Abstract
Introduction
Multiple sclerosis (MS) is the most common chronic inflammatory, demyelinating disease of the CNS and results in neurological disability. Existing immunomodulatory and immunosuppressive approaches lower the number of relapses but do not cure or reverse existing deficits nor improve long-term disability in MS patients.
Areas Covered
Monogenic antibodies were described as treatment options for MS, however the immunogenicity of mouse antibodies hampered the efficacy of potential therapeutics in humans. Availability of improved antibody production technologies resulted in a paradigm shift in MS treatment strategies. In this review, an overview of immunotherapies for MS that use conventional monoclonal antibodies reactive to immune system and their properties and mechanisms of action will be discussed, including recent advances in MS therapeutics and highlight natural autoantibodies (NAbs) that directly target CNS cells.
Expert Opinion
Recent challenges for MS therapy are the identification of relevant molecular and cellular targets, time frame of treatment, and antibody toxicity profiles to identify safe treatment options for MS patients. The application of monoclonal antibody therapies with better biological efficacy associated with minimum side effects possesses huge clinical potential. Advances in monoclonal antibody technologies that directly target cells of nervous system may promote the CNS regeneration field from bench to bedside.
Keywords: multiple sclerosis, immunogenicity, monoclonal antibodies, mAbs, clinical studies, safety
1. Introduction
More than a hundred years ago, Paul Ehlrich, founder of chemotherapy, conceptualized the magic bullet paradigm in cancer research with drugs that specifically target the molecular defect without harming the organism1. Ehrlich's vision was not realized until the development of the hybridoma technology (mAbs) by Köhler and Milstein in 19752. This technology led to the production of monoclonal antibodies (mAbs) in unlimited quantity combined with desired specificity. Recent advances in technology allowed the transition from originally murine to chimeric, humanized antibodies to finally fully human mAbs3, 4. The therapeutic potential of mAbs was first shown in a patient with B-cell lymphoma. The patient was first treated with an anti-idiotype antibody followed by an anti-CD3 mAb (OKT3, Muromonab-CD3). Muromonab-CD3 was the first mAb approved by the US Food and Drug Administration (FDA) for clinical use in humans5.
In this review, we will present an overview of MS, followed by an overview of monoclonal antibodies for the treatment of MS. We will summarize immunotherapies that use conventional monoclonal antibodies and discuss properties and mechanisms of action for monoclonal antibodies currently used for MS patients and in clinical trials. We will also discuss recent advances in MS therapeutics and highlight natural autoantibodies (NAbs) that directly target CNS cells.
1.1. Multiple Sclerosis, an inflammatory CNS disease
MS is a demyelinating disease of the CNS that is most often relapsing, and progresses in the white (and grey) matter of the CNS with unclear pathogenesis. So far, there is no treatment available to stop disease progression or reverse existing disabilities in MS patients. Statistics from the MS Foundation estimated more than 400,000 people in the United States and about 2.5 million people worldwide with MS (for the year 2015; http://j.mp/MS_Statistics). The most common MS subtype, relapsing–remitting MS (RRMS) is present in 80-85 percent of patients and typically begins in the second or third decade of life with a female predominance of 2:1 and most recently 3:1. Patients typically improve spontaneously or respond to corticosteroids administered intravenously; a treatment paradigm using 1 gram methylprednisolone intravenously for each of 3 consecutive days without oral corticosteroid taper was initiated by Moses Rodriguez at the Mayo Clinic in 1983 (known at the Mayo Clinic as the “Rodriguez protocol”). This treatment protocol has gone on to become the standard approach for handling acute exacerbations of MS throughout the world. Unfortunately, the patient's responsiveness to corticosteroids typically fades over time. A certain level of CNS dysfunction may persist between relapses or progresses over time (secondary progressive MS). Some of these deficits that persist after methylprednisolone therapy may respond to plasma exchange6. This was proven to be the case in a double blind placebo controlled trial where 40% of patients improved with true exchange7. Approximately 15-20 percent of MS patients are diagnosed with primary progressive MS, which progresses gradually in the absence of obvious relapses and remissions. Primary progressive MS has a similar incidence among men and women8 and does to respond to currently approved therapies for MS. A definitive cause (or causes) for MS are yet unknown, and a cure is beyond our grasp. However, several disease management strategies for MS were developed, starting in 1993 with the approval of interferon-β1b (Betaseron®) followed by other Food and Drug Administration (FDA) approved treatments such as interferon (IFN)-β1a (Avonex® and Rebif®), glatiramer acetate (Copaxone®), and mitoxantrone. However, none of these approved drugs fundamentally alter the progressive course of MS disease. In addition, each drug presents with its own unique profile of potential adverse side effects. The routine application of magnetic resonance imaging (MRI) of brain and spinal cord for diagnosis and follow-up of MS patients has significantly expanded our understanding of the pathogenesis of MS. This led to the design of different drugs that suppress inflammatory MRI readouts. However, these drugs came with a significant price, i.e., the compromise of the safety of the medication: the more powerful the medication’s ability to suppress the immune system (and therefore immune-mediated attacks during early disease stages) is, the more serious and even deadly were the adverse effects. We believe a paradigm shift is necessary from immunosuppressive/immunomodulatory strategies in MS, which aim to prevent disease exacerbation, towards active regenerative strategies, which intend to repair demyelinated brain and spinal cord lesions.
2. US Food and Drug Administration (FDA)-approved monoclonal antibodies for the treatment of MS
So far, Natalizumab and Alemtuzumab are the only US Food and Drug Administration (FDA)-approved monoclonal antibodies for the treatment of MS.
2.1 Natalizumab (Tysabri)
Natalizumab is a humanized monoclonal antibody that targets the focal adhesion molecule integrin α-4 present on T lymphocytes and other immune cells. Integrin α-4 is essential for T lymphocytes to cross the BBB and enter the CNS lesion site. Natalizumab is effective in an immune-mediated animal model of MS where it prevents T lymphocytes from entering the CNS lesions and thereby lessening the disease9. When administered every four weeks in a phase III trial, natalizumab reduced the exacerbation rate by approximately 68%10. Natalizumab combined with beta-interferon resulted in an additional decrease in disease activity11. However, Natalizumab poses an increased risk of CNS virus infections leading to progressive multifocal leukoencephaolpathy (PML). As of 31st August 2015 there were 588 cases of natalizumab-associated PML among 142,000 patients (http://bit.ly/natpml0815)12.
2.2 Alemtuzumab (Campath-1H)
Alemtuzumab (CAMPATH-1H) is a CD52-specific humanized monoclonal antibody that has six hypervariable loops from the rat immunoglobulin (Ig)G2b CAMPATH-1G combined to a human IgG1 (consisting of the κ light chain of the Bence-Jones protein REI and the heavy chain of a new immunoglobulin)13. The molecule CD52 is a cell-surface glycosylphosphatidylinositol-anchored glycoprotein that is expressed on human lymphocytes, monocytes, and eosinophils, and also on epididymis epithelial cells and mature spermatozoa. The primary indication for Alemtuzumab is the treatment of chronic lymphocytic leukemia. The treatment causes intense depletion of lymphocyte and monocyte populations in the bloodstream within minutes of initial dosing and this effect is maintained for up to one-year post-treatment. Peripheral blood monocytes returned to baseline levels within one month in MS patients treated with alemtuzumab14, and B cell populations within 3–7 months post-treatment15. A phase III trial in patients with RRMS demonstrated lower relapse rates compared to controls but without having an effect on the degree of disability16. An earlier phase II trial was terminated due to development of idiopathic thrombocytopenia in three patients16. Approximately 23% of patients under Alemtuzumab develop autoimmune thyroid disease and may be at risk to develop PML or other potentially fatal opportunistic infections. The main safety concerns include autoimmune thyroid problems and idiopathic thrombocytopenic purpura (ITP) for which patients will require monitoring.
3. The presence of immune cells and their components in CNS
3.1 Role of B cells in MS pathogenesis
An MS plaque is histologically characterized by inflammation, demyelination and gliosis. Infiltration by mononuclear cells, particularly macrophages and T cells is typical of the acute MS lesion. Previous reports indicated that autopsy material from the brains and spinal cords of MS patients had more Ig-containing cells within the plaques than outside and were more common in recent than in old plaques17. Subsequently, it was reported that B cells account for up to 25% of the CSF-infiltrating leukocytes during CNS inflammatory responses18. Other authors suggested a germinal center (GC)-like reaction takes place during the immune attack against CNS structures19. By using a rodent model with an intact blood-brain barrier, it was shown that the trafficking of activated antigen-specific B cells into the brain, retention, and antibody production was possible suggesting that the brain microenvironment supports the development of antigen-directed humoral immunity20. Corcione et al., confirmed this concept by showing that each B-cell subset participating in the GC reaction could be detected in the CSF of MS patients21. To date, the exact target antigens of pathogenic B-cell responses in MS remain unknown. However, it is clear that not all B-cells in MS patients support detrimental autoimmunity, being able to clearly differentiate between pathologically relevant cells from normal B-cells is important. Accordingly, pro-inflammatory GM-CSF-producing B cell subset that co-expresses high levels of TNFα as well as IL-6, induces pro-inflammatory myeloid cell activation in a GM-CSF–dependent manner, and is abnormally increased in patients with MS, thus highlighting the rationale for selective targeting of distinct B cell populations in MS22.
3.2 Presence of immunoglobulins in CNS
Intrathecal production of antibodies after clonal expansion in MS patients was noted by the consistent identification of oligoclonal bands in CSF23. Numerous publications during the last few decades supported the idea that CSF oligoclonal bands correlate to the level of B-cell involvement in MS24. Further studies showed that that presence of these oligoclonal bands in MS generally correlated to a worse outcome25 or disability prognosis in MS26.
