Abstract
Saccular intracranial aneurysm (sIA) aneurysm causes intracranial hemorrhages that are associated with high mortality. Lipid accumulation and chronic inflammation occur in the sIA wall. A major mechanism for lipid clearance from arteries is adenosine triphosphate-binding cassette A1 (ABCA1)-mediated lipid efflux from foam cells to apolipoprotein A-I (apoA-I). We investigated the association of wall degeneration, inflammation, and lipid-related parameters in tissue samples of 16 unruptured and 20 ruptured sIAs using histology and immunohistochemistry. Intracellular lipid accumulation was associated with wall remodeling (p = 0.005) and rupture (p = 0.020). Foam cell formation was observed in smooth muscle cells, in addition to CD68- and CD163-positive macrophages. Macrophage infiltration correlated with intracellular lipid accumulation and apolipoproteins, including apoA-I. ApoA-I correlated with markers of lipid accumulation and wall degeneration (p = 0.01). ApoA-I-positive staining colocalized with ABCA1-positive cells particularly in sIAs with high number of smooth muscle cells (p = 0.003); absence of such colocalization was associated with wall degeneration (p = 0.017). Known clinical risk factors for sIA rupture correlated inversely with apoA-I. We conclude that lipid accumulation associates with sIA wall degeneration and risk of rupture, possibly via formation of foam cells and subsequent loss of mural cells. Reduced removal of lipids from the sIA wall via ABCA1-apoA-I pathway may contribute to this process.
Keywords: Apolipoproteins, Atherosclerosis, Inflammation, Intracranial Aneurysm, Lipid, Smooth Muscle Cell.
INTRODUCTION
Saccular intracranial aneurysms (sIAs) are pouch-like dilatations of large cerebral arteries that may undergo rupture causing devastating intracranial hemorrhages, which despite modern neurointensive therapy, are associated with up to a 40%–50% total mortality (1). Unruptured sIAs are relatively common (2%–3% prevalence) (2, 3). The incidence of hemorrhage from sIA is only 10/100 000 in North America and in most European countries, whereas it is twice as high in Finland (4). Because of the high morbidity and mortality associated with sIA rupture, most unruptured sIAs are treated before rupture occurs. All sIAs do not, however, rupture even during a lifelong follow-up (5). Because the surgical clipping of unruptured sIAs is associated with significant risks, (5%–7% morbidity, 1%–2% mortality (6)), it would be extremely important to identify rupture-prone sIAs from those that do not require intervention.
Chronic inflammation, loss of smooth muscle cells (SMCs), and degeneration of the extracellular matrix are the main characteristics of a rupture-prone sIA wall (7–13). Prior studies suggest that lipid accumulation and oxidative stress are related to and possibly a cause of the degenerative remodeling of the sIA wall (7, 11, 14, 15). Lipid accumulation may trigger cell death and inflammation in the sIA wall (7, 11, 14).
We have previously shown that sIA walls contain apolipoprotein B-100 (apoB-100) (14), which reflects the presence of very low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), or low-density lipoproteins (LDL) (16, 17). These lipoprotein particles transport lipids from the circulation into the vessel walls. Saccular IA wall contains also hydroxynonenal (HNE), reflecting the presence of oxidized lipids (14, 18), and adipophilin, a marker for intracellular lipid accumulation (14, 19). Our prior data suggest that impaired function of the sIA wall endothelium as a barrier to circulating apoB-100 lipoproteins increases lipid accumulation in the sIA wall (7, 14).
In addition to increased influx of lipids contained in apoB-100 lipoproteins, impaired clearance mechanisms of the retained lipids may contribute to their accumulation in the sIA wall. High-density lipoprotein (HDL)-mediated cellular lipid release is considered to be the main clearance mechanism of lipids from the vessel wall (16, 17). Apolipoprotein A-I (apoA-I) is the major protein of HDL particles. By interacting with the adenosine triphosphate-binding cassette A1 (ABCA1) transporter at the surface of the foam cells, apoA-I induces efflux of excess cholesterol from these cells to HDL particles. HDL particles then carry the released cholesterol from the vascular wall back to the liver for excretion from the body; this occurs along a multistep pathway called the reverse cholesterol transport (20). Therefore, the apoA-I-dependent induction of cholesterol efflux from intracellular stores in foam cells macrophage origin or SMC origin, that is, the initial steps occurring in the arterial wall, are critical for the anti-atherosclerotic activity of HDL (21). Apolipoprotein E (apoE) (22), a structural component of apoB-100-containing lipoproteins, can be present also in a small fraction of HDL particles and, by interacting with arterial proteoglycans, it may cause HDL retention in the vessel wall (23).
Here, we studied the association of lipid accumulation and degenerative sIA wall remodeling with the presence of apoA-I containing lipoprotein particles and expression of ABCA1. Our hypothesis was that impaired clearance of lipids via ABCA1 and HDL particles may contribute to the degenerative remodeling of the sIA wall.
MATERIALS AND METHODS
Samples and Clinical Data
We studied a previously published series of 36 tissue samples (16 from unruptured, 20 from ruptured sIAs) resected after clipping of the sIA neck at the Department of Neurosurgery, Helsinki University Central Hospital, Helsinki, Finland (15). The samples were immediately snap-frozen at the operating theatre and handled as previously described (15). Clinical data, including plasma lipid levels, were collected from patient’s medical records, and sIA dimensions were obtained from preoperative computed tomography angiography images. Table 1 shows patient demographics and histological characteristics of the sIAs. The Hospital Ethics Committee approved the study protocol.
TABLE 1.
Clinical and Histological Characteristics of Patients With Unruptured and Ruptured Saccular Intracranial Aneurysms(sIAs)
| Bleeding Status |
||||
|---|---|---|---|---|
| Variable | Unruptured sIAs (n = 16) | Ruptured sIAs (n = 20) | p value | |
| Patient | ||||
| Femalea | 11/16 (69%) | 14/20 (70%) | 1.000 | |
| Age, years (range)b | 55 (36–67) | 52 (24–87) | 0.962 | |
| Patient with multiple (≥2) sIAsa | 10/16 (63%) | 4/20 (20%) | 0.016* | |
| Patient with previous aneurysmal subarachnoidalhemorrhagesa | 2/16 (13%) | 0/20 (0%) | 0.190 | |
| Aneurysm | ||||
| Fundus width (mm)b | 4.8 (3–13) | 4.8 (3–12) | 0.396 | |
| Fundus length (mm)b | 5.8 (2–18) | 7.2 (3–15) | 0.191 | |
| Histology of sIA wall | ||||
| Endothelium not intacta | 9/16 (56%) | 17/20 (85%) | 0.073 | |
| SMC disorganization presenta | 4/16 (25%) | 12/20 (60%) | 0.049* | |
| Intraluminal fresh thrombosis presenta | 0/15 (0%) | 10/18 (56%) | 0.001* | |
| Intraluminal organizing thrombosis presenta | 1/15 (7%) | 10/18 (56%) | 0.004* | |
| Lipid present in extracellular matrixa | 14/16 (88%) | 14/20 (70%) | 0.257 | |
| Neovessels presenta | 7/16 (44%) | 8/20 (40%) | 1.000 | |
| Iron deposition presenta | 6/16 (38%) | 3/20 (15%) | 0.146 | |
| Mast cells presenta | 6/16 (38%) | 3/20 (15%) | 0.146 | |
| Total number of inflammatory cells | ||||
| Tryptase-positive mast cellsb c | 0 (0–24) | 0 (0–32) | 0.262 | |
| CD68-positive macrophagesb c | 7 (0–228) | 67 (0–527) | 0.042* | |
| CD163-positive macrophagesb c | 33 (5–185) | 132 (28–443) | 0.005* | |
| CD3-positive T lymphocytesb c | 8 (0–137) | 30 (2–357) | 0.058 | |
aFisher exact test used, proportions are given.
