Abstract
Background
Although deep neuromuscular block (post-tetanic-count 1-2 twitches) improves surgical conditions during laparoscopic retroperitoneal surgery compared with standard block (train-of-four 1-2 twitches), the quality of surgical conditions varies widely, often related to diaphragmatic contractions. Hypocapnia may improve surgical conditions. Therefore we studied the effect of changes in arterial carbon dioxide concentrations on surgical conditions in patients undergoing laparoscopic surgery under general anaesthesia and deep neuromuscular block.
Methods
Forty patients undergoing elective laparoscopic surgery for prostatectomy or nephrectomy received propofol/remifentanil anaesthesia and deep neuromuscular block with rocuronium. Patients were randomized to surgery under hypocapnic or hypercapnic conditions. During surgery, the surgical conditions were evaluated using the 5-point Leiden-Surgical Rating Scale (L-SRS) ranging from 1 (extremely poor conditions) to 5 (optimal conditions) by the surgeon, who was blinded to group.
Results
Mean (sd) arterial carbon dioxide concentrations were 4.5 (0.6) [range: 3.8–5.6] kPa under hypocapnic and 6.9 (0.6) [6.1–8.1] kPa under hypercapnic conditions. The L-SRS did not differ between groups: 4.84 (0.4) [4-5] in hypocapnia and 4.77 (0.4) [3.9–5] in hypercapnia. Ninety-nine percent of ratings were good or excellent irrespective of treatment.
Conclusions
Deep neuromuscular block provides good to optimal surgical conditions in laparoscopic retroperitoneal urological surgery, independent of the level of arterial .
Clinical trial registration
Keywords: carbon dioxide; hypercapnia; hypocapnia; laparoscopy, nephrectomy, neuromuscular block, prostatectomy, rocuronium, urological surgical procedures
Editor's Key Points.
Operating conditions for laparoscopic retroperitoneal surgery are improved with deep neuromuscular block
However diaphragmatic contractions may still occur and these may be affected by arterial
In this small study, induced hypocapnia had no effect on surgical conditions when deep neuromuscular block was provided
These data suggest that when surgical conditions are good, induced hypocapnia provides no additional benefit
Anaesthetists play an essential role in optimizing surgical conditions. This is especially true for procedures which are performed in a narrow anatomical space, such as laparoscopic retroperitoneal and robot-assisted laparoscopic surgery.1,2 We recently showed that provision of deep neuromuscular block (NMB, 1-2 twitches post tetanic count) significantly improved surgical conditions in laparoscopic retroperitoneal prostatectomies and nephrectomies and greatly reduces the incidence of unacceptable conditions.1 Similar observations were made by others for laparoscopic gynaecological procedures and laryngeal microsurgery.3,4 Despite the improvement of surgical conditions at deep muscle relaxation, the variation in surgical conditions is still high (>20%), indicating that there is still room for improvement.1 Also other studies show that that during deep NMB suboptimal surgical conditions may persist. For example, Fernando and colleagues5 showed already in 1987 that at deep NMB (PTC values 1-2) diaphragmatic contractions still occur. Causes of such diaphragmatic contractions include resistance to neuromuscular blocking agents (diaphragm relaxation is often less intense compared with relaxation of the adductor pollicis longus muscle) and efferent activation from brainstem respiratory centres, as a result of high arterial CO2 levels. The latter item is relevant as CO2-insufflation with high intra-abdominal pressures during laparoscopic surgery coincides with high arterial CO2 concentrations even when end-tidal concentrations suggest values in the normal range.
One possible method to reduce high CO2-related diaphragm contractions is to hyperventilate the patient to (sub)normal arterial levels. This technique has been used previously to reduce the dose of anaesthetic agents and to improve surgical conditions when standard neuromuscular block is applied (TOF 1-2).6–8 We hypothesized that the induction of hypocapnia during laparoscopic surgery would further improve surgical conditions under general anaesthesia with deep neuromuscular block.
