Abstract
Background
Single-dose human fibrinogen concentrate (FCH) might have haemostatic benefits in complex cardiovascular surgery.
Methods
Patients undergoing elective aortic surgery requiring cardiopulmonary bypass were randomly assigned to receive FCH or placebo. Study medication was administered to patients with a 5 min bleeding mass of 60–250 g after separation from bypass and surgical haemostasis. A standardized algorithm for allogeneic blood product transfusion was followed if bleeding continued after study medication.
Results
519 patients from 34 centres were randomized, of whom 152 (29%) met inclusion criteria for study medication. Median (IQR) pretreatment 5 min bleeding mass was 107 (76–138) and 91 (71–112) g in the FCH and placebo groups, respectively (P=0.13). More allogeneic blood product units were administered during the first 24 h after FCH, 5.0 (2.0–11.0), when compared with placebo, 3.0 (0.0–7.0), P=0.026. Fewer patients avoided transfusion in the FCH group (15.4%) compared with placebo (28.4%), P=0.047. The FCH immediately increased plasma fibrinogen concentration and fibrin-based clot strength. Adverse event rates were comparable in each group.
Conclusions
Human fibrinogen concentrate was associated with increased allogeneic blood product transfusion, an unexpected finding contrary to previous studies. Human fibrinogen concentrate may not be effective in this setting when administered according to 5-minute bleeding mass. Low bleeding rates and normal-range plasma fibrinogen concentrations before study medication, and variability in adherence to the complex transfusion algorithm, may have contributed to these results.
Clinical trial registration
ClinicalTrials.gov identifier no. NCT01475669; EudraCT trial no. 2011-002685-20.
Keywords: blood, coagulation; fibrinogen; haemorrhage; surgery, cardiovascular
Editor's key points.
Hypofibrinogenaemia is a common occurrence after complex cardiac surgery.
There is variability in transfusion practices around the world.
Factor concentrates and other haemostatic agents are commonly used in complex cardiovascular surgery.
This study found that fibrinogen concentrate administration was associated with an increase in administration of blood products.
Complex cardiovascular surgery with cardiopulmonary bypass (CPB) is frequently associated with coagulopathy and bleeding.1 Allogeneic blood products are commonly administered despite the inherent risks of transfusion-related acute lung injury, volume overload, and transmission of pathogens.2,3 Compared with allogeneic blood products, coagulation factor concentrates may reduce transfusion volumes and immunogenic or infectious complications; they do not need cross-matching; and they can be prepared and infused more rapidly.4,5
The pathophysiology of bleeding after cardiovascular surgery and CPB is complex. Cardiopulmonary bypass causes greater impairment of fibrin formation than of thrombin generation or platelet function.6 Fibrinogen is the first coagulation factor to reach critically low concentrations in major bleeding,7 which has been associated with increased bleeding and adverse outcomes in cardiovascular surgery and trauma.8–10 Thus, fibrinogen is thought to have a key role in the management of bleeding.
Pasteurized human fibrinogen concentrate (FCH) has been available since 1985, and more than 3 million grams have been administered.11 It is licensed in 18 countries for treatment and prophylaxis of bleeding in patients with acquired hypofibrinogenaemia.12 Human fibrinogen concentrate rapidly and consistently increases plasma fibrinogen concentrations, improving clot strength and speed of clot formation.13,14
Initial investigations showed that FCH reduces transfusion in aortic surgery requiring CPB,15,16 leading to a phase II, single-centre, double-blind, placebo-controlled trial.17,18 The results of that study demonstrated the potential for FCH to reduce bleeding and transfusion in aortic replacement surgery.18 A multicentre phase III study was designed with methodology based on the phase II study. Based on results of the phase II study, the hypothesis was that FCH decreases bleeding independent of the patient's fibrinogen concentration, and consequently, reduces administration of allogeneic blood products compared with placebo. Our objective was to confirm that FCH therapy reduces the need for transfusion among complex cardiovascular surgery patients with a need for coagulation therapy to control bleeding but no confirmation of low plasma fibrinogen concentrations.
Methods
Study design and population
This was a phase III, multinational, multicentre, randomized, double-blind, placebo-controlled study (ClinicalTrials.gov identifier no. NCT01475669; EudraCT trial no. 2011-002685-20). The first patient was enrolled on January 23, 2012; the last patient completed the study on September 11, 2014. The study followed the International Conference on Harmonization Good Clinical Practice guidelines. Patients were enrolled at 34 centres in 11 countries. At each centre, approval was obtained from the ethics committee or institutional review board. Signed informed consent was obtained from patients before participation. A protocol amendment was implemented during the study (Supplementary data, Table S1).
The inclusion criteria at screening were as follows: age ≥18 yr (≥20 yr in Japan); and an elective open surgical procedure on any part of the aorta requiring CPB, with or without other cardiac procedures. Exclusion criteria (Supplementary data, Table S2) included the following: reoperative aortic surgery at the same site as the original procedure; operation for infection; and any known coagulation disorder.
At the start of surgery, patients were randomly assigned 1:1 to receive FCH or placebo using an interactive voice response system. Randomization was stratified by study centre and by surgery (primary or reoperation).
To be eligible for study treatment, randomized patients had to meet the following intraoperative inclusion criteria: first 5 min bleeding mass of 60–250 g; body temperature ≥35°C; activated clotting time within 25% of the baseline value; and blood pH >7.3. The 5min bleeding mass was defined as weight gained by surgical swabs after 5 min of packing of the surgical site; it was initially measured after surgical haemostasis was completed after separation from CPB and heparin reversal. Intraoperative exclusion criteria were as follows: prior use of any haemostatic therapy during the surgical procedure; and any perception that study participation could threaten the patient's safety.
Study assessments were scheduled at screening (within 4 weeks before surgery; Visit 1), surgery (Day 1; Visit 2), discharge from hospital or Day 11 (whichever was sooner; Visit 3), and end of study (Day 46; Visit 4). Baseline characteristics were recorded <48 h before surgery.
