Abstract
We previously demonstrated blood-brain barrier impairment in remote contralateral brain areas in rats at 7 and 30 days after transient middle cerebral artery occlusion (tMCAO), indicating ischemic diaschisis. Here, we focused on effects of subacute and chronic focal cerebral ischemia on the blood-spinal cord barrier (BSCB). We observed BSCB damage on both sides of the cervical spinal cord in rats at 7 and 30 days post-tMCAO. Major BSCB ultrastructural changes in spinal cord gray and white matter included vacuolated endothelial cells containing autophagosomes, pericyte degeneration with enlarged mitochondria, astrocyte end-feet degeneration and perivascular edema; damaged motor neurons, swollen axons with unraveled myelin in ascending and descending tracts and astrogliosis were also observed. Evans Blue dye extravasation was maximal at 7 days. There was immunofluorescence evidence of reduction of microvascular expression of tight junction occludin, upregulation of Beclin-1 and LC3B immunoreactivities at 7 days and a reduction of the latter at 30 days post-ischemia. These novel pathological alterations on the cervical spinal cord microvasculature in rats after tMCAO suggest pervasive and long-lasting BSCB damage after focal cerebral ischemia, and that spinal cord ischemic diaschisis should be considered in the pathophysiology and therapeutic approaches in patients with ischemic cerebral infarction.
Keywords: Astrocytes, Autophagosomes, Blood-spinal cord barrier (BSCB), Middle cerebral artery occlusion (MCAO), Stroke, Subacute and chronic diaschisis, Tract demyelination.
INTRODUCTION
Strokes are the fifth leading cause of death in the United States (1); they are classified as ischemic, intracerebral hemorrhagic, or subarachnoid hemorrhagic; approximately 87% of strokes are ischemic (2). Stroke survivors are at risk for long-term disability; 50% suffer hemiparesis and about 30% are dependent in daily living activities (3).
Cerebrovascular ischemic injuries occur in a time-dependent manner and are categorized as acute (minutes to hours), subacute (hours to days), or chronic (days to months). Numerous studies have identified blood-brain barrier (BBB) disruption after focal cerebral ischemia (4–8). Increased BBB permeability has been shown in patients with acute ischemic stroke (9) and in a rodent model of middle cerebral artery occlusion (MCAO) (10–13), likely due to decreased endothelial cell (EC) tight junction integrity (7). Moreover, BBB leakage may persist for 3–5 weeks after the initial insult (14, 15). This prolonged barrier permeability might extend the ischemic tissue injury in association with brain edema and swelling (16). Pathophysiological processes such as alterations in blood flow and metabolism in brain regions contralateral to the initial ischemic insult have also been identified in patients up to 14 days after cerebral infarcts (17, 18). Brain injuries remote from focal ischemic lesions have been termed “transhemispheric diaschisis” (17, 19–22). Crossed cerebellar, thalamic, and cortical diaschisis have been demonstrated at acute, subacute, and chronic post-stroke stages; these types of diaschisis have been correlated with clinical and recovery-related outcomes in patients (23–25). In a rat MCAO model of transient ischemia (tMCAO), transcortical diaschisis was identified in the neocortex, suggesting widespread degeneration of corticostriatal connections (26, 27). Ipsilateral diaschisis in connected cortical regions distant from the initial damage was demonstrated in a focal cortical rat ischemia model (28). Recently, we showed BBB alterations not only in the ipsilateral hemisphere, but also in contralateral brain areas, 7 and 30 days after tMCAO in rats, indicating subacute and chronic ischemic diaschisis (29, 30). Damaged ECs containing numerous autophagosomes and vascular leakage were identified in the contralateral striatum, motor, and somatosensory cortices. This pervasive and long-lasting BBB damage could have major effects on brain functions.
Spinal cord injury may also result from cerebral infarcts. Neuropathological changes have been demonstrated in the lumbar spinal cord, contralateral to focal cerebral ischemia in rodent models (31–33). The glial cell response in the spinal cord was accompanied by increased expression of various proinflammatory cytokines and markers of oxidative stress 24–72 hours after permanent MCAO (32, 34). It has been proposed that inflammatory changes occurring not only in remote cerebral areas but also in the spinal cord distant from initial focal ischemic insult might be involved in the pathogenesis of secondary neuronal damage linked to antero- and retrograde degeneration (35).
Although the importance of inflammatory processes in post-cerebral infarct spinal cord pathology has been shown, the competence of the blood-spinal cord barrier (BSCB) following ischemic cerebral infarcts has not to our knowledge been investigated. Impairment of any BSCB component may lead to an increasingly toxic environment in the spinal cord. A better understanding of the BSCB function could encourage the development of more effective therapeutics for patients with strokes. We hypothesized that damage from focal cerebral ischemia might extend farther from the initial insult, that is, to the spinal cord. The aim of this study was to evaluate spinal cord diaschisis in subacute and chronic rat models of focal cerebral ischemia.
MATERIALS AND METHODS
Ethics Statement
All described procedures were approved by the Institutional Animal Care and Use Committee at USF and were conducted in compliance with the Guide for the Care and Use of Laboratory Animals.
All animals used in the study were obtained from The Jackson Laboratory, Bar Harbor, Maine. Fifty-seven Sprague Dawley adult male rats weighting 262.8 ± 2.32 g were randomly assigned to 1 of 2 groups: MCAO (n = 32) or control (n = 25). All rats were housed in a temperature-controlled room (23 °C), and maintained on a 12:12 hour dark: light cycle (lights on at 06:00 AM). Food and water were available ad libitum. The study design is schematically represented in Figure 1.
FIGURE 1.
Schematic representation of study design.
Middle Cerebral Artery Occlusion
tMCAO was induced using the intraluminal filament technique previously described in detail (29, 30, 36, 37). Based on our prior standardization of this model there is at least 80% reduction in regional cerebral blood flow (as determined by laser Doppler [Perimed, Järfälla, Sweden]) in the experimental animals during the occlusion period (38–40). Briefly, the tip of the filament was customized using dental cement (GC Corporation, Tokyo, Japan). Body temperature was maintained at 37 ± 0.3 °C during the surgical procedures. A midline skin incision was made in the neck with subsequent exploration of the right common carotid artery (CCA), the external carotid artery, and internal carotid artery. A 4-0 monofilament nylon suture (27.0–28.0 mm) was advanced from the CCA bifurcation until it blocked the origin of the middle cerebral artery (MCA). Animals were allowed to recover from anesthesia during MCAO. At 60 minutes after MCAO, animals were reanesthetized with 1%–2% isoflurane in nitrous oxide/oxygen (69%/30%) using a facemask and reperfused by withdrawal of the nylon thread. A midline incision was made in the neck and the right CCA was isolated. The incisions were then closed and the animals were allowed to recover from anesthesia.
Perfusion and Tissue Preparation
Seven or 30 days after reperfusion, tMCAO rats and controls were killed under CO2 inhalation and perfused transcardially with 0.1 M phosphate buffer ([PB] pH 7.2), followed by 4% paraformaldehyde in PB solution under pressure controlled fluid delivery at 85 mm Hg. The tMCAO rats (7 days post-tMCAO: n = 10; 30 days post-tMCAO: n = 9) and controls (for 7 days post-tMCAO: n = 6; for 30 days post-tMCAO: n = 7) were intravenously injected with 1 mL of 2% Evans Blue dye ([EB] Sigma-Aldrich, St. Louis, MO) in saline solution via the jugular vein 30 minutes prior to perfusion, as previously described (29, 30). The surgical procedure was performed in tMCAO and control rats using the same protocol, including exposure to anesthesia. Rats assayed for Evans Blue extravasation received only PB solution. After perfusion, rat cervical spinal cords were rapidly removed from tMCAO rats and controls for Evans Blue extravasation assay. Rats assayed for electron microscope analysis were randomly chosen from the 7 and 30 days post-tMCAO groups (tMCAO, n = 3; control, n = 2). Rat cervical spinal cords were immediately removed, labeled on right spinal side, and fixed in 4% paraformaldehyde in 0.1 M PB for 16–24 hours at 4 °C. The next day, spinal cords were cut into 1-mm slices, mapped against a diagram of the whole section according to a rat spinal cord atlas (41) and coded from the slices of both spinal cord sides in gray (ventral horn) and white (lateral funiculus) matter. Tissues were then fixed overnight in 2.5% glutaraldehyde in 0.1M PB (Electron Microscopy Sciences, Inc., Hatfield, PA) at 4 °C and stored for further electron microscope processing. Remaining rats were perfused and their cervical spinal cords immediately removed, fixed intact in 4% paraformaldehyde in 0.1 M PB for 24–48 hours and then cryoprotected in 20% sucrose in 0.1 M PB overnight. Coronal spinal cord tissues were cut at 30 μm in a cryostat, thaw-mounted onto slides, and stored at −20 °C for immunohistochemical and histological analyses.
BSCB Permeability
Evans Blue dye, 961 Da, was used as a tracer for assessing BSCB disruption. The Evans Blue extravasation assay was performed as previously described (29, 30, 42). Briefly, after perfusion, the cervical spinal cords were weighed and placed in 50% trichloroacetic acid solution (Sigma). Following homogenization and centrifugation, the supernatant was diluted with ethanol (1:3) and loaded into a 96 well plate in triplicate. The dye was measured with a spectrofluorometer (Gemini EM Microplate Spectrofluorometer, Molecular Devices, Sunnyvale, CA) at excitation of 620 nm and emission of 680 nm (43). Calculations were based on external standards in the same solvent. The Evans Blue content in tissue was quantified from a linear standard curve derived from known amounts of the dye and was normalized to tissue weight (μg/g). All measurements were performed by two experimenters blinded to the experiment.
Electron Microscopy
Ultrastructural analyses were performed in rats 7 and 30 days after tMCAO. Briefly, spinal cord tissue samples were postfixed in 1% osmium tetroxide (Electron Microscopy Sciences, Inc.) in 0.1M PB for 1 hour at room temperature (RT) and then dehydrated in a graded series of acetone dilutions. Tissues were transferred to a 50:50 mix of acetone and LX112 epoxy resin embedding mix (Ladd Research Industries, Burlington, VT) and infiltrated with the mix for 1 hour. The tissues were then transferred to a 100% LX112 embedding mix and infiltrated with fresh changes of this embedding mix. The tissues were further infiltrated overnight in fresh embedding medium at 4 °C. On the following day, the tissues were embedded in a fresh change of resin in tissue capsules. The blocks were polymerized at 70 °C in an oven overnight. The blocks were trimmed and then sectioned with a diamond knife on an LKB Huxley ultramicrotome. Thick sections cut at 0.35 µm were placed on glass slides and stained with 1% toluidine blue stain. Thin sections were cut at 80–90 nm, placed on copper grids, and stained with uranyl acetate and lead citrate.