bMann–Whitney U test used, median and range are given for continuous variables.
cIn standard-sized areas of 0.613 mm2.
*p ≤ 0.05 considered significant.
Histological analysis of this series of sIA samples was previously published (15).
Histology, Immunohistochemistry, and Immunofluorescence
Table 2 shows the primary antibodies and the dilutions used. In brief, frozen sections of the sIAs were fixed, blocked for endogenous peroxidase and nonspecific binding, incubated with primary antibodies, which were subsequently detected with either Vectastain Elite ABC kit (Vector Laboratories, Burlingame, California) or EnVision kit (Dako, Glostrup, Denmark) using diaminobenzidine substrate and Mayer’s Hematoxylin (Sigma, St. Louis, Missouri) background staining, as previously described (15). In negative controls, the polyclonal primary antibodies were omitted or an irrelevant IgG class-specific monoclonal antibody (IgG1 or IgG2a; Serotec, Oxford, United Kingdom) was used. Resected human tonsils and autopsied intracranial arteries served as positive controls. The staining for hematoxylin and eosin, Oil Red O (ORO)-positive neutral lipids, iron deposition (Perls method), CD68-, CD163-, CD3, or tryptase-positive leukocyte infiltrations, CD34-positive neovascularization, CD31-positive luminal endothelium, and α-smooth muscle cell actin (αSMA) were performed as previously described (15).
TABLE 2.
Antibodies
| Epitope | Function | Antibody clone | Dilution | Origina |
|---|---|---|---|---|
| Apolipoprotein A-I | HDL particles | 1C5b | 1:5000 | Monosan |
| Apolipoprotein B-100 | LDL particles | MB47c | 1:10000 | (18, 37) |
| apoE | HDL, VLDL, IDL, or LDL particles | WU E-4b | 1:500 | Santa Cruz Biotechnology |
| HNE | Oxidized lipid | HNEd | 1:300 | (18) |
| Adipophilin | Marker of intracellular lipid accumulation | AP125b | 1:10 | R&D Systems |
| ATP-binding cassette A1 | Removal of intracellular lipids | NB400-105e | 1:100 | Novus Biologicals |
| CD68 | Macrophages | EBM11b | 1:100 | Dako |
| CD163 | Macrophages | 5C6-FATb | 1:100 | Novus Biologicals |
| αSMA | α-SMC actin | 1A4c | 1:100 | Dako |
aMonosan, Uden, The Netherlands; Santa Cruz Biotechnology, Dallas, TX; R&D Systems, Minneapolis, MN; Novus Biologicals, Littleton, CO; Dako, Glostrup, Denmark.
bMouse monoclonal IgG1.
cMouse monoclonal IgG2a.
dGuinea pig polyclonal.
eRabbit polyclonal.
To examine intracellular lipid accumulation further, macrophages (CD163- and CD68-positive), and SMCs (αSMA-positive) were stained with ORO, and their ABCA1 expression was detected by immunofluorescence. Frozen sections were fixed with formalin for 10 minutes, and no lipid-diluting reagents such as Tween were used in the immunohistochemistry protocol; ORO staining was performed before hematoxylin incubation (15). For ABCA1 immunofluorescence double staining for macrophages and SMCs, the sections were fixed for 10 minutes with 4% paraformaldehyde, blocked for 60 minutes for nonspecific binding with 3% normal horse serum and 3% normal goat serum, and then incubated first for 60 minutes in ABCA1 primary antibodies followed by incubation with Alexa 488 (green) F(ab’)2 fragment of goat anti rabbit IgG secondary antibodies (Molecular Probes, Eugene, Oregon). After this, the sections were incubated with primary antibodies against CD163, CD68, or αSMA (Table 1), which were detected by incubation with Alexa 546 (red) F(ab’)2 fragment of goat anti mouse IgG secondary antibodies. All primary and secondary antibodies were diluted to 1:100 in buffer solution (15). To detect nuclei, the sections were incubated for 5 minutes in DAPI (1 mg/ml; Sigma); finally, the slides were mounted with fluorescent mounting medium (Dako).
Histological Analysis
The histological and immunohistochemical stains were photographed with a Zeiss Axioplan 2 imaging microscope (Flokal Bv Micro-optik, Deursen, The Netherlands), and a Progres C7 USB digital camera (Jenoptik, Jena, Germany). The immunofluorescence stainings were photographed using 3DHISTECH Pannoramic 250 FLASH II digital slide scanner at Genome Biology Unit (Research Programs Unit/Faculty of Medicine, Biocenter Finland, University of Helsinki), with the Pannoramic viewer program to create pseudocolored images.
The wall type classification and the analysis of CD68, CD163, CD3, tryptase, CD34, and CD31–positive cells were performed as previously described (15). In brief, the wall type was classified as follows: type A is a wall with intact endothelium and in which SMCs are linearly organized; type B is a thickened wall with disorganized SMCs; type C is a hypocellular wall with myointimal hyperplasia or organizing luminal thrombus; and type D is an extremely thin hypocellular wall with organized luminal thrombus (9). Because only two of the sIAs were representative for type D, they were excluded. Additionally, 2 sIAs could not be classified because of poor orientation of the sections (15). The amount of αSMA-positive cells in the sIA walls was scored as follows: (1) none, (2) a small group and/or few scattered cells, (3) scarce or (4) extensive presence of SMCs with or without areas lacking SMCs, and (5) increased density of SMCs throughout the wall.
The presence and the localization (either intra- or extracellular, either in the wall or in the thrombus), of apoA-I, apoB-100, apoE, HNE, and adipophilin were analyzed. Positively stained areas were measured and expressed as percentages of the total surface area of a sIA wall section (ImageJ, NIH Software) for apoA-I, apoB-100, HNE, and adipophilin. To study further the type of lipoprotein particles in the sIA wall, we analyzed whether staining for apoA-I and apoB100 colocalized with staining for ApoE in adjacent (consecutive) wall sections.
