Abstract
The Heliothinae complex in Argentina encompasses Helicoverpa gelotopoeon (Dyar), Helicoverpa zea (Boddie), Helicoverpa armigera (Hübner), and Chloridea virescens (Fabricius). In Tucumán, the native species H. gelotopoeon is one of the most voracious soybean pests and also affects cotton and chickpea, even more in soybean-chickpea succession cropping systems. Differentiation of the Heliothinae complex in the egg, larva, and pupa stages is difficult. Therefore, the observation of the adult wing pattern design and male genitalia is useful to differentiate species. The objective of this study was to identify the species of the Heliothinae complex, determine population fluctuations of the Heliothinae complex in soybean and chickpea crops using male moths collected in pheromone traps in Tucuman province, and update the geographical distribution of H. armigera in Argentina. The species found were H. gelotopoeon, H. armigera, H. zea, and C. virescens. Regardless of province, county, crop, and year, the predominant species was H. gelotopoeon. Considering the population dynamics of H. gelotopoeon and H. armigera in chickpea and soybean crops, H. gelotopoeon was the most abundant species in both crops, in all years sampled, and the differences registered were significant. On the other hand, according to the Sistema Nacional Argentino de Vigilancia y Monitoreo de Plagas (SINAVIMO) database and our collections, H. armigera was recorded in eight provinces and 20 counties of Argentina, and its larvae were found on soybean, chickpea, sunflower crops and spiny plumeless thistle (Carduus acanthoides). This is the first report of H. armigera in sunflower and spiny plumeless thistle in Argentina.
Keywords: Fabaceae, invasive species, old world bollworm, population fluctuations
The Heliothinae (Lepidoptera: Noctuidae) complex encompasses 381 described species, distributed in 28 genera (Pogue 2013). The most economically important species included in Helicoverpa Hardwick and Chloridea Duncan and (Westwood) are polyphagous. The females lay eggs, and the larval stage can survive and feed on a very wide range of host plant species. Many of these hosts are crops, including many field crops: cotton (Gossypium hirsutum L.; Malvales: Malvaceae), sorghum (Sorghum spp. Moench; Poales: Poaceae), sunflower (Helianthus annuus L.; Asterales: Asteraceae), chickpea (Cicer arietinum L.; Fabales: Fabaceae), alfalfa (Medicago sativa L.; Fabales: Fabaceae), lupins (Lupinus spp. L.; Fabales: Fabaceae), soybean (Glycine max L.; Fabales: Fabaceae), tobacco (Nicotiana tabacum L.; Solanales: Solanaceae), corn (Zea mays L.; Poales: Poaceae), wheat (Triticum spp. L.; Poales: Poaceae), horticultural crops such as tomatoes (Solanum lycopersicum L.; Solanales: Solanaceae), lettuce (Lactuca sativa L.; Asterales: Asteraceae), capsicum (Capsicum annuum L.; Solanales: Solanaceae), various bean crops, and flowers such as chrysanthemum (Chrysanthemum spp. L.; Asterales: Asteraceae), gladiolus (Gladiolus spp. L.; Asparagales: Iridaceae), and rose (Rosa spp. L.; Rosales: Rosaceae) (Cunningham and Zalucki 2014).
The native species of Helicoverpa in South America are divided into two groups: (a) gelotopoeon, including Helicoverpa gelotopoeon (Dyar), Helicoverpa titicacae Hardwick, Helicoverpa bracteae Hardwick, and Helicoverpa atacamae Hardwick; and (b) zea, including only H. zea (Boddie). Species of Chloridea are represented by Chloridea virescens (Fabricius), Chloridea tergemina (Felder and Rogenhofer), and Chloridea subflexa (Guenée) (Mitter et al. 1993, Pastrana 2004, Pogue 2013).
In the gelotopoeon group, species can only be separated by morphological characters of the adults, since the biology and morphology of the immature stages remain unknown (Navarro et al. 2009). Recently, H. armigera (Hübner) has been detected in Brazil (Czepak et al. 2013, Specht et al. 2013, Tay et al. 2013), Paraguay (SENAVE 2013), Argentina (Murúa et al. 2014), Bolivia, Uruguay (Kriticos et al. 2015), Puerto Rico (NAPPO 2014), and the United States (NAPPO 2015). Thus, the presence of H. armigera in northwestern Argentina (NOA) has led to the inclusion of this species in the Heliothinae complex, together with H. gelotopoeon, H. zea, and C. virescens.
Helicoverpa gelotopoeon is a polyphagous pest and has been reported in cotton, alfalfa, sunflower, soybean, chickpea, and corn, to name a few crops it attacks. Larvae cause damage in the vegetative and reproductive plant growth stages. This species occurs in Argentina, Chile, southern Brazil, Paraguay, and Uruguay (Pastrana 2004, Navarro et al. 2009). In Tucumán and other provinces of Argentina, H. gelotopoeon causes severe damage to soybean crops and can be difficult to control with insecticides (Navarro et al. 2009, Scalora et al. 2012). Consequently, it causes a significant economic impact, because Argentina is the third major soybean producer in the world, covering an area of 20.1 million hectares (Bolsa de Cereales 2015). This insect species also affects cotton and chickpea crops, causing more severe problems when they are grown in succession cropping systems (Pastrana 2004, Fichetti et al. 2009, Navarro et al. 2009, Scalora et al. 2012).