3.3 Role of T cells in MS pathogenesis
The most widely used mouse model of MS is experimental autoimmune encephalomyelitis (EAE). Th-1 CD4+ T cells determine this disease model. Due to this reason, this subset of T cells was the main area of MS research for several decades, whereas CD8+ T cells were neglected. The EAE model helped MS researchers understand autoimmunity, immune surveillance, CNS inflammation and immune-mediated tissue destruction and led to the development of three approved medications for treatment in MS27. The report that CD8+ T cells outnumber the CD4+ T-cell subset in all samples from MS patients, regardless of the MS subtype, duration and speed of disease progression, shifted the focus towards role of CD8+ T cells28. Clear evidence regarding the clonal expansion of CD8+ T cells in MS patients29 and MS lesions30 was also documented. The works of Tzartos et al., reported an enrichment of both IL-17+ CD4+ and CD8+ T cells in active MS lesions as well as an important role for IL-17 in MS pathogenesis31.
Finally, B cell depletion, both ex vivo and in vivo, results in significantly diminished pro-inflammatory (Th1 and Th17) responses of both CD4+ and CD8+ T cells32. Taken together, the above-listed studies provide evidence for the role of B- and T- cells in the pathogenesis of MS, and thus a therapeutic opportunity to target these immune cells to control/halt the progress of MS.
4. Non FDA-approved monoclonal antibodies for the treatment of MS: mAbs reactive to B Cells
Some of the FDA-approved drugs show some level of immunogenicity in patients (Table 1), arguing in favor of identifying other therapeutic drugs with low toxicity profiles in humans. As discussed above, recent reports advocate greater involvement of B cells and immunoglobulins in the initiation and propagation of MS lesions at different stages of their ontogeny33. By targeting all B-cell stages including subsets of plasma cells, antibodies against CD2034 or CD1935 could have a better therapeutic efficacy in MS therapy.
Table 1.
FDA approved antibody therapeutics
| Antibody name | Company | Type | Target | Indication | Reported Immunogenicity |
|---|---|---|---|---|---|
|
Rituximab
(Rituxan) |
Genentech (Roche)/Biogen Idec |
Chimeric | CD20 | Non-Hodgkin lymphoma |
11% (2578) |
|
Alemtuzumab
(Campath) |
Ilex Pharma (Genzyme) |
Humanized | CD52 | MS, B cell chronic lymphocytic leukemia |
1.9-8.3% (133- 211) |
|
Ibritumomab
(Zevalin) |
Idec Pharma (Biogen Idec) |
Murine | CD20 | Non-Hodgkin lymphoma |
1.3% (446) |
|
Tositumomab
(Bexxar) |
GSK | Murine | CD20 | Non-Hodgkin lymphoma |
11% (230) |
|
Natalizumab
(Tysabri) |
Biogen Idec | Humanized | α-4 integrin | MS, Crohn's disease |
9% (627) |
|
Ofatumumab
(Arzerra) |
GSK | Human | CD20 | CLL | 0% (79) |
FDA approved (a) antibody therapeutics (adapted from http://1.usa.gov/1Jur44k) showing the level of reported immunogenicity observed in patients.
4.1 Rituximab
The chimeric monoclonal antibody Rituximab (Rituxan®) binds and destroys B cells through binding to a phosphorylated glycoprotein (CD20). Rituximab was primarily used for diseases characterized by overactive B cells. Based on antibody-mediated injury seen in an immune-mediated animal model of MS (EAE), and its assumed predictive value for relapsing and progressive MS, Rituximab was considered as an alternative treatment for MS and studied in more detail. Rituximab lowers the rate of relapses in MS patients by 50 % as determined 48 weeks after administration34. However, the clinical outcome in patients with primary progressive disease was not significantly different36 In addition, Rituximab may also cause PML and is associated with reactivation of dormant prior infections like hepatitis B37. Neuromyelitis optica (NMO) is an inflammatory autoimmune disorder of the CNS, usually relapsing, that causes variable degrees of attack-related disability. The disease varies in responsiveness to current treatments, which mainly consist of immunosuppression. Rituximab was previously listed in consensus statements as an option for the treatment of NMO, however an increasing number of reports have recently demonstrated that this drug is not effective in all patients with NMO38. In some cases, Rituximab caused a rapid exacerbation of NMO following treatment39.
4.2 Ocrelizumab
The fully humanized monoclonal antibody Ocrelizumab targets the phosphorylated glycoprotein CD20 (see Rituximab) on circulating B-lymphocytes but not plasma cells. Similar to Rituximab, Ocrelizumab causes depletion of B-lymphocytes through complement- and antibody-dependent cytotoxicity plus stimulation of apoptosis. An important reason for its development was the detection of anti-chimeric neutralizing antibodies against the chimeric antibody Rituximab in 24% of patients. This disturbing outcome is likely to be reduced in patients treated with the humanized antibody Ocrelizumab. The favorable outcome in a phase II trial comparing Ocrelizumab against interferon beta-1a and placebo led to a phase III study in primary progressive MS and RRMS40. There are, however, safety concerns using Ocrelizumab based on the phase II MS trial noted above with a 41-year old patient dying from brain edema 14 weeks into the trial. A phase III trial of Ocrelizumab in systemic lupus erythematosus was discontinued due to opportunistic infections after methotrexate exposure41. In September of 2015 (http://j.mp/ocrelizumab_1509) Genentech announced positive results from a pivotal Phase III study that evaluated Ocrelizumab in patients with primary progressive multiple sclerosis (PPMS). The study (called ORATORIO) met its primary endpoint, showing treatment with Ocrelizumab significantly reduced the progression of clinical disability sustained for at least 12 weeks compared with placebo, as measured by the Expanded Disability Status Scale (EDSS). In recently concluded OPERA studies (October of 2015), researchers randomized 1,656 patients with RRMS to a 600-mg IV infusion of Ocrelizumab every 6 months or to interferon beta-1a (Rebif) given as 44-mg subcutaneous injections three times per week. The results showed that Ocrelizumab reduced the annualized relapse rate by about 50% over 2 years compared with interferon (0.292 versus 0.156 in OPERA I and 0.290 versus 0.155 in OPERA II, P<0.0001 for both studies). Overall adverse events were similar in both groups in both trials at about 83%, but as expected, infusion-related reactions were higher in the Ocrelizumab group (34.3% versus 9.7%). There were similar rates of serious adverse events (6.9% and 8.7%), with no cases of PML reported (http://bit.ly/OPERA_Trial).
4.3 Ofatumumab
Ofatumumab (Arzerra® or HuMax-CD20) is a fully human monoclonal antibody that targets CD20. This monoclonal antibody attaches to a dissimilar region of the CD20 molecule than the other two CD20-specific monoclonal antibodies, Rituximab and Ocrelizumab. It suppresses the activation of B-lymphocytes and is presently approved by the FDA for treatment of certain cases of chronic lymphocytic leukemia, which are refractory to therapy with Alemtuzumab and Fludarabine and has been used experimentally for the treatment of RRMS. Results from phase I/II trials using Ofatumumab vs placebo for RRMS reported no major safety concerns. Ofatumumab-treatment resulted in a ≥90% reduction in new T1 gadolinium-enhancing lesions for all doses of Ofatumumab ≥30 mg (P < .001)42.
4.4 MEDI-551
MEDI-551 is humanized IgG1k mAb that targets CD19. The antibody had potent effects in deletion of several B-cell lines obtained from patients with B-cell malignancies in vitro43. The mAb is currently investigated in a randomized, double-blind, placebo-controlled Phase I/II study in patients with RRMS (NCT01585766). MEDI-551 is also being investigated in patients with scleroderma and B-cell malignancies. Phase II clinical studies demonstrated an acceptable profile of safety. The most frequent adverse events described were neutropenia, nausea, pyrexia, cytokine release syndrome and fatigue44.
4.5 Tabalumab
Tabalumab (LY2127399) is a high-affinity human antibody with neutralizing activity against membrane-bound and soluble B-cell activating factor (BAFF, CD257)45. BAFF is a survival and maturation factor for B cells belonging to the tumor necrosis factor (TNF) superfamily46. Dysregulated BAFF expression contributes to autoimmune diseases or B-cell malignancies via effects on abnormal B-lymphocyte activation, proliferation, survival, and immunoglobulin secretion. BAFF binds to three different receptors, which are mainly found on mature B cells, so playing a crucial role in B-cell activation and B-cell survival. Eli Lilly and Company performed a phase II clinical trial of multiple subcutaneous doses of Tabalumab in patients with RRMS (NCT00882999). The primary outcome of the study was to look at reduction in MRI lesions at 12, 16, 20, and 24 weeks compared to placebo. No data from this study completed in June 2012 is published yet.
5. Non FDA-approved monoclonal antibodies for the treatment of MS: mAbs reactive to cytokines or chemokines or their receptors
5.1 Daclizumab
Daclizumab (Zenapax®) (anti-CD25) is a humanized monoclonal antibody of IgG1 class, which is attached to the Tac epitope on the α-chain of CD25 (interleukin-2 receptor). Daclizumab successfully ceases the generation of the high-affinity Il-2 receptor. Signaling via the high affinity Il-2 receptor, when activated, stimulates the expansion of activated T lymphocytes47. Daclizumab was primarily used to prevent rejection of transplanted organs. In MS, the mechanism of action is considered to be inhibition of the Il-2 receptor, which blocks the expansion and activation of T lymphocytes that ameliorates the disease. With respect to disease related processes CD25 is also expressed in neoplastic B cells, neuroblastomas and tumor infiltrating lymphocytes. Daclizumab improved the MRI outcome and clinical scores in patients with MS in combination with β-interferon or in monotherapy47. So far, there are no reports of opportunistic infections or other life-threatening adverse effects with Daclizumab. This is in contrast to side effects seen with Natalizumab and Rituximab and may be based on its rather immune modulatory as opposed to immune suppressive effects. It was assumed that Daclizumab eradicates certain CD4+ and CD8+ T lymphocyte populations through increasing levels of CD56+ natural killer cells48. A phase III trial using Daclizumab versus interferon beta-1a demonstrated a 45% reduction in annual relapses compared to interferon beta-1a in patients with RRMS49. The safety profile of Daclizumab was consistent with that seen in the prior phase II trials. Compared to IFN, the drug caused an increased incidence of cutaneous adverse events (37% versus 19%), serious cutaneous events (eczema, drug eruption, pityriasis rubra pilaris, and urticaria) (2% versus 1%), and serious infections (4% versus 2%). Elevations in liver function tests greater than five times upper limit of normal occurred in 6% of patients on Daclizumab as opposed to 3% on IFN. There was one death in the Daclizumab-treated group that was deemed not to be attributable to the medication; there were two deaths in the phase II trials, one from autoimmune hepatitis and one from a psoas abscess whose relation to the drug could not be ruled out49.
