Abstract
The production of amyloid-β peptides in the brains of patients with Alzheimer disease (AD) may contribute to memory loss and impairments in social behavior. Here, an efficient adenylate cyclase activator, forskolin, was orally administered by gavage (100 mg/kg body weight) to 5-month-old transgenic APP/PS1 mice, which serve as an animal model of cerebral amyloidosis. Analyses of nest construction, sociability, and immunohistochemical features were used to determine the effects of forskolin treatment. After a relatively short term of treatment (10 days), forskolin-treated transgenic mice showed restored nest construction ability (p < 0.05) and their sociability (p < 0.01). There was a reduction of Aβ plaque deposition in the cortex and in the hippocampus. Furthermore, expression of transforming growth factor β, glial fibrillary acidic protein, and Iba-1 in the cortex was reduced in the forskolin-treated group, suggesting regulation of the inflammatory response mediated by activated microglia and astrocytes in the brains of the APP/PS1 mice (p < 0.01). Taken together, these findings suggest that forskolin shows neuroprotective effects in APP/PS1 Tg mice and may be a promising drug in the treatment of patients with AD.
Keywords: β-amyloid, Alzheimer disease, APP/PS1 transgenic mice, Cerebral amyloidosis, Forskolin.
INTRODUCTION
Coleus forskohlii is a perennial plant that has traditionally been used to treat convulsions and asthma in India, Burma, and Thailand (1). The plant is of particular interest because it produces forskolin, a pure compound obtained from its root (Fig. 1). Forskolin is mostly reported and available in the market for its beneficial effect on weight loss (2). The reported method of action of forskolin is activation of adenylate cyclase (AC), which increases cyclic adenosine monophosphate (cAMP) levels, an important signal carrier for cell communication. Indeed, increasing cAMP levels was reported to improve long-term memory (3), provide neuroprotection (4), and improve cognition (5). Therefore, forskolin may be a good candidate for studies investigating Alzheimer disease (AD) treatment.
Figure 1.
Molecular structure of forskolin.
AD is a progressive neurodegenerative disease characterized in its early stages by a memory loss and in its late-stage by the loss of the ability of affected patients to respond to their environment. In AD patients, the formation of ß-amyloid plaques and neurofillary tangles in the brain is associated with progressive decline in cognitive function, along with exaggerated neuroinflammation. Decreased AC levels have recently been reported in CSF and postmortem brain tissue samples from patients suffering from dementia (6). Previous studies have shown that there are disruptions of G-protein stimulation of AC activity in AD postmortem brains (7). It has also been reported that the AC signal transduction pathway is affected in AD leading to the suggestion that increasing cAMP levels could benefit AD patients (8). Moreover, stimulation of AC activity in AD was also correlated with histopathological changes in patients (9).
There is no effective cure for AD but research is ongoing to find treatments that may slow the worsening of dementia symptoms and improve the quality of life for AD patients; this may include improving noncognitive behaviors (10). Many natural compounds isolated from medicinal plants including baicalin (11), curcumin (12), and resveratrol (13) have shown promising effects in the alleviation of the disease symptoms. Compounds of the terpenoids family such as ginsenosides, gingkolides, and cannabinoids have also been reported as promising anti-AD agents (14). Further studies even suggested application of diterpenoids as a therapeutic option for neurodegenerative diseases (15). Among them, forskolin is the only diterpene compound reported to activate AC in cells (16). It binds to AC with high affinity, thereby contributing to the increment of cAMP levels in cells (17). It was reported that forskolin activates all mammalian AC by interacting with cytoplasmic domains (C1 and C2) at the catalytic core (18). The acidification of lysosomes by treating microglia with forskolin also contributed to amyloid fibril degradation (19).
Another characteristic of AD is brain neuroinflammation. Many studies have implicated pro- and anti-inflammatory cytokines as contributing to AD pathogenesis. Among them, transforming growth factor β (TGF-β) is an important factor in regulating inflammatory responses by regulating β-amyloid precursor protein synthesis, plaque formation, and microglial responses. In addition, it has been demonstrated that microglia produce Aβ, which causes activation of several inflammatory components (20). It has also been reported that forskolin suppresses tumor necrosis factor (TNF) production in microglia by reducing the nuclear translocation and DNA binding activity of NF-κB (8). Iba1 and glial fibrillary acidic protein (GFAP) are highly specific markers for microglia and astrocytes, respectively, and their levels of expression in the cortex and hippocampus reflect the amount of inflammation in the brain.
The purpose of this study was to use an APP/PS1-21 double transgenic mouse model of cerebral amyloidosis to assess the potential therapeutic effect of forskolin. The choice of this model was supported by the fact that these transgenic mice exhibit prominent AD-like pathology with relatively early deposition of amyloid plaques at 2 months in the cortex, and 2 months later in the hippocampus (21).
MATERIALS AND METHODS
Animals and Treatment
Male APP/PS1-21 mice were obtained from Prof. M. Jucker (Hertie-Institute, Tubingen, Germany). Heterozygous male APP/PS1-21 mice were bred with wild-type C57BL/6J females (Charles River Germany, Sulzfeld, Germany). Offspring were tail snipped and genotyped using PCR with primers specific for the APP sequence (Forward: “GAATTCCGACATGACTCAGG,” Reverse: “GTTCTGCTGCATCTTGGACA”). Mice were kept in a 12-hour dark/12-hour light environment and were given ad libitum access to water and food. The experiments were licensed and approved according to the German Animal Welfare Act (TierSchG) of 2006.