The differentiation of the Heliothinae complex in the egg, larva, and pupa stages is very difficult, but adults can be distinguished by the pattern of wing veins and male genitalia using traditional taxonomic methods (Pogue 2004, Navarro et al. 2009). A further complication is distinguishing between H. armigera and the native H. zea (North and South America), since the latter is estimated to have diverged from the former only approximately 1.5–2 million years ago, and the same pheromone compounds, although in different concentrations, are found in both species (Pogue 2004, Witzgall et al. 2004, Behere et al. 2007). Thus, males of both species are attracted to sex pheromone lures released by females of both species in the field. Yet another complication is the fact that H. armigera and H. zea have been shown to hybridize in the laboratory and could well be doing the same in the field (Hardwick 1965, Laster and Hardee 1995, Laster and Sheng 1995). It is unclear whether H. armigera can hybridize with other endemic Heliothinae species such as H. gelotopoeon. Therefore, genitalic dissection or molecular techniques are necessary to accurately identify adult males collected in pheromone traps (Pogue 2004, Behere et al. 2008, Specht et al. 2013, Tay et al. 2013, Leite et al. 2014, Arneodo et al. 2015, Kriticos et al. 2015). Behere et al. (2008) reported the use of two partial mitochondrial DNA genes [cytochrome oxidase subunit I (COI), cytochrome b (Cyt b)] as markers to differentiate H. armigera, H. punctigera, H. assulta, and H. zea. Arneodo et al. (2015) designed a rapid and simple molecular tool to distinguish H. armigera from H. zea and H. gelotopoeon. The method was validated using Helicoverpa specimens collected across Argentina, and their identity was further corroborated by COI sequence and phylogenetic analysis.
Helicoverpa armigera seems to be well adapted to the climate of agricultural regions of South America and, according to Kriticos et al. (2015) it is spreading rapidly throughout Argentina, Bolivia, Brazil, Paraguay, and Uruguay, and most recently to Puerto Rico. However, no studies have been performed to investigate if H. armigera detected in these non-Brazilian locations were due to natural spread of Brazilian populations, or if they represented separate incursion events.
It is important to mention that H. armigera has developed resistance to insecticides and Cry proteins (Forrester et al. 1993, Armes et al. 1996, Li et al. 2007, Mahon et al. 2007, Gao et al. 2009, Liu et al. 2010, Bird and Downes 2014, Tay et al. 2015). Therefore, it is necessary to know how the Heliothinae complex is distributed in legumes of economic importance in NOA, considering the difficulty of identifying these species.
The objective of this study was to determine population fluctuations of the Heliothinae complex in soybean and chickpea crops using male moths collected in pheromone traps. The distribution of H. gelotopoeon in Tucumán province and an update of the geographical distribution of H. armigera in Argentina are also provided herein.
Materials and Methods
Identification of the Heliothinae Complex
Larvae were collected in chickpea and soybean fields in Tucumán and Santiago del Estero provinces. In chickpea, collections took place on September 12, 2013 in Viclos (27°10′38″S, 64°52′42″W). In soybean, larvae were collected on January 31 and February 26, 2014 in La Cocha (27°45′55″S, 65°30′6″W), on February 7, 2014 in Jiménez (26°52′51″S, 64°42′3″W), and on February 15, 2015 in San Agustín (26°50′21″S, 64°51′32″W). Samples were collected during vegetative and reproductive stages in both crops. One hectare was monitored in each locality. In each hectare, 10–15 plants of soybean or chickpea were sampled at each of ten locations. Larvae were collected using a sheet method as described by Drees and Rice (1985).
The collected larvae were taken to the laboratory and reared on artificial diet, which included dry bean flour, wheat germ (Grandiet, Buenos Aires, Argentina), brewer’s yeast (Calsa, Tucumán, Argentina), vitamin C, sorbic acid (Anedra, Buenos Aires, Argentina), vitamin supplement, amino acids (Ruminal, Buenos Aires, Argentina), and methylparaben (Todo Droga, Córdoba, Argentina) (Murúa et al. 2003), in growth chambers under controlled conditions [27 ± 2°C, 70–75% relative humidity, 14:10 (L:D) h cycles] until adult emergence. This diet has been used to rear other polyphagous lepidopteran species such as Spodoptera frugiperda (J. E. Smith), Diatraea saccharalis (Fabricius), Rachiplusia nu (Guenée), and Chrysodeixis includens (Walker) (Lepidoptera: Noctuidae) (Murúa et al. 2003, 2008; Prieto et al. 2008, Barrionuevo et al. 2012). Only male moths were examined using genitalic observation to confirm the species according to Velasco de Estacul et al. (1969) and Pogue (2004). The females collected were not considered for this study, which might have affected the accuracy of data regarding species composition in the Heliothinae complex, mainly in species with unequal sex ratios. Some insects of each of these populations were deposited as voucher specimens in the collection of Sección Zoología Agrícola, at Estación Experimental Agroindustrial Obispo Colombres, Tucumán province, Argentina.
Population Fluctuations
Male moths were sampled in chickpea and soybean crops from 2013 to 2015 in Tucumán province. Traps were placed in chickpea crops from August to October 2013 in Viclos, and from July to December 2014 in San Agustín. In soybean, traps were placed from January to June 2014 and from January to May 2015 in San Agustín. Yellow traps (Unitrap, ChemTica Internacional S.A., Heredia, Costa Rica) were used and installed according to manufacturer’s recommendations. The lures were changed every 20 or 30 d and the insects were collected every 7 or 10 d. Traps were baited with H. armigera and H. gelotopoeon pheromones (ChemTica Internacional S.A., Heredia, Costa Rica). Growth stages of chickpea and soybean crops were determined based on the description by Soltani et al. (2006) and Fehr et al. (1971), respectively.
