Abstract
Current therapies for treating movement disorders such as Parkinson’s disease are effective but limited by undesirable and intractable side effects. Developing more effective therapies will require better understanding of what causes basal ganglia dys-regulation and why medication-induced side effects develop. Although basal ganglia have been extensively studied in the last decades, its circuit anatomy is very complex, and significant controversy exists as to how the interplay of different basal ganglia nuclei process motor information and output. We have recently identified the importance of an underappreciated collateral projection that bridges the striatal output direct pathway with the indirect pathway. These bridging collaterals are extremely plastic in the adult brain and are involved in the regulation of motor balance. Our findings add a new angle to the classical model of basal ganglia circuitry that could be exploited for the development of new therapies against movement disorders. In this Scientific Perspective, we describe the function of bridging collaterals and other recent discoveries that challenge the simplicity of the classical basal ganglia circuit model. We then discuss the potential implication of bridging collaterals in the pathophysiology of Parkinson’s disease and schizophrenia. Because dopamine D2 receptors and striatal neuron excitability have been found to regulate the density of bridging collaterals, we propose that targeting these projections downstream of D2 receptors could be a possible strategy for the treatment of basal ganglia disorders.
Keywords: Dopamine receptors, bridging collaterals, direct and indirect pathways, Parkinson’s disease, dyskinesia
If the 1980s were the golden age of basal ganglia (BG) research, establishing a model of their circuitry at both structural and functional levels has never been as controversial as today. Before the elaboration of the now “classical” direct/indirect pathway model, several seminal studies had provided anatomical and physiological descriptions of BG networks.1,2 In this early view, the striatum—the input structure of the BG—receives and integrates glutamatergic excitatory projections from the cortex and thalamus as well as neuromodulatory dopaminergic afferents from the midbrain. All of these inputs converge onto dendritic spines of gamma-aminobutyric acid (GABA)ergic inhibitory medium spiny neurons (MSNs), representing 95% of striatal neuron population, and large cholinergic interneurons.2 The direct/indirect pathway model of the BG network then emerged as an attempt to explain the clinical phenomenology of human basal ganglia disorders in general, and of Parkinson’s disease (PD) in particular.1–4 However, single-cell tracing studies5–10 as well as a recent report assessing the functional activity of BG networks in vivo11 have challenged this simplistic view and have suggested that the two output pathways are interconnected to coordinate their actions.
In a recent paper in Neuron,12 we have described the remarkable anatomical plasticity of axon collaterals emerging from the direct pathway that functionally bridge the two striatal output pathways into the external globus pallidus (GPe) and that control the balance of motor coordination. In this Scientific Perspective, we revisit the organization and the functioning of BG networks and incorporate our recent findings. We also discuss the implication of these collaterals and their regulation by dopamine D2 receptors in the context of PD and schizophrenia.
The Classical Model of Basal Ganglia Circuitry
The Direct and Indirect Pathways
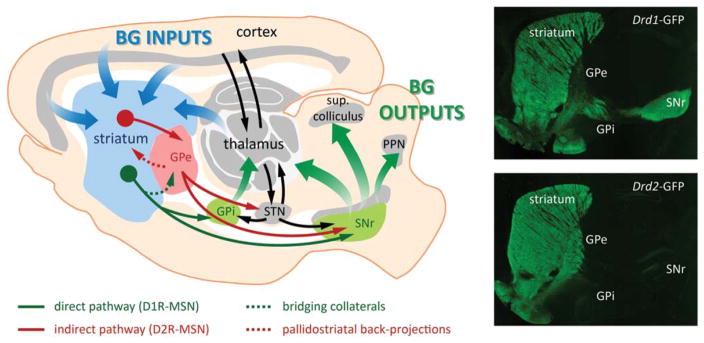
The classical model of BG networks emerged from a study using projection retro-labeling combined with molecular characterization of neuronal populations into the striatum.3 In this model, a dual organization in striatal output projections connect the striatum, as the BG input nucleus, to the output nuclei: MSNs that receive cortical, thalamic, and dopaminergic inputs project to the output nuclei (the internal globus pallidus [GPi] and the substantia nigra pars reticulata [SNr]) through a monosynaptic “direct” pathway and through a polysynaptic “indirect” pathway that involves relays in the GPe and in the subthalamic nucleus (STN) (Fig. 1). Because these striatal output neurons are GABAergic, activation of the direct pathway would promote movement initiation, whereas activation of the indirect pathway would inhibit movement,1,4 an idea that has recently been confirmed with optogenetic tools in freely moving mice.13
FIG. 1.