Methods
The study (with acronym BLISS2) was carried out at Leiden University Medical Centre between February 2013 and September 2015, after approval was obtained of the protocol (protocol number P13.127) by the local medical ethics committee (Commissie Medische Ethiek). The protocol was registered at clinicaltrials.gov (NCT01968447). The study was conducted in accordance with Good Clinical Practice and Good Research Practice guidelines. Eligible patients were approached by one of the investigators and received oral and written information about the study. If a patient was willing and able to participate, oral and written informed consent were obtained. The study had a randomized (hypocapnia against hypercapnia), double-blinded (surgical team and investigators were blinded to treatment allocation but the attending anaesthetist was not blinded) design. Randomization was performed using a computer-generated randomization list obtained from www.randomization.com. The attending anaesthetist, who received the randomization code just before induction of anaesthesia, was responsible for setting the desired tidal volume and respiratory rate on the anaesthesia ventilator (Primus, Dräger Medical Netherlands BV, Zoetermeer, The Netherlands).
ASA class I-III patients were included if they had prostatic or renal disease requiring elective laparoscopic retroperitoneal surgery. Patients were excluded if they were <18 yr of age, ASA class >III, had a known or suspected neuromuscular disorder, allergies to medication to be used during anaesthesia, a (family) history of malignant hyperthermia, renal insufficiency (as defined by glomerular filtration rate of <30 ml h−1), previous retroperitoneal surgery at the site of the current surgery, a BMI >35 kg m−2 or chronic obstructive pulmonary disease (defined as GOLD stage 2 or higher).
Perioperative protocol
All patients received total i.v. anaesthesia with propofol, remifentanil and rocuronium; the patients lungs were ventilated with 40% oxygen in nitrogen. Monitoring was according to local practice and comprised ECG, non-invasive blood pressure (BP) and EEG monitoring (BIS module, Philips, Eindhoven, The Netherlands). BIS values were maintained between 45-55 throughout surgery. In addition, a 22-gauge arterial cannula was placed in the radial artery of the left or right wrist and connected to a Vigileo advanced minimally invasive monitoring system (Edwards Lifesciences, USA), for haemodynamic monitoring and to obtain blood samples for arterial measurements at 15 min intervals.
Neuromuscular monitoring was performed using the TOF cuff device (RGB Medical Services SA, Madrid, Spain).9 The TOF cuff is an upper arm cuff and was applied contralateral to the bp cuff. The cuff incorporates two electrodes that stimulate the ulnar nerve. The evoked neuromuscular activity is recorded by measuring the pressure change induced by the muscular activity from stimulation. A previous study found that the TOF cuff performed equally well with mechanomyography according to the bias and limits of agreement.9 In a few patients we compared the TOF cuff with the TOF watch and observed similar results. Hence, we contend that using the TOF cuff resulted in a reliable assessment of neuromuscular function during anaesthesia. The TOF cuff is easier to use in patients in the lateral position or when the arm is hidden below the surgical drapes.
The TOF cuff was calibrated after induction but before administration of rocuronium. Thereafter, rocuronium 1.0 mg kg−1 was given and a continuous rocuronium infusion was started at 0.2–0.6 mg kg−1 h−1. The depth of neuromuscular block was closely monitored and aimed at 1-2 twitches post-tetanic count (PTC; i.e. a deep NMB). At the end of surgery, the deep NMB was antagonized with a bolus dose of sugammadex of 4 mg kg−1. The patient was extubated when the TOF ratio was >0.9 and the patient was breathing spontaneously and opened his/her eyes on request. For postoperative pain relief, morphine 0.15–0.2 mg kg−1 was given at least 45 min before surgical closure.
All patients were randomized before induction of anaesthesia and the ‘time out’ procedure. Allocation to treatment was performed just before intraabdominal insufflation of carbon dioxide. Patients were randomly assigned to one of two treatment groups. Group 1: hypocapnia during surgery (arterial range 3.5–4.5 kPa [26–34 mm Hg]). The tidal volume of the ventilator was set at 8 ml kg−1 and the respiratory rate at 16 min−1; Group 2: hypercapnia during surgery (arterial range 6.5–7.5 kPa [49–53 mm Hg]). The tidal volume of the ventilator was set at 8 ml kg−1 and the respiratory rate at 11 min−1. Insufflation rates were adjusted such that the target arterial was maintained throughout the laparoscopic procedure. However, the attending anaesthetist could deviate from the protocol at his/her discretion. In both treatment groups PEEP values were kept constant during anaesthesia at 5 mm Hg.