5.2 Secukinumab
Secukinumab (AIN457) is a fully human IgG1k mAb that targets IL-17A50. Th17 cells produce the proinflammatory cytokine IL-17A as well as other effector cytokines, including IL-17F and IL-22. IL-17A is also expressed by multiple lineages of the innate immune system, including mast cells, neutrophils, dendritic cells, γδ-T cells, macrophages and natural killer cells. Inhibition of IL-17A as a therapeutic target would exert different physiological effects than the suppression of Th17 cell activity. Rapid and sustained symptom reductions in psoriasis, rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis have been observed in Secukinumab-treated patients, with no overt safety signals50. In several animal51 and human52 studies, Th17 cells and IL-17A were shown to play an important role in the pathogenesis of MS. A first proof-of-concept study (NCT01051817) enrolled 73 RRMS that were randomized 1:1 to iv. 10 mg/kg Secukinumab or placebo administered for 20 weeks. First results showed a significant reduction of combined unique active lesions during weeks 24–48 (primary outcome) in the Secukinumab-treated group. A phase II adaptive dose-ranging study (NCT01874340) to evaluate the efficacy and safety of Secukinumab in patients with RRMS terminated early. This was due to the development of a fully-human anti-IL17 monoclonal antibody with better potential for treating MS patients.
5.3 Tocilizumab
Tocilizumab (TCZ) is a humanized IgG1k mAb directed against interleukin-6 receptor (IL-6Ra). It represents a treatment strategy for patients with rheumatoid arthritis (RA) and is currently approved in the United States for RA patients who have failed to improve with at least one anti-TNF therapy53. A double-label immunohistochemistry technique showed the presence of IL-6 in acute and chronic active plaques from the brain of six MS patients54. When a panel of Th1/Th2 cytokines and the chemokine CCL2 in serum and CSF from MS patients and healthy controls was analyzed, increased levels of IL-6 (p<0.05) were found in CSF from patients with RRMS. A case study in a 53-year-old Japanese woman with MS and RA reported stabilization of MS when treated with Tocilizumab for the RA55. Clinical trials for Tocilizumab in MS are currently being planned. A very recent study reported that a 48-year-old woman developed MS while on treatment with Tocilizumab for RA56. This case report illustrates that Tocilizumab may cause secondary auto-immunity in CNS. Therefore, anti-IL6 therapy should be considered with caution.
5.4 MOR103
MOR103 is a human IgG1 mAb against granulocyte-macrophage colony-stimulating factor (GM-CSF). The mAb works by blocking GM-CSF interaction with its receptor and thereby preventing subsequent signal transduction57. GM-CSF can act as a pro-inflammatory cytokine mainly by inducing the activation, maturation and differentiation of macrophages and dendritic cells, which are essential for the initiation and propagation of cell-mediated immune responses. Soluble GM-CSF is a potent adjuvant for the generation of immune responses to foreign proteins as well as peptides derived from self-antigens58. GM-CSF is required for recruitment of peripheral myeloid cells into the CNS59. A recent Phase I/II dose-escalation trial of MOR103 evaluated the safety, tolerability and signs of efficacy in RA patients60. There were no strong treatment-emergent adverse events reported, with the most common adverse event being nasopharyngitis. The data supported further investigation of the mAb to GM-CSF in RA patients and potentially in those with other immune-mediated inflammatory diseases. A recent report indicates that a subset of B cells that produce GM-CSF and co-express high levels of TNFα as well as IL-6 contributes to MS pathogenesis. In addition, this subset of cells abnormally increased in patients with MS22. This data implicated a pro-inflammatory B cell/myeloid cell axis in MS and provided the rationale for selective targeting of distinct B cell populations (such as the ones that produce GM-CSF) in MS. A recent clinical trial investigated in a randomized, double-blind, placebo-controlled phase Ib Study, the safety and pharmacokinetics of MOR103 in patients with MS61. The primary objective was to determine the safety and tolerability of MOR103 in patients with RRMS or SPMS. MOR103 administered at 3 different doses at 2-week intervals was well tolerated in patients with MS.
5.5 GNbAC1
GNbAC1 is a humanized mAb directed against Multiple Sclerosis-Associated Retrovirus (MSRV) gene that encodes an envelope protein (Env): MSRV-Env. The antibody is a recombinant DNA–derived humanized mAb of the IgG4/κ isotype and contains human framework regions and the complementarity-determining regions (CDRs) of a parent murine antibody. GNbAC1 selectively binds with high affinity to the extracellular domain of MSRV-Env. The protein was named MSRV because the viral particles were originally isolated from the brain tissue of patients with MS62, 63. Human endogenous retrovirus (HERV) genes represent about 8% of the human genome. MSRV-Env activates a pro-inflammatory and autoimmune cascade through its interaction with Toll-like receptor 4. Due to its pro-inflammatory properties and inhibitory effects on OPC differentiation, the MSRV-Env protein plays a crucial role in MS pathogenesis. In a 10-year blind observational study the presence of MSRV in the CSF of early MS patients was found to be associated with a significantly greater rate of relapse-unrelated unremitting disability and secondary progression of the disease64. It was also reported that brains and peripheral blood mononuclear cells of MS patients hyperexpress MSRV/HERV-W endogenous retrovirus, with the MSRV envelope protein localized to MS plaques65. The particular therapeutic interest of the MSRV-Env target comes from the fact that this protein is highly expressed in MS patients, most specifically in brain demyelinating lesions, and it has no known role in the normal human physiology. Based on these considerations preclinical and early clinical development studies tested the efficacy of GNbAC1 in MSRV-Env induced EAE66. The study showed an absence of safety risk67. Additional in vitro analyses showed an absence of complement or antibody-dependent cytotoxicity as well as a low level of cross-reactivity to human tissues. The first-in-man clinical study in 33 healthy subjects and a long-term clinical study in 10 MS patients showed that GNbAC1 is well tolerated in humans without induction of immunogenicity and that it induces a pharmacodynamic response on MSRV biomarkers68, 69. Pharmacokinetic data confirm a dose-linear pharmacokinetics with an elimination half-life of 15–17 days compatible with monthly iv. infusions69. These data indicate that GNbAC1, a mAb antagonist of MSRV-Env, may represent a new approach to the treatment of MS without an impact on the immune system.
5.6 Ustekinumab (CNTO-1275)
Ustekinumab is a human IgG1 mAb generated via recombinant human IL-12 immunization of human Ig (hu-Ig) transgenic mice70. The mAb binds to the p40 subunit common to IL-12 and IL-23 and prevents their interaction with the IL-12 receptor β1 subunit of the IL-12 and IL-23 receptor complexes71. IL-12 drives Th1 cell differentiation whereas IL-23 drives the IL-17-producing Th17 CD4+ cells72. Ustekinumab is approved for treatment of moderate-to-severe plaque psoriasis73 and has demonstrated efficacy in Crohn disease74 and psoriatic arthritis75. The basis for use of Ustekinumab for MS stems from the following studies: animal models and human disease samples established a strong link between dysregulation of the Th1/Th17 pathways and MS76, administration of IL-12 exacerbated rodent EAE, which was prevented by administration of anti-IL-12 antibodies77, with similar results being reported in common marmosets78. The observation of elevated serum levels of IL-12 in patients with secondary chronic progressive course of MS79. IL-12 and IL-23 induce differentiation of IFNγ and IL17, respectively, which drive tissue inflammation and are upregulated in active MS plaques80. A 1,000-fold compartmentalized release of the p40 subunit of IL-12 that was associated with CSF levels of myelin basic protein (MBP) as a measure of myelin degradation was reported81. In another study that used serial MRI, Ustekinumab delayed white-matter demyelination, prevented T2 lesion accumulation, and suppressed inflammation of pre-existing brain lesions in marmosets with established EAE82. Finally, in a phase I clinical study, single subcutaneous doses of Ustekinumab were shown to be well tolerated in patients with RRMS that supported its investigation in large clinical trials83. In a latter clinical trial, a full dose-ranging, proof-of-concept study to assess the safety, efficacy, and pharmacokinetics of repeated subcutaneous injections of Ustekinumab in patients with RRMS was performed84. The results showed that Ustekinumab is generally well tolerated and did not exacerbate inflammatory demyelination. However, the drug did not show any efficacy in reducing the cumulative number of gadolinium-enhancing T1-weighted lesions in MS. One of the possible failures for this drug to show any efficacy was partially due to the study's inclusion of patients with advanced disease. The authors also consider that it is unlikely that Ustekinumab consistently passed the BBB in this study. Thus, while peripheral IL-12 and IL-23 may have been neutralized, immune cells already in the CNS may not have been affected. This drug is currently discontinued for MS treatment.
