Abstract
Background
Surgical site infections (SSIs) can have devastating consequences for children who undergo spinal instrumentation. Prospective evaluations of prophylactic cefazolin in this population are limited. The purpose of this study was to describe the pharmacokinetics and skeletal muscle disposition of prophylactic cefazolin in a paediatric population undergoing complex spinal surgery.
Methods
This prospective pharmacokinetic study included 17 children with adolescent idiopathic scoliosis undergoing posterior spinal fusion, with a median age of 13.8 [interquartile range (IQR) 13.4–15.4] yr and a median weight of 60.6 (IQR 50.8–66.0) kg. A dosing strategy consistent with published guidelines was used. Serial plasma and skeletal muscle microdialysis samples were obtained during the operative procedure and unbound cefazolin concentrations measured. Non-compartmental pharmacokinetic analyses were performed. The amount of time that the concentration of unbound cefazolin exceeded the minimal inhibitory concentration for bacterial growth for selected SSI pathogens was calculated.
Results
Skeletal muscle concentrations peaked at a median of 37.6 (IQR 26.8–40.0) µg ml−1 within 30–60 min after the first cefazolin 30 mg kg−1 dose. For patients who received a second 30 mg kg−1 dose, the peak concentrations reached a median of 40.5 (IQR 30.8–45.7) µg ml−1 within 30–60 min. The target cefazolin concentrations for SSI prophylaxis for meticillin-sensitive Staphylococcus aureus (MSSA) and Gram-negative pathogens were exceeded in skeletal muscle 98.9 and 58.3% of the intraoperative time, respectively.
Conclusions
For children with adolescent idiopathic scoliosis undergoing posterior spinal fusion, the cefazolin dosing strategy used in this study resulted in skeletal muscle concentrations that were likely not to be effective for intraoperative SSI prophylaxis against Gram-negative pathogens.
Keywords: cefazolin; microdialysis; paediatric; scoliosis; surgery, spinal
Editor's key points.
Prophylactic antibiotic administration is an important component of the prevention of surgical site infection.
Cefazolin is a commonly used prophylactic agent in orthopaedic surgery.
The authors used tissue microdialysis to study cefazolin pharmacokinetics in paediatric patients.
After 30 mg kg−1 doses, skeletal muscle concentrations were adequate for meticillin-sensitive Staphylococcus aureus, but not for Gram-negative organisms.
Surgical site infections (SSIs) cause patient harm and excess health-care costs. In children undergoing spinal instrumentation for correction of scoliosis, SSIs often require repeated surgical procedures, long-term i.v. antibiotics, and prolonged hospitalizations.1–3
Both patient factors, such as a diagnosis of neuromuscular scoliosis, and surgical process factors, such as the timing of administration of preoperative antibiotic prophylaxis, have been associated with increased SSI rates.1,4–6 Although consensus recommendations for best practices have been developed for paediatric spine surgery,7 there have been relatively few prospective evaluations of some of the modifiable risk factors.
Perioperative antibiotic prophylaxis is the cornerstone of SSI prevention. Achieving and maintaining goal tissue concentrations of prophylactic antibiotics near the surgical site are crucial elements to maximize their effectiveness.8,9 Tissue microdialysis is a technique used in clinical pharmacology to sample more directly and continuously the free, unbound drug concentrations, including perioperative antibiotics, in the interstitial fluid of various tissues.10,11
Currently, the only published pharmacokinetic (PK) study of prophylactic cefazolin in children undergoing spinal surgery used total plasma concentrations as a surrogate for tissue drug concentrations.12 Total plasma concentrations, however, may not accurately reflect antimicrobial concentrations at the incision site, especially given the unknown penetration of cefazolin into soft tissue and the unknown relationship between total cefazolin and unbound cefazolin (the active drug) deposition into tissue. Furthermore, the surgical procedure and anaesthesia may alter systemic and local physiology that may affect perioperative antibiotic pharmacokinetics.
The purpose of this study was to determine the pharmacokinetics and skeletal muscle disposition of prophylactic cefazolin using both plasma and microdialysis sampling in a cohort of patients diagnosed with adolescent idiopathic scoliosis (AIS) undergoing posterior spinal fusion (PSF).
Methods
Patients and surgical procedure
The Institutional Review Board at The Children's Hospital of Philadelphia approved this prospective pharmacokinetic study. The study protocol met all applicable safety and reporting guidelines, with informed consent obtained from legal guardians of all patients and informed assent obtained from all patients who were less than 18 yr of age.
Males or females from 10 to 18 yr old with AIS who were undergoing primary posterior spinal fusion with instrumentation and who were going to receive cefazolin as part of their perioperative antibiotic regimen were eligible. Exclusion criteria included a known allergy to cefazolin, anatomical or other abnormalities that precluded insertion of a microdialysis catheter into the selected paraspinal muscle, or known renal or hepatic insufficiency or failure. The cefazolin dosing schedule used 30 mg kg−1 (maximum 2000 mg) given i.v. within 60 min before incision and 30 mg kg−1 (maximum 2000 mg) repeated every 4 h during the procedure, which was consistent with national guidelines.9 This study was designed to evaluate the current standard of care; this was not an intervention trial. Anaesthetic technique was at the discretion of the attending anaesthetist.
