For many years, there was arguably little progress at the front line of airway management, because all we had was our hands, then a classic laryngoscope, and later, a classic laryngeal mask to control the airway. Since then, the airway armamentarium has progressed in quantum leaps, particularly with the introduction of videolaryngoscopy and a wide range of supraglottic airway devices (SADs).1 At present, SADs have collectively enjoyed an unparalleled safety record and are very popular devices in everyday practice,2 with broadening indications. Globally, of the ∼250 million patients undergoing major surgery under general anaesthesia on an annual basis, some 60% receive such a device to maintain a patent airway.3–5 The vast majority of anaesthetics in patients undergoing elective surgery are performed using some form of SAD. Since the initial introduction of the LMA-Classic,6 the evolution in supraglottic airway designs has been a continuous process.7 Consequently, many new characteristics have been added in an attempt to combine efficacy with safety.8,9 Some of these changes were subtle, such as from re-usable to single-use disposable, or progression from classic to flexible SAD. Other changes genuinely added innovations in functions through design, such as facilitation of tracheal intubation or facilitation of stomach decompression via an oesophageal vent.
Anaesthetists are faced with a multitude of different SADs being introduced to their clinical practice, and the problem is that many lack the evidence of efficacy and safety to inform evidence-based decision-making regarding which devices to adopt. In order to introduce new devices into clinical practice or develop an appropriate clinical trial, detailed knowledge of the physical characteristics and potential application is essential. Only by careful analysis of the design of new devices, with appropriate preclinical research and development followed by preclinical testing, can specific hypotheses be generated that are amenable to clinical testing. We demonstrate this through the recently introduced LMA-Protector™, for which limited clinical evidence exists.
Faced with the concern that an increasing number of airway management devices were being introduced into clinical practice with little or no previous evidence of their clinical efficacy or safety, the Airway Device Evaluation Project Team (ADEPT) was formed by the Difficult Airway Society (DAS) in the UK in 2011.10 The ADEPT strategy proposes ‘procurement pathways’ for evaluating airway equipment so that: (i) evidence-based principles should be applied to institutional purchasing decisions regarding airway management products; (ii) products with documented evidence of efficacy and safety should be preferred to those where evidence is lacking; and (iii) anaesthetists should play a more active role in local device and equipment evaluation and in selection and purchasing decisions. Regulators, manufacturers, and clinical researchers need to cooperate to improve the overall successful introduction