Abstract
TCR-αβ+ DN T cells (CD3+ TCR-αβ+ CD4− CD8− NK1.1− CD49b−) represent a minor heterogeneous population in healthy mice and humans. Both pro-inflammatory and regulatory capacities have been attributed to these cells which are expanded in several autoimmune diseases. Importantly, previous work indicates that self-reactive CD8+ T cells become DN after activation by self-antigens, suggesting that self-reactive T cells may exist within the DN T cell population. Here, we demonstrate that in unmanipulated mice, PD-1 expression identifies a subset of DN T cells that display activation-associated markers and a phenotype that strongly suggests they derive from self-reactive CD8+ cells. We show that the majority of the pro-inflammatory cytokines produced by DN T cells are generated by the PD-1+ subset. Finally, using a TCR-activation reporter mouse (Nur77-GFP), we confirm that PD-1+ DN T cells have engaged endogenous antigen in healthy mice during steady state conditions. In conclusion, we provide evidence that indicates that the PD-1+ fraction of DN T cells represents self-reactive cells.
Keywords: Autoimmunity, DN T cells, PD-1, IL-17, Nur77-GFP mice
INTRODUCTION
Central tolerance acts a major protective force against autoimmunity by eliminating the bulk of autoreactive T cells [1]. However, self-reactive lymphocytes are present in healthy mice and humans [2–4]. In healthy individuals these cells display an unresponsive phenotype [3,4], and their abundance is considered low. However, recent reports have proposed that self-reactive T cells may be as frequent as naïve T cells specific for foreign antigens [3]. The presence of self-reactive T cells entails the risk of autoimmunity through the instigation of inflammatory tissue damage and promotion of autoantibody production [5]. Abundance of self-reactive T cells may have prognostic meaning in autoimmunity because their increased frequency has been associated to autoimmune diseases such as vitiligo, diabetes, and multiple sclerosis [4,6,7].
TCR-αβ+ CD4− CD8− double negative T cells (henceforth DN T cells) have been associated with autoimmune conditions and display a pro-inflammatory profile. Their frequency is increased in several autoimmune/inflammatory disorders such as systemic lupus erythematosus (SLE) [8], autoimmune lymphoproliferative syndrome (ALPS) [9], and Sjögren’s syndrome [10]. They promote autoantibody production [11], are found infiltrating target organs in mice and humans [8], are associated with inflammation and disease progression in mouse models of autoimmunity, and produce several pro-inflammatory cytokines [8,12]. However, DN T cells represent a scarce population in normal mice and humans, and their study has proved challenging due to the existence of other T cell subsets with a similar phenotype, such as NKT and Mucosal-Associated Invariant T (MAIT) cells. DN T cells have also been ascribed regulatory properties [13], an observation inconsistent with the pro-inflammatory profile reported in patients and mice with inflammatory diseases.
Previous studies have suggested that under certain conditions, CD8+ T cells may lose CD8 expression and become DN [14–16]. Moreover, we have previously shown that self-reactive CD8 T cells lose CD8 expression after recognition of cognate self-antigen and become DN T cells, supporting the concept that CD8 downregulation may represent an active mechanism of peripheral tolerance [17]. Here, we analyze DN T cells in normal, unmanipulated mice and we show that Programmed cell death 1 (PD-1) expression identifies a subset of DN T cells that displays activation markers, produces pro-inflammatory cytokines, and, importantly, exhibits evidence of TCR-mediated activation. Together, our data indicate that PD-1+ DN T cells represent self-reactive cells.
RESULTS
PD-1 expression distinguishes a distinct subset of DN T cells in normal mice
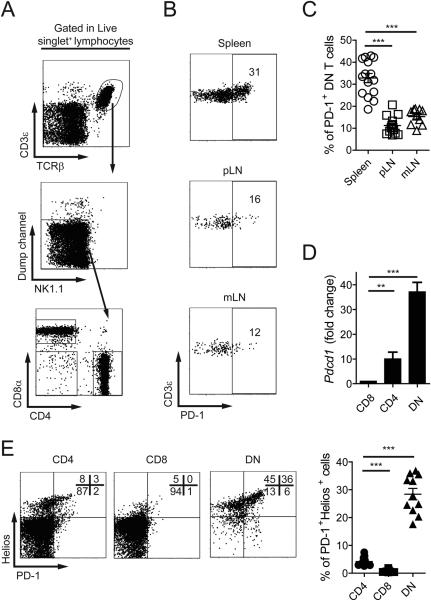
Self-reactive CD8+ T cells become inactivated upon exposure to cognate self-antigen. During this process, they downregulate CD8 and upregulate inhibitory molecules including PD-1 and Helios [17]. As a result, a population of PD-1+ DN T cells is generated. These cells are functionally impaired but survive for at least 60 days in adoptive transfer recipients [17]. This suggests that self-reactive former CD8+ cells may be found within the DN T cell compartment of normal mice. To address this hypothesis, we analyzed the expression of PD-1 in splenic DN T cells of unmanipulated B6 mice. DN T cells were defined as live CD3+TCR-β+CD4−CD8− NK1.1−B220−CD49b− cells to exclude TCR-γδ (TCR-β−) and NKT (NK1.1+CD49b+) cells (Fig. 1A).