The classical model of basal ganglia (BG) circuits. The striatum, the main input nucleus of BG, receives excitatory corticostriatal and thalamic projections. Striatal D1R-MSNs form the direct pathway (green) that projects monosynaptically to the GPi and SNr output nuclei. The D2R-MSNs form the indirect pathway (red) that projects to the GPe, which subsequently projects to the subthalamic nucleus (STN) and SNr output nucleus. Outputs of the BG emerging from GPi and SNr nuclei are directed to the thalamus, superior colliculus, and pendunculopontine nucleus (PPN). Direct pathway bridging collaterals (dotted line, green) project to the GPe, and indirect pathway pallidostriatal axons (dotted line, red) project back to the striatum. These projections highlight the need for revisiting the global model of BG circuits as they play important roles in the control of the behavioral balance. Fluorescent imaging of a brain section from Drd1a-GFP (up) shows the presence of terminals in the GPi and SNr, but also in the GPe. Striatal Drd2-GFP neurons (down) project exclusively to the GPe. Adapted from Ting and Feng.15 MSN, medium spiny neurons; SNr, substantia nigra pars reticulata; GPi, internal globus pallidus; GPe, external globus pallidus.
Dopamine D1 and D2 Receptors
At the molecular level, MSNs from the direct pathway predominantly co-express dopamine D1 receptors (D1R), substance P, and dynorphin, whereas neurons from the indirect pathway express dopamine D2 receptors (D2R), adenosine A2a receptors (A2a), and enkephalin1,14,15 (Fig. 1). Because of this molecular dichotomy, dopaminergic input from the substantia nigra pars compacta (SNc) exerts a dual effect on striatal projection neurons: although D2R-expressing indirect pathway neurons are inhibited by dopamine, D1R-expressing direct pathway neurons are stimulated. In the parkinsonian state, reduced dopamine input from the SNc to the striatum would therefore facilitate indirect pathway neurons while reducing the activation of direct pathway neurons.3 Consequently, increased inhibition from indirect MSNs to the GPe disinhibits the STN, which then overdrives inhibitory output neurons in the GPi and SNr. In parallel, decreased activation of direct MSNs reduces the phasic inhibition on the GPi and SNr, thereby contributing to excessive activation of inhibitory basal ganglia output. These alterations in the function of the BG networks in PD patients result in the inability to initiate movements.1–4
Challenging the Simplicity of the Classical Model
Anatomical Evidence
The direct and indirect circuits are often described not only as functionally opposing but also as anatomically segregated. However, several anatomical studies have challenged this notion of pure anatomical segregation5–10. Reviewing single-neuron labeling studies performed in the rat,12 we found that approximately 40% of MSNs project exclusively to the GPe (“pure” indirect pathway), whereas a small minority (3%) project only to the SNr (“pure” direct pathway). Surprisingly, the remaining 60% of neurons projecting to the SNr possess collateral terminal fields in the GPe.5–7 Similar observations have been made in nonhuman primates.8–10 Because of their unique location at the interface of the two striatal output projections in the GPe, these axon collaterals may form a functional bridge between the two pathways. We therefore termed them “bridging collaterals.”
Neuronal populations in the GPe have been divided into parvalbumin-positive (PV-positive) and PV-negative cells that indistinctively receive inputs from indirect pathway neurons.16 We found that these bridging collaterals also make synaptic contacts with both PV-positive and PV-negative cells in the GPe. Using bacterial artificial chromosome (BAC) transgenic mice that express GFP under the control of the Drd1a or the Drd2 promoter (Drd1a-GFP and Drd2-GFP mice) and that allow us to visualize the axonal projections of both direct (Drd1a-GFP) and indirect (Drd2-GFP) pathways,14 we found that these bridging collaterals are extremely plastic in the adult animal and that they can grow or retract in the GPe within few days after manipulating either striatal neuron activity or dopamine D2R12 levels or function (Fig. 2). Their location at the interface of the two striatal output pathways and their dynamic regulation by dopamine receptors highlight the need for revisiting the simple view of the classical model of BG circuits that presents the direct and indirect pathways as parallel and segregated.
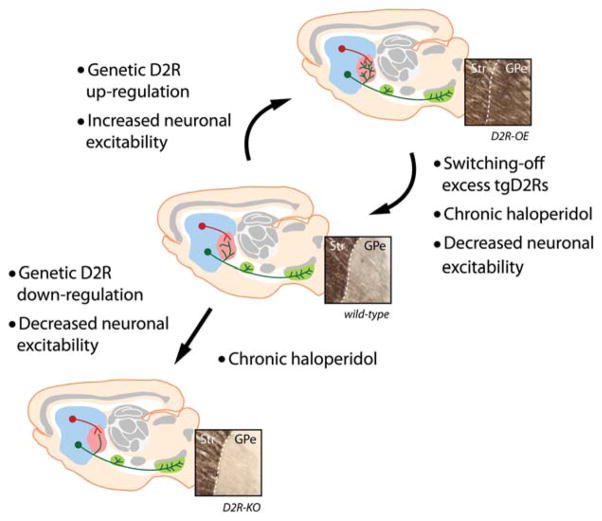
FIG. 2.
Bridging collaterals. Direct pathway axons possess collaterals in the GPe that bridge the two striatal output pathways. These axon branches are highly plastic in the adult brain and are dynamically regulated by dopamine D2Rs function through its effect on neuronal excitability. GPe, external globus pallidus.