of any new SAD into clinical practice and the market place rather than rely on the Conformité Européne (CE) ‘mark’ alone. Fairly often, manufacturers make modifications without notifying the users, and newer versions of SADs automatically replace older versions of stock, such that it is not necessarily the same device as described in earlier device versions and their accompanying publications. Furthermore, no patient should be confronted with new equipment used by anaesthetists who lack knowledge of the basic characteristics of the new device.
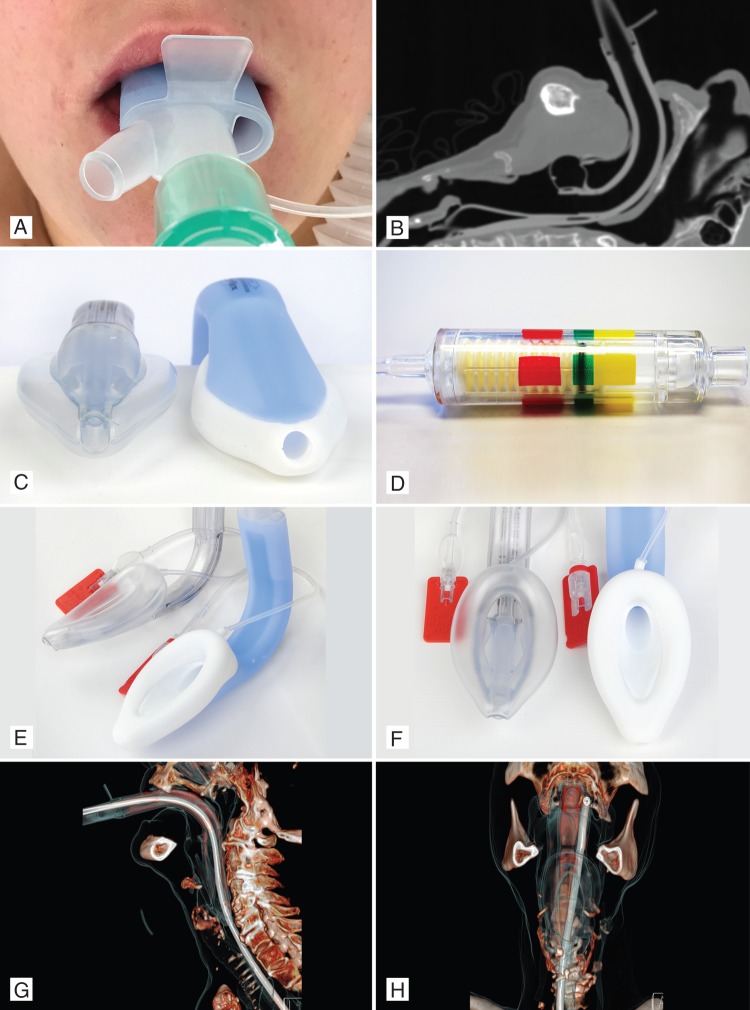
Recently, a new second-generation single-use CE-marked SAD, the LMA-Protector™, was introduced into medical practice (Fig. 1a–h). The LMA-Protector™ shares common features11–15 with previous LMA devices, such as the LMA-ProSeal™ (high oropharyngeal leak pressure, gastric access, and bite block), the LMA-Fastrach™ (fixed-curved tube and guiding handle), the LMA-Unique™ (single use), and the LMA-Supreme™ (a firm, anatomically shaped airway tube; Fig. 1b and c), incorporates a drain tube within its lumen to separate the respiratory and gastrointestinal tracts, and has an oval-shaped tube (Fig. 1e and f) to match the shape of the mouth better and to reduce rotation in the pharynx.
Fig 1.
(a) LMA-Protector™ in situ, showing two ports. (b) Computed tomography scan of LMA-Protector™ in situ. (c) LMA-Supreme™ and LMA-Protector™, showing the 10° slant of the tip of the distal part of the cuff. (d) Cuff Pilot Valve™. (e) and (f) LMA-Supreme™ and LMA-Protector™ lateral (e) and frontal view (f). (g) and (h) Computed tomography reconstruction of LMA-Protector™, showing tissue and gastric tube path in the lateral (g) and frontal view (h). Lateral X-ray image of LMA-Protector™ in situ.
The LMA-Protector™ is made with medical-grade silicone (except the 15 mm connector), which makes it a more flexible and potentially a less traumatic device than the LMA-Supreme™, which is made of polyvinyl chloride. The LMA-Protector™ provides access to, and functional separation of, the ventilation and digestive tracts, with the presence of two drainage channels, which emerge proximally as separate ports and enter a chamber, located behind the cuff bowl. Removal of gastric fluid through the upper oesophageal sphincter can be performed by attaching suction to the male suction port or by insertion of a gastric tube through the female drainage port to the stomach (Fig. 1a).
Primary research assessing the LMA-Protector™ is sparse. For example, while a PubMed search of LMA-Supreme yields ∼101 results, searching for LMA Protector (or similar terms) yields no results. From the above-mentioned properties, it becomes clear that physical properties, including the flexibility (objectively measured), degree of trauma, and efficacy of the gastric drainage system (which might be a combination of the ease with which a gastric tube can be passed and any inherent reflux of material via this channel), must be understood by the anaesthetist when using this new device.
