Abstract
Introduction
Although intracanal application of the triple antibiotic paste (TAP) may offer advantages (e.g., disinfection), this practice has been associated with significant drawbacks, including tooth discoloration. In this study, the color change of dentin was monitored during treatment with distinct TAP pastes and novel tubular-shaped three-dimensional (3D) electrospun scaffolds containing minocycline-MINO or doxycycline-DOX.
Methods
Two TAP pastes (TAPMINO–MINO, metronidazole/MET, and ciprofloxacin/CIP; and TAPDOX–DOX, MET, and CIP), four scaffold-based groups containing MINO or DOX, at distinct concentrations; one antibiotic-free scaffold (Scaffold); and one untreated group (Control) were investigated. Human canines were sectioned at the cemento-enamel junction (CEJ) and tubular-shaped scaffolds or paste were placed into the root canals and sealed. Color measurements (CIEL*a*b* parameters) were performed at baseline and after 1, 3, 7, 14, 21, and 28 days. Color changes were expressed as ΔE* values. In addition, scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) were also performed on the specimens after treatment. Data were analyzed using Repeated-measures ANOVA (alpha=0.05).
Results
All antibiotic-containing groups led to greater discoloration than the antibiotic-free groups. A severe discoloration occurred after 1 day. At the end of the experiment, antibiotic-treated samples exhibited crusts/agglomerates over the dentin surface, which totally or partially obliterated the dentinal tubules. The presence of MINO resulted in greater color change than DOX.
Conclusion
Scaffolds containing MINO or DOX produced similar color change to dentin when compared to their respective TAP systems, although DOX-related discoloration was less pronounced.
Keywords: Electrospinning, scaffold, antibiotic, disinfection, regeneration, discoloration
Dental caries and/or trauma in immature permanent teeth can lead to infection of the dental pulp, with potential negative impact on tooth development. In the past decade, regenerative-based endodontic therapy (i.e., root disinfection with the so-called TAP paste followed by evoked bleeding) has been shown to be a viable treatment option for immature teeth with pulpal necrosis (1) since this technique allows for the replacement of damaged pulp by living tissue and tooth development (2).
An essential stage of regenerative endodontics is disinfection of the root canal via intracanal application of medicaments (e.g., calcium hydroxide or the combination of antibiotics) in the form of a paste (3–5). Although intracanal treatment with TAP may offer advantages such as effective disinfection and lower probability of allergic reactions compared to systemic antibiotics, this practice has been associated with stem cell toxicity (6), development of bacterial resistance (7), and significant tooth discoloration (8–12). It has been demonstrated that TAP produces substantial color change on dentin, especially due to MINO use because it can bind to the calcium ions found in dentin via chelation (13).
Several clinical reports have also confirmed that crown discoloration may appear within 24 h of MINO application (8), and possibly after 1 h of smear layer removal and placement of the TAP paste (12). Interestingly, a case report on a novel approach to eliminating tooth discoloration suggested that sealing the dentinal tubules of the pulp chamber with a bonding agent would be a viable alternative (14), although still not completely efficacious. Furthermore, antibiotics other than MINO (e.g., DOX, cefaclor, among others) were also shown to produce tooth discoloration when incorporated with a paste vehicle (9), thus raising an important concern regarding the use of these antibiotic-containing materials.
From a clinical perspective, the major consequence related to the discoloration produced on anterior teeth treated with TAP is aesthetically driven. Additional treatments such as tooth bleaching and restorative procedures are necessary to improve visual appeal after endodontic therapy, which may increase chair-time and costs for the patient. Thus, alternative therapies producing a lesser degree of tooth discoloration, yet preserving the antimicrobial efficacy, are paramount. In this way, polymer-based antibiotic-containing scaffolds recently have been shown to be a promising method for attaining effective disinfection (4, 15–17). Notably, the antibiotic concentration incorporated into these scaffolds is considerably lower when compared to the concentration used in conventional TAP (4, 18). Therefore, the following hypotheses were tested: (1) the use of novel tubular-shaped 3D antibiotic-containing electrospun scaffolds would produce less or no discoloration on dentin when compared with the clinically advocated TAP paste, and (2) the presence of MINO would produce greater color change when compared to an alternative antibiotic (i.e., DOX).