The Leiden-Surgical rating scale (L-SRS)
During the laparoscopic procedure, the surgical field was rated by one surgeon (R.F.B.), with ample experience in laparoscopic retroperitoneal renal and prostatic surgery, using the Leiden Surgical Rating Scale.1 The L-SRS is a five point scale, which covers the quality of the surgical field for extremely poor to optimal conditions (see Table 1 in Ref.1 for a detailed description of the L-SRS and Table 1). If the rating was 3 or lower, the surgeon and anaesthesia team would negotiate ways to improve the surgical working field. For example, a bolus of 15 mg rocuronium could be given in case of suboptimal neuromuscular block and the infusion rate increased by 20%, or propofol 30 mg could be injected if BIS values were high. The L-SRS has been used in several recent and ongoing studies to assess the influence of anaesthesia on the surgical field in retroperitoneal, laparoscopic, thoracolaparoscopic and microlaryngeal surgeries.1–3,10,11
Table 1.
The Leiden Surgical Rating Scale (L-SRS)
| 1 | Extremely poor conditions: The surgeon is unable to work due to coughing or due to the inability to obtain a visible laparoscopic field because of inadequate muscle relaxation. Additional neuromuscular blocking agents must be given. |
| 2 | Poor conditions: There is a visible laparoscopic field but the surgeon is severely hampered by inadequate muscle relaxation with continuous muscle contractions and/or movements with the hazard of tissue damage. Additional neuromuscular blocking agents must be given. |
| 3 | Acceptable conditions: There is a wide visible laparoscopic field but muscle contractions and/or movements occur regularly causing some interference with the surgeon's work. There is the need for additional neuromuscular blocking agents to prevent deterioration. |
| 4 | Good conditions: There is a wide laparoscopic working field with sporadic muscle contractions and/or movements. There is no immediate need for additional neuromuscular blocking agentsunless there is the fear for deterioration. |
| 5 | Optimal conditions: There is a wide visible laparoscopic working field without any movement or contractions. There is no need for additional neuromuscular blocking agents. |
Sample size calculation
The primary end-point of this study was the L-SRS. Our previous study yielded a mean surgical rating score of 4.7 during deep NMB.1 However, in that study, arterial was not controlled resulting in hypo- or hypercapnic conditions in some patients. Also depth of anaesthesia was not controlled resulting in sometimes-deep levels of anaesthesia (BIS<40), which may have confounded the study outcome to some extent. A realistic a priori estimation of the L-SRS in the current study was 4.1–4.3 in the hypercapnic group and 4.8–4.9 in the hypocapnia group. The estimated mean difference between the treatment groups was therefore conservatively estimated to be 0.5. Assuming a sd of 0.45, a sample size of at least 19 subjects would provide at least 90% power to observe the expected difference at alpha=0.05.
Data and statistical analysis
The following clinical variables were collected on the case record form: surgical variables (L-SRS, retroperitoneal pressure), anaesthesia variables (BIS, TOF or PTC, drug dosages), haemodynamic variables (arterial bp, heart rate, cardiac index) and respiratory variables (arterial and end-tidal , tidal volume, respiratory rate, inspiratory pressure, PEEP). Recurrent measurements were made at 15 min intervals.
Data analysis was based on an intent-to-treat basis. A linear mixed model with an autoregressive covariance structure was used to determine the effect of hypocapnia against hypercapnia on the primary end-point, L-SRS. Secondary end-points were the effect of hypocapnia against hypocapnia on cardiorespiratory variables. These variables were averaged over time to get an indication of their mean value. Treatment effects were evaluated on the average data by t-test. The data were analysed with SPSS (v 22; IBM corporation, Armonk, NY, USA). All data are presented as mean (sd) unless otherwise stated. P-values <0.05 were considered significant.
Results
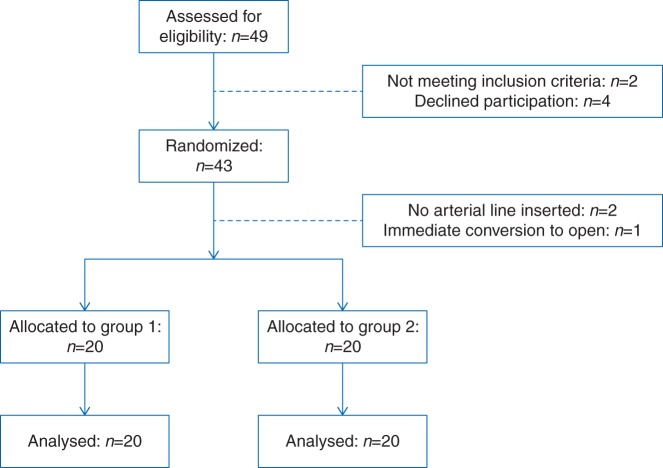
The flow chart of the study is given in Figure 1. After randomization, three randomized patients were not allocated because of inability to insert an arterial line (n=2) and a decision to perform open surgery at the pre-surgical ‘time-out’. Three others replaced these three patients. In total 40 patients in both groups received the allocated treatment and were analysed. Patient characteristics and data obtained at screening did not differ between groups (Table 2).