Another body of evidence that exemplifies the complex organization of BG networks has also been brought at the level of the GPe. In contrast to the general view, the neuronal population of the GPe is not homogenous, and pallidal neurons do not project exclusively downstream within the indirect pathway, as postulated in the classical model.17 Instead, a subset of GPe neurons (named “arkypallidal” neurons by Mallet and colleagues16) makes strong projections back to the striatum while another subset connects both the striatum and the STN.16,18,19 By connecting both direct and indirect MSNs in the striatum, these “back-projections” may provide an additional step in the synchronization of output information between the direct and indirect pathways. Moreover, action inhibition appears to be much more prominent in arkypallidal neurons than in other GPe neurons and matches the time course of striatal suppression during action cancellation.17 As a consequence, arkypallidal neurons and their back-projections to the striatum pathway seem to be directly involved in behavioral inhibition, a critical set of mechanisms for ensuring that behavior is both flexible and well controlled.
Finally, axonal collateralization and functional communication between the two populations of MSNs also has been demonstrated at the level of the striatum.20 The MSN collateralization within the striatum is also regulated by D2Rs, like bridging collaterals. Chronic D2R activation regulates the rate and the strength of recurrent collateral inhibition between D2-D1 and D2-D2 MSNs,21 thereby providing another mechanism by which the dopamine system modulates the balance between both pathways.
Functional Evidence
In the classical basal ganglia model, direct and indirect pathways are two parallel functionally opposing pathways, one promoting movement (direct pathway), the other inhibiting movement (indirect pathway). Direct support for this model comes from a recent optogenetic study by Kravitz et al.,13 using selective stimulation of direct and indirect pathway neurons in freely moving mice. This study unambiguously shows that optical activation of direct pathway MSNs promotes locomotor activity while stimulation of indirect pathway MSNs decreased locomotion, thereby confirming the opposing actions of both pathways. Nevertheless, other models propose coordinated activation of the two pathways, which are simultaneously or sequentially activated during an action11,22–24. In particular, an elegant study by Cui and colleagues11 demonstrated that these two pathways are not mutually exclusive but are rather concurrently activated during movement initiation. In this study, the authors developed an in vivo method that measures neuronal activity of direct and indirect pathway neurons in mice during a behavioral task, using a genetically encoded calcium indicator. Surprisingly, the authors observed increased neuronal activity in both direct and indirect pathways MSNs when mice initiated an action. This concurrent activation was not observed when mice were at rest. Thus it suggests that the two pathways may act in concert to coordinate their opposing effects on output nuclei in order to control timing and synchrony of neural activity during motor action. These studies indicate that the two parallel striatal output networks communicate between each other in order to create a dynamic balance exerting opposing but coordinated actions on the control of movement, cognition, drug addiction and motivational processes13,22–29. In addition, an imbalance between both pathways has been postulated for several brain disorders, including PD1,30.
It must be noted that, in contrast to the calcium imaging study by Cui et al.11, the optogenetic stimulation study by Kravitz and colleagues13 used forced stimulation of one pathway over the other, which may have led to functional imbalance and thus to an exaggerated “all-or-none” behavior. In contrast, during spontaneously initiated movements both pathways work in concert to coordinate antagonist information to output nuclei to balance motor activity. Although the behavioral outcome after direct and indirect pathway optical stimulation is unambiguous, optogenetic activation of striatal direct and indirect pathway neurons produces different cellular responses in basal ganglia output (SNr) neurons, with stimulation of each pathway eliciting both excitations and inhibitions,31 demonstrating the complex nature of the circuit. Nevertheless, inhibited SNr neurons predicted locomotor activation during direct pathway stimulation, whereas excited neurons predicted motor suppression after indirect pathway stimulation, in line with the classical model.
How can we explain these diverse SNr responses after stimulating either pathway? How are these output signals synchronized to finely activate or inhibit output nuclei and to control motor coordination? We believe that the bridging collaterals play an important role in coordinating the functional strengths of both pathways, thereby affecting SNr output activity. Evidence for this comes from in vivo recordings performed in mice in which the density of bridging collaterals was manipulated.12 Optogenetic stimulation of GABAergic indirect pathway MSNs led to a profound and sustained inhibition of spontaneously active pallidal neurons, as expected from the classical model. Surprisingly, stimulating direct pathway neurons also led to sustained inhibition of GPe neurons, though to a lesser extent, but with similar kinetics, as after indirect pathway stimulation, which is consistent with a monosynaptic mode of action. In mice with increased density of bridging collateral, both the amplitude of inhibition and number of cells that are inhibited in the GPe were increased to almost comparable levels as with indirect pathway stimulation. These observations indicate that both direct and indirect pathway output neurons are able to inhibit neurons of the GPe relay nucleus, suggesting that both pathways have the ability to disinhibit SNr neurons (Figure 3). This may explain, at least in part, the observations made in the Freeze et al. paper,31 in which the authors found that direct and indirect MSNs elicit both excitation and inhibition of nigral neurons.