Pharmacokinetic sampling
Blood samples (2 ml of blood collected in lithium heparin tubes) were obtained from an arterial catheter. Blood samples were timed relative to each cefazolin dose (pre-dose, 5, 15, 30, 60, 90, 120, 180, and 240 min) and at the time of skin closure. Plasma samples were separated into two aliquots: one to measure the total (protein bound plus unbound) cefazolin concentration and one to measure the unbound cefazolin concentration. Microdialysate samples were collected continuously every 30 min after the initial dose throughout the operative procedure.
Description of microdialysis
Microdialysis was performed using the 63 microdialysis catheter (M Dialysis AB, Solna, Sweden) inserted percutaneously after induction of anaesthesia into a right-sided paraspinal muscle, with the catheter tip terminating approximately two vertebral bodies superior to the superior edge of the planned incision. The catheters contained polyarylethersulphone membranes with a molecular weight cut-off of 20 kDa, were 30 mm in length, and were perfused with an isotonic solution designed for use in peripheral tissue (Perfusion Fluid T1; M Dialysis AB). A 107 microdialysis pump (M Dialysis AB) with a flow rate of 1 µl min−1 was used for microdialysate sample collection. Each catheter was removed before emergence from anaesthesia. The CHOP Institutional Review Board considered the 63 microdialysis catheter a non-significant risk device.
Relative recovery and calculations of interstitial fluid concentration
In order to quantify the interstitial fluid cefazolin concentrations accurately, retrodialysis was used to calibrate the microdialysis catheter so that the in vivo relative recovery (RR) of cefazolin could be calculated for each patient.10,11 For retrodialysis, the perfusion fluid was prepared by our Investigational Pharmacy and contained cefazolin 20 µg ml−1 and was administered at a perfusion rate of 1 µl min−1 for 20 min before collection of the retrodialysis sample. The in vivo RR was calculated for each patient using the following equation11:
where Cdialysate is the cefazolin concentration in the retrodialysis sample and Cperfusate is the concentration of cefazolin in the perfusion fluid that was run during retrodialysis. After retrodialysis, catheters were flushed with the Perfusion Fluid T1 (not containing cefazolin), followed by a median equilibration period of 25.0 min [interquartile range (IQR) 23.0–30.0 min] before the administration of the first dose of cefazolin. The interstitial concentration of cefazolin was calculated using the following equation:
where the concentration in the microdialysate is the cefazolin concentration measured in the collected specimens.
Sample analysis
Blood samples were centrifuged at 580 g for 10 min. The plasma was separated into two aliquots, stored at −20°C at collection and then at −80°C until analysis. One aliquot was processed further by ultrafiltration of 400 µl of plasma with a Spin-X ultrafiltrate membrane (10 000 MWCO; Corning Inc., Lowell, MA, USA) to measure unbound cefazolin. Non-specific binding of cefazolin to the ultrafiltration membrane was 12% and was corrected for in the final calculations. One total plasma concentration value and one unbound plasma concentration value (out of a total of 268 time points and 536 sample preps) were estimated based on average protein binding for other similar samples.
Cefazolin concentrations in microdialysate (unbound only) and plasma samples (total and unbound) were determined by validated aqueous and plasma assays using high-performance liquid chromatography and tandem mass spectrometry developed and performed at The Children's Hospital of Philadelphia and described elsewhere.13 The lower limit of quantification for the total plasma assay was 1 µg ml−1 (linear range 1–500 µg ml−1), with interday and intraday coefficients of variation <5%. For the unbound cefazolin assay, the lower limit of quantification was 0.1 µg ml−1 (linear range 0.1–100 µg ml−1), with interday and intraday coefficients of variation <5%.
Pharmacokinetic and statistical analysis
Non-compartmental methods were used for pharmacokinetic analyses on the 17 evaluable patients. Maximal plasma and skeletal muscle concentrations (Cmax) and the time to maximal concentrations (Tmax) were determined after each dose of cefazolin. Owing to the nature of microdialysis collection, the Cmax values for the microdialysis samples represent average concentrations throughout the collected time interval.
The area under the concentration–time curve (AUC) for the plasma samples was calculated using the log–linear trapezoid method. For microdialysis samples, the skeletal muscle AUC was calculated by multiplying the measured unbound cefazolin concentration corrected for RR by the time interval of the microdialysis sample collection, then summation of the areas for each interval. Calculation of the AUC0–last allows for a measure of cefazolin exposure in both plasma and skeletal muscle for the duration of the intraoperative sample collection period. Tissue penetration of cefazolin was determined by calculating, and comparing, ratios of AUC0–last for microdialysis samples with AUC0–last for unbound plasma samples.