Forskolin Treatment
Forskolin (>98% pure) was purchased from Carbosynth Ltd. (Compton, Berkshire, United Kingdom). For oral treatment, forskolin was suspended in 1% carboxymethylcellulose ([CMC], Blanose, Hercules-Aqualon, Düsseldorf, Germany) and administered by gavage at a dose of 100 mg/kg. Control mice received an equivalent volume of CMC.
Mice were randomly assigned to groups (n = 6/group, 3 males and 3 females for each group) at the age of 5 months and used for the experimental treatments: daily oral administration of forskolin (100 mg/kg bodyweight, suspended in CMC), or the same volume of 1% CMC by gavage. The experiment lasted 10 days and consisted of 3 groups: Forskolin treatment, vehicle control, and controls without any treatment.
Design and Evaluation of Nest Construction Assay
A nest construction assay was modified (22) to determine the deficits in affiliative/social behavior of APP/PS1 mice and potential changes following treatment. Mice were individually housed for at least 24 hours in clean plastic cages containing 1 cm of wood chip bedding. Mice were tested in counterbalanced groups of mixed genotypes and genders to reduce variability in housing conditions. Two hours prior to the onset of the dark phase, individual cages were supplied with paper towel torn into 5 × 5 cm squared pieces. The next morning, cages were inspected for nest construction. Paper towel nest construction was scored by a 3-point system: 1 = no biting or tears on the paper, 2 = moderate biting and/or tears on the paper but no coherent nest, and 3 = the vast majority of paper torn into pieces and grouped into a corner of the cage (23).
Forskolin-treated and control mice were killed after 10 days of treatment. Nest scores were given by 3 independent observers who were not aware of the treatment categories.
Social Interaction Assay
The procedure to access sociability and the preference for social novelty in mice was applied as described in previous studies (24). The apparatus was made up of a 3-chambered box. Prior to the experiment, 2 dividing walls were made with openings in order to allow free access into each chamber. Moreover, each chamber was cleaned and paper chip bedding was added for each single trial.
The test mouse (ie, transgenic mouse, treated or not) was placed in the middle chamber and allowed to accommodate for 5 minutes. After the accommodation period, a stranger mouse (stranger 1, with which the test mouse was not familiar and had no previous contact) was placed in one of the side chambers. The stranger 1 was placed, respectively, in the left and in the right side chamber between trials. The mice serving as strangers were male C57BL/6J that had previously been accommodated in small cages. The entries to the side chambers were made free and the test mouse was allowed to explore the entire social test box for 10 minutes. We then measured the time spent in each chamber. After the first 10 minutes, each test mouse was submitted to a second 10 minutes session to quantitate social preference toward a new stranger mouse. Thereafter, another unfamiliar and unknown mouse (stranger 2) was introduced in the chamber that was kept empty during the first 10 minute. In this second phase, the test mouse had to choose between the stranger 1 (unfamiliar but with which it was already in contact) and the newly introduced stranger 2 mouse (also unfamiliar). Measures were taken to estimate the amount of time spent in each chamber of the equipment during the second 10-minute session.
Immunohistochemistry, Double Staining, and Image Analysis
For immunohistochemistry, brains were embedded in paraffin and postfixed in 4% paraformaldehyde overnight at 4 °C. Brains were serially sectioned (3 µm) and mounted on silan-covered slides. Hemispheric sections were stained by immunohistochemistry as described previously (25), with antibodies to: β-amyloid (1:100; Abcam, Cambridge, United Kingdom) for Aβ deposition; Iba-1 (1:200; Wako, Neuss, Germany) for activated microglia; GFAP (1:500; Chemicon [Millipore], Billerica, Massachusetts) for astrocytes; antitransforming growth factor β1 (TGF-β1) (1:50, Santa Cruz, Dallas, Texas); and anti-Alzheimer precursor protein A4 (APP) (1:200, Chemicon). Sections were counterstained with hemalum.
To evaluate the spatial relationships between amyloid β plaques and plaque-associated glial cells, we performed double staining with diaminobenzidine (Sigma-Aldrich, Munich, Germany) substrate (brown) and Fast Blue BB salt chromogen substrate solution (blue, Sigma-Aldrich, Munich, Germany) for amyloid plaques; counterstaining was omitted in those preparations.
After immunostaining, hemisphere sections were examined and photographed by light microscopy (Nikon Cool Scope, Nikon, Düsseldorf, Germany). All sections were randomly numbered and analyzed independently by 2 observers who were not aware of treatments. β-Amyloid plaques, Iba-1-positive, TGF-β1-positive, and GFAP-positive cells in cortex and hippocampus were counted. Both small Aβ plaques with a dense core and larger plaques with a dense core and a large halo of diffuse amyloid were counted. Small points or spots of Aβ staining, smaller than a cellular nucleus (around 10 μm) and slightly stained diffuse amyloid without a dense core were classified as unclear deposition and were not counted. Only plaques larger than a cellular nucleus were counted. APP staining was located on cells or outside cells presenting a plaque-like expression pattern.
To evaluate immunostaining data further, area percentages of specific immunoreactivity (IR) in selected regions were determined. Briefly, images of hemisphere cross sections were captured under 5× magnification using Nikon Cool-scope with fixed parameters; the neocortex and hippocampus areas were manually outlined on these photos and further analyzed using the software MetaMorph Offline 7.1 (Molecular Devices, Toronto, Canada). Areas of IR were selected by color threshold segmentation; all parameters were fixed for all images. In addition, ratios of GFAP area to Aβ IR area in the cortex were calculated from double stained images.
