficially into the deepest aspect of layer IV at the septa (arrowheads, Fig. 1D,E), while receding slightly in layer Va subjacent to the barrel centers (asterisks, Fig. 1D,E). Radially-oriented fibers could be followed occasionally from layer Va through layer IV septa (arrow, Fig. 1D); these were largely unbranched as they traversed layer IV, and only infrequently entered barrel centers. In some cases, such radially-oriented fibers continued superficially, towards and sometimes reaching, layer I, where a second, dense terminal-like plexus was formed (Fig. 1C). In many cases, such superficially-located fibers branched obliquely within layers II and III as they angled towards layer I (small arrows, Fig. 1C).
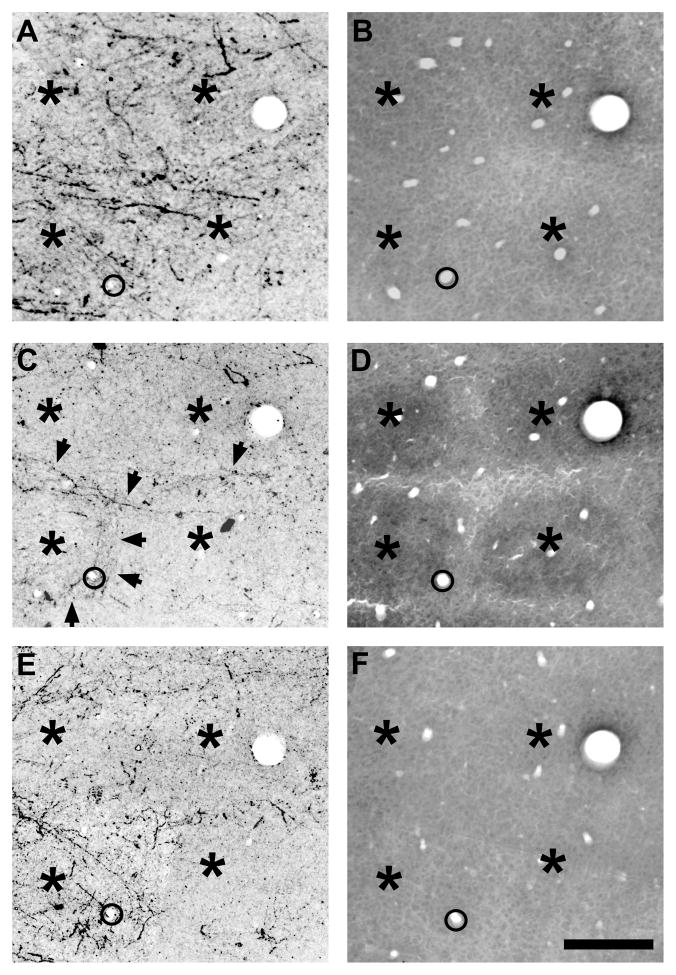
Fig. 1.
Laminar pattern of POm TCA innervation of adult mouse barrel cortex.
A, B: Photomicrographs of adjacent coronal sections through somatosensory thalamus showing a representative injection of PHA-L (asterisk) in the POm nucleus (A). The image in (A) was taken in darkfield optics, so the injection site appears white against a dark background. The adjacent section (B) was processed histochemically for cytochrome oxidase activity to indicate nuclear borders. The dotted line in (A) corresponds to the border between VPM and POm nuclei shown in (B). C, D: Photomicrographs of coronal sections through adult mouse barrel cortex showing laminar innervation patterns of PHA-L-labeled POm TCAs and terminals (dark lines and stipple). C: Pia-to-white matter image through the border (vertical arrow) between the PMBSF and the dysgranular cortex (dysgran). In the PMBSF, labeled axons formed a dense terminal plexus in layer Va; innervation of layer IV was sparse. Some axons extended superficially, and branched obliquely to enter layer I (small arrows, left). Granular and supragranular layers of the dysgranular cortex were densely innervated (arrow, right). In this and subsequent figures, Roman numbers refer to cortical layers. D, E: higher power adjacent coronal sections through layer IV showing PHA-L-labeled axons and terminals (D) or cytochrome oxidase staining (E) to indicate position of intensely reactive barrels (asterisks) and more lightly stained, intervening septa (arrowheads). Septa are narrow in mice, and few axon terminals or fibers (arrow, D) were present in layer IV. Scale bars = 250 μm in A–C; 100 μm in D, E.
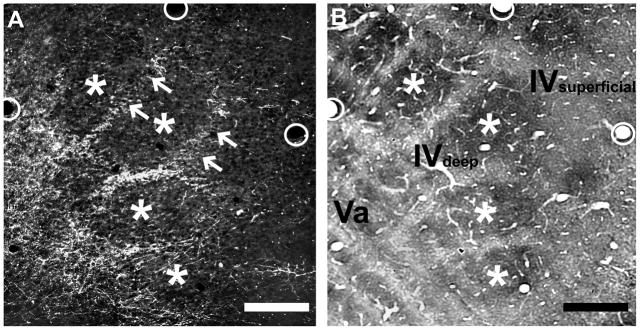
The lack of a clear septal innervation pattern of POm TCA terminations throughout layer IV in the mouse PMBSF, when viewed in coronal sections, was unexpected because in the PMBSF of adult rats, POm TCA terminations form a prominent septal pattern through the full extent of layer IV that also extends superficially into layer III and deeper into layer V (Koralek et al., 1988; Lu and Lin, 1993). To investigate this further in mice, we examined serial sections cut in an oblique, tangential plane through layers IV and V in which the barrels and the intervening septa in layer IV are best visualized. In the superficial half of layer IV, labeled POm TCAs were sparse, although largely confined to the septa where present (Fig. 2A, B). However, in passing through to the deeper half of layer IV, the density of labeled TCAs within the septa progressively increased, yielding a septal innervation pattern that became sharper as the sections approached the layer IV/Va boundary (arrows, Fig. 2A). In passing through to the most superficial aspect of layer Va, essentially at the boundary with layer IV, the septal labeling pattern remained, although labeling within the position of the barrel centers appeared as the plane of section dipped into the layer Va plexus, eventually becoming homogeneously labeled as the plane of section passed squarely through the layer Va plexus. Thus, in mice, POm TCAs are largely concentrated in layer IV septa only in the deepest aspects of layer IV, particularly at the layer IV/Va boundary, and merge into a dense plexus in layer Va that is homogeneous across overlying barrel and septal compartments. In contrast, this pattern differs from published accounts in rats, where septal innervation is found evenly throughout the depth of layer IV, and extends superficially into layer III and deeper into layer V (Koralek et al., 1988; Lu and Lin, 1993).
Fig. 2.
Septal-related patterning of POm TCAs in adult mouse PMBSF.
Photomicrographs of adjacent sections cut in an oblique, tangential plane through middle layers of the PMBSF, showing PHA-L-labeled POm TCAs in darkfield optics (A), or cytochrome oxidase activity (B) which reveals borders between barrels (asterisks) and intervening septa. The plane of the oblique section extends from the upper half of layer IV (layer IV superficial) through the deeper half of layer IV (layer IV deep) into layer Va. PHA-L-labeled POm TCAs formed a dense plexus in layer Va that extended into the septa at the layer IV/Va border and into the deeper half of layer IV, but the density of fibers within the septa decreased significantly in passing into the superficial half of layer IV (arrows). Very few labeled axons were present within the barrels. The circles in the two images show matched positions of the same blood vessels. Scale bars = 100 μm in A, B.
In the dysgranular cortex adjacent to the PMBSF, the pattern of innervation of labeled POm TCAs within layers I–IV is different in comparison with that of the PMBSF (Fig. 1C). Labeled axons form a dense column of innervation, characterized principally by the dense and complete innervation of the full extent of dysgranular layer IV, with both radially oriented as well as more oblique axons that extend through layers I–III (large arrow, Fig. 1C). In infragranular layers, the density of POm TCA innervation is much less in comparison with that of superficial layers, and appears more or less similar to that in deep layers of the PMBSF. The pattern of POm TCA innervation of mouse dysgranular cortex is similar to that described previously in rats (Lu and Lin, 1993).
Developmental patterns of POm TCA innervation of barrel cortex
The developmental progression of POm TCA innervation of barrel cortex in mice and rats was examined in P0–P10 pups by placing tiny deposits of lipophilic carbocyanine dyes (DiI and/or DiD) into POm and/or VPM nuclei. For both species, we used sections cut in the coronal plane to examine basic temporal and spatial features of the laminar innervation pattern, and used sections cut in an oblique, tangential plane through middle layers to examine in more detail the emergence of patterning in relationship to layer IV barrel/septal compartments.
