Abstract
Systemic inflammatory response syndrome describes a heterogeneous group of cytokine storm disorders, with different immunogens and cytokines leading to variations in organ pathology. The severe inflammation generated by the cytokine storm results in widespread organ pathology including alterations in T-and B-lymphocyte counts. This study explores the roles of TLR9 and IFN-γR stimulation in decreasing T- and B-cell lymphopoiesis in a mouse model of hyperinflammation. We demonstrate that early B-cell lymphopoiesis is severely compromised during TLR9 and IFN-γ driven hyperinflammation from the Ly-6D+ common lymphoid progenitor (CLP) stage onwards with different effects inhibiting development at multiple stages. We show that TLR9 signaling directly decreases in vitro B-cell yields while increasing T-cell yields. IFN-γ also directly inhibits B-cell and T-cell differentiation in vitro as well as when induced by TLR9 in vivo. Microarray and RT-PCR analysis of Ly-6D− CLPs point to HOXa9 and EBF-1 as transcription factors altered by TLR9 induced inflammation. Our work demonstrates both cellular and molecular targets that lead to diminished B-cell lymphopoiesis in sustained TLR9 and IFN-γ driven inflammation that may be relevant in a number of infectious and autoimmune/inflammatory settings.
Keywords: B cells, inflammation, cytokines, transcription factors, hematopoiesis
Systemic inflammatory response syndrome is a common endpoint pathologic state initiated by various genetic, infectious, or autoimmune factors. It induces multi-organ pathology including hepatitis, fever, splenomegaly, cytokinemia and pan-cytopenias, and in severe cases, hemodynamic insufficiency and death. [1–4] One common thread across various causes of inflammation is impaired lymphopoiesis in the bone marrow, specifically drastic reductions in B-and T-cell lymphopoiesis.[5–9] Hematopoietic progenitors express several cytokine and inflammatory receptors that might induce diminished lymphopoiesis, yet our knowledge about the effects of signaling through these receptors is incomplete. Since many infectious and autoimmune syndromes result in sustained TLR9 stimulation and IFN-γ production, understanding the effects of hyperinflammation on lymphopoiesis is important for questions of pathogenesis as well as possible therapeutic interventions that might restore homeostasis.
During non-inflammatory conditions, hematopoietic stem cells differentiate towards the lymphoid lineage, losing erythroid and megakaryocytic potentials. Lymphoid committed progenitors with B-cell potential upregulate Flt3 and IL-7Rα and are termed common lymphoid progenitors (CLPs). CLPs are a heterogeneous population; CLPs expressing Ly-6D are exclusively committed to the B-cell lineage in vivo, while the Ly-6D− CLPs can give rise to B, T, NK, or dendritic cells (DC).[10–12] These Ly-6D+ CLPs retain the ability to generate T cells in vitro on OP9-dl1 cultures but do not generate T cells in vivo by intrathymic injection. [11]
Depending on the inflammatory model and which cytokines are produced, different components of hematopoiesis are impacted. In mice, alum-induced inflammation induces migration of B-cell lymphopoiesis due to TNFα induced alterations in the chemokine CXCL12 that normally retains precursors in the marrow.[13–15] In this model IL-1 increases granulopoiesis.[13, 14, 16] Type 1 interferons decrease B-cell lymphopoiesis when administered exogenously at high levels to mice or directly in vitro.[17–19] Another report found that stimulating bulk CLPs through TLR9 leads to lower B-cell yields but higher DC yields.[20, 21] While the developing B-cell compartment in the bone marrow is diminished during systemic inflammation, it is unclear which factors mediate these decreases in vivo and how they act to inhibit B-cell lymphopoiesis. It is also unclear whether these inflammatory mediators are acting directly on the progenitors cells and throughout lymphocyte development.
We have previously characterized a murine model of cytokine storm using repeated in vivo stimulation of TLR9. These mice develop the same signs and symptoms of hyperinflammation including peripheral leukopenias.[1, 2, 22] Prior work from our lab found increased serum levels of IFN-γ, IL-12, IL-6, and IL-18 as well as the anti-inflammatory cytokine IL-10. IFN-γ was essential for disease as IFN-γRKO mice do not develop hepatitis, anemia, or leucopenia.[1] The presence of peripheral lymphopenia in this model prompted us to further investigate whether this complex inflammatory milieu, specifically TLR9 and IFN-γR stimulation, resulted in changes in lymphopoiesis within the bone marrow.
In this study we confirm that systemic inflammation decreases B-celllymphopoiesis at multiple stages of development. We further show that TLR9 signaling directly decreases in vitro B-cell yields from CLPs, Pro and Pre B cells while increasing T-cell yields in a TLR9 intrinsic fashion. However, this TLR9 intrinsic effect is masked in vivo, likely due in part to IFN-γ, which has toxic effects on B- and T-cell development when administered in vitro as well as when induced by TLR9 in vivo. The hyperinflammation in vivo skews the CLP population from the B-cell lineage towards the T-cell lineage but not DC lineage when the CLPs are removed from the inflammatory environment. At a transcriptional level, we show the differential behavior of Ly-6D− and Ly-6D+ CLPs with respect to proliferation and differentiation programs. However, both CLP populations show a statistical decrease in the transcriptional programs of B-cell specification factors, with unchanged T-cell specification programs, suggesting that inflammation induced T-cell skewing may be more due to a loss of B-cell potential that a gain of T-cell potential. Finally, we specifically identified HOXa9 and EBF-1,[23–29] two important B-cell specification transcription factors, as being decreased by TLR9 induced inflammation.
