Abstract
Objective
We examined how serum cholesterol, an established risk factor for cerebrovascular disease (CVD), relates to cognitive function in healthy middle-older aged individuals with no neurologic or CVD history.
Method
A complete lipid panel was obtained from a cohort of one hundred twenty individuals, ages 43–85, who also underwent a comprehensive neuropsychological examination. In order to reduce the number of variables and empirically identify broad cognitive domains, scores from neuropsychological tests were submitted into a factor analysis. This analysis revealed three explainable factors: Memory, Executive Function and Memory/Language. Three separate hierarchical multiple regression analyses were conducted using individual cholesterol metrics (total cholesterol, low density lipoprotein; LDL, high density lipoprotein; HDL, and triglycerides), as well as age, education, medication status (lipid lowering agents), ApoE status, and additional risk factors for CVD to predict neuropsychological function.
Results
The Memory Factor was predicted by a combination of age, LDL, and triglyceride levels; both age and triglycerides were negatively associated with factor score, while LDL levels revealed a positive relationship. Both the Executive and Memory/Language factor were only explained by education, whereby more years were associated with better performance.
Conclusions
These results provide evidence that individual cholesterol lipoproteins and triglycerides may differentially impact cognitive function, over and above other common CVD risk factors and ApoE status. Our findings demonstrate the importance of consideration of vascular risk factors, such as cholesterol, in studies of cognitive aging.
Keywords: cerebrovascular health, cerebrovascular risk, cholesterol, cognition
Cholesterol is a primary risk factor for cardiovascular and cerebrovascular disease (CVD). Abnormally elevated levels of total cholesterol, low-density lipoproteins (LDL), and triglycerides, as well as low levels of high-density lipoproteins (HDL) have all been associated with conditions such as atherosclerosis, coronary artery disease, and hyperlipidemia; all of these can cause vascular-related brain changes, such as small-vessel ischemic infarcts, as well as more serious events such as stroke (Bang, Saver, Liebeskind, Pineda, & Ovbiagele, 2008; Khan, Porteous, Hassan, & Markus, 2007; Staekenborg et al., 2008). High cholesterol has been implicated in age-associated cognitive decline (Morley & Banks, 2010), as well as in the development of pathological disorders such as Alzheimer’s disease (AD) and vascular dementia (Lee et al., 2009; Merched, Xia, Visvikis, Serot, & Siest, 2000; Reitz, Tang, Luchsinger, & Mayeux, 2004), in which cognitive impairment is a defining feature. In broad studies that have examined individuals at risk for CVD, cholesterol levels have been most often tied to reduced performance on neuropsychological measures of executive function (Elias, Elias, D’Agostino, Sullivan, & Wolf, 2005; Gendle, Spaeth, Dollard, & Novak, 2008; Reijmer et al., 2012), although deficits in other cognitive domains have also been reported (Sparks et al., 2010); this is generally consistent with the literature on other common cerebrovascular factors such as blood pressure and diabetes (Bender & Raz, 2012; Dahle, Jacobs, & Raz, 2009).
While the connections between cholesterol, CVD and dementia are fairly well established, the specific relationship to cognition of the individual components of cholesterol, including the lipid transport molecules low-density lipoprotein (LDL) and high-density lipoprotein (HDL), as well as triglycerides, is less clear. Each of these may have a specific impact on cardiovascular health, and thus, may have differential effects on cognitive function. Few studies have specifically examined how LDL, HDL and triglycerides influence neuropsychological function, and while the majority has reported that higher levels are related to worse performance, some have found no statistical relationship (Huang et al., 2009; Packard et al., 2007) and one study reported a positive association in late life (greater than age 85) (West et al., 2008). High LDL levels have been tied to reduced general neuropsychological function and cognitive decline over time (Henderson, Guthrie, & Dennerstein, 2003; Reitz et al., 2010). Higher levels of HDL have been tied to better memory performance and general cognitive function (van den Kommer, Dik, Comijs, Jonker, & Deeg, 2010; van Exel et al., 2002; Wolf et al., 2004), but some studies have not found a relationship with cognition (Gillum & Obisesan, 2011; Yaffe, Barrett-Connor, Lin, & Grady, 2002). High triglycerides have been associated with worse memory and general cognitive decline (de Frias et al., 2007; Morley & Banks, 2010), but there are also reports of a lack of association with cognitive function (Reitz, Luchsinger, Tang, Manly, & Mayeux, 2005). In general, most studies have focused on just one or two cognitive domains, using limited neuropsychological measures to do so, leaving open the question of how cholesterol molecules affect broad categories of cognitive function that are typically affected in cerebrovascular disease and cerebrovascular disease risk. This is important because cholesterol is a potentially modifiable risk factor that if identified early, may prevent worsening cognitive decline.