Materials and Methods
Study Design
This study evaluated the effects of antibiotic-based delivery systems (i.e., TAP pastes containing different combinations of antibiotics or tubular-shaped 3D antibiotic-containing scaffolds) on the color change of human dentin. Eight groups were investigated, according to the material placed into the root canal: two TAP paste groups (TAPMINO—MINO, MET, and CIP; and TAPDOX—DOX, MET, and CIP); four groups of antibiotic-containing scaffolds (MINO or DOX); one group of an antibiotic-free scaffold (Scaffold) to serve as the control for the novel scaffolds; and one group to act as the control for the TAP paste groups (Saline, 0.9% Sodium Chloride Irrigation, Baxter Healthcare Corporation, Deerfield, IL, USA). Forty specimens were tested in total, which were evaluated with a chroma meter at different time intervals up to 28 days and have color change (ΔE*) as the study’s outcome.
Synthesis of Tubular-shaped 3D Antibiotic-containing Electrospun Scaffolds
Polydioxanone polymer solutions (PDS II®, Ethicon, Somerville, NJ, USA) were prepared by dissolving PDS in 1,1,1,3,3,3-hexafluoro-2-propanol at a concentration of 10 wt.%. Each solution was then incorporated with MINO (Sigma-Aldrich, St. Louis, MO, USA) or DOX (Sigma-Aldrich), at 25 or 50 wt% (respective to the total polymer weight). Antibiotic-free PDS solution was also prepared to serve as control. Each solution was spun (i.e., flow rate 2 mL/h, 18 cm distance, and electrical voltage between 15–19 kV) into nanofibers using a conventional electrospinning system and collected on a custom-made grounded Teflon®-coated stainless steel mandrel (2.0±0.02 mm) connected to a high-speed stirrer (BDC6015, Caframo, Wiarton, ON) at 120 revolutions per minute. After processing, the scaffolds (Figures 1A–B) were cut into tubular-shaped specimens (6.0 mm height±0.1mm) and dried under vacuum to remove any remaining solvent (15, 16).
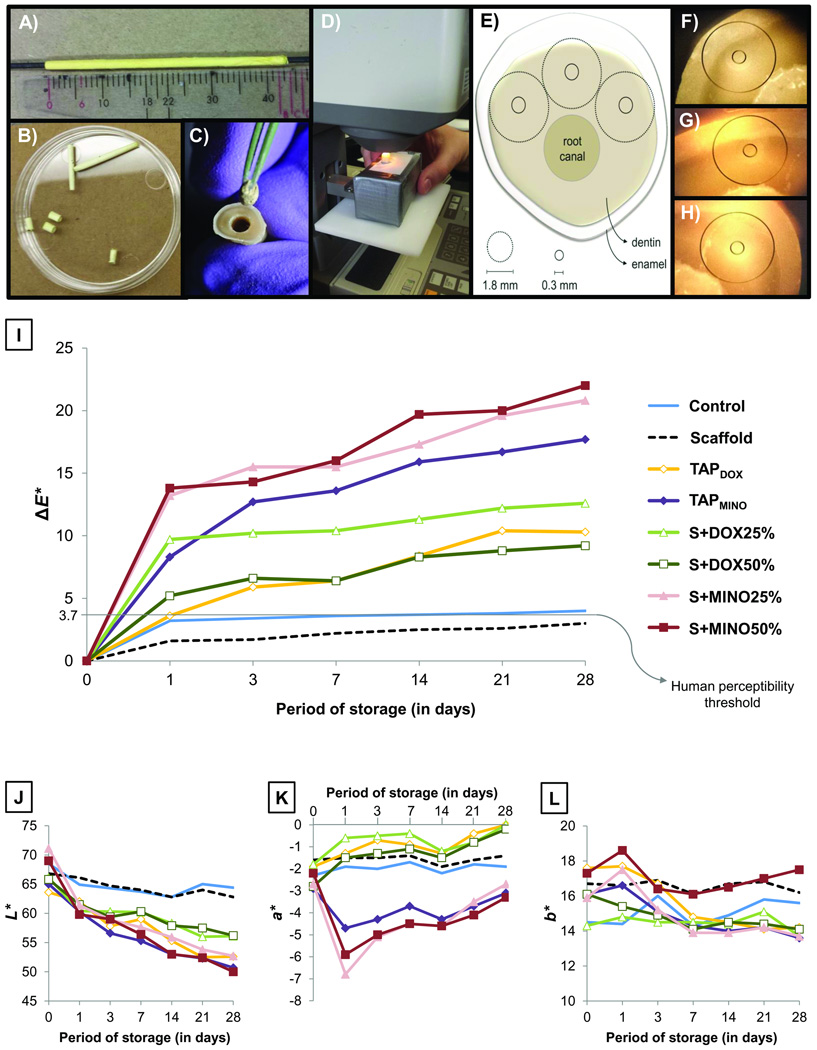
Figure 1.