Developmental sequence in mice
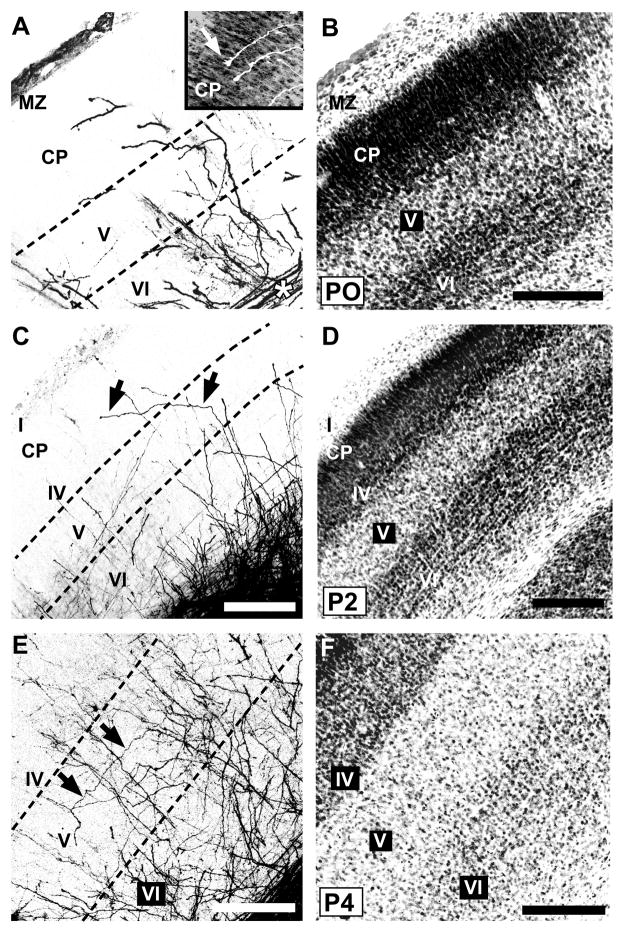
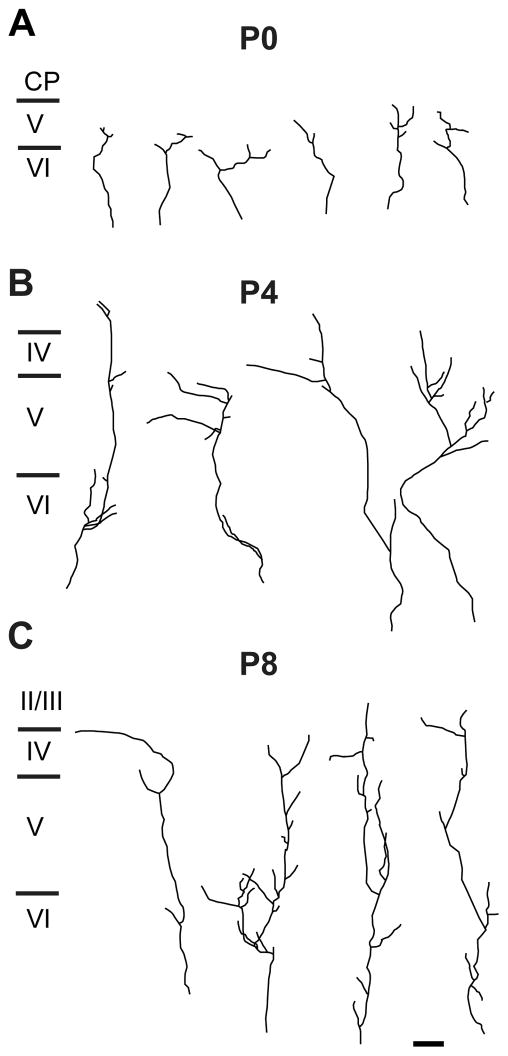
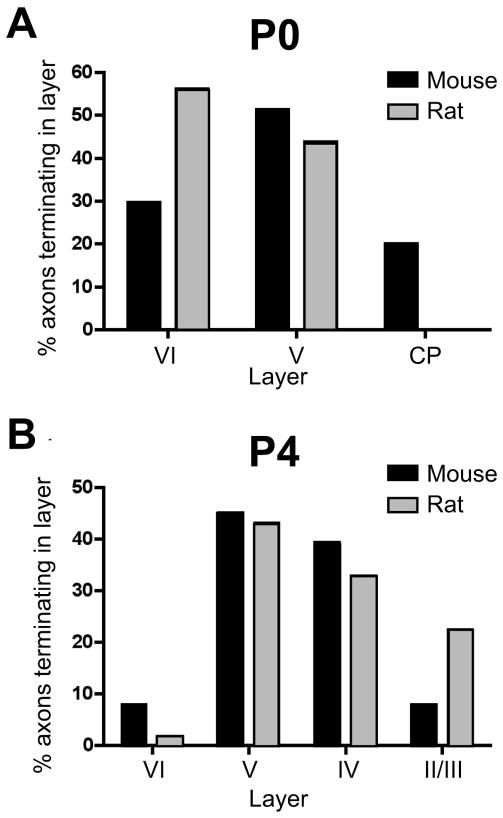
On the day of birth (P0), mouse somatosensory cortex consists of four principal cytoarchitectonically delineated layers (Fig. 3B). From superficial to deep, these are the marginal zone (layer I); a dense, undifferentiated cortical plate; layer V; and layer VI, in accordance with previous descriptions (Rice and Van der Loos, 1977; Senft and Woolsey, 1991; Agmon et al., 1993). Labeled POm TCAs were present within the barrel cortex on P0, forming a dense, tangentially oriented stratum within the subjacent white matter (asterisk, Fig. 3A). Many such axons, or interstitial branches from them, could be followed as they turned radially into the overlying cortex. Labeled POm axons then extended mostly radially or obliquely through layers VI and V, with fewer extending into the dense cortical plate (Figs. 3A; 4A; 10A). Few, however, appeared to reach the marginal zone (layer I). Many axons, including those that penetrated the cortical plate, were capped by growth cones (Fig. 3A, inset). Examination of the intracortical arbors of single TCAs showed that individual arbors were relatively simple—each parent radial axon within layers V and/or VI emitted one or a few, short oblique or horizontal branches (Fig. 4A). The tangential (mediolateral) extent of these simple arbors in the plane of the sections ranged from 36 to 301 μm (mean ± SD: 133 ± 44 μm; median: 119 μm).
Fig. 3.
Developmental progression of laminar innervation by POm TCAs in mouse barrel cortex.
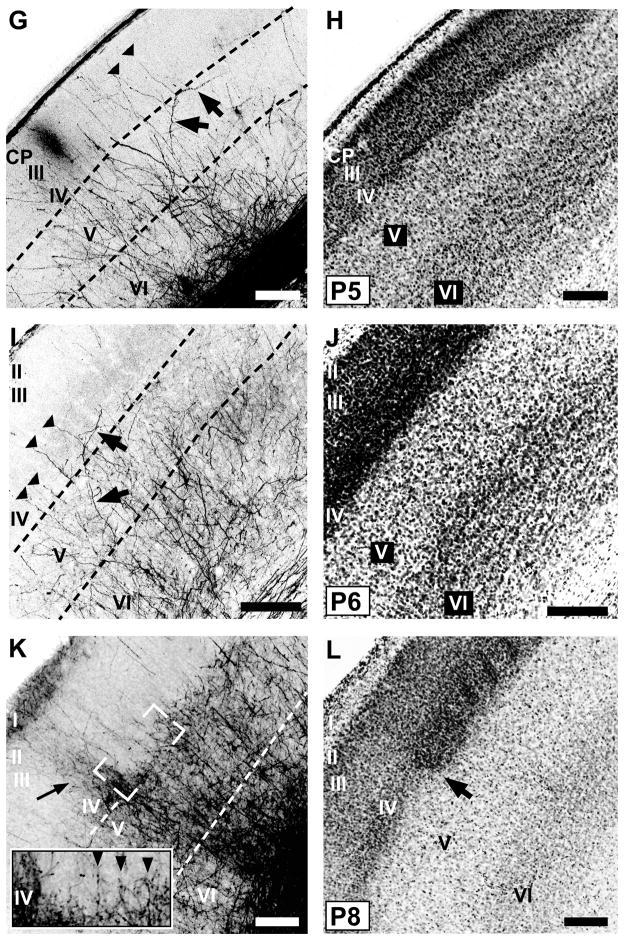
Pairs of confocal microscope images of coronal sections through developing mouse PMBSF at various postnatal ages from birth (P0) through P8 showing POm TCAs labeled by DiI (A,E,G,I,K) or DiD (C), and laminar cytoarchitecture revealed by Nissl counterstaining (B,D,F,H,J,L) of the corresponding dye-labeled sections. Dotted lines in the DiI/D images indicate borders between layers. A, B: At P0, many POm TCAs turned into barrel cortex from a subjacent stratum of labeled axons in the white matter (asterisk). Many axons penetrated the dense cortical plate (CP), and some were tipped by growth cones (inset, arrow; DiI-labeled axons are shown in white against the Nissl counterstaining). MZ, marginal zone. C, D: By P2, many radially oriented POm TCAs in layer V sent an oblique, horizontal branch into layer IV (arrows, C), which has differentiated from the CP by this age (D). E, F: By P4, overall density of labeled POm TCAs had increased in deep layers; long horizontal branches were evident in layer V (arrows, E). G, H: By P5, regularly-spaced, radially oriented branches spanned layer IV and extended into superficial layers, some of which (arrowheads, G) could be traced from long horizontal or oblique branches in layer V (arrows, G). I, J: By P6, the regularly spaced, radial branches that spanned layer IV (arrowheads, I) were evident; fewer labeled axons were found superficially in comparison with younger stages. Some oblique branches arose from layer V and traversed layer IV horizontally (arrows, I). K, L: By P8, features of an adult-like pattern emerged. Brackets (K) indicate position of image through layers IV/Va shown at higher power in inset. Regularly spaced, single fibers penetrated radially through layer IV in the PMBSF (arrowheads, inset). The layer Va plexus appeared scalloped at the border with layer IV. Adjacent to the PMBSF, labeled POm TCAs innervated densely all layers of dysgranular cortex (arrow, K). The cytoarchitectural border between the PMBSF and dysgranular cortex is shown by arrow in (L). Scale bars = 200 μm in B–L.