Given the inconsistencies in the relationship of cholesterol to cognitive function, and the gaps in our knowledge of how cholesterol affects specific domains of neuropsychology, the primary aim of the current study was to determine how the lipid transport molecules LDL and HDL, as well as triglycerides, relate to neuropsychological function. We examined these relationships in a population of community dwelling middle-older aged adults, ages 43–87 who had cholesterol levels ranging from normal to moderately-severely elevated, enabling us to fully understand the effects of individual cholesterol components across a wide range of risk as well as across a broad age range. We also included additional CVD risk factors (blood pressure and diabetes-related factors), as well as APoE status, a cholesterol transport molecule with known risk for cognitive decline and AD, in order to determine if there is a unique effect of cholesterol on cognitive function, over and above other potential significant contributors. We utilized a comprehensive battery of neuropsychological tests in order to thoroughly examine the effects comprehensively across critical cognitive domains, as this has not yet been established. Specific domains included attention, executive function, memory, and language. Based on the existing data with regard to cholesterol and to CVD risk, we predicted that higher levels of LDL cholesterol and triglycerides would be associated with reduced neuropsychological function, with stronger associations in executive function and memory domains. Given the fact that higher levels of HDL are clinically indicative of better health, we expected that lower levels would be associated with reduced performance in the executive function and memory domains. We expected that findings from this study would contribute significantly to the knowledge regarding the role that specific components of cholesterol may play in cognition, and to the larger literature on risk for CVD and cognitive decline. To our knowledge, this is one of the first studies to systematically examine the relationship of cholesterol to cognition, in the context of other common CVD and genetic risk factors.
Methods
Participants
One hundred-twenty participants (74 F/46 M) were recruited from two separate but overlapping studies examining how common cerebrovascular risk factors impact neuropsychological function. Individuals in these studies were recruited based on either the presence of absence of CVD risk factors such as cholesterol, blood pressure, and abnormally regulated glucose. Thirty-one participants were selected from a larger sample recruited by the Harvard Cooperative Program on Aging (HCPA) Claude Pepper Older American Independence Center. Participants in this program were recruited from the community in response to an advertisement asking for healthy community-dwelling older African Americans. Eighty-nine participants were recruited through the Understanding Cardiovascular and Alzheimer’s Risk in the Elderly (UCARE) program, a study investigating how CVD risk impacts brain structure and cognition. Participants in this study were recruited through the Boston University Alzheimer’s Disease Center (BUADC) based on the initial criteria of being neurologically healthy and having a first-degree family relative with dementia. Inclusion criteria for both studies included age of 40–85. Participants were excluded for the following reasons: a history of head trauma of “mild” severity or greater according to the criteria of Fortuny et al. (Fortuny, Briggs, Newcombe, Ratcliff, & Thomas, 1980) (e.g., loss of consciousness for greater than 10 minutes), any history of more than one head injury (due to possible cumulative neuropathological effects), diagnosis of any form of dementia (i.e., Parkinson’s disease, Alzheimer’s disease, vascular dementia), significant neurologic disease such as epilepsy or stroke, any severe psychiatric illness, or history of brain surgery. All participants were literate with at least a 6th grade education. Ninety-two of the participants were right-handed. Mini-mental state examination (MMSE) scores ranged from 23 to 30. These scores are in a range outside of a dementia diagnosis, according to normative data for the two racial groups (Caucasian, African American) in this sample (Bohnstedt, Fox, & Kohatsu, 1994). This study was approved by the IRB committee at the VA Boston Healthcare System, and written informed consent was obtained from all participants.
Serum Cholesterol Measurement
Following informed consent, fasting blood was drawn and processed for analysis of serum levels of cholesterol. The following cholesterol parameters were used: total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides. Current clinical convention considers total cholesterol of less than 200 mg/dL to be “normal,” while 200–239 mg/DL is “mildly elevated,” and total cholesterol levels of 240 or greater to be “severely elevated.” In our sample, total cholesterol levels ranged from 103 to 341 mg/dL. By these standards, 40 (33%) individuals would be considered to have mildly high cholesterol, while only 20 (17%) would be considered to have cholesterol levels in the severe range. Thus, 60 (50%) would be considered to be in the normal and mild range with regard to cholesterol.
Additional Metrics of Cerebrovascular Health
We also obtained additional metrics of cerebrovascular health, including systolic blood pressure (BP) and diabetes-related variables (fasting glucose and hemoglobin A1C), in order to determine if there is a unique association between cholesterol variables and cognition. BP was recorded using a standard sphygmomanometer, in a seated position after five minutes of rest, with the arm at rest at the level of the heart. A second measurement was obtained five minutes later and the average of two values was recorded. Serum measures of fasting glucose as well as hemoglobin A1C (HA1C), which both reflect metabolic health and possible diabetes, were also obtained. HA1C is a form of hemoglobin that can estimate the average plasma glucose concentration over a period of 4–12 weeks. It is therefore an indicator of how regulated individual glucose levels have been and is particularly useful in evaluating patients with diabetes. HA1C levels typically range from 4 to 14.
Medication Usage
Thirty-eight (32%) individuals reported taking lipid-lowering medication; of note, information regarding medication usage was not available for one individual. Because of the significant number of individuals taking lipid-lowering agents, medication usage was included as a variable in subsequent regression analyses.
ApoE status
APoE genotyping was performed on all participants and was therefore available for use in analyses. The well-established procedure for DNA extracting and genotyping is reported in prior papers (Foley et al., 2014). ApoE status was available for all participants; 32 participants tested positive (found to have at least one ApoE 4 gene). Given the documented evidence of a relationship with cholesterol and cognition (Perna, Mons, Rujescu, Kliegel, & Brenner, 2015; Sato & Morishita, 2015), we included APoE status as a variable in all regression models.