Results
CpG induced inflammation preferentially decreases bone marrow B-cell and thymic T-cell progenitors
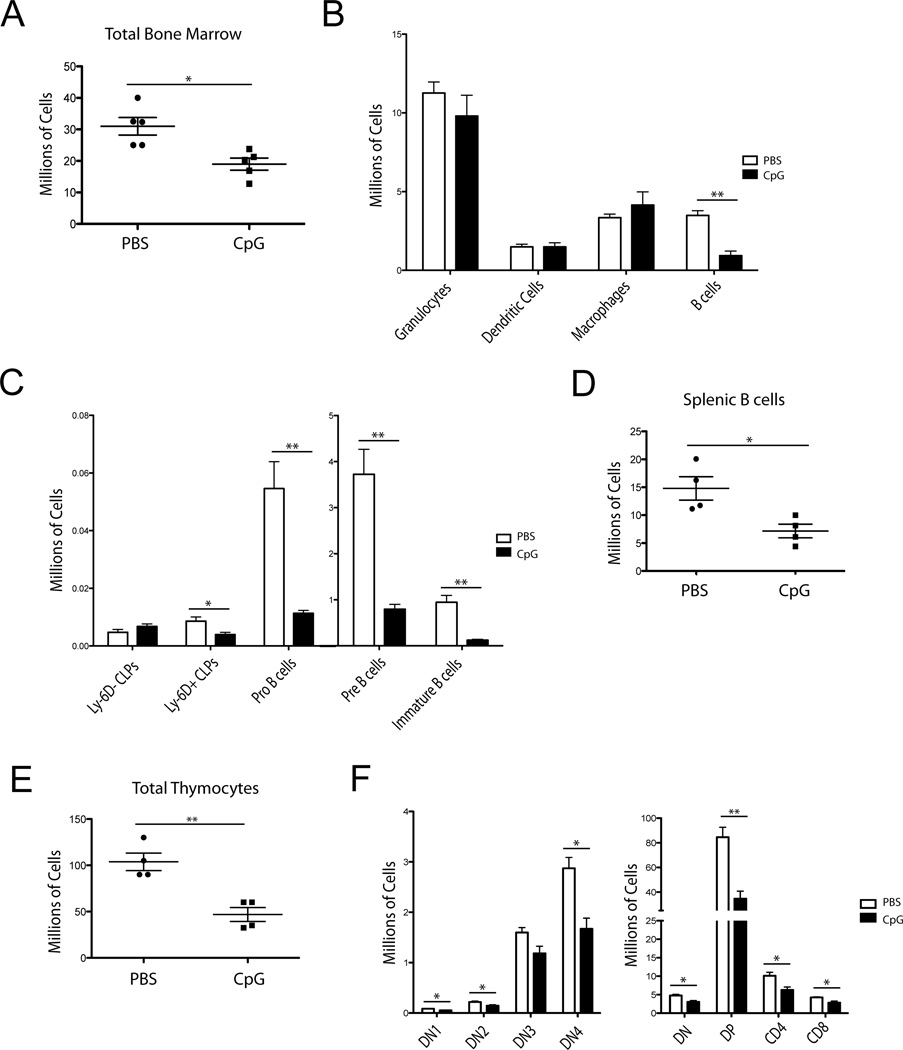
We induced sustained TLR9 inflammation in mice over a 10-day period as previously described.[1, 2, 22] On day 10, we examined the bone marrow and found decreased total cellularity (Fig. 1A). Interestingly, this was driven primarily by reductions in the CD19+ compartment while the other leukocyte cell lineages were grossly intact (Fig. 1B). The remaining loss of bone marrow cells was accounted for by loss of eythroid precursors (data not shown). We then characterized which B-cell precursors were affected by CpG treatment and found that Pro-, Pre-, and Immature B-cells were all decreased approximately ten-fold in CpG treated mice compared to the PBS controls. We further found that B-lineage biased CLPs, identified via surface expression of Ly-6D, were decreased approximately two-fold while numbers of upstream Ly-6D− CLPs were unaffected (Fig. 1C). To ascertain if large numbers of bone marrow B-cell precursors had migrated to the periphery, we also examined the spleen. However, we found decreased splenic B-cell numbers even though total splenic numbers were increased, suggesting that B-cell development was not solely mobilizing to the spleen during inflammation (Fig. 1D). We also examined the thymuses of these mice for T-cell precursors and found that total cellularity was decreased by CpG (Fig. 1E) and that essentially all stages of T-cell development were suppressed (Fig. 1F).
Figure 1. Repeated CpG treatment impairs B-cell lymphopoiesis.
(A– F) C57BL6 mice were treated with PBS or CpG five times over ten days. (A) Bone marrow cell numbers from both tibias and femurs were determined by cell counting with trypan blue. Each dot represents an individual mouse, bars show mean +/− SEM. (B) DCs (CD11c+ NK1.1−, GR-1−), Macrophages (GR-1+ CD11blo), Granulocytes (GR-1+ CD11bhi) and B cells (CD19+) were discriminated by flow cytometry and numbers were calculated using total bone marrow cellularity as determined in (A). Bars show mean +SEM C) CLPs (Lineage−, cKitint, Sca-1low/high, IL-7Rα+, Flt3+) discriminated on expression of Ly-6D, Pro (B220int, Aa4.1+, CD19+, IgM−, CD43+), Pre (B220int, Aa4.1+, CD19+, IgM−, CD43−), and Immature B-cell (B220int, Aa4.1+, CD19+, IgM+) numbers from treated mice. D) Total splenic B cell (CD19+) population numbers. E) Total thymocyte numbers. F) Thymocyte subsets DN1 (CD4−, CD8−, cKit+, CD25−), DN2 (CD4−, CD8−, cKit+, CD25+), DN3 (CD4−, CD8−, cKit−, CD25+), DN4 (CD4−, CD8−, cKit−, CD25−), DN (CD4−, CD8−), DP (CD4+, CD8+), CD4 (CD4+, CD8−), CD8 (CD4−, CD8+). (A–F) Data are representative of 5 independent experiments with 4–5 mice per group. * = p<0.05, ** = p<0.005 by unpaired t-test. Error bars represent SEM.