6. Non FDA-approved monoclonal antibodies for the treatment of MS: mAbs reactive to CNS antigens
6.1 Anti-LINGO-1 antibody (Li81) (BIIB033)
The anti-(LINGO-1 antibody (BIIB033) is a potential treatment for MS and ON patients currently in clinical trials. LINGO-1 ("leucine-rich repeat and Ig domain containing NOGO receptor interacting protein-1") is a functional component (co-receptor) of the Nogo receptor-signaling complex and interacts with the ligand-binding Nogo-66 receptor (NogoR). Lingo-1 is almost exclusively expressed in CNS neurons and oligodendrocytes during both embryonic and postnatal stages85-89. Anti-LINGO-1 antibody demonstrated promotion of spinal cord remyelination in the EAE model. The murine monoclonal antibody mAb3B5 recognizes mouse LINGO-1 and is a surrogate antibody for the human monoclonal antibody Li81 (BIIB033). mAb3B5, improves axon function in mice after experimental spinal cord demyelination. The anti-LINGO-1 antibody Li81 blocks contact points in LINGO-1 required for protein oligomerization. Binding of the antibody’s Fab region (responsible for antigen binding) to LINGO-1 results in the formation of a stable complex comprised of 2 copies of LINGO-1 and 2 copies of the Fab region. The resulting complex blocks epitopes in the LINGO-1 IgG domain that are involved in oligodendrocyte differentiation. Anti-LINGO-1 immunotherapy is intended to stimulate regrowth of the myelin sheath, which is damaged in MS CNS lesions. Serum pharmacokinetics of BIIB033 were determined in control groups and MS cohorts with similar outcomes in both groups. The antibodies serum half-life ranges from 15 to 24 days after intravenous administration. Intravenous doses >3 mg per kg in humans lead to serum concentrations that are predicted to have pharmacological activity, based on remyelination studies in rats. Cerebrospinal fluid (CSF) pharmacokinetics resulted in variable outcomes amongst tested individuals. Ratio of brain and spinal cord to plasma concentrations is likely to be about 0.1% in humans compared to 0.1 to 0.4% in rats.
6.1.1 RENEW Clinical Trial
In April 2015 results from the Phase II clinical trial RENEW (Biogen) were published90. The study tested safety and efficacy of anti-LINGO-1 in acute optic neuritis (AON). AON damages the optic nerve, causing loss of the myelin sheath and axonal injury, and may result in loss of visual function. The most common cause of AON is MS. RENEW (NCT01721161) was a randomized, double-blind, placebo-controlled, parallel-group study in subjects with first unilateral acute optic neuritis episode and was designed to study anti-LINGO-1's ability to enable repair of an optic nerve lesion via axonal remyelination. RENEW is part of the anti-LINGO-1 Phase 2 clinical development program, which includes the SYNERGY trial (see below) in MS. 82 participants with their first episode of AON were given 6 doses of anti-LINGO-1 once every 4 weeks for 20 weeks total or a placebo. RENEW trial studied the effects on remyelination by measuring the latency of nerve conduction between the retina and the visual cortex in the brain using full field visual evoked potential (FF-VEP). The primary endpoint measured FF-VEP latency for the affected eye at week 24 compared to the unaffected eye at baseline. Results demonstrated a 34% improvement in the recovery of optic nerve latency compared to placebo in the per-protocol population, but were not statistically significant (p=0.0504), perhaps due to the small study population. In addition, top-line data showed no effect on change in thickness of the retinal layers (optic nerve neurons and axons), and visual function, as measured by spectral domain optical coherence tomography and low contrast letter acuity, respectively. The retinal ganglion cell layer analysis demonstrated that considerable thinning took place before treatment was administered. As a result, anti-LINGO-1 may not have had an opportunity to provide evidence of neuroprotection in this study. The authors concluded that this insight offers valuable information on the speed of axonal loss following an AON attack, and combined with the positive primary endpoint results, will help inform future studies. The analysis of the intent-to-treat (ITT) population, which includes patients in both arms who did not complete the study, showed a positive trend but did not reach statistical significance. Treatment-related anti-LINGO-1 serious adverse events (SAEs) consisted of two patients with hypersensitivity reactions occurring around the time of infusion and one patient with an asymptomatic elevation in liver transaminases, which resolved after drug discontinuation. No deaths occurred during the trial. No immunogenicity was observed.
6.1.2 SYNERGY Clinical Trial
The clinical trial SYNERGY (Biogen) tested efficacy and safety of anti-LINGO-1 in patients with RRMS or with secondary progressive multiple sclerosis (SPMS)91. The trial started in April 2013 and is due for completion in early 2016. 416 participants receive β-interferon 1a once weekly plus placebo or 3 mg, 10 mg, 30 mg or 100 mg of anti-LINGO-1 per kilogram of weight. Anti-LINGO-1 is given once monthly for 72 weeks. A major focus of this clinical trial is to investigate improvement in cognitive functions.
6.2. Non FDA-approved monoclonal antibodies for the treatment of MS: Natural antibodies reactive both to immune system and CNS myelin
High levels of CSF-IgM binding to MBP were associated with early benign course in MS92. When a mAb to MBP105-120 recognizing the 222-228 epitope of the extracellular domain of high affinity immunoglobulin gamma Fc-receptor I (CD64) was isolated from EBV+ B cell clones of long-term stable RRMS patients, the mAb exerted immunosuppressive activity on MS-derived T cell lines through induction and release of high amounts of IL-10 and decreased levels of IL-12 from activated monocytes93. It is remarkable that B cell clones isolated from patients with long-term stable MS can induce a monoclonal IgM to MBP with immunosuppressive activity. The demonstration that an immunosuppressive IgM mAb is part of the natural human antibody repertoire and its association mainly with stable MS suggests that it may play a role in the mechanism underlying stable course in MS. This hypothesis is further supported by the finding of the presence of significantly high levels of circulating IgM, but not IgG, to CD64 epitope in serum of all MS patients with mAbs to CD64. These IgM antibodies showed the same in vitro immunosuppressive properties as the anti-CD64 222–228 mAb isolated from B cell clones. The finding that these circulating IgM bind to CD64 in its native conformation as expressed on the transfected cell surface, suggests that they bind CD64 in vivo, which may explain their immunosuppressive activity. The in vitro properties of these IgM antibodies confirm that naturally occurring antibodies (NAbs) are mainly protective, providing a potential new therapy for MS and other immune-mediated inflammatory diseases of the CNS.
6.3. Non FDA-approved monoclonal antibodies for the treatment of MS: Natural antibodies reactive to CNS cells for repair of CNS lesions
Even though the existence of NAbs was met with initial skepticism, pioneering work by Avrameas94-96 and Notkins97, 98, established convincing evidence that NAbs are part of the human innate immunoglobulin repertoire 99. NAbs utilize germline-encoded genes directed against foreign antigens, self- and altered self-structures94 and are present in newborns without stimulation by foreign antigens100. In contrast, conventional antibodies require external stimuli for their production. NAbs are polyreactive by definition with few or no somatic mutations in the antibody’s variable light and heavy chain, which are required for high affinity binding of a single antigen. NAbs of the IgM isotype are found in invertebrates and vertebrates. High levels of IgG NAbs and to a lower extent IgM and IgA isotypes are detected in vertebrates101.
In general, NAbs bind their antigen with low affinity but high avidity102, which describes the combined synergistic strength of multiple interactions rather than the sum of interactions between antigen and antibody. In contrast, conventional antibodies, typically of the IgG isotype, undergo affinity maturation and contain somatic mutations to ensure high-affinity antigen binding, which is commonly linked to the antibody’s monospecificity. Accumulating evidence categorize NAbs as natural systemic surveillance molecules that tag damaged cells and foreign pathogens for elimination by the immune system through opsonization or antibody-dependent cellular cytotoxicity. Some NAbs can actively signal in cancer and brain cells. The ability of identified NAbs to detect and sometimes induce apoptosis in tumor cells may play an important function in tumor surveillance103, 104. In mice and humans, another class of NAbs, termed remyelination-promoting antibodies, actively promotes repair in demyelinated spinal cord areas105-107.
6.3.1 HIgM22
The human monoclonal IgM antibody 22 (HIgM22) was discovered at the Mayo Clinic (Rochester, MN, USA). The serum form of the antibody, sHIgM22, was isolated from a Waldenström macroglobulinemia patient with no incidence of any neurologic disease. A recombinant version of sHIgM22, rHIgM22, was developed by cloning the antibody variable region DNA sequence into an expression vector providing the heavy and light chain framework108. GMP-grade rHIgM22 antibody was purified in gram quantities for formal toxicology studies. Both the serum and later the recombinant form of the antibody were able to induce spinal cord remyelination in the TMEV-model of demyelinating disease106, 107. Compared with control-treated animals, mice that received rHIgM22 exhibited fewer lesions (34.3% [rHIgM22] vs. 41.8% [control]) and increased remyelination (59.7% [rHIgM22] vs. 15.8% [control]). MRI studies using the same experimental animal model showed similar increases in remyelination109. Moreover, the study demonstrated that the human IgM antibody was able to penetrate the BBB and enter the CNS. Further research indicates that immunomodulation plays little to no role for the rHIgM22-mediated remyelination effect110 but is rather associated with anti-apoptotic signaling in pre-myelinating oligodendrocytes and reduced caspase-3 activation and caspase gene expression111. rHIgM22-promoted remyelination preserved spinal cord axons and protected functional axon integrity112. Recent data identified a signaling complex in OPCs responsible for rHIgM22-mediated actions including platelet-derived growth factor (PDGF)αR, integrin αvβ3 and the Src family kinases (SFK) Lyn113 suggested an involvement of the astroglial growth factor PDGF in rHIgM22-mediated actions in OPCs. Most important, only mixed glial cultures consisting of astrocytes, OPCs and microglial cells demonstrate observable rHIgM22-mediated OPC proliferation; isolated OPCs do not respond114. This suggests that astrocytic or microglial co-factors or direct contact either provide a necessary microenvironment or co-stimulates the proliferative response. The fact that most glial cell-secreted PDGF derives from astrocytes further supports a role for secreted astrocytic factors in IgM-stimulated OPC proliferation and remyelination.