Fig 1.
Flow chart of the study.
Table 2.
Patient characteristics and data obtained at screening, presented as mean (sd) unless otherwise stated; MAP is mean arterial pressure
| Group 1: hypocapnia (n=20) | Group 2: hypercapnia (n=20) | |
|---|---|---|
| Prostate surgery (n) | 13 | 11 |
| Renal surgery (n) | 7 | 9 |
| ASA class I (n) | 8 | 3 |
| ASA class II (n) | 12 | 17 |
| Gender M/F (n/n) | 17/3 | 14/6 |
| Age (median, range) | 65 (22–80) | 62 (25–72) |
| Weight (kg) | 81 (12) | 81 (17) |
| Height (cm) | 176 (10) | 175 (9) |
| BMI (kg m2) | 26.1 (3.4) | 26.3 (4.3) |
| MAP (mm Hg) | 99 (13) | 99 (14) |
| Heart rate (min−1) | 77 (16) | 74 (14) |
Anaesthesia, and neuromuscular block
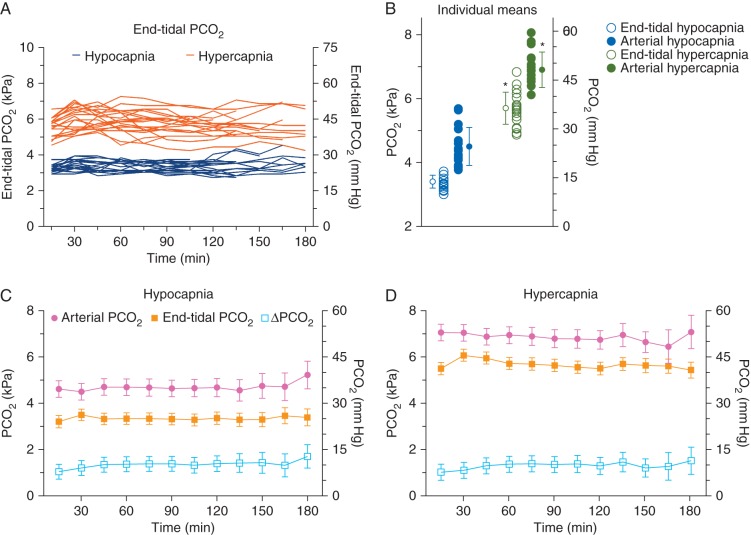
The use of anaesthetics and opioids did not differ between groups with also comparable values of post tetanic count (3.2–3.8), indicative of a similar deep NMB in both treatment groups (Table 3). Figure 2a and b show the individual profiles of the end-tidal over time for the two study groups and the individual mean end-tidal and arterial values. The end-tidal differed by 2.3 kPa between treatments: hypocapnia 3.4 (0.2) kPa with a range of 3.0–3.7 kPa and hypercapnia 5.7 (0.5) kPa with a range of 4.9–6.8 kPa). Similarly, arterial differed by 2.4 kPa between treatments: hypocapnia 4.5 (0.6) kPa (range 3.8–5.6 kPa) and hypercapnia 6.9 (0.6) kPa (range 6.1–8.1 kPa). A clear difference between arterial and end-tidal was present during both hypocapnia and hypercapnia and averaged to 1.2 (0.1) kPa (Fig. 2). As is visible in panels c and d of Figure 2, the difference tended to slowly increase over time.
Table 3.