Figure 1.
PD-1 expression in DN T cells distinguishes two cell subsets. (A) Gating strategy used to define DN T cells. Dump channel includes: B220, TCR-γδ, and CD49b cells. (B, C) Representative dot plots (B) and cumulative data (C) of percentages of PD-1+ DN cells in spleen, axillary and inguinal LN (pLN) and mLN of B6 mice. (D) Pdcd1 mRNA levels in CD4+, CD8+ or DN TCR-αβ+ cells. Results are expressed as fold change over CD8 T cells, and data are the mean from 3 experiments pooling FACS sorted T cells from 10-20 B6 mice. (E) Distribution of CD4+, CD8+ or DN T cells according to the expression of PD-1 and Helios. Cumulative data are expressed as mean ± SEM, pooling data from 3-5 independent experiments (n=3-4). **p<0.01; ***p<0.001
Roughly 30% of splenic DN T cells expressed high levels of PD-1 (Fig. 1B). Interestingly, this population accounted for only 10-15% in lymph nodes (Fig. 1B and C). Accordingly, DN T cells produced 30-40 times more PD-1 (Pdcd1) mRNA than CD8+ and 4-7 times more than CD4+ T cells (Fig. 1D).
MAIT cells are rare in spleens of unmanipulated B6 mice [18,19]. However, they may be confounded with DN T cells because their surface marker expression is similar. To determine whether MAIT cells were an important fraction of the PD-1+ DN population, we measured the frequency of MR1-tetramer+ (Tet+) cells within this subset [18–20]. Only a minute percentage of DN T cells bound MR1-tetramers and we were unable to detect mRNA corresponding to the Vα19-Jα33 TCR rearrangement in PD-1+ DN T cells (Supporting Information Fig. 1A). In agreement with these results, expression of IL-18Rα, found in most MAIT cells [19], was restricted almost exclusively to PD-1− DN T cells (Supporting Information Fig. 1B).
PD-1+ DN cells were not decreased in Cd1d−/− mice, and within DN T cells from WT B6 mice virtually none of the cells were positive for CD1d-Tet, further confirming the absence of NKT cells in the PD-1+ DN subpopulation. These findings were consistent with very low levels of Vα14-Jα18 rearrangement found in PD-1+ DN cells compared to PD-1− DN cells (Supporting Information Fig. 1C and D) [21].
In addition to PD-1, DN T cells derived from self-reactive CD8 T cells express Helios [17], a transcription factor upregulated in self-reactive T cells undergoing tolerance [22] and in T cells chronically exposed to cognate antigen [23]. We analyzed the expression of Helios and observed that in contrast to CD4+ and CD8+ T cells, the majority of DN cells were positive (Fig. 1E). Approximately 10% of CD4+ cells, probably corresponding to Tregs, also expressed high levels of Helios [24]. Other tolerance-associated surface molecules (CTLA-4, LAG-3, BTLA, and TIM-3) were not expressed by DN T cells (Supporting information Fig. 1E). Of note, a fraction of PD-1+ DN T cells expressed 2B4 (CD244, Slamf4), an inhibitory receptor found in memory CD8 T cells and NKT cells [25]. CD160 expression was present in a subset of PD-1− DN cells (Supporting information Fig. 1E).
Altogether, these results indicate that normal mice have a population of DN T cells, that does not contain MAIT or NKT cells, distinguished by the expression of PD-1 and Helios.
PD-1+ DN T cells possess an effector phenotype
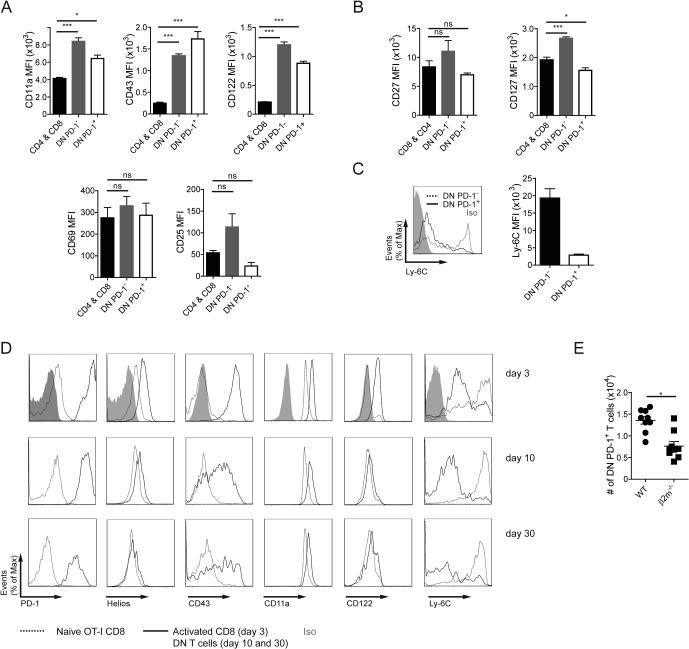
A more thorough analysis was performed to determine whether PD-1+ DN T cells exhibit evidence of cognate antigen encounter. Because these experiments were performed in unmanipulated mice kept in pathogen-free conditions, presumably only antigens derived from commensal microorganisms and self were exposed to the immune system. Analysis of CD44 and CD62L expression revealed that contrary to CD8+ and CD4+, comprised mostly of naïve CD62L+CD44low cells, the DN compartment (PD-1− and PD-1+) was enriched with cells bearing an activated or effector memory/central memory phenotype (CD62L−CD44+/CD62L+CD44+, respectively; Supporting Information Fig. 2A). Interestingly, the majority of PD-1+ DN T cells lacked expression of CD62L.