The pharmacokinetic–pharmacodynamic (PK–PD) factor most closely associated with the antibacterial effectiveness of cephalosporins is the amount of time the concentration of the free drug exceeds the minimal inhibitory concentration (MIC) for bacterial growth (fT>MIC);14 therefore, this value was calculated using relevant minimum inhibitory concentrations required to inhibit growth of 90% of bacteria (MIC90) values.15
Summary data are presented as medians (IQR). Non-parametric tests including the Wilcoxon signed-rank test and the Wilcoxon rank-sum test were used for comparison statistics. Correlation analysis was performed with linear regression. Statistical analyses and plots were performed using R Studio Version 0.98.1091 (R Studio Inc, Boston, Massachusetts, USA), Microsoft Excel for Mac 2011 (Microsoft Corp, Redmond, Washington, USA), and KaleidaGraph Version 4.5.2 (Synergy Software, Reading, Pennsylvania, USA).
Safety and monitoring
After microdialysis catheter placement, each patient had a microdialysis adverse event monitoring form completed during surgery and daily for up to 5 days after surgery or until hospital discharge.
Results
Population characteristics
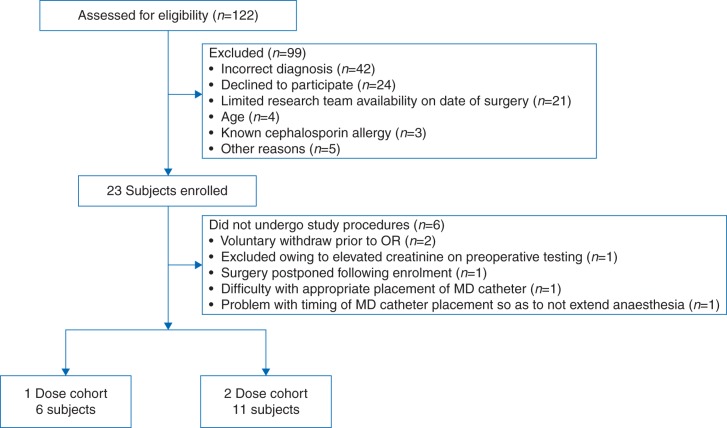
A convenience sample of 23 patients was enrolled in this study, resulting in 17 evaluable patients (Fig. 1 and Table 1). Six patients received one intraoperative dose of cefazolin (i.e. their procedures were <240 min). Eleven patients received two intraoperative doses of cefazolin. All patients were ASA Class II. There were no instances of SSIs in this cohort.
Fig 1.
Flow diagram describing study participants. MD, microdialysis; OR, operating room.
Table 1.
Patient characteristics (n=17). Values are reported as medians (interquartile range) unless otherwise noted. Categorical data were compared using Fisher's exact test and continuous data using Wilcoxon rank-sum test. Estimated circulating blood volume was calculated by multiplying the weight in kilograms by 70 ml kg−1. Estimated circulating blood volume loss was calculated by dividing the estimated blood loss by the estimated circulating blood loss and is reported as a percentage. NA, not assessed
| Variable | One-dose cohort (n=6) | Two-dose cohort (n=11) | P-value |
|---|---|---|---|
| Age (yr) | 13.8 (12.4–14.6) | 13.8 (13.6–17.1) | 0.29 |
| Sex (male/female) | 2/4 | 3/8 | 0.56 |
| Weight (kg) | 61.6 (50.9–65.1) | 58.5 (52.8–67.3) | 1 |
| Height (m) | 1.60 (1.55–1.63) | 1.65 (1.62–1.69) | 0.10 |
| BMI (kg m−2) | 24.5 (19.9–26.0) | 20.3 (19.2–25.3) | 0.88 |
| Time from first cefazolin dose to incision (min) | 3.9 (3.5–4.9) | 8.2 (7.1–11.3) | 0.01 |
| Time from first cefazolin dose to second cefazolin dose (min) | NA | 240 (236–240) | – |
| First cefazolin dose (mg kg−1) | 29.9 (29.7–30.0) | 30.0 (29.3–30.1) | 0.35 |
| Second cefazolin dose (mg kg−1) | NA | 30.0 (29.3–30.1) | – |
| Total fluid received (ml) | 3300 (2300–3753) | 3700 (2826–5618) | 0.22 |
| Crystalloid received (ml) | 3300 (2300–3663) | 3550 (2750–5000) | 0.26 |
| Cellsaver blood received (ml) | 32 (0–91) | 150 (105–313) | 0.01 |
| Packed red blood cells received (ml) | 0 (0–0) | 0 (0–0) | 0.54 |
| Whole blood received (ml) | 0 (0–0) | 0 (0–0) | 0.33 |
| Estimated circulating blood volume (ml) | 4309 (3560–4553) | 4095 (3693–4708) | 1.0 |
| Estimated blood loss (ml) | 500 (500–500) | 700 (450–1000) | 0.33 |