Measurements during anaesthesia and surgery. Values are mean (sd). *P>0.05, **P<0.001
| Group 1: hypocapnia | Group 2: hypercapnia | |
|---|---|---|
| Duration of anaesthesia (min) | 184 (43) | 170 (50) |
| BIS | 41 (3) | 43 (5) |
| Propofol (g) | 1.7 (0.4) | 1.5 (0.5) |
| Remifentanil (μg kg−1 min−1) | 0.21 (0.06) | 0.19 (0.08) |
| Rocuronium (mg) | 223 (65) | 201 (94) |
| Sugammadex (mg) | 317 (77) | 334 (96) |
| Post tetanic count | 3.2 (2.4) | 3.8 (3.3) |
| Retroperitoneal pressure (mm Hg) | 11 (1) | 11 (1) |
| Leiden Surgical Rating Scale | 4.84 (0.4) | 4.77 (0.4)* |
| Mean arterial pressure | 85 (10) | 81 (9)* |
| Heart rate (min−1) | 68 (11) | 69 (9)* |
| Cardiac output (L min−1) | 3.9 (1.1) | 4.4 (1.3)* |
| Arterial (kPa) Arterial range (kPa) |
4.5 (0.6) 3.8–5.6 |
6.9 (0.6)** 6.1–8.1 |
| End-tidal (kPa) End-tidal range (kPa) |
3.4 (0.2) 3.0–3.7 |
5.7 (0.5)** 4.9–6.8 |
| Minute volume (L min−1) | 12.5 (2.1) | 7.1 (1.3)** |
| Respiratory rate (min−1) | 20.3 (3.0) | 12.7 (1.6)** |
| Inspiratory pressure (cm H2O) | 24 (4) | 22 (2)* |
| Peep (cm H2O) | 5 (0.6) | 5 (0.3)* |
Fig 2.
(a) Individual end-tidal values over time. Blue lines hypocapnia, orange lines hypercapnia. (b) Individual mean end-tidal and arterial values and mean of the means (sd). *P<0.001. (c and d) Profiles of the arterial , end-tidal and difference between arterial and end-tidal () under hypocapnic (c) and hypercapnic conditions (d). Data are mean (95% confidence interval).
Rating of surgical conditions
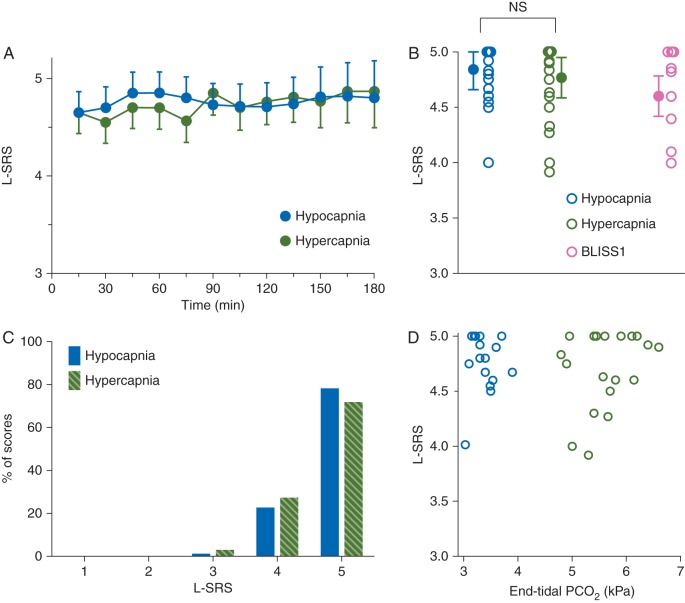
We obtained 9–12 scores per patient. In Figure 3a, the mean scores over time are given for the two treatment groups. The scores did not differ between groups (P=0.59). The mean of the mean L-SRS scores were: hypocapnia 4.84 (0.4) against hypercapnia 4.77 (0.4) (Fig. 3b). The distribution of the individual L-SRS scores is given in Figure 3c, showing that 99% of all scores were good or optimal, irrespective of the arterial conditions. There was no correlation between end-tidal and L-SRS (Fig. 3d, Pearson r=0.02, P=0.9) or between arterial and L-SRS (r=−0.08, P=0.67).
Fig 3.
(a) Mean (95% confidence interval) Leiden-Surgical Rating Scale (L-SRS) scores against time under hypocapnic (blue symbols) and hypercapnic conditions (green symbols). (b) Individual mean L-SRS scores (each symbol is the mean L-SRS of one patient) and mean of the means (sd). In pink the results of the BLISS 1 study performed under normocapnic conditions (arterial 6 kPa or 45 mm Hg). (c) Distribution of the surgical ratings under hypocapnic (blue bars) and hypercapnic (green bars) conditions. (d) Individual mean L-SRS scores vs mean individual end-tidal values in subjects treated with hypocapnia (blue symbol) and hypercapnia (green symbol). Pearson r=0.02, P=0.9.