(A) Image showing the Teflon®-coated stainless steel rod used to prepare the 3D tubular-shaped electrospun scaffolds. (B–C) Images showing the appearance of the 6 mm-long scaffolds and placement into the root canal. (D) Chroma meter used in the present study. (E) Schematic diagram demonstrating the three pre-selected anterior areas of each specimen used to measure the color of dentin. (F–H) Actual experimental view of the pre-selected areas at 0.3 mm. (I) Graph illustrating the progression of ΔE* values with increased days of storage. (J–L) Graphs showing changes in the L*a*, and b* color parameters of each group at each time point tested.
Tooth Sample Preparation and Initial Color Measurements (Baseline)
Forty human canines were sectioned 3 mm above and 5 mm below the CEJ to obtain 8.0 mm-thick (±0.1mm) enamel/dentin samples (8). The smear layer was removed by sample immersion in 2.5% sodium hypochlorite (NaOCl) solution for 3 min, followed by 3 baths of distilled water (DI), and then immersion in 17% ethylenediamine tetraacetic acid (EDTA, Inter-Med, Inc., Racine, WI, USA) for 3 min. The apical foramen of all tooth samples was closed using a bonding system (Adper Scotchbond Multi-Purpose, 3M ESPE, St. Paul, MN, USA) and resin composite (Z100, 3M ESPE). Following that, each specimen was then stored in DI until use. The color of all specimens was evaluated under a chroma meter (model Minolta CR-241, Konica Minolta Sensing Americas, Inc., Ramsey, NJ, USA) considering the aperture size of 0.3 mm (smaller measurable circle, Figure 1E), the 3 most anterior areas of the tooth were measured using the CIEL*a*b* color system and the 3 measurements were then averaged. As shown in Figures 1F–H, the wall of the root canal of each sample was used as the initial parameter to determine the location of the bigger measurable circle (1.8 mm), thus facilitating the measurement of the same area at each time point investigated.
Application of Testing Materials and Final Color Measurements
The 3D tubular-shaped electrospun scaffolds were weighed (S+DOX25% = 9.48±2.36mg [DOX=2.37±0.59mg], S+DOX50% = 7.54±0.91mg [DOX=3.77±0.46mg], S+MINO25% = 6.60±0.86mg [MINO=1.65±0.22mg], and S+MINO50% = 9.62±0.52mg [MINO=4.76±0.26mg]) and directly placed into the root canal, sized to match the diameter, and perfectly (no gaps) adapted to the canal walls (Figure 1C). Standardized preparation of the root canal was performed by instrumentation up to K file #40, Gates Glidden #6 and enlargement with a round bur (ϕ=2.7 mm). Meanwhile, the antibiotic pastes were prepared by mixing the respective antibiotics MET (Sigma-Aldrich), CIP, and MINO [TAPMINO] or DOX [TAPDOX] with saline, at the clinically-used concentration [1g/mL], and then placed into the root canal with a sterile Lentulo spiral filler. One group of specimens was filled with saline (control). The root canals were then temporary sealed (Cavit™, 3M ESPE). All samples were stored inside 24-well plates at 37°C. A wet environment was created to mimic the root canal condition. Briefly, non-sticky wax was placed on the bottom surface of the plate to stabilize the tooth sample. All samples were stored for 28 days and kept in direct contact with the testing materials. Color measurements were taken at days 1, 3, 7, 14, 21, and 28 (Figures 1D–E).
Color Change Analysis
The color change (ΔE*) of each sample (i.e., an average of the 3 pre-selected tested areas, (Figures 1F–H) and at each time interval was assessed using the following formula:
where ΔL*, Δa*, and Δb* corresponded, respectively, to the difference between the test days and baseline L*a*, and b* values.
Scanning Electron Microscopy (SEM) and Energy-dispersive X-ray Spectroscopy (EDS)
After 28 days, 2 samples from each group were randomly allocated for SEM analysis. The teeth were mesio-distally cleaved with a size 5 LeCron instrument in 2 halves. While one of the halves was cleaned with DI water to evaluate the dentin surface, the other half was kept untouched to analyze the overall aspects of the material placed inside the root canal (pastes or scaffolds). The specimens were sputter-coated with Au-Pd prior to imaging (FE-SEM, Model JSM-6701F, JEOL Ltd., Tokyo, Japan). EDS analysis was done to study the chemical composition of the dentin surface and materials immediately after their removal from the canal.
Statistical Analysis
The quantitative color change (ΔE*) data were statistically analyzed (SigmaPlot version 12, Systat Software Inc., San Jose, CA, USA) using Repeated-Measures Analysis of Variance. Time was repeated within each sample, and group was a between-sample factor. The Sidak’s method was used to control the overall significance level of the pair-wise group comparisons at 5% for each time point and location.
