Abstract
Chronic traumatic encephalopathy (CTE) is a neurodegenerative condition associated with repetitive mild traumatic brain injury. In recent years, attention has focused on emerging evidence linking the development of CTE to concussive injuries in athletes and military personnel; however, the underlying molecular pathobiology of CTE remains unclear. Here, we provide evidence that the blood–brain barrier (BBB) is disrupted in regions of dense perivascular p-Tau accumulation in a case of CTE. Immunoreactivity patterns of the BBB-associated tight junction components claudin-5 and zonula occludens-1 were markedly discontinuous or absent in regions of perivascular p-Tau deposition; there was also immunohistochemical evidence of a BBB in these foci. Because the patient was diagnosed premortem clinically as having progressive supranuclear palsy (PSP), we also compromised that the CTE alterations appear to be distinct from those in the brain of a patient with PSP. This report represents the first description of BBB dysfunction in a pathologically proven CTE case and suggests a vascular component in the postconcussion cascade of events that may ultimately lead to development of a progressive degenerative disorder. BBB dysfunction may represent a correlate of neural dysfunction in live subjects suspected of being at risk for development of CTE.
Keywords: Blood–brain barrier, Chronic traumatic encephalopathy, Claudin-5, Tight junctions.
INTRODUCTION
Traumatic brain injury (TBI) is a leading cause of death in children and young adults globally (1). Malignant brain swelling and a breakdown in the integrity of the blood–brain barrier (BBB) are central features of the pathophysiology that evolves after severe TBI (2–4). While major brain injury is a risk in modern contact sports, the number of deaths and legacy of continuing disability emanating from sports-related head injury appear at first glance to be small. A far greater challenge is the occurrence of concussive and subconcussive injuries, which fall within the spectrum of mild TBI. Indeed, in recent years, chronic traumatic encephalopathy (CTE) has come to the fore as a neurologic condition associated with repetitive mild TBI in military personnel and athletes (5). The underlying molecular pathobiology associated with CTE is, however, poorly understood and numerous critiques have highlighted the lack of distinct “in-life” diagnostic criteria for CTE (6, 7). Postmortem microscopic diagnosis of CTE is almost exclusively limited to the use of antibodies directed against phosphorylated tau protein (p-Tau), with p-Tau immunoreactivity observed in a perivascular pattern and at the depths of the sulci in addition to p-Tau immunopositive glial and neuronal profiles in subpial regions and astrocytic p-Tau positive plaques (8). Given the relatively distinctive perivascular nature of p-Tau accumulation in CTE and its association with repetitive mild TBI, it can be hypothesized that the BBB may be involved in some capacity, in the development of CTE.
The BBB plays a critical role in maintaining CNS homeostasis. It has been estimated that a neuron is never more than approximately 20 µm from a capillary. Cerebral endothelial cells are distinguished from systemic endothelial cells by high electrical resistance tight junctions that limit paracellular transport between adjacent cells. Inter-endothelial cell tight junctions are comprised of a series of up to 30 interacting proteins that seal gaps between endothelial cells in the neural microvasculature. Tight junction proteins, both intracellular and membrane-bound, include junctional adhesion molecule-1 (JAM-1); claudins-3, -5, and -12; occludin; tricellulin; zonula occludens (ZO)-1, -2, and -3; and the recently discovered lipolysis-stimulated lipoprotein receptor (LSR). Together they constitute, as the name implies, a tight and highly regulated barrier, even to very small molecules. In addition to regulating the exchange of ions and macromolecules between the blood and the delicate neural microenvironment, cerebral endothelial cells protect the brain by restricting entry of potentially damaging blood–borne agents, such as neurotoxic chemicals, antibodies, pathogens, immune cells, and anaphylatoxins (9). Claudin-5 is the most enriched tight junction protein and is likely involved in mediating size-selective passive diffusion of material at the BBB. In addition, claudin-5 levels have been shown to be dysregulated in many neurological conditions (10).