For analysis of BSCB ultrastructure, microvessels in the right and left gray and white matter of cervical spinal cords of 7 and 30 days post-tMCAO rats and controls were examined by an investigator blinded to the animal groups using mapped/coded sections and photographed with an AMT ActiveVu XR 16 digital camera (Advanced Microscopy Techniques, Woburn, MA) attached to a FEI Morgagni transmission electron microscope (FEI, Inc., Hillsboro, OR) at 60 kV.
Immunohistochemistry
Spinal cord tissues were preincubated with 10% normal goat serum and 0.3% Triton 100X in phosphate-buffered saline (PBS) for 60 minutes at RT. Rabbit polyclonal anti-occludin antibody (occludin, 1:50, Abcam, Cambridge, MA) was applied on the slides overnight at 4 °C. The next day, the slides were rinsed in PBS and incubated with secondary goat anti-rabbit antibody conjugated to fluorescein isothiocyanate (FITC) (1:600, Thermo Fisher Scientific, Waltham, MA) for 2 hours. After rinsing, slides were coverslipped with Vectashield containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA) and examined using an Olympus BX60 epifluorescence microscope. Immunoexpression of occludin was observed in each image and qualitatively analyzed.
Double immunohistochemical staining for autophagosomes using Beclin-1 and LC3B biomarkers was performed to detect an autophagy response within capillary ECs in the ventral horns of both sides of the cervical spinal cords. Briefly, serial spinal cord tissue sections were preincubated in blocking solution as described above and then incubated overnight with rabbit polyclonal anti-Beclin-1 antibody (Beclin-1, 1:500, Pierce, Rockford, IL). The next day, the slides were rinsed in PBS and incubated with secondary goat anti-rabbit antibody conjugated to FITC (1:500, Thermo Fisher Scientific) for 2 hours. After rinsing, slides were again pre-incubated with blocking solution as described above for 60 minutes at RT and rabbit polyclonal anti-LC3B antibody (LC3B, 1:250, Abcam, Cambridge, MA) was applied on the slides overnight at 4 °C. The slides were then rinsed in PBS and incubated with secondary goat anti-rabbit antibody conjugated to Rhodamine (1:1200, Thermo Fisher Scientific) for 2 hours. After rinsing, slides were coverslipped with Vectashield containing DAPI (Vector Laboratories) and examined using an Olympus BX60 epifluorescence microscope. Observation and quantification of Beclin-1 and LC3B fluorescent intensities within capillaries was performed in randomly selected cervical spinal cord sections in both right and left sides of ventral horn. For consistent results, capillaries of diameters from 15 to 20 μm, as measured using NIH ImageJ (version 1.46) software, were used for fluorescent detection of Beclin-1 and LC3B immunoreactivities. Microvessels with diameters below or above this range, as well as longitudinal capillaries, were excluded. Fluorescent images were taken from 3 to 4 sections in the gray ventral horn of the cervical spinal cords of both sides per animal separated by approximately 60–90 μm. Fluorescent Beclin-1 and LC3B intensities were analyzed in same capillary (10–15 capillaries per examined spinal cord areas) using NIH ImageJ software.
In a separate set of spinal cord sections, immunohistochemical staining of astrocytes was performed as previously described (29, 30). Briefly, the tissue sections were preincubated in blocking solution as described above and then incubated overnight with rabbit polyclonal anti-glial fibrillary acid protein (GFAP) primary antibody (1:500, Dako, Glostrup, Denmark) at 4 °C. The next day, secondary goat anti-rabbit antibody conjugated to FITC (1:500, Thermo Fisher Scientific) was applied for 2 hours. After washing, slides were coverslipped with Vectashield containing DAPI (Vector Laboratories) and examined using an Olympus BX60 epifluorescence microscope. Fluorescent images were taken from 3 sections in the gray ventral horn of the cervical spinal cords of both sides per animal separated by approximately 90 μm and fluorescent intensity was analyzed as described above.
Immunohistochemical images of all assessments were analyzed by an investigator blinded to the experiments and animal codes were removed prior to analysis. To avoid bias in the analysis of fluorescence images, the gray matter cervical spinal cord areas in both sides were identified in a section using a 10×/0.30 numerical aperture lens, and then areas of interest were photographed with a 20×/0.50 numerical aperture lens, photographing the slide in a random raster pattern. All image analyses for Beclin-1/LC3B and GFAP were performed by measuring intensity of fluorescent expression (%/μm2) using NIH ImageJ software. For Beclin-1/LC3B immunoexpressions, fluorescent intensity was measured relative to capillary area. For GFAP, fluorescent intensity was measured in the gray matter images. Thresholds for detection of Beclin-1/LC3B and GFAP expressions were adjusted for each image to eliminate background noise.
Histological Analysis and Stereological Counts of Motor Neurons
The separate set of cervical spinal cord sections were stained with 0.1% Luxol fast blue (LFB) (Aldrich Chemical, Milwaukee, WI) and 0.1% Cresyl violet (Sigma-Aldrich) technique using a standard protocol. To determine motor neuron numbers in the gray ventral horn of the cervical spinal cords on both sides, the optical fractionator method of unbiased stereological cell counting techniques (44, 45) was used with a Nikon Eclipse 600 microscope and quantified using Stereo Investigator software (MicroBrightField, Williston, VT). The virtual grid (150 × 150 µm) and counting frame (75 × 75 µm) were optimized to count at least 200 cells per animal with error coefficients <0.05. Outlines of the anatomical structures were done using a 10×/0.45 objective and cell quantification was conducted using a 60×/1.40 objective. The motor neuron numbers (20- to 25-μm diameter) were counted in discrete levels of the cervical spinal cord (n = 7 sections/level/animal separated by ∼60 μm) and presented as average per ventral horn on both spinal cord sides. Motor neuron morphology and myelin staining were also analyzed.
Statistical Analysis
Data are presented as means ± SE. One-way ANOVA with Bonferroni’s Multiple Comparison test using GraphPad Prism software version 5 (GraphPad Software) was performed for statistical analysis. Significance was defined as p < 0.05.
RESULTS
Cervical Spinal Cord Ultrastructure at 7 Days Post-tMCAO
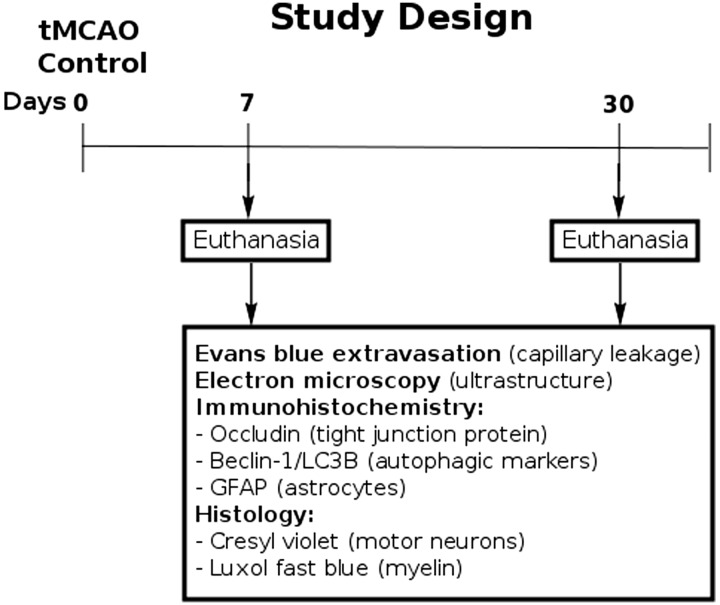
Both sides of the cervical spinal cords in control rats were characterized by normal appearance of capillaries surrounded by astrocyte cell processes and myelinated axons in gray (Fig. 2A) and white matter (Fig. 2D, F). Capillaries consisted of a single layer of ECs surrounded by a basement membrane layer, sometimes enclosed by additional pericyte cytoplasm. Motor neurons and glial cells demonstrated normal morphology with central nuclei in gray matter (Fig. 2B, C). Organelles in all cells were well preserved and mitochondria showed a normal pattern of cristae. White matter oligodendrocytes and astrocytes were morphologically typical with distinct nuclei (Fig. 2E, F). At 7 days after right tMCAO there was dramatic microvascular damage on both sides of the spinal cord in gray and white matter. On the right side (ipsilateral to tMCAO), vacuolated ECs containing large autophagosomes were noted in numerous gray matter (Fig. 2G, H) and white matter (Fig. 2I) capillaries of the ventral cervical spinal cord. Some ECs were necrotic with condensed cytoplasm and detached from the capillary lumen (Fig. 2G). Edematous spaces near capillaries or motor neurons were seen. Also, some axons with myelin disruption and unraveling were apparent (Fig. 2J). On the left (contralateral) side there were also severely damaged vessels in both gray and white matter including EC degeneration and necrosis, endothelial autophagosome accumulation, pericyte degeneration with enlarged mitochondria, astrocyte end-feet degeneration, and extensive perivascular edema (Fig. 2K–M). Dilated endoplasmic reticulum in motor neurons near damaged capillaries with perivascular edema was also noted in addition to some degree of nuclear fragmentation, suggesting apoptotic processes. Numerous swollen axons with and without myelin were surrounded by capillaries in white matter (Fig. 2M, N). Importantly, markedly swollen axons were seen mainly in white matter of the left side compared with the right side at 7 days post-tMCAO.
FIGURE 2.
Electron microscope examination of microvasculature in the rat cervical spinal cord at 7 days after tMCAO. (A–F) Representative areas of gray matter (A–C) and white matter (D–F) of control rat cervical spinal cord demonstrate normal ultrastructural appearances of capillaries, neurons, myelinated axons, and glial cells. (G–N) Seven days post-tMCAO, numerous gray matter capillaries on the right side displayed damaged ECs containing large autophagosomes (G, H). Necrotic EC with condensed cytoplasm was detached from capillary lumen (#) (G). Extracellular edema surrounding the capillary and motor neuron was seen. Similarly to gray matter, white matter capillaries demonstrated EC alterations including vacuolization or necrosis (I). Some demyelinated axons were also seen (J). The left side capillaries appeared more damaged in gray matter (K, L) and white matter (M), as indicated by degenerated and necrotic ECs with autophagosome accumulation, degenerated pericytes with enlarged mitochondria, astrocyte end-feet degeneration, dilated endoplasmic reticulum in motor neurons, and extensive perivascular edema. Numerous swollen axons with and without myelin are surrounded by capillaries with perivascular edema in white matter (M, N). Abbreviations: En, endothelial cell; BM, basement membrane; Ast, astrocyte; m, mitochondrion; c, capillary; A, axon; P, pericyte; Nu, nucleus; Aph, autophagosome; Oligo. Oligodendrocyte; ER, endoplasmic reticulum. #Separation of EC from BM. Asterisks in panels G–M indicate edema. Scale bars: A–F, H–J, L, N = 2 μm; G, K, M = 500 nm.