Results
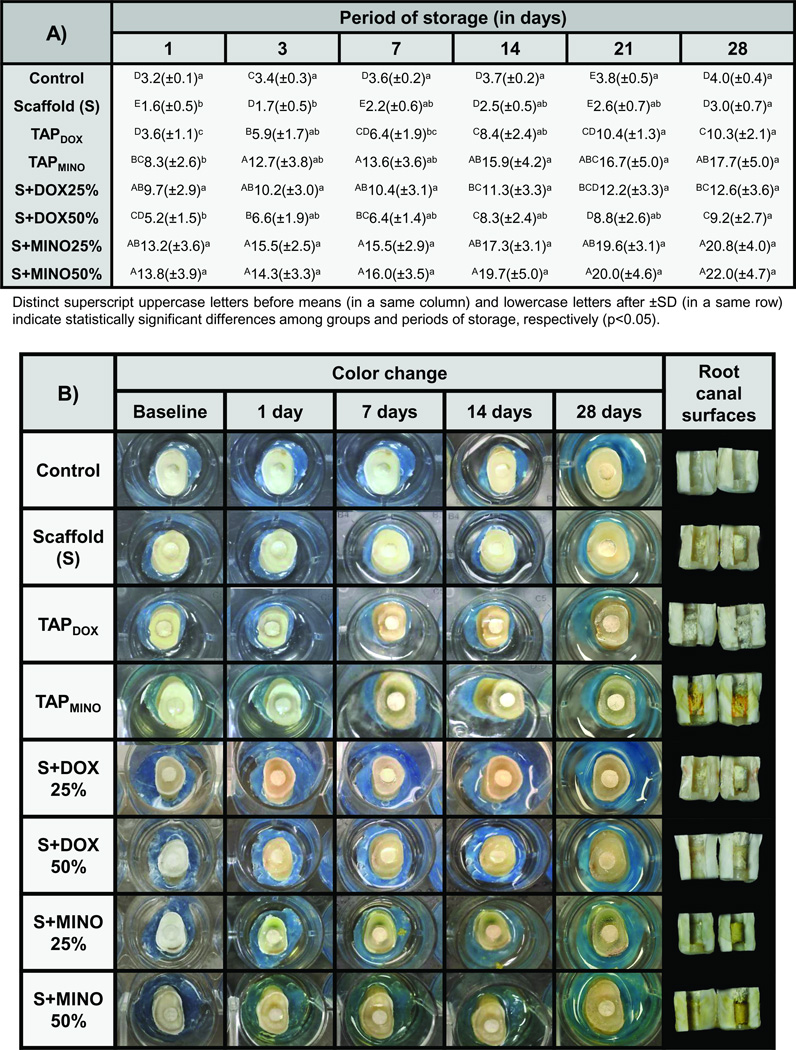
The color change results obtained in the study are shown in Figures 1I and 2A. According to the statistical analysis, both factors “material” and “storage period” were significant (P ≤ .001), although their interaction was not (P = .462). Overall, groups containing MINO exhibited considerably higher color change than groups containing DOX; additionally, all antibiotic-based groups resulted in greater discoloration compared to the antibiotic-free groups (P ≤ .001) (Figure 2A). A severe discoloration (beyond the human perceptibility threshold, i.e., ΔE* = 3.7) (9) occurred after the first day of placement of all antibiotic-containing materials (Figure 1I). Although the discoloration effect clearly increased with greater periods of storage, specimens treated with S+DOX25%, S+MINO25%, and S+MINO50% showed similar ΔE* values after 28 days (Figure 2A), when compared to 1 day of storage (P ≥ .098). Related to the individual color parameters tested (Figures 1J–L), all groups became darker (reduction in the L* axis); however, it was more intensive for groups treated with antibiotics. Specimens treated with MINO-based materials exhibited reduction of the a* axis (i.e., they became greenish), especially after day 1, whereas specimens treated with DOX-based materials slightly increased the a* axis (Figure 1K). The b* axis was not pointedly affected by the tested materials (Figure 1L).
Figure 2.
(A) Table showing the color change (ΔE*) means (± standard deviation/SD) for all groups investigated: control (saline), scaffold (S) based on polydioxanone/PDS, triple antibiotic paste/TAP prepared with doxycycline/DOX or minocycline/MINO, and scaffolds containing 25 or 50 wt.% of DOX or MINO at different periods of storage. (B) Representative macrophotographs showing the color stability/change of specimens after 1, 7, 14, and 28 days of storage.