The distribution of other activation markers was heterogeneous between PD-1− and PD-1+ DN T cells. PD-1+ cells did not express early activation markers such as CD69 or CD25 but had increased levels of CD43, CD122 (IL-2Rβ), and CD11a (LFA-1α) (Fig. 2A). CD127 (IL-7Rα), downregulated upon acquisition of effector phenotype, was decreased only in PD-1+ DN T cells (Fig. 2B). Expression of CD27 was not different between PD-1+ and PD-1− DN T cells. Ly-6C is expressed at high levels by CD8+ memory cells but downregulated in cells with high reactivity towards self-peptide/MHC and in chronically activated T cells [23,26,27]. Whereas PD-1− DN cells expressed high amounts of Ly-6C, their PD-1+ counterparts expressed low levels suggesting chronic exposure to cognate antigen (Fig. 2C).
Figure 2.
PD-1+ DN T cells display features of activated T cells and resemble DN cells derived from self-reactive CD8+ T cells. (A, B) Expression of activation-induced surface markers (A), as well as CD27 and CD127 (B) in CD4+ and CD8+, PD-1−, and PD-1+ DN T cells. (C) Expression of Ly-6C in PD-1− and PD-1+ DN cells. (D) Phenotype of CD8/DN T cells exposed to cognate self-antigen (mOVA mice) at the indicated time points after transfer (representative histograms). (E) Percentage of PD-1+ DN T spleen cells from WT and β2m−/− mice. Cumulative data are expressed as mean ± SEM, pooling results from 1-3 independent experiments (n=3-4) *p<0.05; ***p<0.001
In order to compare the phenotype of naturally occurring DN PD-1+ T cells with that of DN T cells generated by exposure to self-antigen, we adoptively transferred CD8+ OT-I CD45.1+ T cells into mOVA mice that ubiquitously express OVA [17] and analyzed their phenotype at days 3, 10, and 30 after transfer (Supporting Information Fig. 2B). In this model, a large fraction of CD8 T cells loses CD8 expression by day 10 [17]. As shown in Figure 2D, at day 3, prior to CD8 downregulation, cells transferred into mOVA mice upregulated CD11a, CD43, CD122, PD-1, and Helios. Additionally, the expression of Ly-6C was suppressed. These changes are indicative of antigen exposure. By days 10 and 30, DN T cells derived from transferred OT-I cells maintained high expression of CD11a, CD43, CD122, and PD-1 as well as low levels of Ly-6C. Helios gradually returned to baseline suggesting that its expression at high levels indicates recent antigen-driven activation (Fig. 2D).
Because PD-1+ DN T cells found in normal mice may represent previous self-reactive CD8+ T cells, we analyzed whether MHC class I is necessary for their presence. We observed that the numbers of PD-1+ DN T cells decreased by half in MHC class I-deficient mice (β2m−/−) (Fig. 2E). Moreover, cluster analysis of the TCR Vβ repertoire indicated a similar Vβ usage between CD8+ and DN T cells (Supporting Information Fig. 2C).
PD-1+ cells represent the main source of pro-inflammatory cytokines produced by DN T cells
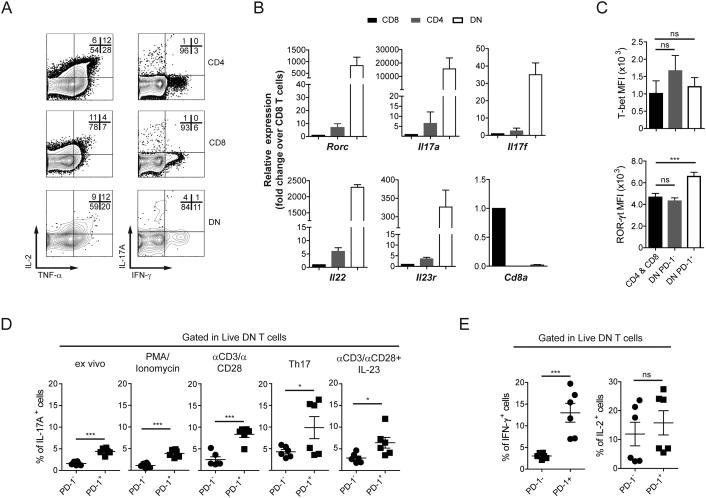
DN T cells have been reported to be a relevant source of IL-17 and other pro-inflammatory cytokines in the context of autoimmunity and infection [8,12,28]. To determine their cytokine production capacity in the steady state, we analyzed cytokine production in freshly isolated DN T cells stimulated for 6 hours with PMA and ionomycin. As shown in Fig. 3A, a relatively high fraction of DN cells produced IL-2, IFN-γ, TNF-α, and IL-17A. Further, FACS-sorted DN T cells were enriched in Th17-related transcripts (Il23r, Il17a, Il17f, Il22, Rorc) in the absence of any ex vivo stimulation (Fig. 3B).