Procedures
Study medication [FCH (CSL Behring, Marburg, Germany) or placebo (0.9% sodium chloride solution)] was infused i.v. during 1–2 min. The dose of FCH was based on FIBTEM maximum clot firmness (MCF) at the end of CPB, targeting a FIBTEM MCF of 22 mm.13,18 Study medication was prepared by a pharmacist or blood bank technologist. The FCH was reconstituted with 50 ml sterile water per gram of fibrinogen. Investigator blinding was maintained by providing placebo at an equivalent volume to the patient's dose of FCH and by using identical opaque syringes. Surgical, anaesthesia, and intensive care unit teams were blinded to fibrinogen concentrations and the results of ROTEM® (Tem International, Munich, Germany) analyses throughout the study after administration of study medication. The 5 min bleeding mass was assessed for the second time approximately 5–10 min after administration of study medication. A standardized transfusion algorithm was followed if this was ≥60 g (Supplementary data, Fig. S1). The 5min bleeding mass was measured after each transfusion step, with further transfusions being administered if bleeding mass remained ≥60 g. Red blood cells were administered to patients with haemoglobin <7 g dl−1, end-organ ischaemia, acute blood loss, or other patient-specific reasons. Antifibrinolytic medication could be given during surgery, according to each site's standard procedure. Administration of hydroxyethyl starch, desmopressin, or aprotinin was not permitted during surgery or within 24 h after study medication. The CPB pump was primed using crystalloid fluid.
Study end points
The primary end point was the number of units of allogeneic blood products (FFP, platelets, and red blood cells) administered during the 24 h after administration of study medication. Secondary end points included the number of units of individual allogeneic blood products, total avoidance of transfusions, need for reoperation, mortality, plasma fibrinogen (central laboratory, Clauss assay), and MCF (FIBTEM assay performed at each site using a ROTEM device). Safety was assessed principally by the incidence of treatment-emergent adverse events (TEAEs) throughout the 46 day observation period, changes in vital signs and laboratory values, and ECG assessments. Viral transmission was investigated by enzyme-linked immunosorbent assays and polymerase chain reaction tests. The TEAEs of special interest included thromboembolic events, allergic reactions to study medication, adverse effects of transfusions, and severe complications of surgery or CPB. All serious adverse events (AEs) were monitored by an independent Data Safety Monitoring Board; all fatal and thromboembolic events were adjudicated by an independent, blinded adjudication committee.
Statistics
Sample size was calculated using a Wilcoxon rank-sum superiority test comparing allogeneic blood product use with FCH vs placebo, with the following assumptions based on the phase II trial: mean treatment difference, 7.12 (sd 12.45) units; 5% of patients in the intention-to-treat analysis with primary efficacy results imputed because of missing data, two-sided significance level of 5%; 90% power; randomization ratio 1:1; and distributional assumptions for a parametric test not met. It was determined that 152 patients would be required.
Efficacy analysis was performed on the intention-to-treat population, comprising all randomized patients who received study medication (identical to the safety population). Imputation was performed for patients dying, withdrawing, or lost to follow-up before 24 h after study medication, before discharge from hospital, or before discharge from the intensive care unit. The imputation procedure assigned the highest observed value in the treatment group for the number of units of allogeneic blood products and duration of hospitalization and intensive care unit stay.
The primary efficacy analysis compared median numbers of units of allogeneic blood products by study group. Given the low numbers of patients per study centre, instead of using a stratified Wilcoxon test (van Elteren test) with stratification by study centre, pooled centres were created within countries with sufficient patients (Canada, Czech Republic, Japan, and UK), and the remaining countries (Austria, Denmark, Finland, Germany, Italy, and Poland) were pooled together. For secondary efficacy analyses, continuous variables were analysed using a Wilcoxon rank sum test with the same pooling of study centres. Categorical variables were analysed by Cochran–Mantel–Haenszel tests. Safety variables were summarized with descriptive statistics by treatment group. Results for variables following the normal distribution are presented as the mean (sd), and variables not following the normal distribution the results are presented as the median [interquartile range (IQR)].
Results
Baseline and surgical characteristics
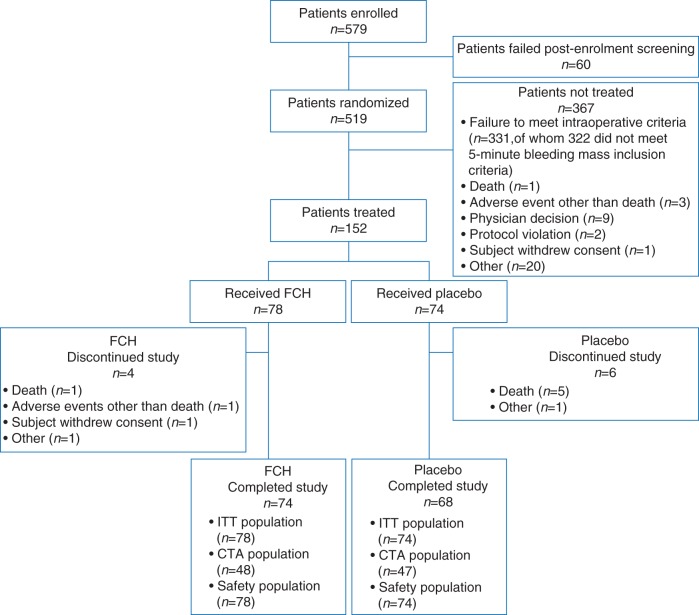
In total, 579 patients consented and were enrolled; of these, 519 were randomized. After confirming fulfilment of the intraoperative eligibility, 152 patients received study medication (Fig. 1). The most common procedure in both groups was thoracic aortic aneurysm repair with or without arch involvement. Of the 367 patients not receiving study medication, 327 (89%) had 5 min bleeding mass <60 g. Most treated patients were male and of white or Asian ethnicity (Table 1). One major difference between study groups was that the initial 5 min bleeding mass (before study medication) was higher in the FCH group than in the placebo group [median 107 (IQR 76–138) vs 91 (71–112) g, respectively; P=0.13]. Otherwise, the groups were comparable. Intraoperative and laboratory assessments were similar between the groups.
