Abstract
The leading cause of morbidity and mortality after surviving the rupture of an intracranial aneurysm is delayed cerebral ischaemia (DCI). We present an update of recent literature on the current status of prevention and treatment strategies for DCI after aneurysmal subarachnoid haemorrhage. A systematic literature search of three databases (PubMed, ISI Web of Science, and Embase) was performed. Human clinical trials assessing treatment strategies, published in the last 5 yr, were included based on full-text analysis. Study data were extracted using tables depicting study type, sample size, and outcome variables. We identified 49 studies meeting our inclusion criteria. Clazosentan, magnesium, and simvastatin have been tested in large high-quality trials but failed to show a beneficial effect. Cilostazol, eicosapentaenoic acid, erythropoietin, heparin, and methylprednisolone yield promising results in smaller, non-randomized or retrospective studies and warrant further investigation. Topical application of nicardipine via implants after clipping has been shown to reduce clinical and angiographic vasospasm. Methods to improve subarachnoid blood clearance have been established, but their effect on outcome remains unclear. Haemodynamic management of DCI is evolving towards euvolaemic hypertension. Endovascular rescue therapies, such as percutaneous transluminal balloon angioplasty and intra-arterial spasmolysis, are able to resolve angiographic vasospasm, but their effect on outcome needs to be proved. Many novel therapies for preventing and treating DCI after aneurysmal subarachnoid haemorrhage have been assessed, with variable results. Limitations of the study designs often preclude definite statements. Current evidence does not support prophylactic use of clazosentan, magnesium, or simvastatin. Many strategies remain to be tested in larger randomized controlled trials.
Clinical trial registration
This systematic review was registered in the international prospective register of systematic reviews. PROSPERO: CRD42015019817.
Keywords: brain ischaemia; subarachnoid haemorrhage; vasospasm, intracranial
Rationale
Aneurysmal subarachnoid haemorrhage (aSAH) is a life-threatening condition with an estimated worldwide annual incidence of 9.1 patients per 100 000.1 The incidence is slightly higher in women and increases linearly with age.2 Although an uncommon form of stroke, it often occurs at a young age, causing either death or severe disability.3 The consequential loss of productive years entails a socioeconomic burden comparable with other more common stroke subtypes.4 Morbidity and mortality are further compromised by the occurrence of delayed cerebral ischaemia (DCI).5–7 Although angiographic cerebral vasospasms were traditionally considered to be the sole cause of DCI, microvascular spasm, microthrombosis, cortical spreading depolarization, and failure of cerebral autoregulation have been implicated as being involved.8–10 Delayed cerebral ischaemia is now usually defined as functional vasospasm including clinical deterioration. Cerebral vasospasm typically occurs between days 4 and 14 after aneurysm rupture and resolves spontaneously after 21 days.11 About two-thirds of patients are affected, of whom only half will be clinically symptomatic.12 To date, there is no medical strategy to prevent the occurrence of DCI. Even nimodipine, a dihydropyridine L-type calcium channel blocker, initially regarded as a potential preventive drug for DCI, has no proven effect on the incidence of angiographic vasospasm; however, it reduces the risk of poor neurological outcome, and oral administration is therefore recommended for all aSAH patients.13,14 After diagnosis of DCI, ‘triple-H’ therapy, a combination of hypervolaemia, induced arterial hypertension, and haemodilution, has traditionally been the cornerstone of treatment. In recent years, however, there has been a paradigm shift supported by a growing body of data towards isolated induced hypertension and euvolaemia.15–17 This shift is already reflected in the latest treatment guidelines, where induction of hypertension is recommended for patients with DCI as tolerated by cardiac output.18 The improvement in DCI management after aSAH has halved the mortality rate in the last two decades.19
Objectives
Since the publication of the American guidelines for the management of aneurysmal subarachnoid haemorrhage in 201218 and the European guidelines in 2013,20 many new clinical studies have been published. The primary goal of this systematic review is to provide an overview of all clinical trials focusing on prevention and treatment of DCI published in the last 5 yr. All studies in human adults who suffered an aSAH treated with an experimental therapy to improve the outcome, the incidence of vasospasm, or DCI were included in this systematic review.
Methods
Protocol and registration
In conjunction with all authors, the review protocol was outlined in April 2015. Inclusion and exclusion criteria, the search time period, and a comprehensive trial evaluation checklist were composed. The protocol has not been published; however, this review is conducted and written in accordance with the PRISMA statement21 and was registered in the international prospective register of systematic reviews, PROSPERO: CRD42015019817.
Eligibility criteria
The search was limited to clinical trials on human adults, published between January 1, 2010 and December 1, 2015. Included studies assessed a possible intervention aiming to reduce the incidence of vasospasm or DCI, or improve clinical outcome, or affect a surrogate outcome [e.g. cloth clearance rate, brain tissue oxygen tension ()]. Interventions could also consist of new monitoring methods to improve standard intensive care. Prospective (randomized and non-randomized) and retrospective studies and case series (with more than five patients) were included. Furthermore, pilot studies and preliminary results were included. Feasibility and safety studies, technical reports, study protocols, and epidemiological studies were excluded.
Information sources and search procedure
A literature search of Entrez PubMed NIH online medical database, Embase online medical databases, and ISI Web of Science was performed in April and December 2015. The following search terms were used: cerebral vasospasm OR delayed cerebral isch(a)emia OR delayed isch(a)emic neurological deficit AND subarachnoid h(a)emorrhage. The reference list of every article was also screened for eligible articles. All searches were restricted to English, German, French, and Dutch languages and limited to clinical trials in human adults.
Data items
A standardized table based on the PICO approach (as recommended by the PRISMA Explanation and Elaboration Document)22 was used to extract the following information: patient characteristics (age, sex, and aneurysm treatment modality), intervention characteristics (starting point, duration, dosage, and mode of application), outcome characteristics (mortality, neurological outcome, and long-term follow-up), and study characteristics (study design, sample size, dropouts, and statistical analyses).
Risk of bias in individual studies
Risk of bias was assessed independently during the data-extraction process by M.V. and A.S. using the Cochrane Collaboration's tool for assessing risk of bias in randomized trials.23 The risks of five types of bias were classified as high, low, or unclear. In the category other bias, differences in baseline characteristics, standard management, and the use of rescue therapy between groups were evaluated. The Cochrane ACROBAT-NRSI tool was used in a similar manner to judge potential bias in non-randomized studies.24 These studies were scored as being at low, moderate, serious, or critical risk of bias for seven different domains.
Quality of evidence for each individual outcome
We have adopted the Grading of Recommendation Assessment, Development, and Evaluation (GRADE) system to rate the quality of evidence for each outcome, across studies.25 This was carried out for all treatment strategies that have been evaluated in at least two trials with a control group included. The quality of evidence for each of the common outcomes was rated based on the type of study (randomized vs observational, and retrospective vs prospective) and this rating was, whenever necessary, modified downward based on five factors (study limitations, imprecision, inconsistency of results, indirectness of evidence, and publications bias) or modified upwards based on three factors (large magnitude of effect, dose–response, and possible confounders). Risk ratios and confidence intervals were calculated using Review Manager 5.3 (http://tech.cochrane.org/revman/download). Results are summarized as a GRADE evidence profile in Tables 1 –6 generated with the GRADE pro GDT software (http://gradepro.org/).
Table 1.
GRADE evidence profile: quality assessment per outcome of DCI prevention options. Should cilostazol vs placebo be used for DCI prevention? CI, confidence interval; DCI, delayed cerebral ischaemia; GRADE, Grading of Recommendation Assessment, Development, and Evaluation; mRS, modified ranking scale; RR, risk ratio. *One cilostazol trial was excluded based on simultaneous changes in nutritional management when introducing cilostazol therapy. †Clinical/symptomatic vasospasm was significantly reduced in only one of the RCTs. ‡The rate of cerebral infarction was reduced in only one of the RCTs. ¶Clinical outcome as measured by mRS was improved in only one of the RCTs. §The mRS was measured in one trial after 3 months and in one trial at discharge
| Quality assessment |
No. of patients |
Effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Cilostazol | Placebo/usual care | Relative (95% CI) | Absolute (95% CI) | Quality | Importance |
| Clinical/symptomatic cerebral vasospasm | ||||||||||||
| 226,27 | Randomized trials* | Not serious | Very serious† | Not serious | Not serious | None | 18/103 (17.5%) | 41/106 (38.7%) | RR 0.46 (0.25–0.84) | 209 fewer per 1000 (from 62 fewer to 290 fewer) | ⨁⨁◯◯ LOW |
IMPORTANT |
| Vasospasm-related cerebral infarction | ||||||||||||
| 226,27 | Randomized trials | Not serious | Very serious‡ | Not serious | Not serious | None | 11/103 (10.7%) | 30/106 (28.3%) | RR 0.38 (0.20–0.72) | 175 fewer per 1000 (from 79 fewer to 226 fewer) | ⨁⨁◯◯ LOW |
CRITICAL |
| Favourable outcome (mRS≤2) | ||||||||||||
| 226,27 | Randomized trials | Not serious | Very serious¶ | Serious§ | Not serious | None | 86/113 (76.1%) | 64/106 (60.4%) | RR 1.41 (0.96–2.05) | 248 more per 1000 (from 24 fewer to 634 more) | ⨁◯◯◯ VERY LOW |
CRITICAL |
Table 2.
GRADE evidence profile: quality assessment per outcome of DCI prevention options. Should clazosentan vs placebo be used for DCI prevention? CI, confidence interval; DCI, delayed cerebral ischaemia; GOSE, extended Glasgow outcome scale; GRADE, Grading of Recommendation Assessment, Development, and Evaluation; RR, risk ratio
| Quality assessment |
No. of patients |
Effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Clazosentan | Placebo/usual care | Relative (95% CI) | Absolute (95% CI) | Quality | Importance |
| Composite end point of mortality and morbidity | ||||||||||||
| 228,29 | Randomized trials | Not serious | Not serious | Not serious | Not serious | Dose–response gradient | 60/1122 (5.3%) | 52/557 (9.3%) | RR 0.56 (0.34–0.93) | 41 fewer per 1000 (from 7 fewer to 62 fewer) | ⨁⨁⨁⨁ HIGH |
IMPORTANT |
| Poor outcome (GOSE≤4) | ||||||||||||
| 228,29 | Randomized trials | Not serious | Not serious | Not serious | Not serious | Dose–response gradient | 129/1146 (11.3%) | 70/572 (12.2%) | RR 0.82 (0.42–1.62) | 22 fewer per 1000 (from 71 fewer to 76 more) | ⨁⨁⨁⨁ HIGH |
CRITICAL |
Table 3.
GRADE evidence profile: quality assessment per outcome of DCI prevention options. Should i.v. magnesium be used for DCI prophylaxis? CI, confidence interval; DCI, delayed cerebral ischaemia; GOSE, extended Glasgow outcome scale; GRADE, Grading of Recommendation Assessment, Development, and Evaluation; mRS, modified ranking scale; RR, risk ratio. *No reduction of clinical/symptomatic vasospasm in any of the trials. †Significant reduction in angiographic vasospasm in only one of both RCTs. ‡In one trial, numbers of angiographic and transcranial Doppler-measured vasospasm were combined. ¶No improvement in outcome as measured by mRS in any of the included trials. §Relevant differences in treatment strategies (fixed dose of magnesium vs target blood concentration). ||No improvement in outcome as measured by GOSE in any of the included trials. #Relevant differences in treatment strategies (fixed dose of magnesium vs target blood concentration)
| Quality assessment |
No. of patients |
Effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Magnesium | Placebo/usual care | Relative (95% CI) | Absolute (95% CI) | Quality | Importance |
| Clinical/symptomatic vasospasm | ||||||||||||
| 230,31 | Randomized trials | Not serious | Not serious* | Not serious | Not serious | None | 51/223 (22.9%) | 44/211 (20.9%) | RR 0.94 (0.42–2.12) | 13 fewer per 1000 (from 121 fewer to 234 more) | ⨁⨁⨁⨁ HIGH |
IMPORTANT |
| Angiographic vasospasm | ||||||||||||
| 230,32 | Randomized trials | Not serious | Serious† | Serious‡ | Not serious | None | 93/133 (69.9%) | 104/131 (79.4%) | RR 0.95 (0.74–1.21) | 40 fewer per 1000 (from 167 more to 206 fewer) | ⨁⨁◯◯ LOW |
CRITICAL |
| Favourable outcome (mRS≤2) | ||||||||||||
| 331–33 | Randomized trials | Not serious | Not serious¶ | Serious§ | Not serious | None | 542/844 (64.2%) | 535/831 (64.4%) | RR 0.99 (0.93–1.07) | 6 fewer per 1000 (from 45 fewer to 45 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| Favourable outcome (GOSE>4) | ||||||||||||
| 330–32 | Randomized trials | Not serious | Not serious|| | Serious# | Not serious | None | 206/304 (67.8%) | 182/292 (62.3%) | RR 1.08 (0.96–1.12) | 50 more per 1000 (from 25 fewer to 75 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
Table 4.