Neuropsychological Assessment
All participants underwent a comprehensive neuropsychological battery that included measures of attention, executive function, and memory. Specific tests included: Digit Span subtest (from the Wechsler Adult Intelligence Scale III) (Wechsler, 1997), the Stroop Color Word Test (SCWT) (Spreen & Strauss, 1998), Trail Making parts A & B (Spreen & Strauss, 1998), Controlled Oral Word Association (COWA) (Spreen & Strauss, 1998), the Logical Memory subtest from the Wechsler Memory Scale, 3rd Edition (Wechsler, 1997), the California Verbal Learning Test, 2nd Edition (CVLT) (Delis, Kramer, Kaplan, & Ober, 2000), and the Boston Naming Test (BNT) (Kaplan, Goodglass, & Weintraub, 2001).
Statistical Analyses
Factor Analysis of Neuropsychological Data
Raw neuropsychological test scores were submitted into a principal components analysis in order to reduce the number of dependent variables and to derive empirical factors representing broad cognitive domains.
Multiple Regression
Three separate hierarchical multiple regression analyses were conducted with each neuropsychological factor score as an outcome (dependent) variable. General covariates (age, education, lipid medication status (yes/no), and ApoE status (yes/no)) were entered first on Step 1, given predictability of their relationship with cholesterol. On Step 2, we entered cerebrovascular covariates (systolic BP, glucose, HA1C), in order to examine how additional risk factors might contribute to variance in cognitive function. Finally, on Step 3, we entered cholesterol variables (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides).
Results
Demographic, cholesterol, and medical data for all participants are presented in Table 1. Participants ranged in age from 43–87, and 74 participants were male. Cerebrovascular risk status (including blood pressure, cholesterol, and glucose regulation) ranged from no risk to moderately severe, thereby representing a sample with a range of risk.
Table 1.
Demographic, Medical and Cholesterol Data
| Mean | Standard Deviation | Range | |
|---|---|---|---|
| Age | 68.10 | 9.61 | 43–87 |
| Education (years) | 14.83 | 2.72 | 9–20 |
| MMSE score (n=114)** | 27.82 | 1.79 | 23–30 |
|
|
|||
| Cholesterol | |||
|
|
|||
| Total Cholesterol | 202.65 | 41.59 | 103.8–341.8 |
| HDL Cholesterol | 61.75 | 17.46 | 22–135 |
| LDL Cholesterol | 119.03 | 33.02 | 52–193 |
| Triglycerides | 101.81 | 45.15 | 31–249 |
| Fasting glucose | 94.30 | 17.39 | 54–156 |
| Hemoglobin A1C | 5.8 | .67 | 4.8–8.4 |
| Systolic Blood Pressure | 132.92 | 17.98 | 91–178 |
HDL=High density lipoprotein; LDL=low density lipoprotein
MMSE was not administered to 6 participants
triglyceride, glucose and HA1C values were each missing for one participant.
Factor Analysis of Neuropsychological Data
Neuropsychological data are presented in Table 2. Raw scores from each of the seven neuropsychological measures were submitted to a factor analysis in order to reduce the number of dependent variables and to derive empirical factors representing broad cognitive domains (Bryant & Yarnold, 1995). Fourteen total variables were entered which included: Digit Span (total score), SCWT (total interference score), Trailmaking A (total time to complete), Trailmaking B (total time to complete), COWA (total number of exemplars produced in 3 minutes), CVLT (total score, short-delay cued recall; SDCR, short-delay free recall; SDFR, long-delay cued recall; LDCR, long-delay free recall; LDFR, total recognition hits); BNT (total score). Because higher scores on Trail Making indicates worse performance, scores on part A and part B were inverted prior to entry into the factor analysis in order to be consistent with all other variables. Thus for all other variables prior to entry into the principal components analysis, higher scores denote better performance. Varimax rotation was used and the minimum eigenvalue for extraction was set at one. Loadings from the rotated solution are presented in Table 3. This analysis revealed a set of three orthogonal and empirically coherent factors containing separate aspects of cognition. The three factors explained a total of 68.52% of the variance. The first factor extracted was called “Memory,” and explained 46 % of the variance, with high loadings from all CVLT recall measures (total, cued and free). The second factor was called “Executive,” explaining 14% of the variance with high loadings from Trailmaking A & B, SCWT, Digit Span, and COWA. The third factor was called “Memory/Language,” explaining 8% of the variance, with loadings from the Logical Memory I & II, the Boston Naming Test, as well as CVLT recognition hits. The relationship between age and factor scores and between education and factor scores was examined using bivariate correlations.
Table 2.
Neuropsychological Data
| Mean | Standard Deviation | Range | |
|---|---|---|---|
| Digit Span total score | 17.39 | 4.09 | 7–28 |
| SCWT total interference score | 90.50 | 25.48 | 37–168 |
| Trailmaking part A | 38.71 | 13.33 | 13–110 |
| Trailmaking part B | 92.77 | 43.83 | 24–246 |
| COWA (total words produced) | 41.43 | 12.68 | 15–76 |
| CVLT: total score, trials 1–5 | 48.56 | 11.46 | 16–71 |
| CVLT SDCR | 11.28 | 2.92 | 4–16 |
| CVLT SDFR | 9.31 | 3.40 | 0–16 |
| CVLT LDCR | 11.18 | 3.23 | 3–16 |
| CVLT LDFR | 10.23 | 3.32 | 0–16 |
| CVLT Recognition Hits | 14.52 | 1.72 | 6–16 |
| Logical Memory I (immediate memory | 42.72 | 9.89 | 17–72 |
| Logical Memory II (delayed memory) | 26.98 | 8.01 | 6–45 |
| Boston Naming Test | 55.49 | 4.49 | 41–60 |
Note: SCWT=Stroop Color-Word Test, COWA=Controlled Oral Word Association, CVLT=California Verbal Learning Test
Table 3.