The data obtained were tested for normality using the Shapiro–Wilk test. The data regarding H. gelotopoeon and H. armigera adults obtained from each trap that did not show normal distribution or homogeneity of variance were subjected to a square root transformation [√(X + 0.5)]. The transformed data were analyzed using a t-test to detect differences between the adults of both species collected on both crops from different years. All the analyses were conducted using the software InfoStat (2006).
Geographical Distribution of H. armigera
Pheromone and light traps were used to update the distribution of this noctuid pest in Tucumán province. As described previously, males were collected using pheromone traps in San Agustín (Cruz Alta). A light trap (A todo campo, Buenos Aires, Argentina) was installed in Las Talitas (26°47′S, 65°11′W) from September to November 2014 and only male moths were selected for further identification. Moreover, larvae of this species were caught directly from sunflower, and spiny plumeless thistle (Carduus acanthoides L.; Asterales: Asteraceae).
To report the geographical distribution of H. armigera in other regions of Argentina, the database of Sistema Nacional Argentino de Vigilancia y Monitoreo de Plagas (SINAVIMO) of the Servicio Nacional de Sanidad y Calidad Agroalimentaria (SENASA) was utilized.
Results
Identification of Heliothinae complex
A total of 880 larvae were collected from 2013 to 2015 on chickpea and soybean crops and 416 male moths were obtained and identified. Species identification used adult males only, because using male genitalic characters are more reliable than using females (Perera et al. 2015). Female obtained were 394 and remainder of larvae died by unknown reasons. The species found were determined as H. gelotopoeon, H. armigera, H. zea, and C. virescens. Regardless of province, county, crop, and year, the predominant species was H. gelotopoeon (Table 1).
Table 1.
Larvae of the Heliothinae complex (Helicoverpa spp. and Chloridea virescens) collected in Viclos, La Cocha, San Agustín and Jiménez counties in chickpea and soybean crops used to identify the adults of each species
| Province | County | Crop | Date | Larvae collected (no.) | Males obtained and identifieda (no.) | H. gelotopoeon | H. armigera | H. zea | C. virescens |
|---|---|---|---|---|---|---|---|---|---|
| Tucumán | Viclos | Chickpea | September 12 2013 | 250 | 96 | 94 | 1 | 0 | 1 |