CpG and IFN-γ stimulation inhibit B cell growth at multiple stages in vitro
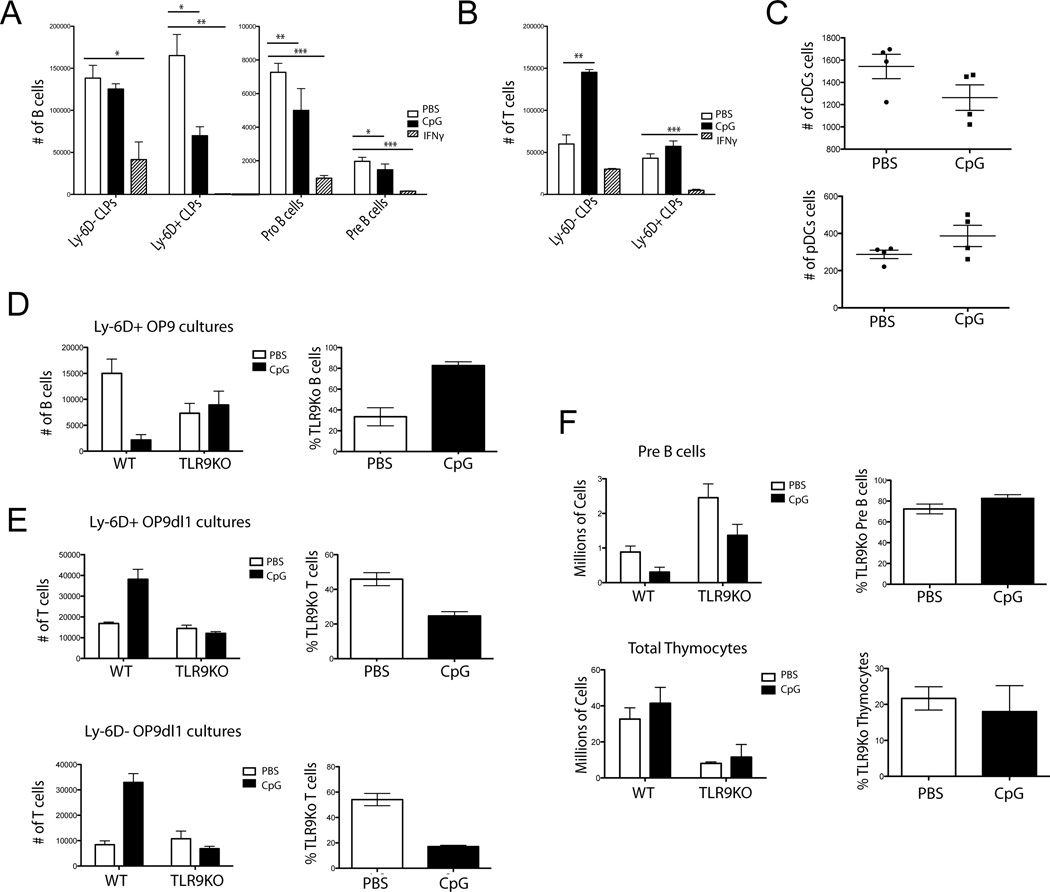
Because IFN-γ is induced by in vivo TLR9 stimulation and B-cell precursors express both TLR9 and IFN-γR, we next investigated which B-cell progenitors were affected by CpG and IFN-γ treatment. We turned to an in vitro system allowing us to isolate direct effects of each factor without the myriad of confounding factors that may be present in vivo. Using the established OP9 co-culture system,[30, 31] Ly-6D− CLPs, Ly-6D+ CLPs, Pro, or Pre B cells were cultured in B-cell promoting conditions and administered PBS, CpG, or IFN-γ. We found significant reductions in the number of B cells generated from Ly-6D+ CLPs, Pro and Pre B cells that received CpG or IFN-γ compared to PBS (Fig. 2A). To test if treatment effects were specific to the B-cell lineage, CLPs were cultured in T-cell promoting conditions. Surprisingly, Ly-6D− CLPs receiving CpG yielded more T-cell precursors while IFN-γ severely decreased T-cell yields (Fig. 2B). This suggests that CpG and IFN-γ are acting via different pathways. We then wanted to know if this skewing of the CLP population in response to CpG was specific to the T-cell lineage. We therefore cultured CLPs in DC promoting culture conditions. While the Ly-6D+ CLPs did not differentiate well into DCs (data not shown), the Ly-6D− CLPs did, with no difference in conventional or plasmacytoid DC (cDC and pDC) yields when CpG was added to cultures (Fig. 2C). This demonstrates that CpG induced increases in T-cell yields are not simply due to an across the board increase in non-B-cell lineage potentials, but rather are specific to the T-cell and not DC fates.
Figure 2. CpG and IFN-γ differentially inhibit B-cell lymphopoiesis.