Pattern Matrix from Factor Analysis of Neuropsychological Variables
| Factor
|
|||
|---|---|---|---|
| Memory | Executive Function | Memory/Language | |
| CVLT, LDCR* | .924 | .163 | .198 |
| CVLT, LDFR | .893 | .227 | .211 |
| CVLT, SDCR | .886 | .227 | .232 |
| CVLT, SDFR | .865 | .141 | .313 |
| CVLT total score (trials 1–5) | .820 | .269 | .264 |
| Trailmaking part B* | −,166 | −.805 | −.139 |
| COWA total score | −.038 | .733 | .382 |
| Digit Span total score | .151 | .723 | .044 |
| SCWT | .365 | .638 | −.064 |
| Trailmaking part A* | .365 | −.542 | −.001 |
| BNT total score | −.002 | .515 | .510 |
| Logical Memory I | .307 | .194 | .796 |
| Logical Memory II | .332 | .180 | .768 |
| CVLT Recognition Hits | .308 | −.119 | .490 |
Note: CVLT=California Verbal Learning Test; LDCR=long-delay cued recall;
LDFR=long-delay free recall; SDCR=short-delay cued recall, SDFR=short-delay free recall; SCWT=Stroop Color-Word Test, COWA=Controlled Oral Word Association, BNT=Boston Naming Test
Relationship of Serum Cholesterol Measures to Neuropsychological Function
The relationship of serum cholesterol markers to neuropsychological function was examined using a series of hierarchical multiple linear regressions. In each regression, demographic and medical variables were entered on Step 1 (age, education, lipid medication status, ApoE status), cerebrovascular variables were entered on Step 2 (systolic BP, glucose, HA1C), and cholesterol variables were entered in the final Step (Step 3; total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides). Each model was examined separately for multicollinearity using the variance inflation factor (VIF) statistic. Using this criteria, total cholesterol was removed from all models due to a low tolerance.
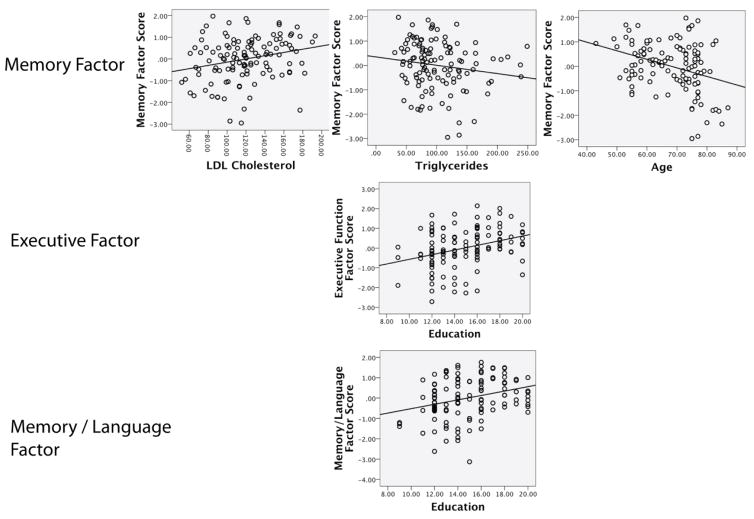
Variables retained in the model predicting the Memory factor score included age, triglycerides, and LDL levels. Greater age and higher triglyceride level were associated with lower factor score and thus reduced performance on tasks of memory, while higher LDL levels were associated with higher factor score and thus better performance on memory tasks. Variables retained in the Executive model included education; as expected, greater education was associated with higher factor score and better performance on tasks of executive function. Variables retained in the Memory/language model included education. As in the executive model, higher levels of education were associated with higher factor score. Regression results are presented in Table 4, and scatter plots of significant results are presented in Figure 1.
Table 4.
Results of multiple regression analyses predicting neuropsychological factor scores
| Variable | Memory | Executive Function | Memory/Language | |||
|---|---|---|---|---|---|---|
|
| ||||||
| B | SE B | B | SE B | B | SE B | |
| Age | −.030 | −.286 | −.026 | −.251 | .005 | .053 |
|
|
||||||
| Education | .013 | .035 | .089 | .237 | .120 | .322 |
|
|
||||||
| Lipid Medication | .214 | .099 | .172 | .079 | .356 | .166 |
|
|
||||||
| ApoE Status | −.037 | −.016 | .149 | .065 | .028 | ,012 |
|
|
||||||
| Systolic Blood Pressure | −.006 | −.106 | .008 | .131 | .000 | .007 |
|
|
||||||
| Fasting Glucose | −.002 | .007 | −.012 | −.209 | −.003 | −.051 |
|
|
||||||
| HA1C | .138 | .165 | −.153 | −.209 | −.186 | −.124 |
|
|
||||||
| LDL | .008 | .250 | ..004 | .115 | .003 | .087 |
|
|
||||||
| HDL | .001 | .022 | .005 | .078 | −.007 | −.125 |
|
|
||||||
| Triglycerides | −.005 | −.229 | .000 | .000 | .002 | .098 |
|
|
||||||
| R2 (adjusted) | .141 | .128 | .119 | |||
| F | 2.91* | 2.70* | 2.57* | |||
p < .01
Total cholesterol was removed from all analyses due to a low tolerance.
Figure 1.