Fig 1.
Patient disposition. CTA, completer of transfusion algorithm (i.e. patients in whom the standardized treatment algorithm was adhered to); FCH, human fibrinogen concentrate; ITT, intention to treat.
Table 1.
Baseline characteristics and perioperative data. *Before the start of surgery. †Measured at the central laboratory. ‡Fibrinogen concentration at the time of measurement of the first 5 min bleeding mass, measured using the Clauss assay (central laboratory). CPB, cardiopulmonary bypass; FCH, human fibrinogen concentrate; IQR, interquartile range; MCF, maximum clot firmness; TAA, thoracic aortic aneurysm; TAAA, thoraco-abdominal aortic aneurysm; TRUST, transfusion risk understanding scoring tool
| Parameter | FCH (n=78) | Placebo (n=74) |
|---|---|---|
| Sex [n (%)] | ||
| Female | 18 (23.1) | 23 (31.1) |
| Male | 60 (76.9) | 51 (68.9) |
| Age [yr; mean (range)] | 63.9 (22–86) | 64.2 (24–86) |
| BMI [kg m−2; mean (sd)] | 25.7 (4.6) | 25.6 (4.6) |
| Race [n (%)] | ||
| Asian | 27 (34.6) | 24 (32.4) |
| White | 51 (65.4) | 49 (66.2) |
| Other | 0 | 1 (1.4) |
| Surgery type [n (%)] | ||
| TAAA repair | 3 (3.8) | 4 (5.4) |
| TAA repair with proximal arch | 32 (41.0) | 36 (48.6) |
| TAA repair without proximal arch | 43 (55.1) | 34 (45.9) |
| Unknown | 0 | 3 (4.1) |
| TRUST score | ||
| Mean (sd) | 2.2 (1.42) | 2.4 (1.59) |
| Plasma fibrinogen* (g litre−1) | ||
| n | 73 | 68 |
| Mean (sd) | 3.03 (0.92) | 3.06 (0.83) |
| FIBTEM MCF* (mm) | ||
| n | 70 | 66 |
| Mean (sd) | 16.3 (4.7) | 16.5 (5.2) |
| Duration of CPB [n (%)] | ||
| <120 min | 13 (16.7) | 10 (13.5) |
| 120–180 min | 30 (38.5) | 25 (33.8) |
| >180 min | 35 (44.9) | 39 (52.7) |
| Duration of circulatory arrest [n (%)] | ||
| 0 | 53 (67.9) | 41 (55.4) |
| >0 and <30 min | 18 (23.1) | 22 (29.7) |
| 30–60 min | 6 (7.7) | 7 (9.5) |
| >60 min | 1 (1.3) | 4 (5.4) |
| Lowest core temperature (°C) | ||
| n | 78 | 74 |
| Mean (sd) | 28.8 (5.4) | 28.1 (5.5) |
| First 5 min bleeding mass (g) | ||
| n | 78 | 74 |
| Median (IQR) | 107.0 (76.0–138.0) | 91.0 (71.0–112.0) |
| Platelet count at first 5 min bleeding mass† (×109 litre−1) | ||
| n | 70 | 69 |
| Mean (sd) | 102.4 (48.76) | 90.4 (35.7) |
| Last platelet count before administration of study medication [n (%)] | ||
| <100×109 litre−1 | 38 (48.7) | 38 (51.4) |
| ≥100×109 litre−1 | 40 (51.3) | 36 (48.6) |
| Plasma fibrinogen before administration of study medication‡ (g litre−1) | ||
| n | 74 | 70 |
| Mean (sd) | 1.86 (0.66) | 1.77 (0.51) |
Efficacy
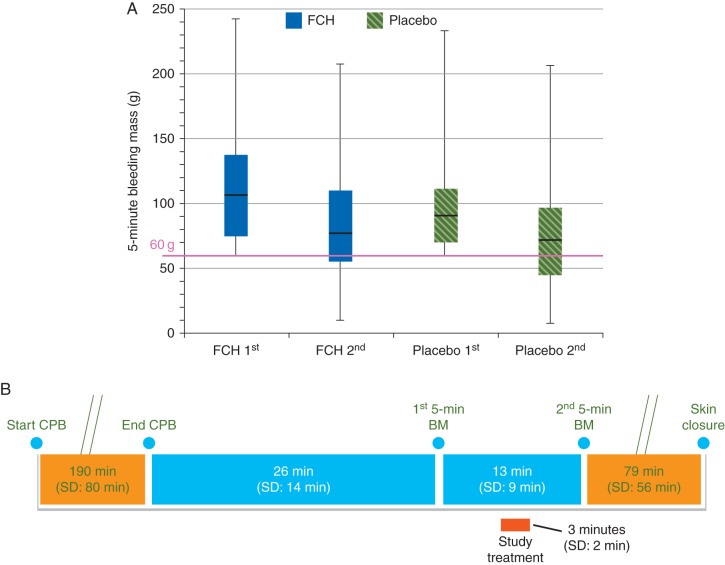
The median decrease in 5 min bleeding mass between the first and second measurements was 20 (IQR 3.0–50) g in the FCH group and 19.5 (1.0–42) g in the placebo group (P=0.32; Fig. 2 and Table 2). The measurements of chest tube drainage at 6, 12, and 24 h after administration of study medication showed numerically less bleeding in the FCH group (Table 2).
Fig 2.
First and second bleeding mass (median, interquartile range and range; a) and the timings of these assessments [mean (sd); b]. BM, bleeding mass; CPB, cardiopulmonary bypass; FCH, human fibrinogen concentrate.
Table 2.