Chronic (30 Days) Post-tMCAO Stage
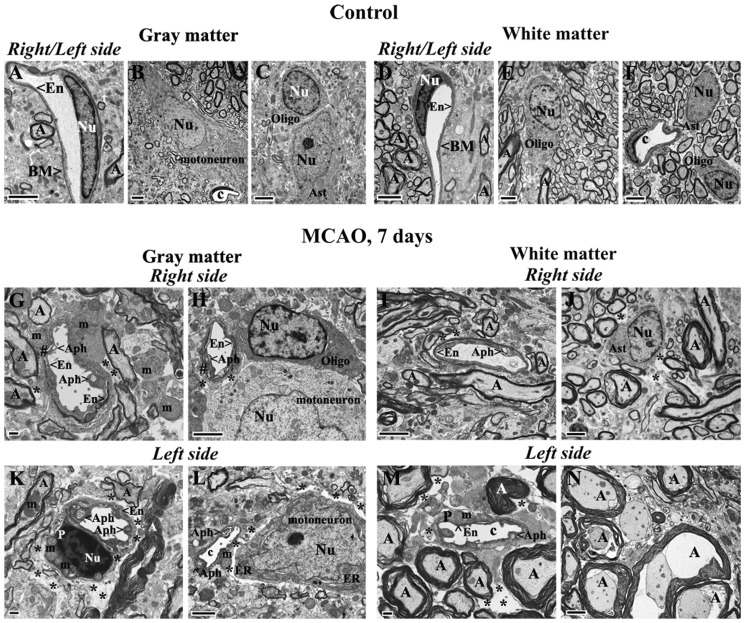
Similar to controls for 7 days post-tMCAO, control cervical spinal cords for 30 days after tMCAO demonstrated normal ultrastructural morphology in gray matter (Fig. 3A–C) and white matter (Fig. 3D–F) on both sides of the cord. Capillary walls showed healthy-appearing ECs and adjacent pericytes (Fig. 3A, D). Motor neurons, astrocytes, oligodendrocytes, and microglia all showed well-defined central nuclei with normal morphology. Myelinated axons of high density were present in white matter on both right and left sides (Fig. 3E, F).
FIGURE 3.
Electron microscope examination of microvasculature in the rat cervical gray and white matters of spinal cord at 30 days after tMCAO. (A–F) Representative areas of gray matter (A–C) and white matter (D–F) of a control rat cervical spinal cord are characterized by normal ultrastructural morphology of capillaries, neurons, myelinated axons, and glial cells. (G–J) Thirty days post-tMCAO, numerous capillaries in gray matter on the right side showed swollen ECs containing autophagosomes and enlarged mitochondria (G). White matter capillaries have expanded mitochondria, vacuolization in ECs and a pericyte (I). Perivascular edema and detachment of ECs from capillary lumen were seen in both gray (G) and white (I) matter. Motor neurons with dilated endoplasmic reticulum in gray matter (H) and many axons with unraveling myelin in white matter (J) were also found on the right side of the cord. Edema surrounding the swollen axons is noted in the white matter (J). (K–N) On the left side of the cord there is edema between a capillary and a degenerated motor neuron (K), and around a motor neuron with dilated endoplasmic reticulum (L) in gray matter. Capillaries in both gray matter (K) and white matter (M, N) contain large autophagosomes or vacuoles. As on the right side, axons with unraveling myelin are seen near capillaries on the left side of the cord (N). Abbreviations: En, endothelial cell; BM, basement membrane; Ast, astrocyte; m, mitochondrion; c, capillary; A, axon; P, pericyte; Nu, nucleus; Aph, autophagosome; Oligo, oligodendrocyte; ER, endoplasmic reticulum; #, separation of EC from BM. Asterisks in panels G, I–N indicate extracellular edema. Scale bars: B, C, E, F, H, J, L = 2 μm; A, D, G, I, K, M, N = 500 nm.
At 30 days after tMCAO, numerous capillaries in gray matter on the right side of the cord revealed swollen ECs containing autophagosomes and enlarged mitochondria (Fig. 3G). Similar to 7 days post-tMCAO, damaged ECs characterized by expanded mitochondria and vacuolization and edematous pericytes were detected in white matter capillaries on the right side (Fig. 3I). Perivascular edema and detachment of ECs from capillary lumen were seen in both gray and white matter. Also, motor neurons with dilated endoplasmic reticulum (Fig. 3H) and many axons with damaged myelin (Fig. 3J) were seen on the right side of the cord; there was also considerable edema. On the left side of cervical spinal cord, large areas of edema between capillary and degenerated motor neurons were apparent in gray matter (Fig. 3K). Some motor neurons demonstrated dilated endoplasmic reticulum (Fig. 3L). Capillaries in both gray matter (Fig. 3K) and white matter (Fig. 3M, N) contained large autophagosomes or vacuoles. Some myelin injury and swollen axons near capillaries were also seen on the left side of the cord (Fig. 3N).
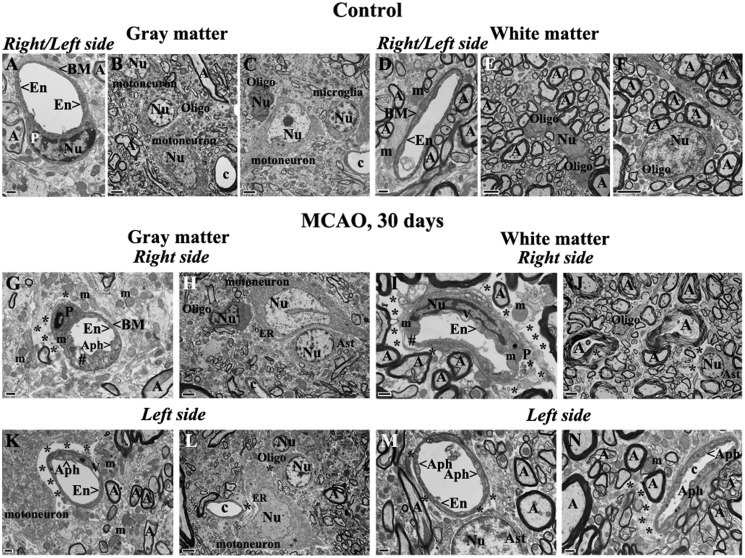
Oligodendrocytes with dark condensed cytoplasm were also detected proximal to edematous spaces near damaged capillaries in gray matter on the right side of the cervical spinal cord 30 days after tMCAO (Fig. 4A). Also, a capillary with 3 layers of ECs and astrocyte end-feet with normal mitochondria was observed on the left cord side of gray matter at same post-tMCAO stage (Fig. 4B). Of note, there was less perivascular edema in this field.
FIGURE 4.
Electron microscope examination of potential repair of microvasculature in the rat cervical spinal cord gray matter at 30 days after tMCAO. (A) Activated-appearing oligodendrocytes characterized by dark condensed cytoplasm are seen near an edematous space and damaged capillary on the right side of cord in gray matter. (B) A capillary with 3 layers of ECs and astrocyte end-feet with normal mitochondria is seen on the left cord side of gray matter. Abbreviations: En, endothelial cell; BM, basement membrane; Ast, astrocyte; m, mitochondrion; c, capillary; A, axon; Oligo, oligodendrocyte. Asterisks in A and B indicate extracellular edema. Scale bars: A = 2 μm; B = 500 nm.
In summary, ultrastructural examination of microvasculature in the cervical spinal cord of rats after 7 (subacute) and 30 (chronic) days right tMCAO insult revealed severe damage of capillary endothelium and pericytes, degeneration of astrocyte end-feet, and extensive perivascular edema in gray and white matter on both sides of the cord. Damaged motor neurons and numerous swollen axons were also observed.
Microvascular Permeability
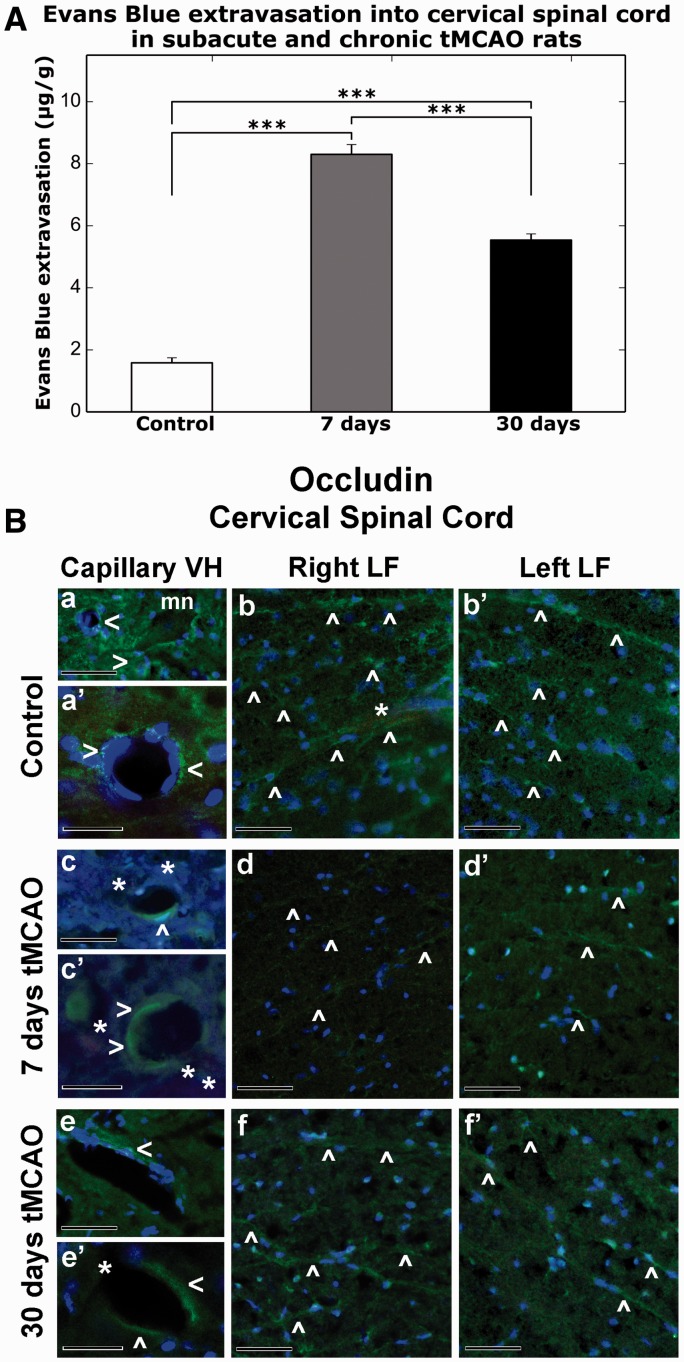
Tissue measurements showed significantly higher EB levels at 7 days (8.30 ± 0.32 μg/g, p < 0.001) and 30 days (5.54 ± 0.20 μg/g, p < 0.001) post-tMCAO versus controls (1.58 ± 0.16 μg/g) (Fig. 5A). Because there were no significant differences in EB extravasation between control rats at 7 days (1.40 ± 0.30 μg/g) and 30 days (1.79 ± 0.16 μg/g), data for controls were pooled. Greater parenchymal EB levels were found at subacute (7 days) versus chronic (30 days) post-ischemic stages (p < 0.001). These results correlated with the EM findings showing ultrastructural abnormalities in capillary endothelia in gray and white matter on both sides of the spinal cord possibly leading to microvascular leakage. Thus, significant vascular leakage in the spinal cord at subacute and chronic ischemia might indicate prolonged BSCB impairment.