| La Cocha | Soybean | January 31 2014 | 200 | 94 | 89 | 4 | 1 | 0 | |
| February 26 2014 | 100 | 54 | 53 | 1 | 0 | 0 | |||
| San Agustín | Soybean | January 15 2015 | 180 | 95 | 95 | 0 | 0 | 0 | |
| Santiago del Estero | Jiménez | Soybean | February 15 2014 | 150 | 77 | 71 | 2 | 3 | 1 |
aOnly male moths obtained from larvae collected were examined using genitalia to confirm the species. Females obtained were not considered for this study.
Population Fluctuations
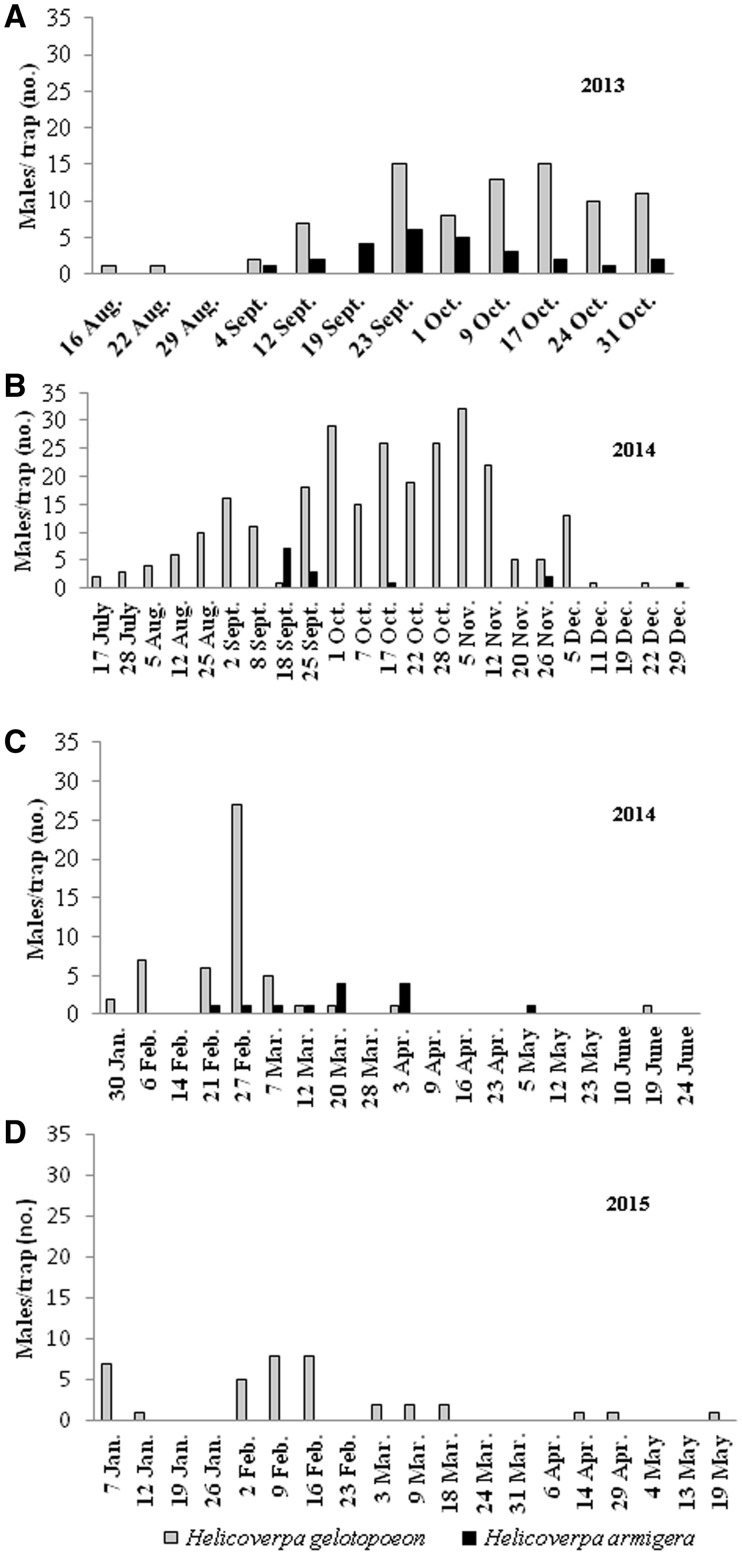
Population fluctuations of H. gelotopoeon and H. armigera males in chickpea and soybean crops are shown in Fig. 1. Considering the total number of moths obtained (H. gelotopoeon, n = 440; H. armigera, n = 53), H. gelotopoeon was the most abundant species in both crops, in all years sampled, and the difference in number of moths between the two crops was statistically significant (t = −6.15; df = 89; P < 0.0001). In chickpea (Fig. 1a and b), the first species that appeared was the native, H. gelotopoeon, and it was present, in general, during the entire crop cycle in 2013 and 2014. The largest collections of H. gelotopoeon and H. armigera were from October to November and from September to October in 2013, respectively, and adults were only collected in some weeks from September to November in 2014.
Fig. 1.
Population fluctuations of H. gelotopoeon and H. armigera adults, in chickpea and soybean crops, from 2013 to 2015, in Tucumán province, Argentina. Chickpea crop: (A) Viclos county; (B) San Agustín county. Soybean crop: (C, D) San Agustín county.
In soybeans (Fig. 1c and d), the largest collection of H. gelotopoeon was recorded in February in both years and, in general, this pest was present during all months in 2015. Male H. armigera individuals were only recorded in 2014 from mid-February to April. In 2014, male H. gelotopoeon specimens were observed before H. armigera.
Geographical Distribution of H. armigera
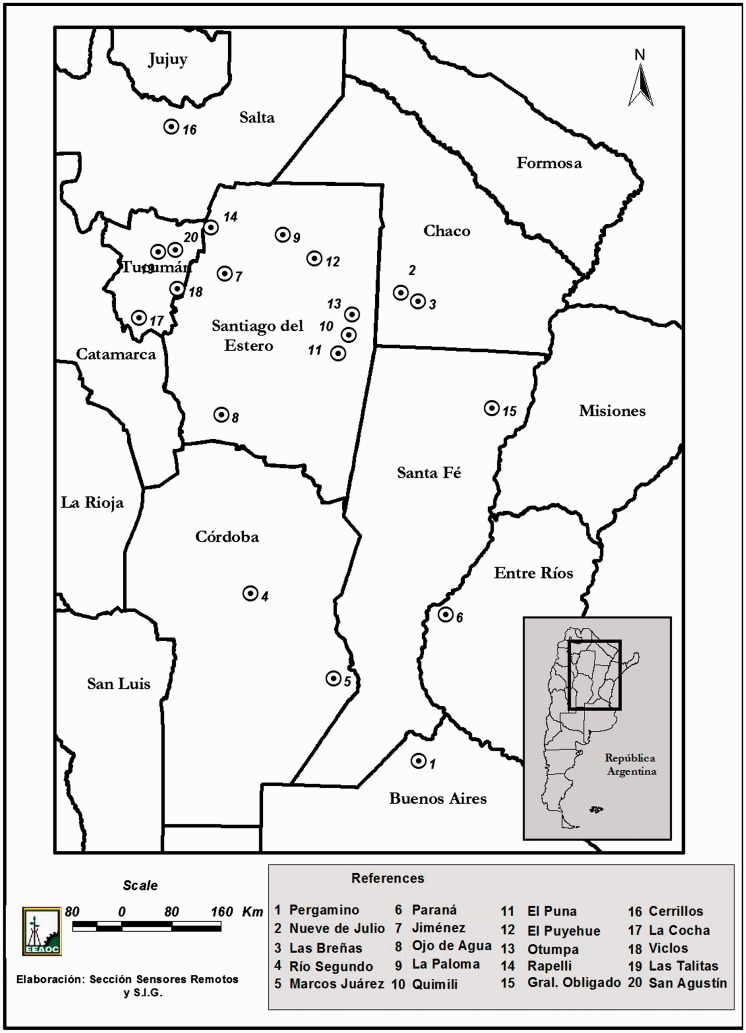
Considering our collections, H. armigera was found in two counties (Las Talitas and San Agustín) of Tucumán province and one county (Jiménez) of Santiago del Estero province, and these were new records for both provinces. Larvae of this species were recorded on soybean, chickpea, sunflower, and spiny plumeless thistle. This is the first report of H. armigera in sunflower and spiny plumeless thistle in Argentina (Table 2 and Fig. 2).
Table 2.