| Estimated circulating blood volume loss (%) | 11.6 (11.0–11.0) | 19.7 (10.4–24.1) | 0.23 |
| Urine output (ml) | 290 (258–435) | 760 (650–1080) | 0.04 |
| Urine output (ml kg−1 h−1) | 1.4 (1.1–1.8) | 3.1 (1.8–4.1) | 0.09 |
| Total surgical time (min) | 215 (190–223) | 285 (268–304) | <0.001 |
| Procedure start temperature (°C) | 34.9 (34.7–5.1) | 34.7 (34.5–35.2) | 0.48 |
| Procedure end temperature (°C) | 37.1 (36.8–37.5) | 36.8 (36.4–37.2) | 0.39 |
Pharmacokinetic parameters
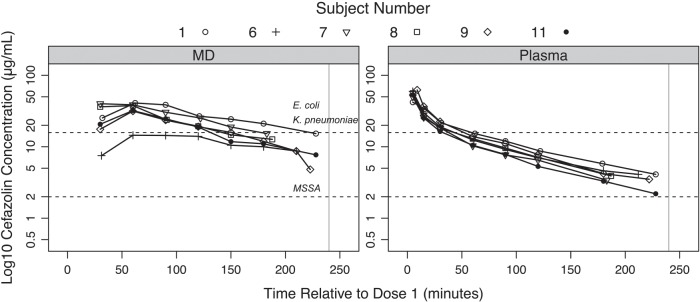
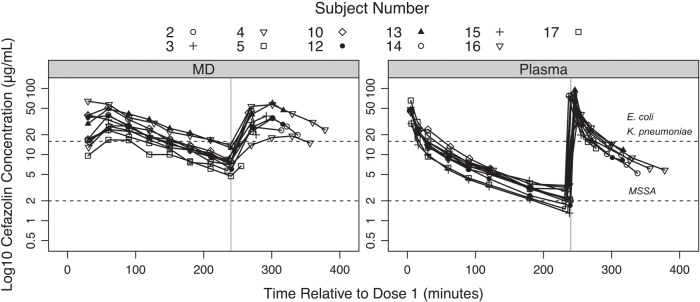
The median time from the administration of the first dose of cefazolin to incision for all patients was 6.9 (IQR 3.9–10.0) min. After administration of the first cefazolin dose, unbound plasma cefazolin concentrations peaked at the first measured time point (Cmax1 and Tmax1; Table 2 and Fig. 2). For those patients who received a second dose, the Cmin measured before Dose 2 was 7.1 (IQR 6.2–8.0) µg ml−1. The second dose was administered 240.0 (IQR 235.8–240.4) min after the first dose and produced a second unbound plasma peak (Cmax2) that was significantly higher than that following the first dose (Table 2 and Fig. 3).
Table 2.
Pharmacokinetic parameters for cefazolin for all patients. Values are reported as medians (interquartile range) except for Tmax values for microdialysis samples, which are reported as median values of the collection time interval during which the peak was likely to be collected, because the measured microdialysate concentrations represent average concentrations during the collected time frame and are not precise point estimates. Calculations for clearance and t½ are based on the measured total (unbound plus protein-bound) cefazolin concentrations in plasma. AUC, area under the concentration–time curve; Cmax, maximal concentration; MD, microdialysis; t½, half-life; Tmax, time to maximal concentration. *P=0.003
| Parameter | All patients (n=17) | One-dose cohort (n=6) | Two-dose cohort (n=11) |
|---|---|---|---|
| Tmax1 unbound plasma (min) | 5.0 (5.0–5.3) | 5.0 (5.0–5.0) | 5.0 (5.0–5.8) |
| Cmax1 unbound plasma (µg ml−1) | 47.7 (43.3–54.0) | 54.7 (50.8–58.5) | 46.0* (43.2–47.7) |
| Tmin1 unbound plasma (µg ml−1) | – | – | 236.0 (233.0–238.5) |
| Cmin1 unbound plasma (µg ml−1) | – | – | 2.1 (1.9–2.9) |
| Tmax1 MD (min) | 30.0–60.0 | 30.0–60.0 | 30.0–60.0 |
| Cmax1 MD (µg ml−1) | 37.6 (26.8–40.0) | 34.8 (31.6–39.4) | 38.4 (25.4–44.4) |
| Tmin1 MD (min) | – | – | 210–239 |
| Cmin1 MD (µg ml−1) | – | – | 7.1 (6.2–8.0) |
| Tmax2 unbound plasma (min) | – | – | 5.0 (5.0–5.5) |
| Cmax2 unbound plasma (µg ml−1) | – | – | 74.2* (54.7–78.3) |
| Tmax2 MD (min) | – | – | 30–60 |
| Cmax2 MD (µg ml−1) | – | – | 40.5 (30.8–45.7) |
| Unbound plasma AUC0–infinity (µg min ml−1) | 2935.9 (2654.8–3466.8) | 3576.4 (3159.7–3825.7) | 2765.0 (2329.9–3004.0) |
| Unbound plasma AUC0–last (µg min ml−1) | 3537.0 (2921.7–4546.9) | 3039.2 (2740.3–3405.8) | 4141.3 (3529.8–4625.6) |
| MD AUC0–last (µg min ml−1) | 5792.2 (4306.4–6986.6) | 4183.8 (3918.6–5010.0) | 6328.9 (5643.6–7172.9) |
| MD/unbound plasma AUC0–last ratio | 1.69 (1.17–1.94) | 1.37 (1.00–1.85) | 1.83 (1.32–2.07) |
| Clearance (total plasma; litre h−1 kg−1) | 0.06 (0.06–0.07) | 0.06 (0.06–0.07) | 0.06 (0.06–0.07) |
| t½ (total plasma; h) | 1.57 (1.47–1.71) | 1.51 (1.46–1.67) | 1.64 (1.49–1.70) |
Fig 2.