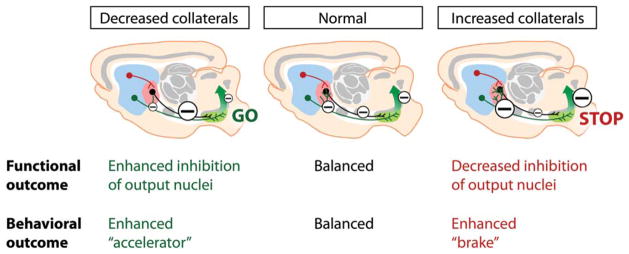
FIG. 3.
Functional and behavioral consequences of bridging collaterals’ plasticity. The density of bridging collaterals controls the functional weight on output nuclei (here, SNr). Because all output projections of BG networks are GABAergic, increased collaterals leads to increased inhibition of GPe neurons, which relieves tonic inhibition of SNr neurons, thus increasing the output inhibition of downstream nuclei (“enhanced brake” or “STOP”). Conversely, decreased collaterals induce decreased GPe inhibition, thus increasing inhibition of SNr neurons and relieving tonic inhibition on downstream nuclei (“enhanced accelerator” or “GO”). Large minuses = increased inhibition, small minuses = decreased inhibition. SNr, substantia nigra pars reticulata; BG, basal gangia; GABA, gamma-aminobutyric acid; GPe, external globus pallidus.
Behavioral Evidence
Could this increase in GPe inhibition affect the behavioral balance of the BG circuitry? In the classical model, the direct and indirect pathways underlie opposing functions, although other models propose a coordinated function of the two pathways that are simultaneously or sequentially activated during an action.22–24 The Ca imaging study by Cui et al.11 suggests that a high level of coordination between striatal output pathways is required. The concurrent activation of both direct and indirect MSNs in the striatum transmits simultaneous and opposing information to the SNr. By connecting these two opposing outputs downstream the striatum with a one-synapse delay (via the GPe), the bridging collaterals may shape the timing and weight of antagonistic information to the SNr, thereby controlling the balance of activation/inhibition in the BG network during movement.
Because bridging collaterals can functionally reinforce or weaken one pathway over the other, regulating the density of their axonal branches should influence the balance of motor behaviors toward over-inhibition (high density of collaterals; indirect pathway is favored) or overactivation (low density of collaterals; direct pathway is favored) (Figure 3). To verify this hypothesis, we repeated the optogenetic experiment in freely moving mice, using the open field experiment designed by Kravitz and colleagues.13 In two mouse models with excess bridging collaterals, D2R-overexpressing mice and Kir2.1AAA mice (see below for further details on these models), optogenetic stimulation of the direct pathway does not any longer lead to locomotor initiation as observed in wild-type mice, but in contrast to inhibition of locomotor activity when collaterals were in excess.12 This suggests that increased bridging collaterals not only lead to increased inhibition of the GPe after direct pathway stimulation, but they also disrupt behavioral activation that is normally induced by the direct pathway. This process therefore reinforces the disinhibitiory strength of the indirect pathway. We therefore postulate that the density of bridging collaterals into the GPe represents an efficient way for regulating the level of synchronization of the output signals to the SNr nucleus.
As described in the next paragraph, bridging collaterals are highly plastic in the adult animal, and their density is regulated by MSN excitability and D2R function. Regulating the extent of bridging collateral branches through modulation of MSN activity or D2R function could therefore represent a mechanism to regulate the level of lateral inhibition in the BG output circuits while learning new movements. In consequence, behavioral training that engages striatal activity or activates the dopamine system over long periods, such as motor learning, may be in the position to alter the functional balance of the two striatal output pathways.
In addition to bridging collaterals, the previously mentioned lateral inhibition between D1- and D2-MSNs at the level of the striatum20,21 may be another way for regulating the synchronization of the striatal output pathways. As observed for bridging collaterals, chronic D2R activation was found to be regulating axonal collateralization between direct and indirect MSNs. Therefore, D2Rs may play a central role in regulating the strength of both forms of lateral inhibition in an effort to coordinate striatal output information.
Remarkable Plasticity of Bridging Collaterals in the Adult Brain
Lessons From the Developmental Brain
During development, neuronal activity is an important mechanism for regulating the growth and refining of axonal projections and for establishing proper connectivity between structures.32–34 Axonal connections are formed in a two-phase scheme. First, a coarse axonal circuitry is established during an early phase, presumably as a result of predetermined genetic programs that will target specific structures but with no apparent functional organization. Then this coarse immature network is refined by neuronal activity during a later phase while the brain interacts with its environment. Because the adult brain is constantly exposed to environmental changes, axonal networks are thought to be refined at the functional and anatomical level to allow behavioral adaption. In the basal ganglia, learning-induced plasticity plays a central role in the selection of actions based on updated representations of current context. Whether development-like axonal refinement could happen in the BG circuitry is, however, unknown.