Results are given as arithmetic means of plaque/cell counts or area percentages of IR to interest areas on cross-sections and SEM.
Statistical Analysis
Differences of plaque/cell counts, area percentages, nesting scores, and behavioral data were analyzed by exact nonparametric Mann–Whitney U (Graph Pad Prism 6.0 software). For all statistical analyses, p < 0.05 was considered significant.
RESULTS
Forskolin Improved Nest Construction Capacity in APP/PS1 Tg-AD Mice
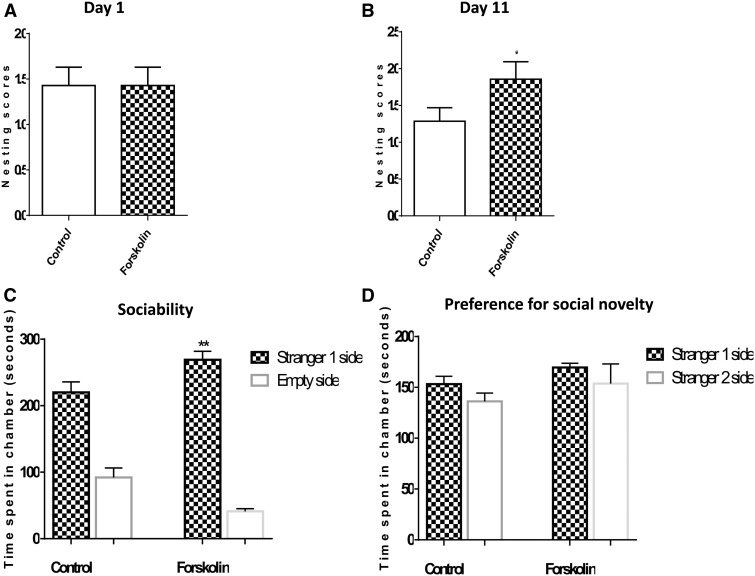
In our previous study, we showed that nesting ability of APP/PS1 transgenic mice was impaired in comparison to nontransgenic mice (26). At the beginning of our experimental treatments (day 1), no difference in nesting performance was observed between forskolin-treated and groups (control = 1.429 ± 0.20; forskolin = 1.429 ± 0.20, p = 0.50, Fig. 2A). However, after 10 days of treatment (day 11), forskolin restored the impairment observed in nontreated mice by increasing nest-building performance as indicated by significant differences in nesting scores (control = 1.286 ± 0.184; forskolin = 1.857 ± 0.236, p < 0.05, Fig. 2B). After 10 days of treatment with forskolin, paper towels were torn into pieces and grouped at the corner of the cage. In contrast to the forskolin-treated group, control mice did not destroy the paper towels and they were found not grouped in the corner. After obtaining these positive results on the nesting assay, we conducted the social interaction impairment assay.
FIGURE 2.
Effect of forskolin treatment on behavior and sociability impairments. (A, B) APP/PS1 mice were treated for with forskolin in 1% carboxymethylcellulose (CMC) or with CMC alone for 10 days. They were assessed for nesting behavior along with untreated nontransgenic mice. There was no significant difference between the forskolin-treated and control groups at day 1 (n = 6/group) (A). At day 10 there was a significant increase in nesting score in the forskolin-treated compared with control mice (n = 6/group) (p < 0.05) (B). (C) Tested mice spent more time with the stranger 1 mice than empty side; forskolin-treated mice spent more time versus controls (p < 0.01). (D) Times spent in the chamber were not significantly different between control and forskolin group for the preference for social novelty test.
Forskolin Improved the Social Interaction Capacity of APP/PS1 Tg-AD Mice
The time spent in the chambers was evaluated for sociability, that is, the preference for a stranger mouse versus an empty chamber, and the preference for a novel stranger versus the first stranger mouse. Forskolin-treated APP/PS1 mice demonstrated a significant preference (vs control mice) for spending time in the chamber containing the stranger 1 mouse in comparison to the time spent exploring the empty chamber (Fig. 2C). When the test mouse was to choose between the first stranger mouse 1 and a second stranger mouse 2, both control and forskolin-treated mice did not demonstrate any significant preference for social novelty (Fig. 2D).