Figure 3.
PD-1+ DN T cells are the major producers of cytokines within the DN TCR-αβ+ subset. (A) IL-2, TNF-α, IFN-γ, and IL-17A production from CD4, CD8, and DN T cells after ex vivo stimulation with PMA/Ionomycin. (B) mRNA of Th17 signature genes. Cd8a expression is shown as control. (C) Expression of T-bet and ROR-γt in CD4 and CD8, PD-1−, and PD-1+ DN T cells from spleens of B6 mice. (D, E) Percentage of cytokine+ cells within PD-1− and PD-1+ DN cells measured as GFP abundance in IL-17A-GFP reporter mice directly ex vivo, after PMA/Ionomycin or after αCD3/αCD28 stimulation for 3 days (D), or quantified by intracellular cytokine staining after PMA/Ionomycin (IFN-γ and IL-2; E). Flow cytometry plots are representative of 2-4 independent experiments (n= 3). Cumulative data (B-E) pooling results from several experiments; ns: not significant; *p<0.05; **p<0.01; ***p<0.001
To investigate whether the pro-inflammatory capacity of DN T cells was asymmetrically divided between PD-1− and PD-1+ cells, we evaluated RAR-related orphan receptor (ROR)-γt and T-bet, as well as cytokine production in both DN cell subsets. As shown in Fig. 3C, ROR-γt expression (quantified as MFI) was significantly higher in PD-1+ DN cells than in CD4+ and CD8+, and in PD-1− DN T cells. Accordingly, PD-1+ DN T cells from an Il17a-GFP reporter mouse produced more IL-17A than PD-1− counterparts when measured ex vivo, as well as after stimulation with PMA/ionomycin (6 h), or anti-CD3/CD28 in the presence or absence of IL-23 or other Th17-promoting cytokines (Fig. 3D) [28]. Production of IFN-γ was also significantly higher in PD-1+ DN T cells (Fig. 3E).
PD-1+ DN cells have been exposed to endogenous cognate antigen
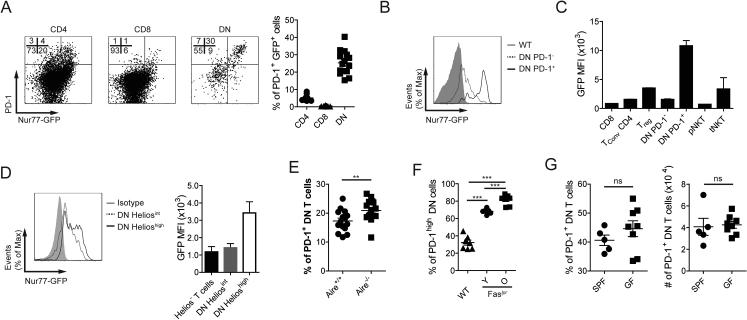
The phenotype of PD-1+ DN T cells strongly suggested that these cells have been exposed to cognate antigen, probably of self-origin. In order to confirm this and rule out other explanations to their effector phenotype (i.e. bystander activation in response to cytokines or other pro-inflammatory cues), we analyzed in vivo TCR signaling using Nur77-GFP reporter mice. In this system, previously used to detect self-reactive lymphocytes [29–31], GFP expression is upregulated in lymphocytes whose antigen receptor (TCR or BCR) has been engaged and the abundance of GFP can be used as a surrogate marker of signaling strength [32].
First, we confirmed that GFP expression in DN T cells behaved like that of CD4 and CD8 (i.e. it increased upon TCR activation and decreased when T cells were deprived of MHC signals; Supporting Information Fig. 2D). In order to determine whether DN T cells had been exposed to cognate antigen, we analyzed their GFP content directly ex vivo. As shown in Figure 4A, GFP expression in DN T cells mostly paralleled PD-1 expression, where PD-1+ cells were GFP+ and vice versa. No equivalent population was observed in CD4+ and CD8+ T cells. A comparison of the GFP levels between PD-1− and PD-1+ DN T cells confirmed that PD-1+ cells had significantly more GFP and its abundance was even higher than in other cells known to encounter self-antigens (e.g. Treg or tNKT cells; Fig. 4B and C) [32]. Of note, GFP expression was higher in Helioshigh DN T cells than in Heliosint DN T cells (Fig. 4D). These results demonstrate that PD-1+ DN T cells have received a signal through the TCR. The high levels of GFP expression suggest that the signal was strong and recent. In line with the idea that PD-1+ DN T cells are self-reactive, we found that the frequency of these cells was significantly increased in mice that accumulate autoreactive T cells as a consequence of defects in central and peripheral tolerance (Aire−/− and Faslpr mice, respectively; Fig. 4E and F) [1,33].