De Marco Garcia and colleagues33 have recently shown that during embryonic development decreasing neuronal excitability impairs proper axonal path finding of cortical interneurons. In this study, the authors used overexpression of potassium inwardly rectifying 2.1 (Kir2.1) channels to suppress neuronal excitability within caudal ganglionic eminence–derived interneurons that form local inhibitory networks of the cortex. They found that disrupting neuronal activity leads to changes in the expression of Dlx1 (a transcription factor involved in neuronal differentiation) and its associated downstream targets, which leads to impaired dendritic branching and axonal path finding.33 In a previous study,35 we used this Kir2.1 strategy in adult mice to determine whether changes in neuronal activity could alter the structural properties of striatal neurons. In contrast to the studies described, we used a dominant negative form of Kir2.1 channels (Kir2.1AAA) that increases neuronal excitability.36 Increasing neuronal excitability of MSNs in these mice led to a profound rearrangement of their dendritic arborization. Increased excitability of MSNs has been discussed as an important factor in PD. In particular, the dendritic atrophy and spine loss observed in patients or PD animal models have been associated with increased membrane excitability.37–39 The mechanism for striatal hyperexcitability in these animal models is unknown, although downregulation of potassium channels, including Kir and Kv channels, could play a role in this process.40,41 In addition, striatal hyperexcitability may be responsible for dendritic atrophy and spine loss in PD.42 Our Kir2.1AAA experiments provide direct evidence that MSN excitability can shape dendritic arbor morphology, as observed during development.
Neuronal Excitability and Bridging Collaterals
In the adult brain, structural remodeling is generally observed at the level of dendritic branches and spines. In analogy to what is observed during development, we questioned whether structural refining of axonal projections also can occur in the adult BG axonal circuitry.12 Using selective viral expression of Kir2.1 and Kir2.1AAA channels to respectively suppress or increase neuronal activity in the direct or indirect pathway, we observed a specific remodeling of direct pathway collaterals into the GPe. Remarkably, only bridging collaterals were affected by these manipulations, leaving the “classical” terminal fields (ie, GPi and SNr for the direct pathway; GPe for the indirect pathway) unchanged. In contrast to what is traditionally observed during development,32–34 the anatomical rearrangement of direct pathway collaterals is not cell-autonomous but rather depends on neuronal excitability of indirect pathway neurons.
Why this structural reorganization is specific for the bridging collaterals of the direct pathway and why it does not affect the classical terminal fields of both pathways are unclear. We believe that this is related to the non–cell-autonomous nature of the mechanism. Changes in activity of the indirect pathway may lead to the release of attractive or repulsive molecules within the GPe that are specifically recognized by direct pathway bridging collaterals. These molecules could be released directly by indirect pathway terminals or indirectly by GPe target neurons in response to changes in electrical activity. In summary, the observation of plastic bridging collaterals suggests that active remodeling of axonal projections can exist in networks of the adult central nervous system that are a priori not known for their structural plasticity.
Dynamic Regulation by Dopamine D2Rs
Because neuronal excitability of indirect pathway neurons is critical for the growth of direct pathway collaterals, regulatory mechanisms that would be specific to this neuronal population may be involved. Striatopallidal neurons of the indirect pathway predominantly express D2Rs, and we previously showed that D2Rs regulate neuronal excitability of these neurons.35 In particular, we found that chronic overexpression of D2R increases excitability of the indirect pathway via downregulation of Kir2 channels. Could dopamine D2Rs be involved in the selective reorganization of bridging collaterals in the adult brain? Using transgenic mice in which D2R is either overexpressed (D2R-OE) or knocked down (D2R-KD), we found that D2Rs bidirectionally and selectively regulate the extent of direct pathway bridging collaterals by altering neuronal excitability12 (Fig. 2). For instance, in D2R-OE mice, a twofold increase in the density of bridging collaterals occurs compared with control littermates, whereas in D2R-KD mice very few collaterals can be observed.
Excess collaterals in adult D2R-OE mice could be reversed to normal density after restoring normal level of excitability, which suggests a remarkable level of structural plasticity. Could dynamic changes in D2R expression also affect the extent of direct pathway collateral branches during adulthood? Using D2R-OE mice, in which D2R overexpression can be switched off during adulthood with doxycycline, we found that the amount of bridging collaterals progressively returned to normal density as D2R expression was progressively restored. Most remarkably, switching off D2R expression—to allow the retraction of collaterals—followed by reinstatement of D2R overexpression led to the regrowth of axonal terminal fields to nearly original levels. These observations show that bridging collaterals are dynamically regulated by D2Rs and that they can undergo an extreme level of structural plasticity in the adult BG circuitry.
Bridging Collaterals Are Sensitive to Haloperidol
Another remarkable proof of structural plasticity in the adult BG circuitry is brought by the complete retraction of excessive bridging collaterals in D2R-OE mice after chronic treatment with haloperidol (Figure 2), a D2R blocker that is commonly used as antipsychotic medication in humans. Using a same-subject longitudinal design in a subgroup of mice that was tested before and after chronic haloperidol treatment, we found that chronic pharmacological blockade of D2Rs completely restored the altered locomotor balance. Because D2R dysfunction has been associated with the pathogenesis of PD but also schizophrenia or drug-induced dyskinesia, we postulate that bridging collateral function and density may be relevant for these brain disorders. In the next paragraph, we therefore discuss how bridging collaterals and their dynamic regulation by D2Rs may be critically involved in patients with PD, schizophrenia, and antipsychotic-induced tardive dyskinesia.