A) Ly-6D− and Ly-6D+ CLPs, Pro and Pre B cells were sorted from WT mice and grown in vitro on OP9 stromal cells with 0.6 µg CpG, 100 pg IFN-γ or PBS and cell numbers were determined by flow cytometry. Bars depict a representative mean + SEM from 3 independent experiments with 4 replicates per experiment. B) Ly-6D− and Ly-6D+ CLPs were sorted and grown on OP9-dl1 co-cultures with CpG, 100 pg IFN-γ, or PBS and cell numbers were determined by flow cytometry. Bars depict mean + SEM representative from 3 independent experiments with 4 replicates per experiment C) CLPs were plated in DC promoting culture conditions with CpG or PBS and cell numbers were determined by flow cytometry. Each dot shows an individual mouse. Data are representative of 3 experiments with 4 replicates per experiment. D) 250 each WT CD45.1 and TLR9KO CD45.2 Ly-6D+ CLPs were mixed and grown on OP9 stromal cells with CpG or PBS and cell numbers were determined by flow cytometry. Left graph represents absolute numbers of CD19+ cells per well as counted by flow cytometry. Data were modeled using genotype and PBS/CpG treatment as independent variables in a 2-way ANOVA looking for a statistically significant interaction term to determine a differential effect of CpG on genotype for cell counts (p=0.0064 interaction, 2-way ANOVA). Graph on right represents the same data as % TLR9KO cells per well. Bars represent mean + SEM. Mixed cultures are representative of 5 experiments with 4 replicates per group. E) Cultures were set up as in part D but on OP9dl1 stromal cells. Top represents plated Ly-6D+ CLPs and the bottom Ly-6D− CLPs. Statistical analysis was performed as in part D. T-cell OP9dl1 co-cultures Ly-6D+ (p=0.0027 interaction, 2-way ANOVA) and Ly-6D− (p<0.0001 interaction, 2-way ANOVA). Graphs on right represent the same data as % TLR9KO cells per well. Bars represent mean + SEM. Mixed cultures are representative of 5 experiments with 4 replicates per group. F) Mixed bone marrow chimeras were made using 50:50 WT and TLR9 congenically marked bone marrow. After reconstitution, mice were treated with repeated CpG dosing regimen and cell numbers were determined by flow cytometry. Left graphs represent numbers of Pre B cells from tibias and femurs (p=0.3736 interaction, 2-way ANOVA) or thymocytes (p=0.6601 interaction, 2-way ANOVA). Right graphs represent % TLR9KO Pre B cells or Thymocytes from treated mice. Mixed chimeras are representative of 2 experiments with 7 mice per group. For OP9 experiments B cells are defined as being CD19+ B220+, T cells as being CD90+ NK1.1−, cDCs as CD11c+ CD11b+ B220− NK1.1− and pDCs as CD11c+ B220+ CD11b− NK1.1−.* = p<0.05, ** = p<0.005, *** = p<0.0005, unpaired t-test.
TLR9 signaling directly inhibits B-, but increases T-cell differentiation in vitro
To interrogate whether the CpG-induced inhibition of B-cell lymphopoiesis was acting in a TLR9 intrinsic manner, we performed similar in vitro experiments using mixed cultures of equal numbers of TLR9 sufficient and TLR9 deficient (TLR9KO) CLPs. In the B-cell promoting conditions, we found that only the TLR9 sufficient progenitors produced lower B-cell yields while the TLR9KO cells yielded similar numbers in the PBS and CpG treated groups (Fig. 2D). We performed the same experiment in T-cell promoting conditions and found that only the TLR9 sufficient CLPs led to increased T-cell yields with CpG treatment, suggesting that TLR9 signaling directly alters the lineage potential of all CLP populations in vitro (Fig. 2E). Contrasting this result with the loss of thymocytes in vivo suggests that while CLPs may have an intrinsic TLR9 driven increased T-cell potential, non-CLP cells, such as thymic stromal cells, are responding to TLR9 induced signals that result in loss of T-cells in vivo.
TLR9 extrinsic factors contribute to the decrease in B lymphopoiesis in vivo
While in vitro experiments demonstrate the potential of direct TLR9 signals to diminish B-cell lymphopoiesis, the possibility remained that other signals in vivo may contribute in a TLR9 receptor extrinsic fashion. To determine if TLR9 signaling was the primary and direct cause of the lymphopoiesis defect in vivo, we made mixed bone marrow chimeras. We injected lethally irradiated TLR9KO hosts with mixed 50:50 congenically marked WT/TLR9KO bone marrow and waited 10 weeks for reconstitution before administering the 10-day CpG regimen. In contrast to the in vitro data, we found that the TLR9KO B-cell progenitors were not spared, as Pre-B-cell numbers from mixed chimeras receiving CpG demonstrated similar decreases in numbers of both wild type (WT) and TLR9KO precursors (Fig. 2F). This does not rule out that in vivo, a TLR9 intrinsic component decreases B-cell yields, but rather suggests that TLR9 induced cytokines also inhibit B-cell lymphopoiesis and mask the TLR9 intrinsic effect observed in vitro. Intriguingly, thymocyte loss was not seen in these mice in either TLR9 sufficient or deficient cells (Fig. 2F). Since the host mouse was TLR9 deficient, these results suggest a non-hematopoetic cell is affected by TLR9 induced inflammation resulting in thymocyte loss. Consistent with a CLP extrinsic mechanism for TLR9 mediated thymocyte loss, both WT and TLR9KO marrow contributed equally to thymopoiesis (Fig. 2F). Additionally, while the results were not significant, there was a trend toward greater thymocyte numbers in CpG exposed mice, consistent with the in vitro data.