GRADE evidence profile: quality assessment per outcome of DCI prevention options. Should simvastatin be used for DCI prevention? CI, confidence interval; DCI, delayed cerebral ischaemia; GRADE, Grading of Recommendation Assessment, Development, and Evaluation; RR, risk ratio. *Two trials were excluded because they lacked a placebo/control group. †Incidence of vasospasm differed significantly in only one of the two trials
| Quality assessment |
No. of patients |
Effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Simvastatin | Placebo/usual care | Relative (95% CI) | Absolute (95% CI) | Quality | Importance |
| Clinical/symptomatic vasospasm | ||||||||||||
| 234,35 | Randomized trials* | Not serious | Serious† | Not serious | Not serious | None | 69/410 (16.8%) | 75/431 (17.4%) | RR 0.96 (0.67–1.37) | 7 fewer per 1000 (from 57 fewer to 64 more) | ⨁⨁⨁◯ MODERATE |
IMPORTANT |
Table 5.
GRADE evidence profile: quality assessment per outcome of DCI prevention options. Is 80 mg simvastatin more effective in preventing DCI compared with 40 mg simvastatin? CI, confidence interval; DCI, delayed cerebral ischaemia; GRADE, Grading of Recommendation Assessment, Development, and Evaluation; mRS, modified ranking scale; RCT, randomized controlled trial; RR, risk ratio. *One RCT and one prospective cohort study. †The prospective cohort study was at critical risk for bias because of confounding. ‡Reduction of clinical vasospasm in only one trial. ¶One RCT and one prospective cohort study. §The prospective cohort study was at critical risk for bias because of confounding
| Quality assessment |
No. of patients |
Effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Simvastatin 80 mg | Simvastatin 40 mg | Relative (95% CI) | Absolute (95% CI) | Quality | Importance |
| Clinical/symptomatic vasospasm | ||||||||||||
| 236,37 | Observational studies* | Serious† | Serious‡ | Not serious | Not serious | None | 19/162 (11.7%) | 19/158 (12.0%) | RR 0.96 (0.48–1.89) | 5 fewer per 1000 (from 63 fewer to 107 more) | ⨁◯◯◯ VERY LOW |
IMPORTANT |
| Favourable outcome (mRS≤2) after 3 months | ||||||||||||
| 236,37 | Observational studies¶ | Serious§ | Not serious | Not serious | Not serious | All plausible residual confounding would reduce the demonstrated effect dose–response gradient | 118/162 (72.8%) | 125/158 (79.1%) | RR 0.93 (0.83–1.05) | 55 fewer per 1000 (from 40 more to 134 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
Table 6.
GRADE evidence profile: quality assessment per outcome of DCI prevention options. Should a lumbar drainage be used in preventing DCI compared with no CSF diversion? CI, confidence interval; CSF, cerebrospinal fluid; DCI, delayed cerebral ischaemia; GRADE, Grading of Recommendation Assessment, Development, and Evaluation; RR, risk ratio
| Quality assessment |
No. of patients |
Effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | lumbar drainage | No drainage | Relative (95% CI) | Absolute (95% CI) | Quality | Importance |
| Clinical/symptomatic vasospasm | ||||||||||||
| 238,39 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 46/231 (19.9%) | 82/213 (38.5%) | RR 0.40 (0.26–0.61) | 231 fewer per 1000 (from 150 fewer to 285 fewer) | ⨁⨁⨁⨁ HIGH |
IMPORTANT |
Summary measures and synthesis of results
Whenever possible, the occurrence of the primary outcome parameters is presented as a percentage per treatment group, with the respective P-value. As a result of heterogeneity in outcome definition, a meta-analysis could not be performed.
Risk of bias across studies
Publication bias was not assessed. Selective reporting bias was assessed with the above-mentioned tools.
Results
Study selection
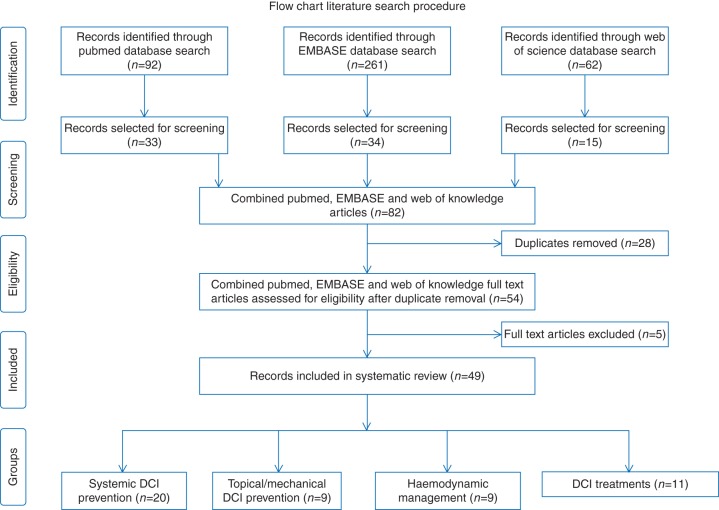
The PubMed search revealed 92 hits, and the EMBASE and Web of Science searches revealed 261 and 62 matches, respectively. Review articles and meta-analyses were removed, and the remaining articles were subsequently evaluated for relevance based on title and abstract. Feasibility and safety studies, technical reports, study protocols, and epidemiological studies were excluded. Consequently, a total of 33, 34, and 15 articles were selected. After removal of duplicates (n=28), another five articles were excluded after full-text analysis. Four articles contained no interventional step in the study protocol, and one article examined the effect of pre-admission statin use. Overall, a total of 141 initially identified articles were removed. The details of the search procedures and its results are summarized in Fig. 1. After full-text analysis, the 49 included studies could be divided into 38 trials assessing a preventive strategy and 11 trials assessing a therapy for diagnosed DCI or cerebral vasospasm (cVS). Preventive strategies could be subdivided into three categories: systemic drugs (n=20), topical/mechanical treatments (n=10), and haemodynamic management (n=8). Delayed cerebral ischaemia/cVS treatment strategies could be divided in three categories: induced hypertension (n=1), intraventricular nicardipine (n=1), and interventional neuroradiological treatments (n=9). Results are schematically depicted per category in four overview tables (see Tables 7 –10).
Fig 1.
Flow diagram of the search procedure and results. A total of 415 studies were identified in the three searched databases. Based on title and abstract, all articles not meeting the predefined inclusion criteria were excluded (n=333). Duplicates between databases (n=28) were removed. After full-text screening, an additional five studies were excluded. Four articles contained no interventional step in the study protocol, and one article examined the effect of pre-admission statin use.
Table 7.
Summary of all 20 studies examining systemic DCI prevention. aSAH, aneurysmal subarachnoid haemorrhage; BI, Barthel index; b.d., bis in die/twice daily; CCS, case–control study; CIV, vasospasm-related cerebral infarction; co, coiling; cp, clipping; CS, case series; cVS, cerebral vasospasm; DCI, delayed cerebral ischaemia; DIND, delayed ischaemic neurological deficit; EPA, eicosapentaenoic acid; EPO, erythropoietin; EVAS, Eicosapentaenoic acid for cerebral Vasospasm after Aneurysmal Subarachnoid; GOS, Glasgow outcome scale; GOSE, extended Glasgow outcome scale; IMASH, intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage; MASH-2, magnesium sulphate for aneurysmal subarachnoid hemorrhage-2; MBI, modified Barthel index; mRS, modified ranking scale; ns, not specified; OR, odds ratio; , brain tissue oxygen tension; R-CO, retrospective cohort study; RCT, randomized controlled trial; RRR, relative risk reduction; SF-36, short-form 36; TCD, transcranial Doppler; UFH, unfractionated heparin
| Authors (year) | Study type | Aneurysm treatment modality | Treatment | Sample size (treatment/control) | Sample size after dropouts | Primary outcome parameters | Secondary outcome parameters | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Suzuki and colleagues (2011)26 | RCT | cp | Cilostazol 100 mg orally b.d. for 14 days | 49/51 | 49/51 | Clinical cVS, CIV, mRS at discharge | ns | cVS (37.3 vs 22.4%; P=0.183), CIV (27.5 vs 10.2%; P=0.091). Favourable outcome, mRS≤2 at discharge (79.6 vs 47.1; P=0.007) | Cilostazol improved outcome after aSAH without reducing the incidence of cVS or infarction |
| Senbokuya and colleagues (2013)27 | RCT | cp | Cilostazol 100 mg orally b.d. for 14 days | 54/55 | 54/55 | Clinical cVS | Angiographic cVS, CIV, mRS after 1, 3, and 6 months | Clinical cVS (13.0 vs 40.0%; P=0.0021), angiographic cVS (50 vs 76.4%; P=0.0055), CIV (11.1 vs 29.1%; P=0.0304). No difference in mRS | Cilostazol decreased the incidence of symptomatic and angiographic cVS without improving outcome |
| Kimura and colleagues (2015)40 | CCS | cp | Cilostazol 100 mg orally b.d. for 14 days plus combined enteral and parenteral nutrition | 62/68 | 62/68 | Clinical/ angiographic cVS, CIV, mRS at discharge | ns | Symptomatic cVS (11.3 vs 36.8%; P=0.001), angiographic cVS (33.9 vs 51.5%; P=0.051), CIV (6.4 vs 30.9%; P=0.0006), mRS was significantly better in the treatment group | Cilostazol in combination with enteral and parenteral nutrition decreased the incidence of symptomatic cVS and improved clinical outcome |
| Macdonald and colleagues (2011), CONSCIOUS-228 | RCT | cp | Clazosentan i.v. 5 mg h−1 for a maximum of 14 days | 768/389 | 762/381 | Mortality, CIV, DIND | Poor outcome (GOSE≤4) at week 12 | Mortality/morbidity after 6 weeks (21 vs 25%; RRR=17%; P=0.10). Poor outcome (29 vs 25%; RRR=18%; P=0.10) | Clazosentan failed to improve mortality and morbidity or functional outcome |
| Macdonald and colleagues (2012), CONSCIOUS-329 | RCT | co | Clazosentan i.v. 5 mg h−1 or 10 mg h−1 for a maximum of 14 days | 189/194/188 (placebo/low dose/high dose) | 176/184/176 (placebo/low dose/high dose) | Mortality, CIV, DIND | Poor outcome (GOSE≤4) week 12 | Mortality/morbidity (high-dose 15% vs low-dose 24% vs placebo 27%; P=0.007 and P=0.340). Poor outcome in 24% of the placebo group, in 25% of the 5 mg h−1 group (OR: 0.918; P=0.748), and in 28% of the 15 mg h−1 group (OR: 1.337; P=0.266) | Premature stop after completion of CONCSCIOUS-2. Clazosentan 15 mg h−1 significantly reduced cVS-related morbidity and all-cause mortality. The drug did not improve neurological outcome |
| Yoneda and colleagues (2014), EVAS41 | RCT | cp | EPA 900 mg t.i.d. | 81/81 | 80/80 | Clinical cVS, CIV | Favourable outcome (mRS≤2) after 1 and 6 months | Clinical cVS (15 vs 30%; P=0.022), CIV (7 vs 21%; P=0.012). Favourable outcome at 1 month (78 vs 73%; P=0.47), favourable outcome 6 months (88 vs 85%; P=0.65) | The incidence of symptomatic cVS and related infarction was significantly lower in the EPA group compared with control, without a beneficial effect on outcome |
| Helbok and colleagues (2012)42 | CS | cp/co | EPO 30 000 IU daily for 3 days | 6 | 6 | ns | EPO infusion significantly increased | EPO increased in poor-grade aSAH patients with severe cVS | |
| Zhao and colleagues (2011)43 | RCT | cp | Fasudil i.v. 30 mg t.i.d. for 14 days vs nimodipine i.v. 1–2 mg h−1 | 63/66 | 55/59 | Clinical cVS, CIV on CT. Favourable outcome (GOS>4) after 1 month | ns | Clinical cVS (14.3 vs 24.1% P=0.244), CIV on CT (21.8 vs 23.7%; P=0.040). Favourable outcome (74.5 vs 61.7%; P=0.040) | The occurrence of CT low-density areas was similar between both groups. Clinical outcome was significantly more favourable in the fasudil group |