Forskolin Reduced Amyloid Plaque Deposition in Cortex and Hippocampus in APP/PS1 Tg-AD Mice
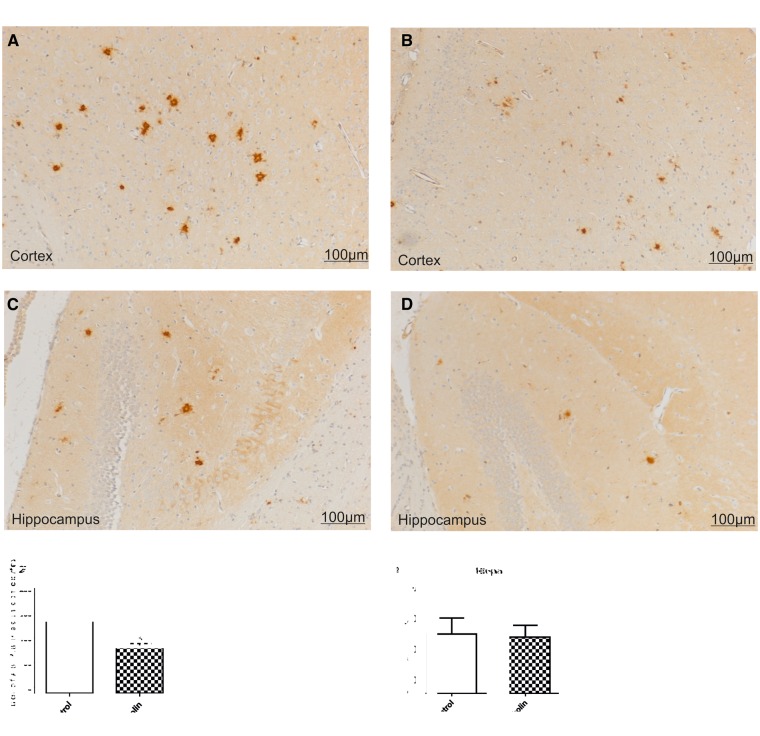
Amyloid β plaques were distributed throughout hippocampus and cortex of APP/PS1 transgenic mice (Fig. 3D). Some of these plaques were small, dense core plaques and the others were rather larger plaques with a dense core and a large halo of diffuse amyloid. In the hippocampus, plaque density was much lower than in the cortex. Forskolin reduced plaque numbers in cortex versus CMC control (CMC, 181.9 ± 11.44; forskolin, 128.00 ± 10.89, p < 0.01, n = 6/group) (Fig. 3A, B, E). Analysis of Aβ area percentages was determined using the MetaMorph software. Following treatment with forskolin, the Aβ areas were significantly reduced in brains of APP/PS1 transgenic mice in comparison to the nontreated control mice, in cortex (control, 0.36% ± 0.02%; forskolin, 0.31% ± 0.01%, p < 0.05, Fig. 3F). There was no significant difference between the forskolin-treated and the CMC group in plaque numbers in the hippocampus (control, 8.429 ± 2.287, forskolin, 8.286 ± 1.861, p = 0.48, Fig. 3G). Nevertheless, Aβ plaques in the hippocampus in treated mice generally were smaller than those in the control mice (Fig. 3D), and in the forskolin-treated group Aβ areas in the hippocampus were decreased versus controls (control, 0.20% ± 0.098%, forskolin, 0.147% ± 0.8650%, p < 0.05, Fig. 3H).
FIGURE 3.
Effect of forskolin on Aβ deposition. Representative photomicrographs of coronal sections through cortex and hippocampus show reduction of Aβ deposition following forskolin treatment. (A–D) There were numerous, relatively large Aβ plaques in the cortex of a control mouse (A) as compared with the forskolin-treated group (B). There were larger Aβ deposits in the hippocampus of a mouse from the control group (C), as compared with the forskolin-treated group (D). (E–H) Arithmetic means of plaque counts and of IR area percentages. In the cortex of mice treated with forskolin there were fewer Aβ plaques (E) and smaller IR area percentages of Aβ staining (F) than in control mice. The percentage area of Aβ in the hippocampus (H) was also reduced for the forskolin-treated group. *p < 0.05.
Forskolin Reduces the Brain Microglial Response
Iba-1-positive microglia were aggregated and surrounded amyloid deposits both in cortex and hippocampus in APP/PS1 transgenic mice (Fig. 4A–D). Forskolin treatment resulted in a reduction of Iba-1 IR area in both cortex and hippocampus (cortex control, 0.2988% ± 0.02%; cortex forskolin, 0.1408% ± 0.016%; hippocampus control, 0.1053% ± 0.008%, hippocampus forskolin, 0.082% ± 0.007%, p < 0.05) (Fig. 4E, F). The reduction of the number of Iba-1-positive cells in the cortex and hippocampus following forskolin treatment indicated reduced microglial activation.
FIGURE 4.
Effect of forskolin on microglia. (A–D) Representative photomicrographs of coronal sections through cortex and hippocampus show reduction of microglial activation following treatment with forskolin. Many Iba-1-positive cells are seen in the cortex (A) and hippocampus (C) of control mice. Most of the Iba-1-positive microglia appear to surround Aβ plaques. There are fewer Iba-1-positive cells in the cortex (B) of the forskolin-treated group. There were large numbers of Iba-1-positive cells in the hippocampus, in both control (C) and forskolin-treated group (D). (E, F) The IR area percentage of Iba-1 staining (E) was reduced in the forskolin-treated group (n = 6/group) and in the hippocampus (F). **p < 0.01; *p < 0.05.
Transforming Growth Factor β
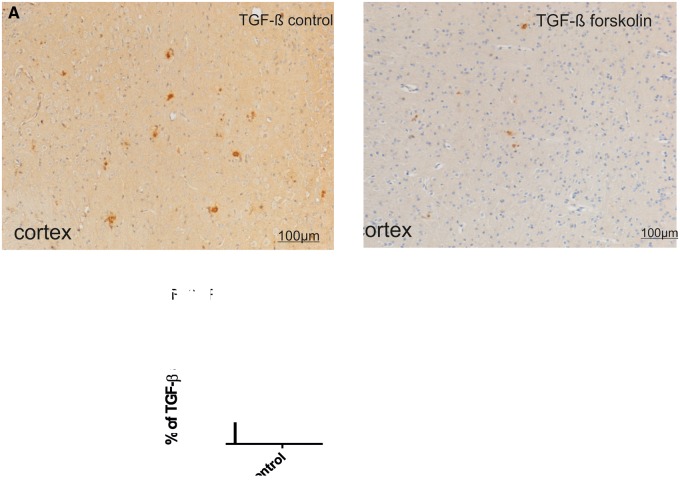
We could not detect TGF-β1 IR in cortex or hippocampus of APP/PS1 transgenic or nontransgenic mice up to 3 months of age (25). However, 2 months later there was increased TGF-β1 IR in transgenic mice that was mainly located on or around Aβ plaques (Fig. 5A, B). TGF-β1 IR was more concentrated at the edges of plaques and diffuse branches and less in the center of plaques. After 10 days of forskolin treatment, TGF-β1 IR was significantly reduced in the cortex of APP/PS1 mice (control, 0.266% ± 0.032%; forskolin, 0.131% ± 0.024%, p < 0.05, Fig 5A–C).