Type 2 but not type 1 interferons mediate decreases in B-cell lymphopoiesis
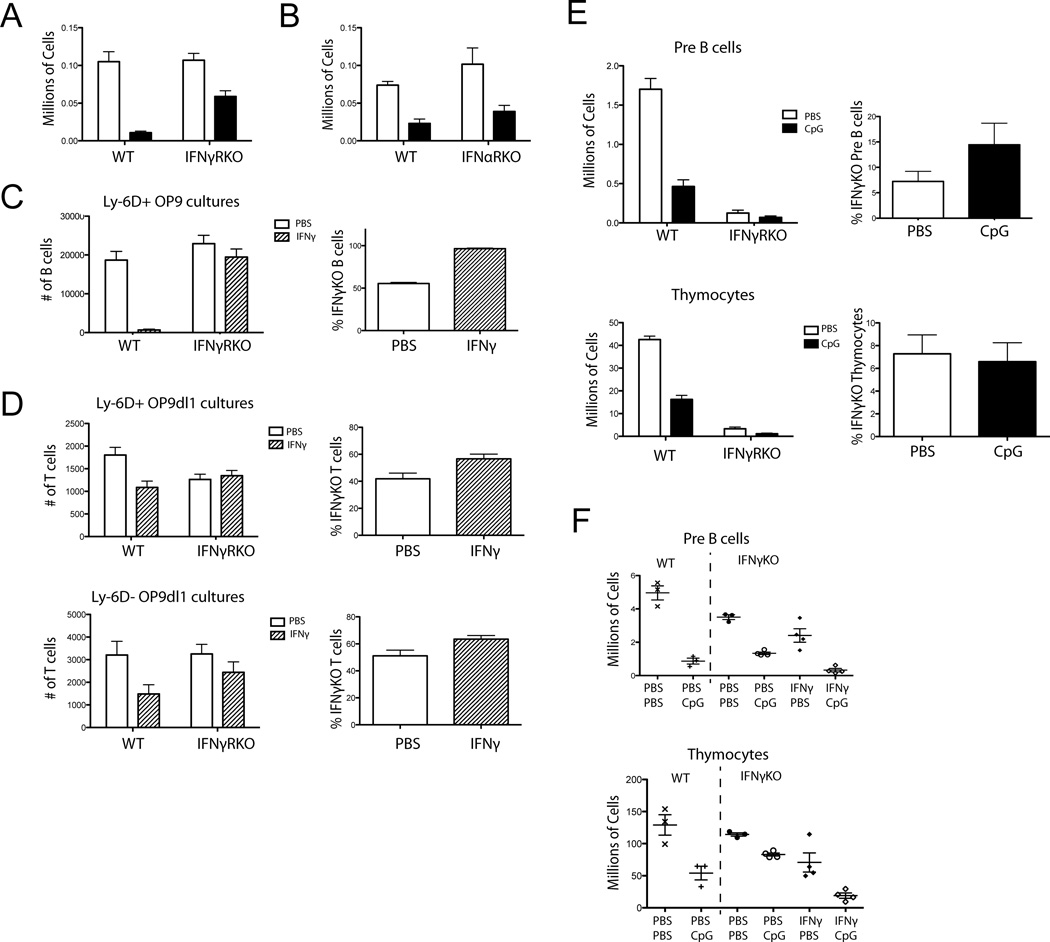
Because IFN-γ inhibited CLP development in vitro (Fig. 2A), we investigated whether IFN-γ contributed to B-cell lymphopoiesis defects in vivo. We injected WT or IFN-γ receptor deficient (IFN-γRKO) mice with CpG or PBS and found that the IFN-γRKO mice were protected as they had significantly smaller decreases in B-cell progenitors compared to WT mice (Fig. 3A). This suggests that IFN-γ is one of the main mediators induced by CpG leading to the decreased B-cell progenitors in vivo, and a potential candidate for masking the TLR9 intrinsic effect. This is not true of all TLR9 induced cytokines, as mice lacking the IFN-α receptor gene (IFN-αRKO) were not protected compared to the WT mice (Fig. 3B).
Figure 3. IFN-γ directly inhibits B-cell development in vitro and in vivo.