| Simard and colleagues (2013)44 | R-CO | cp | UFH i.v. 8 U kg−1 h−1 for 14 days | 43/43 | 43/43 | Angiographic or clinical cVS, CIV | ns | Clinical cVS (9 vs 47%; P=0.0002). Infarction on CT (0 vs 21%; P=0.003) | Heparin infusion caused significantly fewer patients to develop symptomatic cVS and related infarctions |
| Soppi and colleagues (2012)45 | RCT | cp/co | Nimodipine 6×60 mg oral vs i.v. 2 mg h−1 | 84/87 | 81/83 | DIND | Ischaemic lesions on MRI, GOS, and mRS after 12 months | DIND (20 vs 16%; P=0.61), ischaemic lesions (34 vs 34%; P=0.99). No significant difference in clinical outcome | No difference in occurrence of MRI ischaemic lesions and long-term clinical outcome was shown between enteral and i.v. nimodipine use |
| Westermaier and colleagues (2010)30 | RCT | cp/co | Mg i.v. for 10 days, target concentration (2.0–2.5 mmol litre−1) | 55/55 | 54/53 | CIV on CT within 10 days | DIND, TCD/angio-defined cVS, GOS after 6 months | CIV (22 vs 51%; P=0.0020), DIND (17 vs 28% P=0.1491), cVS (67 vs 85%; P=0.0279). No significant improvement of clinical outcome after 6 months | Magnesium-treated patients showed a lower rate of clinical and radiographical cVS. However, no difference in clinical outcome was seen between both groups. |
| Wong and colleagues (2010), IMASH31 | RCT | cp/co | Mg i.v. (target concentration maxiumum 2.5 mmol litre−1) | 169/158 | 159/154 | Favourable outcome (GOSE≥5) after 6 months | Clinical cVS first 2 weeks, mRS, BI, SF-36 at 6 months | Favourable outcome (64 vs 63%; OR 1.0). None of the secondary outcome parameters reached statistical significance | Magnesium did not improve outcome after 6 months in any of the subgroups |
| Dorhout Mees and colleagues (2012), MASH-233 | RCT | cp/co | Mg i.v. 64 mmol daily for 20 days | 606/597 | 604/596 | Poor outcome (mRS≥4) after 3 months | ns | Poor outcome (26.2 vs 25.3%; RR=1.03), mRS was not significantly different (P=0.95) | Magnesium i.v. did not decrease the incidence of cVS or improve neurological outcome 3 months after aSAH |
| Bradford and colleagues (2013)32 | RCT | cp/co | Mg i.v. for 12 days (target concentration 1.60–2.50 mmol litre−1) | 81/81 (high range/normal range) | 78/79 (high range/normal range) | Angiographic cVS | GOSE and mRS after 90 days, ICU LOS, duration of mechanical ventilation | cVS (50.6 vs 64.1%; P=0.09). GOS and mRS were similar in both groups (P=0.12, P=0.14). None of the secondary outcome parameters differed significantly |
Magnesium i.v. did not improve clinical outcome |
| Gomis and colleagues (2010)46 | RCT | cp/co | Methylprednisolone i.v. 16 mg kg−1 daily for 3 days | 49/46 | 40/38 | Clinical cVS, dypodensities on CT (days 4–10) | GOS and FOS after 1 yr | Symptomatic cVS (28.8 vs 31.5%; P=0.7). Hypodensities on CT (24.4 vs 18.4%; P=0.8). GOS (P=0.08). FOS (P=0.02) | High-dose i.v. methylprednisolone improved functional outcome 1 yr after aSAH as measured by the FOS |
| Ghodsi and colleagues (2015)47 | RCT | cp | Meloxicam 7.5 mg b.d. for 7 days | 40/41 | 40/41 | TCD cVS, mortality, LOS, GOS at discharge | ns | TCD cVS (32.5 vs 44.0%; P=0.564), mortality (15.0 vs 21.95%; P=0.569), LOS (17.4 vs 18%; P=0.145), GOS (P=0.618) | Prophylactic use of meloxicam did not decrease mortality or the incidence of TCD vasospasm and had no effect on clinical outcome as measured by GOS |
| Garg and colleagues (2013)34 | RCT | cp | Simvastatin 80 mg for 14 days | 19/19 | 17/18 | Clinical, angiographic or TCD cVS | GOS, mRS, MBI after 1, 3, and 6 months | Clinical cVS (26.3 vs 42.1%; P=0.31), TCD cVS (12.8 vs 26.3%; P=0.7). None of the secondary outcome parameters differed significantly | Simvastatin showed no benefit in reducing symptomatic vasospasm and improving functional outcome |
| Kirkpatrick and colleagues (2014), STASH35 | RCT | cp/co | Simvastatin 40 mg for 21 days | 391/412 | 379/403 | Favourable outcome (mRS≤2) at discharge and 6 months | Mortality at 6 months, DIND requiring rescue therapy | Favourable outcome at discharge (62 vs 62%; P=0.608). Favourable outcome 6 months (71.5 vs 71.7%; P=0.809). None of the secondary outcome parameters differed significantly |
Simvastatin did not improve outcome in aSAH patients |
| Wong and colleagues (2015), HDS-SAH trial36 | RCT | cp/co | Simvastatin 80 mg vs 40 mg for 21 days | 124/131 (40 mg/80 mg) | 124/131 (40 mg/80 mg) | DIND | Symptomatic cVS, CIV, favourable outcome (mRS≤2) at 3 months | DIND (27 vs 24%; P=0.586). Symptomatic cVS (15 vs 12%; P=0.589), CIV (16 vs 17%; P=0.886). Favourable outcome (73 vs 72%; P=0.770) | Simvastatin showed no benefit on the incidence of delayed ischaemic deficits or on the rate of favourable outcome at 3 months |
| Woo and colleagues (2015)37 | P-CO | cp/co | Simvastatin 20 mg/40 mg/80 mg for 14 days | 22/35/32 | 22/34/32 | mRS at 1 and 6 months | Symptomatic cVS | mRS at 1 month (77.3 vs 85.3 vs 87.1%; P=0.661), mRS at 3 months (90.9 vs 91.2 vs 87.1%; P=0.958). Symptomatic cVS (36.4 vs 8.8 vs 3.2%; P=0.003) | Prophylactic treatment with simvastatin reduced the incidence of symptomatic vasospasm significantly at a dose of 40 and 80 mg a day, without affecting clinical outcome as measured by the mRS |
Table 8.
Summary of all nine studies examining topical/mechanical DCI prevention. ACA, anterior cerebral artery; BI, Barthel index; CBF, cerebral blood flow; CCS, case–control study; CIV, vasospasm-related cerebral infarction; co, coiling; cp, clipping; CS, case series; CSF, cerebrospinal fluid; cVS, cerebral vasospasm; DCI, delayed cerebral ischaemia; DIND, delayed ischaemic neurological deficit; GOS, Glasgow outcome scale; MCA, middle cerebral artery; mRS, modified ranking scale; NPRI, nicardipine prolonged release implants; ns, not specified; , arterial partial carbon dioxide pressure; PPV, papaverine; r-tPA, recombinant tissue plasminogen activator; StiO2, brain tissue oxygen saturation; TCD, transcranial Doppler; UK, urokinase
| Authors (year) | Study type | Aneurysm treatment modality | Treatment | Sample size (treatment/control) | Sample size after dropouts | Primary outcome parameters | Secondary outcome parameters | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Webb and colleagues (2010)48 | CS | cp/co | Nicardipine intraventricular 4 mg every 8–12 h | 64 | 42 | TCD-measured mean flow velocities | ns | Mean CBF reduction of 26.3 cm s−1 in the MCA and 7.4 cm s−1 in the ACA | Nicardipine intraventricular is associated with a significant reduction in mean cerebral blood flow velocity as measured by TCD |
| Kasuya (2011)49 | CS | cp | Nicardipine implants | 136 | 136 | DIND, angiographic cVS, CIV CT | ns | DIND (8.2%), angiographic cVS (24.6%), CIV CT (12.4%) | The incidence of DIND and cVS was low compared with the overall incidence in control groups in similar studies50 |
| Schneider and colleagues (2011)51 | CCS | cp | Nicardipine implants | 27/27/27 (endovascular/no NCRI/NCRI) | 21/24/22 (endovascular/no NCRI/NCRI) | Angiographic cVS, CIV on CT, favourable outcome (mRS≤2) 1 yr | ns | Angiographic cVS (48 vs 44 vs 11%). Cerebral infarction on CT (28 vs 22 vs 7%). Favourable outcome (48 vs 50 vs 77%) | A reduction of the incidence of radiographical vasospasm and cerebral ischaemia is seen after NPRI implantation. The use of NCRI also showed a favourable 1 yr clinical outcome |
| Westermaier and colleagues (2014)52 | CS | cp/co | Transient hypercapnia | 6 | 6 | TCD-measured mean flow velocities | StiO2 | of 50 and 60 mm Hg resulted in mean CBF of 124 and 143% of baseline, StiO2 increased by 104 and 111% of baseline | Induced hypercapnia can enhance CBF and StiO2, with a slow return to baseline without a rebound effect |
| Al-Tamimi and colleagues (2012), LUMAS38 | RCT | cp/co | Insertion of a lumbar drain | 105/105 (drain/no drain) | 87/89 (drain/no drain) | DIND | Favourable outcome (mRS≤2) day 10 and 6 months | DIND (21.0 vs 35.2%; P=0.021). Favourable outcome day 10 (55.2 vs 37.5%; P=0.009). Favourable outcome 6 months (80.2% vs 81.4%; P=0.83) | Lumbar drain insertion decreased the incidence of DIND and improved short-term outcome |
| Park and colleagues (2015)39 | RCT | cp | 5–10 ml h−1 CSF drainage via LD during 14 days | 126/108 (drain/no drain) | 126/108 (drain/no drain) | Clinical cVS, need for angioplasty, CIV on MRI, GOS (4–5) discharge and 6 months, mortality | ns | Symptomatic cVS (19 vs 42%; P=<0.001). Need for angioplasty (17 vs 38%; P=0.001). CIV on MRI (17 vs 38%; P=0.001). GOS (4–5) discharge (69 vs 58%; P=0.084) and 6 months (82 vs 71%; P=0.001). Mortality (5 vs 10%; P=0.273) | CSF drainage via an LD significantly reduced the incidence of symptomatic vasospasm, the need for angioplasty, and the incidence of infarction and improved clinical outcome after 6 months |
| Kim and colleagues (2014)53 | P-CO | cp | Intracisternal irrigation with PPV or UK | 40/39/42 (PPV/UK/simple drainage) | 40/39/42 (PPV/UK/simple drainage) | Serum and CSF concentration of ICAM-1 and VCAM-1 | Angiographic cVS, symptomatic cVS, favourable outcome (GOS 4–5) at 6 months | Angiographic cVS (17.5 vs 23.8 vs 20.5%). Symptomatic cVS (15.0 vs 26.0 vs 17.9%). Favourable outcome (82.5 vs 76.2 vs 82.0%) |
Both cisternal irrigation with PPV and UK reduced the incidence of angiographic and symptomatic vasospasm compared with simple drainage, but there was no difference in outcome |
| Litrico and colleagues (2013)54 | RCT | cp/co | Intraventricular fibrinolysis with r-tPA | 11/8 | 11/8 | Mortality at 1 month | Days to intraventricular blood clearance as measured by the Graeb score in CT imaging GOS at 3 months and 1 yr | Mortality (45.5 vs 62.5%; P=0.65). Blood clearance rate (4.25 vs 10.67; P=0.001). No difference in clinical outcome | Intraventricular fibrinolysis reduced time to blood clearance without affecting mortality |
| Etminan and colleagues (2013)55 | RCT | cp/co | Intraventricular fibrinolysis with r-tPA and low-frequency head rotation | 30/30 | 30/30 | GOS at discharge and 3 months | Cloth clearance rate, radiographical cVS | GOS did not differ between both groups. Clot clearance rate was significantly faster in the r-tPA group | Intraventricular fibrinolysis increased the cloth clearance rate without improving functional outcome as measured by GOS |
| Kramer and colleagues (2014)56 | RCT | co | Intraventricular fibrinolysis with r-tPA | 6/6 | 6/6 | (safety/feasibility), clearance rate of intracranial blood | Angiographic cVS, DCI, mRS and BI after 6 months | More rapid reduction of bleeding volume (P=0.009). None of the secondary outcome parameters differed significantly | Intraventricular r-tPA accelerates clearance of subarachnoid and intraventricular blood, especially when administered early |
Table 9.