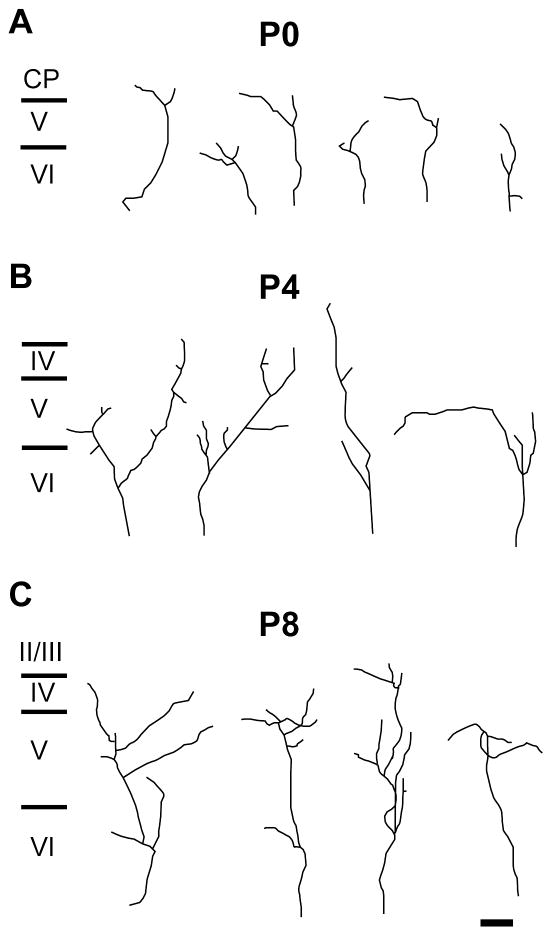
Fig. 4.
Morphology of single POm TCA arbors in developing mouse barrel cortex.
Representative examples of single, DiI-labeled POm TCAs in the PMBSF at P0 (A), P4 (B) and P8 (C). Individual arbors were selected from a total of 35 axons at P0 (n = 6 mice); from a total of 51 axons at P4 (n = 8 mice); and from a total of 43 axons at P8 (n = 9 mice). Scale bar = 100 μm in C (applies to A–C).
By P2, a nascent layer IV could be discerned at the bottom of the dense cortical plate (Fig. 3D). A notable feature at this age was the presence of labeled axons that traversed layer VI and part of layer V more or less radially, but then made an abrupt, oblique turn superficially, continuing on, and through, layer IV (arrows, Fig. 3C). Many of these labeled axons could be followed tangentially through layer IV for at least 200 μm. The density of labeled POm TCAs in superficial layers was quite sparse.
By P4, the density of axons in layers IV–VI had further increased (Figs. 3E,F; 4B). Labeled axons appeared more or less uniformly distributed in deep layers, and whereas many were radially oriented, tangentially oriented axons were also apparent in layer V (arrows, Fig. 3E). Examination of single TCAs at P4 showed that most parent axons first branched within layer VI (Fig. 4B). Daughter branches then either continued obliquely into layer V, where further, short branches were emitted mostly in layer Vb, or continued as a relatively simple branch that penetrated or traversed layer IV. At P4, the tangential (mediolateral) extent of POm TCA arbors in the plane of the sections had increased from earlier stages, and ranged from 59 to 572 μm (mean ± SD: 208 ± 66 μm; median: 197 μm). By P5–P6 (Fig. 3G–J), numerous, mostly radially-oriented labeled axons now penetrated layer IV at intervals that appeared somewhat regularly spaced (arrowheads, Fig. 3G, I). Many such radial, layer IV axons appeared as branches of obliquely oriented axons within layer Va (arrows, Fig. 3G). In other cases, oblique layer V axons penetrated layer IV tangentially (arrows, Fig. 3I). In superficial layers at P6, the density of labeled POm TCAs remained quite sparse.
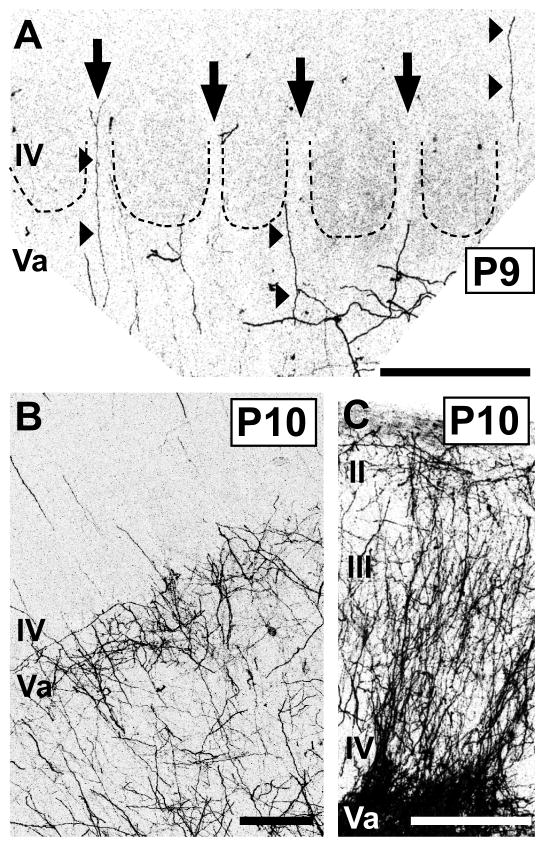
By P7–P8, several adult-like features in the pattern of POm innervation became apparent (Fig. 3K,L), and these patterns were even sharper by P9–P10 (Fig. 5). By P8, the first hint of the layer Va plexus became evident (Fig. 3K), becoming prominent by P9–10 (Fig. 5B). At the superficial aspect of this plexus, near the layer IV/Va border, the axon distribution appeared, in places, scalloped, as it does in the adult, with only a few, radial axons projecting upwards, into or through layer IV, at the peaks (Fig. 3K, arrowheads; Fig. 5A, arrows). Such radial axons traversed layer IV mostly through the septa when examined at high magnification (Fig. 5A). Examination of single TCAs at P8 (Fig. 4C) showed that branching appeared to have increased in layer Va in comparison with younger stages, with primarily radial branches that then entered or traversed layer IV. At P8, the tangential (mediolateral) extent of POm TCA arbors in the plane of the slices was similar to that at P4, ranging from 38 to 490 μm (mean ± SD: 252 ± 66 μm; median: 204 μm). Additionally, at these stages, the stark difference in innervation pattern of the dysgranular cortex in comparison with that of the PMBSF emerged (Figs. 3K,L; 5C). By P8, labeled axons were densely and uniformly distributed in dysgranular layer IV (Fig. 3K), and extended, as in a column, superficially up to layer I, a pattern which was just evident at P8 (arrow, Fig. 3K) but became strong by P10 (Fig. 5C).
Fig. 5.
Features of the adult-like pattern of laminar innervation by POm TCAs emerge at the beginning of the second postnatal week in mice.
Confocal images of carbocyanine dye-labeled POm TCAs in middle layers of PMBSF (A, B) or in dysgranular cortex (C). A: High-power image of a section through layers IV/Va taken from a P9 mouse. Dotted lines demarcate outlines of individual barrels; the barrels are visible by refringence. Septa are indicated by arrows. A few, radially oriented DiD-labeled axons entered or traversed layer IV through the septa (arrowheads); the origins of many could be identified as extensions from oblique or horizontal branches in the layer Va plexus. In this animal, the injection was small and very few axons were labeled, affording great clarity in the pattern. B: Image taken from a P10 mouse in which the DiI injection site was larger, resulting in a greater density of DiI-labeled POm TCAs. At this stage, the dense layer Va plexus was adult-like, as was the sparse innervation of layer IV. C: Image taken from same animal as that shown in (B); the POm TCA innervation of dysgranular cortex was dense and adult-like. Scale bars = 200 μm in A–C.