FIGURE 5.
Quantitative analysis of Evans blue (EB) parenchymal extravasation and occludin microvascular immunostaining patterns in the rat cervical spinal cord at 7 days and 30 days after tMCAO. (A) Quantitative measurement of parenchymal EB content showed higher extravasated EB levels at 7 days and 30 days post-tMCAO versus control (p < 0.001). Levels at 7 days were greater than at 30 days (***p < 0.001). (B) Immunofluorescence staining for occludin (a, a′) within ventral horn capillaries (green, arrowheads) and (b, b′) in lateral funiculus of both sides of the cervical spinal cord (green, arrowheads) show typical tight junction expression patterns in control rats. Capillaries with strong occludin immunostaining were noted near motor neurons (mn) in ventral horn (a). The EB is within microvessels in white matter lateral funiculus (b, light red, asterisk). At 7 days post-tMCAO, weak occludin immunoreactivity was observed in (c, c′, green, arrowheads) portions of numerous ventral horn capillaries with detection of perivascular EB leakage (light red, asterisks). Occludin immunostaining was identified in only a few microvessels in the lateral funiculus of both sides of the spinal cord in these animals (d, d′, green, arrowheads). At 30 days after tMCAO, limited occludin immunoexpression (e, e′, green, arrowheads) was determined in ventral horn capillaries, which some of them are leaky for EB (light red, asterisks), similar to 7 days post-stroke. Microvessels with enhanced occludin immunostaining in both sides of lateral funiculus areas were noted in these animals similar to controls (f, f″, green, arrowheads). Scale bars: a, b, b′, c, d, d′, e, f, f′ = 50 µm; a′, c′, e′ = 25 µm.
Microvascular Occludin
Microvascular occludin immunostaining demonstrated strong expression of occludin in capillary lumens of ventral horns (Fig. 5B, a, a′) and in numerous microvessels of right and left lateral funiculi (Fig. 5B, b, b′) in controls. EB was visible within microvessels in a white matter lateral funiculus (Fig. 5B, b, asterisk). At 7 days post-tMCAO, weak occludin immunoexpression was observed in portions of numerous ventral horn capillaries showing perivascular EB leakage (Fig. 5B, c, c′, asterisks). In the lateral funiculi of both sides of the spinal cord in these animals, occludin immunostaining was identified in only a few microvessels (Fig. 5B, d, d′). At 30 days after tMCAO, limited occludin immunostaining was seen in ventral horn capillaries, some of which showed EB leakage, similar to 7 days post--infarct findings (Fig. 5B, e, e′). Interestingly, microvessels with enhanced occludin immunostaining in both sides of lateral funiculi areas were noted in these animals, similar to controls (Fig. 5B, f, f′). Thus, the extensive vascular leakage determined via EB extravasation into parenchyma of the cervical spinal cord at subacute and chronic ischemia might result from reduction of occludin “tightness” between ECs, mainly in animals at 7 days post-tMCAO.
Autophagosomes in Spinal Cord Endothelium
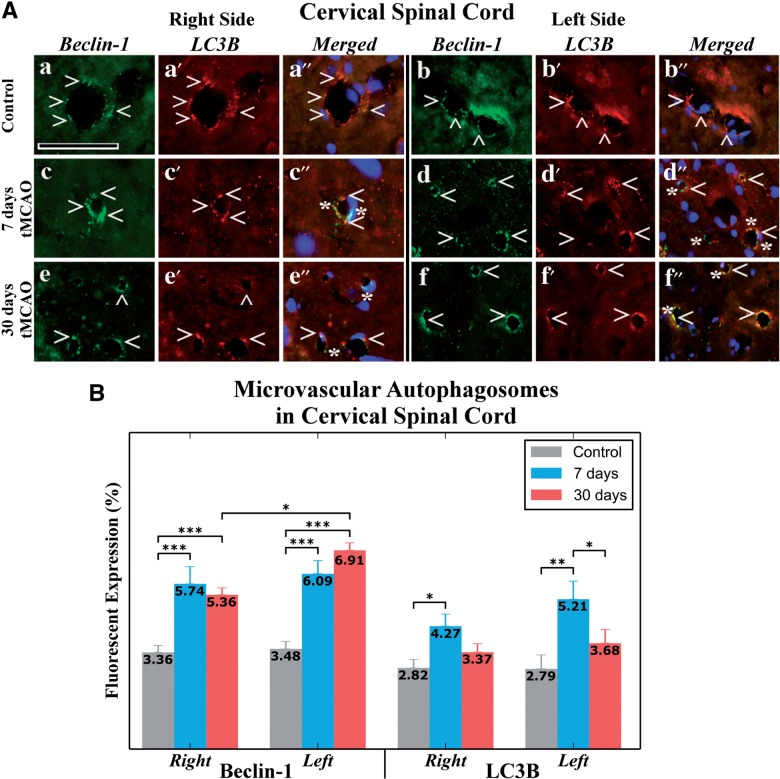
Because autophagosome accumulations were observed by EM in numerous EC capillaries at 7 and 30 days after tMCAO, immunohistochemical analysis of capillary Beclin-1 and LC3B expression and intensity measures were performed. There was a significant increase in capillary Beclin-1 immunoreactivity on the right (7 days: 5.74% ± 0.6%; 30 days: 5.36% ± 0.32%) and left (7 days: 6.09% ± 0.46%; 30 days: 6.91% ± 0.33%) side of the spinal cord (Fig. 6A, c–f, B) versus controls (right side: 3.36% ± 0.24%; left side: 3.48% ± 0.26%) (Fig. 6A, a, b, B) (p < 0.001). Importantly, significantly higher Beclin-1 immunostaining was noted in capillaries on the left versus the right side at 30 days post-tMCAO (p < 0.05). By contrast, significant upregulation of capillary LC3B immunostaining was seen only at 7 days post-tMCAO on the right (p < 0.05, 4.27% ± 0.42%) and left (p < 0.01, 5.21% ± 0.63%) sides versus controls (right: 2.82% ± 0.29%; left: 2.79% ± 0.48%) (Fig. 6A, c′, d′, B). Moreover, higher LC3B fluorescent expression was observed on the left side of ventral horn at 7 days versus 30 days post-tMCAO (p < 0.05). In control animals, LC3B and Beclin-1 expression on both sides of the spinal cord were similar. Comparative reduction of LC3B versus Beclin-1 was identified after 7 and 30 days tMCAO (Fig. 6B); at 30 days post-infarct, LC3B immunostaining was less than half of Beclin-1 on the left side of the cervical spinal cord. In merged Beclin-1/LC3B images representing in controls, double immunostaining demonstrates overlap between these autophagic biomarkers in capillary endothelium in both sides of the cervical spinal cord (Fig. 6A, a″, b″), whereas some capillaries show lack of Beclin-1/LC3B overlay immunoexpression after 7 days (Fig. 6A, c″, d″) and 30 days (Fig. 6A, e″, f″) post-tMCAO. In these merged images, Beclin-1 protein expression is apparent without overlap with LC3B. Because Beclin-1 is an essential protein inducing the autophagy process by autophagosome formation, and LC3B is required for autophagy activity (leading to autolysosome formation for removal of various intracellular components), reduced LC3B processing might indicate impaired autophagy in the capillary endothelium resulting in increased numbers of nonprocessing autophagosomes.
FIGURE 6.
Autophagosome analysis in capillary endothelium of the ventral horn cervical spinal cord in rats 7 and 30 days after tMCAO. (A) Immunofluorescence staining of spinal cord in control rats for Beclin-1 (green, arrowheads) (a, b) and LC3B (red, arrowheads) (a′, b′) showed typical expression patterns in capillary endothelium in both sides of the spinal cord. In merged Beclin-1/LC3B images, double immunostaining demonstrates overlap between these autophagic biomarkers (orange, arrowheads) (a″, b″). At 7 days after tMCAO, extensive Beclin-1 (green, arrowheads) (c, d) and LC3B (red, arrowheads) (c′, d′) staining is shown in capillaries in the right and left sides of the spinal cord. Some capillaries showed lack of Beclin-1/LC3B overlay immunostaining; Beclin-1 protein immunostaining (green, asterisks) is clearly seen in merged images on both sides of the cord (c″, d″). Similarly, greater Beclin-1 (green, arrowheads) (e, f) and (e′, f′) LC3B (red, arrowheads) immunostaining is seen in the right and the left side capillaries 30 days after tMCAO versus controls. In merged images, omitted overlap between Beclin-1 and LC3B immunoexpressions (green, asterisks) was determined in the right (e″) and the left (f″) side capillaries. Nuclei are stained with DAPI in the merged images (a″–f″). Scale bar in a–f″ is 50 µm. (B) Quantitative analysis of Beclin-1 and LC3B immunostaining in capillary endothelium in control rats showed similar patterns on each side. Increases in Beclin-1 immunostaining on the right and left side spinal cord capillaries at 7 and 30 days post-tMCAO was noted versus that in controls (p < 0.001). Higher Beclin-1 immunostaining was noted in capillaries on the left versus the right side at 30 days post-tMCAO (p < 0.05). Nevertheless, there was upregulation of LC3B expression only at 7 days post-tMCAO in capillaries on the right (p < 0.05) and left (p < 0.01) sides versus controls. Higher LC3B expression was detected on the left side of ventral horn at 7 days versus 30 days post-tMCAO (p < 0.05). *p < 0.05; **p < 0.01; ***p < 0.001.