Representative macrophotographs of tooth samples treated with the distinct antibiotic(s) delivery methods at each time point investigated, except after 3 and 21 days of storage, are presented in Figure 2B. While untreated specimens (control) and those treated with the antibiotic-free scaffold (S) exhibited no dentin discoloration, specimens treated with antibiotic-containing materials suffered a visually circumferential discoloration. The images also show that samples treated with MINO-based materials became greenish, whereas those treated with DOX became brownish. With regard to the root canal surfaces at the end of the experiment, surfaces treated with TAPMINO demonstrated the most dentin discoloration.
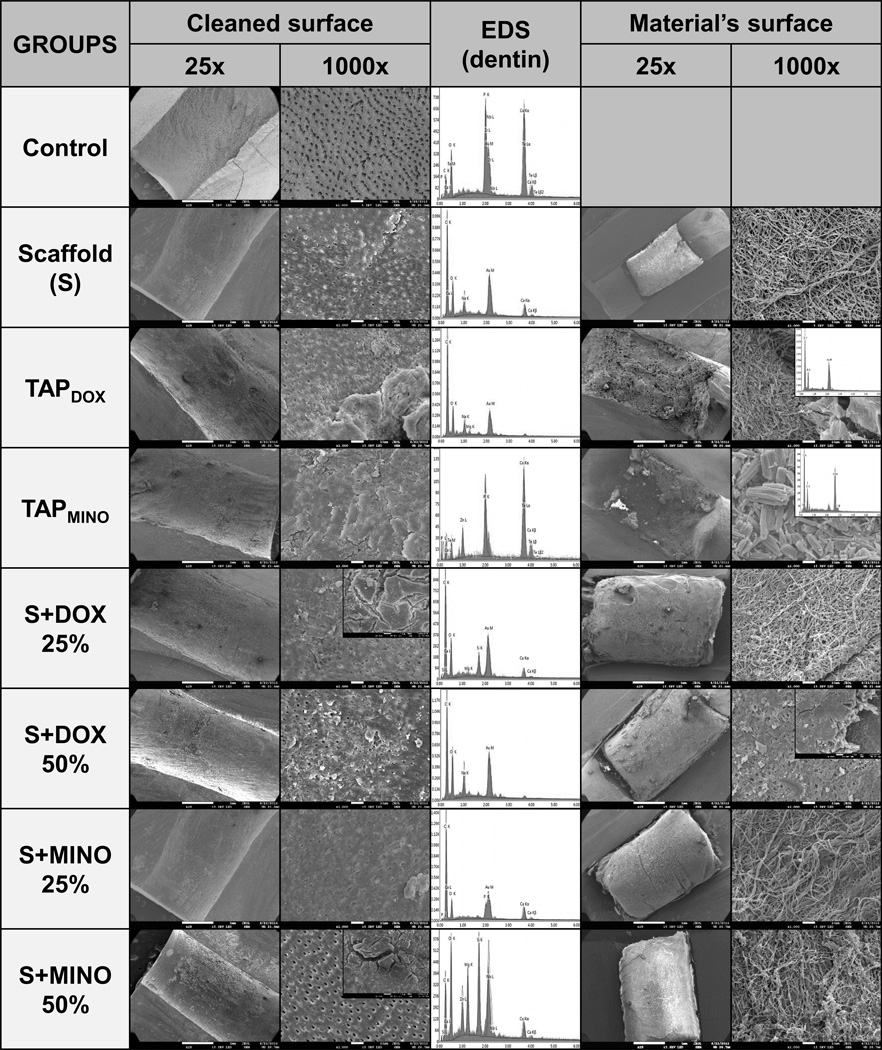
Figure 3 shows the SEM micrographs and EDS spectra of the dentin surface after direct contact with the tested materials for 28 days, as well as the surface of the materials used. Untreated specimens presented completely open dentinal tubules (no obliteration), and demonstrated intense detection of calcium and phosphorus. In contrast, specimens treated with scaffolds and TAPDOX demonstrated partial obliteration of the tubules and a vast amount of organic residues (carbon-based compounds). Specimens treated with TAPMINO exhibited nearly complete obliteration of the tubules and presence of elements, such as zinc, calcium, phosphorus, and tellurium. Other chemical elements were also observed on the S+MINO50%-treated specimens. Thick crusts could be seen attached to the dentin surface of all specimens treated with antibiotics. Regarding the material’s surface, all scaffolds appeared to be moderately intact after 28 days of storage. Within the TAPDOX paste, ferule-shaped powder composed of carbon and oxygen was observed, whereas rod-like powder composed of carbon, oxygen, and chlorine was noted within the TAPMINO paste.