ABCA1-positive cells were scored semiquantitatively as follows: (1) none, (2) a few, (3) a small dense group and/or few scattered positively stained cells, (4) a dense accumulation in a half of the wall area and/or numerous scattered cells in the whole wall area, or (5) a dense accumulation in the whole wall area. The number of ORO-positive lipids containing CD163, CD68, or αSMA -positive foam cells was scored semiquantitatively as follows: (1) none, (2) a few (<5), (3) a small dense group and/or solitary scattered, or (4) numerous and widely spread double-positive cells. Colocalization of CD163-, CD68-, or αSMA-positive cells with ABCA1-positive cells was assessed from immunofluorescence stainings, and the presence of ORO and CD163-, or CD68-, or αSMA double-positive foam cells in the same wall area of with ABCA1-positive cells was observed from adjacent sections.
Statistics
Data analysis was performed using the IBM SPSS Statistics Software, version 21 (IBM Corporation). For categorical variables, proportions were calculated, and the Fisher exact test was used. For continuous variables, median and range were calculated, and Mann–Whitney U (MWU) test, Kruskall–Wallis (KW) multiple comparison test, and Spearman correlation tests were used. p values <0.05 were considered significant.
RESULTS
Lipid Accumulation and Wall Degeneration
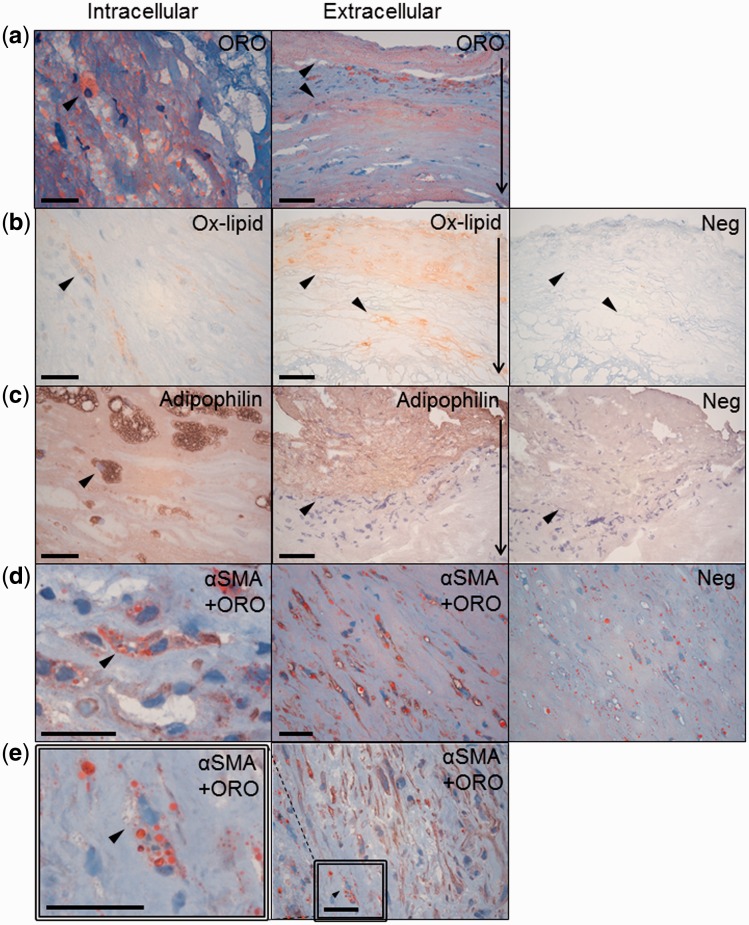
Accumulation of neutral lipids (ORO staining) and oxidized lipids (HNE), as well as expression of adipophilin were associated with wall degeneration and loss of mural SMCs (Fig. 1 and Tables 3 and 4). Lipid accumulation in mural SMCs was a common phenomenon in sIAs; αSMA-positive foam cells were found in 16/36 (44%) of the sIA walls (Fig. 1). Adipophilin-positive area was more extensive in ruptured sIAs than in unruptured ones (Table 4), suggesting that increased intracellular lipid uptake had occurred ruptured sIAs.
FIGURE 1.
Intracellular and extracellular staining of lipid accumulation markers (arrows) in saccular intracranial aneurysm (sIA) walls. (a) Neutral lipids (Oil Red O, ORO) in red. (b) Oxidized lipids (Ox-lipids, HNE) (c) and adipophilin in brown. (d) ORO-positive lipid accumulation in SMCs (α-smooth muscle cell actin, αSMA) in brown. (e) Lipid accumulation in cells other than SMCs (αSMA-negative, ORO-positive). Irrelevant primary antibodies served as negative controls (adjacent section to panels showing extracellular staining). The scale bars correspond to 25 μm in panels showing intracellular and 50 μm in panels showing extracellular stainings. In the latter, the thin arrows at the right-hand margin show orientation of the sIA wall from the adventitial to luminal side.
TABLE 3.
Positive Staining for Apolipoproteins, HNE, and Adipophilin in the Saccular Intracranial Aneurysm Walls
| Staining pattern |
||||||
|---|---|---|---|---|---|---|
| Variable | Total n (%) | Only intracellularly n (%) | Intracellularly, total | Intra- and extracellularly n (%) | Wall and thrombus n (%) | Stained wall area median (%, range) |
| Apolipoprotein A-I | 36/36 (100) | 2/36 (5.6) | 18/36 (50) | 16/36 (44) | 20/22 (91) | 56 (0–100) |
| Apolipoprotein B-100 | 36/36 (100) | 3/36 (8.3) | 34/36 (94) | 32/36 (88) | 14/22 (64) | 16 (0–89) |
| Apolipoprotein E for consistency | 35/36 (97) | 28/36 (78) | ||||
| HNE | 35/36 (97) | 5/36 (14) | 34/36 (94) | 29/36 (81) | 14/22 (64) | 10 (0–92) |
| Adipophilin | 35/36 (97) | 7/36 (19) | 29/36 (81) | 22/36 (61) | 18/22 (82) | 13 (0–87) |
TABLE 4.
Association of Apolipoproteins A-I and B-100, HNE, and Adipophilin of Saccular Intracranial Aneurysms (sIAs) With Wall Type and Wall Rupture
| Wall type | Bleeding status | ||||||
|---|---|---|---|---|---|---|---|
| Variable | A (n = 9) | B (n = 12) | C (n = 11) | p value | Unruptured sIAs (n = 16) | Ruptured sIAs (n = 20) | p value |
| Positively stained area (%) | Positively stained area (%) | ||||||
| Apolipoprotein A-I | 38 (0–69) | 51 (2–84) | 86 (35–100) | 0.011* | 57 (0–100) | 56 (6–100) | 0.604 |
| Apolipoprotein B-100 | 7 (0–17) | 15 (0–56) | 32 (8–89) | 0.001* | 9 (0–89) | 23 (0–56) | 0.158 |
| HNE | 8 (0–22) | 8 (0–55) | 43 (4–92) | 0.034* | 7 (0–92) | 20 (0–88) | 0.089 |
| Adipophilin | 4 (0–21) | 8 (0–58) | 30 (0–87) | 0.005* | 4 (0–87) | 21 (0–80) | 0.020* |
Kruskal–Wallis and Mann–Whitney U tests were used; median and range are given for continuous variables.
p ≤ 0.05 considered significant.