Efficacy and safety results. AE, adverse event; FCH, human fibrinogen concentrate; FFP, fresh frozen plasma; IQR, interquartile range; TEAE, treatment-emergent adverse event
| Parameter | FCH (n=78) | Placebo (n=74) | P-value |
|---|---|---|---|
| Primary end point | |||
| Total number of units of allogeneic blood product during first 24 h after study medication | |||
| Median (IQR) | 5.0 (2.0–11.0) | 3.0 (0.0–7.0) | 0.026 |
| Secondary end points | |||
| Number of patients with total avoidance of allogeneic blood product transfusion | |||
| n (%) | 12 (15.4) | 21 (28.4) | 0.047 |
| Units of packed red blood cells administered (first 24 h) | |||
| Median (IQR) | 1.0 (0.0–3.0) | 0.0 (0.0–2.0) | 0.101 |
| Units of FFP administered (first 24 h) | |||
| Median (IQR) | 4.0 (0.0–6.0) | 0.0 (0.0–4.0) | 0.017 |
| Units of platelet concentrate administered (first 24 h) | |||
| Median (IQR) | 1.0 (0.0–2.0) | 1.0 (0.0–1.0) | 0.089 |
| Blood loss after administration of study medication: 5 min bleeding mass (g) | |||
| Second 5 min bleeding mass [median (IQR)] | 78.0 (55.0–110.0) | 72.0 (45.0–96.0) | 0.195 |
| Decrease from first 5 min bleeding mass [median (IQR)] | 20.0 (3.0–50.0) | 19.5 (1.0–42.0) | 0.319 |
| Blood loss after administration of study medication: chest tube drainage volume [ml; median (IQR)] | |||
| 6 h | 260.0 (155.0–410.0) | 297.5 (200.0–455.0) | 0.241 |
| 12 h | 405.0 (245.0–600.0) | 447.5 (320.0–700.0) | 0.137 |
| 24 h | 590.0 (400.5–839.5) | 682.5 (530.0–1050.0) | 0.120 |
| Adverse events | |||
| Pretreatment AE | 27 (34.6) | 26 (35.1) | – |
| Any TEAE | 75 (96.2) | 67 (90.5) | – |
| Severe TEAE | 42 (53.8) | 38 (51.4) | – |
| Treatment-related TEAE | 10 (12.8) | 10 (13.5) | – |
| Serious TEAE | 27 (34.6) | 25 (33.8) | – |
| Treatment-related serious TEAE | 4 (5.1) | 4 (5.4) | – |
| TEAE leading to death | 1 (1.3) | 5 (6.8) | – |
| TEAE of special interest | 32 (41.0) | 32 (43.2) | – |
| TEAE leading to discontinuation | 1 (1.3) | 0 | – |
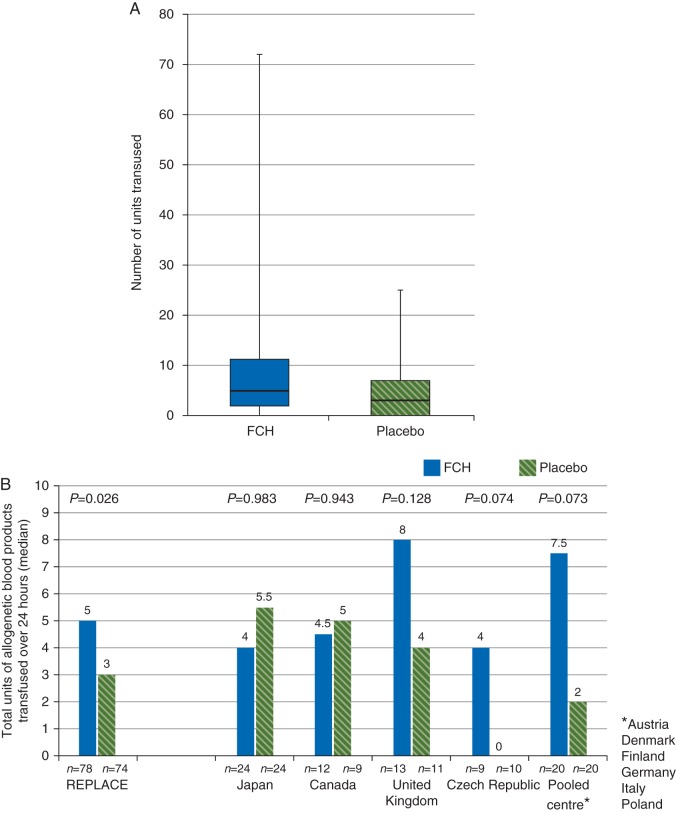
The primary analysis showed a median total of 5.0 (IQR2.0–11.0) units of allogeneic blood products administered in the 24 h after study medication in the FCH group, compared with 3.0 (0.0–7.0) units in the placebo group (P=0.026; Fig. 3). In the FCH group, values were imputed for two patients withdrawn from the study within 24 h after receiving study medication. Considerable variability in the primary end point was evident when comparing pooled study centres (Fig. 3).
Fig 3.
Transfusion of allogeneic blood products within 24 h of administering study medication. (a) Median, interquartile range and range. (b) Variability by pooled centre. *Austria, Denmark, Finland, Germany, Italy, and Poland. FCH, human fibrinogen concentrate.
Exploratory post hoc analyses of the primary end point were performed with the following: (i) no imputation of missing data (n=152); (ii) patients for whom the standardized transfusion algorithm was followed (n=104); and (iii) patients for whom the standardized transfusion algorithm was not followed (n=48). The results of analysis (i) were similar to those of the primary analysis. Median 24 h administration of allogeneic blood products in population (iii) (FCH, 11.0 units; placebo, 8.0 units) was considerably higher than that of population (ii) (FCH, 3.5 units; placebo, 1.0 units).