Summary of all nine studies examining haemodynamic management. AEs, adverse events; ALISAH, albumin in subarachnoid hemorrhage; CI, cardiac index; co, coiling; cp, clipping; cVS, cerebral vasospasm; DCI, delayed cerebral ischaemia; DIND, delayed ischaemic neurological deficit; GEDI, median global end-diastolic volume index; GOS, Glasgow outcome scale; IABC, intra-aortic balloon counterpulsation; ICU, intensive care unit; LOS, length of stay; MAP, mean arterial pressure; mRS, modified ranking scale; NIHSS, National Institute of Health Stroke Scale; ns, not specified; NV/HV/NT/IH, normovolaemia/hypervolaemia/no-hypertension/induced hypertension; P-CO, prospective cohort study; PE, pulmonary embolism; R-CO, retrospective cohort study; RCT, randomized controlled trial; SF-36, short-form 36; TCD, transcranial Doppler; WFNS, World Federation of Neurosurgical Societies
| Authors (year) | Study type | Aneurysm treatment modality | Treatment | Sample size (treatment/control) | Sample size after dropouts | Primary outcome parameters | Secondary outcome parameters | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Rondeau and colleagues (2012)57 | RCT | co | Prophylactic dobutamine increased CI vs norepinephrine increased MAP | 17/18 | 17/18 | Angiographic cVS | DCI, duration of mechanical ventilation, ICU LOS | Angiographic cVS (28 vs 27%; P=1), DCI (41 vs 50%; P=0.24), median duration of mechanical ventilation (8 vs 19 days; P=0.01), median ICU LOS (11 vs 21 days; P=0.01) | Increased CI with dobutamine did not lower the incidence of cVP compared with norepinephrine-induced hypertension |
| Togashi and colleagues (2015), IMPROVES58 | RCT | cp/co | Prophylactic hypervolaemia or induced hypertension for 10 days | 5/5/5/5 (NV/HV/NT/IH) | 5/5/5/5 (NV/HV/NT/IH) | (treatment compliance/feasibility), mRS at 6 months | Composite score of a neuropsychological testing battery | mRS volume group (1.7 vs 1.6; P=0.87), mRS pressure group (1.9 vs 1.4; P=0.43). Composite score of volume group (75 vs 68; P=0.64). Composite score of pressure group (85 vs 57; P=0.04) | Neither hypervolaemia nor induced hypertension was able to improve 6 month clinical outcome as measured by mRS. Hypervolaemia was associated with a considerable increase in adverse events. Patients treated with induced hypertension performed significantly worse in the neuropsychological testing |
| Bulters and colleagues (2013)59 | RCT | cp | Prophylactic IABC | 35/36 | 35/36 | GOS/SF-36 after 6 months | ns | No difference in GOS and SF-36 between groups | The use of IABC in aSAH patients had no clinical benefit as measured by the GOS and SF-36 |
| Ibrahim and Macdonald (2013)60 | R-CO | cp/co | Colloid infusion or no colloid infusion | 41/82 | 41/82 | DIND, CIV | NIHSS, mRS, GOS | DIND (17.1 vs 22.0%; P=0.64), delayed infarcts (51.2 vs 47.6%; P=0.71), NIHSS≥10 (9.7 vs 1.2%; P=0.042), no difference in mRS or GOS | No difference in DIND or infarct incidence was seen between cohorts. Patients who received colloids scored worse on the NIHSS. Colloid administration and a positive fluid balance can be potentially deleterious in aSAH patients |
| Yoneda and colleagues (2013)61 | P-CO | cp/co | PiCCO monitoring | 204 | 204 | Passive monitoring of GEDI and CI | ns | GEDI, CI, and systemic vascular resistance index (days 3–6) were independently related to DCI onset (P=0.023, P=0.013, and P=0.003) | PiCCO monitoring could help to facilitate haemodynamic management of aSAH patients |
| Tagami and colleagues (2014)62 | P-CO | cp/co | PiCCO monitoring | 180 | 180 | DCI, PE within 14 days | Identification of variables associated with DCI and PE onset | GEDI is independently related to DCI onset between days 1 and 7 after SAH and PE occurrence between days 4 and 7. GEDI threshold is associated with DCI (<822 ml m−2) and PE (>921 ml m−2) | Maintaining GEDI slightly above normal might be beneficial in preventing DCI |
| Mutoh and colleagues (2014)63 | RCT | cp/co | PiCCO monitoring vs conventional monitoring | 80/80 | 80/80 | DCI within 21 days | Favourable outcome (mRS 0–3) after 3 months | DCI (33 vs 42%; P=0.33), mRS did not differ significantly. Subgroup analysis of WFNS (4–5) DCI (5 vs 14%; P=0.036). Favourable outcome (52 vs 36%; P=0.026) | Patients with high-grade aSAH who received early goal-directed therapy had a significant lower incidence of DCI and a higher incidence of favourable outcome after 3 months |
| Suarez and colleagues (2012)64 and (2015), ALISAH65 | P-CO | ns | Up to 1.875 g kg−1 prophylactic albumin daily for 7 days | 20/20/7 (0.625, 1.25, and 1.875 g kg−1) | 16/16/6 (0.625, 1.25, and 1.875 g kg−1) | Incidence of serious AEs | TCD cVS, DCI, CIV | 171 AEs, TCD cVS (75 vs 55 vs 29%), DCI (20 vs 15 vs 14%), cerebral infarctions (45 vs 27 vs 25%) | Albumin appears to be safe in a dose up to 1.25 g kg−1 and may exert neuroprotective effects |
Table 10.
Summary of all 11 studies examining DCI treatments. CIV, vasospasm-related cerebral infarction; co, coiling; cp, clipping; CS, case series; DCI, delayed cerebral ischaemia; IAS, intra-arterial spasmolysis; ICU, intensive care unit; LOS, length of stay; mRS, modified ranking scale; ns, not specified; , brain tissue oxygen tension; RCT, randomized controlled trial; TBA, transluminal balloon angioplasty; TCD, transcranial Doppler
| Authors (year) | Study type | Aneurysm treatment modality | Treatment | Sample size (treatment/control) | Sample size after dropouts | Primary outcome parameters | Secondary outcome parameters | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Intraventricular nicardipine | |||||||||
| Lu and colleagues (2012)66 | CCS | cp/co | Intraventricular nicardipine in patients with suspected cerebral vasospasm based on TCD or clinical deficits | 14/14 | 14/14 | mRS and GOS after 30 and 90 days | TCD-measured mean flow velocities | No difference in mRS or GOS between groups. Reduction of the mean flow velocity of 38.2 cm s−1 in right MCA and 28.8 cm s−1 in left MCA | Intraventricular nicardipine decreased the mean MCA flow velocity but did not lead to an improvement in clinical outcome (mRS and GOS) after 30 or 90 days |
| Induced hypertension | |||||||||
| Gathier and colleagues (2015)67 | RCT | cp/co | Norepinephrine-induced hypertension | 18/18 | 13/12 | CBF measured by CT perfusion imaging | ns | CBF change (−8.5 vs 0.1; P=0.25) | Induced hypertension did not increase CBF measured by CT perfusion imaging in this small sample |
| Interventional neuroradiological treatments | |||||||||
| Suzuki and colleagues (2012)68 | CS | cp/co | IAS with papaverine or colforsin | 98/133 (papaverine/colforsin) | 22/22 (patients who received spasmolysis) | Favourable outcome (mRS≤2) at discharge | CIV | mRS≤2 in patients with cVS (34 vs 66%; P=0.032), CIV (85.2 vs 62.1%; P=0.039) | Spasmolysis with colforsin resulted in a better mRS compared with papaverine |
| Sherif and colleagues (2015)69 | CS | cp/co | Combined IAS with milrinone and nimodipine | 16 | 16 | Effectiveness in resolving angiographic vasospasm, pre- and postinterventional TCD flow velocities | Favourable clinical outcome (mRS≤2), neurological status, mortality | 87.5% showed DSA improvement, mean TCD flow reduced significantly (P=0.001). Favourable outcome (68.5%). Mortality (6.25%) | Combined IAS with milrinone and nimodipine was able to resolve angiographic vasospasm and improve TCD flow velocities |
| Saito and colleagues (2015)70 | CS | cp/co | IAS with fasudil | 38 | 38 | Arterial circulation time in DSA before and after IAS | ns | Significant reduction of the circulation time (P=0.005) | Intra-arterial spasmolysis with fasudil significantly reduced the arterial cerebral circulation time as measured during DSA |
| Iwabuchi and colleagues (2015)71 | CS | cp/co | IAS with fasudil | 92 | 92 | GOS at discharge, effectiveness in resolving angiographic cVS | ns | High proportion of patients with GOS 5 at discharge (83%). Fasudil infusion improved angiographic cVS in all patients | Intra-arterial spasmolysis with fasudil was able to improve angiographic cVS and resulted in a high proportion of patients with favourable outcome |
| Aburto-Murrieta and colleagues (2012)72 | CCS | cp/co | IAS with nimodipine or TBA | 22/8 (IAS/TBA) | 22/8 (IAS/TBA) | Favourable outcome (mRS≤2) | ns | Favourable outcome (45 vs 25%; P=0.42) | No difference in clinical outcome measured by mRS was observed between IAS with nimodipine and TBA |
| Albanese and colleagues (2010)73 | CS | cp/co | Prolonged intra-arterial verapamil | 12 patients; 36 treated vessels | 12 patients; 36 treated vessels | Effectiveness in resolving angiographic cVS | mRS at discharge | Verapamil infusion resolved angiographic cVS completely in 32 vessels and partly in 4 of 36 treated vessels | Continuous spasmolysis with verapamil is safe and shown to be effective in resolving medically refractory vasospasm |
| Musahl and colleagues (2011)74 | CS | cp/co | Continuous intra-arterial nimodipine | 6 | 6 | Neurological status, TCD, , mRS | ns | Neurological deficits improved in all patients or returned to normal. mRS 0–2 in 1, mRS 3–4 in 2, and mRS 5 in 1 patient | Continuous spasmolysis with nimodipine appears safe and effective in treating refractory vasospasm |
| Bele and colleagues (2015)75 | CCS | cp/co | Continuous intra-arterial nimodipine | 21/20 | 19/20 | Favourable outcome (GOS>4) at 6 months | Incidence of CIV | 6 months GOS>4 (76 vs 10.0%), CIV (42.6 vs 75.0%) | Continuous intra-arterial nimodipine improved long-term clinical outcome as measured by GOS and reduced the incidence of ischemia |
| Mortimer and colleagues (2015)76 | R-CO | cp/co | Intensive medical and endovascular management vs no specific treatment (no cVS aSAH control group) | 17/63 (intensive management/no specific treatment) | 16/62 (intensive management/no specific treatment) | Favourable outcome at discharge (GOS>4). Favourable outcome at 90 days (GOS>4). Favourable outcome at 90 days (mRS≤2) | LOS in hospital, LOS in ICU | Discharge GOS>4 (47.1 vs 55.5%; P=0.591), 90 days GOS>4 (94.1 vs 82.5%; P=0.444), 90 days mRS≤2 (88.2 vs 81.0%; P=0.755), LOS (27.1 vs 21.5 days; P=0.039), ICU LOS (2.7 vs 2.4 days; P<0.0001) | Vasospasm treatment with induced hypertension and endovascular therapy resulted in a similar clinical outcome (GOS) compared with a no-vasospasm control group |
Study characteristics
In the final analysis, 26 randomized controlled trials,26–36,38–41,43,45–47,54–59,63,67 five case–control studies,40,51,66,72,75 five prospective cohort studies,37,53,61,64 three retrospective cohort studies,44,60,76 and 10 case series42,48,49,52,68–71,73,74 were included. A summary of the most relevant data per study is depicted in Tables 7 –10. Fourteen studies included only clipped patients,26–28,34,39–41,43,44,47,49,51,53,59 three only coiled patients,29,56,57 and 31 studies included patients treated by either coiling or clipping.30–33,35–38,42,45,46,48,52,54,55,58,60–63,66–74 Clipping is the treatment of an intracerebral aneurysm by operative closure of the aneurysm neck by placing a titanium clip. During coiling the aneurysm is treated by endovascularly filling the aneurysm cavity with titanium coils. One study did not specify the aneurysm treatment modality.64 It is noteworthy that outcome assessment suffered from a large heterogeneity in definition. This precludes adequate comparison of study results and makes pooling of DCI data impossible. The applied definitions of clinical or angiographic vasospasm, DCI, delayed ischaemic neurological deficit (DIND), and favourable and unfavourable outcome across different studies are summarized in Supplementary Table S3.
Risk of bias within and across studies
The results of both bias assessors proved to be congruous and are presented in Supplementary Tables S1 and S2. Of all 26 randomized trials, nine trials were labelled at low risk for all bias domains.28,29,33,35,36,38,55,56,77 Of the remaining trials, nine suffered from performance bias, mostly attributable to the lack of a placebo group or blinding.26,27,30,41,43,45,54,57,63 Eleven studies were labelled at unclear risk of selection bias because they did not specify how randomization or concealment was achieved.26,32,34,39,41,43,46,47,57,59,63 Attrition or reporting bias was not identified because all trials adequately documented dropouts, and missing values were well balanced between the studied groups. In three studies, a relevant difference in standard management between groups was identified.26,27,46 Four out of 18 observational studies were labelled as being at moderate risk of bias.61,62,64,76 Five trials were considered at critical risk, mainly because of the combination of confounding and selection bias, and missing data.37,48,69–71 All other trials were at serious risk for bias because of confounding and selection bias inherent to the study design.42,44,49,52,53,60,73,74 Of the five included case–control studies, two were labelled as moderate risk40,51 and three as serious risk.66,72,75
Quality of evidence for each individual outcome
Prophylactic therapy with cilostazol, clazosentan, magnesium, simvastatin, and cerebrospinal fluid (CSF) drainage with lumbar drainage was assessed in two or more included trials. Intraventricular fibrinolysis was excluded because of relevant inter-study treatment differences [one of the included trials also applied kinetic energy in combination with intraventricular recombinant tissue plasminogen activator (r-tPA)].
Results of individual studies
Per category, the individual treatments are listed alphabetically.