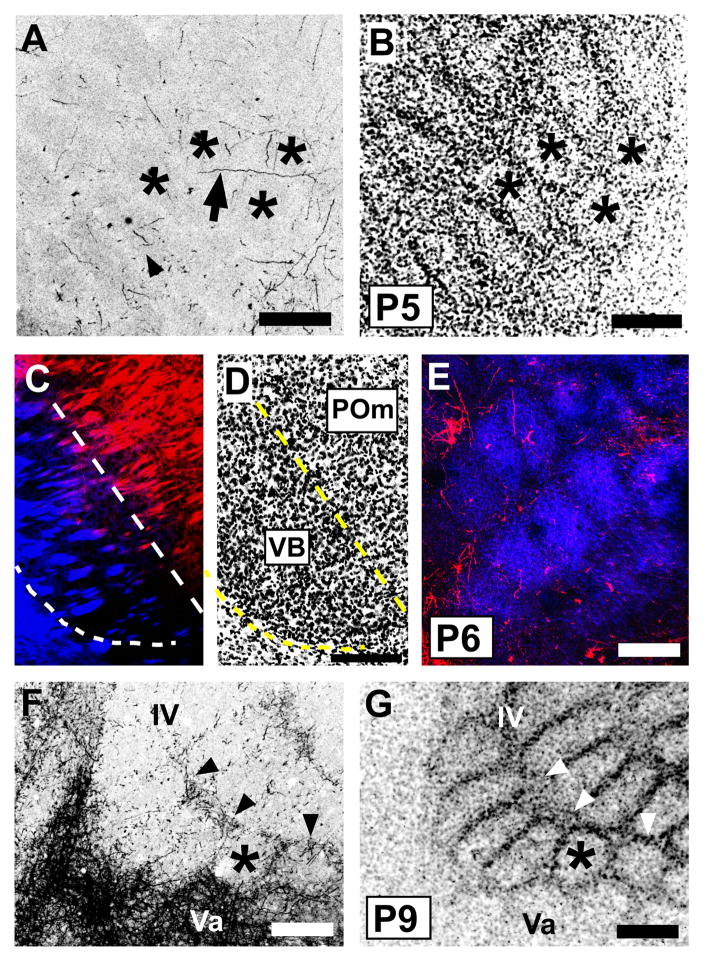
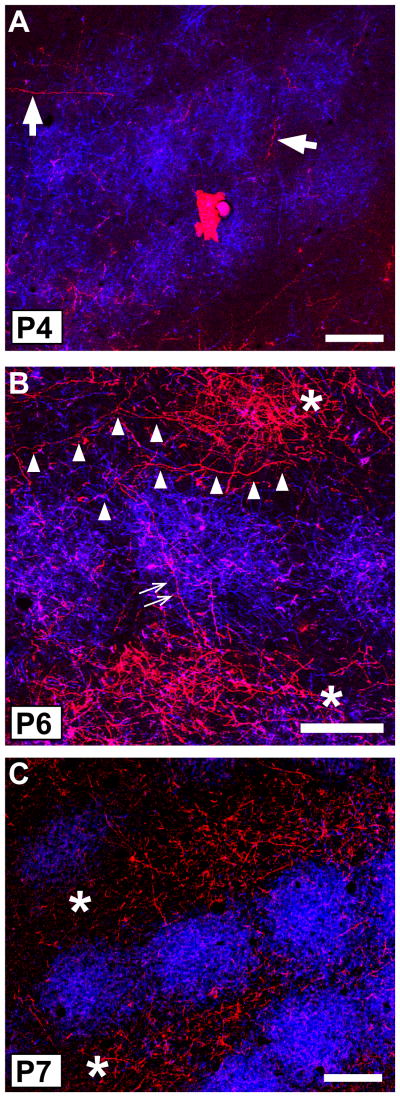
We examined in more detail the emergence of the layer IV/Va septal innervation pattern in sections cut in the tangential plane. Previous studies have shown that cytoarchitecturally, barrels appear in mouse layer IV between P3 and P4, with septa following by P5 (Welker and Woolsey, 1974; Rice and Van der Loos, 1977), which we confirmed (Fig. 6B). Only occasional POm TCA labeling was present in sections through granular and supragranular layers prior to P4, and in such sparseness, we were unable to discern evidence of incipient septal-like patterning in the distribution of the few labeled POm axons present at these stages (data not shown). By P4–P5 (Fig. 6A,B), radially cut axons or longer segments of obliquely or horizontally oriented axons were relatively sparsely distributed in layer IV. Such labeled axons were present in both septa and barrels (arrow, Fig. 6A). By P6–7, the major change appeared to be a slightly increased density of labeled POm TCAs in layer IV, with a hint of greater concentration within the septa in comparison to fewer labeled axon segments found within barrel centers. This was particularly evident in brains in which we colabeled VPM TCAs with a different carbocyanine dye, revealing in single sections both the barrel innervation by VPM TCAs and the incipient septal innervation by POm TCAs (Fig. 6C–E). By P8–10, the septal innervation pattern emerged clearly. Single, tangential sections that spanned the deeper half of layer IV–Va showed numerous labeled axons in the interbarrel layer IV septa that separated arcs, with more prominent labeling in the wider septa that separated barrel rows (arrowheads, Fig. 6F, G). A few axons were also present within the barrels. Inspection of serial, tangential sections that spanned the upper half of layer IV through layer Va revealed a near absence of labeled POm TCAs in the upper portion of layer IV (Fig. 7A, B), but in passing through to the deeper portion of layer IV, a clear septal innervation pattern progressively emerged (Fig. 7C,D). At the layer IV/Va boundary, labeling within the position of the barrels increased as the section dipped into the layer Va plexus (Fig. 7E, F).
Fig. 6.
Emergence of a septal-related pattern of POm TCA innervation in developing mouse barrel cortex.
A, B: Confocal microscope images of a tangential section through layer IV taken from a P5 mouse showing DiI-labeled POm TCAs (A) and barrel/septal cytoarchitecture revealed by Nissl-counterstaining (B). Asterisks show the same barrels in the two images; barrels are also visible in (A) by refringence. Short axon-fragments were found in both barrels (arrowhead) and septa; occasional longer, horizontally oriented axons were also seen in the septa (arrow). No overt septal pattern was yet evident at this age. C, D: A representative pair of adjacent, confocal images of coronal sections through the somatosensory thalamus of a P6 mouse in which two different carbocyanine dyes (C) were used simultaneously to label VPM (DiD, blue) and POm (DiI, red) TCAs. Nuclear borders were revealed cytoarchitecturally by Nissl staining (D); dotted lines delineate the extent of the VPM nucleus. E: Confocal image of a tangential section through layer IV taken from a mouse in which VPM and POm nuclei were both labeled as shown in (C). At P6, whisker-related clusters of VPM TCAs (blue) were well-formed as expected. Sparsely-distributed POm TCAs were found in both barrels and septa. F, G: Confocal images of an oblique, tangential section spanning the upper half of layer IV (upper right in the image) through layer Va (lower left in the image) taken from a P9 mouse showing DiI-labeled POm TCAs (F) and layer IV barrel/septal cytoarchitecture revealed by Nissl counterstaining (G). By this age, the dense layer Va plexus of POm TCAs was evident, as was a mature-like septal-pattern of innervation in the lower half of layer IV, particularly along barrel rows (arrowheads). The asterisks denote the position of the same, representative barrel in the two images. Scale bars = 200 μm in A, B, D–G (that in D also applies to C).
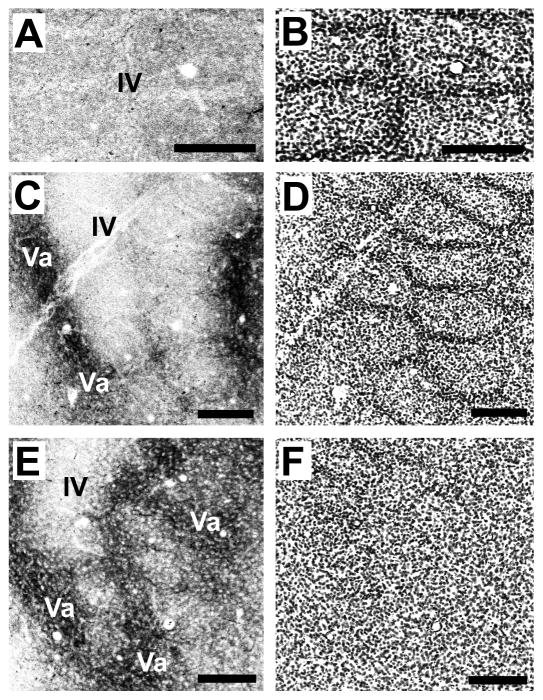
Fig. 7.
A mature-like septal-innervation pattern by POm TCAs is evident in mice by P10.
Confocal images of an adjacent series of tangential sections spanning the full depth of layer IV (A–D) through the layer IV/Va border (E,F) taken from a P10 mouse showing DiD-labeled POm TCAs (A, C, E) and barrel/septal cytoarchitecture revealed by Nissl counterstaining (B, D, F). A mature-like pattern was fully resolved by this age. Labeled POm TCAs were very sparse in the upper half of layer IV (A, B), but in passing progressively deeper, they became increasingly concentrated in the septa, particularly along barrel rows (C,D). At the layer IV/Va border, the septal-innervation pattern was prominent, with labeled POm TCAs also appearing in the position of the barrels as the plane of the section dips into the layer Va plexus (E, F). Scale bars = 200 μm in A–F.
Developmental sequence in rats
At P0, labeled POm TCAs were present within S1 cortex where, like mice, they formed a stratum in the white matter (asterisk, Fig. 8A), emitting branches into the overlying cortical layers. Long tangential branches (~300 μm long) were observed in layers V and VI (large arrows, Fig. 8A), some of which were capped by growth cones (arrowheads, Fig. 8A). In contrast to mice, only an occasional axon penetrated the dense cortical plate (Fig. 8A). Analysis of the intracortical arbors of single rat POm TCAs confirmed that at P0, the majority terminated deeper in barrel cortex in comparison with those in P0 mice (Figs. 9A; 10A), a significant difference in comparison with mice (p < 0.001). The morphology of single arbors in rats, however, was similar to those in mice (cf Figs. 4A, 9A). The tangential (mediolateral) extent of the arbors ranged from 12 to 479 μm (mean ± SD: 121 ± 40 μm; median: 125 μm).
Fig. 8.
Developmental progression of laminar innervation by POm TCAs in rat barrel cortex.