Additional efficacy results were consistent with the primary analysis (Table 2). The percentage of patients avoiding transfusion of allogeneic blood products was higher in the placebo group (21/74, 28.4% vs 12/78, 15.4%; P=0.047). The median number of units of FFP transfused throughout the first 24 h was significantly higher for the FCH group (P=0.017). There were no statistically significant differences in transfusions of platelets or red blood cells.
Seven patients (9.0%) in the FCH group and three (4.1%) in the placebo group required reoperation. Surgical bleeding was the reason for reoperation in all of these patients except one in the FCH group.
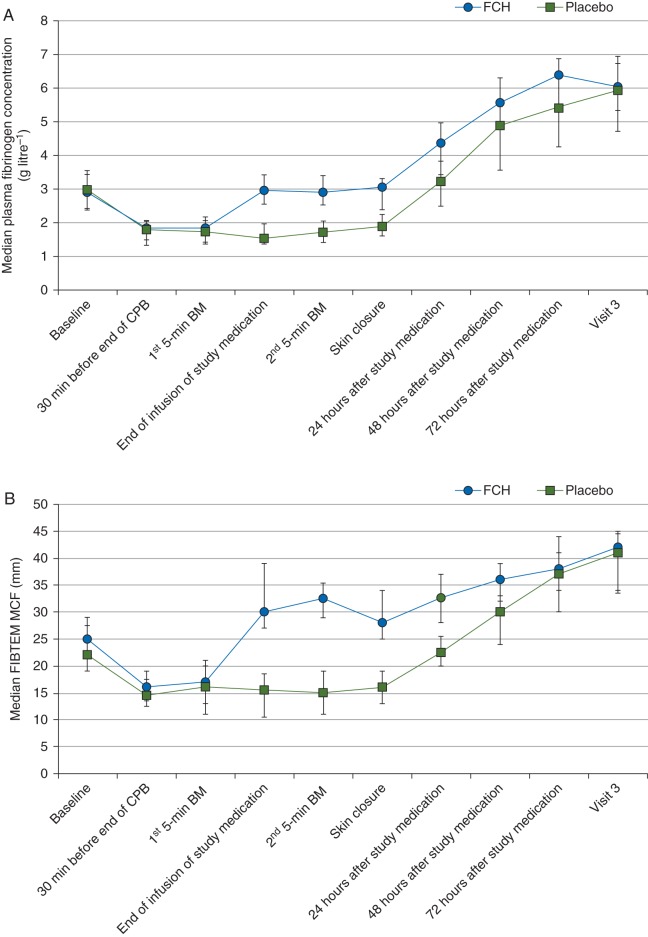
The mean dose in the FCH group was 6.29 (sd 1.97) g. Treatment with FCH consistently achieved the target FIBTEM MCF of 22 mm, corresponding to an immediate increase in the plasma fibrinogen concentration (Fig. 4). There was no increase in the fibrinogen concentration after administration of placebo. The difference between the two treatment groups decreased as time passed, and median plasma fibrinogen concentrations were similar in the two groups at Visit 3.
Fig 4.
Changes through time in plasma fibrinogen concentration (measured by the Clauss assay; a) and FIBTEM MCF (b). Error bars represent interquartile ranges. CPB, cardiopulmonary bypass; FCH, human fibrinogen concentrate; MCF, maximum clot firmness.
Further coagulation data (Supplementary data, Table S3) show few differences between the two study groups in values of coagulation factors or laboratory measures of coagulation, either before or after study treatment. Pretreatment data suggest a lack of pure coagulopathic bleeding in the study population. Post-treatment ROTEM data for the FCH group show that, in addition to increased strength of the fibrin-based clot (FIBTEM MCF), strength of the clot formed without platelet inhibition (EXTEM MCF) was increased vs placebo, and that the speed of clot formation (clotting time and clot formation time in the EXTEM assay) was increased.
Safety
Similar percentages of patients in both treatment groups reported any TEAE, treatment-related TEAEs, and serious TEAEs (Table 2). The most common TEAEs (≥20% in either group) were atrial fibrillation, pleural effusion, and anaemia (Supplementary data, Table S4). There was no between-group difference in the frequency of TEAEs of special interest or TEAEs related to study medication (Supplementary data, Table S5).
Thromboembolic events were reported in 6/78 patients (7.7%) in the FCH group and 10/74 patients (13.5%) in the placebo group. No difference was apparent between the two groups in the type, severity, or outcome of these events. No systematic differences between groups were observed in vital signs, clinical chemistry, haematology, or ECG assessments.
Six patients died during the study (FCH, one; placebo, five). Two deaths (one per group) occurred between 1 and 10 days after study medication; three (placebo) between 10 and 30 days; and one (placebo) occurred >30 days after study medication. Causes of death were cerebrovascular accident (FCH group) and ischaemic cerebral infarction, brain injury, infection, sepsis, and cardiac tamponade (placebo group). All deaths except one (placebo) were considered by the investigator as unrelated to study medication.
Discussion
REPLACE was the first prospective, multinational, multicentre phase III study assessing the effects of FCH on bleeding and transfusion of allogeneic blood products in cardiovascular surgery. When given to randomized patients as a single bolus according to 5 min bleeding mass, FCH was associated with an increase in administration of blood products. This unexpected finding, contrary to previous studies, might be attributable to low bleeding rates, the fact that many patients had plasma fibrinogen concentrations that would not trigger therapy in clinical practice, and variability in adherence to the complex transfusion algorithm. Treatment with FCH achieved the target FIBTEM MCF of 22 mm, reflecting an immediate, consistent increase in the plasma fibrinogen concentration that was not associated with any adverse safety findings.