Prevention of delayed cerebral ischaemia
Systemic delayed cerebral ischaemia prevention
Cilostazol
Cilostazol is a selective inhibitor of phosphodiesterase-3 and exerts a vasodilatory and antithrombotic effect. Cilostazol 100 mg orally twice daily for 14 days was able to improve clinical outcome measured by the modified ranking scale (mRS) at discharge.26 In another randomized controlled trial (RCT) applying the same dose and length of application, the incidence of symptomatic and angiographic vasospasm decreased significantly without affecting clinical outcome assessed by mRS after 6 months.27 Finally, the introduction of early combined enteral and parenteral nutrition together with oral cilostazol reduced the incidence of symptomatic vasospasm and cerebral infarction seen on computed tomography (CT).40 These results were reflected in a better clinical outcome at discharge measured by mRS. No serious adverse reactions to cilostazol, such as haemorrhagic complications or refractory hypotension, were reported.
Clazosentan
Clazosentan is an endothelin receptor antagonist that has been tested to reduce vasospasm-related morbidity and all-cause mortality in three large RCTs, the so called ‘Clazosentan to Overcome Neurological Ischaemia and Infarction studies’ (CONSCIOUS-1, -2, and -3). CONSCIOUS-1 (published before 2010) showed a dose-dependent reduction in moderate or severe angiographic vasospasm, and a dose of 15 mg h−1 resulted in a 65% risk reduction.78 These promising results were explored further in aSAH patients treated either by surgical clipping (CONSCIOUS-2) or endovascular coiling (CONSCIOUS-3). In the first study, patients were randomly assigned to receive clazosentan 5 mg hourly (n=768) or placebo (n=389) for up to 14 days.28 The trial failed to show a significant difference in mortality and morbidity or the incidence of new cerebral infarcts and DIND. These negative results have led to the premature halt of the CONSCIOUS-3 trial.29 Nevertheless, this study managed to enrol 571 aSAH patients in three different dosage tiers (placebo, n=189; clazosentan 5 mg h−1, n=194; and clazosentan 15 mg h−1, n=188). The highest dosage tier presented with a significantly reduced incidence of vasospasm-related morbidity and all-cause mortality compared with the placebo group (15 vs 27%; P=0.007). No effect on clinical outcome measured by the extended Glasgow outcome scale (GOSE) was detected. It is argued by the authors that this study was underpowered to detect a difference because of its premature discontinuation.
Eicosapentaenoic acid
Omega-3 fatty acids, such as eicosapentaenoic acid (EPA), have established protective cardio- and cerebrovascular properties.79 The long-term use of EPA led to a 20% relative risk reduction in recurrent stroke.80 Reduction in platelet reactivity and a vasodilatory effect made it an interesting potential treatment in aSAH patients. In the efficacy of Eicosapentaenoic acid for cerebral Vasospasm after aneurysmal subarachnoid hemorrhage study (EVAS), clipped patients were randomized to receive EPA 900 mg three times daily or no treatment.41 The treatment group presented with a significantly lower incidence of symptomatic vasospasm and cerebral infarction but without a difference in clinical outcome measured by mRS. The latter finding was interpreted as a result of the success of rescue therapy given to patients with DCI, masking the sole effect of EPA. In this study, no serious EPA-related adverse reactions were reported.
Erythropoietin
The erythropoiesis-stimulating hormone erythropoietin (EPO) has been shown to reduce the severity of vasospasm.81 In a case series of seven patients with cerebral vasospasm, the infusion of 30 000 IU of EPO for 3 days consecutively increased brain tissue oxygen tension significantly over baseline.42 The effect was apparent within hours, and both restoration of cerebral autoregulation and an anti-inflammatory potency have been postulated as possible underlying mechanisms.
Fasudil
Fasudil is a Rho-kinase inhibitor exerting a vasodilatory effect. In a Japanese study, fasudil reduced the relative incidence of symptomatic vasospasm by 30% and angiographic vasospasm by 38%.82 The drug has therefore become the standard prophylactic treatment in Japan because nimodipine is not approved there.83 In a randomized open-label clinical trial, the use of fasudil 30 mg as a daily i.v. bolus was compared with nimodipine i.v.43 Neither the incidence of clinical vasospasm nor the occurrence of CT hypodensities differed significantly between groups. However, a more favourable clinical outcome after 1 month, as measured by a Glasgow outcome scale (GOS) >4, was observed in the fasudil group.
Heparin
Anti-inflammatory features capable of affecting endothelial dysfunction have been attributed to the mixture of polysaccharide chains in unfractionated heparin. Alongside its better-known anticoagulant properties, this makes heparin an interesting potential DCI preventive treatment. In a retrospective case–control study, the postoperative administration of unfractionated heparin 8 U kg−1 h−1 was compared with a control group receiving unfractionated heparin 5000 U s.c. twice daily.44 The incidence of symptomatic vasospasm was 9% in the heparin group compared with 47% in the control group (P=0.0002). No haemorrhagic complications were reported. These positive results have to be seen in relation to the study limitations. Patients were not randomized, both groups were treated by different neurosurgeons, and no long-term clinical outcome was provided.
Nimodipine i.v
The dihydropyridine calcium channel blocker nimodipine is recommended for all aSAH patients. The evidence for efficacy of this drug is largely based on a single large RCT.84 Nimodipine 60 mg six times daily for 21 days after aSAH reduced the incidence of poor outcome significantly as measured by GOS. In most institutions, if oral use is not applicable, nimodipine tablets are ground and administered via a nasogastric tube. Although i.v. nimodipine has been branded by the manufacturer, the pharmacodynamics are very different, and i.v. application is not recommended based on a Cochrane review.14 No difference in incidence of DIND and clinical outcome (GOS and mRS) was seen in a randomized trial comparing oral nimodipine with a nimodipine infusion.45 Aberrant to treatment guidelines, oral nimodipine was used for only 16 days instead of 21 days. Furthermore, relevant side-effects, such as hypotension, are not mentioned. Although small in size, this study presents the use of i.v. nimodipine as an equivalent mode of administration in instances of impaired enteral absorption, excessive nausea, and decreased level of consciousness.
Magnesium
As a physiological calcium antagonist, magnesium exerts a vasodilatory effect. Four large trials have recently been published applying magnesium i.v. The first trial showed that an elevated serum concentration of 2.0–2.5 mmol litre−1 could reduce the incidence of ischaemia on CT.30 However, no long-term clinical outcome was reported. The intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage failed to show any beneficial effects on outcome as measured by the GOSE after continuous i.v. magnesium therapy.31 In the magnesium sulphate for aneurysmal subarachnoid hemorrhage-2 (MASH-2) trial, a fixed dose of magnesium sulphate (64 mmol) failed to show a beneficial effect on clinical outcome measured by mRS after 3 months.33 The last study compared a high-range magnesium concentration by continuous infusion with a normal-range control group, without showing a difference in radiological vasospasm.32
Methylprednisolone and meloxicam
Accumulating data reveal the role of endothelial injury and inflammation in the development of DCI. Therefore, an immunosuppressant strategy might become an interesting approach. In a French RCT, high-dose methylprednisolone i.v. significantly improved functional outcome at 1 yr after aSAH.46 There was no difference between both groups in the incidence of symptomatic vasospasm or hypodensities on CT imaging. These results are difficult to interpret because the incidence of DCI and ischaemic infarction is directly correlated with poor functional outcome.85 In contrast, the prophylactic use of the non-steroidal anti-inflammatory drug, meloxicam, had no effect on clinical outcome measured by GOS and was not able to reduce the incidence of transcranial Doppler (TCD)-measured vasospasm in a single double-blind RCT.47
Simvastatin
In animal research, statins have been shown to increase cerebral blood flow (CBF) via the expression of cerebral endothelial nitric oxide synthase.86 The application of simvastatin 80 mg for 14 days after aSAH did not affect either the incidence of clinical vasospasm or the clinical outcome compared with placebo.34 In the Simvastatin in Aneurysmal Subarachnoid Haemorrhage (STASH) study, patients received oral simvastatin 40 mg once daily during 21 days, or placebo.35 At 6 months, the incidence of favourable outcome (mRS≤2) did not differ between groups (P=0.809). Also, none of the secondary outcome parameters, such as 6 month mortality, incidence of DIND, or the need for rescue therapy, differed significantly. A higher dose of simvastatin (80 mg) for 21 days was not able to decrease the incidence of DCI either.36 In a recent Korean trial, however, simvastatin 80 mg was able to reduce the incidence of DIND compared with lower doses without affecting mRS-measured clinical outcome.37 This study lacked a control group, and it is unclear which patients received rescue therapy.
Topical or mechanical prevention of delayed cerebral ischaemia
Intraventricular nicardipine
Local application of calcium channel blockers provides the advantage of bypassing systemic side-effects, in particular hypotension. Different types and routes of application have been studied. One such application method is via a pre-existing external ventricular drain inserted for CSF drainage and intracranial pressure monitoring. In a retrospective analysis, intraventricular nicardipine 4 mg every 8–12 h significantly reduced mean CBF velocities in the middle and anterior cerebral arteries of patients with clinical vasospasm.48 The effect persisted over a 24 h period. This was a pilot trial examining sonographic vasospasm as a surrogate outcome parameter. The authors gave no insights into the use of additional rescue therapy, clinical outcome, or the incidence of vasospasm-related infarctions.
Nicardipine controlled release implants
Surgical exposure of the aneurysm and the surrounding vessels most prone to vasospasm development allows the topical application of prophylactic vasodilatory agents. In this context, the most commonly studied drug is nicardipine applied via nicardipine implants. These offer the possibility of releasing the drug for up to 2 weeks in a controlled fashion. This is naturally applicable only in patients treated by surgical clipping, and effects on the contralateral side may not be as distinct. In a Japanese series, a low incidence of DIND was seen in Fisher grade 3 aSAH patients who were treated by surgical clipping and nicardipine 4 mg slow-release pellet implantation.49 In a controlled trial of Fisher grade 3 haemorrhages, a group treated by surgical clipping and nicardipine pallet implantation alongside the exposed vessels was compared with a coiling and a clipping, without nicardipine group.51 The nicardipine group showed the lowest incidence of cerebral infarction and a better 1 yr outcome measured by mRS.
Subarachnoid blood clearance
The positive correlation between the amount of subarachnoid blood and the risk of developing vasospasm has long been established. Accordingly, techniques to increase blood clearance have been assessed. One simple method is to use lumbar drainage. In a British RCT, the lumbar drainage group had a significantly lower incidence of DIND and a better outcome (mRS≤2) at day 10.38 However, the latter effect vanished after 6 months. Lumbar drainage of CSF also significantly reduced the incidence of symptomatic vasospasm and the incidence of infarction.39 This trial could, however, be susceptible to multiple types of bias because the manuscript does not provide insights into how randomization or allocation concealment was achieved or whether patients and assessors were blinded or not.
A more invasive alternative is cisternal irrigation. Experimental data have shown that thrombolytic agents, such as urokinase, quicken the dissolution of subarachnoid blood and thus help to prevent vasospasm. When comparing three irrigation modalities, passive spontaneous drainage proved inferior compared with irrigation with papaverine or urokinase.53 The incidence of vasospasm in the papaverine group was not significantly different from the urokinase group (P=0.328) but was significantly lower than in the simple drain group (P=0.035). There was no significant difference in GOS after 6 months between the three subgroups. These results are compromised by the lack of randomization and a non-drainage aSAH control group. The application of r-tPA 6 mg daily via an external ventricular drain has been proved to increase blood clearance in aSAH patients with third and fourth ventricle obstruction. This intervention, however, did not decrease mortality or improve functional outcome defined by GOS, compared with simple passive drainage (n=8).54 In a small randomized trial, treatment with intraventricular r-tPA 2 mg every 12 h resulted in a rapid reduction of blood volume as seen on consecutive CT scans.56 An unusual approach to accelerate blood clearance is kinetic energy. Clot clearance rate was significantly faster in a study where treatment with intraventricular fibrinolysis was combined with low-frequency movement in a rotational bed as measured by a software volumetric analysis of CT slices.55 Functional outcome measured by GOS at discharge and after 3 months did not differ between groups. This study was most probably underpowered to detect a long-term difference in clinical outcome.
Transient hypercapnia
In physiological conditions, the partial pressure of carbon dioxide is a major determinant of CBF regulation, and a local increase has a strong vasodilatory effect. This effect was conformed in a small aSAH cohort of six patients.52 An increase in CBF of 124 and 143% above baseline, respectively, could be achieved by inducing hypercapnia of ∼6.66 and 8 kPa.
Haemodynamic management
Blood pressure and fluid management
Prophylactic triple-H therapy is not recommended. Nevertheless, trials have been conducted examining the preventive properties of single component of the triad. When norepinephrine-induced hypertension was compared with dobutamine-induced elevated cardiac index as a preventive strategy, the incidence of radiographic vasospasm was shown to be equal in both groups.57 However, for unclear reasons, the median duration of mechanical ventilation and the median intensive care unit length of stay were significantly lower in the dobutamine group.
An interesting approach to overcome systemic effects of catecholamine infusion is the treatment with intra-aortic balloon counterpulsation (IABC). This technique has been shown to increase CBF in animal models. In a small RCT of clipped patients, no improvement in clinical outcome after 6 months measured by GOS and SF-36 was shown when induced hypertension was compared with IABC as a prophylactic measure.59 Yet, there could be a setting for the use of IABC when cerebral vasospasm is refractory to induced hypertension or when catecholamine treatment is causing side-effects in patients with diffuse vasospasm not eligible for angioplasty. Furthermore, this treatment could prove to be useful in patients with neurogenic stunned myocardium, in whom catecholamine treatment may be ineffective or even harmful.