We sought to examine the pattern of expression of claudin-5 in relation to p-Tau in a confirmed case of CTE. Here, we report evidence of localized BBB dysfunction in an individual originally diagnosed clinically with progressive supranuclear palsy (PSP) and in whom CTE was confirmed at postmortem brain examination.
MATERIALS AND METHODS
Clinical History and Diagnosis
Briefly, the clinical details are as follows and are provided in more detail in a related manuscript (11). A 56-year-old man presented for the first time in 2011 after an episode of acute confusion. He had a 5-year history of cognitive dysfunction ranging from memory and organizational problems to difficulty managing his day-to-day working life. He was a former rugby union player with a very long career that began in his teens and extended to the age of 50. As noted in the clinical details in another manuscript, the subject experienced numerous head injuries during his playing career that would likely have amounted to a clinical diagnosis of concussion (11). While it is acknowledged that the retrospective history of concussion is fraught, in this case, the history was very clear and the individual’s on-field behavior was noted for its physicality as was reported by numerous playing colleagues as well as by family members.
There was no history of participation in other sports such as boxing or martial arts. Notably, the subject was diagnosed with PSP in life based on the cognitive profile and diagnostic motor features such as vertical gaze palsy and axial rigidity with falls. His clinical deterioration continued and after a period of hospitalization, he died 1 year later of hypostatic pneumonia. Neuropathology (as reported elsewhere), and outlined here in Figure 1, confirmed the diagnosis of CTE (11).
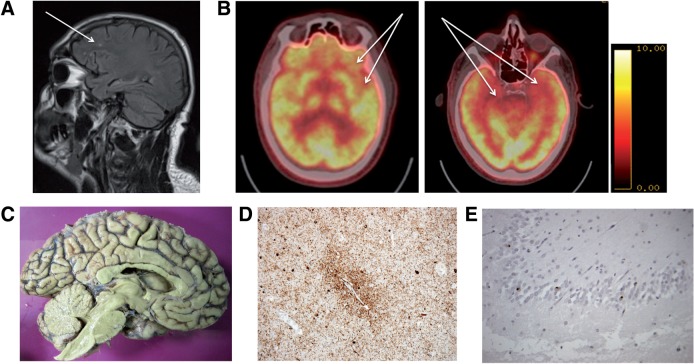
FIGURE 1.
MRI and PET imaging. (A) Fluid attenuated inversion recovery sequence, sagittal orientation. Arrow indicates white matter abnormalities in an area of tissue sampling (left frontal). (B) Fludeoxyglucose (18F), FDG-PET imaging. Arrows indicate areas of hypometabolism in the left inferior frontal and anterior temporal (left) and bilateral medial temporal areas (right). (C) Medial view of the left cerebral hemisphere showing minimal anterior frontal atrophy. (D) Perivascular phospho-Tau deposition. (E) Cytoplasmic TDP-43 accumulation in dentate fascia neurons.
Immunofluorescence
We sought to explore the hypothesis that the diagnostic perivascular distribution of p-Tau was related to a compromise of the BBB. To explore this hypothesis, we examined the integrity of the BBB using previously described techniques pioneered in our laboratory.