Figure 4.
PD-1+ DN T cells have encountered endogenous antigen. (A) Distribution of CD4+, CD8+, and DN T cells as defined in Fig. 1A in relation to PD-1 and GFP expression from spleens of Nur77-GFP reporter mice. (B, C) Representative histogram (B) and cumulative data (C) of GFP levels in several T cell populations from spleen (CD8, TconvCD4, Treg, DN PD-1−, DN PD-1+, pNKT) and thymus (tNKT) of Nur77-GFP mice. TconvCD4: CD4+CD25− T cells; Treg: CD4+CD25+ T cells; pNKT: NK1.1+ T cells; tNKT: CD1d/αGalCer-Tetramer+CD44low NK1.1lowCD24+. (D) Representative histogram (left) and cumulative data (right) of GFP (measured with αGFP) levels in DN T cells in relation to their expression of Helios. (E, F) Percentages of PD-1+ DN T spleen cells in WT and Aire−/− littermate mice (E), or WT, and young (Y: 5-6 week-old) and old (O: 20-27 week-old) B6.Faslpr mice (F). (G) Frequency and numbers of PD-1+ DN T cells were measured in SPF and GF sex- and age-matched mice. Data are expressed as mean ± SEM. Flow cytometry plots are representative of 2-4 independent experiments (n= 3). Cumulative data (A, B, D and F) pools results from several experiments; one experiment representative of 2 (C; n=3), one experiment (G; n=5-8) or pooled data from five experiments (E; n=1-4). ns: not significant; *p<0.05; **p<0.01; ***p<0.001
Besides self-antigens, APC from unmanipulated B6 mice housed in specific pathogen-free (SPF) facilities can cross-present microbiota-derived molecules. To exclude the possibility that PD-1+ DN T cells were being primed by microbiome-derived antigens, we analyzed the frequency and phenotype of PD-1+ DN T cells in germ-free (GF) mice. We did not observe a reduction in the proportion or numbers of PD-1+ DN T cells in GF mice (Fig. 4G). Accordingly, expression of activation markers (CD11a, Ly-6C, CD44) did not change significantly in cells derived from GF mice, when compared to cells from mice housed in SPF conditions (Supporting Information Fig. 2E). These findings argue against DN T cells being activated by foreign antigen in normal unmanipulated B6 mice. If DN T cells were microbiota-dependent, their population would experience a reduction as observed in MAIT [34] and NKT cells [35] (Supporting Information Fig. 2F).
DISCUSSION
Here we have shown that the presence of PD-1 distinguishes a subset of DN T cells in normal mice. The evidence provided argues that PD-1+ DN cells have acquired an effector phenotype (activation markers, cytokine production, expression of Rorc) most probably induced by cognate antigen exposure. The remarkable phenotypic similarities between these cells identified in unmanipulated mice and those generated by exposing CD8+ T cells to known cognate antigen presented as self, suggest that PD-1+ DN T cells (PD-1+ Helios+ Ly-6C−) may represent self-reactive former CD8+ T cells that have undergone an inactivation process in the periphery.
Our data indicates that PD-1+ DN T cells represent a separate population, different from other unconventional or scarce T cell subsets such as MAIT or NKT cells. The presence of both of these cell types in the PD-1+ DN T cell fraction was ruled out by tetramer staining, measurement of invariant TCR-specific mRNA, and by analyses of their phenotype (i.e. CD127, CD69, CD49b, NK1.1, IL-18Rα). Moreover, contrary to what occurs to MAIT and NKT cells, the PD-1+ DN T cell population was unaffected by GF conditions.
Determining the self-reactive nature of PD-1+ DN T cells represents a challenge because no specific technology has been developed for this purpose. No group of cognate antigens are known to be specific for PD-1+ DN T cells. In addition, the use of tetramers developed for CD8+ cells may not bind to DN T cells due to the lack of the coreceptor [3,36]. For this reason, we approached the problem in a comprehensive manner. Based on the following data, we propose that PD-1+ DN T cells are self-reactive: a) they express high levels of Helios and PD-1; b) their phenotype is very similar to the one of CD8+ T cells that have encountered antigen as self; c) they express high levels of activation markers in the steady state; d) they lack Ly-6C and CD127; e) absence of microbiota does not affect their levels or phenotype; f) they show evidence of TCR-mediated activation (high GFP levels in the Nur77-GFP reporter background).