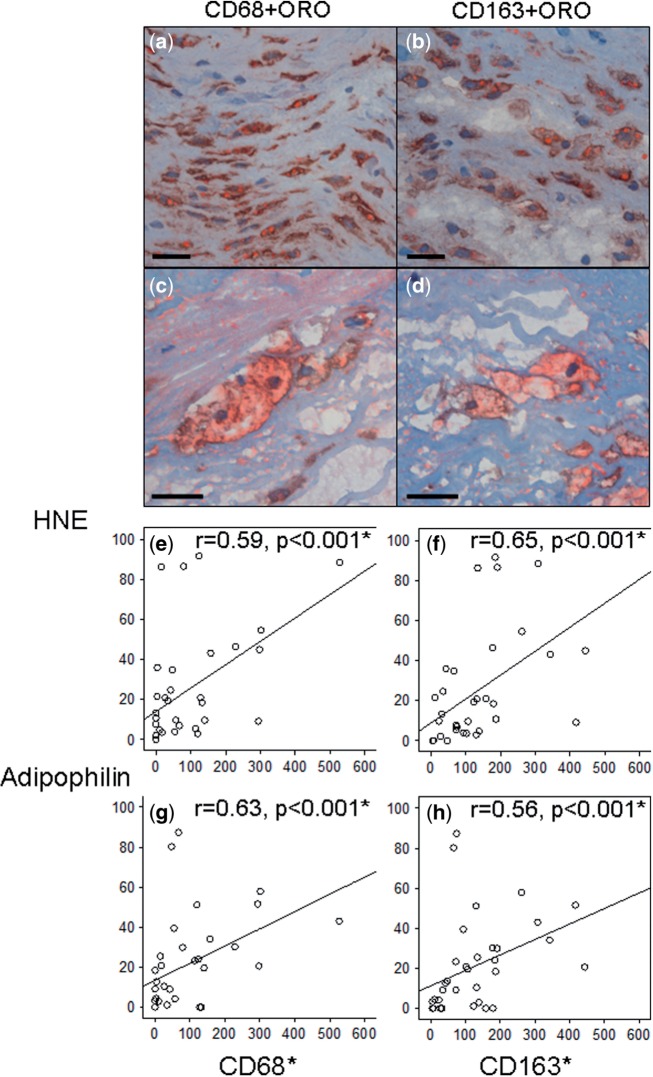
Macrophage (CD68-positive and CD163-positive) infiltration positively correlated with HNE accumulation, as well as with adipophilin expression (r = 0.56–0.65, p < 0.001, Spearman; Fig. 2). CD68-positive foam cells were present in 26/36 sIA walls and CD163-positive foam cells were also detected (Fig. 2). Different foam cell types were often present in the same sIA, but CD68-positive (presumably) macrophages appeared most often (20/36 sIAs) as the dominant foam cell type. Notably, the presence of αSMA-positive foam cells was associated with the loss of SMCs (p = 0.011, Fisher).
FIGURE 2.
Positive correlations of CD68- and CD163-positive macrophages with lipid accumulation in saccular intracranial aneurysm (sIA) walls. (a–d) ORO-positive lipid (red) containing foam cells that are positive for CD68 and CD163, suggesting macrophage origin. Scale bars, 25 μm. (e–h) The numbers of macrophages (in standard-sized area of 0.613 mm2) are higher in the sIA walls with extensive oxidized lipid accumulation and adipophilin expression. Spearman’s test was used.
Apolipoproteins and Wall Degeneration
Accumulation of apoB-100 (reflecting the presence of VLDL, IDL, or LDL) was found in all sIA walls and associated with wall degeneration (p = 0.001, KW) and macrophage infiltration (CD68, r = 0.66, p < 0.001; CD163, r = 0.70, p = 0.005; Spearman). ApoA-I (reflecting the presence of HDL) was also found in all sIAs, and associated positively with wall degeneration although it did not significantly differ between ruptured and unruptured sIAs (Fig. 3 and Tables 3 and 4). ApoE (reflecting mostly the presence of VLDL and IDL), was found in 97% of sIA walls.
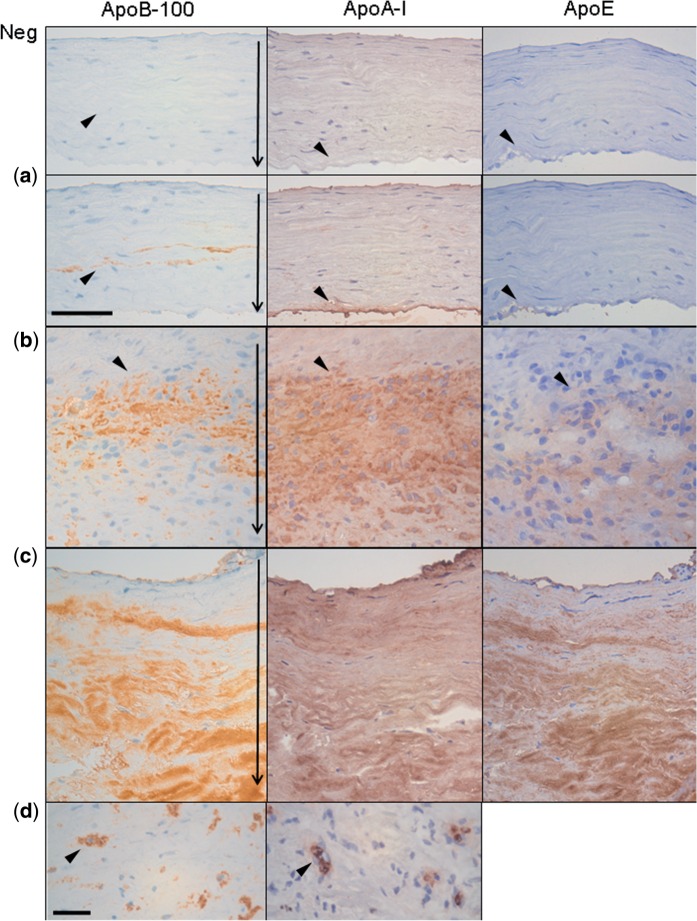
FIGURE 3.
apoA-I, apoB-100, and apoE in degenerated saccular intracranial aneurysms (sIAs, adjacent sections; see arrowheads). (a–c) Apolipoproteins in type A (a), type B (b), and type C walls (c) (9). (d) Intracellular stainings apoA-I and apoB-100. The thin arrows in the panels showing apoB-100 staining point to the sIA wall orientation similarly in Figure 1. Scale bars: a–c, 50 μm; d, 25 μm.