FIGURE 5.
Effect of forskolin on TGF-β IR. (A, B) TGF-β1 IR in the cortex of 5-month-old APP/PS1 transgenic mice. After 10 days of forskolin treatment, TGF-β1 IR was reduced in cortex of treated (B) versus control (A) mice. (C) There was a significant reduction in area of TGF-β1 IR following 10 days of forskolin treatment (n = 6/group). **p < 0.01.
Astrocytes
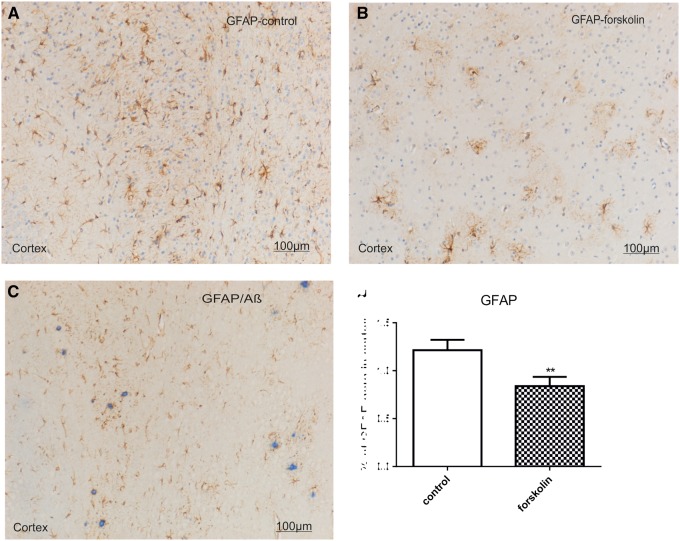
Many GFAP-positive astrocytes were distributed throughout the cortex and hippocampus in the mice (Fig. 6A, B). In forskolin-treated mice GFAP IR area values in the cortex were less than in the control group (control, 1.215% ± 0.107%; forskolin, 0.677% ± 0.133%, p = 0.01, Fig. 6D). In preparations double stained for GFAP and Aβ, many GFAP-positive cells appeared to be clustered around amyloid plaque deposits (Fig. 6C). There was no significant difference in the ratio of Aβ-associated GFAP-positive astrocytes to Aβ IR areas.
FIGURE 6.
Double staining of for Aβ and GFAP. (A, B) Representative photomicrographs of coronal section through the cortex of transgenic mice reveal GFAP-positive astrocytes (brown) that appear to be clustered around plaques in a control mouse (A) and in a forskolin-treated mouse (B). Nuclei are stained with hemalum. (C) Representative coronal section shows that many most GFAP-positive astrocytes appear to surround Aβ plaques (blue [fast blue chromogen]). (D) GFAP IR area values in the cortex were less in the forskolin versus the control group (n = 6/group). **p = 0.01.
DISCUSSION
In this work, we describe beneficial effects of oral treatment with forskolin in a transgenic mouse model of cerebral amyloidosis. After 10 days of treatment, forskolin effectively attenuated microglial and astrocytes activation in the cortex and reduced Aβ deposition in cortex and hippocampus. Moreover, forskolin restored the capacity of mice to construct nests as well as their social interaction behavior, which are important affiliative/social behaviors that are impaired in APP/PS1 transgenic mice.
Natural compounds are promising therapeutic options for the treatment of AD and other neurodegenerative diseases (14). This is the case of diterpenoids from Croton tonkinensis (27) and Salvia miltiorrhiza (15), which showed neuroprotective effects by inhibiting acetylcholinesterase. Another new diterpene, CBNU06, showed neuroprotective effects against β-amyloid-induced toxicity through the inhibition of NF-κB signaling pathway in PC12 cells (28). Forskolin is the only diterpene compound reported to activate AC in cells (16); therefore, it is a good potential candidate for AD treatment (29). The second messenger cAMP has been reported to be involved in the complex mechanisms regulating memory (30) and to be disrupted in the brains of AD patients (31). Moreover, activation of the cAMP/PKA (protein kinase A) pathway has been proposed as a mechanism for improving age-related cognitive deficits based on studies of hippocampal function (32). Over the last decade, studies have focused on AC activators as targets for the treatment of neurodegenerative diseases. Forskolin demonstrated potent antidepressant activity in a rat forced swimming model (33) and prevented induced seizures in mice (34). Further, it can be considered as an indirect inhibitor of the transcription factor NF-κB. Indeed, the cAMP produced after activation of AC by forskolin inhibits NF-κB (35), which not only blocks inflammation in the brain, but also directly contributes to inhibit the production of Aβ peptides (36). Another study revealed that rolipram could improve cognitive performances of normal rats by activating AC (37).