PD-1+ DN T cells display high levels of activation-induced molecules (i.e. CD11a, CD43, CD44) and low levels of surface receptors downregulated upon TCR engagement (i.e. CD62L and CD127). Interestingly, the low levels of CD62L may explain their relative high concentration in the spleen, the only secondary lymphoid organ where homing does not involve CD62L [37]. Helios is upregulated upon TCR activation [38] and its levels remain high when antigen is chronically present and displayed [23]. Because we cannot dismiss that other unknown signals may regulate the expression of these molecules, we analyzed a mouse model that has been shown to specifically report (by GFP expression) TCR activity not influenced by other environmental cues [32]. We found that, similar to known self-reactive T cells (Tregs), PD-1+ DN T cells have elevated GFP levels. This provided us with strong evidence supporting the autoreactive nature of PD-1+ DN T cells.
PD-1+ DN T cells are actively exposed to TCR signaling and transcribe Th17-related genes. Further, a relatively large fraction of them produces pro-inflammatory cytokines. These features (i.e. their autoreactivity and their pro-inflammatory capacity) indicate that they possess pathogenic potential. DN T cells are expanded in some systemic autoimmune diseases such as SLE and Sjögren’s syndrome and they have been proposed by our group and others to play a role in these conditions [8,10,16]. Their presence in healthy mice and humans is not associated to any pathology, which indicates that in normal conditions their pathogenic potential is neutralized. This may be related to their low frequency [39] and/or to the presence of mechanisms that limit their activation. CD8 downregulation, as well as their high expression of molecules with inhibitory ability (i.e. PD-1 and Helios), may limit their expansion and pathogenicity. Why these cells expand in the setting of autoimmunity may be related to defects in the expression or function of the inhibitory molecules that keep them in check in normal individuals. A recent report suggested that SLE disease activity and prognosis is influenced by the expression of inhibitory molecules in CD8 T cells including PD-1 [40]. The question of whether DN T cells are inadequately controlled in systemic autoimmune conditions is the focus of current investigation.
MATERIALS AND METHODS
Mice
Mice 8-14 weeks (unless indicated otherwise) of age were used. B6, Nur77-GFP, IL-17A-GFP, OT-I, CD45.1, Act-mOVA (mOVA), β2m−/−, Faslpr (all B6 background) were obtain from Jackson Laboratories (Bar Harbor, ME). Cd1d−/− mice were kindly provided by Dr. Lydia Lynch. Aire deficient (Aire−/−) mice were generously provided by Drs. Christophe Benoist, Noriyiku Fujikado and Matthew Meredith. All mice were housed and bred in a SPF facility at BIDMC following IACUC guidelines. GF mice were a generous gift from Drs. Dennis Kasper, Francesca S. Gazzaniga and Isaac Kasper and they were negative for microbiota presence by the day of the experiment.
Flow cytometry
Spleen and lymph nodes were dissociated in full RPMI 1640. Unless otherwise indicated, for surface epitopes, cells were stained in PBS + 2% FCS for 30 min after blocking Fc receptors with TruStain fcX (Biolegend). All antibodies were from Biolegend or eBioscience (for complete antibody list see Supporting information), except anti-IL-18Rα (R&D). Samples were acquired in a modified LSRII (BD Biosciences, San Jose, CA) and analyzed with FlowJo (TreeStar, Ashland, OR).
For intracellular staining, cells were stained for surface antigens and further processed with the Cytofix/Cytoperm (BD) or Foxp3/Transcription Factor Staining buffer (eBioscience) kits as per manufacturer’s instructions. Cytokines were measured after stimulating the cells with PMA (50 ng/mL) plus Ionomycin (250 ng/mL, Sigma) in the presence of Brefeldin A (BD) for 5-7 hours.
For tetramer staining, cells were incubated for 1h at RT (CD1d-Tet) prior to surface staining or 30 min (MR1-Tet) at RT together with antibody cocktail for surface staining. MR1-Tetramers were synthesized and loaded as previously described [18–20].
Tissue culture and T cell isolation
For in vitro stimulation, T cells were purified by magnetic selection (Dynabeads), and cultured on flat-bottom 96-well plates in full RPMI with plate-bound anti-CD3/anti-CD28 (2 μg/mL) at a density of 4-5×105 cells/well for 16 or 72h. When indicated, splenocytes were stimulated in the presence of IL-23 (10 ng/mL) or IL-23 plus IL-6 (25 ng/mL) and TGF-β1 (2.5 ng/mL) for 3 days.
For adoptive transfers, CD8+ cells from spleen and lymph nodes of OT-I.CD45.1 mice were magnetically isolated with Dynabeads (purity≥95%). 5×106 cells in PBS were injected by tail vein injection into mOVA.CD45.2 or B6 mice.