A recently concluded phase I clinical trial115 evaluated safety, tolerability, pharmacokinetics and immunogenicity of a single intravenous dose of rHIgM22 in patients with MS (NCT01803867). This was a multi-center, double-blind, randomized, placebo-controlled study designed to evaluate safety, tolerability, pharmacokinetics, and immunogenicity of a single dose of rHIgM22 in participants with any type of MS who were clinically stable for at least three months. All 72 participants remained on their existing MS treatment regimens, including disease-modifying therapies. The trial, which followed participants for up to six months after receiving a single dose of rHIgM22, found no dose-limiting toxicities at any of the five dose levels studied. Furthermore, rHIgM22 was detected in the cerebral spinal fluid (CSF) two days after intravenous injection (i.e., ≥0.05 ng/ml) in all 14 rHIgM22-treated patients at two dose levels. Even 29 days after treatment, rHIgM22 was measurable in the CSF of 5 out of 12 patients116. This human data demonstrates that IgM antibodies are able to cross the BBB and persist in the CSF (>40 % of patients) for almost a month after treatment. Recruitment for a phase Ib study (NCT02398461) started in October of 2015, in patients experiencing a clinical acute relapse (new or worsening neurological symptoms attributable to MS preceded by a stable or improving neurological state of at least 30 days) and with at least one new, active lesion (damaged area) on MRI scans. The primary outcome of the trial will be the safety and tolerability of a single dose of rHIgM22 in relapsing MS subjects. The estimated completion date for this study is November 2016.
6.3.2 HIgM12
The beneficial effects of mAbs targeting myelin/oligodendrocytes in demyelinating disease models were harnessed to identify monoclonal IgMs targeting neuronal cells. These antibodies differ from remyelination-promoting antibodies, in that they had no impact on the extent of remyelination in vivo or Ca2+ influx in glial cells in vitro. Two neuron-binding antibodies (sHIgM12, sHIgM42) stimulated neurite extension117 and a recombinant form of sHIgM12, rHIgM12, was generated118. Binding of HIgM12 to the neuronal surface led to membrane reorganization and stimulated neurite outgrowth119. Peripheral administration of rHIgM12 as a single bolus improved motor function in chronically demyelinated mice120. A single dose of rHIgM12 improved brainstem NAA concentrations, a biomarker for density of spinal cord axons, in a model of progressive MS121. We identified gangliosides122 and polysialic acid (PSA)123 attached to the neural cell adhesion molecule (NCAM) as the antigens for rHIgM12. Within the CNS, NCAM is the major polysialylated molecule (> 95%) with long, negatively charged α2′-8′-linked sialic acid homopolymers (n>10 sialic acid residues). PSA is abundantly present in the developing brain and its early expression is tightly linked to critical developmental events including neuronal precursor migration (neuroblasts), axonal sprouting and oligodendrocyte progenitor proliferation. PSA-NCAM, is expressed at the axonal surface and acts as a negative regulator of myelination, presumably by preventing myelin-forming cells from attaching to the axon. PSA-NCAM, normally absent from adult brain, is re-expressed on demyelinated axons in the plaques. Within shadow plaques, remyelinated axons do not express PSA-NCAM. The antibody is currently under development as a therapeutic agent for MS.
7. Expert Opinion
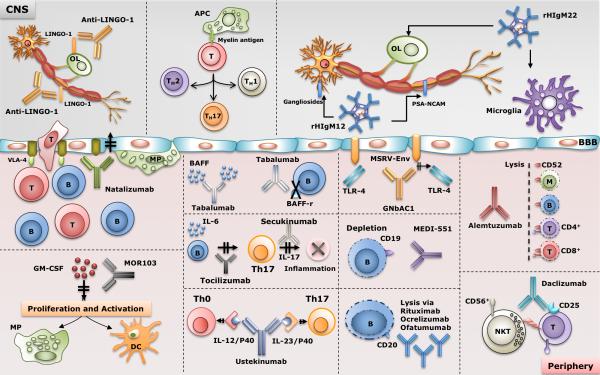
The outlook for MS patients continue to improve as more safe and effective therapies become available. In addition, the availability of agents with efficacies higher than the first-line agents makes it likely that the long-term outcomes in MS may be favorably affected. Figure 1 provides a schematic summary of proposed mechanisms of action of mAbs for MS therapy. Most of the antibody therapies approved target the immune system. Accordingly, monoclonal antibody therapies that target immune cells, cytokines or chemokines or their receptors are helpful in managing MS, however, these treatments do not prevent or reverse long-term disabilities. There is an immediate need to identify new treatment strategies for basically all neurologic diseases including MS and neurodegenerative diseases. In demyelinating diseases like MS, the therapeutic focus should be on strategies that actively induce brain lesion repair and stimulation of remyelination. Future prospects for remyelination therapies are encouraging. Preclinical research has identified multiple targets affecting remyelination in experimental animal models of demyelinating disease, and early stage human clinical trials have started. Potential combinatorial therapeutic approaches could include agents that target the immune system to eliminate deleterious immune system-mediated injury and perhaps anti-LINGO antibodies or rHIgM22 that stimulate remyelination. Indeed, human monoclonal IgMs that target the CNS may enhance the permissive environment for regenerating lost myelin.
Figure 1. Schematic of the proposed mechanisms of action of existing and emerging therapies for MS.
Massive activation of the immune system is followed by the migration of activated immune cells (T and B lymphocytes as well as macrophages) across the disrupted blood-brain barrier (BBB). APC’s would then present CNS antigens to the activated T lymphocytes, which leads to to further differentiation of the naïve T lymphocytes to other groups such as Th1, Th2 and Th17 lymphocytes. Antibodies such as alemtuzumab, daclizumab, natalizumab, rituximab, ocrelizumab, ofatumumab, MOR103, tabalumab, secukinumab, ustekinumab, MED-551 and GNbAC1work mostly on immune cells, cytokines or their receptors at the periphery. rHIgM22 and anti-LINGO-1 antibodies work at the level of CNS and affect OPC’s and OL’s to enhance the process of CNS repair (remyelination). rHIgM22 binds astrocytes in culture and induces a Ca2+-influx in astrocytes. rHIgM12 is a poly-specific antibody that binds to either gangliosides or PSA_NCAM and works at the level of neurons and promotes neurite extension.
OL: oligodendrocyte, OPC: oligodendrocyte progenitor cell, MP: macrophage, APC: antigen presenting cell, Th1: Type 1 helper T cells, Th2: Type 2 helper T cells, Th17: Type 17 helper T cells (produce IL-17), T: T cell, NKT: natural killer T cell, B: B cell, M: monocyte, VCAM-1: vascular cell adhesion protein, VLA-4: very late antigen-4 (Integrin α4β1), CD19: B-lymphocyte antigen cluster of differentiation 19, CD20: B-lymphocyte antigen cluster of differentiation 20, CD52: cluster of differentiation 52 (CAMPATH-1 antigen), TLR-4: Toll like receptor 4, MSRV-Env: Multiple Sclerosis-Associated Retrovirus-Envelope protein, BAFF: B-cell activating factor, PSA-NCAM: Polysialylated-neural cell adhesion molecule, Gagliosides: GD1a, GT1b.
Reparative human monoclonal antibodies are a potential novel class of therapeutics for MS patients with the ability to actively repair CNS lesions in animal models of MS. The importance of blood brain barrier (BBB) breakdown in the context of therapeutic approaches is important. There are several anatomical features between the vascular and the nervous systems that have been recognized, with the interface between the two systems called as the neurovascular unit (NVU). The NVU is comprised of endothelial cells, astrocytes, neurons and pericytes. A major component of NVU is BBB, formed by endothelial cells and isolates CNS from blood circulation. Almost every aspect of CNS function is affected due to this interface. The term ‘barrier’ gives the notion of a rigid, inaccessible obstruction between the vascular and nervous systems. However, the barrier is in fact a region of dynamic molecular transport to constantly regulate the flow of essential components such as amino acids, across endothelial walls and maintains the homeostasis of the microenvironment124. Following CNS injury, BBB is compromised and promotes accumulation of pro-inflammatory cytokines and cell lytic products. The resulting inflammation may attenuate the efflux activity of the BBB, but also ameliorate the permeability to bring in adaptive immune cells. Under these conditions, breakdown of BBB may in fact be beneficial, as this will result in an increased influx of natural autoantibodies or drugs with reparative properties to target and protect intrinsic cells of the CNS125. Nonetheless, the emergence of new molecules or techniques that would promote easier and safer crossing of the BBB without increasing the risk of CNS side effects is of great importance and would be crucial for the therapy of MS. The very low amount of the human antibody rHIgM22 necessary for stimulation of remyelination in mice, and an open BBB during acute phases of the human disease, are encouraging. In contrast to available immunomodulatory therapies, antibody-mediated CNS repair strategies with no- or minimal- toxicity gives hope for MS patients.
Article Highlights.
. Both B cells and T cells play a role in the pathogenesis of MS.
. Monoclonal antibodies (mAbs) have shown efficacy in the early phases of the disease.
. Currently approved mAbs and few that are in clinical trials come with safety concerns and secondary side effects.
. Naturally occurring monoclonal antibodies (NAbs) represent novel strategy to treat MS.
. NAbs are of germline origin with little or no somatic mutations. They are often polyreactive and bind with rather low affinity to one or multiple structurally unrelated antigens.
. NAbs that target CNS cells to promote remyelination represent a paradigm shift in MS therapeutics.
. Most known remyelination-promoting antibodies are of the IgM isotype and bind to myelin and oligodendrocytes. Their molecular targets are cell surface SLs and, to some extent, cytoskeletal proteins.
Acknowledgments
B Wootla, M Rodriguez, J Watzlawik, H Dasari, M Abdelrahim, J Henley and A Warrington are employees of the Mayo Clinic, which has issued and owns patents for antibodies that promote remyelination and CNS repair. The work in this paper was supported by grants from the NIH (R01 GM092993, R01 NS048357 and R21 NS073684) and the National Multiple Sclerosis Society (CA 1060A). This work was also supported by the European Regional Development Fund (FNUSA-ICRC), Mayo Clinic Center for Translational Science Activities (CTSA) and Mayo Clinic CTSA grant number UL1 TR000135 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH) through a High-Impact Pilot and Feasibility Award (HIPFA) and Novel Methodology Award (NMDA) Additional support was provided from the Mayo Clinic Center for Multiple Sclerosis and Demyelinating Diseases (CMSDD) through a gift from Dr. and Mrs. Moon Park. The authors also acknowledge with thanks support from the Applebaum, Hilton, Peterson and Sanford Foundations, the Minnesota Partnership Award for Biotechnology and Medical Genomics, the MnDRIVE Robotics, Sensors, and Advanced Manufacturing program and the McNeilus family. No writing assistance was utilized in the production of this manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Ehrlich P. Aus Theorie und Praxis der Chemotherapie. W. Klinkhardt; Leipzig: 1911. [Google Scholar]
- 2.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–7. doi: 10.1038/256495a0. * First described the technology to produce on-demand monoclonal antibodies with desired specificties.