Slides of sectioned brain tissue (8 µm) from individuals diagnosed with PSP and CTE, and sectioned tissue from a control brain, were provided by the Dublin Brain Bank. Tissue sections were permeabilized using 0.5% Triton-X (Cat. #T8787-100ML, Sigma, St. Louis, MO) for 20 minutes followed by blocking in 5% Normal Goat Serum (Cat. #G9023-10ML, Sigma) for 40 minutes. Double-labeled immunofluorescence was carried out on the sections with overnight incubations at 4°C in primary antibodies: rabbit anti-claudin-5 (1:250 dilution; Cat. #34-1600, Source Bioscience, Nottingham, UK) and mouse anti-phospho-Tau (1:250 dilution; Cat. #90206, Innogenetics, Ghent, Belgium). After incubation, sections were washed in PBS followed by incubation in fluorescently conjugated secondary antibodies: goat anti-rabbit Cy3 conjugate (1:1000; Cat. #ab6939, Abcam, Cambridge, UK) and goat anti-mouse Alexa Fluor 488 conjugate (1:1000; Cat. #A-11001, Source Bioscience), for 3 hours at room temperature. Sections were washed with PBS and counterstained with Hoechst staining solution (Sigma; B2261-25MG) at a dilution of 1:5000 for 30 seconds. Labeling of tissues for fibrinogen and human IgG used rabbit antihuman fibrinogen antibodies (Dako, Glostrup, Denmark) and rabbit antihuman IgG (Abcam). Imaging of stained sections was carried out at room temperature using a Zeiss LSM-710 confocal microscope and a Zeiss T-PMT camera using 10X and 40X objectives.
Neuroimaging and Postmortem Review
We retrospectively examined brain MRI and PET scans obtained during life. Fluid attenuated inversion recovery (FLAIR) sequences indicated global atrophy and white matter abnormalities from the area of postmortem tissue sampling; however, these white matter changes were in keeping with a standard pattern of involutional age-related change that might be expected in an individual of similar age to this patient (Fig. 1A). We subsequently reexamined FDG-PET scans from this patient and identified evidence of very severe hypometabolism in the region of tissue sampling (left inferior and anterior temporal lobes) (Fig. 1B). A medial view of the left cerebral hemisphere at autopsy showed minimal anterior frontal atrophy (Fig. 1C). Characteristic perivascular p-Tau accumulation was evident and led to a diagnosis of CTE (Fig. 1D). Additionally, cytoplasmic TDP-43 accumulation in the cytoplasm of the dentate fascia neurons was evident, adding to the CTE diagnosis (12).
RESULTS
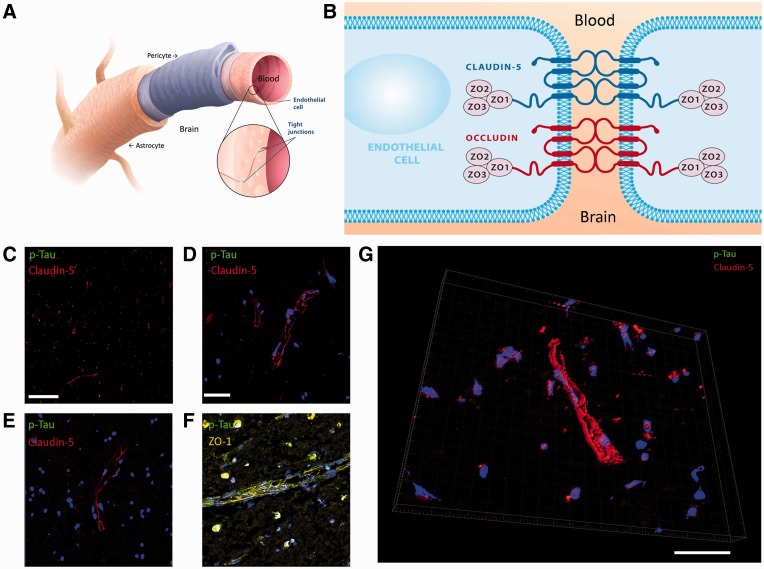
The cellular and molecular architecture of brain microvascular tight junctions is depicted diagrammatically in Figure 2A, B. Claudin-5 immunoreactivity was clearly visible, and it was expressed exclusively in the vasculature of a normal-aged matched healthy control brain section; there was minimal detection of p-Tau in the normal human brain (Fig. 2C–F). Zonula occludens-1 (ZO-1) immunoreactivity was similarly equally abundant in the normal brain microvasculature (Fig. 2F). Three-dimensional reconstruction of confocal Z-stack images highlighted the spatial pattern of claudin-5 expression in the brain (Fig. 2G).