Scatterplots of significant regression results.
Discussion
The present study demonstrates that in a large sample of generally healthy adults, individual serum lipoproteins and triglycerides are differentially related to neuropsychological function., Age, LDL, and triglycerides emerged as significant predictors of the Memory factor, but while higher triglycerides were associated with poorer performance as expected, higher LDL levels were associated with better performance on neuropsychological measures of memory. The only predictor of both the Executive and Memory/Language factors was education, in which higher education was associated with better performance. HDL cholesterol did not emerge as a significant predictor of any cognitive factor, nor did any medical or CVD risk factors. These results demonstrate that serum lipoproteins and triglycerides may have differential effects on cognition, and specifically, memory, over and above LLA usage, APoE status, blood pressure, and diabetes-related variables.
The Memory factor was explained by a combination of age, LDL, and triglycerides, and as expected, age was negatively associated with performance, while triglycerides were negatively associated with factor scores. However, higher LDL levels were associated with better scores on this factor, which primarily contains measures of immediate and delayed list learning. A positive relationship LDL and cognitive function has been reported previously; West et al. (2008) report that higher LDL cholesterol was associated with better memory performance, only in individuals aged 85 or greater (West et al., 2008). To our knowledge, however, positive associations have not been found in younger populations, and as such, this finding was unexpected in our study, as higher levels of cerebrovascular risk factors typically lead to poorer performance on cognitive tests. Several years of research that have implicated portions of the frontal lobes, including frontal-subcortical connections as well as cortical volume, as being initial targets of elevated cerebrovascular disease (CVD) risk (Delano-Wood et al., 2008; Leritz et al., 2010; Raz, Rodrigue, Kennedy, & Acker, 2007; van Es et al., 2008). In fact, even subclinical elevations can result in subtle damage to these brain regions, which are particularly vulnerable to CVD-related consequences such as reduced blood flow resulting from blocked arteries, resulting in reduced tissue integrity (Leritz et al., 2010; Salat et al., 2012) (Williams et al., 2013). However, it is becoming clearer that when specifically examining individual CVD risk factors, including the individual components of cholesterol, may have a signature impact on brain structure (Leritz et al., 2011; Salat et al., 2012). It may be the molecular properties of LDL target brain regions or brain networks that are important in memory. LDL, a lipid transport protein, is of particular relevance to the potential development of CVD as it can facilitate the deposition of excess serum cholesterol into artery walls, resulting in plaques and in cases of very high levels of LDL, atherosclerosis (Badimon & Vilahur, 2012; Weber & Noels, 2011). Atherosclerosis is a primary contributor to CVD, as it can lead to ischemia, stroke, and by itself has been independently associated with cognitive decline and dementia (Yuan, Wen, Li, & Liu, 2013; Zheng et al., 2012). However, it may be that LDL negatively affects the brain only when elevated levels are sustained for a long enough duration so as to disrupt the neurovascular environment and subsequent cognitive function. Taken together with our results, these findings suggest that LDL may promote better memory under certain circumstances, and it may be that there are optimal levels that support cognitive, and particularly memory function.
The negative relationship of triglycerides with the Memory factor was consistent with our predictions, as well as prior work, including a recent study reporting an association between high triglycerides and poorer memory performance (de Frias et al., 2007; Morley & Banks, 2010; van den Kommer et al., 2010). However, this is in contrast to findings of reduced executive function, and taken together with LDL findings, provides support for the idea that the individual components of cholesterol may have a specific effect on memory function. Similar to LDL, higher triglycerides are a known contributor to atherosclerosis and cerebrovascular disease, but they are a specific source of excess fats and calories that can accumulate in blood vessels throughout the body, including the brain (Talayero & Sacks, 2011). Given the known relationship between CVD and poorer cognitive function, it is highly plausible that higher levels contribute to reduced cognitive performance via direct tissue damage as a result of reduced blood flow.
The fact that education was the only predictor of the Executive Function and Memory/Language factor scores is somewhat surprising, as we expected that cholesterol measures would be most strongly associated with this domain given prior findings with regard to the impact of cerebrovascular risk factors on cognitive function (Bender & Raz, 2012; Debette et al., 2011). In our sample, which consisted of a broad range of cholesterol levels, cholesterol variables accounted for the most variance in memory factors, suggesting that varying levels of triglycerides and the individual cholesterol lipoproteins have at least an initial impact on memory function. Perhaps, as CVD risk increases, with higher levels over long durations, additional domains, such as executive function, become affected. Cholesterol is implicated in dementia syndromes such as Alzheimer’s disease, with memory loss as a defining feature, and it may be that the individual lipoproteins initially target specific structural brain regions that are especially sensitive to these disease processes, such as the hippocampus, that support memory function.