Because forskolin is a potent inhibitor of NF-κB, it could be of therapeutic utility for the treatment of AD by directly inhibiting the production and deposition of Aβ peptides in the brain. This mechanism of action was also reported with other diterpene compounds such as oridonin in our previous study (38). Therefore, reduced Aβ deposition observed in this study might directly attribute of NF-κB inhibition and its effects on Aβ production.
Deficits in noncognitive behaviors and social interaction are primary diagnostic indicators of AD and other neurological diseases (39). Early in the course of AD, 50% of patients exhibit depression symptoms with a concomitant paucity of social behavior and memory recall (40). Given the primacy of cognitive/noncognitive impairments, deposition of amyloid-β, and neuroinflammation in the brains of AD patients, we considered these clinical manifestations to be essential components for translation of mouse models of AD (41). Cognitive impairments in animal models are normally tested by many methods including Morris water maze (42). However, evidence of cognitive impairments appear rather only at 8 months of age (43), and, therefore, cannot be used to analyze the potential of treatments at an early stage of the disease. We used APP/PS1 transgenic mice because they exhibit a relatively early deposition of amyloid plaques at 2 months in the cortex, and 2 months later in the hippocampus (21). To evaluate the effect of a treatment on the early manifestations of amyloid deposition, we used nest construction and social interaction assays, which have previously been used in many studies (44, 45).
Nest construction ability is a common affiliative and social behavior of mice. Nests are built to facilitate reproduction and shelter (46). We have demonstrated impairment of nesting ability of APP/PS1 versus control mice (26). This is likely the consequence of Aβ deposition and neuroinflammation in the peripheral cortex and the hippocampus (22), that is, the brain regions responsible for social memory that are impaired in AD (47). Impaired social behaviors have also been reported in other transgenic mouse models of AD (48, 49). In this study, nest construction ability was significantly improved by forskolin treatment after 10 days of treatment.
Sociability was defined in this study as the tendency to approach and remain proximal to an unfamiliar stranger mouse. Therefore, the test mouse had the choice between avoidance of the stranger mouse by remaining in the center chamber and exploring or being in contact with the stranger mouse. After treatment with forskolin, the treated mice spent more time in the side of the cage containing the stranger (ie, unfamiliar) mice versus the time spent in the empty side than did the control mice. Previous work in social recognition has shown that C57BL/6J, DBA/2J, and FVB/NJ mice exhibited similar sociability (24). On the other hand, in the social novelty assay, which is defined as the tendency to initiate social contacts with a new mouse versus a previous familiar mouse, no significant difference was observed between the control and the forskolin-treated group. This could be interpreted as a demonstration that the mice preferred to remain with the familiar stranger 1 mice, rather than going into contact or approaching a new unfamiliar stranger mice 2. It was also an indication of the ability of the forskolin-treated mice to discriminate between 2 strangers and to recognize only the one that was familiar and already previously encountered. Considering the short term of treatment with forskolin (ie, 10 days), a reduction of Aβ plaque deposition in the cortex and hippocampus of mice, together with attenuation of inflammatory reaction by the brain immune cells may explain the improved nest construction capacity and social behavior.
Forskolin has shown efficient anti-inflammatory activity on macrophages and microglia in animal models of rheumatoid arthritis through attenuating TNF production and activation of AC (50). This suggests the potential effect of forskolin in neurodegenerative diseases because microglia are regarded as the main immune cells of the brain that play an important role in the clearance of amyloid β plaques (19). Our present histological and immunohistochemical data show beneficial effects of forskolin on Iba-1-positive cells and their association with Aβ plaques.
TGF-β1 is a polypeptide that is reported to play a key role in the aggravation of inflammation. Although increased TGF-β1 has been reported in aged transgenic mouse AD models, the specific role of TGF-β1 in AD remains elusive. Some studies have suggested a protective effect of TGF-β1 in AD by promoting Aβ clearance through activation of microglia (51). Several studies further mentioned that TGF-β1 increased the production of APP and subsequent Aβ generation in murine and human astrocyte cultures (36). Many other studies reported that transgenic mice overexpressing TGF-β1 in astrocytes elicit Aβ deposition (52) and that inhibition of TGF-β1 may provide protection against neurodegeneration through the alleviation of microglia-mediated inflammation (53). Here, we observed a decrease of TGF-β1 IR in the cortex of transgenic mice treated with forskolin along with other indicators of reduced inflammation, that is, the reduction of astrocyte GFAP and astrocyte association with plaques.
Neuropathological alterations in the hippocampus in APP/PS1 transgenic mice occur later than in the cortex, that is, deposition of Aβ plaques starts at 5 months of age. Because there is an exponential increase in Aβ deposition at around 5 months of age, we started the treatment at that age but this explains why the reductions in Aβ plaque numbers were not significant in the hippocampi of forskolin-treated mice when treatment was given at that time point.
In summary, the results obtained in this study showed the protective effects of forskolin in APP/PS1 transgenic mice. Forskolin treatment restored impairment in nesting ability and sociability. It also reduced or inhibited Aβ accumulation in the cortex, reduced microglial activation and TGF-β1 and GFAP IR in the cortex. Taken together, our findings suggest that forskolin may be considered a promising drug for AD patient therapy.
ACKNOWLEDGMENT
The authors would like to thank Prof. M. Jucker for providing male transgenic APP/PS1 mice for breeding.