Measurements after surgery
NMB reversal was by sugammadex 4 mg kg−1 and resulted in optimal extubation conditions (train of four ratio >0.9) within 3 min in all patients. Postoperative pain scores were similar between groups (data not shown).
Discussion
In this study we found no effect of arterial on surgical conditions as rated by the Leiden-Surgical Rating Scale during retroperitoneal laparoscopic surgery with deep neuromuscular block. To increase the possible difference between treatments we compared hypocapnic conditions against hypercapnic (rather than normocapnic) conditions induced by differences in respiratory rate. We cannot reject the null-hypothesis as no differences were observed in L-SRS (mean L-SRS hypocapnia 4.84, hypercapnia 4.77; Fig. 3a-d) between the two CO2 conditions.
The many physiological and pharmacological effects of hypocapnia made us believe that decreasing arterial CO2 concentrations would improve surgical conditions. These effects include deepening of the depth of anaesthesia, enhanced muscle relaxation, inhibition of abdominal muscle reflexes and a reduced efferent output from the brainstem to the diaphragm.6–8,12 Our inability to detect a significant difference between L-SRS scores does not imply that any of these effects of hypocapnia did not occur. It simply indicates that hypocapnia, irrespective of the physiological changes it causes, had no significant effect on the quality of the surgical field. We therefore infer from our data that in case of suboptimal surgical condition, increasing ventilation is without effect and additional relaxation is required.
We induced hypocapnia by increasing respiratory rates rather than increasing tidal volume. We did not allow high tidal volumes (>8 ml kg−1) to prevent any pressure-related damage to the lungs.13 Despite the relative high insufflation rates in hypocapnia peak inspiratory pressures were considered acceptable (Table 3) and none of the patients experienced any harm from hypocapnia.
Two recent studies have been published that use the L-SRS to assess the quality of the surgical field in deep NMB. Yoo and colleagues2 observed a mean score of 4 (range 3–5) during deep NMB against a mean score of 3 (range 2–5) during a moderate block in robotic laparoscopic prostate surgery. Kim and colleagues4 showed improved conditions during deep block compared with a moderate block in microlaryngeal surgery with 92% of patients with scores 4 or 5 at PTC 1-2 and 78% at TOF 1-2. Furthermore, just 3% of patients at deep NMB exhibited vocal cord movement during surgery compared with 39% of patients at moderate NMB. These studies indicate not only that a deep NMB is associated with superior surgical conditions in various complex surgeries but also show the practical usefulness of the L-SRS in scoring the quality of surgical field in different surgeries. Screening the various public clinical trial registries showed that additional studies that assess surgical conditions during anaesthesia using the L-SRS are underway, including one study on the effect of deep NMB in bariatric surgery (clinicaltrials.gov identifier NCT02553629). Apart from the 5-point L-SRS that we developed, other scoring systems were used in clinical studies, such as a 4-point scale by Dubois and colleagues3 with similar end-points as used by us (Table 1), a 4-point scale to assess the surgical space conditions by Staehr-Rye and colleagues14 (only the worst score per patient is reported), and a 100-point visual analogue scale by Blobner and colleagues.15 While we do not feel that one scoring system is superior to the other, we do express the need for a uniform scoring system that allows comparison between studies.
There are some limitations to our study. (1) The target at hypocapnia was not reached in all patients. Hyperventilation to relatively low levels of is more difficult in laparoscopic surgeries with continuous intracorporal CO2 insufflation, than in open surgeries. Greater frequencies than applied would have been required to reach the target values. As these high rates often hindered the surgeon we accept these deviations from target. (2) Scoring in our current and previous study was performed by one surgeon (R.B.), with large experience in the tested surgeries. This eliminates inter-observer variability but other surgeons may rate the surgical field differently, especially surgeons with less experience or surgeons trained in other subspecialties. This is illustrated by our previous observation that there was just moderate agreement between the ratings of our expert surgeon and eight other laparoscopy-skilled surgeons who rated video images of the surgical procedure.1 Further validation of the L-SRS in other specialties is therefore necessary. (3) The ability to effectively antagonize a deep NMB in an acceptable time frame is essential in clinical practice. Sugammadex is currently the only reversal agent that allows rapid and safe recovery from a deep block without consequences such as residual curarization. However, the restricted use or availability of sugammadex in some hospitals makes application of a deep NMB not always practical.