- 3.Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol. 2005 Sep;23(9):1117–25. doi: 10.1038/nbt1135. [DOI] [PubMed] [Google Scholar]
- 4.Rees AR. The antibody molecule : from anti-toxins to therapeutic antibodies. Oxford University Press; New York, NY: 2014. [Google Scholar]
- 5.Smith SL. Ten years of Orthoclone OKT3 (muromonab-CD3): a review. J Transpl Coord. 1996 Sep;6(3):109–19. doi: 10.7182/prtr.1.6.3.8145l3u185493182. quiz 20-1. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez M, Karnes WE, Bartleson JD, et al. Plasmapheresis in acute episodes of fulminant CNS inflammatory demyelination. Neurology. 1993 Jun;43(6):1100–4. doi: 10.1212/wnl.43.6.1100. [DOI] [PubMed] [Google Scholar]
- 7.Weinshenker BG, O'Brien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999 Dec;46(6):878–86. doi: 10.1002/1531-8249(199912)46:6<878::aid-ana10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med. 2000 Sep 28;343(13):938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 9.Yednock TA, Cannon C, Fritz LC, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992 Mar 5;356(6364):63–6. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 10.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. The New England journal of medicine. 2006 Mar 2;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 11.Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. The New England journal of medicine. 2006 Mar 2;354(9):911–23. doi: 10.1056/NEJMoa044396. [DOI] [PubMed] [Google Scholar]
- 12.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. The New England journal of medicine. 2012 May 17;366(20):1870–80. doi: 10.1056/NEJMoa1107829. ** Independent Swedish registry that estimated the incidence of PML among natalizumab-treated patients with multiple sclerosis.
- 13.Cheetham GM, Hale G, Waldmann H, et al. Crystal structures of a rat anti-CD52 (CAMPATH-1) therapeutic antibody Fab fragment and its humanized counterpart. J Mol Biol. 1998 Nov 20;284(1):85–99. doi: 10.1006/jmbi.1998.2157. [DOI] [PubMed] [Google Scholar]
- 14.Freedman MS, Kaplan JM, Markovic-Plese S. Insights into the Mechanisms of the Therapeutic Efficacy of Alemtuzumab in Multiple Sclerosis. J Clin Cell Immunol. 2013 Jul 8;4(4) [PMC free article] [PubMed] [Google Scholar]
- 15.Hill-Cawthorne GA, Button T, Tuohy O, et al. Long term lymphocyte reconstitution after alemtuzumab treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012 Mar;83(3):298–304. doi: 10.1136/jnnp-2011-300826. [DOI] [PubMed] [Google Scholar]
- 16.Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012 Nov 24;380(9856):1829–39. doi: 10.1016/S0140-6736(12)61768-1. [DOI] [PubMed] [Google Scholar]
- 17.Esiri MM. Multiple sclerosis: a quantitative and qualitative study of immunoglobulin-containing cells in the central nervous system. Neuropathol Appl Neurobiol. 1980 Jan-Feb;6(1):9–21. doi: 10.1111/j.1365-2990.1980.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 18.Cepok S, Jacobsen M, Schock S, et al. Patterns of cerebrospinal fluid pathology correlate with disease progression in multiple sclerosis. Brain. 2001 Nov;124(Pt 11):2169–76. doi: 10.1093/brain/124.11.2169. [DOI] [PubMed] [Google Scholar]
- 19.Colombo M, Dono M, Gazzola P, et al. Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J Immunol. 2000 Mar 1;164(5):2782–9. doi: 10.4049/jimmunol.164.5.2782. ** First suggested to a compartmentalized clonal expansion of B cells in MS.
- 20.Knopf PM, Harling-Berg CJ, Cserr HF, et al. Antigen-dependent intrathecal antibody synthesis in the normal rat brain: tissue entry and local retention of antigen-specific B cells. J Immunol. 1998 Jul 15;161(2):692–701. [PubMed] [Google Scholar]
- 21.Corcione A, Casazza S, Ferretti E, et al. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A. 2004 Jul 27;101(30):11064–9. doi: 10.1073/pnas.0402455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Rezk A, Miyazaki Y, et al. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med. 2015 Oct 21;7(310):310ra166. doi: 10.1126/scitranslmed.aab4176. [DOI] [PubMed] [Google Scholar]
- 23.Qin Y, Duquette P, Zhang Y, et al. Clonal expansion and somatic hypermutation of V(H) genes of B cells from cerebrospinal fluid in multiple sclerosis. J Clin Invest. 1998 Sep 1;102(5):1045–50. doi: 10.1172/JCI3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cross AH, Wu GF. Multiple sclerosis: oligoclonal bands still yield clues about multiple sclerosis. Nat Rev Neurol. 2010 Nov;6(11):588–9. doi: 10.1038/nrneurol.2010.142. [DOI] [PubMed] [Google Scholar]
- 25.Amato MP, Ponziani G. A prospective study on the prognosis of multiple sclerosis. Neurol Sci. 2000;21(4 Suppl 2):S831–8. doi: 10.1007/s100720070021. [DOI] [PubMed] [Google Scholar]
- 26.Joseph FG, Hirst CL, Pickersgill TP, et al. CSF oligoclonal band status informs prognosis in multiple sclerosis: a case control study of 100 patients. J Neurol Neurosurg Psychiatry. 2009 Mar;80(3):292–6. doi: 10.1136/jnnp.2008.150896. [DOI] [PubMed] [Google Scholar]
- 27.Steinman L, Zamvil SS. Virtues and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends Immunol. 2005 Nov;26(11):565–71. doi: 10.1016/j.it.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Booss J, Esiri MM, Tourtellotte WW, et al. Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis. J Neurol Sci. 1983 Dec;62(1-3):219–32. doi: 10.1016/0022-510x(83)90201-0. [DOI] [PubMed] [Google Scholar]
- 29.Monteiro J, Hingorani R, Pergolizzi R, et al. Clonal dominance of CD8+ T-cell in multiple sclerosis. Ann N Y Acad Sci. 1995 Jul 7;756:310–2. doi: 10.1111/j.1749-6632.1995.tb44529.x. [DOI] [PubMed] [Google Scholar]
- 30.Babbe H, Roers A, Waisman A, et al. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000 Aug 7;192(3):393–404. doi: 10.1084/jem.192.3.393. ** Demonstrates clonal expansion and dominance of CD8+ T cells in the CSF of patients with early MS.
- 31.Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008 Jan;172(1):146–55. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bar-Or A, Fawaz L, Fan B, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol. 2010 Apr;67(4):452–61. doi: 10.1002/ana.21939. [DOI] [PubMed] [Google Scholar]
- 33.Wootla B, Denic A, Keegan BM, et al. Evidence for the role of B cells and immunoglobulins in the pathogenesis of multiple sclerosis. Neurol Res Int. 2011;2011:780712. doi: 10.1155/2011/780712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. The New England journal of medicine. 2008 Feb 14;358(7):676–88. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 35.Hammer O. CD19 as an attractive target for antibody-based therapy. MAbs. 2012 Sep-Oct;4(5):571–7. doi: 10.4161/mabs.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawker K, O'Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Annals of neurology. 2009 Oct;66(4):460–71. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- 37.Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol. 2010 Apr;47(2):187–98. doi: 10.1053/j.seminhematol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Mealy MA, Wingerchuk DM, Palace J, et al. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: multicenter study of treatment efficacy. JAMA Neurol. 2014 Mar;71(3):324–30. doi: 10.1001/jamaneurol.2013.5699. * Summarizes that B-cell depletion therapies are not always succesful in NMO.