Figure 3.
SEM micrographs and EDS spectra showing the morphology and superficial chemical composition of dentin (cleaned surface) and the tested materials (material’s surface) after 28 days of experiment. Untreated specimens (control) did not show dentinal tubules obliteration; calcium and phosphorus were intensively detected. Specimens treated with scaffolds and TAPDOX demonstrated partial dentinal tubules obliteration and a vast amount of organic residues (carbon-based compounds). Specimens treated with TAPMINO exhibited nearly complete dentinal tubules obliteration and the presence of elements, such as zinc, calcium, phosphorus, and tellurium. Thick crusts could be seen attached to the dentin surface of all specimens treated with antibiotics. Regarding the material’s surface, all scaffolds appeared to be moderately intact after 28 days of storage.
Discussion
The first hypothesis of this study was to determine whether the use of novel tubular-shaped 3D antibiotic-containing scaffolds would produce less or no discoloration on dentin when compared with the traditionally used TAP paste (i.e., TAP prepared with MINO). Based on the reported findings, this hypothesis was rejected. Surprisingly, both antibiotic vehicles (i.e., scaffold or paste) led to similar ΔE* values at the end of the experiment (Figure 1A). Considering the scaffold vehicle carried a much smaller amount (fewer milligrams per scaffold) of antibiotics than the TAP vehicle (1 g/mL), the former was supposed to provoke less discoloration than the latter. Additionally, even the more concentrated scaffold group (S+MINO50%) did not cause greater discoloration compared to the less concentrated group (S+MINO25%). Thus, it can be suggested that antibiotic concentration does not seem to be the major factor related to tooth discoloration, but more importantly, the nature/composition of the antibiotic(s) used emerges as the leading determinant. This inference can also be reinforced by the results obtained for the DOX-containing groups: a similar color change was obtained regardless of the vehicle and concentration factors. The replacement of MINO with DOX resulted in a significant reduction in the total color change although, from an aesthetics standpoint, still clinically undesirable since it would require additional procedures (e.g., bleaching) in order to treat the resultant discoloration. Even so, and despite this unsatisfactory finding, it is worth mentioning that DOX has a lower discoloration potential compared to MINO, thus the second study hypothesis was accepted.
A recent study by Akcay et al. (9) found similar results to the ones reported herein, i.e., the presence of MINO was associated with greater discoloration when compared to DOX. This might account for the ability of MINO to bind to the calcium ions of dentin, causing a chelation reaction that stains the substrate (13); alternatively, DOX, has not yet been shown to have the same ability. Nonetheless, a chemical reaction between a root canal irrigant containing DOX (BioPure MTAD, Dentsply) and distinct concentrations of NaOCl leads to red-purple staining of human teeth (19). It can be suggested that the DOX used in this study somehow interacted with residual NaOCl molecules left in the dentin, thus leading to staining (Figure 2B). Considering that both MINO and DOX are derivatives of the same substance (i.e., tetracycline), there is also the possibility that DOX has some chelation potential, similar to MINO. This should be the focus of future research using appropriated methodologies.
Several studies have suggested modifying the formulation of the TAP paste by replacing MINO with alternative antibiotics (9, 20–22) or reducing treatment duration from 21–28 days to 24–48 h (8). Nonetheless, both strategies do not seem to be completely effective in avoiding severe dentin discoloration as demonstrated by the collected data. Moreover, using a double antibiotic paste (DAP) constituted of MET and CIP has been considered. Although this strategy would effectively prevent dentin discoloration (9), questions remain regarding its overall disinfection potential and clinical prognosis since only a few clinical cases have used this antibiotic combination. Worth mentioning, calcium hydroxide has been used as an intracanal medicament for regenerative endodontics (23, 24), and according to a recent study (10), it did not produce significant color change on dentin-enamel samples; consequently, calcium hydroxide may be considered for disinfection purposes as it does not jeopardize the color appearance.