Semi-logarithmic concentration–time profile for unbound cefazolin in microdialysate and plasma samples of all patients who received one dose of cefazolin. The horizontal lines indicate various concentration targets and associated surgical site infection pathogen(s). The vertical line indicates 240 min, the time when cefazolin was dosed again for those patients receiving two doses. E., Escherichia; K., Klebsiella; MD, microdialysate; MSSA, meticillin-sensitive Staphylococcus aureus.
Fig 3.
Semi-logarithmic concentration–time profile for unbound cefazolin in microdialysate and plasma samples of all patients who received two doses of intraoperative cefazolin. The horizontal lines indicate various concentration targets and associated surgical site infection pathogen(s). The vertical line indicates 240 min, the time when cefazolin was dosed again. E., Escherichia; K., Klebsiella; MD, microdialysate; MSSA, meticillin-sensitive Staphylococcus aureus.
After administration of the first cefazolin dose, muscle concentrations of cefazolin peaked between 30 and 60 min (Table 2 and Figs 2 and 3). For those patients who received two doses, peak muscle concentrations were also seen between 30 and 60 min, and the Cmax for each dose was similar (Table 2 and Fig. 3).
Tissue penetration
The median in vivo RR was 84.5% (IQR 77.8–89.3%). The skeletal muscle to unbound plasma AUC0–last ratio was similar between those who received one and two doses of cefazolin (Table 2).
Amount of time the concentration of free drug exceeds the minimal inhibitory concentration (fT>MIC90)
For all patients, approximately 98–100% of the sampling time period showed unbound cefazolin concentrations in both plasma and skeletal muscle of >2 µg ml−1 (Table 3). This percentage decreased for both groups for concentrations >16 and >32 µg ml−1 in plasma or skeletal muscle, respectively (Table 3).
Table 3.
Percentage of time that unbound (free) cefazolin concentrations were above different concentration targets. The concentration targets of 2 µg mL−1 and 16 µg mL−1 represent estimated minimum inhibitory concentrations required to inhibit growth of 90% of bacteria (MIC90) of bacteria that are potentially susceptible to cefazolin and known to cause SSIs in patients with adolescent idiopathic scoliosis undergoing posterior spinal fusion procedures. These calculations therefore represent the amount of time the concentration of free cefazolin exceeds the MIC90 (fT>MIC90). All reported values are median (interquartile range) percentages. MSSA, meticillin-sensitive Staphylococcus aureus; SSI, surgical site infection
| Target concentration (µg ml−1) | SSI pathogen | All patients (n=17) |
One-dose cohort (n=6) |
Two-dose cohort (n=11) |
|||
|---|---|---|---|---|---|---|---|
| Unbound plasma | Skeletal muscle | Unbound plasma | Skeletal muscle | Unbound plasma | Skeletal muscle | ||
| 2 | MSSA | 100 (100–100) | 98.9 (98.6–99.4) | 100 (100–100) | 98.8 (98.3–98.9) | 100 (95.3–100) | 99.1 (98.8–99.4) |
| 16 | Escherichia coli, Klebsiella pneumoniae | 22.1 (19.2–25.9) | 58.3 (45.9–86.8) | 22.4 (20.9–23.5) | 71.3 (47.6–88.1) | 22.1 (18.9–27.9) | 58.3 (46.0–62.5) |
| 32 | 8.4 (7.0–9.1) | 13.0 (0.0–25.1) | 7.1 (7.0–7.7) | 12.6 (0.0–27.7) | 8.9 (8.3–10.2) | 13.0 (0.0–22.2) | |
Correlations
There were minimal correlations between the skeletal muscle to unbound plasma AUC0–last ratio and estimated blood loss (r2=0.21), percentage of circulating blood volume lost (r2=0.16), total fluids administered (r2=0.15), BMI (r2=0.14), or urine output during the procedure (r2=0.04). There were minimal correlations between the percentage of circulating blood volume lost and total clearance (r2=0.02), half-life (t½; r2=0.19), and volume of distribution (Vd) of the central compartment (r2=0.09). There were moderate correlations between the total clearance and the skeletal muscle to unbound plasma AUC0–last ratio (r2=0.32), fT>MIC90 for >16 (r2=0.33) and >32 µg ml−1 (r2=0.37), respectively.
Protein binding
In this study, the median percentage protein binding of cefazolin in all collected plasma samples was 90.0% (IQR 85.6–92.3%).