REFERENCES
- 1.Shivaprasad HN, Gopalakrishna S, Mariyanna B, et al. Effect of Coleus forskohlii extract on cafeteria diet-induced obesity in rats. Pharmacognosy Res 2014;6:42–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virgona N, Taki Y, Umegaki K. A rapid HPLC with evaporative light scattering method for quantification of forskolin in multi-herbal weight-loss solid oral dosage forms. Pharmazie. 2010;65:322–6 [PubMed] [Google Scholar]
- 3.Barad M, Bourtchouladze R, Winder DG, et al. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A 1998;95:15020–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block F, Schmidt W, Nolden-Koch M, et al. Rolipram reduces excitotoxic neuronal damage. Neuroreport 2001;12:1507–11 [DOI] [PubMed] [Google Scholar]
- 5.Reneerkens OA, Rutten K, Steinbusch HWM, et al. Selective phosphodiesterase inhibitors: A promising target for cognition enhancement. Psychopharmacology (Berl) 2009;202:419–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han P, Caselli RJ, Baxter L, et al. Association of pituitary adenylate cyclase-activating polypeptide with cognitive decline in mild cognitive impairment due to Alzheimer disease. JAMA Neurol 2015;72:333–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garlind A, Johnston JA, Algotsson A, et al. Decreasedβ-adrenoceptor-stimulated adenylyl cyclase activity in lymphocytes from Alzheimer’s disease patients. Neurosci Lett 1997;226:37–40 [DOI] [PubMed] [Google Scholar]
- 8.Chong YH, Shin SA, Lee HJ, et al. Molecular mechanisms underlying cyclic AMP inhibition of macrophage dependent TNF-alpha production and neurotoxicity in response to amyloidogenic C-terminal fragment of Alzheimer’s amyloid precursor protein. J Neuroimmunol 2002;133:160–74 [DOI] [PubMed] [Google Scholar]
- 9.Ohm T, Bohl J, Lemmer B. Reduced basal and stimulated (isoprenaline, Gpp(NH)p, forskolin) adenylate cyclase activity in Alzheimer’s disease correlated with histopathological changes. Brain Res 1991;540:229–36 [DOI] [PubMed] [Google Scholar]
- 10.Liljegren M, Naasan G, Temlett J, et al. Criminal behavior in frontotemporal dementia and Alzheimer disease. JAMA Neurol 2015;94158:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, Li X, Gao P, et al. Baicalin attenuates Alzheimer-like pathological changes and memory deficits induced by amyloid β1-42 protein. Metab Brain Dis 2015;30:537–44 [DOI] [PubMed] [Google Scholar]
- 12.Lim GP, Chu T, Yang F, et al. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci 2001;21:8370–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granzotto A, Zatta P. Resveratrol and Alzheimer’s disease: Message in a bottle on red wine and cognition. Front Aging Neurosci 2014;6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo KY, Park SY. Terpenoids as potential anti-Alzheimer’s disease therapeutics. Molecules 2012;17:3524–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren Y, Houghton PJ, Hider RC, et al. Novel diterpenoid acetylcholinesterase inhibitors from Salvia miltiorhiza. Planta Med 2004;70:201–4 [DOI] [PubMed] [Google Scholar]
- 16.Seamon KB, Padgett W, Daly JW. Forskolin: Unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A 1981;78:3363–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Insel PA, Ostrom RS. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol 2003;23:305–14 [DOI] [PubMed] [Google Scholar]
- 18.Yan SZ, Huang ZH, Andrews RK, et al. Conversion of forskolin-insensitive to forskolin-sensitive (mouse-type IX) adenylyl cyclase. Mol Pharmacol 1998;53:182–7 [DOI] [PubMed] [Google Scholar]
- 19.Majumdar A, Cruz D, Asamoah N, et al. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol Biol Cell 2007;18:1490–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagatsu T, Sawada M. Inflammatory process in Parkinson’s disease: Role for cytokines. Curr Pharm Des 2005;11:999–1016 [DOI] [PubMed] [Google Scholar]
- 21.Holcomb L, Gordon MN, McGowan E, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med 1998;4:97–100 [DOI] [PubMed] [Google Scholar]
- 22.Wesson DW, Wilson DA. Age and gene overexpression interact to abolish nesting behavior in Tg2576 amyloid precursor protein (APP) mice. Behav Brain Res 2011;216:408–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Zug C, Qu H, et al. Hesperidin ameliorates behavioral impairments and neuropathology of transgenic APP/PS1 mice. Behav Brain Res 2015;281:32–42 [DOI] [PubMed] [Google Scholar]
- 24.Moy SS, Nadler JJ, Perez A, et al. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes Brain Behav 2004;3:287–302 [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Zhang ZY, Fauser U, et al. FTY720 ameliorates experimental autoimmune neuritis by inhibition of lymphocyte and monocyte infiltration into peripheral nerves. Exp Neurol 2008;210:681–90 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z-Y, Schluesener HJ. Oral administration of histone deacetylase inhibitor MS-275 ameliorates neuroinflammation and cerebral amyloidosis and improves behavior in a mouse model. J Neuropathol Exp Neurol 2013;72:178–85 [DOI] [PubMed] [Google Scholar]