Prevention of aspiration requires a good-quality oropharyngeal airway seal (Fig. 1g and h) within the laryngopharynx (first seal) and oesophagus (second seal). Airway seal pressures using the device therefore need to be studied. Venting of the stomach with a gastric tube channel allows stomach gas and contents to escape. The internal volume of the drainage pathway for sizes 3, 4, and 5 of the LMA-Protector™ (Table 1) is about five times more (31, 41, and 42 ml, respectively) than those of the LMA-Supreme™ (6, 6.5, and 7 ml, respectively). Table 1 details the physical characteristics of the LMA-Protector™ and the LMA-Supreme™. Notably, the LMA-Protector™ has two gastric tubes and no mask aperture bars, is dynamically flexible, and may be used as an intubation conduit for tracheal tubes. This is an aspect that can be readily assessed, using research methodologies that are well established.16,17 Both the LMA-Supreme™ and the LMA-Protector™ have an elliptical cuff at the distal drainage orifice and a 10° slant (Fig. 1c), allowing the cuff to follow the contours of the upper oesophageal sphincter, which may improve placement and seal.14 Furthermore, the incorporation of the gastric channel into the tip of the mask also reinforces the tip and may prevent spontaneous folding on itself. Once inflated, with the device in its correct position, the distal cuff may provide a tight apposition to the oesophageal opening and may prevent reflux (Fig. 1b). The length of the integral bite block may differ depending which size is used. In the LMA-Supreme™ and LMA-Protector™, there is no difference between a size 4 and a size 5 (identical cuff size); however, the bite block in a size 4 is shorter than in a size 5, adding to the overall length of the device in the latter. Ideally, there should be a minimum of 1 cm gap between the fixation tab and the patient's upper lip. If that is not the case, a larger size laryngeal mask may be needed. If the fixation tab is more than 2.5 cm from the upper lip after fixation, it may be advisable to use a smaller size of laryngeal mask. The LMA-Protector™ will be available with the pilot balloon or with the Integrated Cuff Pilot™ (Fig. 1d). In contrast to the LMA-Supreme™, the LMA-Protector™ does not provide paediatric sizes of the device as yet.
Table 1.
List of specifications for LMA-Supreme™ and LMA-Protector™. CPR, cardiopulmonary resuscitation; LMA, laryngeal mask airway; MR, magnetic resonance; N/A, not applicable; TT, tracheal tube
| Specification | LMA-Supreme™ | LMA-Protector™ |
|---|---|---|
| Indication for use as airway device | ||
|
|
|
| Available LMA sizes | 1, 1.5, 2, 2.5, 3, 4, and 5 | 3, 4, and 5 |
| Material, both cuff and tube | Polyvinyl chloride | Medical-grade silicone |
| Number of uses | Single use | Single use |
| Functional separation of ventilation and digestive tracts | Yes | Yes |
| Number of ventilation tubes | Two lateral (ventilation paths divided by drain tube) | One central (maximal internal diameter 13 mm) |
| Number of gastric tubes | One | Two (male suction port and female drainage port) |
| Position of proximal gastric drainage orifice | Behind 15 mm connector | Two separate ports on each side of airway tube |
| Position of drainage tube | Runs in middle of airway tube | On each side of airway tube |
| Design of distal drainage orifice | Elongated cuff, 10° slant | Elongated cuff, 10° slant |
| Drainage chamber behind cuff bowl | N/A | Yes, two drainage channels continue distally and enter chamber |
| Nominal length of internal ventilation pathway of LMA sizes 3, 4, and 5 (cm) | 16, 17, and 19 | 16, 18, and 20 |
| Nominal length of internal drainage pathway of LMA sizes 3, 4, and 5 (cm) | 22, 24, and 26 | 18, 21, and 23 |
| Internal volume of ventilatory pathway for LMA sizes 3, 4, and 5 (ml) | 18, 23, and 26 | 18, 22, and 23 |
| Internal volume of drainage pathway for LMA sizes 3, 4, and 5 (ml) | 6, 6.5, and 7 | 31, 41, and 42 |
| Design of cuff | Enlarged air-inflatable cuff, high airway seal cuff, reinforced tip | Enlarged air-inflatable cuff, high airway seal cuff, reinforced tip |