In most cases, apoA-I, apoB-100, and apoE were abundant in the extracellular matrix (Fig. 3). Intracellular staining was also observed for apoB-100 and apoA-I (Table 3). Accumulation of apoB-100 associated strongly with lack of intact CD31-positive endothelium (p = 0.001, MWU), and with the presence of organized thrombus on the luminal surface (p = 0.009, MWU). Accumulations of apoB-100 and HNE associated with density of mural neovessels (r = 0.64, p < 0.001 and r = 0.46, p = 0.005, Spearman) and with intramural microhemorrhages (strong iron depositions; p = 0.012 and 0.018, MWU). ApoA-I staining pattern (Fig. 3) was more diffuse and widespread in the sIA wall (median 56% of surface area) than staining for apoB-100 (16%), HNE (10%), or adipophilin (13%, Table 3). Within the wide apoA-I-positive wall areas, 35/36 sIAs had smaller, local apoE-containing areas, which were predominantly positive also for apoB-100, suggesting the presence of VLDL and/or LDL particles. However, we detected also areas positive for apoA-I and apoE, but not for apoB-100, suggesting a potential extracellular retention of HDL particles via apoE. ApoAI-positive cells were localized in all the 18/36 sIAs in the same wall areas with apoE-positive cells in adjacent sample sections, suggesting that intracellularly accumulated apoA-I-positive staining could be originated either from HDL or from VLDL or IDL.
Association of ABCA1 and apoA-I With Lipid Accumulation, Degenerative Remodeling, and Inflammation
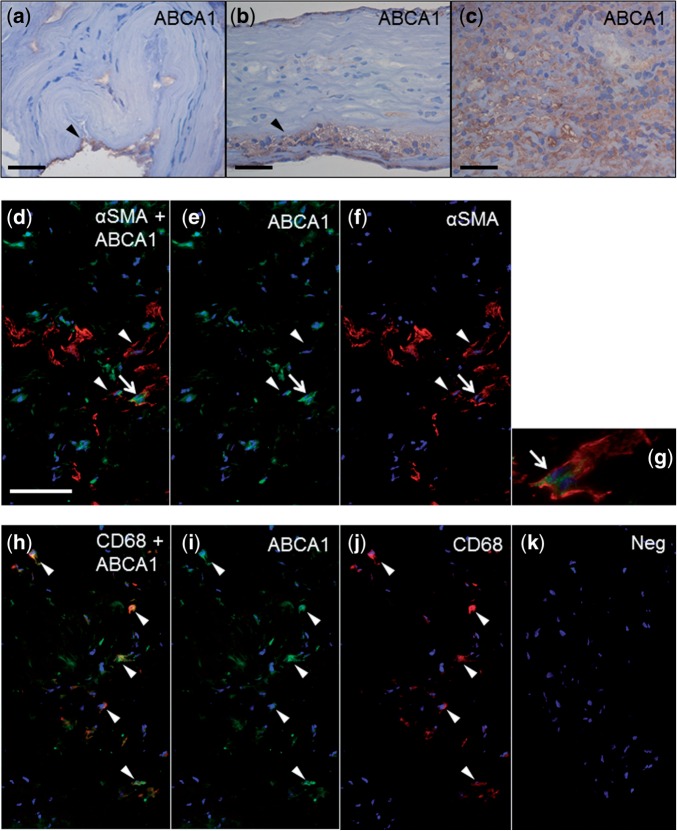
Expression of ABCA1 was present in CD68-positive macrophages and SMCs (Fig. 4), and the extent of its expression in the sIA wall was associated with wall degeneration (p = 0.005, Fisher) and rupture (p = 0.022, Fisher), as well as with lipid oxidation and intracellular lipid accumulation (HNE, p = 0.004; adipophilin, 0.019; KW) and macrophage infiltration (CD68, p = 0.005; CD163, p = 0.001; KW). ApoA-I-positive staining colocalized with ABCA1-positive cells in 18/36 (50%) of the sIAs, reflecting the known potential of apoA-I to remove intracellularly accumulated lipids through the ABCA1 transporter. The presence of ABCA1-positive cells in apoA-I-positive wall areas associated with a high number of SMCs (p = 0.003, Fisher), whereas absence of this colocalization was associated with wall degeneration (p = 0.017, Fisher).
FIGURE 4.
Extent and intracellular localization of ATP-binding cassette 1 (ABCA1) expression in saccular intracranial aneurysm (sIA) walls. (a-c) A few (a), a small dense group (b), and a dense accumulation (c) of ABCA1-positive cells (arrowheads). (d–g) ABCA1-expression in αSMA-positive SMCs (arrowheads). White arrow points to a ABCA1- and αSMA-double-positive cell presented with digital magnification in (g). (h–j) CD68-positive macrophage-like cells (arrowheads). (k) Negative control, in which the primary antibody was omitted. Scale bars: a, 25 μm; b, c, 50 μm; d–j, 100 μm.
Clinical Risk Factors, Wall Remodeling, Inflammation, and Lipid Accumulation
Current smoking did not correlate in the studied samples with sIA wall remodeling. Smokers had significantly less macrophage infiltration in their sIA walls (CD68 and CD163; p = 0.041 for both, MWU). Smoking was not associated with accumulation of lipids (ORO and adipophilin), apoB-100, HNE, or ABCA1 in the sIA wall. Smokers had less apoA-I in the sIA wall (40% vs 67%, p = 0.055, MWU), but the difference appeared only as a statistical trend.
The plasma levels of total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides were available from 21/36 patients and they did not associate with the extent of apoA-I, apoB-100, HNE, or adipophilin staining in the sIA walls, nor did they associate with ORO-positive lipid accumulation, ABCA1 expression, inflammatory cell infiltration, wall degeneration, or rupture. However, higher total cholesterol and LDL cholesterol values were associated with the presence of αSMA-positive foam cells in the sIA wall (p = 0.009 and p = 0.028, MWU).
We did not find a clear correlation or an association with the cumulative effect of the known risk factors for sIA rupture (PHASES score: population, hypertension, age, size, earlier SAH, and site (24)) and wall remodeling or macrophage infiltration. PHASES score did not associate either with accumulation of neutral lipids (ORO), adipophilin or oxidized lipids (HNE). PHASES score did, however, correlate inversely with apoA-I (r = −0.54, p = 0.001, Spearman).
DISCUSSION
We demonstrated an association of lipid accumulation with formation of SMC-derived foam cells, loss of mural SMCs, inflammation and degenerative remodeling of the sIA wall, and with eventual rupture of the sIA wall. In addition, we comprehensively analyzed the presence of the key apolipoproteins known to be involved in the accumulation of lipids in atherosclerotic arterial walls in sIAs.