The antimicrobial potential of novel TAP-mimic scaffolds has recently been demonstrated against an Actinomyces naeslundii (4) biofilm, a very prevalent microorganism found in necrotic permanent immature teeth (25). The formation of calcium-enriched agglomerates over the dentin surface was also seen in a former study causing staining of the substrate, a phenomenon that was attributed to the presence of MINO (4). It can be speculated that the aforementioned agglomerates would affect not only the tooth color, but also the structural and morphological characteristics of dentin. These characteristics could have implications in the regenerative outcome. In this study all specimens treated with antibiotic(s) exhibited the presence of crusts/agglomerates partially covering the dentin surface (Figure 3). These agglomerates demonstrated a heterogeneous composition (EDS analyses), although calcium elements could also be detected corroborating the results of a previous study (4). Once again, it can be noticed that even the less concentrated scaffold groups resulted in the formation of these crusts, denoting that the contact of the antibiotic(s) with the substrate, regardless of the concentration or vehicle used, is sufficient to form these mineralized agglomerates. Considering that MINO and DOX have a very small structure (please note the SEM micrographs showing the shape and size of the antibiotic powders), it can be speculated that antibiotics can interact easily with the dentin components, forming complex agglomerates that may serve as an antibiotic(s) reservoir. Although one might view this reservoir ability as an advantage as it pertains to the antibacterial potential of the treatment, the application of antibiotics into the root canal is known to negatively affect the survival of stem cells (6), and it changes the morphological characteristics of radicular dentin (26), possibly limiting their use. It is shown in the SEM micrographs that specimens treated with antibiotics revealed the dentinal tubules as obliterated by these crusts, considerably reducing dentin permeability. Conversely, the specimens that did not receive any antibiotic treatment exhibited no tubule obliteration (Control) or only slight obliteration (Scaffold) (Figure 3).
The use of antibiotic-containing scaffolds during regenerative endodontic therapy is quite new in dentistry and should be investigated further in order to clarify the effects of these biomaterials on dentin properties. Although the experimental scaffolds containing MINO or DOX did not prevent dentin discoloration, this strategy carries clinical potential for the treatment of necrotic teeth. The replacement of MINO by other antibiotics, such as Augmentin (27), is under investigation as a potential discoloration-free strategy. The method used in this study to measure color change was based on the CIEL*a*b* color system, which is a device-independent system. Although it has been considered the universal method to assess color changes that are easily perceptible to the human eye, one should bear in mind that the use of an alternative system such as the RGB color space, might have produced different results. Nonetheless, the combination of the quantitative (ΔE*) and qualitative (macrophotographs) data allowed for a satisfactory collection of important information regarding the dentin discoloration potential of antibiotics used for regenerative endodontics.
Taken together, scaffolds containing MINO or DOX produced similar color changes to dentin when compared to their respective TAP paste systems. Materials containing DOX suffered less discoloration than materials prepared with MINO. Both drugs were associated with the formation of crusts/agglomerates over the radicular dentin surface.
Acknowledgments
The authors are grateful to Mr. George J. Eckert for his help on statistics. M.C.B. acknowledges funding from an International Development Funds (IDF) Grant from Indiana University Purdue University (IUPUI/OVCR), start-up funds from Indiana University School of Dentistry (IUSD), and the NIH-NIDCR (Grant # DE023552)
Footnotes
The authors deny any conflicts of interest related to this study.
Margaret Louise A. Porter, DMD
Eliseu A. Münchow, DDS, MSD
Maria T. P. Albuquerque, DDS, MSD
Kenneth J. Spolnik, DDS, MSD
Anderson T. Hara, DDS, MSc, PhD
Marco C. Bottino, DDS, MSc, PhD
References
- 1.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377–390. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009;35:1343–1349. doi: 10.1016/j.joen.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Raju SM, Yadav SS, Kumar MS. Revascularization of immature mandibular premolar with pulpal necrosis - a case report. J Clin Diagn Res. 2014;8:ZD29–ZD31. doi: 10.7860/JCDR/2014/8963.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albuquerque MT, Ryan SJ, Munchow EA, et al. Antimicrobial Effects of Novel Triple Antibiotic Paste-Mimic Scaffolds on Actinomyces naeslundii Biofilm. J Endod. 2015 Apr 24; doi: 10.1016/j.joen.2015.03.005. pii: S0099-2399(15)00237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196–200. doi: 10.1097/00004770-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Ruparel NB, Teixeira FB, Ferraz CC, et al. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012;38:1372–1375. doi: 10.1016/j.joen.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Bottino MC, Arthur RA, Waeiss RA, et al. Biodegradable nanofibrous drug delivery systems: effects of metronidazole and ciprofloxacin on periodontopathogens and commensal oral bacteria. Clin Oral Investig. 2014;18:2151–2158. doi: 10.1007/s00784-014-1201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JH, Kim Y, Shin SJ, et al. Tooth discoloration of immature permanent incisor associated with triple antibiotic therapy: a case report. J Endod. 2010;36:1086–1091. doi: 10.1016/j.joen.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 9.Akcay M, Arslan H, Yasa B, et al. Spectrophotometric analysis of crown discoloration induced by various antibiotic pastes used in revascularization. J Endod. 2014;40:845–848. doi: 10.1016/j.joen.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Lenherr P, Allgayer N, Weiger R, et al. Tooth discoloration induced by endodontic materials: a laboratory study. Int Endod J. 2012;45:942–949. doi: 10.1111/j.1365-2591.2012.02053.x. [DOI] [PubMed] [Google Scholar]
- 11.Petrino JA, Boda KK, Shambarger S, et al. Challenges in regenerative endodontics: a case series. J Endod. 2010;36:536–541. doi: 10.1016/j.joen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed HM, Abbott PV. Discolouration potential of endodontic procedures and materials: a review. Int Endod J. 2012;45:883–897. doi: 10.1111/j.1365-2591.2012.02071.x. [DOI] [PubMed] [Google Scholar]
- 13.Tanase S, Tsuchiya H, Yao J, et al. Reversed-phase ion-pair chromatographic analysis of tetracycline antibiotics. Application to discolored teeth. J Chromatog B Biomed Sci Appl. 1998;706:279–285. doi: 10.1016/s0378-4347(97)00563-x. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds K, Johnson JD, Cohenca N. Pulp revascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration: a case report. Int Endod J. 2009;42:84–92. doi: 10.1111/j.1365-2591.2008.01467.x. [DOI] [PubMed] [Google Scholar]
- 15.Bottino MC, Kamocki K, Yassen GH, et al. Bioactive nanofibrous scaffolds for regenerative endodontics. J Dent Res. 2013;92:963–969. doi: 10.1177/0022034513505770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bottino MC, Yassen GH, Platt JA, et al. A novel three-dimensional scaffold for regenerative endodontics: materials and biological characterizations. J Tissue Eng Regen Med. 2013 Mar 8; doi: 10.1002/term.1712. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Kim HW, Knowles JC, Kim HE. Development of hydroxyapatite bone scaffold for controlled drug release via poly(epsilon-caprolactone) and hydroxyapatite hybrid coatings. J Biomed Mater Res B Appl Biomater. 2004;70:240–249. doi: 10.1002/jbm.b.30038. [DOI] [PubMed] [Google Scholar]
- 18.Kamocki K, Nor JE, Bottino MC. Dental pulp stem cell responses to novel antibiotic-containing scaffolds for regenerative endodontics. Int Endod J. 2014 Nov 25; doi: 10.1111/iej.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tay FR, Mazzoni A, Pashley DH, et al. Potential iatrogenic tetracycline staining of endodontically treated teeth via NaOCl/MTAD irrigation: a preliminary report. J Endod. 2006;32:354–358. doi: 10.1016/j.joen.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Sato I, Ando-Kurihara N, Kota K, et al. Sterilization of infected root-canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J. 1996;29:118–124. doi: 10.1111/j.1365-2591.1996.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 21.Tawfik H, Abu-Seida AM, Hashem AA, et al. Regenerative potential following revascularization of immature permanent teeth with necrotic pulps. Int Endod J. 2013;46:910–922. doi: 10.1111/iej.12079. [DOI] [PubMed] [Google Scholar]
- 22.Trope M. Treatment of the immature tooth with a non-vital pulp and apical periodontitis. Dent Clin North Am. 2010;54:313–324. doi: 10.1016/j.cden.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Cehreli ZC, Isbitiren B, Sara S, Erbas G. Regenerative endodontic treatment (revascularization) of immature necrotic molars medicated with calcium hydroxide: a case series. J Endod. 2011;37:1327–1330. doi: 10.1016/j.joen.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 24.Lee BN, Moon JW, Chang HS, et al. A review of the regenerative endodontic treatment procedure. Restor Dent Endod. 2015;40:179–187. doi: 10.5395/rde.2015.40.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagata JY, Soares AJ, Souza-Filho FJ, et al. Microbial evaluation of traumatized teeth treated with triple antibiotic paste or calcium hydroxide with 2% chlorhexidine gel in pulp revascularization. J Endod. 2014;40:778–783. doi: 10.1016/j.joen.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 26.Yassen GH, Chu TM, Eckert G, et al. Effect of medicaments used in endodontic regeneration technique on the chemical structure of human immature radicular dentin: an in vitro study. J Endod. 2013;39:269–273. doi: 10.1016/j.joen.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Bezgin T, Yilmaz AD, Celik BN, et al. Efficacy of platelet-rich plasma as a scaffold in regenerative endodontic treatment. J Endod. 2015;41:36–44. doi: 10.1016/j.joen.2014.10.004. [DOI] [PubMed] [Google Scholar]