These novel data correlate with the EM observations and suggest that extensive EC autophagosome accumulations in spinal cord capillaries likely affect cell integrity and thus contribute to the BSCB dysfunction.
Spinal Cord Astrocyte Reactivity in tMCAO Rats
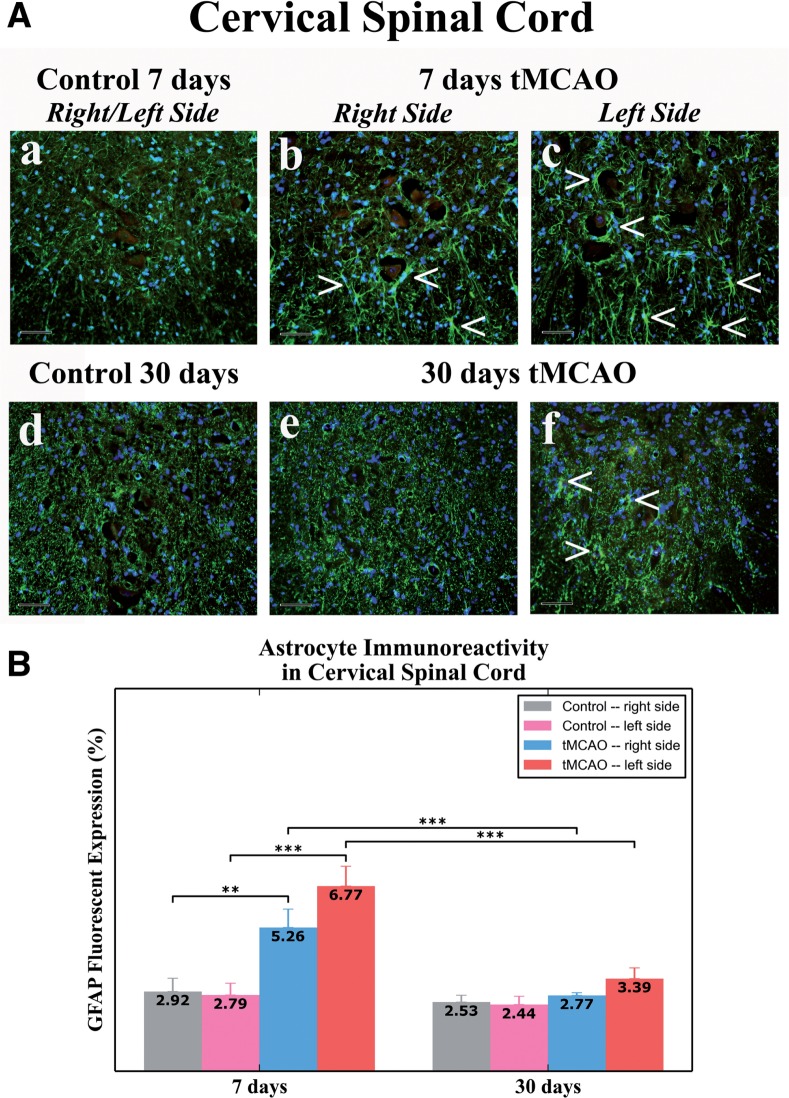
In 7 day post-tMCAO rats, significantly elevated GFAP fluorescence expression was identified in right (5.26%± 0.67%, p < 0.01) and left (6.77% ± 0.73%, p < 0.001) sides of ventral horn gray matter of the cervical spinal cord versus control animals (right: 2.92% ± 0.48%; left: 2.79% ± 0.43%) (Fig. 7A, a–c, B). Although there was higher GFAP immunoreactivity on the left side of the spinal cord, there were no statistical differences compared to the right side. GFAP-positive cells were mostly distinguished proximal to motor neurons in both sides of the cervical spinal cord (Fig. 7A, b, c), likely indicating spinal cord astrocyte reactivity at the subacute stage.
FIGURE 7.
Astrocytes in the cervical spinal cord ventral horn in rats 7 and 30 days after tMCAO. (A) Immunohistochemical analysis of GFAP immunoexpression in the right or left sides of control rats for (a) 7 days and (d) 30 days tMCAO demonstrated normal appearance of parenchymal GFAP positive cells (green). At 7 days after tMCAO, increased GFAP immunoreactivity (green, arrowheads), indicating astrogliosis, was determined in ventral horn parenchyma mostly surrounding motor neurons in both (b) the right and (c) the left side of the spinal cord. At 30 days after tMCAO, greater GFAP fluorescent expression (green, arrowheads) was observed in the (f) panel versus the right (e) side. The nuclei are stained with DAPI; scale bars, 50 µm. (B) Quantitative analysis of GFAP immunostaining in the cervical spinal cord parenchyma of control rats showed a similar extent on both sides. In 7 days post-tMCAO rats, there was an increase of GFAP expression on the right (p < 0.01) and the left (p < 0.001) sides versus controls. There was greater GFAP immunostaining on the left versus the right side of the cord. There were no significant differences between 30 days tMCAO and control rats in GFAP immunoexpression on both sides of the spinal cord, although there was greater GFAP immunostaining on the left side at 7 versus 30 days post-tMCAO. **p < 0.01; ***p < 0.001.
In rats at 30 days post-tMCAO, no significant differences were found in GFAP expression in either right (2.77% ± 0.10%) or left (3.39% ± 0.39%) sides of cervical ventral horn versus controls (right: 2.53% ± 0.25%; left: 2.44% ± 0.30%) (Fig. 7A, d–f, B). However, slightly greater GFAP immunoreactivity was noted in the left side versus the right side (Fig. 7A, e, f). Thus, significant upregulation of GFAP immunoreactivity in the gray matter ventral horn of the cervical spinal cord in tMCAO rats was mainly demonstrated in both sides at subacute (7 days) post-infarct phase, with higher GFAP fluorescent expression in the left side; astrocyte reactivity was also evident in the left side of the spinal cord in rats at 30 days after tMCAO.
Spinal Cord Histology and Stereological Counts of Motor Neurons
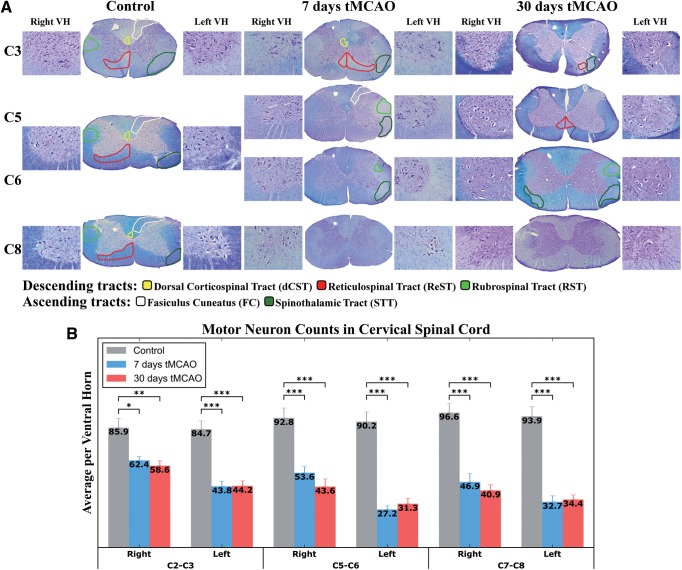
The projections from the brain to the spinal cord or from the spinal cord to the brain consist mainly of 3 descending tracts: dorsal corticospinal tract (dCST), reticulospinal tract (ReST), and the rubrospinal Tract (RST), and 2 ascending tracts: fasciculus cuneatus (FC, tract of Burdach) and the spinothalamic tract (STT) (46, 47) (Fig. 8A). The zones of the descending (motor) tracts indicated on the right side and the ascending (sensory) tracts on the left side of the cervical spinal cord in control rats demonstrated normal myelin staining. In rats at 7 and 30 days after tMCAO, pale areas in white matter spinal cord (ie, weakly stained by LFB) were identified through C3–C6 cervical levels (Fig. 8A). However, at the C8 level, white matter myelin abnormalities were not identified. At 7 days post-tMCAO, there was distinct myelin pallor primarily on the left side of the spinal cord (ie, contralateral to the tMCAO insult) in areas of the descending tracts, that is, in C3, ReST; in C5, C6, RST and the ascending tracts; in C3, STT; in C5, FC, STT; and in C6, STT (Fig. 8A). Some degree of myelin pallor was seen in dCST at C3 level on the right side of the cervical spinal cord, ipsilateral to tMCAO.
FIGURE 8.
Histological analysis of the cervical spinal cord in rats 7 and 30 days after tMCAO. (A) Cervical spinal cord sections were stained with Luxol fast blue (LFB) and Cresyl violet (cv) for analysis of myelin and motor neurons. Zones of the descending tracts are indicated on the right side and the ascending tracts on the left side of the cervical spinal cord in control rats demonstrated normal myelin staining (middle part). At 7 days post-tMCAO, areas of myelin pallor in white matter spinal cord, weakly stained by LFB, were primarily present on the left side of the spinal cord, contralateral to tMCAO insult, of the descending (ReST and RST) and the ascending (STT) tracts. Some degree of demyelination was seen in dCST at C3 level on the right side of the cervical spinal cord, ipsilateral to tMCAO. (right part) In rats after 30 days tMCAO, demyelinated areas were similarly observed in the descending (ReST and RST) and the ascending (FC and STT) tracts on the left side of the spinal cord. Also, ReST and STT were affected on the right side. Abbreviations: descending tracts: dorsal Corticospinal Tract (dCST), Reticulospinal Tract (ReST), Rubrospinal Tract (RST); ascending tracts: Fasciculus Cuneatus (FC, tract of Burdach), Spinothalamic Tract (STT), ventral horn (VH). Mark hole on coronal spinal cord sections indicates the right side. Magnification: coronal spinal cord section is 4×, inserts on both sides of coronal sections are 20×. (B) Stereological motor neuron counts on both the right and left sides of discrete levels of the cervical spinal cord demonstrated substantial cell loss in rats at 7 and 30 days after tMCAO versus controls. Motor neuron numbers were significantly (p < 0.001) decreased on the left side of the spinal cord primarily at C5-C6 and C7-C8 levels in animals at subacute (7 days) and chronic (30 days) tMCAO stages. C2-C3, C5-C6, and C7-C8 are levels of the cervical spinal cord. *p < 0.05; **p < 0.01; ***p < 0.001.