Lipid Accumulation as a Cause of Degenerative Wall Remodeling and Rupture
Lipids accumulate in sIAs both intracellularly and extracellularly, and lipid accumulation is associated with wall degeneration (11, 14, 15). In this study, lipid accumulation was observed in sIA walls irrespective of the plasma lipid levels, as also observed in our prior study (14). This intriguing observation suggests a significant contribution of local inflammation as a direct or indirect driver of influx and accumulation of lipids derived from circulating apoB-100-containing lipoproteins. ApoB-100 is the main protein of the lipoprotein particles that carry lipids to the vascular wall, that is, LDL, and also IDL and VLDL (16, 17). In accordance with the above findings, we found apoB-100 in all sIA walls and the extent of it associated with degenerative wall remodeling.
We previously demonstrated the intracellular presence of HNE, a marker of oxidized lipids (18), in SMCs of the sIA wall, which may reflect the uptake of cytotoxic oxidized lipids by SMCs (11, 14). In this study, we demonstrate that lipid accumulation in mural SMCs of the sIA wall is not uncommon (observed in 44% of samples), and that formation of SMC-derived lipid-laden foam cells is associated with loss of mural SMCs. Intracellular lipid accumulation disturbs the homeostasis of the lipid-laden cells (25), raising the possibility that the observed loss of mural SMCs reflects, at least partly, the death of SMC-derived foam cells. Another intriguing possibility is induction of SMCs death by nearby macrophage-derived foam cells. Such a scenario has been suggested to take place in the foam cell-containing thickened intima of human saphenous vein grafts after coronary artery bypass surgery, that is, in a tissue with a morphology resembling that of unstable plaques of coronary arteries (26). Adipophilin is a protein attached to intracellular lipid droplets (19). Besides intracellularly located adipophilin, also extracellular adipophilin was observed. Importantly, extracellularly accumulated adipophilin was more widespread in ruptured than in unruptured sIAs reflecting death of lipid-laden cells, macrophages, and SMCs (14, 27, 28), and release of their intracellular, adipophilin-covered lipid-droplets, into the extracellular matrix (14, 17, 25).
We and others have previously demonstrated that loss of SMCs is characteristic to ruptured sIA walls (8–10, 13, 15, 29) in an experimental model that loss of mural SMCs indeed leads to aneurysm growth and rupture (13). Taken together, our current findings and prior literature strongly implicate lipid accumulation as one of the key factors that cause sIA wall degeneration and rupture; however, experimental models are needed to demonstrate firmly a causal relation.
Inflammation and Lipid Accumulation
Finding considerable amounts of apoB-100, HNE, and adipophilin in those sIAs that also contained high numbers of CD68- and CD163-positive macrophages, suggested that lipid accumulation, the presence of oxidized lipids, and intracellular uptake of lipids may be an important trigger of the inflammatory response in sIA walls. Macrophages are capable of phagocytizing extracellularly modified LDL particles (30), especially if opsonized by antibodies reacting to oxidatively or otherwise modified lipoproteins (31). We previously found that patients with unruptured sIAs have higher titers of circulating oxidized lipid-reacting antibodies than patients with aneurysmal subarachnoid hemorrhages (14). Thus, by removing the cytotoxic lipids, macrophage activity may reduce lipid burden, SMC foam cell formation and the subsequent loss of mural SMCs, thus reducing the degenerative remodeling and risk of rupture. In atherosclerotic plaques, phagocytosis of accumulated extracellular lipids and apoptotic cell debris by macrophages prevents the formation of a necrotic lipid core and stabilizes the vascular wall (17, 30, 32).
Why Do Lipids Accumulate in the sIA Wall—Enhanced Entry or Impaired Clearance?
Lipoproteins may enter intact vascular wall with a normal endothelial barrier function via endothelial cell transcytosis (22). Importantly, endothelial dysfunction increases lipoprotein influx via endothelial cell junctions, and more severe damage of endothelium may allow unrestricted entry of lipoproteins into the vessel wall (22). Many sIA walls lack a functionally intact endothelium or have undergone total endothelial erosion (7). ApoB-100 was, indeed, more widespread in sIA walls that lacked an intact endothelium. ApoA-I, apoB-100, HNE, and adipophilin were detected in larger amounts in the sIA walls with organized thrombus, suggestive of an earlier endothelial erosion and loss of endothelial barrier function that makes the aneurysm wall more permeable and more susceptible to hydraulic convection of plasma components into the IA wall. Lipids, especially cholesterol, may also originate from the thrombus itself, particularly from erythrocytes, trapped and lysed in the intramural or intraluminal thrombus since they contain high cholesterol levels in their cell membranes (33). Another potential source of lipids are leaky mural neovessels (33), detected earlier also in sIAs (15) through which lipoprotein particles and erythrocytes may enter the sIA wall. The distributions of apoB-100 and HNE are associated with the presence of neovessels and microhemorrhages (15), suggesting that the neovessels in the sIA wall indeed contribute to the lipid accumulation in the sIA similarly to the diseased arterial wall in atherosclerosis (34).
ApoB-100-containing lipoproteins can bind to proteoglycans (35) and thus accumulate in the sIA wall extracellular matrix (27). As in our prior studies (11, 14), we also found intracellular staining for apoB-100 and HNE in the sIAs. Such intracellular staining for apoB-100 has also been reported in atherosclerotic aortas (36) and aortic aneurysms (37). Under normal conditions, apoB-100 is broken down in lysosomes after ingestion (38). Oxidation or other kinds of modification of LDL can, however, disturb the normal function of lysosomes (39). Indeed, following internalization of an oxidized LDL, its apoB-100 component is poorly degraded within lysosomes, and the reduction in proteolysis has been shown to correlate with the degree of oxidation, that is, with the degree of apoB-100 crosslinking with HNE (40). Thus, we can infer that the intracellular staining pattern of apoB-100 in the sIA walls suggests ingestion and poor degradation of oxidized LDL by sIA wall cells, particularly since intracellular staining for HNE was also detected.
Here, we demonstrated for the first time that apoA-I is also present in sIAs and associates with degenerative wall remodeling. ApoA-I is the main apolipoprotein of HDL, which induce the clearance of intracellularly accumulated lipids from the vascular wall, thereby initiating the reverse cholesterol transport pathway (22). Interaction of lipid-free or lipid-poor apoA-I particles with the cholesterol efflux transporter ABCA1 present in the foam cells leads to formation of the mature HDL particles found in circulation (22, 41).
In contrast to apoB-100, the distribution of apoA-I in the sIA walls was independent of the presence of endothelial damage. The smaller size of apoA-I-containing HDL particles facilitates their diffusion through the endothelium (22). ApoA-I does not bind to the proteoglycans (22) and therefore did not contribute to HDL accumulation in the sIA wall. We did, however, observe localized distribution of apoA-I in most sIA walls. Of interest, the apoA-I retention has been reported in atherosclerotic carotid plaques, in which HDL particles were shown to bind to matrix proteoglycans via apoE (23). A similar event may also occur in sIAs. ApoA-I was also present in apoE-negative areas of the sIA walls; therefore, ApoE-mediated retention of HDL particles may only in part explain the presence of apoA-I in the sIA wall. Moreover, we found apoA-I in wider areas in more degenerated and lipid-containing sIAs walls, which may be due to inflammation and increased permeability of the walls. Enhanced vascular permeability facilitates entry of plasma HDL and promotes macrophage-reverse cholesterol transport from skin in mice (42). Since apoA-I is namely involved in clearance of accumulated lipids, our finding raises the possibility that the cholesterol efflux function of apoA-I in the sIA wall may be impaired.