FIGURE 2.
Tight junction expression at the blood–brain barrier (BBB) in a normal human control brain sample. (A) Schematic representation of the human BBB. (B) Schematic representation of tight junction molecules associated with the BBB. (C) Claudin-5 expression (red) and p-Tau (green) in normal control brain (10X objective; scale bar: 100 µm). (D, E) Claudin-5 (red) and p-Tau (green) expression in normal control brain sample (40X objective; scale bar: 20 µm [applies to E–G]). (F) Zonula occludens-1 (yellow) and p-Tau (green) expression. (G) Three-dimensional rendered image of claudin-5 (red) and p-Tau (green) expression at the BBB.
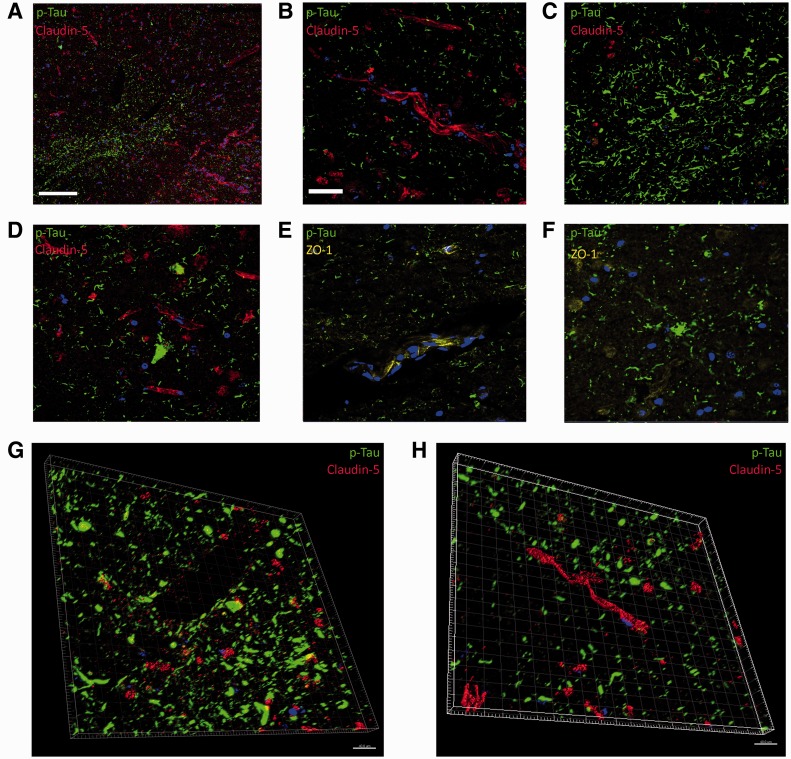
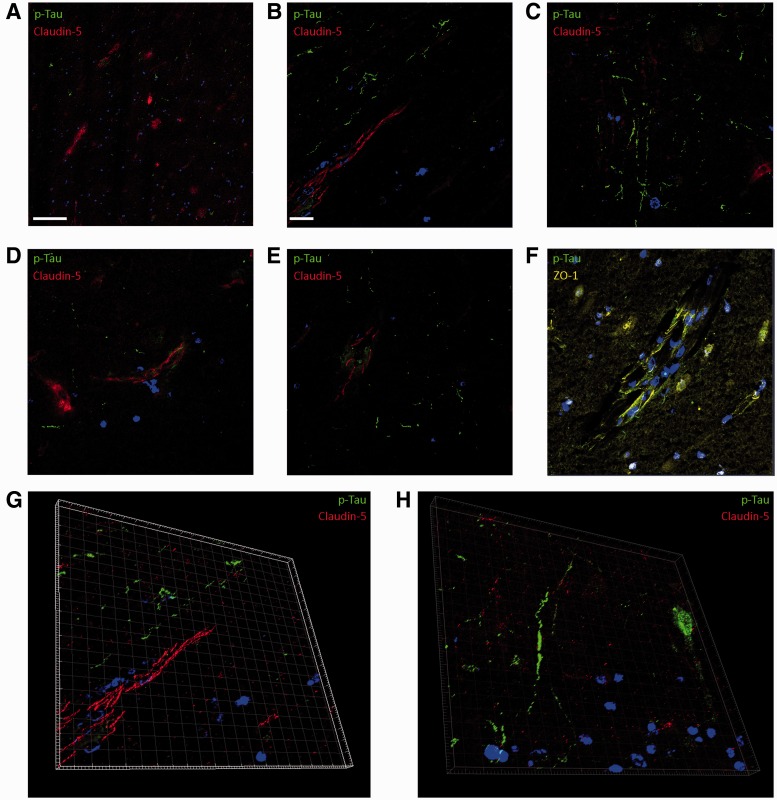
Detailed analysis of sections of the left medial frontal lobe of the CTE case showed an aberrant immunoreactivity pattern of claudin-5 in relation to p-Tau. In regions of dense perivascular p-Tau expression, claudin-5 was almost exclusively absent or expression was nonlinear and not localized to the tight junction (Fig. 3A). However, this pattern of claudin-5 expression was not pervasive throughout the section, and in regions displaying p-Tau tangles similar to those observed in Alzheimer disease, claudin-5 staining was identical to that observed in the normal control brain sample (Fig. 3B). Areas of dense perivascular p-Tau associated with larger vessels showed little or no positive immunoreactivity for claudin-5 (Fig. 3C). Similarly, in regions displaying dense p-Tau positive astrocytic plaques, claudin-5 staining was absent (Fig. 3D). The pattern of ZO-1 