Previous single-centre studies of FCH as haemostatic therapy in patients undergoing complex cardiovascular surgery reported positive efficacy results. Initial studies investigated FCH in ascending aorta replacement and elective thoraco-abdominal aortic aneurysm surgery, where a single bolus of fibrinogen concentrate reduced the need for transfusion compared with the control group.15,16 In the randomized, placebo-controlled, single-centre phase II study, which the REPLACE study was based upon, FCH reduced 24 h administration of allogeneic blood products compared with placebo (median 2 vs 13 units, P<0.001).18 Human fibrinogen concentrate also reduced transfusion among 29 coronary artery bypass graft patients with decreased platelet function.19 In a more recent study in 116 patients undergoing complex cardiac surgery with CPB, avoidance of transfusions was achieved in 67.2% of patients receiving first-line treatment with FCH (median dose 4 g), vs 44.8% in the placebo group.20
Our study was of a similar design to the preceding, single-centre phase II study,18 but conducted in multiple centres in different countries. Although the hypothesis that FCH leads to a reduction in transfusion of allogeneic blood products in these experimental conditions was rejected, evidence from other studies (including randomized controlled trials)13,15,16,18–21 shows this effect. We do not have a definitive explanation for the difference in results between REPLACE and the preceding phase II study but, as discussed below, multiple factors may be considered. Future studies of FCH in cardiac surgery would benefit from efforts to address these factors, and the lack of cell salvage data is a further weakness of our study that could be addressed.
Variability
The inclusion of a large number of centres in multiple countries, as opposed to the single-centre setting, allowed for increased variability in clinical practice. There were differences among countries and among centres within each country in the complexity of surgical procedures, the extent to which study procedures departed from routine practice, and in adherence to the study protocol; all these could have influenced the study outcome. As an example, the randomization failure rate (i.e. patients not meeting the bleeding mass criterion) ranged from 18.7% in Japan to 81.4% in the pooled centre. These increases in variability increased the level of ‘noise’, making the detection of a treatment effect less likely.
Adherence to study protocol
Overall adherence to the transfusion algorithm was only 68% in REPLACE (ranging across pooled centres from 100% in the Czech Republic to 47.6% in Canada), compared with 87% in the preceding phase II study (Supplementary data, Fig. S2). Allogeneic blood product transfusion in instances where the algorithm was not followed was three times higher than where the algorithm was followed. The algorithm was designed specifically for use in this study to demonstrate a treatment effect with FCH, and the authors do not consider it to be applicable in routine clinical practice. It is possible that the algorithm was too complex to follow in the multicentre setting, particularly in patients with severe bleeding. The high rate of non-adherence to the algorithm increased variability in the primary end point (number of units transfused). Although protocol deviations occurred to a similar extent in both study groups, they differed between centres, increasing overall variability within the study and decreasing the likelihood of detecting the true effect of FCH.
In REPLACE, the mean time from the end of CPB to the first 5 min bleeding mass was prolonged: 25.6 (sd 13.5) min, compared with 11.3 (7.9) min in the phase II study. Surgical haemostasis was likely to be ongoing during this period, leading to 62% of randomized patients not fulfilling the bleeding criteria to receive study medication. In the phase II study, only 19% of randomized patients failed to meet the bleeding criteria. It is encouraging that haemostasis could be achieved by surgical means alone in a significant proportion of patients. However, outside the confines of a clinical trial, an increased tendency towards clinically significant bleeding is likely, and this would increase the need for procoagulant therapy. Therefore, in the ‘real world’ setting, it is possible that the treatment effect of FCH could be higher than in the clinical trial setting. The time between the end of the first and start of the second 5 min bleeding mass measurement was also prolonged in REPLACE: 12.5 (sd 9.4) min, compared with 8.1 (3.3) min in the phase II study. The protocol stated that the second 5 min bleeding mass should have occurred immediately after study drug infusion. Surgical haemostasis may have occurred during the period between first and second bleeding mass measurements, and consequently, the second 5 min bleeding mass may have provided a less accurate evaluation of the efficacy of study medication in the REPLACE study. The differences between REPLACE and the preceding phase II study in timings of study assessments could have contributed to the differences in the effects of FCH on bleeding and transfusion of allogeneic blood products.
Criteria for administering therapy
The use of 5 min bleeding mass to assess bleeding and trigger the administration of study drug must be questioned. The procedure involved the surgeon packing swabs around suture lines to collect blood and not suctioning any blood for 5 min, then weighing the swabs to quantify blood collected. The surgical packing alone may have reduced or stopped surgical bleeding, introducing potential variability not accounted for in the study design. The arbitrary 5 min bleeding mass threshold of 60 g may have aggravated random pretreatment differences between the two groups. By chance, patients in the placebo arm had a lower median first 5-minute bleeding mass than those in the FCH group [91.0 g (IQR: 71.0–112.0 g) vs. 107.0 g (IQR: 76.0–138.0 g); P=0.134]. Thus, in the placebo group, a smaller change in bleeding mass was required to meet the criteria for avoidance of transfusion (i.e. 5 min bleeding mass <60 g). Although the median reduction in bleeding mass was ∼20 g in both groups, avoidance of transfusion was higher in the placebo group than in the FCH group (28.4 vs 15.4%). Future research might identify a better method to assess bleeding while the sternum is open and surgery is ongoing.
Fibrinogen supplementation is indicated in patients with hypofibrinogenaemia. A single definition of hypofibrinogenaemia in this context is not universally agreed, but less than 1.5–2.0 g litre−1 has been proposed. Our patients receiving FCH had a mean pretreatment fibrinogen concentration of 1.9 (sd 0.66) g litre−1; therefore, it is possible that the potential effect of FCH was reduced. Based on results of the preceding phase II study, we were not expecting an issue in this regard.