Optimal fluid management after aSAH can be challenging, and evidence is lacking. A post hoc analysis of the CONSCIOUS-1 data showed that the use of colloids for volume expansion was associated with a worse performance in National Institute of Health Stroke Scale and mRS.60 In a randomized pilot trial, prophylactic hypervolaemia caused an overall four-fold increase in the risk of adverse events.58 This supports the existing evidence that hypervolaemia is not desirable in aSAH patients.87–89 In the search for a quantifiable threshold to guide fluid management, the Japanese SAH PiCCO® study group has explored the value of a pulse contour cardiac output device to guide haemodynamic management. Interestingly, in a sample of aSAH patients the median global end-diastolic volume index (GEDI) and the cardiac index were independently related to DCI onset.61 In a subset of this cohort, the patients struck by DCI presented with a significantly lower GEDI during the first 7 days.62 A threshold value of 822 ml m−2, slightly above the reference range (680–800 ml m−2), proved protective against the development of DCI. These results suggest that maintaining GEDI slightly above normal might be beneficial in DCI prevention. Furthermore, invasive haemodynamic monitoring and an early goal-directed fluid therapy based on pulse contour monitoring proved to be beneficial in a subgroup of aSAH patients with World Federation of Neurosurgical Societies grade 4 and 5.63 These patients had a significantly lower incidence of DCI and higher incidence of favourable functional outcome at 3 months measured by mRS compared with the treatment group receiving volume management based only on fluid balance and central venous pressure.
Albumin
Human albumin has been shown to exert neuroprotective effects in animal models.90 The albumin in subarachnoid hemorrhage (ALISAH) study investigated the safety and tolerability of four different dosages of 25% human albumin in aSAH patients.64 The highest dosage tier group experienced a high dropout rate mainly because of adverse effects, voluntary dropouts, and a protocol violation. However, in the two lowest dose groups, a dose-related effect was seen because patients who received the higher dose performed consistently better on GOS and mRS after 3 months. Additionally, subjects in the higher dosage group experienced less TCD vasospasm, DCI, and cerebral infarctions.65 The authors conclude that a dose of albumin up to 1.25 g kg−1 day−1 for 7 days is safe and may have neuroprotective effects. A randomized placebo-controlled trial called ALISAH II is planned to provide better support for this statement.
Treatment of delayed cerebral ischaemia
Intraventricular nicardipine
Intraventricular injection of nicardipine was examined in one trial to treat TCD-diagnosed vasospasm. In this retrospective case–control study, intraventricular nicardipine was able to lower the mean flow velocity significantly in patients with suspected vasospasm.66 However, this effect was observed only in the right-sided middle cerebral artery (P=0.041). No significant difference in clinical outcomes as measured by the mRS or GOS was seen between the treatment and control group after 30 and 90 days. This study had shortcomings, and both groups were not well matched for GCS and Fisher grade.
Induced hypertension
Evidence accumulates in favour of euvolaemic hypertension as a monotherapy to treat insufficient cerebral perfusion after aSAH. However, it remains unclear how to achieve this condition. Unfortunately, studies are scarce, and only one trial was found. In the recently finished hypertension induction in the management of aneurysmaL subarachnoid haemorrhage with secondary ischaemia (HIMALAIA) study, patients with DCI were randomized to receive either induced hypertension or no induced hypertension.91 The first published pilot data of this study showed that noradrenaline-induced hypertension was not able to increase CBF measured by computed tomography perfusion imaging.67 This is the first randomized trial of its kind, and further clinical results are highly anticipated.
Interventional neuroradiological treatments
Confronted with vasospasm refractory to medical treatment, rescue strategies have emerged out of the field of interventional neuroradiology. Intra-arterial spasmolysis (IAS) has a transient effect but is able to treat more distally located and diffuse spasms, whereas transluminal balloon angioplasty (TBA) has a longer-lasting effect and is used to treat proximal vasospasm. There is a lack of clinical studies, and selection criteria for endovascular treatment are not well defined. To date, no guideline exists, leading to a large inter-institutional variation.92,93 Nine studies were found assessing endovascular treatment of vasospasm. A handful of vasodilating drugs have been described to perform IAS. When comparing papaverine with colforsin, the latter proved to be associated with a better outcome (mRS≤2) in a retrospective cohort study.68 Unfortunately, both cohorts were collected in different time periods, leading to bias. Nonetheless, these data support an existing tendency to abandon papaverine as a vasodilator based on previous reports of deleterious effects, such as increased hypertension and neurotoxicity.94 The combination of milrinone and nimodipine appeared to have good spasmolytic properties measured by an increased post-interventional arterial circulation time.69 Intra-arterial fasudil resulted in good angiographic improvement of vasospasm and could effectively prevent neurological deficits in a case series of aSAH patients with angiographic vasospasm.70,71 When TBA was compared with IAS (nimodipine), no effect on patient outcome measured by mRS was observed.72 It is, however, questionable to what extent both methods can be compared, because they have different indications. To overcome the transient nature of IAS treatment effects, necessitating multiple sessions, drugs can be delivered through an indwelling microcatheter. These prolonged or continuous infusions have been described using verapamil or nimodipine. In a case series, prolonged intra-arterial verapamil infusion proved to be effective in restoring flow and resolving angiographic vasospasm.73 Comparably, continuous local intra-arterial administration of nimodipine improved the clinical condition in six aSAH patients with refractory vasospasm.74
Two trials providing clinical outcome data after endovascular treatment were found.75 In the first trial, patients who received prolonged intra-arterial nimodipine were compared with a non-endovascular-treated historical cohort. Here, the nimodipine group presented with a better clinical outcome as measured by the GOS after 6 months. This trial was, however, labelled as being at serious risk for bias, mainly because of confounding and the lack of specification of treatments applied in the control group. In the second trial, treatment of vasospasm with induced hypertension and endovascular therapy resulted in a similar clinical outcome (GOS) to an aSAH cohort with mild to no cVS.76
Discussion
Summary of evidence
In the present systematic review, an update is given on all clinical studies published in the last 5 yr assessing potential treatment strategies to overcome DCI and improve functional outcome in aSAH patients. A total of 49 studies were identified that met our inclusion criteria. The risk of bias in each individual study was assessed. Quality of evidence analysis according to outcome parameters was performed for five treatment strategies.
An overview of existing DCI recommendations from American and European guidelines and new developments from recent literature are provided in Table 11.
Table 11.
Overview of recommendations concerning DCI prevention and treatment and new developments in the last 5 yr. aSAH, aneurysmal subarachnoid haemorrhage; CSF, cerebrospinal fluid; DCI, delayed cerebral ischaemia
| AHA/ASA Guidelines | European Guidelines | New developments | |
|---|---|---|---|
| Systemic DCI prevention | Oral nimodipine should be administered to all patients with aSAH (class I, level A) | Nimodipine should be administered orally to prevent DCI (class I, level A). If oral administration is not possible, nimodipine should be applied i.v. | The preventive use of clazosentan, magnesium, or simvastatin cannot be recommended (class I, level A). There is low-quality evidence that prophylactic cilastozol improves post-aSAH outcome (class IIa, level B) |
| Magnesium sulphate is not recommended for the prevention of DCI (class I, level A) | |||
| Topical/mechanical DCI prevention | Prophylactic hypervolaemia or balloon angioplasty before the development of angiographic spasm is not recommended (class III, level B) | Not discussed | There is weak evidence that CSF diversion with a lumbar drain can be used to optimize outcome after aSAH (class IIa, level B) |
| Blood pressure and fluid management | Maintenance of euvolaemia is recommended to prevent DCI (class I, level B) | No statements on preventive haemodynamic management | There is weak evidence that pulse contour cardiac monitoring could improve overall haemodynamic management in aSAH patients (class IIa, level B) |
| Induced hypertension | Induction of hypertension is recommended for patients with DCI unless cardiac status precludes it (class I, level B) | There is no evidence from controlled studies for induced hypertension or hypervolaemia to improve outcome in patients with delayed ischaemic deficit (class IV, level C) | No studies examining therapeutic hypertension were included |
| Interventional neuroradiological treatments | Cerebral angioplasty, selective intra-arterial vasodilator therapy, or both, is reasonable in patients with symptomatic cerebral vasospasm who are not responding rapidly to hypertensive therapy (class IIb, level B) | Not discussed | Intra-arterial spasmolysis or balloon angioplasty are valuable techniques in patients with symptomatic cerebral vasospasm who are refractory to induced hypertension (class IIb, level B) |
There is low-quality evidence that cilostazol is able to reduce the incidence of clinical vasospasm, reduce the rate of infarction, and improve mRS-measured outcome. The evidence quality was downgraded based on contradictory results between the two included RCTs.26,27 Cilostazol is an interesting prophylactic that warrants further larger trials.
The endothelin receptor blocker clazosentan was tested in three large RCTs and failed to improve clinical outcome, although a dose of 15 mg h−1 was able to reduce the incidence of vasospasm.29 This benefit was overshadowed by a high incidence of adverse events (mainly pulmonary complications, hypotension, and anaemia). Based on combined data of CONSCIOUS-1 and -2, clazosentan cannot be recommended to improve GOSE-measured outcome.
High-quality evidence exists that magnesium does not reduce the incidence of clinical vasospasm. Furthermore, i.v. treatment did not reduce the incidence of infarction or improve clinical outcome. These results are supported by a recent meta-analysis.95 Possible reasons for failure of magnesium treatment, such as insufficient blood–brain barrier penetrance, have been postulated. Whether intrathecal use proves beneficial has to be examined.33
Although the evidence quality is moderate, prophylactic daily administration of simvastatin 40 mg cannot be recommended to reduce the incidence of symptomatic vasospasm. A higher dose of 80 mg, however, was able to reduce the rate of vasospasm in a single trial. Nonetheless, this study had to be labelled at critical risk for bias because of confounding and a lack of a control group.
Methylprednisolone might be able to improve clinical outcome, but this result is based on a single small monocentric study.46 The trial was labelled at high risk for bias based on a relevant difference in standard therapy between groups. Prophylactic use of methylprednisolone or meloxicam cannot be recommended.
The initial positive reports on the use of eicosapentaenoic acid, erythropoietin, and heparin are based on single studies. The EPO and heparin trials were labelled at serious risk for bias because of confounding and selection bias. Only eicosapentaenoic acid was tested in a randomized trial; however, it was unclear whether blinding was applied or not. For now, there is insufficient evidence to recommend any of these treatments, and further research is needed.
Subsequently, fasudil i.v. and nimodipine i.v. have shown similar protective effects. Unfortunately, fasudil was compared only with nimodipine i.v., and a comparison with oral nimodipine, as guidelines recommend,18 appears paramount. Administration of nimodipine i.v. proved comparable with oral use in a single non-blinded RCT. As i.v. use is substantially more expensive, oral use remains first choice.
Moreover, topical application of vasodilatory therapy and, especially, the implantation of nicardipine drug capsules resulted in a notable reduction in clinical and radiographical vasospasm. Nicardipine implants are slowly proving to be a promising adjuvant in clipped patients; this statement is based on a high-quality but small case–control study, and a randomized trial assessing this treatment further is underway.96 Intraventricular injection of nicardipine was able to reduce TCD-measured flow velocities in vasospastic vessels, but without any effect on hard outcomes.48,66 The included intraventricular nicardipine case–control study lacked adequate matching and was therefore rated as being at serious risk for bias.
Both the use of lumbar drainage and intraventricular thrombolytic drugs are associated with a higher subarachnoid blood clearance rate. So far, no consistent benefits on long-term clinical outcome have been shown. However, based on data of two RCTs, there is high-quality evidence that the application of lumbar drainage is associated with a lower incidence of clinical vasospasm. In one trial, clinical outcome measured by GOS improved in the lumbar drainage group, but this study lacked an adequate randomization procedure, increasing bias susceptibility.
Furthermore, as evidence points towards hypertension as the most effective ‘H’ in ‘triple-H’, more institutions apply euvolaemic hypertension as a first step to treat symptomatic vasospasm. Hypertension increases CBF and is able to reverse neurological deficits in two-thirds of patients.97 At present, when hypertension is induced, the sympathomimetic drugs used and treatment goals differ greatly between institutions. The HIMALAIA trial is the first to randomize DCI patients in an induced hypertension group or no hypertension. Publication of the clinical data is expected soon.
Pulse contour cardiac output devices seem to be helpful in guiding blood pressure and fluid management in the aSAH patient. However, the quality of evidence remains weak because there are only observational trials and one relatively small non-blinded randomized trial to support this statement.