Microdialysis catheter safety
Eight patients had minor bleeding (defined as one to three drops of blood) during placement of the catheters. No patients had subcutaneous bleeding, haematoma formation, or accidental venous puncture. There were no instances of catheter disruption or displacement during the surgical procedures and no complications attributable to the microdialysis catheters during the follow-up period.
Discussion
This study prospectively evaluated the pharmacokinetics and skeletal muscle disposition of prophylactic cefazolin using both plasma and microdialysis sampling in a paediatric cohort with AIS undergoing PSF. Skeletal muscle concentrations of cefazolin achieved with this dosing strategy are likely to be effective for intraoperative SSI prophylaxis against meticillin-sensitive Staphylococcus aureus (MSSA) but might not be effective for intraoperative SSI prophylaxis against Gram-negative pathogens. This has potential implications for perioperative antibiotic dosing guidelines.
The exact tissue concentrations of cefazolin required for effective SSI prophylaxis are unknown. For reference, treatment of active Gram-positive and Gram-negative infections with cefazolin requires a goal fT>MIC of 30–40 and 60–70%, respectively, with maximal killing effects around four to five times MIC.14 Our conclusions are based on a conservative estimate for prophylaxis that a concentration of cefazolin equal to the MIC90 be achieved for at least 60–70% of the dosing interval. However, achieving these concentrations at the time of the incision and maintaining them throughout the entire procedure are perhaps the correct targets.16
Major surgery has the potential to induce changes in systemic physiology and local conditions within the surgical site that may lead to altered pharmacokinetics and tissue penetration of cefazolin.17 One advantage of the present study is that we measured skeletal muscle concentrations of cefazolin for the entire PSF operation in close proximity to the surgical site. Consistent with a previous study in adult patients, we found no significant relationship between blood loss during PSF and various PK parameters, including Vd of the central compartment and t½.18
Although the patients in our study had a relatively low estimated blood loss, we found minimal correlation between blood loss and tissue penetration of cefazolin, as measured by the skeletal muscle to unbound plasma AUC0–last ratio. Our data on blood loss are consistent with two previous studies (one paediatric study with a larger blood loss and one adult study with a similar blood loss to the patients in our study) that found no significant correlation between intraoperative blood loss and total cefazolin plasma concentrations.12,18 However, another study of adult patients with a blood loss more than twice what was seen in our study found a significant correlation between blood loss and the change in tissue concentrations of cefazolin.19 The degree of blood loss may be important for tissue penetration of antibiotics during major surgery, but we did not observe this in our cohort.
It is important to achieve and maintain adequate tissue concentrations of cefazolin during a surgical procedure to have maximal efficacy.8,9 For the present study, we separated the analysis and compared those patients who received one dose of cefazolin with those who received two doses during their procedure because of differences in their drug exposure. Not surprisingly, the Cmax1 was significantly lower than the Cmax2 for the unbound plasma fraction in patients receiving two doses of cefazolin. However, there was no significant difference between Cmax1 and Cmax2 for the skeletal muscle concentrations in these patients. The time course for the attainment of maximal tissue concentration for the first and second doses of cefazolin was between 30 and 60 min after administration. Although microdialysis is superior to other assessment methods for antibiotic tissue concentrations, samples contain average cefazolin concentrations throughout the collection interval and, therefore, we cannot be more precise about describing the rate of increase of skeletal muscle concentrations. This time course of cefazolin distribution into tissue following the first dose is similar to that described in other paediatric and adult surgical populations.13,17,20,21
Our findings reinforce that both the timing and the dose of cefazolin are important considerations when using this antibiotic for intraoperative SSI prophylaxis. In this study, the median time from the first dose of cefazolin to incision was 6.9 min, which may not have been long enough to allow for adequate tissue concentrations before incision for common SSI pathogens. Even before the 4 h time point, tissue concentrations of cefazolin fell below concentrations that are likely to be effective against Gram-negative organisms and were approaching threshold concentrations that are likely to be effective against MSSA, emphasizing the importance of intraoperative repeated dosing. For populations at high risk for Gram-negative infections, such as those with neuromuscular scoliosis,1,4,22–24 repeated dosing may have to be even more frequent or consideration be given for use of a continuous infusion of cefazolin. Alternatively, additional Gram-negative antimicrobial coverage may be required for these populations, as suggested recently in the literature.7,25
Finally, although we did not assess cefazolin clearance before or after surgery, one study reported no change in clearance when comparing preoperative and intraoperative PK analyses in an adult population undergoing elective spinal fusion with internal fixation.18 We speculate that this is also true for children and that the current recommended standard dosing of cefazolin every 6–8 h for perioperative SSI prophylaxis or treatment doses for skin and soft tissue infections treated with cefazolin may not be sufficient. These are important areas for future research.
The most common side-effect related to the microdialysis catheters was minor bleeding (defined as one to three drops of blood) during insertion of the catheter. There were no instances of significant bleeding (defined as >1 ml of blood), haematoma formation, or concern for infection related to the microdialysis catheter. Microdialysis is a research method that is feasible to use during paediatric surgical procedures.