Our findings regarding the apparently differential relationship of cholesterol to cognition may be, in part, reflective of the complex nature of lipids. While clinical data support the belief that cholesterol is traditionally thought of as a negative entity, cholesterol molecules do in fact play critical roles in metabolism throughout the body, and especially in the brain. Critical to optimal brain function is an optimal balance between serum cholesterol and brain-derived cholesterol, which is secondarily influenced by lipid transport proteins (LDL, HDL), and triglycerides (Babiker et al., 2005). Brain cholesterol metabolism can be indirectly measured in the blood as oxysterols, and a disproportionate ratio between brain and serum cholesterol metabolism has been linked to the development of dementia and other neurodegenerative conditions (Hughes, Rosano, Evans, & Kuller, 2013). This suggests that cholesterol levels, particularly as they interact with brain cholesterol metabolites, may play an important role in maintaining adequate brain and cognitive health (Leoni & Caccia, 2013; Presecki et al., 2011; van den Kommer et al., 2009). Moreover, a recent study found that serum markers of such imbalance are more associated with CVD than AD (Hughes et al., 2012). It may be that our findings of a positive relationship between LDL and memory function are at least partially reflective of this, and suggest that perhaps there are ideal levels of this particular lipid transport molecule needed to maximally support neural and cognitive function. Interestingly, APoE status did not contribute to the variance in cognitive function. APoE is in part responsible for the formation of lipoproteins, and in transporting cholesterol molecules to the liver for removal and excretion. Possession of an e4 allele can increase cholesterol levels and CVD risk by hampering the removal of cholesterol, triggering the accumulation of cholesterol in the blood, which eventually contributes to atherosclerosis and vascular dysfunction. Thus, it would not be surprising if APoE status played a role in the relationship between cholesterol and cognition, particularly given it’s known risk for AD and the fact that memory function is often times one of the first neuropsychological complaints in dementia syndromes such as AD and dementia due to CVD. However, it is also likely that the relationship between cholesterol and cognitive function is very complex, and that the positive and negative influences of cholesterol on memory function may fluctuate over time, and with a greater number of CVD risk factors (Perna et al., 2015). Indeed, we cannot comment on whether this relationship between LDL and memory function is beneficial for long-term cognitive health, in part due to the cross-sectional nature of the study. However, they do suggest a potential functional relationship between cholesterol and cognitive function. In a prior study, we reported that a factor score containing total cholesterol and LDL was associated with increased thickness of the cerebral cortex in several brain regions (Leritz et al., 2011). Taken together, these collective findings point to a possible link between optimal LDL levels, brain structure, and cognition. Future work with longitudinal and larger samples, including a closer look at how APoE status affects the relationship between cholesterol and cognition, will be needed to confirm this speculation.
Limitations of the current study include our recruitment strategy, which was biased to increase the overall prevalence of cardiovascular risk. First-degree family relatives of an individual with dementia likely have increased risk, and a large portion of our sample was comprised of African Americans, who as a racial group are believed to have increased risk for CVD. We consider this to be a strength, however, as it speaks to the relationship between individual cholesterol molecules and cognition in an enriched sample. However, this may lessen the overall generalizability of our findings. An additional limitation is that duration of lipid medication is unknown for our participants. This could be a potentially important variable, as it may be that a longer duration of LLA use may present an additional protective effect on neural structure and cognitive decline. Ongoing work will collect this important variable in a larger and a longitudinal sample.
In summary, we present results suggesting that LDL and triglycerides may have differential effects on neuropsychological performance that may reflect both beneficial and deleterious properties of cholesterol in relation to brain and cognitive health. These results were independent of HDL, as well as of use of LLA’s, APOE status and additional CVD risk factors. Thus, the associations here appear to be unique and suggest that consideration of individual variables may be important in future studies. Future longitudinal work in our laboratory will determine if the direction of these associations persist over time.
Acknowledgments
This work was supported by the National Institute of Neurologic Disorders and Stroke (K23NS062148 & R01NS086882), the National Institute of Nursing Research (R01NR010827), the National Institute on Aging, and by Medical Research Service VA Merit Review Awards
References
- Babiker A, Dzeletovic S, Wiklund B, Pettersson N, Salonen J, Nyyssonen K, et al. Patients with atherosclerosis may have increased circulating levels of 27-hydroxycholesterol and cholestenoic acid. Scand J Clin Lab Invest. 2005;65(5):365–375. doi: 10.1080/00365510510025746. [DOI] [PubMed] [Google Scholar]
- Badimon L, Vilahur G. LDL-cholesterol versus HDL-cholesterol in the atherosclerotic plaque: inflammatory resolution versus thrombotic chaos. Ann N Y Acad Sci. 2012;1254:18–32. doi: 10.1111/j.1749-6632.2012.06480.x. [DOI] [PubMed] [Google Scholar]