A) IFN-γRKO and WT mice were treated with CpG or PBS with 5 doses over 10 days. Numbers of Pro B cells were determined by flow cytometry. (p=0.0349 interaction, 2-way ANOVA). Bars show mean + SEM. Data are representative of 3 experiments with 4 mice per group. B) IFN-αRKO and WT mice were treated with PBS or CpG 5 times over 10 days. Numbers of Pro B cells were determined by flow cytometry. Data are representative of 2 experiments with 4 mice per group.. (p=0.2607 interaction, 2-way ANOVA). C) 250 each WT and IFN-γRKO progenitors were sorted, mixed and grown on OP9 cells with PBS or IFN-γ and cell numbers were determined by flow cytometry Left graph represents numbers of B-cells recovered per well. Data were modeled using genotype and PBS/CpG treatment as independent variables in a 2-way ANOVA looking for a statistically significant interaction term to determine a differential effect of CpG on genotype for cell counts (p=0.0006 interaction, 2-way ANOVA). Graph on right represents the same data as % IFN-γRKO cells per well. Bars show mean + SEM. Data are representative of 2 experiments with 7 replicates per group. D) Cultures were set up as in part C but on OP9dl1 stromal cells. Top represents plated Ly-6D+ CLPs and the bottom Ly-6D− CLPs. Data were analyzed as in part C: T-cell OP9dl1 co-cultures Ly-6D+ (p=0.0067 interaction, 2-way ANOVA) and Ly-6D− (p=0.3643 interaction, 2-way ANOVA). Graphs on right represent the same data as % IFN-γRKO cells per well. Mixed cultures are representative of 2 experiments with 7 replicates per group. E) Mixed 90% WT 10% IFN-γRKO bone marrow chimeras were given repeated CpG or PBS injections. Left graphs represent numbers of Pre B cells from tibias and femurs as determined by flow cytometry. (p<0.0001 interaction, 2-way ANOVA) or Thymocytes (p<0.0001 interaction, 2-way ANOVA). Right graphs represent % TLR9KO Pre B cells or Thymocytes from treated mice. Bars show mean + SEM. Data are representative of 2 experiments with 9–10 mice per group. F) IFN-γKO mice were given repeated PBS, CpG, IFN-γ, or CpG and IFN-γ injections. For comparison WT mice were injected with PBS or CpG. Each dot shows one mouse. Data are representative of 3 experiments with 3 or 4 mice per group. Statistical modeling was done on the four groups of IFN-γKO mice by 2-way ANOVA for Pre B cells (p=0.8555 interaction, p<0.0001 PBS vs. CpG, p=0.0010 PBS vs. IFN-γ) and Thymocytes (p=0.2556 interaction, p=0.0005 PBS vs. CpG, p<0.0001 PBS vs. IFN-γ).
Interferon gamma receptor signaling directly inhibits B- and T-cell development in vitro and in vivo
To test if the decreases we saw in vitro with IFN-γ administration were IFN-γR intrinsic, we used the same mixed culture strategy as before but with WT and IFN-γRKO cells. In these mixed cultures, we found that only the WT cells yielded fewer B-cell in response to IFN-γ, as the IFN-γR KO cell yields were similar to cultures receiving PBS (Fig. 3C). In contrast to TLR9 exposed CLPs, IFNγ exposed CLPs had a decreased T-cell potential highlighting the different roles between these two inflammatory signals during T-cell lymphopoeisis (Fig. 3D). We then investigated whether a cell intrinsic IFN-γR response is the dominant force leading to decreased B-cell progenitors in vivo by making mixed IFN-γRKO and WT congenically marked bone marrow chimeras. We used a 90% WT and 10% IFN-γRKO mix since mice generated with fewer IFN-γ responsive cells do not make the full panel of cytokines normally seen in the sustained TLR9 inflammation model (unpublished observations). We found that there was a statistically significant differential in Pre B-cell numbers in the CpG treated mice such that the IFN-γRKO B cells were less affected than WT (Fig. 3E). This suggests that there is a measurable cell intrinsic IFN-γR signaling event contributing to decreased B-cell lymphopoiesis during sustained TLR9 inflammation in vivo. In these chimeric mice, we saw a loss of thymocytes in both IFNγR sufficient and deficient cells (Fig. 3E). However, since in these experiments, the host was a TLR9 sufficient mouse, this likely reflects the non-hematopoetic TLR9 signals noted previously (Fig. 2F).
IFN-γ and CpG act via distinct pathways to inhibit B- and T-cell lymphopoiesis in vivo
Because TLR9 and IFN-γR differentially affected B- and T-cell development in vitro, we next investigated whether these two pathways might interact in vivo. Previous work in innate cells has shown that IFN-γR and TLR9 signaling synergize at the epigenetic level.[32–34] To address this question, we used IFN-γ deficient mice (IFN-γKO) that are capable of responding to IFN-γ but are unable to produce it, and examined how CpG, IFN-γ or the combination of both stimuli affect B-cell lymphopoiesis. The dose of IFN-γ was selected and confirmed to replicate the serum levels of IFN-γ seen in WT mice treated with the repeated CpG protocol (data not shown). We observed that both CpG and IFN-γ have independent capacities to reduce Pre B-cell numbers (Fig. 3F). However, statistical modeling did not reveal a significant interaction effect, suggesting that they are acting via additive and not synergistic pathways, corroborating our in vitro data. Evaluation of thymopoiesis yielded similar results. IFNγ treatment alone resulted in lower thymocyte numbers, concordant with in vitro data. Similarly CpG treatment resulted in thymocyte loss, again likely due to non-hematopoeitic effects of CpG induced inflammation on the thymic environment. As with the B-cell data, the effects of IFN-γ and CpG appear to not interact in a synergistic manner (Fig. 3F).