At 30 days post-tMCAO rats, myelin pallor areas were noted in the descending tracts as ReST (C3 and C5) and RST (C6) and the ascending tracts as FC (C5) and STT (C3 and C6) on the left side of the spinal cord (Fig. 8A). Interestingly, ReST and STT were also affected on the right side.
Stereological motor neuron counts in both the right and left sides of discrete levels of the cervical spinal cord demonstrated substantial cell losses in rats at 7 and 30 days after tMCAO versus controls (Fig. 8B). Motor neuron numbers were decreased on the left side of the spinal cord primarily at C5-C6 and C7-C8 levels in animals at subacute (7 days) and chronic (30 days) tMCAO stages (p < 0.001). The reduced appearance of motor neurons was observed in each ventral horn side/level of the cervical spinal cord via Cresyl violet counterstaining of the Nissl substance (Fig. 8A, inserts).
Thus, histological analysis of the cervical spinal cord demonstrated myelin pallor in the descending (ReST and RST) and ascending (STT) tracts, mainly affecting the left side of the spinal cord, contralateral to the tMCAO insult in rats at 7 and 30 days post-tMCAO. However, some degree of myelin pallor was also observed in these animals on the right side of the upper level cervical spinal cord, ipsilateral to tMCAO. Additionally, significant motor neuron loss was determined at different levels of the spinal cord on both sides at subacute (7 days) and chronic (30 days) tMCAO stages.
DISCUSSION
This study focused on BSCB status and related pathological processes in the cervical spinal cord remote from an initial brain ischemic insult at 2 time points following experimental focal cerebral ischemia in rats. BSCB damage was detected not only in the gray matter of the cervical spinal cord but also in white matter on both sides of the cord at both time points. The BSCB pathological alterations included: (1) vacuolated ECs containing large autophagosomes, (2) pericyte degeneration with enlarged mitochondria, (3) astrocyte end-feet degeneration and extensive perivascular edema, (4) damaged motor neurons and swollen axons with myelin unraveling, (5) EB extravasation, (6) reduction of tight junction occludin immunoreactivity, (7) upregulation of microvascular Beclin-1 and LC3B immunoreactivity at 7 days and reduction of LC3B versus Beclin-1 immunoreactivity at 30 days, (8) parenchymal astrogliosis, (9) myelin pallor in descending and ascending tracts, and (10) motor neuron loss. These alterations in the cervical spinal cord indicate pervasive and long-lasting BSCB damage that likely would severely affect spinal cord function.
Substantial vascular damage represents a major pathologic feature of subacute and chronic tMCAO. The findings of vascular damage in the cervical spinal cord remote from the focal cerebral ischemia site indicate ongoing pathological vascular changes in association with subacute and chronic ischemic diaschisis. The spinal cord capillary leakage and ultrastructural alterations induced by focal cerebral ischemia suggest that there is an extended period of microvascular permeability, as we identified in brain regions distant from initial tMCAO damage (29, 30); it is thus possible that widespread blood-CNS barrier impairment persists following cerebral ischemic events. Also, EB extravasation may result from decreased tight junction protein expression, as has been shown for cerebral microvessels under hypoxic conditions (48–50). Because there are no reports regarding vascular tight junction integrity in the spinal cord after tMCAO, we analyzed the immunoreactivity of occludin, one of several tight junction proteins, and demonstrated reduced immunoexpression within capillaries in the ventral horn and in microvessels of lateral funiculus of both sides of the cervical spinal cord, mainly in animals at 7 days post-tMCAO. Lost tightness between ECs might account for paracellular vascular leakage, as determined by EB extravasation into the spinal cord parenchyma.
Additionally, studies on rabbit spinal cord ischemia have demonstrated a decline in motor function, edema formation in gray and white matter and increased numbers of necrotic neurons in the lumbar spinal cord during reperfusion after an initial ischemic insult via aortic occlusion (51–53). Moreover, blood flow reduction and severe vascular permeability for EB-albumin were noted in this rabbit model, suggesting progressive BSCB breakdown (52).
In this study, autophagosome accumulation within spinal cord capillary endothelium was identified at the ultrastructural level and was confirmed by upregulation of immunoreactivity of the autophagy biomarker Beclin-1 in microvessels on both sides of the cervical spinal cord at both post-tMCAO time points. Importantly, significantly higher Beclin-1 expression was noted in the left (contralateral) versus right side of spinal cord in chronic (30 days) tMCAO rats. Immunoreactivity of another autophagy marker, LC3B, was increased on both sides of the spinal cord at 7 days post-tMCAO compared to controls but there were reductions of LC3B on both sides of the cord in rats at 30 days after tMCAO. This was verified via double immunostaining for both autophagic biomarkers. The lack of overlap between Beclin-1 and LC3B expression was evident in numerous capillary endothelia at both time points. Beclin-1 is an essential protein inducing the autophagy process by autophagosome formation (54, 55). LC3B, as one of several members of the mammalian LC3 family, which is tightly associated with the autophagosome membranes, is indispensable for autophagic activity leading to autolysosome formation for removal of various intracellular components (56–58). Therefore, it is possible that lower LC3B expression indicates impaired autophagy processing in the endothelium via diminished ability of the autophagosome to fuse with the lysosome, resulting in increased numbers of nonprocessing autophagosomes. Although autophagy plays an important role in cell survival by degradation of cytoplasmic components through the autophagosome-lysosomal pathway (59), excessive autophagic processes might induce cellular death (60). Together, our data demonstrated for the first time upregulation of Beclin-1, indicating intensified autophagy, and decreased LC3B protein expression at the later stage, suggesting an impaired autophagy process in post-tMCAO spinal cord capillaries. The endothelial autophagosome accumulation might be a result of blocking traffic to lysosomes without concomitant changes in autophagosome biogenesis, resulting in a reduction of the degradative activity of cytoplasmic components needed to maintain cell survival. Together with our EM findings these data suggest that EC autophagosome accumulations in spinal cord capillaries likely affect cell integrity that may be impaired in spinal cord capillaries following focal cerebral ischemia. Because many EC are unable to self-repair through autophagocytosis, they may die and slough off the capillaries, contributing to the impaired BSCB demonstrated by EB extravasation.
Additionally, our results showed a significant increase of GFAP immunoreactivity proximate to motor neurons on both sides of the spinal cord in rats at 7 days post-stroke with higher expression in the left side; astrocyte reactivity was also present at 30 days post-tMCAO. These results indicating reactive astrogliosis bilaterally in the cervical spinal cord of rats in subacute tMCAO and unilaterally (left side) in chronic tMCAO rats likely point to prolonged post-ischemic inflammation. Inflammation has been shown to play a major role in the pathogenesis of ischemic stroke (4, 61); activated astrocytes (and microglia) contribute to neuroinflammation by the secretion of various proinflammatory cytokines.
Wu and Ling showed increased activated microglia in the lumbar spinal cord, specifically in the dorsal and ventral horns contralateral to focal cerebral ischemia, in rats with permanent MCAO beginning at 24 hours and transforming into amoeboid form at day 3 post-infarct (31). Activated microglia emerged near degenerated motoneurons in the dorsal lumbar spinal cord, while ventral horn motoneurons remained ultrastructurally intact. These authors also showed that hypertrophied astrocytes appeared at 7 days post-MCAO, replacing perineuronal microglia (32). Similarly, cortical lesions associated with contralateral lumbar spinal cord microglia priming lacked significant reactive astrogliosis or lower motor neuron degeneration in a tMCAO mouse model at 24 and 72 hours post-reperfusion (33). The glial cell response in the spinal cord was accompanied by increased expression of proinflammatory cytokines and markers of oxidative stress at 24–72 hours after permanent MCAO (32, 34). Inflammatory changes in the spinal cord distant from the initial focal ischemic insult might also be involved in the pathogenesis of secondary neuronal damage linked to antero- and retrograde degeneration (35). However, these studies primarily focused on the lumbar spinal cord at acute or early subacute stages of MCAO. Although the microglial response was not examined in this study, our results indicate substantial astrogliosis in the cervical spinal cord mainly in rats at 7 days after tMCAO; these might lead to motor neuron damage and BSCB impairment. These data correlated with our EM findings showing astrocyte end-feet degeneration and extensive perivascular edema as well as degenerated motor neurons with dilated endoplasmic reticulum not only at 7 but also at 30 days post-tMCAO.
Potentially long-lasting BSCB damage might affect post-stroke motor neuron survival. We demonstrated significant motor neuron loss at different levels of the cervical spinal cord on both sides of the ventral horn at subacute (7 days) and chronic (30 days) tMCAO stages. Moreover, histological analysis of the cervical spinal cord demonstrated areas of myelin pallor of the descending (ReST and RST) and ascending (STT) tracts, mainly affecting the left side of the spinal cord, contralateral to the tMCAO, in the post-tMCAO rats. Some degree of myelin pallor was also observed in these animals on the right side of the upper level cervical spinal cord, ipsilateral to tMCAO. This may represent secondary demyelination.
Although the cortex and striatum are the areas most affected by MCAO (62, 63), some studies have shown extensive neuronal post-ischemic damage in the thalamus, brainstem, and spinal cord accompanied by degeneration of corticofugal axonal fibers extending caudally along descending tracts indicating remote changes (31, 64–66). Wu and Ling suggested that selective lesions of dorsal horn neurons in the lumbar spinal cord in rats with permanent MCAO might be due to their trans-synaptic de-afferentation, mediated by ischemic degeneration of the descending axons of the corticospinal tract (31). Another study by Iizuka et al (64) using the Fink-Heimer silver staining method to trace degenerating axons showed degenerate corticospinal axons in the ventral portion of contralateral dorsal funiculus at low cervical (C7) and lumbar (L6) segments in rats at 1–6 weeks after MCAO. However, our results demonstrated myelin pallor of descending dCST on the right (ipsilateral) side of the cervical (C3) spinal cord in rats at 7 days post-tMCAO, which contrasts with results from the above mentioned study. Possibly, the BBB alterations we determined at 7 days after tMCAO (29) in contralateral brain areas (striatum, motor and somatosensory cortices) from tMCAO insult induce damage of descending corticospinal fibers, which cross over to ipsilateral spinal cord. However, it is unclear why demyelinated dCST was not observed on the contralateral spinal cord side in subacute tMCAO rats or why there was no evidence of myelin abnormality of this tract in the cervical spinal cord at 30 days after tMCAO. Although the crossed corticospinal tract is located in the ventral part of the dorsal column of the spinal cord of most mammals (67), no topographical organization of the CST has been noted in the cervical spinal cord of the adult rat (68). Interestingly, Liu et al (69) noted that normal rats had predominantly unilateral innervation of CST and corticorubral tract axons and that bilateral innervation occurred through axonal sprouting of the uninjured CST and corticorubral tract in rats 28 days after permanent MCAO. The authors suggested that axonal restructuring occurred as compensatory innervation to “rewire the denervated spinal cord”. Also, anterograde axonal degeneration of different descending tracts might take place over dCST damage in the cervical spinal cord in rats at 7 and 30 days after tMCAO. Although Iizuka et al (64) showed that no degenerated axons were detected in other white matter areas of the spinal cord besides corticospinal axons in 1–6 weeks post-tMCAO rats, our analysis demonstrated myelin abnormalities in areas of the descending ReST and RST, mainly affecting the left side of the cord at 7 days and 30 days post-tMCAO. Consistent with the RST projections from the brain to the spinal cord (47, 70, 71), axon degeneration likely moves from the damaged hemisphere, crossing over at the tegmental decussation, and permeating the contralateral left side of the spinal cord as shown here.