Geographical distribution of H. armigera in Argentina
| Province | Sampling site | Elevation (masl) | Stage collected | Collection/host plant | Month/year |
|---|---|---|---|---|---|
| Buenos Aires | 1 Pergaminoa | 56 | Adults | Pheromone trap/soybean crops | July 2014 |
| Chaco | 2 Nueve de Juliob | 50 | Adults | Cotton and soybean | July 2014 |
| 3 Las Breñasb | 105 | Adults | Pheromone trap | October 2014 to January 2015 | |
| Córdoba | 4 Río Segundoa | 335 | Adults | Pheromone traps | June 2014 |
| 5 Marcos Juáreza | 112 | Adults | Light trap near cereals and oleaginous crops | November 2014 | |
| Entre Ríos | 6 Paranáa | 77 | Adults | Pheromone traps near to soybean and corn crops | July 2014 |
| Santiago del Estero | 7 Jiméneza | 1,030 | Larvae | Soybean | January 2014 |
| 8 Ojo de Aguaa | 600 | Adults | Pheromone traps on soybean crops | February 2015 | |
| 9 La Palomaa | 606 | Adults | Pheromone traps on soybean crops | February 2015 | |
| 10 Quimilia | 137 | Adults | Pheromone traps | June 2015 | |
| 11 El Punaa | 130 | Adults | Pheromone traps | June 2015 | |
| 12 El Puyehuea | 191 | Adults | Pheromone traps | June 2015 | |
| 13 Otumpaa | 155 | Adults | Pheromone traps | June 2015 | |
| 14 Rapellib | 395 | Larvae | Chickpea | October 2014 to January 2015 | |
| Santa Fé | 15 General Obligadoa | 21 | Adults | Cotton | May 2014 |
| Salta | 16 Cerrillosa | 1,217 | Adults | Chickpea | November 2014 |
| Tucumán | 17 La Cocha | 397 | Adultsc | Chickpea | November 2013 |
| Larvae | Soybean | February 2014 | |||
| 18 Viclos | 345 | Adultsc and larvae | Chickpea | September 2013 | |
| Larvae | Spiny plumeless thistle | September 2014 | |||
| 19 Las Talitas | 591 | Adults | Light trap near to sugarcane crops and lemon tree fields | October 2014 | |
| 20 San Agustín | 1,190 | Adults | Pheromone traps on soybean crops | January–June 2014 | |
| Larvae | Sunflower | January 2015 |
Database of SINAVIMO.
Data reported by Arneodo et al. (2015).
Data used to report the first time of H. armigera collection in Argentina (Murúa et al. 2014).
Fig. 2.
Geographical distribution of H. armigera in different provinces of Argentina.
Taking into account SINAVIMO database, the reports of Arneodo et al. (2015), and our own collections, H. armigera was recorded in eight provinces and 20 counties in Argentina (Table 2 and Fig. 2).
Discussion
In Tucumán province, Argentina, H. gelotopoeon, the native species, was the most abundant and predominant Heliothinae in chickpea and soybean crops. Our results showed that H. gelotopoeon and H. armigera had a seasonal distribution in both crops during all years studied in Tucumán province, being more prevalent in September and October on chickpea and during February and March on soybean.
The influence of crops on the abundance of H. armigera was reported by Maelzer and Zalucki (1999). The authors found that alfalfa and corn always had positive effects, whereas sorghum presented a negative effect on the light-trap catch in the second and third generations.
Considering crop phenology, Ahmed and Khalique (2012) observed that the appearance of H. armigera always coincided with the initiation of flowering of chickpea, irrespective of the fact of how early or late the crop started flowering. In our study, an increase in population of both species was detected from September to October, coinciding with the beginning of the podding stage (R3) of chickpea. In soybean, H. armigera was not abundant and the first adults were detected at R2, by the end of February, whereas H. gelotopoeon adults were found from V3, in the beginning of January, in both years.
The results in this study indicate that H. armigera is widely distributed and the species has been adapted to elevations ranging from 20 to 1,200 masl in Argentina. Zhou et al. (2000), Rochester et al. (2002), and Feng et al. (2004) reported that H. armigera adults are highly mobile. They can move many hundreds of kilometers between regions and extensively between fields within regions. Helicoverpa armigera seems to be well adapted to the climate of agricultural regions of South America. According to Kriticos et al. (2015), this insect species has spread rapidly throughout Argentina, Bolivia, Brazil, Paraguay, Uruguay, and most recently to Puerto Rico. However, no studies have been conducted to investigate whether H. armigera detected in these non-Brazilian locations were due to natural spread of Brazilian populations or they represented separate incursion events. In addition, H. armigera has been detected in Brazil in October 2008 (Sosa-Gomez et al. 2016), meaning that the presence of this species in other countries could be due to late detection more than to fast spreading. Also, the detection in Brazil does not mean that this country has been the entrance of this insect species to Latin American countries. Helicoverpa armigera could have invaded South America through other countries where its identification might have been neglected.
So far, in Argentina, larvae of H. armigera have been found on chickpea (Arneodo et al. 2015, SINAVIMO 2015), soybean, cotton (SINAVIMO 2015), sunflower and spiny plumeless thistle. Studies performed in Brazil indicate a widespread distribution of H. armigera throughout Midwest and Northeast Brazil in a variety of crops, particularly dicotyledons, beans (Phaseolus vulgaris L. Fabales: Fabaceae), soybean, and cotton, and to a lesser extent on monocotyledons, e.g., millet (Pennisetum glaucum L. Poales: Poaceae), sorghum, and corn (Leite et al. 2014).
Considering the importance of H. gelotopoeon in the region, few studies on the bioecology, crop damage, chemical control, natural enemies, host plants, oviposition preference or monitoring resistance to insecticides and Bt cultivars, have been performed. Our study is the first to provide information about its seasonal distribution.