Translational Perspectives for Basal Ganglia Disorders
Implication for Parkinson’s Disease
In the classical model of PD, reduced dopamine input from the SNc to the striatum has a dual effect, facilitating indirect pathway neurons via decreased D2R inhibition and inhibiting direct pathway function via decreased D1R activation. Increased inhibition from indirect striatal neurons to the GPe disinhibits the STN, which then overdrives inhibitory output neurons in the GPi and SNr.6,8 In parallel, decreased activation of direct pathway striatal output neurons would effectively reduce the inhibitory influences on the GPi and SNr, thereby contributing to excessive activation of inhibitory basal ganglia output. The akinetic state is thought to result from these changes. Based on our recent discovery of collateral regulation by D2Rs, one would expect that presynaptic dopamine depletion and the resulting decreased activation of D2Rs should lead to a lower density of bridging collaterals. In addition, decreased excitability of striatopallidal neurons has recently been reported in one preclinical PD model,43 which would also predict reduced collaterals. Conversely, increased excitability of striatopallidal neurons has been observed in several other preclinical studies, through cholinergic modulation of potassium Kir2 and Kv channels.40,41,44 Down-regulation of Kir2.1 channel function in striatopallidal neurons40 therefore may have the opposite effect on collaterals as decreased D2R activation. In consequence, the resulting net effect on bridging collateral density and how this affects the strengths of the direct and indirect pathways needs to be determined experimentally, using models of PD.
Altered bridging collateral density also may affect BG functions beyond its conceptualization into the classical BG model. In the previous paragraph, we argued that the density of bridging collaterals into the GPe represents an efficient way for regulating the level of synchronization of BG output. Human studies performed in PD patients, which are supported by animal models, have found a dramatic increase in correlated activity between the GPe and the subthalamic nucleus.45,46 Computer modeling suggests that striatopallidal inhibition is well situated to regulate this synchronous activity.47 In addition, hypokinesia induced by blockade of D1R or D2R correlated with selective changes in beta and gamma frequency power and synchrony in the BG.48 Specifically, the D2R blocker raclopride does not affect gamma power fluctuations with delta phase, presumably because some gamma activity is nested within the delta band. Instead, blocking D1R with SCH23390 can reverse the phase of delta at which gamma is at maximum, indicating that D1R-expressing direct pathway neurons may be more involved in regulating the nesting phenomenon. Interestingly, Dejean and colleagues48 have suggested that axon collaterals of the direct pathway in the GPe (ie, bridging collaterals) could be involved in the synchrony of gamma and delta frequencies within the BG network of PD patients.48 Bridging collaterals may participate in GPe-STN synchronization. If this is the case, changes in their density should affect synchronization, an idea that could be relatively easily tested in animal models.
Implication for Schizophrenia and Antipsychotic-Induced Tardive Dyskinesia
Positron emission tomography imaging studies suggest increased D2Rs function in drug-naïve patients.49–51 Increased dopaminergic function is observed at the presynaptic level with increased amphetamine-induced dopamine release52 and increased uptake of [18F]-fluorodopa, a measure of presynaptic dopamine storage and turnover.53 At the postsynaptic level, occupancy of D2Rs is increased in the striatum of patients. Because some of these changes are already present in prodromal subjects, who are at high risk for developing schizophrenia, this suggests an abnormality in the dopamine system early in the disease process.54 Importantly, D2R occupancy and amphetamine-induced dopamine release correlate with positive (psychotic) symptom severity and predict treatment response to antipsychotic medication, thereby demonstrating a tight relationship between D2R hyperfunction and psychosis. Based on these observations and our recent findings, we hypothesize that bridging collaterals are increased in drug-naïve patients and that this increase is involved in the generation of positive symptoms. In contrast, in patients treated with D2R blockers, such as haloperidol, bridging collateral density should be normalized, and this normalization is important for treatment response. Consistent with this idea, full efficacy with antipsychotic medication is achieved after days to weeks of treatment,55 a duration that correlates with haloperidol-induced retraction of bridging collaterals in mice. Human brain imaging studies performed in drug-naïve and antipsychotic-treated patients could be designed to address the potential role of bridging collaterals plasticity in the efficacy of treatments.
Prolonged treatment with haloperidol—and possibly other typical antipsychotics—may even lead to prolonged retraction of bridging collaterals in humans, depending on the dose, achieved receptor occupancy, and duration of treatment. Just as excess collaterals can induce an imbalance in the treatment of relevant information by overweighting the indirect pathway, a low density of collaterals (or even absence) can lead to the opposite, yet disturbed, phenotype by enhancing the net activity of the direct pathway. This mechanism could explain delayed abnormal involuntary movements that persist even after the discontinuation of the medication, namely, tardive dyskinesia (TD).56 Emergence of TD in the presence of antipsychotics and frequent increase in TD after discontinuation of antipsychotics would be consistent with the relatively unopposed effects of direct pathway MSNs on BG output nuclei by reduced influence of collaterals on indirect pathway. The hypothesis that decreased bridging collaterals contribute to antipsychotic-induced TD can be easily tested using Drd1a-GFP mice. Prolonged treatment with high doses of typical antipsychotic medication, such as haloperidol, would be expected to lead to decreased bridging collateral density and thereby to enhance direct pathway influence on BG output nuclei. These anatomical changes could then be associated with TD in the animal model.