Confocal microscope images of coronal sections through developing rat PMBSF at various postnatal ages showing DiD-labeled POm TCAs (A,C) and corresponding laminar cytoarchitecture revealed by Nissl counterstaining (B,D), or showing dual labeling of VPM and POm TCAs (E–G). Dashed lines in the DiD images indicate borders between layers. A, B: At P0, POm TCAs entered barrel cortex from a white matter stratum of labeled axons (asterisk). Many axons were tipped by growth cones (arrowheads), but unlike mice, few penetrated the dense cortical plate (CP). Most axons were radially oriented; some however took long, horizontal or oblique trajectories into layer V (arrows). Small double arrows indicate a retrogradely-labeled corticothalamic cell. MZ, marginal zone. C, D: By P2, labeled axons remained mostly restricted to infragranular layers and avoided layer IV, which has emerged from the CP by this age. E: By P4, whisker-related clusters of VPM TCAs (DiD, blue) within layer IV barrels (asterisks) were well formed as expected. Branches of DiI-labeled POm TCAs (red) had by this age entered and extended through layer IV, although they occupied both septa and barrels (arrowheads). Other labeled POm TCAs took long, oblique trajectories through infragranular layers, spanning several barrel-widths (large arrows). Some radial axons extended into superficial layers (small double arrows), reaching layer I. F, G: Confocal images through middle layers taken from a P6 rat in which the VPM and POm TCA projections were labeled simultaneously. In (F), the channel showing DiD-labeled POm axons (red) is merged with that showing DiI-labeled VPM TCAs (blue), while in (G), the channel showing POm TCAs is displayed separately. Labeled POm TCAs remained in both septa (arrowhead) and barrels (arrows), but they appeared to be receding somewhat from barrels in comparison with younger ages. The dysgranular cortex was densely innervated by this age. Scale bars = 200 μm (A–G).
Fig. 9.
Morphology of single POm TCA arbors in developing rat barrel cortex.
Representative examples of single, DiI-labeled POm TCAs in the PMBSF at P0 (A), P4 (B) and P8 (C). Individual arbors were selected from a total of 48 axons at P0 (n = 8 rats); from a total of 58 axons at P4 (n = 9 rats); and from a total of 38 axons at P8 (n = 7 rats). Scale bar = 100 μm in C (also applies to A–C).
Fig. 10.
Comparison between rats and mice in the extent of advancement of POm TCAs into barrel cortex during development.
Graphs showing laminar distributions of single-axon endpoints (the most superficial point of each axon examined) plotted as a percentage of the total number of axons analyzed at P0 (A) and P4 (B) for both mice (black bars) and rats (gray bars). At P0, a substantial proportion of POm TCAs in mice penetrated the CP while none of the axons in our sample from rats did. A Fisher exact probability test showed that radial advancement of POm TCAs into the cortex of P0 mice was significantly different in comparison with that in P0 rats (p < 0.001). By P4, a greater proportion of rat POm TCAs extended into superficial layers in comparison with mice, and by this age, there were no significant differences in radial advancement of POm TCAs between species (p > 0.1). The numbers of axons and animals used for each species and age in this analysis are given in the legends to Figures 4 and 9.
By P2, layer IV had differentiated from the cortical plate (Fig. 8D). However, few axons penetrated layer IV (Fig. 8C). This lag between appearance of layer IV and extension of POm TCAs into it contrasts with mice, where a greater fraction of the POm axons was present within the cortical plate and thus were already present within layer IV as it emerged by P2.
By P3–P4, POm TCAs now penetrated layer IV and often extended into superficial layers, sometimes reaching layer I (Figs. 8E; 9B; 10B). Thus, the radial advancement into the cortex was now similar to that in mice (Fig. 10B; p > 0.1). Morphologically, single arbors exhibited terminal-like tufts of branches in layer V (Fig. 9B) or extended horizontally in layer V for relatively long distances (arrows, Fig. 8E). The tangential (mediolateral) extent of single arbors ranged from 46 to 680 μm (mean ± SD: 252 ± 71 μm; median: 223 μm). Within layer IV, many of the axons extended radially through the full thickness of the layer (arrowheads, Fig. 8E; Fig. 9B). By these ages, layer IV barrels and septa become evident cytoarchitecturally (Rice et al., 1985) and VPM projections are already organized into whisker-related clusters occupying barrel centers (asterisks, Fig. 8E) as expected (Erzurumlu and Jhaveri, 1990; Schlaggar and O’Leary, 1994; Catalano et al., 1996; Rhoades et al., 1996). However, labeled POm TCAs did not yet display any obvious parcellation to septa at P4; instead they traversed both barrel center and septal compartments (Fig. 8E; Fig. 11A).
Fig. 11.
Emergence of septal-related pattern of POm TCA innervation in developing rat barrel cortex.
Representative confocal microscope images of tangential sections through the middle of layer IV taken at three developmental ages from rats in which the VPM and POm TCA projections were labeled simultaneously by focal injection of DiD into the VPM nucleus (blue) and DiI into the POm nucleus (red). A: At P4, whisker-related clusters of VPM TCAs within barrels were well delineated as expected by this age. Labeled POm axons were sparse in layer IV, and were present in both barrels and septa (arrows). B: By P6, the density of POm TCA innervation in the septa had increased greatly (asterisks). Long, horizontally oriented POm TCAs were found along septa (arrowheads), or traversed barrels (small double arrows), spanning two rows. C: By P7, a mature-like septal-innervation pattern had resolved, in which the majority of labeled POm TCAs were restricted to the septa (asterisks). Scale bars = 200 μm in A–C.
From P5 through P8, the mature P0m TCA innervation pattern resolved both in the PMBSF (Figs. 8F,G; 9C; 11B,C; 12) and in the dysgranular cortex (Fig. 8F,G). At P6, POm TCAs were concentrated in layer IV septa (asterisks, Fig. 11B), although some remained within the barrels (Fig. 8F,G; 11B). By P7–P8, POm axons appeared largely confined to septa, and were present throughout the depth of layer IV (Fig. 11C; 12C, D). This pattern is unlike that in mice, in which the septal innervation pattern was evident only at the layer IV/Va border. Additionally, serially-ordered, tangential sections spanning lower layer III through layer Va showed that, unlike mice, labeled POm TCAs extended from the layer IV septa superficially into layer III (Fig. 12A, B), and deeper into layer Va (Fig. 12E, F), where axons remained concentrated within septal-like columns (see also Koralek et al, 1988). Inspection of single POm TCA arbors at P8 showed that most were fairly radial in their intracortical trajectory, with terminal-like tufts at the layer V/VI border or side branches that spread more evenly across layers IV–VI (Fig. 9C). Unlike mice, there did not appear in the single-fiber morphology to be an elaboration of terminal-like arbors in layer Va. Overall, the tangential (mediolateral) extent of single arbors ranged from 78 to 612 μm (mean ± SD: 230 ± 47 μm; median: 197 μm).
Fig. 12.
Septa-related columns of POm TCA terminations spanning layers III through Va in rats. Photomicrographs of serially-ordered tangential sections spanning lower layer III (A, B), through layer IV (C, D) to layer Va (E, F) showing PHA-L-labeled POm TCAs (A, C, E) or cytochrome oxidase staining of the adjacent sections (B, D, F) to indicate the more intensely reactive barrels in layer IV (asterisks, D). PHA-L was injected into the POm nucleus on P6, the animal was killed on P8. Asterisks indicate the barrels in layer IV (C, D), or their corresponding positions in layer III (A, B) and layer Va (E, F). In layer IV, TCAs (arrows) are largely confined to septa. Labeled TCAs remain concentrated within septal-like columns in layer III and in layer Va, with fewer axons present above and below the position of the barrels (asterisks). The circle in the lower left corner of each image shows a blood vessel common to all sections. Scale bar = 200 μm in F (applies to A–F).
DISCUSSION
We describe here the organization of POm thalamocortical axon terminations in adult mouse PMBSF and compare such patterns to those described previously in adult rats (Koralek et al., 1988; Chmielowska et al., 1989; Lu and Lin, 1993; Deschenes et al., 1998). We then characterize, in both mice and rats, the developmental sequence during the first ten postnatal days leading to the mature pattern. Our major findings are represented schematically in Figure 13 and are summarized as follows. First, subtle differences between adult mice and rats are evident in the laminar innervation pattern. In mice, the POm innervation of layer IV is sparse except at the layer IV/Va border, where a clear septa-related innervation pattern is evident. In layer Va, a dense plexus extends uniformly across the overlying layer IV barrel and septal compartments. In rats, according to previous descriptions, POm TCAs also innervate layer IV septa, but unlike mice, the axons occupy the full width of layer IV and extend superficially into lower layer III, and deeper, into layer Va, all as a column, a pattern which we confirmed (e.g. Fig. 12). The innervation of layer Va in rats, unlike mice, does not appear uniform in published accounts: the density of POm axons in layer V is greater subjacent to the septa, and sparser in layer V subjacent to barrel centers (Koralek et al., 1988; Lu and Lin, 1993). Second, in both species, POm TCAs are already present within the cortical layers at birth, but species differences are apparent. In mice, POm axons extend through the full thickness of the cortex, penetrate the dense cortical plate, and are present within layer IV as it differentiates. In contrast, in rats, POm axons are restricted to deep layers, and do not enter layer IV until 1–2 days after its differentiation. Third, in both species, no clear layer IV septa innervation pattern emerges until several days after the cytoarchitectural appearance of barrels, and well after the emergence of whisker-related clusters of TCAs from the VPM nucleus. Initially POm axons traverse barrel center and septal compartments, but then undergo a steady, directed progression of growth that is similar in both species. At all ages, single arbors can span distances in the mediolateral dimension that exceed the widths of single barrels. Finally, the major features of the mature pattern resolve earlier in rats (~P5–8) than in mice (~P7–9).