immunostaining in the CTE brain recapitulated that observed of claudin-5 (Fig. 3E, F). Three-dimensional rendered images derived from confocal z-stack imaging of claudin-5 and p-Tau show the spatial pattern of perivascular p-Tau in relation to claudin-5 (Fig. 3G, H).
FIGURE 3.
Blood–brain barrier (BBB) tight junction molecule and p-Tau immunoreactivity in patient with chronic traumatic encephalopathy (CTE). (A) Claudin-5 expression (red) and p-Tau (green) (10X objective; scale bar: 100 µm). (B) Claudin-5 (red) and p-Tau (green) expression in CTE brain (40X objective; scale bar: 20 µm, applies to C–F). (C) Aberrant claudin-5 (red) expression and p-Tau (green) (40X objective). (D) Claudin-5 (red) and p-Tau (green) expression in astrocytic plaque in human CTE brain. (E, F) Zonula occludens-1 (yellow) and p-Tau (green) expression. (G, H) Three-dimensional rendered image of claudin-5 (red) and p-Tau (green) expression at the human BBB in CTE brain. Scale bars: 20 µm.
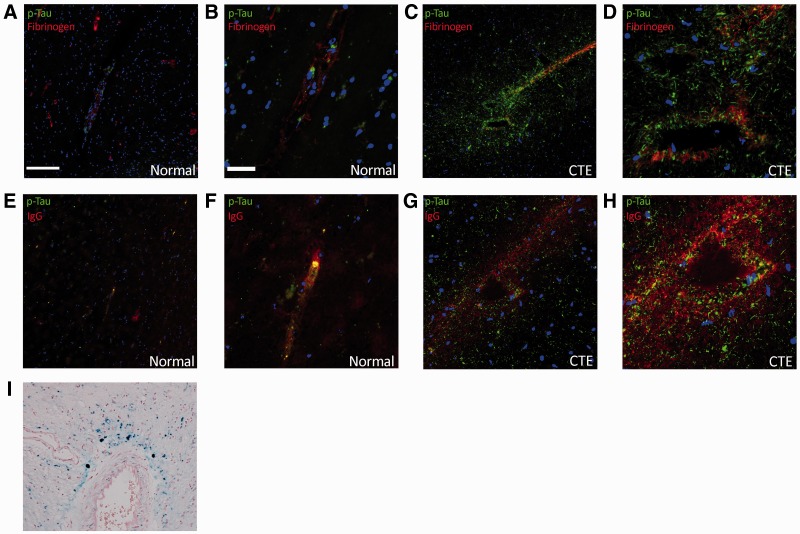
In order to evaluate the integrity of the BBB, we stained sections for the blood component fibrinogen and human IgG as has previously been reported in postmortem samples of TBI (13). Extravasation of these systemic components was evident in regions of perivascular p-Tau deposition in the CTE patient brain (Fig. 4C, D, G, H), but not in the normal control brain sample (Fig. 4A, B, E, F). Additionally, white matter perivascular hemosiderin deposition as depicted by Perls Prussian blue stain was evident in this CTE case, adding to the evidence of BBB dysfunction (Fig. 4I).