Limitations of this study include the fact that data collection was limited to the intraoperative period, and our conclusions may not be applicable to all other paediatric surgical populations. Although the largest study of its kind in the paediatric literature, our study was performed on a relatively small sample size. In addition, as there are no paediatric studies linking tissue concentrations to wound infections, we have to rely on this inference from adult studies26,27 and extrapolated PK–PD principles. Although the microdialysis catheters were placed adjacent to the surgical site, the measured cefazolin concentrations may not entirely reflect those located in the surgical field or at the site of hardware insertion. Finally, this study was not powered to determine the association between PK variability and development of an SSI.
The appropriate administration of antibiotic prophylaxis has been shown to decrease SSI rates both for general paediatric surgical populations28 and specifically for children undergoing spinal fusion procedures.4,6 General statements in dosing guidelines9 and the increasing practice of intrawound antibiotic administration7,25,29,30 during spinal instrumentation highlight the importance of achieving adequate tissue concentrations of an appropriate antibiotic to decrease the incidence of SSIs in these populations. Our results suggest that the dosing strategy of cefazolin used in this study is likely to be adequate for AIS patients undergoing PSF for intraoperative SSI prophylaxis against MSSA but might not prevent infections from some common Gram-negative pathogens. Future investigations on optimizing intraoperative and postoperative tissue concentrations of antibiotics used for prophylaxis are warranted and should focus on achieving appropriate PK–PD objectives.
Authors’ contributions
Study design: A.S.H., M.T.S., A.F.Z., E.F., J.P.D., T.K.
Conduct of study: A.S.H., W.N.S., J.M.F., M.T.S., G.S.M., J.P.D., T.K.
Data collection: A.S.H., M.T.S., T.K.
Data analysis: A.S.H., G.S.M., J.S.G., A.F.Z., E.F., T.K.
Manuscript preparation: A.S.H., W.N.S., J.M.F., M.T.S., G.S.M., J.S.G., A.F.Z., E.F., J.P.D., T.K.
Declaration of interest
None declared.
Funding
Pediatric Orthopaedic Society of North America (POSNA; DePuy Spine Research Grant).
Acknowledgements
The authors wish to acknowledge and thank Janice Prodell and Kerry Costlow, who helped with the data collection.
References
- 1.Labbé AC, Demers AM, Rodrigues R, Arlet V, Tanguay K, Moore L. Surgical-site infection following spinal fusion: a case control study in a children's hospital. Infect Control Hosp Epidemiol 2003; 24: 591–5 [DOI] [PubMed] [Google Scholar]
- 2.Hedequist D, Haugen A, Hresko T, Emans J. Failure of attempted implant retention in spinal deformity delayed surgical site infections. Spine (Phila Pa 1976) 2009; 34: 60–4 [DOI] [PubMed] [Google Scholar]
- 3.Ho C, Skaggs DL, Weiss JM, Tolo VT. Management of infection after instrumented posterior spine fusion in pediatric scoliosis. Spine (Phila Pa 1976) 2007; 32: 2739–44 [DOI] [PubMed] [Google Scholar]
- 4.Linam WM, Margolis PA, Staat MA et al. . Risk factors associated with surgical site infection after pediatric posterior spinal fusion procedure. Infect Control Hosp Epidemiol 2009; 30: 109–16 [DOI] [PubMed] [Google Scholar]
- 5.Ali MHM, Koutharawu DN, Miller F et al. . Operative and clinical markers of deep wound infection after spine fusion in children with cerebral palsy. J Pediatr Orthop 2010; 30: 851–7 [DOI] [PubMed] [Google Scholar]
- 6.Milstone AM, Maragakis LL, Townsend T et al. . Timing of preoperative antibiotic prophylaxis: a modifiable risk factor for deep surgical site infections after pediatric spinal fusion. Pediatr Infect Dis J 2008; 27: 704–8 [DOI] [PubMed] [Google Scholar]
- 7.Vitale MG, Riedel MD, Glotzbecker MP et al. . Building consensus: development of a best practice guideline (BPG) for surgical site infection (SSI) prevention in high-risk pediatric spine surgery. J Pediatr Orthop 2013; 33: 471–8 [DOI] [PubMed] [Google Scholar]
- 8.Kirby JP, Mazuski JE. Prevention of surgical site infection. Surg Clin North Am 2009; 89: 365–89 [DOI] [PubMed] [Google Scholar]
- 9.Bratzler DW, Dellinger EP, Olsen KM et al. . American Society of Health-System Pharmacists; Infectious Disease Society of America; Surgical Infection Society; Society for Healthcare Epidemiology of America. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Sys Pharm 2013; 70: 195–283 [DOI] [PubMed] [Google Scholar]
- 10.Chaurasia CS, Müller M, Bashaw ED et al. . AAPS-FDA Workshop White Paper: microdialysis principles, application, and regulatory perspectives. Pharm Res 2007; 24: 1014–25 [DOI] [PubMed] [Google Scholar]