ABCA1 expression was present in both mural SMCs as well as in macrophages, and its extent associated with lipid accumulation and macrophage infiltration, but not with SMC score. This suggests that lipid accumulation in the sIA wall and subsequent formation of foam cells of macrophage and SMC origin upregulates ABCA1 expression in macrophages, but not necessarily in SMCs. Interestingly, Allahverdian et al recently showed that ABCA1 expression by intimal SMCs is reduced in advanced atherosclerotic lesions, which was not observed in myeloid lineage inflammatory cells (43). SMCs in sIAs may also be relatively unable to release their intracellular lipid-load by ABCA1-mediated efflux as compared to inflammatory cells. We additionally found that ABCA1 and apoA-I were not always present in the same sIA wall areas, which suggests lack of their interaction and thus dysfunction of this ABCA1-mediated lipid clearance mechanism in those areas. Our observation that colocalization of ABCA1 expression and apoA-I associated with high number of SMCs in the sIA wall, whereas the lack of this colocalization associated with wall degeneration, implies that ABCA1-mediated efflux of lipids from sIA wall cells to apoA-I particles is a mechanism to remove intracellularly accumulated lipid from the sIA wall. Furthermore, this clearance of intracellular lipids may importantly reduce formation of foam cells from mural SMCs, and subsequent loss of functioning SMCs from the sIA wall.
Correlation With Clinical Risk Factors
Here, we studied a highly selected series of patient-derived samples. Because samples were collected during surgery only in the cases when it was feasible, caution is necessary when interpreting associations of clinical risk factors and histopathological findings. It needs also to be noticed that we only had a limited amount of aneurysmal tissue available and accordingly could not investigate the whole aneurysm sac, a fact which may have affected the observed associations with clinical risk factors that are likely to have an effect on the entire aneurysm. Nevertheless, we demonstrate several important correlations between lipid accumulation, apolipoproteins and clinical risk factors. The known risk factors for sIA rupture, namely smoking and PHASES score, which sums up the cumulative effect of other known risk factors than smoking (24), negatively correlated with apoA-I in the sIA wall. This supports the interpretation that apoA-I has a protective role in sIA wall degeneration, probably via anti-atherosclerotic, anti-inflammatory, and anti-oxidant functions (22, 41). Interestingly, smokers had not only less apoA-I, but they also had fewer macrophages in their sIAs. Although smoking did not seem to affect lipid accumulation, it may inhibit the various protective functions of apoA-I, for example via activation of mast cells and subsequent release of proteases that degrade apoA-I (44), and so block its ability to induce efflux from foam cells and to act as an anti-inflammatory component in the arterial wall (45).
Another important observation is that PHASES score did not associate with lipid accumulation, which nevertheless is clearly associated with wall degeneration and rupture. This suggests that those factors that predispose to lipid accumulation are not included in the current PHASES score, which could be improved by adding imaging biomarkers or other biomarkers that demonstrate lipid accumulation to the sIA wall.
Our findings strongly implicate lipid accumulation as a key factor promoting the degeneration of the sIA wall. Lipids have not been considered as a risk factor for sIA rupture and subsequent intracranial hemorrhage. Our results call for more studies not only on the subject with special emphasis on plasma lipid and apolipoprotein levels, but also on the potential effects of lipid-lowering therapies or other pharmacotherapies that may affect foam cell formation, thereby influencing the risk of sIA rupture. Furthermore, studies in experimental aneurysm models should focus also on lipid accumulation and its role as a potential cause of inflammation and wall degeneration. It is worth noting that in this study and in our prior study, lipid accumulation in the sIA wall was also observed in patients with normolipemia.
CONCLUSIONS
Entry and accumulation of lipids derived from circulating apoB-100-containing lipoproteins in the sIA wall, and subsequent formation of SMC-derived lipid-laden foam cells, are associated with degeneration and rupture of the sIA wall. Accumulation of lipids occurs to at least some extent in all sIA walls, and may be aggravated by impairment of the ABCA1 and apoA-I-mediated cellular lipid clearance mechanism in the sIAs. Experimental studies that investigate the role of lipid accumulation in degeneration and rupture of the sIA wall are warranted.
ACKNOWLEDGMENTS
We are grateful to Nancy Lim, Mari Jokinen, and Kaisa Laajanen for their excellent technical assistance. We also thank Mikko Mäyränpää and Petri Mattila for kindly providing the used control tissues.