In conclusion, our study shows that deep NMB provides good to optimal surgical conditions in laparoscopic retroperitoneal urological surgery, which were independent of the level of arterial .
Authors' contributions
Study design/planning: M.B., C.M., L.A., A.D.
Study conduct: M.B., C.M., M.H., R.B., A.D.
Data analysis: M.B., A.D.
Writing paper: M.B., C.M., M.H., L.A., A.D.
Revising paper: all authors
Declaration of interest
A.D. has received speaker fees from MSD BV, The Netherlands.
Funding
This study was supported in part by MSD BV, The Netherlands and institutional and departmental funds.
References
- 1.Martini CH, Boon M, Bevers RF, Aarts LP, Dahan A. Evaluation of surgical conditions during laparoscopic surgery in patients with moderate vs deep neuromuscular block. Br J Anaesth 2014; 112: 498–505 [DOI] [PubMed] [Google Scholar]
- 2.Yoo YC, Kim NY, Dhin S et al. . The intraocular pressure under deep versus moderate neuromuscular blockade during low-pressure robot assisted laparoscopic radical prostatectomy in a randomized trial. PLoS ONE 2015; 10: e0135412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubois PE, Putz L, Jamart J, Marotta ML, Gourdin M, Donnez O. Deep neuromuscular block improves surgical conditions during laparoscopic hysterectomy; a randomised controlled trials. Eur J Anaesthesiol 2014; 31: 430–6 [DOI] [PubMed] [Google Scholar]
- 4.Kim HJ, Lee K, Park WK et al. . Deep neuromuscular block improves the surgical conditions of laryngeal microscopy. Br J Anaesth 2015; 115: 868–72 [DOI] [PubMed] [Google Scholar]
- 5.Fernando PU, Viby-Mogensen J, Bonsu AK, Tamilarasan A, Muchhal KK, Lambourne A. Relationship between post tetanic count and response to carinal stimulation during vecuronium-induced neuromuscular blockade. Acta Anaesthesiol Scand 1987; 31: 593–6 [DOI] [PubMed] [Google Scholar]
- 6.Gray TC, Jackson Rees G. The role of apnoea in anesthesia for major surgery. Br Med J 1952; 2: 891–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dundee JW. Influence of controlled respiration on dosage of thiopentone and d-tubocurarine chloride required for abdominal surgery. Br Med J 1963; 2: 893–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downes H. Hyperventilation and abdominal reflex inhibition in the rat. Anesthesiology 1963; 24: 615–9 [DOI] [PubMed] [Google Scholar]
- 9.Rodiera J, Serradell A, Alvarez-Gómez JA, Aliaga L. The cuff method: a pilot study of a new method of monitoring neuromuscular function. Acta Anaesthesiol Scand 2005; 49: 1552–8 [DOI] [PubMed] [Google Scholar]
- 10.Özdemir-van Bunschot DM, Scheffer GJ, Dahan A et al. . Comparison of the effectiveness of low pressure pneumoperitoneum with profound muscle relaxation during laparoscopic donor nephrectomy to optimize the quality of recovery during the early post-operative phase: study protocol for a randomized controlled clinical trial. Trials 2015; 16: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veelo D, Gisbertz S, Hannivoort R et al. . The effect of on-demand vs deep neuromuscular relaxation on rating of surgical and anaesthesiologic conditions in patients undergoing thoracolaparoscopic esophagetomy (DEPTH trial): study protocol for a randomized controlled trial. Trials 2015; 16: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz RL, Wolf CE. Neuromuscular and electromyographic studies in man: effects of hyperventilation, carbon dioxide inhalation and d-tubocurarine. Anesthesiology 1964; 25: 781–7 [DOI] [PubMed] [Google Scholar]
- 13.Futier E, Constantin JM, Paugam-Burtz C et al. . A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Eng J Med 2013; 369: 428–37 [DOI] [PubMed] [Google Scholar]
- 14.Staehr-Rye AK, Rasmussen LS, Rosenberg J et al. . Surgical space conditions during low-pressure laparoscopic cholecystectomy with deep versus moderate neuromuscular blockade: a randomized clinical study. Anesth Analg 2014; 119: 1084–92 [DOI] [PubMed] [Google Scholar]
- 15.Blobner M, Frick CG, Stäuble RB et al. . Neuromuscular blockade improves surgical conditions (NISCO). Surg Endosc 2015; 29: 627–36 [DOI] [PubMed] [Google Scholar]