FIGURE 4.
Fibrinogen and IgG extravasation in normal brain (A, B, E, F) and in the brain of a patient with chronic traumatic encephalopathy (CTE) (C, D, G–I). (A–D) Fibrinogen (red) and p-Tau (green) expression in normal human brain (A, B) and CTE (C, D) samples. (E–H) Human IgG (red) and p-Tau (green) staining in normal human brain (E, F) and CTE brain (G, H) samples. (I) White matter perivascular hemosiderin deposition in the CTE patient brain demonstrated with Perl’s stain. (A, E, G, I) 10X objective; scale bar: 100 µm; (B, D, F, H) 40X objective, scale bar: 20 µm.
The CTE patient was originally clinically diagnosed as having PSP, and areas of the brain in this patient not displaying perivascular p-Tau showed p-Tau-positive tangles similar to those observed in PSP. Therefore, we performed a similar analysis on the brain of a patient with PSP. In that patient, areas of p-Tau immunoreactivity did not necessarily correlate with aberrant claudin-5 or ZO-1 staining (Fig. 5A–H). These observations bolster the concept that BBB dysfunction may represent a distinct pathological feature of the tauopathy observed in CTE; furthermore, the globose tangles, typical of PSP, were not observed in the CTE patient.
FIGURE 5.
Endothelial cell tight junction immunoreactivity in the brain of a patient with PSP. (A, B) Normal claudin-5 expression (red) and p-Tau (green) (10X objective; scale bar: 100 µm (A); (40X objective; scale bar: 20 µm) (B). (C) Aberrant claudin-5 (red) expression and p-Tau (green) in PSP brain (40X objective; scale bar: 20 µm). (D, E) Linear claudin-5 (red) and p-Tau (green) expression in PSP brain. (F) Zonula occludens-1 (yellow) and p-Tau (green) expression (scale bar [as in b: 20 µm). (G, H) Three-dimensional rendered image of claudin-5 (red) and p-Tau (green) expression at the BBB.
DISCUSSION
Currently, there is a paucity of information on the underlying mechanism(s) that lead to the distinctive pattern of perivascular Tau phosphorylation characteristic of CTE. Here, we show BBB dysfunction in a patient with CTE that may shed light on these mechanisms. We demonstrate for the first time that the signature perivascular accumulation of p-Tau is associated with an identifiable compromise in the integrity of components and the function of the BBB.
BBB dysfunction transcends a range of neurological conditions, none so evidently as the malignant cerebral edema associated with severe TBI (13). The pathology of TBI has a very clear vascular component, most often manifested through shearing of small vessels (14). The degree to which these traumatically sheared vessels undergo repair and reconstitution of their endothelial lining (the functional BBB) is unclear. We show here that the BBB is compromised through claudin-5 and ZO-1 loss many years after repetitive TBI. Additionally, the BBB compromise observed is distinctly associated with perivascular p-Tau accumulation. Previously, it has been shown that cerebral vascular malformations, which are devoid of a responsive functioning BBB, are also associated with perivascular p-Tau deposition (15).