| Design of tube | Firm, anatomically shaped oval tube. Semi-rigid fixed-curved tube (2 lateral grooves in airway tube) | Anatomically shaped oval tube. Flexible-curved tube |
| Cross-section of airway tube | Elliptical | Elliptical |
| Mask aperture bars (n) | Two ‘fins’ to prevent epiglottic downfolding | None |
| Guiding handle/fixation tab | Yes | Yes |
| Colour of cuff/tube | Transparent/transparent | White/blue transparent |
| Built-in bite block, long version | Yes | Yes |
| Minimal interdental gap for sizes 3, 4, and 5 (mm) | 19, 20, and 20 | 28, 32, and 32 |
| 15 mm connector, ISO 5356-1 | Fixed | Detachable |
| Latex free | Yes | Yes |
| Phthalates (DHEP) free | No | Yes |
| Inflation valve, Luer cone, ISO 594-1 | Yes | Yes |
| Cuff pilot | Separate cuff pilot balloon | Separate cuff pilot balloon or Integrated Cuff Pilot™ |
| Maximal cuff pressure (cm H2O) | 60 | 60 |
| Magnetic resonance imaging | MR conditional (metallic spring) | MR conditional (metallic spring); MR compatible Integrated Cuff Pilot™ |
|
Requires special technique8 | Yes |
| N/A | TT: 6.5, 7.5, and 7.5 | |
| Maximal orogastric tube through drainage tube for LMA sizes 3, 4, and 5 | 14, 16, and 16 Fr | 16, 18, and 18 Fr |
The use of the LMA-Supreme™ as an intubation conduit for an tracheal tube is not easy to perform and needs extra adjuncts, such as a bronchoscopic/Aintree Intubation Catheter-guided technique.18 The airway lumen of the LMA-Protector™ (maximal internal diameter 13 mm) is wide enough to pass an adequately sized adult tracheal tube directly, without the use of an intermediate exchange catheter (i.e. a maximal size tracheal tube of 6.5, 7.5, and 7.5 for the devices 3, 4, and 5). As such, using the LMA-Protector™ as a conduit for fibreoptic-assisted rescue intubation should be easier to accomplish than using an LMA-Supreme™ or LMA-ProSeal™. However, these hypotheses require investigation through appropriate statistically powered studies. Maximal diameters of orogastric tubes that can be inserted through the gastric channels of the respective sizes 3, 4, and 5 of the LMA-Supreme™ are 14, 16, and 16 Fr, compared with 16, 18, and 18 Fr for the LMA-Protector™.
Laryngeal masks have a prominent place in the most recent ASA and Obstetric Anaesthetists' Association and Difficult Airway Society guidelines for difficult airway management.19,20 As for all medical devices, end-users of SADs need to know the instructions for use and the manoeuvres to correct incorrect mask positions.
The role of the LMA-Protector™ as a routine second-generation SAD in anaesthesia has not yet been studied. We agree with the concerns of Pandit and colleagues10 that an increasing number of airway management devices are being introduced into clinical practice with little or no previous evidence of their clinical efficacy and safety. The purpose of this article is to show how, by careful analysis of the design and structure of the device, a range of specific hypotheses can be generated that are amenable to clinical testing. In this editorial, by providing information about the similarities and differences of the physical characteristics of a new airway device, we hope that anaesthetists are better informed before they introduce the device into clinical practice.
Authors' contributions
All authors approved the final manuscript and attest to the integrity of the original data and the analysis reported in this manuscript.
Declaration of interest
M.W.S. and S.R.L. have previously undertaken postmarketing clinical use and evaluation of the LMA-Protector™. J.J.P. sits as elected Member of Council, Royal College of Anaesthetists, and sat on The Association of Anaesthetists of Great Britain and Ireland Working Parties on development of guidelines for managing the obese patient and minimal monitoring during anaesthesia. He is Scientific Officer of the Difficult Airway Society and an editor of Anaesthesia. The views expressed are his own and not those of these organizations.
Funding
Departmental funds.