CpG-induced inflammation skews CLPs away from the B-cell fate and enhances T-cell differentiation
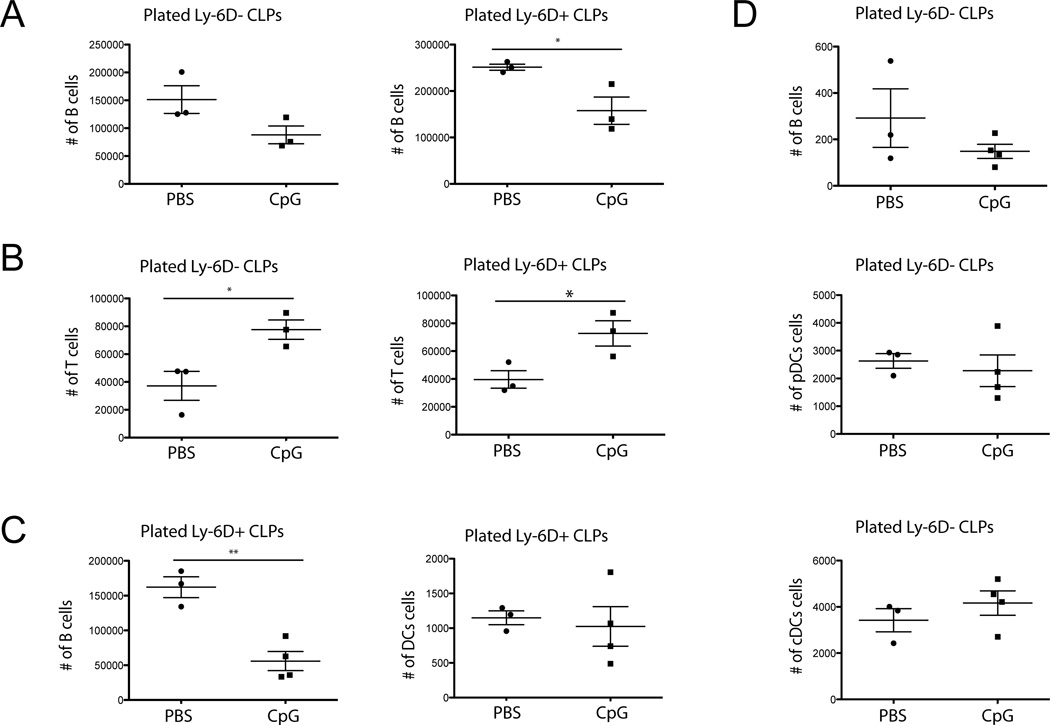
The in vitro data suggest that TLR9 signaling skews precursors away from the B cell lineage, with contrasting effects on T cell fates. To determine if the complex in vivo TLR9 induced inflammatory milieu alters CLP population potentials, we sorted Ly-6D+ and Ly-6D− CLPs from mice receiving repeated CpG or PBS injections and placed them in the OP9 culture system under B- or T-cell promoting conditions. No further CpG or inflammatory cytokines were given during the in vitro culture process. We found that Ly-6D+ CLPs isolated from CpG treated mice yielded significantly fewer B cells than those from the PBS treated mice (Fig. 4A). In contrast, we found that the CLPs exposed to the hyperinflammatory environment demonstrated enhanced T-cell potential (Fig. 4B). We also tested DC potential and while Ly-6D+ CLPs, generated minimal DCs even under DC promoting conditions they did generate B cells, which showed similar results to the B-cell conditions (Fig. 4C). Ly-6D− CLPs however, gave rise to very few B cells but surprisingly generated similar numbers of pDCs and cDCs regardless of prior inflammatory exposure (Fig. 4D).
Figure 4. CpG induced inflammation skews CLPs away from the B-cell fate.
(A, B) CLPs were sorted from WT mice treated with repeated CpG or PBS. A) Sorted CLPs were grown on B-cell OP9 cultures and the number of B cells was determined by flow cytometry. B) Sorted CLPs were grown on T-cell OP9-dl1 cultures and the number of T cells was determined by flow cytometry. Each dot shows one mouse. Data are representative of 5 experiments with 3 mice per group. (C, D) Sorted CLPs were grown on OP9 cultures with DC culture conditions. C) Ly-6D+ CLPs. D) Ly-6D− CLPs. Numbers of B clls and DCs were determined by flow cytometry. Each dot shows one mouse. Data are representative of 2–5 experiments with 3–4 mice per group. * = p<0.05, ** = p<0.005, unpaired t-test.
CpG induced inflammation alters the B-cell transcriptional program
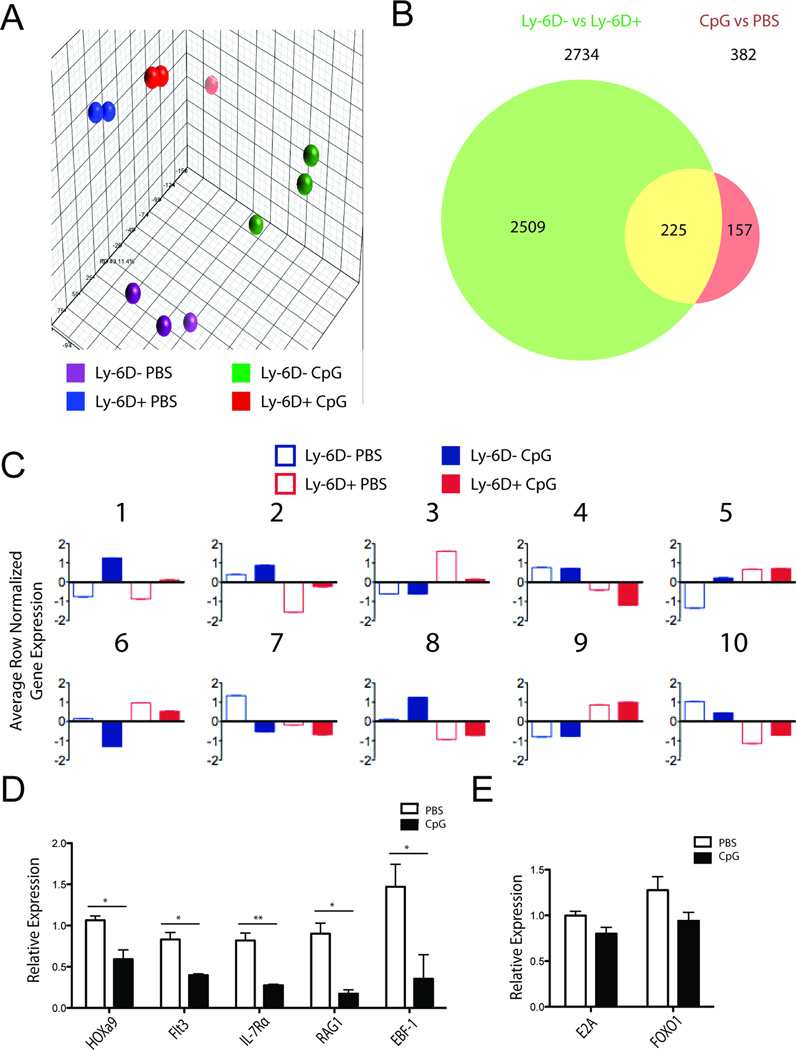
We next assayed the transcriptional program of CLPs during inflammation for factors that might explain the decreased B-cell potential of CLPs. To get a global transcriptional view, we analyzed mRNA expression from Ly-6D− and Ly-6D+ CLPs from either repeated CpG or PBS treated mice by microarray. Principle component analysis demonstrated that all four groups cluster independently, suggesting different transcriptional programs based on both stage of development and the presence of inflammation (Fig. 5A). Considering differentially expressed genes using a false discovery rate of 20% (Benjamini-Hochberg), we found 2734 genes differentially expressed in Ly-6D− vs Ly-6D+ CLPs and 382 genes differentially expressed between PBS and CpG treatments, 225 of which were in both lists (Fig. 5B). Thus, approximately 60% (225/382) of the genes altered by sustained TLR9 induced inflammation are also genes altered during the transition from the Ly-6D− to Ly-6D+ stage of B-cell development.