Claudin-5 is the most enriched tight junction protein at the BBB, and studies in rodents show that deletion of this gene is embryonically lethal, suggesting dosage sensitivity of the protein product. In addition, claudin-5 has 2 extracellular domains that interact homotypically with claudin-5 molecules on adjacent endothelial cells (Fig. 2B). These domains are susceptible to environmental stimuli, and when certain residues are phosphorylated, claudin-5 localization can be discontinuous at the tight junction (16).
The patterns of claudin-5 and ZO-1 expression observed in regions of perivascular p-Tau raise interesting questions. For example, CTE may be triggered by a trauma-induced primary vascular insult (which may be repeated) before a perturbed BBB has reconstituted. A clinical correlate of a compromised BBB may be observed in the so-called “second impact syndrome”; indeed, the posttraumatic malignant brain swelling of childhood often is attributed to a perceived immaturity of the BBB. Alternatively, the primary TBI-related stretch insult to axons may impair the BBB, which in turn, could disrupt the balance between tau phosphorylation and kinase activity.
Given the importance of the BBB in maintaining neural homeostasis and taking the present findings of BBB disruption into consideration, we suggest that it may now be time to examine BBB integrity as a correlate of cognitive dysfunction in individuals suspected of being at risk for CTE.
REFERENCES
- 1.Hardman JM, Manoukian A. Pathology of head trauma. Neuroimaging Clin N Am 2002;12:175–87 [DOI] [PubMed] [Google Scholar]
- 2.Berg J, Tagliaferri F, Servadei F. Cost of trauma in Europe. Eur J Neurol 2005;12(suppl 1):85–90 [DOI] [PubMed] [Google Scholar]
- 3.Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 2004;(suppl 43):28–60 [DOI] [PubMed] [Google Scholar]
- 4.Ukaszewicz AC, Soyer B, Payen D. Water, water, everywhere: Sodium and water balance and the injured brain. Curr Opin Anaesthesiol 2011;24:138–43 [DOI] [PubMed] [Google Scholar]
- 5.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 2012;4(134):134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mez J, Solomon TM, Daneshvar DH, et al. Assessing clinicopathological correlation in chronic traumatic encephalopathy: Rationale and methods for the UNITE study. Alzheimers Res Ther 2015;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundman M, Doraiswamy PM, Morey RA. Neuroimaging assessment of early and late neurobiological sequelae of traumatic brain injury: Implications for CTE. Front Neurosci 2015;9:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omalu B. Chronic traumatic encephalopathy. Prog Neurol Surg 2014;28:38–49 [DOI] [PubMed] [Google Scholar]
- 9.Daneman R. The blood–brain barrier in health and disease. Ann Neurol 2012;72:648–72 [DOI] [PubMed] [Google Scholar]
- 10.Keaney J, Campbell M. The dynamic blood-brain barrier. FEBS J 2015;282:4067–79 [DOI] [PubMed] [Google Scholar]
- 11.Stewart W, McNamara PH, Lawlor B, et al. Chronic traumatic encephalopathy: A potential late and under recognized consequence of rugby union? QJM 2016;109:11–5 [DOI] [PubMed] [Google Scholar]
- 12.McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016;131:75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hay JR, Johnson VE, Young AM, et al. Blood-brain barrier disruption is an early event that may persist for many years after traumatic brain injury in humans. J Neuropathol Exp Neurol 2015;74:1147–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shenton ME, Hamoda HM, Schneiderman JS, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 2012;6:137–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kon T, Mori F, Tanji K, et al. Abnormal tau deposition in neurons, but not in glial cells in the cerebral tissue surrounding arteriovenous malformation. Neuropathology 2012;32:267–71 [DOI] [PubMed] [Google Scholar]
- 16.Nitta T, Hata M, Gotoh S, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 2003;161:653–60 [DOI] [PMC free article] [PubMed] [Google Scholar]