Real-Time PCR
Splenocytes and lymph node cells from 10-25 mice were enriched in T cells by magnetic selection. CD4+, CD8+, DN T (total, PD-1− or PD-1+) cells as defined in Fig. 1A, and enriched MAIT (eMAIT: mLN CD4+/DN T cells expressing TCR Vβ8.2/8.3 and IL-18Rα) or NKT (eNKT: mLN T cells containing 1/3 CD1d-Tetramer+ cells) cells were sorted in a FACSAria (≥95% purity). Samples were washed and lysed in TRIzol Reagent (Life Technologies). RNA was isolated and cDNA synthetized from 1 μg RNA with EcoDry Premix (Clontech, CA). Quantitative PCR was performed using FastStart Universal SYBR Master (Roche, IN) in a StepOne Plus RT-PCR System (Applied Biosystems). To measure the relative amount of Cα, Vα14-Jα18 and Vα19-Jα33 TCR chains, sorted populations as described above were processed with PureLink RNA Micro Kit (Invitrogen) and cDNA synthesized from 500 ng RNA with the iScript™ cDNA Synthesis Kit (Bio-Rad). Quantitative PCR was performed as before or using custom TaqMan assay with previously described primers [41].
Statistical analyses
Statistical analysis was performed using Graphpad Prism. Groups were compared using Student’s t-test, ANOVA (Bonferroni’s or Tukey’s post-test) or their non-parametric equivalents when required.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of CD1d-tetramers. We are grateful to Drs. Christophe Benoist, Lydia Lynch and Dennis Kasper for providing several mouse strains.
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R21 AR063262) (J.C.C.) and by the National Institute of Allergy and Infectious Diseases (R01 AI085567) (G.C.T.) of the National Institutes of Health, and by a grant from the Alliance for Lupus Research (J.C.C.). S.A.A. was supported by a Postdoctoral Fellowship Award from the Arthritis Foundation.
N.R.R, S.A.A., and L.F. performed experiments. N.R.R. and J.C.C. analyzed the data. B.S.M., A.J.C. and J.M. designed and synthesized the MR1-Tetramers and advice regarding MAIT cells. N.R.R., J.M.M.V., G.C.T., and J.C.C. prepared the manuscript.
List of abbreviations
- DN T cell
Double negative T cell
- GF
Germ free
- MAIT cell
Mucosal Associated Invariant T cell
- PD-1
Programmed cell death 1
- ROR-γt
RAR-related orphan receptor γt
- SLE
Systemic Lupus Erythematosus
- SPF
Specific-pathogen-free
Footnotes
All the authors declare no conflict of interest.
REFERENCES
- 1.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–39. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–4. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu W, Jiang N, Ebert PJR, Kidd BA, Müller S, Lund PJ, Juang J, et al. Clonal Deletion Prunes but Does Not Eliminate Self-Specific αβ CD8(+) T Lymphocytes. Immunity. 2015;42:929–41. doi: 10.1016/j.immuni.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeda Y, Nishikawa H, Sugiyama D, Ha D, Hamaguchi M, Saito T, Nishioka M, et al. Detection of self-reactive CD8+ T cells with an anergic phenotype in healthy individuals. Science. 2014;346:1536–40. doi: 10.1126/science.aaa1292. [DOI] [PubMed] [Google Scholar]
- 5.Tsokos GC. Systemic lupus erythematosus. N. Engl. J. Med. 2011;365:2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 6.Zang YCQ, Li S, Rivera VM, Hong J, Robinson RR, Breitbach WT, Killian J, et al. Increased CD8+ cytotoxic T cell responses to myelin basic protein in multiple sclerosis. J. Immunol. 2004;172:5120–7. doi: 10.4049/jimmunol.172.8.5120. [DOI] [PubMed] [Google Scholar]
- 7.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, Roep BO, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J. Clin. Invest. 2004;113:451–63. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crispín JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol. 2008;181:8761–6. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bleesing JJ, Brown MR, Dale JK, Straus SE, Lenardo MJ, Puck JM, Atkinson TP, et al. TcR-alpha/beta(+) CD4(−)CD8(−) T cells in humans with the autoimmune lymphoproliferative syndrome express a novel CD45 isoform that is analogous to murine B220 and represents a marker of altered O-glycan biosynthesis. Clin. Immunol. 2001;100:314–24. doi: 10.1006/clim.2001.5069. [DOI] [PubMed] [Google Scholar]
- 10.Alunno A, Bistoni O, Bartoloni Bocci E, Caterbi S, Bigerna B, Pucciarini A, Tabarrini A, et al. IL-17-producing double-negative T cells are expanded in the peripheral blood, infiltrate the salivary gland and are partially resistant to corticosteroid therapy in patients with Sjögren’s syndrome. Reumatismo. 2013;65:192–8. doi: 10.4081/reumatismo.2013.192. [DOI] [PubMed] [Google Scholar]
- 11.Shivakumar S, Tsokos GC, Datta SK. T cell receptor alpha/beta expressing double-negative (CD4-/CD8-) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J. Immunol. 1989;143:103–12. [PubMed] [Google Scholar]
- 12.Sherlock JP, Joyce-Shaikh B, Turner SP, Chao C-C, Sathe M, Grein J, Gorman DM, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat. Med. 2012;18:1069–76. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 13.D’Acquisto F, Crompton T. CD3+CD4-CD8- (double negative) T cells: saviours or villains of the immune response? Biochem. Pharmacol. 2011;82:333–40. doi: 10.1016/j.bcp.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Mehal WZ, Crispe IN. TCR ligation on CD8+ T cells creates double-negative cells in vivo. J. Immunol. 1998;161:1686–93. [PubMed] [Google Scholar]