We therefore hypothesize that the two types of imbalance (caused by excess or absence of bridging collaterals) may be observed in the brains of drug-naïve and drug-treated schizophrenia patients. We propose to design new imaging studies in humans and rodent models to address the potential relevance of bridging collaterals in the generation of positive symptoms and in antipsychotic-induced TD in patients with schizophrenia.
Closing Remarks: Opening New Venues for Therapies
We are aware of the mostly speculative nature of the last few paragraphs that discuss the clinical importance of altered density of bridging collaterals. They are speculative because no clinical studies have been done to determine the density of bridging collaterals, both in schizophrenia and PD. Our hope is that this perspective will inspire clinicians to look at them in patient populations. The long-term benefits could be significant because, if bridging collaterals turn out to be important for BG diseases, mechanistic ways to alter their density independent of targeting the dopamine system (by targeting growth factors, for example) may be possible. By all means, levodopa and D2R blockers have been the most successful drugs for PD and schizophrenia, but they come at a high cost at the level of side effects and low efficacy in certain patient populations. Moreover, we must move on to novel, more targeted therapies rather than those that were developed 60 y ago. Selective remodeling of BG subnetworks could represent such a novel and promising therapeutic approach.
Acknowledgments
Funding agencies: M.C. is an INSERM Young Investigator and is supported by INSERM and ANR (ANR-12-PDOC-0004). C.K. was funded by NIMH (MH086404 and R01MH093672) and the Silvio O.Conte Center for Schizophrenia Research. U.J.K. was supported in part by NIH (R01NS064439), Parkinson’s Disease Foundation, and Michael J Fox Foundation for Parkinson Research.
We thank Tim Cheung for his critical comment on the manuscript.
Footnotes
Relevant conflicts of interest/financial disclosures: Nothing to report. Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Albin RL, Young AB, Penny JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 3.Gerfen CR, Engber TM, Mahan LC, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 4.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchi Y, Wilson CJ, Emson PC. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci. 1990;10:3421–3438. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y, Richard S, Parent A. The organization of the striatal output system: a single-cell juxtacellular labeling study in the rat. Neurosci Res. 2000;38:49–62. doi: 10.1016/s0168-0102(00)00140-1. [DOI] [PubMed] [Google Scholar]
- 7.Fujiyama F, Sohn J, Nakano T, et al. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur J Neurosci. 2011;33:668–677. doi: 10.1111/j.1460-9568.2010.07564.x. [DOI] [PubMed] [Google Scholar]
- 8.Levesque M, Parent A. The striatofugal fiber system in primates: a reevaluation of its organization based on single-axon tracing studies. Proc Natl Acad Sci USA. 2005;102:11888–11893. doi: 10.1073/pnas.0502710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadjar A, Brotchie JM, Guigoni C, et al. Phenotype of striatofugal medium spiny neurons in parkinsonian and dyskinetic nonhuman primates: a call for a reappraisal of the functional organization of the basal ganglia. J Neurosci. 2006;26:8653–8661. doi: 10.1523/JNEUROSCI.2582-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parent A, Charara A, Pinault D. Single striatofugal axons arborizing in both pallidal segments and in the substantia nigra in primates. Brain Res. 1995;698:280–284. doi: 10.1016/0006-8993(95)01017-p. [DOI] [PubMed] [Google Scholar]
- 11.Cui G, Jun SB, Jin X, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cazorla M, de Carvalho FD, Chohan MO, et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014;81:153–164. doi: 10.1016/j.neuron.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kravitz AV, Freeze BS, Parker PR, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valjent E, Bertran-Gonzalez J, Herve D, Fisone G, Girault JA. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 2009;32:538–547. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallet N, Micklem BR, Henny P, et al. Dichotomous organization of the external globus pallidus. Neuron. 2012;74:1075–1086. doi: 10.1016/j.neuron.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gittis AH, Berke JD, Bevan MD, et al. New roles for the external globus pallidus in basal ganglia circuits and behavior. J Neurosci. 2014;34:15178–15183. doi: 10.1523/JNEUROSCI.3252-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastro KJ, Bouchard RS, Holt HA, Gittis AH. Transgenic mouse lines subdivide external segment of the globus pallidus (GPe) neurons and reveal distinct GPe output pathways. J Neurosci. 2014;34:2087–2099. doi: 10.1523/JNEUROSCI.4646-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bevan MD, Booth PA, Eaton SA, Bolam JP. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. J Neurosci. 1998;18:9438–9452. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuhma N, Tanaka KF, Hen R, Rayport S. Functional connectome of the striatal medium spiny neuron. J Neurosci. 