Despite the low frequency of H. armigera, the results from the current study show that this pest is widely distributed in Argentina. Because of that, it is important to intensify the monitoring and correctly identify the different species of the Heliothinae complex using taxonomic methods or molecular techniques.
The sustainable management of this species poses a significant challenge for Argentinean entomology in the coming years, especially considering its polyphagous feeding habit, great dispersal ability, and numerous reports of resistance to insecticides and Bt crops (Asokan et al. 2012, Li et al. 2007, Gao et al. 2009, Liu et al. 2010, Nair et al. 2013, Bird and Downes 2014).
It is necessary to address different studies, as mentioned, in order to contribute to future studies regarding management for H. gelotopoeon and H. armigera in Argentina.
Acknowledgments
We thank, Lucas Fadda and David González, from EEAOC, for their assistance in the collection of material and excellent technical support in the laboratory; Javier Carreras Baldres (SrySig, EEAOC) for his assistance in the preparation of the map; SENASA NOA-Sur for providing database of H. armigera pheromone traps; Lic. E. Willink for his constructive comments on an earlier draft of the manuscript; Dr. Fernando Navarro [CONICET, Instituto Superior de Entomología Dr. Abraham Willink (INSUE), Facultad de Ciencias Naturales, Universidad Nacional de Tucumán] for the taxonomic identification of the Heliothinae complex adults, for his unconditional support, and for sharing memorable moments.
Funding
This study was supported by EEAOC, CONICET, Consejo de Investigaciones de la Universidad Nacional de Tucumán (CIUNT no. G535/26), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Project no. 308947/2014-2).
References Cited
- Ahmed K., Khalique F. 2012. Oviposition and larval development of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in relation with chickpea, Cicer arietinum L. (Fabaceae) crop phenology. Pak. J. Zool. 44: 1081–1089. [Google Scholar]
- Armes N. J., Jadhav D. R., DeSouza K. R. 1996. A survey of insecticide resistance in Helicoverpa armigera in the Indian subcontinent. Bull. Entomol. Res. 86: 499–514. [Google Scholar]
- Arneodo J. D., Balbi E. I., Flores F. M., Sciocco-Cap A. 2015. Molecular Identification of Helicoverpa armigera (Lepidoptera: Noctuidae: Heliothinae) in Argentina and development of a novel PCR-RFLP method for its rapid differentiation from H. zea and H. gelotopoeon. J. Econ. Entomol. 108: 2505–2510. [DOI] [PubMed] [Google Scholar]
- Asokan R., Nagesha S. N., Manamohan M., Krishnakumar N. K., Mahadevaswamy H. M., Rebijith K. B., Prakash M. N., Sharath Chandra G. 2012. Molecular diversity of Helicoverpa armigera Hübner (Noctuidae: Lepidoptera) in India. Orient. Insects. 46: 130–143. [Google Scholar]
- Barrionuevo M. J., Murúa M. G., Goane L., Meagher R., Navarro F. 2012. Life table studies of Rachiplusia nu (Guenée) and Chrysodeixis (= Pseudoplusia) includens (Walker) (Lepidoptera: Noctuidae) on artificial diet. Fla. Entomol. 95: 944–951. [Google Scholar]
- Behere G. T., Tay W. T., Russel D. A., Batterham P. 2008. Molecular markers to discriminate among four pest species of Helicoverpa (Lepidoptera: Noctuidae). Bull. Entomol. Res. 98: 599–603. [DOI] [PubMed] [Google Scholar]
- Behere G. T., Tay W. T., Russel D. A., Heckel D. G., Appleton B. R., Kranthi K. R., Batterham P. 2007. Mitochondrial DNA analysis of field populations of Helicoverpa armigera (Lepidoptera: Noctuidae) and of its relationship to H. zea. BMC Evol. Biol. 7: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird L. J., Downes S. J. 2014. Toxicity and cross-resistance of insecticides to Cry2Ab-resistant and Cry2Ab-susceptible Helicoverpa armigera and Helicoverpa punctigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 107: 1923–1930. [DOI] [PubMed] [Google Scholar]
- Bolsa de Cereales. 2015. Panorama Agrícola Semanal. Estimaciones Agrícolas. Relevamiento al 29/01/2015. http://www.bolsadecereales.com.ar.
- Cunningham J. P., Zalucki M. P. 2014. Understanding heliothine (Lepidoptera: Heliothinae) pests: what is a host plant? J. Econ. Entomol. 107: 881–896. [DOI] [PubMed] [Google Scholar]
- Czepak C., Albernaz K. C., Vivan L. M., Guimarães H. O., Carvalhais T. 2013. First reported occurrence of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Brazil. Pesq. Agropec. Trop. 43: 110–113. http://www.revistas.ufg.br/index.php/pat/article/view/23691/13928 [Google Scholar]
- Drees B. M., Rice M. E. 1985. The vertical beat sheet: a new device for sampling soybeans insects. J. Econ. Entomol. 78: 1507–1510. [Google Scholar]
- Fehr W. R., Caviness C. E., Burmood D. T., Pennington J. S. 1971. Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sci. 11: 929–931. [Google Scholar]
- Feng H. Q., Wu K. M., Cheng D. F., Guo Y. Y. 2004. Northward migration of Helicoverpa armigera (Lepidoptera: Noctuidae) and other moths in early summer observed with radar in northern China. J. Econ. Entomol. 97: 1874–1883. [DOI] [PubMed] [Google Scholar]
- Fichetti P., Avalos S., Mazzuferi V., Carreras J. 2009. Lepidópteros asociados al cultivo de garbanzo (Cicer arietinum L.) en Córdoba (Argentina). Bol. Sanid. Veg., Plagas. 35: 49–58. http://www.magrama.gob.es/ministerio/pags/Biblioteca/Revistas/pdf_Plagas%2FBSVP_35_01_49_58.pdf. [Google Scholar]
- Forrester N. W., Cahill M., Bird L. J., Layland J. K. 1993. Management of pyrethroid and endosulfan resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) in Australia. Bull. Ent. Res. 1: 1–132. [Google Scholar]
- Gao Y., Wu K., Gould F., Shen Z. 2009. Cry2Ab tolerance response of Helicoverpa armigera (Lepidoptera: Noctuidae) populations from Cry1Ac cotton planting region. J. Econ. Entomol. 102: 1217–1223. [DOI] [PubMed] [Google Scholar]
- Hardwick D. F. 1965. The corn earworm complex. Mem. Entomol. Soc. Can. 97: 5–247. [Google Scholar]
- InfoStat. 2006. InfoStat versión 2006, Manual del Usuario. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina, 1st ed. Editorial Brujas, Córdoba, Argentina.