- 15.Pestano GA, Zhou Y, Trimble LA, Daley J, Weber GF, Cantor H. Inactivation of misselected CD8 T cells by CD8 gene methylation and cell death. Science. 1999;284:1187–91. doi: 10.1126/science.284.5417.1187. [DOI] [PubMed] [Google Scholar]
- 16.Crispín JC, Tsokos GC. Human TCR-alpha beta+ CD4- CD8- T cells can derive from CD8+ T cells and display an inflammatory effector phenotype. J. Immunol. 2009;183:4675–81. doi: 10.4049/jimmunol.0901533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez-Rodríguez N, Apostolidis SA, Penaloza-MacMaster P, Martín Villa JM, Barouch DH, Tsokos GC, Crispín JC. Programmed cell death 1 and Helios distinguish TCR-αβ+ double-negative (CD4-CD8-) T cells that derive from self-reactive CD8 T cells. J. Immunol. 2015;194:4207–14. doi: 10.4049/jimmunol.1402775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, Eckle SBG, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J. Exp. Med. 2013;210:2305–20. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahimpour A, Koay HF, Enders A, Clanchy R, Eckle SBG, Meehan B, Chen Z, et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 2015;212:1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbett AJ, Eckle SBG, Birkinshaw RW, Liu L, Patel O, Mahony J, Chen Z, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509:361–5. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 21.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–9. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 22.Ross EM, Bourges D, Hogan TV, Gleeson PA, van Driel IR. Helios defines T cells being driven to tolerance in the periphery and thymus. Eur. J. Immunol. 2014;44:2048–58. doi: 10.1002/eji.201343999. [DOI] [PubMed] [Google Scholar]
- 23.Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, Wherry EJ. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity. 2014;40:289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010;184:3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waggoner SN, Kumar V. Evolving role of 2B4/CD244 in T and NK cell responses during virus infection. Front. Immunol. 2012;3:377. doi: 10.3389/fimmu.2012.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 2012;188:4866–75. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin B, Auffray C, Delpoux A, Pommier A, Durand A, Charvet C, Yakonowsky P, et al. Highly self-reactive naive CD4 T cells are prone to differentiate into regulatory T cells. Nat. Commun. 2013;4:2209. doi: 10.1038/ncomms3209. [DOI] [PubMed] [Google Scholar]
- 28.Riol-Blanco L, Lazarevic V, Awasthi A, Mitsdoerffer M, Wilson BS, Croxford A, Waisman A, et al. IL-23 receptor regulates unconventional IL-17-producing T cells that control bacterial infections. J. Immunol. 2010;184:1710–20. doi: 10.4049/jimmunol.0902796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards DM, Ruggiero E, Hofer A-C, Sefrin JP, Schmidt M, von Kalle C, Feuerer M. The Contained Self-Reactive Peripheral T Cell Repertoire: Size, Diversity, and Cellular Composition. J. Immunol. 2015;195:2067–79. doi: 10.4049/jimmunol.1500880. [DOI] [PubMed] [Google Scholar]
- 30.Stritesky GL, Xing Y, Erickson JR, Kalekar LA, Wang X, Mueller DL, Jameson SC, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4679–84. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489:160–4. doi: 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 2011;208:1279–89. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurts C, Heath WR, Kosaka H, Miller JF, Carbone FR. The peripheral deletion of autoreactive CD8+ T cells induced by cross-presentation of self-antigens involves signaling through CD95 (Fas, Apo-1) J. Exp. Med. 1998;188:415–20. doi: 10.1084/jem.188.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–9. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 35.Wei B, Wingender G, Fujiwara D, Chen DY, McPherson M, Brewer S, Borneman J, et al. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J. Immunol. 2010;184:1218–26. doi: 10.4049/jimmunol.0902620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao Z, Mescher MF, Jameson SC. Detuning CD8 T cells: down-regulation of CD8 expression, tetramer binding, and response during CTL activation. J. Exp. Med. 2007;204:2667–77. doi: 10.1084/jem.20062376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 38.Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6:e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohlén C, Kalos M, Cheng LE, Shur AC, Hong DJ, Carson BD, Kokot NCT, et al. CD8(+) T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J. Exp. Med. 2002;195:1407–18. doi: 10.1084/jem.20011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKinney EF, Lee JC, Jayne DRW, Lyons PA, Smith KGC. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523:612–6. doi: 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, Bendelac A, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J. Exp. Med. 1999;189:1907–21. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.