Finally, endovascular treatment has become an integral part of the management of patients with medically refractory vasospasm because it can produce angiographic resolution. It is, however, not always clear to what extent this translates into fewer infarctions and better outcome. Revascularization effects can cause additional damage, and procedural risks have to be taken into account. To date, only a limited number of clinical trials exist, mostly comparing different endovascular techniques. Recently, two trials have been published that provide clinical outcome data after endovascular treatment. Prolonged intra-arterial nimodipine and maximal medical plus endovascular vasospasm treatment were able to render a better or similar outcome, comparable with DCI-spared aSAH patients. Both trials have their limitations, and naturally, because of its use as a rescue therapy, the effectiveness and efficacy of endovascular therapy are difficult to assess.
The majority of data presented are from small and often not randomized trials. Of all 49 included studies, only nine (18%) had a sample size per group exceeding 100 patients (of which seven were RCTs), and 26 trials (53%) even had a group sample size smaller than 50 patients. This shortness of high-quality evidence complicates multidisciplinary decision-making in aSAH patients. Although reporting scales of neurological outcome, such as the GOS and mRS, are already widely used, we advocate a further standardization of the definitions of DCI and DIND to enable comparability of trials in aSAH research.
In contrast to traumatic brain injury guidelines, to our knowledge there have been no reports of the effect of aSAH guideline adherence or reports on the effect of guideline introduction. There has been a survey of European practices, pointing out a large variability in practice patterns.98 We believe that a study to examine guideline adherence and its effects on aSAH treatment outcome could prove to be extremely interesting.
Limitations
Only three databases were assessed. Although our search query was restricted to four languages, only articles in English met our selection criteria. Some aSAH studies have been published in, for example, Chinese, Japanese, and Korean, and were not included. Given that we also included non-randomized trials and case series, many studies are highly susceptible to selection bias. Nevertheless, we decided to include these publications because they can make a useful contribution to systematic reviews.99 The aforementioned heterogeneity in definition of outcomes limited the possibility of pooling data and precluded the summary of all data in a forest plot. We acknowledge that one of the major limitations of this manuscript is the limited time frame (from January 2010 to December 2015) out of which eligible studies were selected. This was done because the vast majority of trials on which the latest guidelines are based were published before 2010. The reader has to be aware that all recommendations in this manuscript are based solely on trials published after 2010.
Conclusion
In conclusion, even though aSAH mortality is in decline, there is much potential for improvement in the field of DCI treatment and prevention. There is high-quality evidence that does not support prophylactic use of clazosentan, magnesium, or simvastatin. Many treatments discussed in this manuscript remain to be tested in larger RCTls. We advocate the development of standardized definitions of DCI and DIND to improve future trial comparability. Further effort has to be put into the development and assessment of valuable new strategies to improve aSAH patient outcome.
Authors' contributions
Literature search: M.V.
Hits screened and reviewed: M.V., A.H., A.S., M.C.
Manuscript drafting: M.V.
Manuscript revision and editing: A.H., H.C., A.S., R.R., M.C.
Manuscript approval: M.V., A.H., H.C., A.S., R.R., M.C.
Supplementary material
Supplementary material is available at British Journal of Anaesthesia online.
Declaration of interest
None declared.
Supplementary Material
References
- 1.de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 2007; 78: 1365–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007; 369: 306–18 [DOI] [PubMed] [Google Scholar]
- 3.Rinkel GJ, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol 2011; 10: 349–56 [DOI] [PubMed] [Google Scholar]
- 4.Dodel R, Winter Y, Ringel F et al. Cost of illness in subarachnoid hemorrhage: a German longitudinal study. Stroke 2010; 41: 2918–23 [DOI] [PubMed] [Google Scholar]
- 5.Kassell NF, Torner JC, Jane JA, Haley EC Jr, Adams HP. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 2: surgical results. J Neurosurg 1990; 73: 37–47 [DOI] [PubMed] [Google Scholar]
- 6.Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 1994; 25: 1342–7 [DOI] [PubMed] [Google Scholar]
- 7.Proust F, Hannequin D, Langlois O, Freger P, Creissard P. Causes of morbidity and mortality after ruptured aneurysm surgery in a series of 230 patients. The importance of control angiography. Stroke 1995; 26: 1553–7 [DOI] [PubMed] [Google Scholar]
- 8.Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KT. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth 2012; 109: 315–29 [DOI] [PubMed] [Google Scholar]
- 9.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 2014; 10: 44–58 [DOI] [PubMed] [Google Scholar]
- 10.Budohoski KP, Guilfoyle M, Helmy A et al. The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2014; 85: 1343–53 [DOI] [PubMed] [Google Scholar]
- 11.Harders AG, Gilsbach JM. Time course of blood velocity changes related to vasospasm in the circle of Willis measured by transcranial Doppler ultrasound. J Neurosurg 1987; 66: 718–28 [DOI] [PubMed] [Google Scholar]
- 12.Dabus G, Nogueira RG. Current options for the management of aneurysmal subarachnoid hemorrhage-induced cerebral vasospasm: a comprehensive review of the literature. Interv Neurol 2013; 2: 30–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feigin VL, Rinkel GJ, Algra A, Vermeulen M, van Gijn J. Calcium antagonists in patients with aneurysmal subarachnoid hemorrhage: a systematic review. Neurology 1998; 50: 876–83 [DOI] [PubMed] [Google Scholar]
- 14.Dorhout Mees SM, Rinkel GJ, Feigin VL et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2007; 3: CD000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treggiari MM, Deem S. Which H is the most important in triple-H therapy for cerebral vasospasm? Curr Opin Crit Care 2009; 15: 83–6 [DOI] [PubMed] [Google Scholar]
- 16.Raabe A, Beck J, Keller M, Vatter H, Zimmermann M, Seifert V. Relative importance of hypertension compared with hypervolemia for increasing cerebral oxygenation in patients with cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg 2005; 103: 974–81 [DOI] [PubMed] [Google Scholar]
- 17.Gura M, Elmaci I, Cerci A, Sagiroglu E, Coskun KK. Haemodynamic augmentation in the treatment of vasospasm in aneurysmal subarachnoid hemorrhage. Turk Neurosurg 2012; 22: 435–40 [DOI] [PubMed] [Google Scholar]
- 18.Connolly ES Jr, Rabinstein AA, Carhuapoma JR et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43: 1711–37 [DOI] [PubMed] [Google Scholar]
- 19.Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology 2010; 74: 1494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis 2013; 35: 93–112 [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Altman DG, Gøtzsche PC et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Br Med J 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterne JAC, Higgins J, Reeves BC. on behalf of the development group for ACROBATNRSI. A Cochrane Risk Of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACROBAT-NRSI). In: Collaboration Collaboration, ed, Version 1.0.0, 24 September 2014. Available from http://handbook.cochrane.org/front_page.htm (accessed 4 May 2016) [Google Scholar]
- 25.Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011; 64: 380–2 [DOI] [PubMed] [Google Scholar]
- 26.Suzuki S, Sayama T, Nakamura T et al. Cilostazol improves outcome after subarachnoid hemorrhage: a preliminary report. Cerebrovasc Dis 2011; 32: 89–93 [DOI] [PubMed] [Google Scholar]
- 27.Senbokuya N, Kinouchi H, Kanemaru K et al. Effects of cilostazol on cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a multicenter prospective, randomized, open-label blinded end point trial. J Neurosurg 2013; 118: 121–30 [DOI] [PubMed] [Google Scholar]
- 28.Macdonald RL, Higashida RT, Keller E et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol 2011; 10: 618–25 [DOI] [PubMed] [Google Scholar]
- 29.Macdonald RL, Higashida RT, Keller E et al. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke 2012; 43: 1463–9 [DOI] [PubMed] [Google Scholar]
- 30.Westermaier T, Stetter C, Vince GH et al. Prophylactic intravenous magnesium sulfate for treatment of aneurysmal subarachnoid hemorrhage: a randomized, placebo-controlled, clinical study. Crit Care Med 2010; 38: 1284–90 [DOI] [PubMed] [Google Scholar]
- 31.Wong GK, Poon WS, Chan MT et al. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage (IMASH): a randomized, double-blinded, placebo-controlled, multicenter phase III trial. Stroke 2010; 41: 921–6 [DOI] [PubMed] [Google Scholar]
- 32.Bradford CM, Finfer S, O'Connor A et al. A randomised controlled trial of induced hypermagnesaemia following aneurysmal subarachnoid haemorrhage. Crit Care Resusc 2013; 15: 119–25 [PubMed] [Google Scholar]
- 33.Dorhout Mees SM, Algra A, Vandertop WP et al. Magnesium for aneurysmal subarachnoid haemorrhage (MASH-2): a randomised placebo-controlled trial. Lancet 2012; 380: 44–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garg K, Sinha S, Kale SS et al. Role of simvastatin in prevention of vasospasm and improving functional outcome after aneurysmal sub-arachnoid hemorrhage: a prospective, randomized, double-blind, placebo-controlled pilot trial. Br J Neurosurg 2013; 27: 181–6 [DOI] [PubMed] [Google Scholar]
- 35.Kirkpatrick PJ, Turner CL, Smith C, Hutchinson PJ, Murray GD. Simvastatin in aneurysmal subarachnoid haemorrhage (STASH): a multicentre randomised phase 3 trial. Lancet Neurol 2014; 13: 666–75 [DOI] [PubMed] [Google Scholar]
- 36.Wong GK, Chan DY, Siu DY et al. High-dose simvastatin for aneurysmal subarachnoid hemorrhage: multicenter randomized controlled double-blinded clinical trial. Stroke 2015; 46: 382–8 [DOI] [PubMed] [Google Scholar]
- 37.Woo SW, Kim JH, Kang HI, Kim DR, Moon BG, Kim JS. High-dose simvastatin is effective in preventing cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a prospective cohort study in Korean patients. J Korean Neurosurg Soc 2015; 58: 328–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Tamimi YZ, Bhargava D, Feltbower RG et al. Lumbar drainage of cerebrospinal fluid after aneurysmal subarachnoid hemorrhage: a prospective, randomized, controlled trial (LUMAS). Stroke 2012; 43: 677–82 [DOI] [PubMed] [Google Scholar]
- 39.Park S, Yang N, Seo E. The effectiveness of lumbar cerebrospinal fluid drainage to reduce the cerebral vasospasm after surgical clipping for aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc 2015; 57: 167–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura H, Okamura Y, Chiba Y et al. Cilostazol administration with combination enteral and parenteral nutrition therapy remarkably improves outcome after subarachnoid hemorrhage. Acta Neurochir Suppl 2015; 120: 147–52 [DOI] [PubMed] [Google Scholar]
- 41.Yoneda H, Shirao S, Nakagawara J, Ogasawara K, Tominaga T, Suzuki M. A prospective, multicenter, randomized study of the efficacy of eicosapentaenoic acid for cerebral vasospasm: the EVAS study. World Neurosurg 2014; 81: 309–15 [DOI] [PubMed] [Google Scholar]
- 42.Helbok R, Shaker E, Beer R et al. High dose erythropoietin increases brain tissue oxygen tension in severe vasospasm after subarachnoid hemorrhage. BMC Neurol 2012; 12: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao J, Zhou D, Guo J et al. Efficacy and safety of fasudil in patients with subarachnoid hemorrhage: final results of a randomized trial of fasudil versus nimodipine. Neurol Med Chir (Tokyo) 2011; 51: 679–83 [DOI] [PubMed] [Google Scholar]
- 44.Simard JM, Aldrich EF, Schreibman D, James RF, Polifka A, Beaty N. Low-dose intravenous heparin infusion in patients with aneurysmal subarachnoid hemorrhage: a preliminary assessment. J Neurosurg 2013; 119: 1611–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soppi V, Karamanakos PN, Koivisto T et al. A randomized outcome study of enteral versus intravenous nimodipine in 171 patients after acute aneurysmal subarachnoid hemorrhage. World Neurosurg 2012; 78: 101–9 [DOI] [PubMed] [Google Scholar]