- Kriticos D. J., Ota N., Hutchison W. D., Beddow J., Walsh T., Tay W. T., Borchert D. M., Paula-Moreas S. V., Czepak C., Zalucki M. P. 2015. The potential distribution of invading Helicoverpa armigera in North America: is it just a matter of time? PLoS One 10: e0133224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laster M. L., Hardee D. D. 1995. Intermating compatibility between North American Helicoverpa zea and Heliothis armigera (Lepidoptera: Noctuidae) from Russia. J. Econ. Entomol. 88: 77–80. [Google Scholar]
- Laster M. L., Sheng D. D. 1995. Search for hybrid sterility for Helicoverpa zea in crosses between the North American H. zea and H. armigera (Lepidoptera: Noctuidae) from China. J. Econ. Entomol. 88: 1288–1291. [Google Scholar]
- Leite N. A., Alves-Pereira A., Corrêa A. S., Zucchi M. I., Omoto C. 2014. Demographics and genetic variability of the new world bollworm (Helicoverpa zea) and the old world bollworm (Helicoverpa armigera) in Brazil. PLoS One 9: e113286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. P., Wu K. M., Gould F., Wang J. K., Miao J., Gao X. W., Guo Y. Y. 2007. Increasing tolerance to Cry1Ac cotton from cotton bollworm, Helicoverpa armigera, was confirmed in Bt cotton farming area of China. Ecol. Entomol. 32: 366–375. [Google Scholar]
- Liu F., Xu Z., Zhu Y. C., Huang F., Wang Y., Li H., Gao C., Zhou W., Shen J. 2010. Evidence of field-evolved resistance to Cry1Ac-expressing Bt cotton in Helicoverpa armigera (Lepidoptera: Noctuidae) in northern China. Pest. Manag. Sci. 66: 155–161. [DOI] [PubMed] [Google Scholar]
- Maelzer D. A., Zalucki M. P. 1999. Analysis of long-term light-trap data for Helicoverpa spp. (Lepidoptera: Noctuidae) in Australia: the effect of climate and crop host plants. Bull. Entomol. Res. 89: 455–463. [Google Scholar]
- Mahon R. J., Olsen K. M., Garsia K. S., Young S. R. 2007. Resistance to Bacillus thuringiensis toxin Cry2Ab in a strain of Helicoverpa armigera (Lepidoptera: Noctuidae) in Australia. J. Econ. Entomol. 100: 894–902. [DOI] [PubMed] [Google Scholar]
- Mitter C., Poole R. W., Matthews M. 1993. Biosystematics of the Heliothinae (Lepidoptera: Noctuidae). Annu. Rev. Entomol. 38: 207–225. [Google Scholar]
- Murúa M. G., Scalora F. S., Navarro F. R., Cazado L. E., Casmuz A., Villagrán M. E., Lobos E., Gastaminza G. 2014. First record of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Argentina. Fla. Entomol. 97: 854–856. [Google Scholar]
- Murúa M. G., Vera M. T., Abraham S., Juaréz M. L., Prieto S., Head G. P., Willink E. 2008. Fitness and mating compatibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) populations from different host plant species and regions in Argentina. Ann. Entomol. Soc. Am. 101: 639–649. [Google Scholar]
- Murúa M. G., Virla E. G., Defagó V. 2003. Evaluación de cuatro dietas artificiales para la cría de Spodoptera frugiperda (Lep.: Noctuidae) destinada a mantener poblaciones experimentales de himenópteros parasitoides. Bol. Sanid. Veg., Plagas. 29: 43–51. [Google Scholar]
- Nair R., Kalia V., Aggarwal K. K., Gujar G. T. 2013. Variation in the cadherin gene sequence of Cry1Ac susceptible and resistant Helicoverpa armigera (Lepidoptera: Noctuidae) and the identification of mutant alleles in resistant strains. Curr. Sci. 104: 215–223. [Google Scholar]
- NAPPO. North American Plant Protection Organization’s Phytosanitary Alert System. 2014. Detection of Old World Bollworm (Helicoverpa armigera) in Puerto Rico. http://www.pestalert.org/oprDetail.cfm?oprID=600.
- NAPPO. North American Plant Protection Organization’s Phytosanitary Alert System. 2015. Helicoverpa armigera (Old World Bollworm) - Detection in Florida. http://www.pestalert.org/oprDetail.cfm?oprID=629.