References
- 1.Greenland KB. Art of airway management: the concept of ‘Ma’ (Japanese: ‘when less is more’). Br J Anaesth 2015; 115: 809–12 [DOI] [PubMed] [Google Scholar]
- 2.Fourth National Audit Project of the Royal College of Anaesthetists and Difficult Airway Society . Major complications of airway management in the United Kingdom. Report and Findings. March 2011. ISBN 978-1-9000936-03-3 Royal College of Anaesthetists. London Available from http://www.rcoa.ac.uk/nap4/ (accessed 10 October 2015)
- 3.Woodall NM, Cook TM. National census of airway management techniques used for anaesthesia in the UK: first phase of the 4th National Audit Project at the Royal College of Anaesthetists. Br J Anaesth 2011; 106: 266–71 [DOI] [PubMed] [Google Scholar]
- 4.Weiser TG, Regenbogen SE, Thompson KD et al. An estimation of the global volume of surgery; a modelling strategy based on available data. Lancet 2008; 372: 139–44 [DOI] [PubMed] [Google Scholar]
- 5.Sury MR, Palmer JH, Cook TM, Pandit JJ. The state of UK anaesthesia: a survey of National Health Service activity in 2013. Br J Anaesth 2014; 113: 575–84 [DOI] [PubMed] [Google Scholar]
- 6.Brain AIJ. The laryngeal mask—a new concept in airway management. Br J Anaesth 1983; 55: 801–5 [DOI] [PubMed] [Google Scholar]
- 7.Van Zundert TCRV, Brimacombe JR, Ferson DZ, Bacon DR, Wilkinson DJ. Archie Brain: celebrating 30 years of development in laryngeal mask airways. Anaesthesia 2012; 67: 1375–85 [DOI] [PubMed] [Google Scholar]
- 8.Van Zundert T. Improvements towards safer extraglottic airway devices. PhD thesis The Netherlands: Maastricht University, 2015 [Google Scholar]
- 9.Cook TM, Kelly FE. Time to abandon the ‘vintage’ laryngeal mask airway and adopt second-generation supraglottic airway devices as first choice. Br J Anaesth 2015; 115: 497–9 [DOI] [PubMed] [Google Scholar]
- 10.Pandit JJ, Popat MT, Cook TM et al. The Difficult Airway Society ‘ADEPT’ guidance on selecting airway devices: the basis of a strategy for equipment evaluation. Anaesthesia 2011; 66: 726–37 [DOI] [PubMed] [Google Scholar]
- 11.Van Zundert A, Brimacombe J. The LMA Supreme™ – a pilot study. Anaesthesia 2008; 63: 209–10 [DOI] [PubMed] [Google Scholar]
- 12.Seet E, Rajeev S, Firzo T et al. Safety and efficacy of laryngeal mask airway Supreme versus laryngeal mask airway ProSeal: a randomized controlled trial . Eur J Anaesthesiol 2010; 27: 602–7 [DOI] [PubMed] [Google Scholar]
- 13.Maitra S, Khanna P, Baidya DK. Comparison of laryngeal mask airway Supreme and laryngeal mask airway Pro-Seal for controlled ventilation during general anaesthesia in adult patients. Eur J Anaesthesiol 2014; 31: 266–73 [DOI] [PubMed] [Google Scholar]
- 14.Gaitini L, Vaida S. Is the newly designed distal tip of the LMA Supreme an advantage or a disadvantage? J Clin Anesth 2015; 27: 181–2 [DOI] [PubMed] [Google Scholar]
- 15.Wong DT, Yang JJ, Jagannathan N. Brief review: the LMA Supreme™ supraglottic airway. Can J Anesth 2012; 59: 483–93 [DOI] [PubMed] [Google Scholar]
- 16.Pandit JJ, MacLachlan K, Dravid RM, Popat MT. Comparison of times to achieve tracheal intubation with three techniques using the laryngeal or intubating laryngeal mask airway. Anaesthesia 2002; 57: 128–32 [DOI] [PubMed] [Google Scholar]
- 17.Danha RF, Thompson JL, Popat MT, Pandit JJ. Comparison of fibreoptic-guided orotracheal intubation through classic and single-use laryngeal mask airways. Anaesthesia 2005; 60: 184–8 [DOI] [PubMed] [Google Scholar]
- 18.Van Zundert TCRV, Wong DT, Van Zundert AAJ. The LMA-Supreme™ as an intubation conduit in patients with known difficult airways: prospective evaluation study. Acta Anaesthesiol Scand 2013; 57: 77–81 [DOI] [PubMed] [Google Scholar]
- 19.Apfelbaum JL, Hagberg CA, Caplan RA, Connis RT, Nickinovich DG. Practice guidelines for management of the difficult airway. An updated report by the American Society of Anesthesiologists's Task Force on Management of the Difficult Airway. Anesthesiology 2013; 118: 251–70 [DOI] [PubMed] [Google Scholar]
- 20.Mushambi MC, Kinsella SM, Popat M et al. Obstetric Anaesthetists' Association and Difficult Airway Society guidelines for the management of difficult and failed tracheal intubation in obstetrics. Anaesthesia 2015; 70: 1286–306 [DOI] [PMC free article] [PubMed] [Google Scholar]