Compared to the dCST and RST, ReST connectivity is more complex. It has been shown that cortical neurons bilaterally target the reticular formation and reticulospinal neurons connect with ipsilateral motor neurons directly or indirectly via segmental interneurons (72–74). Because there is no evidence of direct corticomotor neuronal synaptic connections in the cervical rat spinal cord (75), Umeda et al (76) investigated descending pathways involved in functional connections with motor neurons using a hemidecorticate rat model. They showed at least 2 indirect descending corticomotor pathways: the cortico-reticulo-spinal and cortico-spinal pathways (76). In hemidecorticate rats, CST originated from the undamaged sensorimotor cortex, connecting to motor neurons on the opposite spinal cord side in addition to the normal descending cortical motor pathway. Also, these connections were mediated via spinal interneurons and reticulospinal neurons. As we showed in this study demyelinated ReST on both contralateral and ipsilateral sides of the cervical spinal cord in 7 and 30 days post-tMCAO rats, it is possible that axonal degeneration is spreading from the tMCAO damaged hemisphere, passing through both sides of the reticular formation, and ultimately causing secondary demyelination in both the left and right ReST. Subacute and chronic cerebral diaschisis likely contributes to the myelin abnormality observed.
With respect to the ascending pathways, it is possible that axonal degeneration with secondary demyelination travels retrograde from the cerebral hemispheres towards the cell bodies of the STT and FC in the spinal cord. Iizuka et al (65) demonstrated that MCAO of the somatosensory cortex in rats caused retrograde neuronal degeneration in the thalamus, possibly as a secondary effect from thalamocortical fiber damage in the infarcted region. Interestingly, the STT in this study was the most consistently abnormal tract following tMCAO; the potential cause of this secondary demyelination may originate in the cerebral hemispheres and travel to cervical STT axons via the thalamus. STT abnormalities were observed on both the contralateral and ipsilateral sides of the cervical (C6) spinal cord at 30 days post-tMCAO rats. Right STT myelin loss may indicate ipsilateral progression of degeneration from the right tMCAO hemisphere (77), whereas left STT demyelination may represent ipsilateral damage flow from the left hemisphere damaged via diaschisis (29, 30). However, it is unclear why more STT abnormalities were observed on the left side of the spinal cord contralateral to the tMCAO hemisphere versus the ipsilateral side of the cord.
With respect to the FC (tract of Burdach), axonal damage and demyelination may also be progressing in the retrograde direction from the brain to the spinal cord. The FC contralateral to the tMCAO hemisphere showed myelin pallor at the C5 level at both time points. Because FC fibers cross to the opposite side as they ascend to the thalamus (78), it is possible that a right tMCAO hemisphere triggers axonal degeneration in a retrograde fashion down ascending axons and decussates, which results in secondary demyelination in the FC on the left side of the cervical spinal cord. Together, demyelinated descending and ascending pathways, as indicated by histological analyses, were supported by our ultrastructural examination showing damaged motor neurons and numerous swollen axons in the cervical spinal cord of rats at both time points. Future studies are needed to confirm axonal degeneration projections using anterograde and retrograde tracing systems.
The appearance of oligodendrocytes with dark cytoplasm and multilayers of ECs in capillary surrounded by morphologically normal astrocyte end-feet were noted at 30 days post-tMCAO; these findings may indicate spontaneous reparative processes. Because our investigations on post-stroke pathological microvascular alterations including BSCB have just been initiated, many questions remain. Specifically, protein expression for autophagosomes and tight junctions identified in our study by immunofluorescence techniques should be confirmed via additional assays such as Western blot. Also, behavioral tests of motor function in post-stroke animals in correlation with BSCB condition are needed. Additionally, the early post-ischemic timing of BSCB damage needs to be defined. These and other questions will be answered in our future studies.
In summary, we show altered BSCB integrity in subacute and chronic post-ischemic rats on both sides of the cervical spinal cord, that is, areas remote from the initial cerebral ischemic insult, indicating that spinal cord diaschisis occurs at early and remains extended up to 30 days post-ischemia. The widespread microvascular impairment in the gray and white matter of the cervical spinal cord aggravated motor neuron deterioration and could have to motor dysfunction following cerebral ischemia. More structural capillary alterations were observed on the left side of the spinal cord, likely accompanied and/or due to denervation by the damaged descending tracts crossing over from the tMCAO injured hemisphere. Importantly, substantial right side microvessel pathology could also indicate damaged neuronal connective pathways from the opposite hemisphere, initially unaffected by tMCAO. Hence, BSCB impairment in ischemic stroke may have significant input on disease pathology, indicating that spinal cord diaschisis should be considered in revised disease pathology and therapeutic approaches targeting BSCB repair in stroke.
ACKNOWLEDGMENTS
We gratefully acknowledge Maria C.O. Rodrigues, MD, PhD, from the Department of Internal Medicine, Ribeirão Preto School of Medicine, University of Sao Paulo, Sao Paulo, Brazil, and Diana G. Hernandez-Ontiveros, PhD. from USF, for help in performing the EB assay.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: A report from the American Heart Association. Circulation 2015;131:e29–322 [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: A report from the American Heart Association. Circulation 2013;127:e6–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly-Hayes M, Beiser A, Kase CS, et al. The influence of gender and age on disability following ischemic stroke: The Framingham study. J Stroke Cerebrovasc Dis 2003;12:119–26 [DOI] [PubMed] [Google Scholar]
- 4.del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thromb Res 2000;98:73–81 [DOI] [PubMed] [Google Scholar]
- 5.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab 2003;23:879–94 [DOI] [PubMed] [Google Scholar]
- 6.Persidsky Y, Ramirez SH, Haorah J, et al. Blood-brain barrier: Structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol 2006;1:223–36 [DOI] [PubMed] [Google Scholar]
- 7.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis 2008;32:200–19 [DOI] [PubMed] [Google Scholar]
- 8.Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: Critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis 2010;38:376–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dankbaar JW, Hom J, Schneider T, et al. Dynamic perfusion-CT assessment of early changes in blood brain barrier permeability of acute ischaemic stroke patients. J Neuroradiol 2011;38:161–6 [DOI] [PubMed] [Google Scholar]
- 10.Preston E, Sutherland G, Finsten A. Three openings of the blood-brain barrier produced by forebrain ischemia in the rat. Neurosci Lett 1993;149:75–8 [DOI] [PubMed] [Google Scholar]
- 11.Yang GY, Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke 1994;25:1658–64 discussion 1664–5 [DOI] [PubMed] [Google Scholar]
- 12.Belayev L, Busto R, Zhao W, et al. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res 1996;739:88–96 [DOI] [PubMed] [Google Scholar]
- 13.Kuroiwa T, Ting P, Martinez H, et al. The biphasic opening of the blood-brain barrier to proteins following temporary middle cerebral artery occlusion. Acta Neuropathol 1985;68:122–9 [DOI] [PubMed] [Google Scholar]
- 14.Strbian D, Durukan A, Pitkonen M, et al. The blood-brain barrier is continuously open for several weeks following transient focal cerebral ischemia. J Neurosci 2008;153:175–81 [DOI] [PubMed] [Google Scholar]
- 15.Abo-Ramadan U, Durukan A, Pitkonen M, et al. Post-ischemic leakiness of the blood-brain barrier: A quantitative and systematic assessment by Patlak plots. Exp Neurol 2009;219:328–33 [DOI] [PubMed] [Google Scholar]
- 16.Gerriets T, Walberer M, Ritschel N, et al. Edema formation in the hyperacute phase of ischemic stroke. Laboratory investigation. J Neurosurg 2009;111:1036–42 [DOI] [PubMed] [Google Scholar]
- 17.Dobkin JA, Levine RL, Lagreze HL, et al. Evidence for transhemispheric diaschisis in unilateral stroke. Arch Neurol 1989;46:1333–6 [DOI] [PubMed] [Google Scholar]
- 18.Baron JC. Pathophysiology of acute cerebral ischemia: PET studies in humans. Cerebrovasc Dis 1991;1:22–31 [Google Scholar]
- 19.Andrews RJ. Transhemispheric diaschisis. A review and comment. Stroke 1991;22:943–9 [DOI] [PubMed] [Google Scholar]
- 20.González-Aguado E, Martí-Fábregas J, Martí-Vilalta JL. [The phenomenon of diaschisis in cerebral vascular disease]. Rev Neurol 2000;30:941–5 [PubMed] [Google Scholar]
- 21.Meyer JS, Obara K, Muramatsu K. Diaschisis. Neurol Res 1993;15:362–6 [DOI] [PubMed] [Google Scholar]
- 22.Nguyen DK, Botez MI. Diaschisis and neurobehavior. Can J Neurol Sci 1998;25:5–12 [DOI] [PubMed] [Google Scholar]
- 23.Takasawa M, Watanabe M, Yamamoto S, et al. Prognostic value of subacute crossed cerebellar diaschisis: Single-photon emission CT study in patients with middle cerebral artery territory infarct. Am J Neuroradiol 2002;23:189–93 [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Karonen JO, Nuutinen J, et al. Crossed cerebellar diaschisis in acute ischemic stroke: A study with serial SPECT and MRI. J Cereb Blood Flow Metab 2007;27:1724–32 [DOI] [PubMed] [Google Scholar]
- 25.Seitz RJ, Azari NP, Knorr U, et al. The role of diaschisis in stroke recovery. Stroke 1999;30:1844–50 [DOI] [PubMed] [Google Scholar]