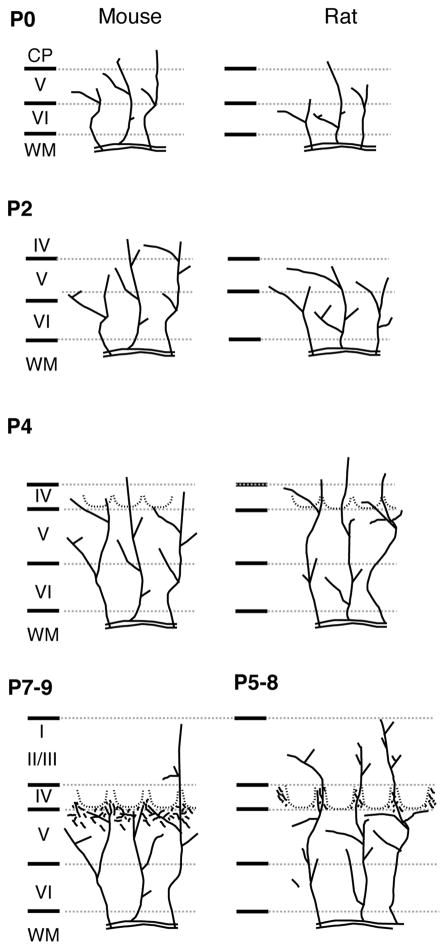
Fig. 13.
A schematic summary of the developmental sequence of POm TCA innervation of barrel cortex in mice (left column) and rats (right column). At P0, POm TCAs are present within the cortical layers in both species, but in mice, many more extend superficially, into the CP, than in rats, where they are mostly confined to deep layers. By P2, layer IV has emerged in both species; POm TCAs are present in layer IV in mice, but are mostly confined to infragranular layers in rats. By P4, barrel cytoarchitecture (dotted lines) and barrel-clusters of VPM TCAs (not shown) are both evident. In both species, POm axons penetrate layer IV and are present within barrels and septa; a greater proportion of POm TCAs in rats now extends superficially in comparison with mice. The major features of a mature-like pattern of POm TCA innervation emerge slightly earlier in rats (P5–8) in comparison with mice (P7–9). WM, white matter.
Advantages and limitations of the approaches used
The use of carbocyanine dyes in the fixed developmental material allowed us to achieve high temporal resolution of innervation patterns as well as enabled us to use two different dyes simultaneously to compare directly the layer IV compartmental termination patterns of the lemniscal and paralemniscal thalamic projection systems. Contamination by other fiber systems inadvertently passing through the intrathalamic dye deposits is unlikely. Previous studies have ruled out contamination by certain brainstem afferents (Catalano et al., 1996); contamination by afferents from the zona incerta (Porter and White, 1983; Saper, 1985; Lin et al., 1990) is also unlikely since it lies some distance from our injection target site where POm TCAs to the PMBSF originate (Nothias et al., 1988; Chiaia et al., 1991; Fabri and Burton, 1991; Diamond et al., 1992; Lu and Lin, 1993; Deschenes et al., 1998; Bureau et al., 2006). Contamination by afferents from some of the intralaminar nuclei is possible, but would be negligible because of the sparseness of their innervation (Zhang and Deschenes, 1998). We observed some retrogradely-labeled corticothalamic neurons, but it is unlikely that they affected significantly the overall patterns attributed to POm TCAs, since our single-fiber analyses were based strictly on axon arbors that could be traced from the white matter stratum.
Comparison between adult mice and rats in POm innervation of S1
Our data indicate differences between rats and mice in laminar organization of paralemniscal input to barrel cortex, supporting recent studies showing functional differences between rats and mice in intrinsic barrel cortex circuitry through which paralemniscal information is processed (Bureau et al., 2006). In rats, ascending connections from layer Va to layer II are functionally strong within septal columns, but are much weaker within barrel columns (Shepherd and Svoboda, 2005). Such disparity in intrinsic connection strength in rats matches an anatomical disparity in the density of POm innervation of septal and barrel columns in layer Va (Koralek et al., 1988; Lu and Lin, 1993; present study). In contrast, in mice, the strength of functional connectivity of layer Va projections to layer II within barrel and septal columns is equally robust (Bureau et al., 2006); such similarity matches our observations that POm TCA innervation of layer Va is uniform across barrel and septal columns. Thus, it would appear that such species differences in intrinsic barrel cortex circuitry subserving paralemniscal information processing is a continuation of anatomical differences already evident at the very first stage in the transfer of information from the POm thalamus to barrel cortex. Although the significance of such differences is unknown, one possibility is that mice have different demands on sensory-motor integration that underlies active whisking in comparison with rats. The POm nucleus transmits signals related to whisker movement (Yu et al., 2006). The speed of whisker movements is faster in mice than in rats, a reflection of species differences in the composition of fiber types of which the intrinsic whisker muscles are composed (Jin et al., 2004). Thus, the uniform innervation of layer Va across barrel and septal columns may indicate that in mice, POm TCA inputs have more direct access to, and thus can rapidly modulate, infragranular motor output circuits distributed across barrel and septal columns (Wise and Jones, 1977; Porter and White, 1983; Crandall et al., 1986; Hoffer et al., 2005; Mitchell and Macklis, 2005). In rats, such integration might occur more indirectly through POm TCA innervation of layers Va and IV within septal columns that is then relayed via intrinsic circuitry that couples layers IV and Va (Feldmeyer et al., 2005) or that couples infragranular motor output neurons occupying septal columns with those occupying barrel columns (Frick et al., 2007). The POm TCA innervation of layer IV appeared very sparse in mice in comparison with the denser innervation through the full extent of layer IV septa in rats (Lu and Lin, 1993). Septa are much narrower in mice in comparison with rats (Welker and Woolsey, 1974), and thus it is possible that the impression of sparseness is partly an illusion simply of the much more limited space through which POm axons can traverse. This could explain why, in tangential sections, we found evidence of a clear septa-related innervation pattern principally in the deepest aspects of layer IV, where the septa are widest (Rice et al., 1985). Alternatively, there may be a genuine paucity of POm TCA innervation of layer IV in mice in comparison with rats (see also Bureau et al., 2006). Future quantitative studies comparing the density of POm TCA synapses within layer IV septa in rats with that of mice, or direct investigation of functional connectivity between POm and layer IV septa in mice, will be necessary to resolve this.
Developmental patterns of POm TCA innervation of barrel cortex
The single-fiber analyses showed that POm TCAs underwent a progressive, directed growth over time, with few, early appearing inappropriate branches within barrel centers eliminated at later stages. We found no evidence of exuberant growth followed by regression. Such a pattern of directed growth is consistent with that described for the development of other types of circuitry, including VPM TCAs to barrel cortex (Agmon et al., 1995; Catalano et al., 1996), geniculocortical inputs to cat visual cortex (Antonini and Stryker, 1993), and intrinsic barrel cortex projections (Rhoades et al., 1996; Miller et al., 2001; Bureau et al., 2004). Nevertheless, the complexity of single POm TCA arbors in adult rats (Deschenes et al., 1998) far exceeds the typical morphology we observed at P8. This difference likely reflects the continued maturation of POm TCA arbors beyond the times we examined, similar to the extended period of elaboration of VPM TCA arbors (Jensen and Killackey, 1987; Agmon et al., 1993; Catalano et al., 1996; Rebsam et al., 2002).
While thalamic inputs from VPM and POm terminate in largely non-overlapping territories within barrel cortex, the factors that drive such partitioning developmentally remain largely unexplored. One idea has been that VPM afferents arrive first, forming a barrel/septa-template to which later-arriving POm TCAs conform. This is based principally on the observations that barrel centers are discernible cytoarchitecturally earlier than septa (Rice and Van der Loos, 1977) and that VPM TCAs are present within infragranular layers at birth and rapidly (within ~1–2 days) form whisker-related clusters (Wise and Jones, 1978; Erzurumlu and Jhaveri, 1990; Senft and Woolsey, 1991; Agmon et al., 1993; Schlaggar and O’Leary, 1994; Catalano et al., 1996; Rebsam et al., 2002). Our data demonstrate that POm TCAs are also well-represented within infragranular layers by birth in both rats and mice, in agreement with a previous study of POm TCAs in developing rats (Galazo et al., 2007), and exhibit single-fiber morphology and a laminar innervation pattern very similar to those reported for VPM axons (Catalano et al., 1996; Rebsam et al., 2002; Lee et al., 2005). Thus, at birth, both sets of TCAs from POm and VPM nuclei are present and extend equally into the barrel cortex, which dispels the notion that there is a major temporal disparity in the arrival and positioning of the two sets of axons at the beginning of postnatal life. We cannot, however, rule out the possibility that VPM axons may have arrived prior to POm axons during embryonic development. The fact that the two sets of axons commingle within the cortex at birth raises the possibility that some competitive interaction occurs between them that influences the segregation process (Goodman and Shatz, 1993). However, such interactions are unlikely to be mutually competitive, since in both species, the emergence of septal patterning by POm TCAs followed by several days barrel-related patterning by VPM TCAs. In the case of rats, the main front of POm TCAs entered layer IV several days after whisker-related clusters are evident in layer IV, which may indicate that in this species, POm TCAs conform to a barrel/septa template previously established in layer IV by VPM TCAs. Such a template may also influence connectivity of other septa-directed connections, including callosal and intrinsic axons, both of which display a timecourse of layer IV innervation and patterning similar to that which we describe here for POm TCAs (Ivy and Killackey, 1981; Rhoades et al., 1996). In the case of mice, the POm TCAs were present within layer IV contemporaneously with the period in which VPM TCAs colonize layer IV and form their whisker-related clusters, suggesting that some repulsive interaction gradually leads to the retraction of the majority of POm axons from layer IV. This early expulsion of POm axons from layer IV may explain why the septal compartments are relatively so much smaller in mice than in rats. In any case, future studies in which VPM TCAs are selectively prevented from colonizing the barrel cortex will be necessary to determine whether VPM TCA patterning influences subsequent septal patterning by POm TCAs, but the converse is unlikely.