2011;31:1183–1192. doi: 10.1523/JNEUROSCI.3833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalchandani RR, van der Goes MS, Partridge JG, Vicini S. Dopamine D2 receptors regulate collateral inhibition between striatal medium spiny neurons. J Neurosci. 2013;33:14075–14086. doi: 10.1523/JNEUROSCI.0692-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- 23.Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- 24.Nambu A. Seven problems on the basal ganglia. Curr Opin Neurobiol. 2008;18:595–604. doi: 10.1016/j.conb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Durieux PF, Bearzatto B, Guiducci S, et al. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- 26.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobo MK, Covington HE, 3rd, Chaudhury D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat Neurosci. 2012;15:1281–1289. doi: 10.1038/nn.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maia TV, Frank MJ. From reinforcement learning models to psychiatric and neurological disorders. Nat Neurosci. 2011;14:154–162. doi: 10.1038/nn.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of basal ganglia output by direct and indirect pathway projection neurons. J Neurosci. 2013;33:18531–18539. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catalano SM, Shatz CJ. Activity-dependent cortical target selection by thalamic axons. Science. 1998;281:559–562. doi: 10.1126/science.281.5376.559. [DOI] [PubMed] [Google Scholar]
- 33.De Marco Garcia NV, Karayannis T, Fishell G. Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature. 2011;472:351–355. doi: 10.1038/nature09865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–1026. doi: 10.1038/nature03409. [DOI] [PubMed] [Google Scholar]
- 35.Cazorla M, Shegda M, Ramesh B, Harrison NL, Kellendonk C. Striatal D2 receptors regulate dendritic morphology of medium spiny neurons via Kir2 channels. J Neurosci. 2012;32:2398–2409. doi: 10.1523/JNEUROSCI.6056-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNeill TH, Brown SA, Rafols JA, Shoulson I. Atrophy of medium spiny I striatal dendrites in advanced Parkinson’s disease. Brain Res. 1988;455:148–152. doi: 10.1016/0006-8993(88)90124-2. [DOI] [PubMed] [Google Scholar]
- 38.Tseng KY, Kasanetz F, Kargieman L, Riquelme LA, Murer MG. Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. J Neurosci. 2001;21:6430–6439. doi: 10.1523/JNEUROSCI.21-16-06430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaja-Milatovic S, Milatovic D, Schantz AM, et al. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology. 2005;64:545–547. doi: 10.1212/01.WNL.0000150591.33787.A4. [DOI] [PubMed] [Google Scholar]
- 40.Shen W, Tian X, Day M, et al. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci. 2007;10:1458–1466. doi: 10.1038/nn1972. [DOI] [PubMed] [Google Scholar]
- 41.Azdad K, Chavez M, Don Bischop P, et al. Homeostatic plasticity of striatal neurons intrinsic excitability following dopamine depletion. PLoS One. 2009;4:e6908. doi: 10.1371/journal.pone.0006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Day M, Wang Z, Ding J, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 43.Fieblinger T, Graves SM, Sebel LE, et al. Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat Commun. 2014;5:5316. doi: 10.1038/ncomms6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fino E, Glowinski J, Venance L. Effects of acute dopamine depletion on the electrophysiological properties of striatal neurons. Neurosci Res. 2007;58:305–316. doi: 10.1016/j.neures.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- 46.Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 47.Best J, Park C, Terman D, Wilson C. Transitions between irregular and rhythmic firing patterns in excitatory-inhibitory neuronal networks. J Comput Neurosci. 2007;23:217–235. doi: 10.1007/s10827-007-0029-7. [DOI] [PubMed] [Google Scholar]
- 48.Dejean C, Arbuthnott G, Wickens JR, Le Moine C, Boraud T, Hyland BI. Power fluctuations in beta and gamma frequencies in rat globus pallidus:association with specific phases of slow oscillations and differential modulation by dopamine D1 and D2 receptors. J Neurosci. 2011;31:6098–6107. doi: 10.1523/JNEUROSCI.3311-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meyer-Lindenberg A, Miletich RS, Kohn PD, et al. Reduced pre-frontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 52.Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol Psychiatry. 2009;65:1091–1093. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Kumakura Y, Cumming P, Vernaleken I, et al. Elevated [18F]fluorodopamine turnover in brain of patients with schizophrenia:an [18F]fluorodopa/positron emission tomography study. J Neurosci. 2007;27:8080–8087. doi: 10.1523/JNEUROSCI.0805-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 55.Sherwood M, Thornton AE, Honer WG. A meta-analysis of profile and time-course of symptom change in acute schizophrenia treated with atypical antipsychotics. Int J Neuropsychopharmacol. 2006;9:357–366. doi: 10.1017/S1461145705005961. [DOI] [PubMed] [Google Scholar]
- 56.Remington G. Tardive dyskinesia:eliminated, forgotten, or overshadowed? Curr Opin Psychiatry. 2007;20:131–137. doi: 10.1097/YCO.0b013e328017f6b1. [DOI] [PubMed] [Google Scholar]