The safety results of REPLACE are consistent with previous findings that FCH is well tolerated. No new safety concerns emerged, and there was no increase in thromboembolic events or mortality compared with placebo. The results are aligned with published postmarketing safety surveillance data, showing the incidences of all AEs and thromboembolic events to be 1 in 6200 and 1 in 23 300 standard doses, respectively.22
In conclusion, the administration of a single bolus of FCH to treat bleeding after complex cardiac surgery, without selecting patients by fibrinogen concentration, was associated with an increase in the transfusion of allogeneic blood products. The main difference was in transfusion of FFP rather than red cells or platelets. The percentage of patients with total avoidance of allogeneic blood product transfusion was also lower in the FCH group than in the placebo group. Considering that the mean plasma fibrinogen concentration before FCH administration was relatively high at 1.9 g litre−1, the use of 5 min bleeding mass to determine the need for FCH administration must be questioned. Human fibrinogen concentrate produced a rapid, consistent increase in plasma fibrinogen to the target value and was well tolerated. The primary efficacy finding of this study, which was not consistent with previous reports, does not answer the question whether FCH would be efficacious if given to correct low levels of fibrinogen activity. The highly variable environment of the multicentre setting and elements of the study design that were applicable only in the single-centre setting (e.g. complexity of the treatment algorithm) might have contributed to the study outcome. Our results might help the design of future studies.
Authors' contributions
Study design: N.R.-M., D.S.S., R.G.
Protocol design: J.H.L.
Advisor for study protocol and management of the study: Y.U.
UK site principal investigator: R.G.
Patient recruitment: C.D.M., A.S., A.A.K., R.B., Y.O., R.G.
Data collection: C.D.M., A.S., A.A.K., R.B., Y.O., R.G.
Study conduct: R.R.
Study monitoring: J.H.L., D.S.S., R.R.
Data analysis: N.R.-M., D.S.S., R.R., R.G.
Data evaluation: J.H.L., D.S.S., R.R.
Writing the manuscript: J.H.L., D.S.S., R.R.
Editing and approval of the manuscript: N.R.-M., J.H.L., C.D.M., A.S., A.A.K., R.B., Y.O., Y.U., D.S.S., R.R., R.G.
Supplementary material
Supplementary material is available at British Journal of Anaesthesia online.
Declaration of interest
N.R.-M. serves on steering committees and advisory boards and is Principal Investigator for CSL Behring and Merck. J.H.L. serves on steering committees for Boehringer Ingelheim, CSL Behring, Grifols, Instrumentation Laboratories, and Janssen. C.D.M. has received research support and honoraria from CSL Behring, Medicines Company, and Fresenius Kabi. A.S. has received honoraria from CSL Behring, Grifols, B. Braun, and Fresenius Kabi in addition to educational grant funding from Orion. A.A.K. has received educational grant funding from CSL Behring, Brightwake, and Pharamacosmos. R.B. and Y.O. have no interest to declare. Y.U. serves on steering committees for CSL Behring in Japan and has received honoraria from CSL Behring for consultation. D.S.S. and R.R. are full-time employees and shareholders of CSL Behring. R.G. serves on steering committees for CSL Behring, serves on advisory boards for Octapharma, and has been paid to speak on behalf of CSL Behring, Octapharma, and Roche.
Funding
CSL Behring GmbH, Marburg, Germany.
Supplementary Material
Acknowledgements
Editorial assistance was provided by Meridian HealthComms Ltd, funded by CSL Behring.
The authors would like to thank study contributors from the following centres:
Vienna General Hospital, Austria (Principal Investigator, Professor Andrea Lassnigg);
University Foundation of Cardiology, Porto Alegre, Brazil (Principal Investigator, Dr Paulo Prates);
Heart Institute (InCor), Clinics Hospital, Sao Paulo, Brazil (Principal Investigator, Dr Ludhmila Hajjar);
Hamilton Health Science, Hamilton, ON, Canada (Principal Investigator, Dr Andre Lamy);
University of Toronto—Saint Michael's Hospital, Toronto, ON, Canada (Principal Investigator, Dr C. David Mazer);
Toronto General Hospital, Toronto, ON, Canada (Principal Investigator, Dr Terrence Yau);
Providence Health, St Paul's Hospital, Vancouver, BC, Canada (Principal Investigator, Dr Jian Ye);
Quebec Heart Institute, Quebec, QC, Canada (Principal Investigator, Dr Francois Dagenais);
University of Ottawa Heart Institute, Ottawa, ON, Canada (Principal Investigator, Dr Sean Dickie);
Montreal Heart Institute, Montreal, QC, Canada (Principal Investigator, Dr Antoine Rochon);
Centre of Cardiovascular and Transplantation Surgery, Brno, Czech Republic (Principal Investigator, Dr Robert Wagner);
University Hospital Ostrava, Ostrava-Poruba, Czech Republic (Principal Investigator, Dr Radim Brat);
Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (Principal Investigator, Dr Peter Skov Olsen);
Helsinki University Hospital, Helsinki, Finland (Principal Investigator, Dr Alexey Schramko);
Franziskus Hospital, Bielefeld, Germany (Principal Investigator, Dr Niels Rahe-Meyer);
JW Goethe University Hospital, Frankfurt, Germany (Principal Investigator, Dr Arndt Kiessling);
University Hospital of Munich, Munich, Germany (Principal Investigator, Dr Patrick Möhnle);
St Orsola Malpighi General Hospital, Bologna, Italy (Principal Investigator, Dr Marco Di Eusanio);
University Hospital of Udine, Udine, Italy (Principal Investigator, Dr Blanca Martinez);
San Raffaele Hospital, Milan, Italy (Principal Investigator, Dr Giovanni Landoni);
National Cerebral and Cardiovascular Centre, Osaka, Japan (Principal Investigator, Dr Kenji Minatoya);
Tenri Hospital, Nara, Japan (Principal Investigator, Dr Kazuo Yamanaka);
Nagoya University Hospital, Nagoya, Japan (Principal Investigator, Professor Akihiko Usui);
Kobe University Hospital, Kobe, Japan (Principal Investigator, Professor Yutaka Okita);
Tohoku University Hospital, Miyagi, Japan (Principal Investigator, Professor Yioshikatsu Saiki);
Keio University Hospital, Shinjuku, Japan (Principal Investigator, Professor Hideyuki Shimizu);
Kyoto University Hospital, Kyoto, Japan (Principal Investigator, Dr Keiichi Kanda);
Kurume University Hospital, Kurume, Japan (Principal Investigator, Professor Hiroyuki Tanaka);
Hamamatsu University Hospital, Hamamatsu, Japan (Principal Investigator, Professor Norihiko Shiiya);
Institute of Cardiology, Warsaw, Poland (Principal Investigator, Professor Jacek Rozanski);
Provincial Hospital, Szczecin, Poland (Principal Investigator, Professor Miroslaw Brykczynski);
University Hospital of Southampton, Southampton, UK (Principal Investigator, Dr Ravi Gill);
Papworth Hospital, Cambridge, UK (Principal Investigator, Dr Andrew Klein);
Glenfield Hospital, Leicester, UK (Principal Investigator, Dr Aamer Ahmed); and
Liverpool Heart and Chest Hospital, Liverpool, UK (Principal Investigator, Dr Seema Agarwal).