- Bang OY, Saver JL, Liebeskind DS, Pineda S, Ovbiagele B. Association of serum lipid indices with large artery atherosclerotic stroke. Neurology. 2008;70(11):841–847. doi: 10.1212/01.wnl.0000294323.48661.a9. [DOI] [PubMed] [Google Scholar]
- Bender AR, Raz N. Age-related differences in memory and executive functions in healthy APOE varepsilon4 carriers: the contribution of individual differences in prefrontal volumes and systolic blood pressure. Neuropsychologia. 2012;50(5):704–714. doi: 10.1016/j.neuropsychologia.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnstedt M, Fox PJ, Kohatsu ND. Correlates of Mini-Mental Status Examination scores among elderly demented patients: the influence of race-ethnicity. J Clin Epidemiol. 1994;47(12):1381–1387. doi: 10.1016/0895-4356(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Bryant FB, Yarnold PR. Principle-components analysis and exploratory and confirmatory factor analysis. In: Grimm LG, Yarnold PR, editors. Reading and understanding multivariate statistics. Washington, D.C: American Psychological Association; 1995. pp. 99–136. [Google Scholar]
- Dahle CL, Jacobs BS, Raz N. Aging, vascular risk, and cognition: blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychol Aging. 2009;24(1):154–162. doi: 10.1037/a0014283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias CM, Bunce D, Wahlin A, Adolfsson R, Sleegers K, Cruts M, et al. Cholesterol and triglycerides moderate the effect of apolipoprotein E on memory functioning in older adults. J Gerontol B Psychol Sci Soc Sci. 2007;62(2):P112–118. doi: 10.1093/geronb/62.2.p112. [DOI] [PubMed] [Google Scholar]
- Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano-Wood L, Bondi MW, Jak AJ, Horne NR, Schweinsburg BC, Frank LR, et al. Stroke risk modifies regional white matter differences in mild cognitive impairment. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer J, Kaplan E, Ober B. California Verbal Learning Test-Second Edition. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Elias PK, Elias MF, D’Agostino RB, Sullivan LM, Wolf PA. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosom Med. 2005;67(1):24–30. doi: 10.1097/01.psy.0000151745.67285.c2. [DOI] [PubMed] [Google Scholar]
- Foley JM, Salat DH, Stricker NH, Zink TA, Grande LJ, McGlinchey RE, et al. Interactive effects of apolipoprotein E4 and diabetes risk on later myelinating white matter regions in neurologically healthy older aged adults. Am J Alzheimers Dis Other Demen. 2014;29(3):222–235. doi: 10.1177/1533317513517045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuny LA, Briggs M, Newcombe F, Ratcliff G, Thomas C. Measuring the duration of post traumatic amnesia. Journal of Neurology, Neurosurgery and Psychiatry. 1980;43(5):377–379. doi: 10.1136/jnnp.43.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendle MH, Spaeth AM, Dollard SM, Novak CA. Functional relationships between serum total cholesterol levels, executive control, and sustained attention. Nutr Neurosci. 2008;11(2):84–94. doi: 10.1179/147683008X301469. [DOI] [PubMed] [Google Scholar]
- Gillum RF, Obisesan TO. High-density lipoprotein cholesterol, cognitive function and mortality in a U.S. national cohort. Lipids Health Dis. 2011;10:26. doi: 10.1186/1476-511X-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Guthrie JR, Dennerstein L. Serum lipids and memory in a population based cohort of middle age women. J Neurol Neurosurg Psychiatry. 2003;74(11):1530–1535. doi: 10.1136/jnnp.74.11.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CQ, Dong BR, Wu HM, Zhang YL, Wu JH, Lu ZC, et al. Association of cognitive impairment with serum lipid/lipoprotein among Chinese nonagenarians and centenarians. Dement Geriatr Cogn Disord. 2009;27(2):111–116. doi: 10.1159/000194660. [DOI] [PubMed] [Google Scholar]
- Hughes TM, Kuller LH, Lopez OL, Becker JT, Evans RW, Sutton-Tyrrell K, et al. Markers of cholesterol metabolism in the brain show stronger associations with cerebrovascular disease than Alzheimer’s disease. J Alzheimers Dis. 2012;30(1):53–61. doi: 10.3233/JAD-2012-111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TM, Rosano C, Evans RW, Kuller LH. Brain cholesterol metabolism, oxysterols, and dementia. J Alzheimers Dis. 2013;33(4):891–911. doi: 10.3233/JAD-2012-121585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia, MD: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Khan U, Porteous L, Hassan A, Markus HS. Risk factor profile of cerebral small vessel disease and its subtypes. J Neurol Neurosurg Psychiatry. 2007;78(7):702–706. doi: 10.1136/jnnp.2006.103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Lim TS, Shin HW, Yong SW, Nam HS, Sohn YH. Serum cholesterol levels and the risk of multiple system atrophy: a case-control study. Mov Disord. 2009;24(5):752–758. doi: 10.1002/mds.22459. [DOI] [PubMed] [Google Scholar]
- Leoni V, Caccia C. 24S-hydroxycholesterol in plasma: a marker of cholesterol turnover in neurodegenerative diseases. Biochimie. 2013;95(3):595–612. doi: 10.1016/j.biochi.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Milberg WP, Williams VJ, Chapman CE, Grande LJ, et al. Variation in blood pressure is associated with white matter microstructure but not cognition in African Americans. Neuropsychology. 2010;24(2):199–208. doi: 10.1037/a0018108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, et al. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage. 2011;54(4):2659–2671. doi: 10.1016/j.neuroimage.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merched A, Xia Y, Visvikis S, Serot JM, Siest G. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer’s disease. Neurobiol Aging. 2000;21(1):27–30. doi: 10.1016/s0197-4580(99)00103-7. [DOI] [PubMed] [Google Scholar]
- Morley JE, Banks WA. Lipids and cognition. J Alzheimers Dis. 2010;20(3):737–747. doi: 10.3233/JAD-2010-091576. [DOI] [PubMed] [Google Scholar]