REFERENCES
- 1.Nieuwkamp DJ, Setz LE, Algra A, et al. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: A meta-analysis. Lancet Neurol 2009;8:635–42 [DOI] [PubMed] [Google Scholar]
- 2.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007;369:306–18 [DOI] [PubMed] [Google Scholar]
- 3.Vlak MH, Algra A, Brandenburg R, et al. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol 2011;10:626–36 [DOI] [PubMed] [Google Scholar]
- 4.Ingall T, Asplund K, Mahonen M, et al. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke 2000;31:1054–61 [DOI] [PubMed] [Google Scholar]
- 5.Korja M, Lehto H, Juvela S. Lifelong rupture risk of intracranial aneurysms depends on risk factors: A prospective Finnish cohort study. Stroke 2014;45:1958–63 [DOI] [PubMed] [Google Scholar]
- 6.Kotowski M, Naggara O, Darsaut TE, et al. Safety and occlusion rates of surgical treatment of unruptured intracranial aneurysms: A systematic review and meta-analysis of the literature from 1990 to 2011. J Neurol Neurosurg Psychiatry 2013;84:42–8 [DOI] [PubMed] [Google Scholar]
- 7.Frösen J, Tulamo R, Paetau A, et al. Saccular intracranial aneurysm: Pathology and mechanisms. Acta Neuropathol 2012;123:773–86 [DOI] [PubMed] [Google Scholar]
- 8.Kataoka K, Taneda M, Asai T, et al. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke 1999;30:1396–401 [DOI] [PubMed] [Google Scholar]
- 9.Frösen J, Piippo A, Paetau A, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: Histological analysis of 24 unruptured and 42 ruptured cases. Stroke 2004;35:2287–93 [DOI] [PubMed] [Google Scholar]
- 10.Tulamo R, Frösen J, Junnikkala S, et al. Complement activation associates with saccular cerebral artery aneurysm wall degeneration and rupture. Neurosurgery 2006;59:1069–76 [DOI] [PubMed] [Google Scholar]
- 11.Tulamo R, Frösen J, Junnikkala S, et al. Complement system becomes activated by the classical pathway in intracranial aneurysm walls. Lab Invest 2010;90:168–79 [DOI] [PubMed] [Google Scholar]
- 12.Tulamo R, Frösen J, Paetau A, et al. Lack of complement inhibitors in the outer intracranial artery aneurysm wall associates with complement terminal pathway activation. Am J Pathol 2010;177:3224–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marbacher S, Marjamaa J, Bradacova K, et al. Loss of mural cells leads to wall degeneration, aneurysm growth, and eventual rupture in a rat aneurysm model. Stroke 2014;45:248–54 [DOI] [PubMed] [Google Scholar]
- 14.Frösen J, Tulamo R, Heikura T, et al. Lipid accumulation, lipid oxidation, and low plasma levels of acquired antibodies against oxidized lipids associate with degeneration and rupture of the intracranial aneurysm wall. Acta Neuropathol Commun 2013;1:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ollikainen E, Tulamo R, Frösen J, et al. Mast cells, neovascularization, and microhemorrhages are associated with saccular intracranial artery aneurysm wall remodeling. J Neuropathol Exp Neurol 2014;73:855–64 [DOI] [PubMed] [Google Scholar]
- 16.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317–25 [DOI] [PubMed] [Google Scholar]
- 17.Kovanen PT. Mast cells: Multipotent local effector cells in atherothrombosis. Immunol Rev 2007;217:105–22 [DOI] [PubMed] [Google Scholar]
- 18.Palinski W, Ylä-Herttuala S, Rosenfeld ME, et al. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low-density lipoprotein. Arteriosclerosis 1990;10:325–35 [DOI] [PubMed] [Google Scholar]
- 19.Heid HW, Moll R, Schwetlick I, et al. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res 1998;294:309–21 [DOI] [PubMed] [Google Scholar]
- 20.Navab M, Reddy ST, Van Lenten BJ, et al. HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nat Rev Cardiol 2011;8:222–32 [DOI] [PubMed] [Google Scholar]
- 21.Lee-Rueckert M, Blanco-Vaca F, Kovanen PT, et al. The role of the gut in reverse cholesterol transport–Focus on the enterocyte. Prog Lipid Res 2013;52:317–28 [DOI] [PubMed] [Google Scholar]
- 22.Annema W, von Eckardstein A, Kovanen PT. HDL and atherothrombotic vascular disease. Handb Exp Pharmacol 2015;224:369–403 [DOI] [PubMed] [Google Scholar]
- 23.O'Brien KD, Olin KL, Alpers CE, et al. Comparison of apolipoprotein and proteoglycan deposits in human coronary atherosclerotic plaques: Colocalization of biglycan with apolipoproteins. Circulation 1998;98:519–27 [DOI] [PubMed] [Google Scholar]
- 24.Greving JP, Wermer MJ, Brown RD, Jr, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol 2014;13:59–66 [DOI] [PubMed] [Google Scholar]
- 25.Tabas I. Consequences of cellular cholesterol accumulation: Basic concepts and physiological implications. J Clin Invest 2002;110:905–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kockx MM, De Meyer GR, Bortier H, et al. Luminal foam cell accumulation is associated with smooth muscle cell death in the intimal thickening of human saphenous vein grafts. Circulation 1996;94:1255–62 [DOI] [PubMed] [Google Scholar]
- 27.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32:2045–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol 2015;12:10–7 [DOI] [PubMed] [Google Scholar]
- 29.Frösen J. Smooth muscle cells and the formation, degeneration, and rupture of saccular intracranial aneurysm wall–A review of current pathophysiological knowledge. Transl Stroke Res 2014;5:347–56 [DOI] [PubMed] [Google Scholar]
- 30.Hansson GK. Atherosclerosis–An immune disease: The Anitschkov Lecture 2007. Atherosclerosis 2009;202:2–10 [DOI] [PubMed] [Google Scholar]
- 31.Ylä-Herttuala S, Palinski W, Butler SW, et al. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb 1994;14:32–40 [DOI] [PubMed] [Google Scholar]
- 32.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: From mice to humans. Immunity 2013;38:1092–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 2005;25:2054–61 [DOI] [PubMed] [Google Scholar]
- 34.Lehti S, Sjövall P, Käkelä R, et al. Spatial distributions of lipids in atherosclerosis of human coronary arteries studied by time-of-flight secondary ion mass spectrometry. Am J Pathol 2015;185:1216–33 [DOI] [PubMed] [Google Scholar]
- 35.Sneck M, Kovanen PT, Öörni K. Decrease in pH strongly enhances binding of native, proteolyzed, lipolyzed, and oxidized low-density lipoprotein particles to human aortic proteoglycans. J Biol Chem 2005;280:37449–54 [DOI] [PubMed] [Google Scholar]
- 36.Niendorf A, Rath M, Wolf K, et al. Morphological detection and quantification of lipoprotein(a) deposition in atheromatous lesions of human aorta and coronary arteries. Virchows Arch A Pathol Anat Histopathol 1990;417:105–11 [DOI] [PubMed] [Google Scholar]
- 37.Yomantas S, Elner VM, Schaffner T, et al. Immunohistochemical localization of apolipoprotein B in human atherosclerotic lesions. Arch Pathol Lab Med 1984;108:374–8 [PubMed] [Google Scholar]
- 38.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 1986;232:34–47 [DOI] [PubMed] [Google Scholar]
- 39.Lougheed M, Zhang HF, Steinbrecher UP. Oxidized low density lipoprotein is resistant to cathepsins and accumulates within macrophages. J Biol Chem 1991;266:14519–25 [PubMed] [Google Scholar]
- 40.Jessup W, Mander EL, Dean RT. The intracellular storage and turnover of apolipoprotein B of oxidized LDL in macrophages. Biochim Biophys Acta 1992;1126:167–77 [DOI] [PubMed] [Google Scholar]
- 41.Rosenson RS, Brewer HB, Jr, Ansell BJ, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol 2016;13:48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kareinen I, Cedo L, Silvennoinen R, et al. Enhanced vascular permeability facilitates entry of plasma HDL and promotes macrophage-reverse cholesterol transport from skin in mice. J Lipid Res 2015;56:241–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allahverdian S, Chehroudi AC, McManus BM, et al. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014;129:1551–9 [DOI] [PubMed] [Google Scholar]
- 44.Helske S, Syväranta S, Kupari M, et al. Possible role for mast cell-derived cathepsin G in the adverse remodeling of stenotic aortic valves. Eur Heart J 2006;27:1495–504 [DOI] [PubMed] [Google Scholar]
- 45.Nguyen SD, Maaninka K, Lappalainen J, et al. Carboxyl-terminal cleavage of apolipoprotein A-I by human mast cell chymase impairs its anti-inflammatory properties. Arterioscler Thromb Vasc Biol 2016;36:274–84 [DOI] [PMC free article] [PubMed] [Google Scholar]