- 11.Joukhadar C, Müller M. Microdialysis: current applications in clinical pharmacokinetic studies and its potential role in the future. Clin Pharmacokinet 2005; 44: 895–913 [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Sosa FH, Polly D, Bowen JR et al. . Serum cefazolin levels during spinal fusion: effect of blood loss and duration of surgery. J Spinal Disord 1993; 6: 296–9 [DOI] [PubMed] [Google Scholar]
- 13.Himebauch AS, Nicolson SC, Sisko M et al. . Skeletal muscle and plasma concentrations of cefazolin during cardiac surgery in infants. J Thorac Cardiovasc Surg 2014; 148: 2634–41 [DOI] [PubMed] [Google Scholar]
- 14.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998; 26: 1–10 [DOI] [PubMed] [Google Scholar]
- 15.Andes DR, Craig WA. Cephalosporins. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas and Bennett’ Principles and Practice of Infectious Diseases, 7th Edn Philadelphia: Churchill Livingstone Elsevier, 2010; 323–40 [Google Scholar]
- 16.Bratzler DW, Houck PM, for the Surgical Infection Prevention Guidelines Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the national surgical infection prevention project. Clin Infec Dis 2004; 38: 1706–15 [DOI] [PubMed] [Google Scholar]
- 17.Andreas M, Zeitlinger M, Hoeferl M et al. . Internal mammary artery harvesting influences antibiotic penetration into presternal tissue. Ann Thorac Surg 2013; 95: 1323–9 [DOI] [PubMed] [Google Scholar]
- 18.Polly DW Jr, Meter JJ, Brueckner R, Asplund L, van Dam BE. The effect of intraoperative blood loss on serum cefazolin level in patients undergoing instrumented spinal fusion: a prospective, controlled study. Spine (Phila Pa 1976) 1996; 21: 2363–7 [DOI] [PubMed] [Google Scholar]
- 19.Swoboda SM, Merz C, Kostuik J, Trentler B, Lipsett PA. Does intraoperative blood loss affect antibiotic serum and tissue concentrations? Arch Surg 1996; 131: 1165–71 [DOI] [PubMed] [Google Scholar]
- 20.Hutschala D, Skhirtladze K, Kinstner C et al. . In vivo microdialysis to measure antibiotic penetration into soft tissue during cardiac surgery. Ann Thorac Surg 2007; 84: 1605–10 [DOI] [PubMed] [Google Scholar]
- 21.Douglas A, Udy AA, Wallis SC et al. . Plasma and tissue pharmacokinetics of cefazolin in patients undergoing elective and semielective abdominal aortic aneurysm open repair. Antimicrob Agents Chemother 2011; 55: 5238–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cahill PJ, Warnick DE, Lee MJ et al. . Infection after spinal fusion for pediatric spinal deformity: thirty years of experience at a single institution. Spine (Phila Pa 1976) 2010; 35: 1211–7 [DOI] [PubMed] [Google Scholar]
- 23.Sponseller PD, LaPorte DM, Hungerford MW, Eck K, Bridwell KH, Lenke LG. Deep wound infections after neuromuscular scoliosis surgery: a multicenter study of risk factors and treatment outcomes. Spine (Phila Pa 1976) 2000; 25: 2461–6 [DOI] [PubMed] [Google Scholar]
- 24.Aleissa S, Parsons D, Grant J, Harder J, Howard J. Deep wound infection following pediatric scoliosis surgery: incidence and analysis of risk factors. Can J Surg 2011; 54: 263–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glotzbecker MP, Riedel MD, Vitale MG et al. . What's the evidence? Systematic literature review of factors and preventive strategies for surgical site infections following pediatric spinal surgery. J Pediatr Orthop 2013; 33: 479–87 [DOI] [PubMed] [Google Scholar]
- 26.Forse RA, Karam B, MacLean LD, Christou NV. Antibiotic prophylaxis for surgery in morbidly obese patients. Surgery 1989; 106: 750–6 [PubMed] [Google Scholar]
- 27.Zelenitsky SA, Ariano RE, Harding GKM, Silverman RE. Antibiotic pharmacodynamics in surgical prophylaxis: an association between intraoperative antibiotic concentrations and efficacy. Antimicrob Agents Chemother 2002; 46: 3026–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah GS, Christensen RE, Wagner DS, Pearce BK, Sweeney J, Tait AR. Retrospective evaluation of antimicrobial prophylaxis in prevention of surgical site infection in the pediatric population. Paediatr Anaesth 2014; 24: 994–8 [DOI] [PubMed] [Google Scholar]
- 29.Gans I, Dormans JP, Spiegel DA et al. . Adjunctive vancomycin powder in pediatric spine surgery is safe. Spine (Phila Pa 1976) 2013; 38: 1703–7 [DOI] [PubMed] [Google Scholar]
- 30.Armaghani SJ, Menge TJ, Lovejoy SA, Mencio GA, Martus JE. Safety of topical vancomycin for pediatric spinal deformity: nontoxic serum levels with supratherapeutic drain levels. Spine (Phila Pa 1976) 2014; 39: 1683–7 [DOI] [PubMed] [Google Scholar]