- Packard CJ, Westendorp RG, Stott DJ, Caslake MJ, Murray HM, Shepherd J, et al. Association between apolipoprotein E4 and cognitive decline in elderly adults. J Am Geriatr Soc. 2007;55(11):1777–1785. doi: 10.1111/j.1532-5415.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- Perna L, Mons U, Rujescu D, Kliegel M, Brenner H. Apolipoprotein E e4 and Cognitive Function: A Modifiable Association? Results from Two Independent Cohort Studies. Dement Geriatr Cogn Disord. 2015;41(1–2):35–45. doi: 10.1159/000440697. [DOI] [PubMed] [Google Scholar]
- Presecki P, Muck-Seler D, Mimica N, Pivac N, Mustapic M, Stipcevic T, et al. Serum lipid levels in patients with Alzheimer’s disease. Coll Antropol. 2011;35(Suppl 1):115–120. [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21(2):149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Reijmer YD, van den Berg E, Dekker JM, Nijpels G, Stehouwer CD, Kappelle LJ, et al. Development of vascular risk factors over 15 years in relation to cognition: the Hoorn Study. J Am Geriatr Soc. 2012;60(8):1426–1433. doi: 10.1111/j.1532-5415.2012.04081.x. [DOI] [PubMed] [Google Scholar]
- Reitz C, Luchsinger J, Tang MX, Manly J, Mayeux R. Impact of plasma lipids and time on memory performance in healthy elderly without dementia. Neurology. 2005;64(8):1378–1383. doi: 10.1212/01.WNL.0000158274.31318.3C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Tang MX, Luchsinger J, Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol. 2004;61(5):705–714. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch Neurol. 2010;67(12):1491–1497. doi: 10.1001/archneurol.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Williams VJ, Leritz EC, Schnyer DM, Rudolph JL, Lipsitz LA, et al. Inter-individual variation in blood pressure is associated with regional white matter integrity in generally healthy older adults. Neuroimage. 2012;59(1):181–192. doi: 10.1016/j.neuroimage.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Morishita R. The roles of lipid and glucose metabolism in modulation of beta-amyloid, tau, and neurodegeneration in the pathogenesis of Alzheimer disease. Front Aging Neurosci. 2015;7:199. doi: 10.3389/fnagi.2015.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks DL, Kryscio RJ, Connor DJ, Sabbagh MN, Sparks LM, Lin Y, et al. Cholesterol and cognitive performance in normal controls and the influence of elective statin use after conversion to mild cognitive impairment: results in a clinical trial cohort. Neurodegener Dis. 2010;7(1–3):183–186. doi: 10.1159/000295660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. New York: Oxford University Press; 1998. [Google Scholar]
- Staekenborg SS, van Straaten EC, van der Flier WM, Lane R, Barkhof F, Scheltens P. Small vessel versus large vessel vascular dementia: risk factors and MRI findings. J Neurol. 2008;255(11):1644–1651. doi: 10.1007/s00415-008-0944-1. discussion 1813–1644. [DOI] [PubMed] [Google Scholar]
- Talayero BG, Sacks FM. The role of triglycerides in atherosclerosis. Curr Cardiol Rep. 2011;13(6):544–552. doi: 10.1007/s11886-011-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Kommer TN, Dik MG, Comijs HC, Fassbender K, Lutjohann D, Jonker C. Total cholesterol and oxysterols: early markers for cognitive decline in elderly? Neurobiol Aging. 2009;30(4):534–545. doi: 10.1016/j.neurobiolaging.2007.08.005. [DOI] [PubMed] [Google Scholar]
- van den Kommer TN, Dik MG, Comijs HC, Jonker C, Deeg DJ. The role of lipoproteins and inflammation in cognitive decline: Do they interact? Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.05.024. [DOI] [PubMed] [Google Scholar]
- van Es AC, van der Grond J, de Craen AJ, Admiraal-Behloul F, Blauw GJ, van Buchem MA. Risk factors for cerebral microbleeds in the elderly. Cerebrovasc Dis. 2008;26(4):397–403. doi: 10.1159/000151680. [DOI] [PubMed] [Google Scholar]
- van Exel E, de Craen AJ, Gussekloo J, Houx P, Bootsma-van der Wiel A, Macfarlane PW, et al. Association between high-density lipoprotein and cognitive impairment in the oldest old. Ann Neurol. 2002;51(6):716–721. doi: 10.1002/ana.10220. [DOI] [PubMed] [Google Scholar]
- Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17(11):1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale - Third Edition. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- West R, Beeri MS, Schmeidler J, Hannigan CM, Angelo G, Grossman HT, et al. Better memory functioning associated with higher total and low-density lipoprotein cholesterol levels in very elderly subjects without the apolipoprotein e4 allele. Am J Geriatr Psychiatry. 2008;16(9):781–785. doi: 10.1097/JGP.0b013e3181812790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams VJ, Leritz EC, Shepel J, McGlinchey RE, Milberg WP, Rudolph JL, et al. Interindividual variation in serum cholesterol is associated with regional white matter tissue integrity in older adults. Hum Brain Mapp. 2013;34(8):1826–1841. doi: 10.1002/hbm.22030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H, Hensel A, Arendt T, Kivipelto M, Winblad B, Gertz HJ. Serum lipids and hippocampal volume: the link to Alzheimer’s disease? Ann Neurol. 2004;56(5):745–748. doi: 10.1002/ana.20289. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Barrett-Connor E, Lin F, Grady D. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol. 2002;59(3):378–384. doi: 10.1001/archneur.59.3.378. [DOI] [PubMed] [Google Scholar]
- Yuan J, Wen G, Li Y, Liu C. The occurrence of cerebrovascular atherosclerosis in Alzheimer’s disease patients. Clin Interv Aging. 2013;8:581–584. doi: 10.2147/CIA.S44160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Mack WJ, Chui HC, Heflin L, Mungas D, Reed B, et al. Coronary artery disease is associated with cognitive decline independent of changes on magnetic resonance imaging in cognitively normal elderly adults. J Am Geriatr Soc. 2012;60(3):499–504. doi: 10.1111/j.1532-5415.2011.03839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]