- 27.Dao TT, Le TVT, Nguyen PH, et al. SIRT1 inhibitory diterpenoids from the vietnamese medicinal plant croton tonkinensis. Planta Med 2010;76:1011–4 [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Lim JY, Sul D, et al. Neuroprotective effects of the new diterpene, CBNU06 againstβ-amyloid-induced toxicity through the inhibition of NF-κB signaling pathway in PC12 cells. Eur J Pharmacol 2009;622:25–31 [DOI] [PubMed] [Google Scholar]
- 29.Hatjis CG. Forskolin: Unique diterpene activator of adenylate cyclase in pregnant and nonpregnant guinea pig myometrial membranes. Am J Obstet Gynecol 1986;155:1202–8 [DOI] [PubMed] [Google Scholar]
- 30.Davis RL, Cherry J, Dauwalder B, et al. The cyclic AMP system and Drosophila learning. Mol Cell Biochem 1995;149-150:271–8 [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto-Sasaki M, Ozawa H, Saito T, et al. Impaired phosphorylation of cyclic AMP response element binding protein in the hippocampus of dementia of the Alzheimer type. Brain Res 1999;824:300–3 [DOI] [PubMed] [Google Scholar]
- 32.Ramos BP, Birnbaum SG, Lindenmayer I, et al. Dysregulation of protein kinase A signaling in the aged prefrontal cortex: New strategy for treating age-related cognitive decline. Neuron 2003;40:835–45 [DOI] [PubMed] [Google Scholar]
- 33.Sun M-K, Alkon DL. Open space swimming test to index antidepressant activity. J Neurosci Methods 2003;126:35–40 [DOI] [PubMed] [Google Scholar]
- 34.Alasbahi RH, Melzig MF. Plectranthus barbatus: A review of phytochemistry, ethnobotanical uses and pharmacology part 2. Planta Med 2010;76:753–65 [DOI] [PubMed] [Google Scholar]
- 35.Minguet S, Huber M, Rosenkranz L, et al. Adenosine and cAMP are potent inhibitors of the NF-κB pathway downstream of immunoreceptors. Eur J Immunol 2005;35:31–41 [DOI] [PubMed] [Google Scholar]
- 36.Gray CW, Patel AJ. Regulation of β-amyloid precursor protein isoform mRNAs by transforming growth factor-β1 and interleukin-1β in astrocytes. Mol Brain Res 1993;19:251–6 [DOI] [PubMed] [Google Scholar]
- 37.Birnbaum SG, Yuan PX, Wang M, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science 2004;306:882–4 [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z-Y, Daniels R, Schluesener HJ. Oridonin ameliorates neuropathological changes and behavioural deficits in a mouse model of cerebral amyloidosis. J Cell Mol Med 2013;17:1566–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Haan W, Mott K, van Straaten EC, et al. Activity dependent degeneration explains hub vulnerability in Alzheimer’s disease. PLoS Comput Biol 2012;8:e1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Starkstein SE, Jorge R, Mizrahi R, et al. The construct of minor and major depression in Alzheimer’s disease. Am J Psychiatry 2005;162:2086–93 [DOI] [PubMed] [Google Scholar]
- 41.Van Dam D, De Deyn PP. Animal models in the drug discovery pipeline for Alzheimer’s disease. Br J Pharmacol 2011;164:1285–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bromley-Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J Vis Exp 2011;53:e2920, doi:10.3791/2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Filali M, Lalonde R, Rivest S. Cognitive and non-cognitive behaviors in an APPswe/PS1 bigenic model of Alzheimer’s disease. Genes Brain Behav 2009;8:143–8 [DOI] [PubMed] [Google Scholar]
- 44.García-Mesa Y, López-Ramos JC, Giménez-Llort L, et al. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J Alzheimers Dis 2011;24:421–54 [DOI] [PubMed] [Google Scholar]
- 45.Torres-Lista V, Giménez-Llort L. Impairment of nesting behaviour in 3xTg-AD mice. Behav Brain Res 2013;247:153–7 [DOI] [PubMed] [Google Scholar]
- 46.Deacon RMJ. Assessing nest building in mice. Nat Protoc 2006;1:1117–9 [DOI] [PubMed] [Google Scholar]
- 47.Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci 2001;2:51–61 [DOI] [PubMed] [Google Scholar]
- 48.Filali M, Lalonde R, Rivest S. Anomalies in social behaviors and exploratory activities in an APPswe/PS1 mouse model of Alzheimer’s disease. Physiol Behav 2011;104:880–5 [DOI] [PubMed] [Google Scholar]
- 49.Schwert GW. Anomalies and market efficiency. Soc Sci Res Netw 2002;1:939–74 [Google Scholar]
- 50.BHC. Rheumatoid arthritis. Better Health Channel\State Government of Victoria 2013;164:1285–300 [Google Scholar]
- 51.Wyss-Coray T, Lin C, Yan F, et al. TGF- β1 promotes microglial amyloid- β clearance and reduces plaque burden in transgenic mice. Nat Med 2001;7:612–8 [DOI] [PubMed] [Google Scholar]
- 52.Wyss-Coray T, Masliah E, Mallory M, et al. Amyloidogenic role of cytokine TGF- β1 in transgenic mice and in Alzheimer’s disease. Nature 1997;389:603–6 [DOI] [PubMed] [Google Scholar]
- 53.Shen W-X, Chen J-H, Lu J-H, et al. TGF-β1 protection against Aβ1–42-Induced neuroinflammation and beurodegeneration in rats. Int J Mol Sci 2014;15:22092–108 [DOI] [PMC free article] [PubMed] [Google Scholar]