The presence of POm TCAs within layer IV septa in rats and their eventual recession from layer IV in mice may have implications for differences between these species in the stability of the barrel pattern in the face of certain perturbations. In both rats and mice, elevation of serotonin levels within the PMBSF leads to excessive growth of VPM TCAs into layer IV septa and fusion of barrels (Vitalis et al., 1998; Lieske et al., 1999; Lotto et al., 1999; Boylan et al., 2000; Rebsam et al., 2002). Surprisingly, in rats, but not in mice, VPM TCAs spontaneously recede from septa at ~P6, reverting to a normal barrel pattern, even in the continued presence of elevated serotonin levels (Boylan et al., 2000), an effect that cannot be attributed to neural activity or insensitivity to serotonin (Rhoades et al., 1994; Boylan et al., 2001). It is therefore striking that the timing of such recovery in VPM patterning correlates well with the timing of the appearance of mature septal patterning and density of innervation by POm TCAs in rats, suggesting that septal patterning of POm afferents actively participates in the restoration of the barrel pattern. Conversely, the lack of recovery in VPM patterning in mice with elevated serotonin levels (Vitalis et al., 1998) might reflect the normal paucity of POm axons in layer IV at comparable ages.
Acknowledgments
Grant Sponsor: The Mount Sinai School of Medicine; National Institutes of Health, U.S. Public Health Service; grant number: NS34659
We thank Dr. Patrick R. Hof for comments on the manuscript and Dr. Youngchao Ge for help with statistical design.
LITERATURE CITED
- Abdel-Majid RM, Leong WL, Schalkwyk LC, Smallman DS, Wong ST, Storm DR, Fine A, Dobson MJ, Guernsey DL, Neumann PE. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nat Genet. 1998;19:289–291. doi: 10.1038/980. [DOI] [PubMed] [Google Scholar]
- Agmon A, Yang LT, O’Dowd DK, Jones EG. Organized growth of thalamocortical axons from the deep tier of terminations into layer IV of developing mouse barrel cortex. J Neurosci. 1993;13:5365–5382. doi: 10.1523/JNEUROSCI.13-12-05365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmon A, Yang LT, Jones EG, O’Dowd DK. Topological precision in the thalamic projection to neonatal mouse barrel cortex. J Neurosci. 1995;15:549–561. doi: 10.1523/JNEUROSCI.15-01-00549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahissar E, Sosnik R, Haidarliu S. Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature. 2000;406:302–306. doi: 10.1038/35018568. [DOI] [PubMed] [Google Scholar]
- Ahissar E, Sosnik R, Bagdasarian K, Haidarliu S. Temporal frequency of whisker movement. II. Laminar organization of cortical representations. J Neurophysiol. 2001;86:354–367. doi: 10.1152/jn.2001.86.1.354. [DOI] [PubMed] [Google Scholar]
- Antonini A, Stryker MP. Development of individual geniculocortical arbors in cat striate cortex and effects of binocular impulse blockade. J Neurosci. 1993;13:3549–3573. doi: 10.1523/JNEUROSCI.13-08-03549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan CB, Bennett-Clarke CA, Crissman RS, Mooney RD, Rhoades RW. Clorgyline treatment elevates cortical serotonin and temporarily disrupts the vibrissae-related pattern in rat somatosensory cortex. J Comp Neurol. 2000;427:139–149. doi: 10.1002/1096-9861(20001106)427:1<139::aid-cne9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Boylan CB, Kesterson KL, Bennett-Clarke CA, Chiaia NL, Rhoades RW. Neither peripheral nerve input nor cortical NMDA receptor activity are necessary for recovery of a disrupted barrel pattern in rat somatosensory cortex. Dev Brain Res. 2001;129:95–106. doi: 10.1016/s0165-3806(01)00163-8. [DOI] [PubMed] [Google Scholar]
- Bureau I, Shepherd GM, Svoboda K. Precise development of functional and anatomical columns in the neocortex. Neuron. 2004;42:789–801. doi: 10.1016/j.neuron.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Bureau I, von Saint Paul F, Svoboda K. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol. 2006;4:e382. doi: 10.1371/journal.pbio.0040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano SM, Robertson RT, Killackey HP. Early ingrowth of thalamocortical afferents to the neocortex of the prenatal rat. Proc Natl Acad Sci U S A. 1991;88:2999–3003. doi: 10.1073/pnas.88.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano SM, Robertson RT, Killackey HP. Individual axon morphology and thalamocortical topography in developing rat somatosensory cortex. J Comp Neurol. 1996;367:36–53. doi: 10.1002/(SICI)1096-9861(19960325)367:1<36::AID-CNE4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Rhoades RW, Fish SE, Killackey HP. Thalamic processing of vibrissal information in the rat: II. Morphological and functional properties of medial ventral posterior nucleus and posterior nucleus neurons. J Comp Neurol. 1991;314:217–236. doi: 10.1002/cne.903140203. [DOI] [PubMed] [Google Scholar]
- Chmielowska J, Carvell GE, Simons DJ. Spatial organization of thalamocortical and corticothalamic projection systems in the rat SmI barrel cortex. J Comp Neurol. 1989;285:325–338. doi: 10.1002/cne.902850304. [DOI] [PubMed] [Google Scholar]
- Crandall JE, Korde M, Caviness VS., Jr Somata of layer V projection neurons in the mouse barrelfield cortex are in preferential register with the sides and septa of the barrels. Neurosci Lett. 1986;67:19–24. doi: 10.1016/0304-3940(86)90201-6. [DOI] [PubMed] [Google Scholar]
- Deschenes M, Veinante P, Zhang ZW. The organization of corticothalamic projections: reciprocity versus parity. Brain Res Rev. 1998;28:286–308. doi: 10.1016/s0165-0173(98)00017-4. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus. J Comp Neurol. 1992;318:462–476. doi: 10.1002/cne.903180410. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Parham C. The afferent connections of the lateral agranular field of the rat motor cortex. J Comp Neurol. 1983;217:76–88. doi: 10.1002/cne.902170404. [DOI] [PubMed] [Google Scholar]
- Dufour A, Seibt J, Passante L, Depaepe V, Ciossek T, Frisen J, Kullander K, Flanagan JG, Polleux F, Vanderhaeghen P. Area specificity and topography of thalamocortical projections are controlled by ephrin/Eph genes. Neuron. 2003;39:453–465. doi: 10.1016/s0896-6273(03)00440-9. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Dev Brain Res. 1990;56:229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- Fabri M, Burton H. Topography of connections between primary somatosensory cortex and posterior complex in rat: a multiple fluorescent tracer study. Brain Res. 1991;538:351–357. doi: 10.1016/0006-8993(91)90455-5. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Roth A, Sakmann B. Monosynaptic connections between pairs of spiny stellate cells in layer 4 and pyramidal cells in layer 5A indicate that lemniscal and paralemniscal afferent pathways converge in the infragranular somatosensory cortex. J Neurosci. 2005;25:3423–3431. doi: 10.1523/JNEUROSCI.5227-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Feldmeyer D, Helmstaedter M, Sakmann B. Monosynaptic connections between pairs of L5A pyramidal neurons in columns of juvenile rat somatosensory cortex. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm074. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Galazo MJ, Martinez-Cerdeno V, Porrero C, Clasca F. Embryonic and postnatal development of the layer I-directed (“matrix”) thalamocortical system in the rat. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm059. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Godement P, Vanselow J, Thanos S, Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987;101:697–713. doi: 10.1242/dev.101.4.697. [DOI] [PubMed] [Google Scholar]
- Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Hoffer ZS, Arantes HB, Roth RL, Alloway KD. Functional circuits mediating sensorimotor integration: quantitative comparisons of projections from rodent barrel cortex to primary motor cortex, neostriatum, superior colliculus, and the pons. J Comp Neurol. 2005;488:82–100. doi: 10.1002/cne.20579. [DOI] [PubMed] [Google Scholar]
- Inan M, Lu HC, Albright MJ, She WC, Crair MC. Barrel map development relies on protein kinase A regulatory subunit II beta-mediated cAMP signaling. J Neurosci. 2006;26:4338–4349. doi: 10.1523/JNEUROSCI.3745-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince-Dunn G, Hall BJ, Hu SC, Ripley B, Huganir RL, Olson JM, Tapscott SJ, Ghosh A. Regulation of thalamocortical patterning and synaptic maturation by NeuroD2. Neuron. 2006;49:683–695. doi: 10.1016/j.neuron.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Ivy GO, Killackey HP. The ontogeny of the distribution of callosal projection neurons in the rat parietal cortex. J Comp Neurol. 1981;195:367–389. doi: 10.1002/cne.901950302. [DOI] [PubMed] [Google Scholar]