- 26.Reinecke S, Lutzenburg M, Hagemann G, et al. Electrophysiological transcortical diaschisis after middle cerebral artery occlusion (MCAO) in rats. Neurosci Lett 1999;261:85–8 [DOI] [PubMed] [Google Scholar]
- 27.Neumann-Haefelin T, Witte OW. Periinfarct and remote excitability changes after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab 2000;20:45–52 [DOI] [PubMed] [Google Scholar]
- 28.Carmichael ST, Tatsukawa K, Katsman D, et al. Evolution of diaschisis in a focal stroke model. Stroke 2004;35:758–63 [DOI] [PubMed] [Google Scholar]
- 29.Garbuzova-Davis S, Rodrigues MCO, Hernandez-Ontiveros DG, et al. Blood-brain barrier alterations provide evidence of subacute diaschisis in an ischemic stroke rat model. PLoS One 2013;8:e63553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garbuzova-Davis S, Haller E, Williams SN, et al. Compromised blood-brain barrier competence in remote brain areas in ischemic stroke rats at chronic stage. J Comp Neurol 2014;522:3120–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu YP, Ling EA. Transsynaptic changes of neurons and associated microglial reaction in the spinal cord of rats following middle cerebral artery occlusion. Neurosci Lett 1998;256:41–4 [DOI] [PubMed] [Google Scholar]
- 32.Wu YP, Ling EA. Induction of microglial and astrocytic response in the adult rat lumbar spinal cord following middle cerebral artery occlusion. Exp Brain Res 1998;118:235–42 [DOI] [PubMed] [Google Scholar]
- 33.Moisse K, Welch I, Hill T, et al. Transient middle cerebral artery occlusion induces microglial priming in the lumbar spinal cord: A novel model of neuroinflammation. J Neuroinflam 2008;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu D, Ng Y-K, Gan P, et al. Permanent occlusion of the middle cerebral artery upregulates expression of cytokines and neuronal nitric oxide synthase in the spinal cord and urinary bladder in the adult rat. Neuroscience 2004;125:819–31 [DOI] [PubMed] [Google Scholar]
- 35.Block F, Dihné M, Loos M. Inflammation in areas of remote changes following focal brain lesion. Prog Neurobiol 2005;75:342–65 [DOI] [PubMed] [Google Scholar]
- 36.Borlongan CV, Hadman M, Sanberg CD, et al. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 2004;35:2385–9 [DOI] [PubMed] [Google Scholar]
- 37.Tajiri N, Acosta S, Glover LE, et al. Intravenous grafts of amniotic fluid-derived stem cells induce endogenous cell proliferation and attenuate behavioral deficits in ischemic stroke rats. PLoS One 2012;7:e43779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsukawa N, Yasuhara T, Hara K, et al. Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci 2009;10:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yasuhara T, Matsukawa N, Hara K, et al. Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem Cells Dev 2009;18:1501–14 [DOI] [PubMed] [Google Scholar]
- 40.Borlongan CV, Kaneko Y, Maki M, et al. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev 2010;19:439–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson C, Paxinos G, Kayalioglu G, et al. Atlas of the rat spinal cord In: Watson C, Paxinos G, Kayalioglu G, editors. The Spinal Cord: A Christopher and Dana Reeve Foundation Text and Atlas. Cambridge, Massachusetts: Academic Press; 2009. p. 238–307 [Google Scholar]
- 42.Borlongan CV, Lind JG, Dillon-Carter O, et al. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res 2004;1010:108–16 [DOI] [PubMed] [Google Scholar]
- 43.Ay I, Francis JW, Brown RH., Jr. VEGF increases blood-brain barrier permeability to Evans blue dye and tetanus toxin fragment C but not adeno-associated virus in ALS mice. Brain Res 2008;1234:198–205 [DOI] [PubMed] [Google Scholar]
- 44.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec 1991;231:482–97 [DOI] [PubMed] [Google Scholar]
- 45.Morganti JM, Nash KR, Grimmig BA, et al. The soluble isoform of CX3CL1 is necessary for neuroprotection in a mouse model of Parkinson’s disease. J Neurosci 2012;32:14592–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kayalioglu G. Projections from the spinal cord to the brain In: Watson C, Paxinos G, Kayalioglu G, editors. The Spinal Cord: A Christopher and Dana Reeve Foundation Text and Atlas. Cambridge, Massachusetts: Academic Press; 2009. p. 148–67 [Google Scholar]
- 47.Watson C, Harvey, Alan R. Projections from the brain to the spinal cord In: Watson C, Paxinos G, Kayalioglu G, editors. The Spinal Cord: A Christopher and Dana Reeve Foundation Text and Atlas. Cambridge, Massachusetts: Academic Press; 2009. p. 168–79 [Google Scholar]
- 48.Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol 2002;282:H1485–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koto T, Takubo K, Ishida S, et al. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol 2007;170:1389–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kago T, Takagi N, Date I, et al. Cerebral ischemia enhances tyrosine phosphorylation of occludin in brain capillaries. Biochem Biophys Res Commun 2006;339:1197–203 [DOI] [PubMed] [Google Scholar]
- 51.Jacobs TP, Shohami E, Baze W, et al. Deteriorating stroke model: Histopathology, edema, and eicosanoid changes following spinal cord ischemia in rabbits. Stroke 1987;18:741–50 [DOI] [PubMed] [Google Scholar]
- 52.Jacobs TP, Kempski O, McKinley D, et al. Blood flow and vascular permeability during motor dysfunction in a rabbit model of spinal cord ischemia. Stroke 1992;23:367–73 [DOI] [PubMed] [Google Scholar]
- 53.DeGirolami U, Zivin JA. Neuropathology of experimental spinal cord ischemia in the rabbit. J Neuropathol Exp Neurol 1982;41:129–49 [DOI] [PubMed] [Google Scholar]
- 54.Hsieh Y-C, Athar M, Chaudry IH. When apoptosis meets autophagy: Deciding cell fate after trauma and sepsis. Trends Mol Med 2009;15:129–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Itakura E, Mizushima N. Atg14 and UVRAG: Mutually exclusive subunits of mammalian Beclin 1-PI3K complexes. Autophagy 2009;5:534–6 [DOI] [PubMed] [Google Scholar]
- 56.Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000;19:5720–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu J, Dang Y, Su W, et al. Molecular cloning and characterization of rat LC3A and LC3B–two novel markers of autophagosome. Biochem Biophys Res Commun 2006;339:437–42 [DOI] [PubMed] [Google Scholar]
- 58.Hansen TE, Johansen T. Following autophagy step by step. BMC Biol 2011;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uchiyama Y, Shibata M, Koike M, et al. Autophagy-physiology and pathophysiology. Histochem Cell Biol 2008;129:407–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reggiori F, Klionsky DJ. Autophagy in the eukaryotic cell. Eukaryotic Cell 2002;1:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol 2006;66:232–45 [DOI] [PubMed] [Google Scholar]
- 62.Nagasawa H, Kogure K. Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke 1989;20:1037–43 [DOI] [PubMed] [Google Scholar]
- 63.Popp A, Jaenisch N, Witte OW, et al. Identification of ischemic regions in a rat model of stroke. PLoS One 2009;4:e4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iizuka H, Sakatani K, Young W. Corticofugal axonal degeneration in rats after middle cerebral artery occlusion. Stroke 1989;20:1396–402 [DOI] [PubMed] [Google Scholar]
- 65.Iizuka H, Sakatani K, Young W. Neural damage in the rat thalamus after cortical infarcts. Stroke 1990;21:790–4 [DOI] [PubMed] [Google Scholar]
- 66.Dihné M, Grommes C, Lutzenburg M, et al. Different mechanisms of secondary neuronal damage in thalamic nuclei after focal cerebral ischemia in rats. Stroke 2002;33:3006–11 [DOI] [PubMed] [Google Scholar]
- 67.Nudo RJ, Masterton RB. Descending pathways to the spinal cord, III: Sites of origin of the corticospinal tract. J Comp Neurol 1990;296:559–83 [DOI] [PubMed] [Google Scholar]
- 68.Jeffery ND, Fitzgerald M. Lack of topographical organisation of the corticospinal tract in the cervical spinal cord of the adult rat. Brain Res 1999;833:315–8 [DOI] [PubMed] [Google Scholar]
- 69.Liu Z, Li Y, Qu R, et al. Axonal sprouting into the denervated spinal cord and synaptic and postsynaptic protein expression in the spinal cord after transplantation of bone marrow stromal cell in stroke rats. Brain Res 2007;1149:172–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Antal M, Sholomenko GN, Moschovakis AK, et al. The termination pattern and postsynaptic targets of rubrospinal fibers in the rat spinal cord: A light and electron microscopic study. J Comp Neurol 1992;325:22–37 [DOI] [PubMed] [Google Scholar]
- 71.Raineteau O, Fouad K, Bareyre FM, et al. Reorganization of descending motor tracts in the rat spinal cord. Eur J Neurosci 2002;16:1761–71 [DOI] [PubMed] [Google Scholar]
- 72.Alstermark B, Ogawa J, Isa T. Lack of monosynaptic corticomotoneuronal EPSPs in rats: Disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J Neurophysiol 2004;91:1832–9 [DOI] [PubMed] [Google Scholar]
- 73.Newman DB, Hilleary SK, Ginsberg CY. Nuclear terminations of corticoreticular fiber systems in rats. Brain Behav Evol 1989;34:223–64 [DOI] [PubMed] [Google Scholar]
- 74.Jankowska E, Hammar I, Slawinska U, et al. Neuronal basis of crossed actions from the reticular formation on feline hindlimb motoneurons. J Neurosci 2003;23:1867–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang H-W, Lemon RN. An electron microscopic examination of the corticospinal projection to the cervical spinal cord in the rat: Lack of evidence for cortico-motoneuronal synapses. Exp Brain Res 2003;149:458–69 [DOI] [PubMed] [Google Scholar]
- 76.Umeda T, Takahashi M, Isa K, et al. Formation of descending pathways mediating cortical command to forelimb motoneurons in neonatally hemidecorticated rats. J Neurophysiol 2010;104:1707–16 [DOI] [PubMed] [Google Scholar]
- 77.Namjoshi DR, McErlane SA, Taepavarapruk N, et al. Network actions of pentobarbital in the rat mesopontine tegmentum on sensory inflow through the spinothalamic tract. J Neurophysiol 2009;102:700–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feldman SG, Kruger L. An axonal transport study of the ascending projection of medial lemniscal neurons in the rat. J Comp Neurol 1980;192:427–54 [DOI] [PubMed] [Google Scholar]