- Navarro F. R., Saini E. D., Leiva P. D. 2009. Clave pictórica de polillas de interés agrícola, agrupadas por relación de semejanza. INTA, EEA PERGAMINO, Pergamino, Buenos Aires, Argentina.
- Pastrana J. A. 2004. Los Lepidópteros Argentinos: sus plantas hospedadoras y otros sustratos alimenticios. Sociedad Entomológica Argentina Ediciones, Tucumán, Argentina.
- Perera O. P., Allen K. C., Jain D., Purcell M., Little N. S., Lutrell G. 2015. Rapid identification of Helicoverpa armigera and Helicoverpa zea (Lepidoptera: Noctuidae) using ribosomal RNA internal transcribed spacer 1. J. Insect. Sci. 15: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue M. G. 2004. A new synonym of Helicoverpa zea (Boddie) and differentiation of adult males of H. zea and H. armigera (Hübner) (Lepidoptera: Noctuidae: Heliothinae). Ann. Entomol. Soc. Am. 97: 1222–1226. [Google Scholar]
- Pogue M. G. 2013. Revised status of Chloridea Duncan and (Westwood), 1841, for the Heliothis virescens species group (Lepidoptera: Noctuidae: Heliothinae) based on morphology and three genes. Syst. Entomol. 38: 523–542. [Google Scholar]
- Prieto S., Murúa M. G., Juárez M. L., Willink E. 2008. Estudio de la distribución temporal y espacial de poblaciones de Diatraea saccharalis Fabricius (Smith) en diferentes plantas hospederas de provincias del norte de Argentina, p. 155. In Actas, 7th Congreso Argentino de Entomología, 21–24 October 2008, Huerta Grande, Córdoba, Argentina.
- Rochester W. A., Zalucki M. P., Ward A., Miles M., Murray D. A. H. 2002. Testing insect movement theory: empirical analysis of pest data routinely collected from agricultural crops. Comput. Electron. Agric. 35: 139–149. [Google Scholar]
- Scalora F., Casmuz A., Cazado L., Socías G., Tolosa G., Aralde M., Guchea M. A., Fadda L., Gómez M., Gómez H., et al. 2012. Evaluación de diferentes insecticidas para el control de la oruga bolillera, H. gelotopoeon Dyar (Lepidoptera: Noctuidae), pp. 147–151. In M.R. Devani, F. Ledesma, and J.R. Sánchez (eds.), El cultivo de la soja en el noroeste argentino. Campaña 2011/2012. Publicación Especial EEAOC nº 45. EEAOC, Las Talitas, Tuc, Argentina. http://www.eeaoc.org.ar/upload/publicaciones/archivos/286/20121122085717000000.pdf.
- SENAVE. Servicio Nacional de Calidad y Sanidad Vegetal y de Semillas. 2013. Alerta tras ingreso de peligrosa plaga agrícola. Color ABC, 17 October 2013. http://www.abc.com.py/edicion-impresa/economia/senave-en-alerta-tras-ingreso-de-peligrosa-plaga-agricola-629240.html.
- SINAVIMO. Sistema Nacional Argentino de Vigilancia y Monitoreo de Plagas. 2015. Helicoverpa armigera http://www.sinavimo.gov.ar/plaga/helicoverpa-armigera.
- Soltani A., Hammer G. L., Torabi B., Robertson M. J., Zeinali E. 2006. Modeling chickpea growth and development: phenological development. Field Crops Res. 99: 1–13. [Google Scholar]
- Sosa-Gómez, Specht D. R., A., Paula-Moraes S. V., Lopes-Lima A., Yano S. A. C., Micheli A., Morais E. G. F., Gallo P., Pereira P. R. V. S., Salvadori J. R., et al. 2016. Timeline and geographical distribution of Helicoverpa armigera (Hübner) (Lepidoptera, Noctuidae: Heliothinae) in Brazil. Revista Brasileira de Entomologia. 60: 101–104. [Google Scholar]
- Specht A., Sosa-Gómez D. R., Paula-Moraes S. V., Yano S. A. C. 2013. Identificação morfológica e molecular de Helicoverpa armigera (Lepidoptera: Noctuidae) e ampliação de seu registro de ocorrência no Brasil. Pesq. Agropec. Bras. 48: 689–692. [Google Scholar]
- Tay W. T., Mahon, Heckel R. J. D. G., Walsh T. K., Downes S., James W. J., Lee W. J. S. F., Reineke A., Williams A. K., Gordon K. H. J. 2015. Insect resistance to Bacillus thuringiensis toxin Cry2Ab is conferred by mutations in an ABC transporter subfamily A protein. PLoS Genet. 11: e1005534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay W. T., Soria M. F., Walsh T., Thomazoni D., Silvie P., Behere G. T. 2013. A brave New World for an Old World Pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. PLoS One 8: e80134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco de Estacul M., Barral J. M., Orfila R. N. 1969. Taxonomía, especificidad y caracteres biológicos diferenciados del complejo de especies denominadas “oruga del capullo” del algodón, “oruga de la espiga” del maíz, “oruga del brote” del tabaco y “bolillera” del lino. Rev. Invest. Agropec. Serie 5, Patología Vegetal. 6: 19–68. [Google Scholar]
- Witzgall P., Lindblom T., Bengtsson M., Tóth M. 2004. The Pherolist. http://www.pherolist.slu.se.
- Zhou X. F., Applebaum S. W., Coll M. 2000. Overwintering and spring migration in the bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) in Israel. Environ. Entomol. 29: 1289–1294. [Google Scholar]