- Jensen KF, Killackey HP. Terminal arbors of axons projecting to the somatosensory cortex of the adult rat. I. The normal morphology of specific thalamocortical afferents. J Neurosci. 1987;7:3529–3543. doi: 10.1523/JNEUROSCI.07-11-03529.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin TE, Witzemann V, Brecht M. Fiber types of the intrinsic whisker muscle and whisking behavior. J Neurosci. 2004;24:3386–3393. doi: 10.1523/JNEUROSCI.5151-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama GH, Robertson RT. Development of geniculocortical projections to visual cortex in rat: evidence early ingrowth and synaptogenesis. J Comp Neurol. 1993;335:123–148. doi: 10.1002/cne.903350109. [DOI] [PubMed] [Google Scholar]
- Kim U, Ebner FF. Barrels and septa: separate circuits in rat barrels field cortex. J Comp Neurol. 1999;408:489–505. [PubMed] [Google Scholar]
- Koralek KA, Jensen KF, Killackey HP. Evidence for two complementary patterns of thalamic input to the rat somatosensory cortex. Brain Res. 1988;463:346–351. doi: 10.1016/0006-8993(88)90408-8. [DOI] [PubMed] [Google Scholar]
- Lee LJ, Iwasato T, Itohara S, Erzurumlu RS. Exuberant thalamocortical axon arborization in cortex-specific NMDAR1 knockout mice. J Comp Neurol. 2005;485:280–292. doi: 10.1002/cne.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieske V, Bennett-Clarke CA, Rhoades RW. Effects of serotonin on neurite outgrowth from thalamic neurons in vitro. Neuroscience. 1999;90:967–974. doi: 10.1016/s0306-4522(98)00501-6. [DOI] [PubMed] [Google Scholar]
- Lin CS, Nicolelis MA, Schneider JS, Chapin JK. A major direct GABAergic pathway from zona incerta to neocortex. Science. 1990;248:1553–1556. doi: 10.1126/science.2360049. [DOI] [PubMed] [Google Scholar]
- Lotto B, Upton L, Price DJ, Gaspar P. Serotonin receptor activation enhances neurite outgrowth of thalamic neurones in rodents. Neurosci Lett. 1999;269:87–90. doi: 10.1016/s0304-3940(99)00422-x. [DOI] [PubMed] [Google Scholar]
- Lu SM, Lin RC. Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens Mot Res. 1993;10:1–16. doi: 10.3109/08990229309028819. [DOI] [PubMed] [Google Scholar]
- Lund RD, Mustari MJ. Development of the geniculocortical pathway in rats. J Comp Neurol. 1977;173:289–306. doi: 10.1002/cne.901730206. [DOI] [PubMed] [Google Scholar]
- Maier DL, Mani S, Donovan SL, Soppet D, Tessaroll L, McCasland JS, Meiri KF. Disrupted cortical map and absence of corticals barrels in growth-associated protein (GAP)-43 knockout mice. Proc Natl Acad Sci USA. 1999;96:9397–9402. doi: 10.1073/pnas.96.16.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Blake NM, Erinjeri JP, Reistad CE, Sexton T, Admire P, Woolsey TA. Postnatal growth of intrinsic connections in mouse barrel cortex. J Comp Neurol. 2001;436:17–31. [PubMed] [Google Scholar]
- Mitchell BD, Macklis JD. Large-scale maintenance of dual projections by callosal and frontal cortical projection neurons in adult mice. J Comp Neurol. 2005;482:17–32. doi: 10.1002/cne.20428. [DOI] [PubMed] [Google Scholar]
- Nothias F, Peschanski M, Besson JM. Somatotopic reciprocal connections between the somatosensory cortex and the thalamic Po nucleus in the rat. Brain Res. 1988;447:169–174. doi: 10.1016/0006-8993(88)90980-8. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Stanfield BB, Cowan WM. Evidence that the early postnatal restriction of the cells of origin of the callosal projection is due to the elimination of axonal collaterals rather than to the death of neurons. Brain Res. 1981;227:607–617. doi: 10.1016/0165-3806(81)90012-2. [DOI] [PubMed] [Google Scholar]
- Porter LL, White EL. Afferent and efferent pathways of the vibrissal region of primary motor cortex in the mouse. J Comp Neurol. 1983;214:279–289. doi: 10.1002/cne.902140306. [DOI] [PubMed] [Google Scholar]
- Rebsam A, Seif I, Gaspar P. Refinement of thalamocortical arbors and emergence of barrel domains in the primary somatosensory cortex: a study of normal and monoamine oxidase a knock-out mice. J Neurosci. 2002;22:8541–8552. doi: 10.1523/JNEUROSCI.22-19-08541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades RW, Bennett-Clarke CA, Shi MY, Mooney RD. Effects of 5-HT on thalamocortical synaptic transmission in the developing rat. J Neurophysiol. 1994;72:2438–2450. doi: 10.1152/jn.1994.72.5.2438. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Crissman RS, Bennett-Clarke CA, Killackey HP, Chiaia NL. Development and plasticity of local intracortical projections within the vibrissae representation of the rat primary somatosensory cortex. J Comp Neurol. 1996;370:524–535. doi: 10.1002/(SICI)1096-9861(19960708)370:4<524::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Rice FL. Gradual changes in the structure of the barrels during maturation of the primary somatosensory cortex in the rat. J Comp Neurol. 1985;236:496–503. doi: 10.1002/cne.902360406. [DOI] [PubMed] [Google Scholar]
- Rice FL, Van der Loos H. Development of the barrels and barrel field in the somatosensory cortex of the mouse. J Comp Neurol. 1977;171:545–560. doi: 10.1002/cne.901710408. [DOI] [PubMed] [Google Scholar]
- Rice FL, Gomez C, Barstow C, Burnet A, Sands P. A comparative analysis of the development of the primary somatosensory cortex: interspecies similarities during barrel and laminar development. J Comp Neurol. 1985;236:477–495. doi: 10.1002/cne.902360405. [DOI] [PubMed] [Google Scholar]
- Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Hypothalamocortical projections. J Comp Neurol. 1985;237:21–46. doi: 10.1002/cne.902370103. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, O’Leary DD. Early development of the somatotopic map and barrel patterning in rat somatosensory cortex. J Comp Neurol. 1994;346:80–96. doi: 10.1002/cne.903460106. [DOI] [PubMed] [Google Scholar]
- Senft SL, Woolsey TA. Growth of thalamic afferents into mouse barrel cortex. Cereb Cortex. 1991;1:308–335. doi: 10.1093/cercor/1.4.308. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Svoboda K. Laminar and columnar organization of ascending excitatory projections to layer 2/3 pyramidal neurons in rat barrel cortex. J Neurosci. 2005;25:5670–5679. doi: 10.1523/JNEUROSCI.1173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogori T, Grove EA. Fibroblast growth factor 8 regulates neocortical guidance of area-specific thalamic innervation. J Neurosci. 2005;25:6550–6560. doi: 10.1523/JNEUROSCI.0453-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreafico R, Barbaresi P, Weinberg RJ, Rustioni A. SII-projecting neurons in the rat thalamus: a single- and double-retrograde-tracing study. Somatosens Res. 1987;4:359–375. doi: 10.3109/07367228709144614. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- Vercelli A, Repici M, Garbossa D, Grimaldi A. Recent techniques for tracing pathways in the central nervous system of developing and adult mammals. Brain Res Bull. 2000;51:11–28. doi: 10.1016/s0361-9230(99)00229-4. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Callebert J, Launay JM, Price DJ, Seif I, Gaspar P. Effects of monoamine oxidase A inhibition on barrel formation in the mouse somatosensory cortex: determination of a sensitive developmental period. J Comp Neurol. 1998;393:169–184. doi: 10.1002/(sici)1096-9861(19980406)393:2<169::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Welker C, Woolsey TA. Structure of layer IV in the somatosensory neocortex of the rat: description and comparison with the mouse. J Comp Neurol. 1974;158:437–453. doi: 10.1002/cne.901580405. [DOI] [PubMed] [Google Scholar]
- Wise SP, Jones EG. Cells of origin and terminal distribution of descending projections of the rat somatic sensory cortex. J Comp Neurol. 1977;175:129–157. doi: 10.1002/cne.901750202. [DOI] [PubMed] [Google Scholar]
- Wise SP, Jones EG. Developmental studies of thalamocortical and commissural connections in the rat somatic sensory cortex. J Comp Neurol. 1978;178:187–208. doi: 10.1002/cne.901780202. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT. Changes in the visual system of monocularly sutured or enucleated kittens demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Yu C, Derdikman D, Haidarliu S, Ahissar E. Parallel thalamic pathways for whisking and touch signals in the rat. PLoS Biol. 2006;4:e124. doi: 10.1371/journal.pbio.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Deschenes M. Projections to layer VI of the posteromedial barrel field in the rat: a reappraisal of the role of corticothalamic pathways. Cereb Cortex. 1998;8:428–436. doi: 10.1093/cercor/8.5.428. [DOI] [PubMed] [Google Scholar]