- 46.Gomis P, Graftieaux JP, Sercombe R, Hettler D, Scherpereel B, Rousseaux P. Randomized, double-blind, placebo-controlled, pilot trial of high-dose methylprednisolone in aneurysmal subarachnoid hemorrhage. J Neurosurg 2010; 112: 681–8 [DOI] [PubMed] [Google Scholar]
- 47.Ghodsi SM, Mohebbi N, Naderi S, Anbarloie M, Aoude A, Habibi Pasdar SS. Comparative efficacy of meloxicam and placebo in vasospasm of patients with subarachnoid hemorrhage. Iran J Pharm Res 2015; 14: 125–30 [PMC free article] [PubMed] [Google Scholar]
- 48.Webb A, Kolenda J, Martin K, Wright W, Samuels O. The effect of intraventricular administration of nicardipine on mean cerebral blood flow velocity measured by transcranial Doppler in the treatment of vasospasm following aneurysmal subarachnoid hemorrhage. Neurocrit Care 2010; 12: 159–64 [DOI] [PubMed] [Google Scholar]
- 49.Kasuya H. Clinical trial of nicardipine prolonged-release implants for preventing cerebral vasospasm: multicenter cooperative study in Tokyo. Acta Neurochir Suppl 2011; 110: 165–7 [DOI] [PubMed] [Google Scholar]
- 50.Barth M, Capelle HH, Weidauer S et al. Effect of nicardipine prolonged-release implants on cerebral vasospasm and clinical outcome after severe aneurysmal subarachnoid hemorrhage: a prospective, randomized, double-blind phase IIa study. Stroke 2007; 38: 330–6 [DOI] [PubMed] [Google Scholar]
- 51.Schneider UC, Dreher S, Hoffmann KT, Schmiedek P, Kasuya H, Vajkoczy P. The use of nicardipine prolonged release implants (NPRI) in microsurgical clipping after aneurysmal subarachnoid haemorrhage: comparison with endovascular treatment. Acta Neurochir (Wien) 2011; 153: 2119–25 [DOI] [PubMed] [Google Scholar]
- 52.Westermaier T, Stetter C, Kunze E et al. Controlled transient hypercapnia: a novel approach for the treatment of delayed cerebral ischemia after subarachnoid hemorrhage? J Neurosurg 2014; 121: 1056–62 [DOI] [PubMed] [Google Scholar]
- 53.Kim JH, Yi HJ, Ko Y, Kim YS, Kim DW, Kim JM. Effectiveness of papaverine cisternal irrigation for cerebral vasospasm after aneurysmal subarachnoid hemorrhage and measurement of biomarkers. Neurol Sci 2014; 35: 715–22 [DOI] [PubMed] [Google Scholar]
- 54.Litrico S, Almairac F, Gaberel T et al. Intraventricular fibrinolysis for severe aneurysmal intraventricular hemorrhage: a randomized controlled trial and meta-analysis. Neurosurg Rev 2013; 36: 523–30; discussion 30–1 [DOI] [PubMed] [Google Scholar]
- 55.Etminan N, Beseoglu K, Eicker SO, Turowski B, Steiger HJ, Hanggi D. Prospective, randomized, open-label phase II trial on concomitant intraventricular fibrinolysis and low-frequency rotation after severe subarachnoid hemorrhage. Stroke 2013; 44: 2162–8 [DOI] [PubMed] [Google Scholar]
- 56.Kramer AH, Roberts DJ, Holodinsky J et al. Intraventricular tissue plasminogen activator in subarachnoid hemorrhage patients: a prospective, randomized, placebo-controlled pilot trial. Neurocrit Care 2014; 21: 275–84 [DOI] [PubMed] [Google Scholar]
- 57.Rondeau N, Cinotti R, Rozec B et al. Dobutamine-induced high cardiac index did not prevent vasospasm in subarachnoid hemorrhage patients: a randomized controlled pilot study. Neurocrit Care 2012; 17: 183–90 [DOI] [PubMed] [Google Scholar]
- 58.Togashi K, Joffe AM, Sekhar L et al. Randomized pilot trial of intensive management of blood pressure or volume expansion in subarachnoid hemorrhage (IMPROVES). Neurosurgery 2015; 76: 125–34; discussion 34–5; quiz 35 [DOI] [PubMed] [Google Scholar]
- 59.Bulters DO, Birch AA, Hickey E et al. A randomized controlled trial of prophylactic intra-aortic balloon counterpulsation in high-risk aneurysmal subarachnoid hemorrhage. Stroke 2013; 44: 224–6 [DOI] [PubMed] [Google Scholar]
- 60.Ibrahim GM, Macdonald RL. The effects of fluid balance and colloid administration on outcomes in patients with aneurysmal subarachnoid hemorrhage: a propensity score-matched analysis. Neurocrit Care 2013; 19: 140–9 [DOI] [PubMed] [Google Scholar]
- 61.Yoneda H, Nakamura T, Shirao S et al. Multicenter prospective cohort study on volume management after subarachnoid hemorrhage: hemodynamic changes according to severity of subarachnoid hemorrhage and cerebral vasospasm. Stroke 2013; 44: 2155–61 [DOI] [PubMed] [Google Scholar]
- 62.Tagami T, Kuwamoto K, Watanabe A et al. Optimal range of global end-diastolic volume for fluid management after aneurysmal subarachnoid hemorrhage: a multicenter prospective cohort study. Crit Care Med 2014; 42: 1348–56 [DOI] [PubMed] [Google Scholar]
- 63.Mutoh T, Kazumata K, Terasaka S, Taki Y, Suzuki A, Ishikawa T. Early intensive versus minimally invasive approach to postoperative hemodynamic management after subarachnoid hemorrhage. Stroke 2014; 45: 1280–4 [DOI] [PubMed] [Google Scholar]
- 64.Suarez JI, Martin RH, Calvillo E et al. The Albumin in Subarachnoid Hemorrhage (ALISAH) multicenter pilot clinical trial: safety and neurologic outcomes. Stroke 2012; 43: 683–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suarez JI, Martin RH, Calvillo E, Bershad EM, Venkatasubba Rao CP. Effect of human albumin on TCD vasospasm, DCI, and cerebral infarction in subarachnoid hemorrhage: the ALISAH study. Acta Neurochir Suppl 2015; 120: 287–90 [DOI] [PubMed] [Google Scholar]
- 66.Lu N, Jackson D, Luke S, Festic E, Hanel RA, Freeman WD. Intraventricular nicardipine for aneurysmal subarachnoid hemorrhage related vasospasm: assessment of 90 days outcome. Neurocrit Care 2012; 16: 368–75 [DOI] [PubMed] [Google Scholar]
- 67.Gathier CS, Dankbaar JW, van der Jagt M et al. Effects of induced hypertension on cerebral perfusion in delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a randomized clinical trial. Stroke 2015; 46: 3277–81 [DOI] [PubMed] [Google Scholar]
- 68.Suzuki S, Sato M, Ota S et al. Intraarterial colforsin may improve the outcome of patients with aneurysmal subarachnoid hemorrhage: a retrospective study. World Neurosurg 2012; 78: 295–9 [DOI] [PubMed] [Google Scholar]
- 69.Sherif C, Wambacher B, Loyoddin M et al. Repeated combined endovascular therapy with milrinone and nimodipine for the treatment of severe vasospasm: preliminary results. Acta Neurochir Suppl 2015; 120: 203–7 [DOI] [PubMed] [Google Scholar]
- 70.Saito A, Inoue M, Kon H et al. Effectiveness of intraarterial administration of fasudil hydrochloride for preventing symptomatic vasospasm after subarachnoid hemorrhage. Acta Neurochir Suppl 2015; 120: 297–301 [DOI] [PubMed] [Google Scholar]
- 71.Iwabuchi S, Hayashi M, Yokouchi T et al. Prophylactic intra-arterial administration of fasudil hydrochloride for vasospasm following subarachnoid haemorrhage. Acta Neurochir Suppl 2015; 120: 167–9 [DOI] [PubMed] [Google Scholar]
- 72.Aburto-Murrieta Y, Marquez-Romero JM, Bonifacio-Delgadillo D, López I, Hernández-Curiel B. Endovascular treatment: balloon angioplasty versus nimodipine intra-arterial for medically refractory cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Vasc Endovascular Surg 2012; 46: 460–5 [DOI] [PubMed] [Google Scholar]
- 73.Albanese E, Russo A, Quiroga M, Willis RN Jr, Mericle RA, Ulm AJ. Ultrahigh-dose intraarterial infusion of verapamil through an indwelling microcatheter for medically refractory severe vasospasm: initial experience. Clinical article. J Neurosurg 2010; 113: 913–22 [DOI] [PubMed] [Google Scholar]
- 74.Musahl C, Henkes H, Vajda Z, Coburger J, Hopf N. Continuous local intra-arterial nimodipine administration in severe symptomatic vasospasm after subarachnoid hemorrhage. Neurosurgery 2011; 68: 1541–7; discussion 7 [DOI] [PubMed] [Google Scholar]
- 75.Bele S, Proescholdt MA, Hochreiter A et al. Continuous intra-arterial nimodipine infusion in patients with severe refractory cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a feasibility study and outcome results. Acta Neurochir (Wien) 2015; 157: 2041–50 [DOI] [PubMed] [Google Scholar]
- 76.Mortimer AM, Steinfort B, Faulder K et al. The detrimental clinical impact of severe angiographic vasospasm may be diminished by maximal medical therapy and intensive endovascular treatment. J Neurointerv Surg 2015; 7: 881–7 [DOI] [PubMed] [Google Scholar]
- 77.Wong GK, Boet R, Poon WS et al. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage: an updated systemic review and meta-analysis. Crit Care 2011; 15: R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Macdonald RL, Kassell NF, Mayer S et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 2008; 39: 3015–21 [DOI] [PubMed] [Google Scholar]
- 79.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med 2011; 364: 2439–50 [DOI] [PubMed] [Google Scholar]
- 80.Tanaka K, Ishikawa Y, Yokoyama M et al. Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial. Stroke 2008; 39: 2052–8 [DOI] [PubMed] [Google Scholar]
- 81.Turner JD, Mammis A, Prestigiacomo CJ. Erythropoietin for the treatment of subarachnoid hemorrhage: a review. World Neurosurg 2010; 73: 500–7 [DOI] [PubMed] [Google Scholar]
- 82.Shibuya M, Suzuki Y, Sugita K et al. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg 1992; 76: 571–7 [DOI] [PubMed] [Google Scholar]
- 83.Evidence-based guidelines for the management of aneurysmal subarachnoid hemorrhage. English Edition. Neurol Med Chir (Tokyo) 2012; 52: 355–429 [DOI] [PubMed] [Google Scholar]
- 84.Pickard JD, Murray GD, Illingworth R et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. Br Med J 1989; 298: 636–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vergouwen MD, Etminan N, Ilodigwe D, Macdonald RL. Lower incidence of cerebral infarction correlates with improved functional outcome after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab 2011; 31: 1545–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McGirt MJ, Lynch JR, Parra A et al. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke 2002; 33: 2950–6 [DOI] [PubMed] [Google Scholar]
- 87.Lennihan L, Mayer SA, Fink ME et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke 2000; 31: 383–91 [DOI] [PubMed] [Google Scholar]
- 88.Rinkel GJ, Feigin VL, Algra A, van Gijn J. Circulatory volume expansion therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2004; 4: CD000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Egge A, Waterloo K, Sjøholm H, Solberg T, Ingebrigtsen T, Romner B. Prophylactic hyperdynamic postoperative fluid therapy after aneurysmal subarachnoid hemorrhage: a clinical, prospective, randomized, controlled study. Neurosurgery 2001; 49: 593–605; discussion -6 [DOI] [PubMed] [Google Scholar]
- 90.Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke 2001; 32: 553–60 [DOI] [PubMed] [Google Scholar]
- 91.Gathier CS, van den Bergh WM, Slooter AJ; HIMALAIA-Study Group. HIMALAIA (Hypertension Induction in the Management of AneurysmaL subArachnoid haemorrhage with secondary IschaemiA): a randomized single-blind controlled trial of induced hypertension vs. no induced hypertension in the treatment of delayed cerebral ischemia after subarachnoid hemorrhage. Int J Stroke 2014; 9: 375–80 [DOI] [PubMed] [Google Scholar]
- 92.Bulsara KR, Günel M, Amin-Hanjani S, Chen PR, Connolly ES, Friedlander RM. Results of a national cerebrovascular neurosurgery survey on the management of cerebral vasospasm/delayed cerebral ischemia. J Neurointerv Surg 2015; 7: 408–11 [DOI] [PubMed] [Google Scholar]
- 93.Hollingworth M, Chen PR, Goddard AJ, Coulthard A, Söderman M, Bulsara KR. Results of an international survey on the investigation and endovascular management of cerebral vasospasm and delayed cerebral ischemia. World Neurosurg 2015; 83: 1120–6.e1 [DOI] [PubMed] [Google Scholar]
- 94.Smith WS, Dowd CF, Johnston SC et al. Neurotoxicity of intra-arterial papaverine preserved with chlorobutanol used for the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 2004; 35: 2518–22 [DOI] [PubMed] [Google Scholar]
- 95.Dorhout Mees SM, Algra A, Wong GK et al. Early magnesium treatment after aneurysmal subarachnoid hemorrhage: individual patient data meta-analysis. Stroke 2015; 46: 3190–3 [DOI] [PubMed] [Google Scholar]
- 96.Hanggi D, Etminan N, Macdonald RL et al. NEWTON: nimodipine microparticles to enhance recovery while reducing toxicity after subarachnoid hemorrhage. Neurocrit Care 2015; 23: 274–84 [DOI] [PubMed] [Google Scholar]
- 97.Treggiari MM. Hemodynamic management of subarachnoid hemorrhage. Neurocrit Care 2011; 15: 329–35 [DOI] [PubMed] [Google Scholar]
- 98.Velly LJ, Bilotta F, Fàbregas N, Soehle M, Bruder NJ, Nathanson MH. Anaesthetic and ICU management of aneurysmal subarachnoid haemorrhage: a survey of European practice. Eur J Anaesthesiol 2015; 32: 168–76 [DOI] [PubMed] [Google Scholar]
- 99.Chambers D, Rodgers M, Woolacott N. Not only randomized controlled trials, but also case series should be considered in systematic reviews of rapidly developing technologies. J Clin Epidemiol 2009; 62: 1253–60 e4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.