- 39.Dai Y, Lu T, Wang Y, et al. Rapid exacerbation of neuromyelitis optica after rituximab treatment. J Clin Neurosci. 2015 Dec 15; doi: 10.1016/j.jocn.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 40.Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011 Nov 19;378(9805):1779–87. doi: 10.1016/S0140-6736(11)61649-8. [DOI] [PubMed] [Google Scholar]
- 41.Hutas G. Ocrelizumab, a humanized monoclonal antibody against CD20 for inflammatory disorders and B-cell malignancies. Curr Opin Investig Drugs. 2008 Nov;9(11):1206–15. [PubMed] [Google Scholar]
- 42.Sorensen PS, Lisby S, Grove R, et al. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology. 2014 Feb 18;82(7):573–81. doi: 10.1212/WNL.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 43.Ward E, Mittereder N, Kuta E, et al. A glycoengineered anti-CD19 antibody with potent antibody-dependent cellular cytotoxicity activity in vitro and lymphoma growth inhibition in vivo. Br J Haematol. 2011 Nov;155(4):426–37. doi: 10.1111/j.1365-2141.2011.08857.x. [DOI] [PubMed] [Google Scholar]
- 44.Suresh T, Lee LX, Joshi J, et al. New antibody approaches to lymphoma therapy. J Hematol Oncol. 2014;7:58. doi: 10.1186/s13045-014-0058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manetta J, Bina H, Ryan P, et al. Generation and characterization of tabalumab, a human monoclonal antibody that neutralizes both soluble and membrane-bound B-cell activating factor. J Inflamm Res. 2014;7:121–31. doi: 10.2147/JIR.S67751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito Y, Miyagawa Y, Onda K, et al. B-cell-activating factor inhibits CD20-mediated and B-cell receptor-mediated apoptosis in human B cells. Immunology. 2008 Dec;125(4):570–90. doi: 10.1111/j.1365-2567.2008.02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bielekova B, Richert N, Howard T, et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon beta. Proc Natl Acad Sci U S A. 2004 Jun 8;101(23):8705–8. doi: 10.1073/pnas.0402653101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A. 2006 Apr 11;103(15):5941–6. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kappos L, Wiendl H, Selmaj K, et al. Daclizumab HYP versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med. 2015 Oct 8;373(15):1418–28. doi: 10.1056/NEJMoa1501481. [DOI] [PubMed] [Google Scholar]
- 50.Patel DD, Lee DM, Kolbinger F, et al. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis. 2013 Apr;72(Suppl 2):ii116–23. doi: 10.1136/annrheumdis-2012-202371. [DOI] [PubMed] [Google Scholar]
- 51.Mardiguian S, Serres S, Ladds E, et al. Anti-IL-17A treatment reduces clinical score and VCAM-1 expression detected by in vivo magnetic resonance imaging in chronic relapsing EAE ABH mice. Am J Pathol. 2013 Jun;182(6):2071–81. doi: 10.1016/j.ajpath.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matusevicius D, Kivisakk P, He B, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999 Apr;5(2):101–4. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 53.Navarro-Millan I, Singh JA, Curtis JR. Systematic review of tocilizumab for rheumatoid arthritis: a new biologic agent targeting the interleukin-6 receptor. Clin Ther. 2012 Apr;34(4):788–802. e3. doi: 10.1016/j.clinthera.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maimone D, Guazzi GC, Annunziata P. IL-6 detection in multiple sclerosis brain. J Neurol Sci. 1997 Feb 27;146(1):59–65. doi: 10.1016/s0022-510x(96)00283-3. [DOI] [PubMed] [Google Scholar]
- 55.Sato H, Kobayashi D, Abe A, et al. Tocilizumab treatment safety in rheumatoid arthritis in a patient with multiple sclerosis: a case report. BMC Res Notes. 2014;7:641. doi: 10.1186/1756-0500-7-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beauchemin P, Carruthers R. MS arising during Tocilizumab therapy for rheumatoid arthritis. Mult Scler. 2016 Jan 7; doi: 10.1177/1352458515623862. [DOI] [PubMed] [Google Scholar]
- 57.Steidl S, Ratsch O, Brocks B, et al. In vitro affinity maturation of human GM-CSF antibodies by targeted CDR-diversification. Molecular immunology. 2008 Nov;46(1):135–44. doi: 10.1016/j.molimm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 58.Disis ML, Bernhard H, Shiota FM, et al. Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood. 1996 Jul 1;88(1):202–10. [PubMed] [Google Scholar]
- 59.Codarri L, Gyulveszi G, Tosevski V, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011 Jun;12(6):560–7. doi: 10.1038/ni.2027. * Describes that GM-CSF serves a nonredundant function in the initiation of autoimmune inflammation regardless of helper T cell polarization.
- 60.Behrens F, Tak PP, Ostergaard M, et al. MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann Rheum Dis. 2015 Jun;74(6):1058–64. doi: 10.1136/annrheumdis-2013-204816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Constantinescu CS, Asher A, Fryze W, et al. Randomized phase 1b trial of MOR103, a human antibody to GM-CSF, in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2015 Aug;2(4):e117. doi: 10.1212/NXI.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perron H, Geny C, Laurent A, et al. Leptomeningeal cell line from multiple sclerosis with reverse transcriptase activity and viral particles. Res Virol. 1989 Nov-Dec;140(6):551–61. doi: 10.1016/s0923-2516(89)80141-4. [DOI] [PubMed] [Google Scholar]
- 63.Perron H, Garson JA, Bedin F, et al. Molecular identification of a novel retrovirus repeatedly isolated from patients with multiple sclerosis. The Collaborative Research Group on Multiple Sclerosis. Proc Natl Acad Sci U S A. 1997 Jul 8;94(14):7583–8. doi: 10.1073/pnas.94.14.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sotgiu S, Mameli G, Serra C, et al. Multiple sclerosis-associated retrovirus and progressive disability of multiple sclerosis. Mult Scler. 2010 Oct;16(10):1248–51. doi: 10.1177/1352458510376956. [DOI] [PubMed] [Google Scholar]
- 65.Mameli G, Astone V, Arru G, et al. Brains and peripheral blood mononuclear cells of multiple sclerosis (MS) patients hyperexpress MS-associated retrovirus/HERV-W endogenous retrovirus, but not Human herpesvirus 6. J Gen Virol. 2007 Jan;88(Pt 1):264–74. doi: 10.1099/vir.0.81890-0. [DOI] [PubMed] [Google Scholar]
- 66.Perron H, Dougier-Reynaud HL, Lomparski C, et al. Human endogenous retrovirus protein activates innate immunity and promotes experimental allergic encephalomyelitis in mice. PLoS One. 2013;8(12):e80128. doi: 10.1371/journal.pone.0080128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Curtin F, Perron H, Kromminga A, et al. Preclinical and early clinical development of GNbAC1, a humanized IgG4 monoclonal antibody targeting endogenous retroviral MSRV-Env protein. MAbs. 2015;7(1):265–75. doi: 10.4161/19420862.2014.985021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Derfuss T, Curtin F, Guebelin C, et al. A phase IIa randomised clinical study of GNbAC1, a humanised monoclonal antibody against the envelope protein of multiple sclerosis-associated endogenous retrovirus in multiple sclerosis patients. Mult Scler. 2015 Jun;21(7):885–93. doi: 10.1177/1352458514554052. [DOI] [PubMed] [Google Scholar]
- 69.Derfuss T, Curtin F, Guebelin C, et al. A phase IIa randomized clinical study testing GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis associated endogenous retrovirus in multiple sclerosis patients - a twelve month follow-up. J Neuroimmunol. 2015 Aug 15;285:68–70. doi: 10.1016/j.jneuroim.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 70.Benson JM, Peritt D, Scallon BJ, et al. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs. 2011 Nov-Dec;3(6):535–45. doi: 10.4161/mabs.3.6.17815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo J, Wu SJ, Lacy ER, et al. Structural basis for the dual recognition of IL-12 and IL-23 by ustekinumab. J Mol Biol. 2010 Oct 8;402(5):797–812. doi: 10.1016/j.jmb.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 72.Annunziato F, Cosmi L, Liotta F, et al. Human Th1 dichotomy: origin, phenotype and biologic activities. Immunology. 2014 Oct 5; doi: 10.1111/imm.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008 May 17;371(9625):1665–74. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 74.Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn's disease. Gastroenterology. 2008 Oct;135(4):1130–41. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 75.Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014 Jun;73(6):1000–6. doi: 10.1136/annrheumdis-2013-204741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lovett-Racke AE, Yang Y, Racke MK. Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis? Biochim Biophys Acta. 2011 Feb;1812(2):246–51. doi: 10.1016/j.bbadis.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leonard JP, Waldburger KE, Goldman SJ. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med. 1995 Jan 1;181(1):381–6. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brok HP, van Meurs M, Blezer E, et al. Prevention of experimental autoimmune encephalomyelitis in common marmosets using an anti-IL-12p40 monoclonal antibody. J Immunol. 2002 Dec 1;169(11):6554–63. doi: 10.4049/jimmunol.169.11.6554. [DOI] [PubMed] [Google Scholar]
- 79.Nicoletti F, Patti F, Cocuzza C, et al. Elevated serum levels of interleukin-12 in chronic progressive multiple sclerosis. J Neuroimmunol. 1996 Oct;70(1):87–90. doi: 10.1016/s0165-5728(96)00101-4. [DOI] [PubMed] [Google Scholar]
- 80.Woodroofe MN, Cuzner ML. Cytokine mRNA expression in inflammatory multiple sclerosis lesions: detection by non-radioactive in situ hybridization. Cytokine. 1993 Nov;5(6):583–8. doi: 10.1016/s1043-4666(05)80008-0. [DOI] [PubMed] [Google Scholar]
- 81.Fassbender K, Ragoschke A, Rossol S, et al. Increased release of interleukin-12p40 in MS: association with intracerebral inflammation. Neurology. 1998 Sep;51(3):753–8. doi: 10.1212/wnl.51.3.753. [DOI] [PubMed] [Google Scholar]
- 82.t Hart BA, Brok HP, Remarque E, et al. Suppression of ongoing disease in a nonhuman primate model of multiple sclerosis by a human-anti-human IL-12p40 antibody. J Immunol. 2005 Oct 1;175(7):4761–8. doi: 10.4049/jimmunol.175.7.4761. [DOI] [PubMed] [Google Scholar]
- 83.Kasper LH, Everitt D, Leist TP, et al. A phase I trial of an interleukin-12/23 monoclonal antibody in relapsing multiple sclerosis. Curr Med Res Opin. 2006 Sep;22(9):1671–8. doi: 10.1185/030079906X120931. [DOI] [PubMed] [Google Scholar]
- 84.Segal BM, Constantinescu CS, Raychaudhuri A, et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008 Sep;7(9):796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- 85.Carim-Todd L, Escarceller M, Estivill X, et al. LRRN6A/LERN1 (leucine-rich repeat neuronal protein 1), a novel gene with enriched expression in limbic system and neocortex. Eur J Neurosci. 2003 Dec;18(12):3167–82. doi: 10.1111/j.1460-9568.2003.03003.x. [DOI] [PubMed] [Google Scholar]
- 86.Mi S, Hu B, Hahm K, et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat Med. 2007 Oct;13(10):1228–33. doi: 10.1038/nm1664. * Describes CNS reactive Lingo-1 antagonist has efficacy in EAE model.
- 87.Mi S, Lee X, Shao Z, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nature neuroscience. 2004 Mar;7(3):221–8. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- 88.Mi S, Miller RH, Lee X, et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nature neuroscience. 2005 Jun;8(6):745–51. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- 89.Mi S, Miller RH, Tang W, et al. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann Neurol. 2009 Mar;65(3):304–15. doi: 10.1002/ana.21581. [DOI] [PubMed] [Google Scholar]
- 90.Biogen. Anti-LINGO-1 Phase 2 Acute Optic Neuritis - RENEW. 2015 http://bit.ly/1PQm8I8.