References
- 1.Sniecinski RM, Levy JH. Bleeding and management of coagulopathy. J Thorac Cardiovasc Surg 2011; 142: 662–7 [DOI] [PubMed] [Google Scholar]
- 2.Nascimento B, Goodnough LT, Levy JH. Cryoprecipitate therapy. Br J Anaesth 2014; 113: 922–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion 2012; 52(Suppl 1): 65S–79S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy JH, Szlam F, Tanaka KA, Sniecienski RM. Fibrinogen and hemostasis: a primary hemostatic target for the management of acquired bleeding. Anesth Analg 2012; 114: 261–74 [DOI] [PubMed] [Google Scholar]
- 5.Tanaka KA, Esper S, Bolliger D. Perioperative factor concentrate therapy. Br J Anaesth 2013; 111(Suppl 1): i35–49 [DOI] [PubMed] [Google Scholar]
- 6.Solomon C, Rahe-Meyer N, Sørensen B. Fibrin formation is more impaired than thrombin generation and platelets immediately following cardiac surgery. Thromb Res 2011; 128: 277–82 [DOI] [PubMed] [Google Scholar]
- 7.Hiippala ST, Myllylä GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth Analg 1995; 81: 360–5 [DOI] [PubMed] [Google Scholar]
- 8.Gielen C, Dekkers O, Stijnen T et al. . The effects of pre- and postoperative fibrinogen levels on blood loss after cardiac surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2014; 18: 292–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlsson M, Ternström L, Hyllner M, Baghaei F, Nilsson S, Jeppsson A. Plasma fibrinogen level, bleeding, and transfusion after on-pump coronary artery bypass grafting surgery: a prospective observational study. Transfusion 2008; 48: 2152–8 [DOI] [PubMed] [Google Scholar]
- 10.Reinhöfer M, Brauer M, Franke U, Barz D, Marx G, Lösche W. The value of rotation thromboelastometry to monitor disturbed perioperative haemostasis and bleeding risk in patients with cardiopulmonary bypass. Blood Coagul Fibrinolysis 2008; 19: 212–9 [DOI] [PubMed] [Google Scholar]
- 11.Levy JH, Welsby I, Goodnough LT. Fibrinogen as a therapeutic target for bleeding: a review of critical levels and replacement therapy. Transfusion 2014; 54: 1389–405 [DOI] [PubMed] [Google Scholar]
- 12.Costa-Filho R, Hochleitner G, Wendt M, Teruya A, Spahn DR. Over 50 years of fibrinogen concentrate. Clin Appl Thromb Hemost 2016; 22: 109–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon C, Pichlmaier U, Schoechl H et al. . Recovery of fibrinogen after administration of fibrinogen concentrate to patients with severe bleeding after cardiopulmonary bypass surgery. Br J Anaesth 2010; 104: 555–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolliger D, Szlam F, Molinaro RJ, Rahe-Meyer N, Levy JH, Tanaka KA. Finding the optimal concentration range for fibrinogen replacement after severe haemodilution: an in vitro model. Br J Anaesth 2009; 102: 793–9 [DOI] [PubMed] [Google Scholar]
- 15.Rahe-Meyer N, Pichlmaier M, Haverich A et al. . Bleeding management with fibrinogen concentrate targeting a high-normal plasma fibrinogen level: a pilot study. Br J Anaesth 2009; 102: 785–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahe-Meyer N, Solomon C, Winterhalter M et al. . Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg 2009; 138: 694–702 [DOI] [PubMed] [Google Scholar]
- 17.Rahe-Meyer N, Hanke A, Schmidt DS, Hagl C, Pichlmaier M. Fibrinogen concentrate reduces intraoperative bleeding when used as first-line hemostatic therapy during major aortic replacement surgery: results from a randomized, placebo-controlled trial. J Thorac Cardiovasc Surg 2013; 145: S178–85 [DOI] [PubMed] [Google Scholar]
- 18.Rahe-Meyer N, Solomon C, Hanke A et al. . Effects of fibrinogen concentrate as first-line therapy during major aortic replacement surgery: a randomized, placebo-controlled trial. Anesthesiology 2013; 118: 40–50 [DOI] [PubMed] [Google Scholar]
- 19.Solomon C, Schöchl H, Hanke A et al. . Haemostatic therapy in coronary artery bypass graft patients with decreased platelet function: comparison of fibrinogen concentrate with allogeneic blood products. Scand J Clin Lab Invest 2012; 72: 121–8 [DOI] [PubMed] [Google Scholar]
- 20.Ranucci M, Baryshnikova E, Crapelli GB et al. . Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery. J Am Heart Assoc 2015; 4: e002066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson M, Ternström L, Hyllner M et al. . Prophylactic fibrinogen infusion reduces bleeding after coronary artery bypass surgery. A prospective randomised pilot study. Thromb Haemost 2009; 102: 137–44 [DOI] [PubMed] [Google Scholar]
- 22.Solomon C, Gröner A, Ye J, Pendrak I. Safety of fibrinogen concentrate: analysis of more than 27 years of pharmacovigilance data. Thromb Haemost 2015; 113: 759–71 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.