- 91.Cadavid D, Edwards K, Hupperts R, et al. BIIB033, Anti-LINGO-1 Antibody, for Treatment of Relapsing Forms of Multiple Sclerosis: Baseline Data of the Phase 2 SYNERGY Trial. Poster N° - P7204 ∣ 67th AAN Annual Meeting ∣ American Academy of Neurology® April 18-25; Washington, DC, USA. 2015 April 18-25; [cited Poster N° - P7.204 ∣ 67th AAN Annual Meeting ∣ American Academy of Neurology® April 18-25, Washington, DC, USA Available from: [Google Scholar]
- 92.Annunziata P, Pluchino S, Martino T, et al. High levels of cerebrospinal fluid IgM binding to myelin basic protein are associated with early benign course in multiple sclerosis. J Neuroimmunol. 1997 Jul;77(1):128–33. doi: 10.1016/s0165-5728(97)00074-x. [DOI] [PubMed] [Google Scholar]
- 93.Annunziata P, Cioni C, Cantalupo L, et al. Immunosuppressive monoclonal antibody to CD64 from patients with long-term stable multiple sclerosis. J Neuroimmunol. 2013 Mar 15;256(1-2):62–70. doi: 10.1016/j.jneuroim.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 94.Avrameas S. Natural autoantibodies: from 'horror autotoxicus' to 'gnothi seauton'. Immunology today. 1991 May;12(5):154–9. doi: 10.1016/S0167-5699(05)80045-3. ** Article discussing the idea of natural autoantibodies and their protective role in physiology.
- 95.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Current opinion in immunology. 1995 Dec;7(6):812–8. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 96.Dighiero G, Lymberi P, Mazie JC, et al. Murine hybridomas secreting natural monoclonal antibodies reacting with self antigens. J Immunol. 1983 Nov;131(5):2267–72. [PubMed] [Google Scholar]
- 97.Haspel MV, Onodera T, Prabhakar BS, et al. Multiple organ-reactive monoclonal autoantibodies. Nature. 1983 Jul 7-13;304(5921):73–6. doi: 10.1038/304073a0. [DOI] [PubMed] [Google Scholar]
- 98.Prabhakar BS, Saegusa J, Onodera T, et al. Lymphocytes capable of making monoclonal autoantibodies that react with multiple organs are a common feature of the normal B cell repertoire. J Immunol. 1984 Dec;133(6):2815–7. [PubMed] [Google Scholar]
- 99.Lacroix-Desmazes S, Kaveri SV, Mouthon L, et al. Self-reactive antibodies (natural autoantibodies) in healthy individuals. J Immunol Methods. 1998 Jul 1;216(1-2):117–37. doi: 10.1016/s0022-1759(98)00074-x. [DOI] [PubMed] [Google Scholar]
- 100.Merbl Y, Zucker-Toledano M, Quintana FJ, et al. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J Clin Invest. 2007 Mar;117(3):712–8. doi: 10.1172/JCI29943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Avrameas S, Selmi C. Natural autoantibodies in the physiology and pathophysiology of the immune system. J Autoimmun. 2013 Mar;41:46–9. doi: 10.1016/j.jaut.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 102.Avrameas S, Ternynck T. The natural autoantibodies system: between hypotheses and facts. Molecular immunology. 1993 Aug;30(12):1133–42. doi: 10.1016/0161-5890(93)90160-d. [DOI] [PubMed] [Google Scholar]
- 103.Brandlein S, Rauschert N, Rasche L, et al. The human IgM antibody SAM-6 induces tumor-specific apoptosis with oxidized low-density lipoprotein. Molecular cancer therapeutics. 2007 Jan;6(1):326–33. doi: 10.1158/1535-7163.MCT-06-0399. [DOI] [PubMed] [Google Scholar]
- 104.Vollmers HP, Brandlein S. Natural antibodies and cancer. New biotechnology. 2009 Jun;25(5):294–8. doi: 10.1016/j.nbt.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 105.Bieber AJ, Warrington A, Asakura K, et al. Human antibodies accelerate the rate of remyelination following lysolecithin-induced demyelination in mice. Glia. 2002 Mar 1;37(3):241–9. doi: 10.1002/glia.10033. [DOI] [PubMed] [Google Scholar]
- 106.Warrington AE, Asakura K, Bieber AJ, et al. Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc Natl Acad Sci U S A. 2000 Jun 6;97(12):6820–5. doi: 10.1073/pnas.97.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Warrington AE, Bieber AJ, Ciric B, et al. A recombinant human IgM promotes myelin repair after a single, very low dose. Journal of neuroscience research. 2007 Apr;85(5):967–76. doi: 10.1002/jnr.21217. [DOI] [PubMed] [Google Scholar]
- 108.Mitsunaga Y, Ciric B, Van Keulen V, et al. Direct evidence that a human antibody derived from patient serum can promote myelin repair in a mouse model of chronic-progressive demyelinating disease. Faseb J. 2002 Aug;16(10):1325–7. doi: 10.1096/fj.01-0994fje. [DOI] [PubMed] [Google Scholar]
- 109.Pirko I, Ciric B, Gamez J, et al. A human antibody that promotes remyelination enters the CNS and decreases lesion load as detected by T2-weighted spinal cord MRI in a virus-induced murine model of MS. FASEB J. 2004 Oct;18(13):1577–9. doi: 10.1096/fj.04-2026fje. [DOI] [PubMed] [Google Scholar]
- 110.Ciric B, Van Keulen V, Paz Soldan M, et al. Antibody-mediated remyelination operates through mechanism independent of immunomodulation. J Neuroimmunol. 2004 Jan;146(1-2):153–61. doi: 10.1016/j.jneuroim.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 111.Howe CL, Bieber AJ, Warrington AE, et al. Antiapoptotic signaling by a remyelination-promoting human antimyelin antibody. Neurobiol Dis. 2004 Feb;15(1):120–31. doi: 10.1016/j.nbd.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 112.Wootla B, Denic A, Watzlawik JO, et al. Antibody-Mediated Oligodendrocyte Remyelination Promotes Axon Health in Progressive Demyelinating Disease. Mol Neurobiol. 2015 Sep 26; doi: 10.1007/s12035-015-9436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Watzlawik J, Holicky E, Edberg DD, et al. Human remyelination promoting antibody inhibits apoptotic signaling and differentiation through Lyn kinase in primary rat oligodendrocytes. Glia. 2010 Nov 15;58(15):1782–93. doi: 10.1002/glia.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Watzlawik JO, Warrington AE, Rodriguez M. PDGF is Required for Remyelination-Promoting IgM Stimulation of Oligodendrocyte Progenitor Cell Proliferation. PLoS One. 2013;8(2):e55149. doi: 10.1371/journal.pone.0055149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Acorda Therapeutics Inc An Intravenous Infusion Study of rHIgM22 in Patients With Multiple Sclerosis. Clinical Trials Identifier - NCT01803867. 2015 Weblink - http://1.usa.gov/1N2gsHJ.
- 116.Greenberg BM, Rodriguez M, Kantarci OH, et al. Safety and Tolerability of the Remyelinating Therapeutic Antibody rHIgM22 in Patients with Stable Multiple Sclerosis. Poster N° - P4339 ∣ ACO P5130, 67th AAN Annual Meeting ∣ American Academy of Neurology® April 18-25; Washington, DC, USA. 2015 April 18-25; [cited Poster N° - P4.339 ∣ ACO P5130, 67th AAN Annual Meeting ∣ American Academy of Neurology® April 18-25, Washington, DC, USA Available from: [Google Scholar]
- 117.Warrington AE, Bieber AJ, Van Keulen V, et al. Neuron-binding human monoclonal antibodies support central nervous system neurite extension. J Neuropathol Exp Neurol. 2004 May;63(5):461–73. doi: 10.1093/jnen/63.5.461. [DOI] [PubMed] [Google Scholar]
- 118.Van Keulen VP, Ciric B, Radhakrishnan S, et al. Immunomodulation using the recombinant monoclonal human B7-DC cross-linking antibody rHIgM12. Clin Exp Immunol. 2006 Feb;143(2):314–21. doi: 10.1111/j.1365-2249.2005.02992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu X, Warrington AE, Wright BR, et al. A human IgM signals axon outgrowth: coupling lipid raft to microtubules. Journal of neurochemistry. 2011 Oct;119(1):100–12. doi: 10.1111/j.1471-4159.2011.07416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Denic A, Macura SI, Warrington AE, et al. A single dose of neuron-binding human monoclonal antibody improves spontaneous activity in a murine model of demyelination. PLoS One. 2011;6(10):e26001. doi: 10.1371/journal.pone.0026001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wootla B, Denic A, Watzlawik JO, et al. A single dose of a neuron-binding human monoclonal antibody improves brainstem NAA concentrations, a biomarker for density of spinal cord axons, in a model of progressive multiple sclerosis. J Neuroinflammation. 2015;12:83. doi: 10.1186/s12974-015-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu X, Denic A, Jordan LR, et al. A natural human IgM that binds to gangliosides is therapeutic in murine models of amyotrophic lateral sclerosis. Dis Model Mech. 2015 Aug 1;8(8):831–42. doi: 10.1242/dmm.020727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Watzlawik JO, Kahoud RJ, Ng S, et al. Polysialic acid as an antigen for monoclonal antibody HIgM12 to treat multiple sclerosis and other neurodegenerative disorders. Journal of neurochemistry. 2015 Sep;134(5):865–78. doi: 10.1111/jnc.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Banerjee S, Bhat MA. Neuron-glial interactions in blood-brain barrier formation. Annu Rev Neurosci. 2007;30:235–58. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bielekova B, Becker BL. Monoclonal antibodies in MS: mechanisms of action. Neurology. 2010 Jan 5;74(Suppl 1):S31–40. doi: 10.1212/WNL.0b013e3181c97ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]