Figure 5. Ly-6D expression and CpG treatment induce transcriptional profile changes.
A) Principle component analysis from microarray of Ly-6D+ and Ly-6D− CLPs from CpG and PBS treated mice. B) Venn diagram of significant genes from 2-way comparison of microarray data sets. False discovery rate was set to 20%. C) Results of K means clustering using k=10 of differentially expressed genes with a false discovery rate of 20%. Average row normalized gene expression for each population in each cluster is presented. (D, E) Ly-6D− CLPs from three PBS and three CpG treated mice were analysed by qRT-PCR. D) HOXa9 and its downstream targets. E) E2A and its unique target FOXO1. Data show mean + SEM from 3 individual samples. Expression of the gene relative to β-actin is shown. * = p<0.05, ** = p<0.005, Student’s t-test
We also performed K-means clustering to identify 10 clusters of genes that change expression levels in similar patterns across developmental stage as well as inflammatory exposure (Fig. 5C, gene contents of each cluster available as supplemental data). Five major patterns were noted: genes that are changed in the same direction by CpG exposure in both Ly-6D− and Ly-6D+ CLPs (Clusters 1, 2, 6, and 7); genes that are unchanged by CpG exposure in Ly-6D− CLPs but are altered in Ly-6D+ CLPs (Clusters 3 and 4); genes that are unchanged by CpG exposure in Ly-6D+ CLPs but are altered in Ly-6D− CLPs (Clusters 5 and 8); genes that are unaltered by CpG exposure, but change with developmental stage (Cluster 9); and genes that have decreased CpG induced expression in Ly-6D− CLPs but increased expression in Ly-6D+ CLPs (Cluster 10).
We next submitted the gene lists from each cluster to Ingenuity Pathway Analysis (IPA) using the Upstream Analysis module to look for transcriptional regulators associated with each list. The top 10 regulators with p-value less than 0.001 for each cluster are shown in Table 1. A number of functional themes arose from this analysis. Not surprisingly, genes in Cluster 9 that change only with developmental stage suggested lymphocyte fate specification functions. However, Clusters 3, 8, and 10 also suggest hematopoietic specification functions as well. Intriguingly, these three clusters also happen to be patterns in which Ly-6D− and Ly-6D+ CLPs behave differently, suggesting different stages have different susceptibilities to altered development due to inflammation. Growth and proliferation were also a common theme amongst many clusters suggesting inflammation may affect lymphopoiesis via these pathways as well. Cytokine signaling was also a common theme, and intriguingly, Cluster 4 (e.g. genes only affected in the Ly-6D+ stage) were related to IL-12 signaling perhaps suggesting a role for this CpG induced cytokine in inhibiting B-cell development.
Table 1. Functional program of gene clusters suggested by Ingenuity Pathway Upstream Regulator Analysis.
Regulators in bold contribute to the functional theme.
| Cluster | Functional Theme | Predicted Upstream Transcriptional Regulators |
|---|---|---|
| 1 | Cytokine Signaling |
IFNG, IL5, IL27, CD38, IL10, MYC, STAT3, DYSF, REL, IL4 |
| 2 | Growth and proliferation |
MYCN, MYC, IL3, PRKAG3, IL4, GNRH2, IFNG |
| 3 | Lymphopoiesis, Cell cycle |
TCF3, CDKN1A, CHEK1, YY1, CDKN2A, TBX2, TADA3, SNAI1 |
| 4 | IL-12 Signaling | LXR, IL12 |
| 5 | Cell cycle |
TP53, E2F4, CDKN1A, RB1, CDKN2A, E2F1, E2F2, EP400, CSF2, BX2 |
| 6 | Inflammatory signaling, Homeobox genes |
IL2, NFKBIB, DMD, FOXO1, HOXA7, HOXA9, FFAR1, DLG1, BTNL2, RELA |
| 7 | Growth |
IGFBP2, BRCA1, PTEN, AKT, NGFR |
| 8 | Growth, Myelopoeisis | Map4K4, MYC, SPI1, LIPE, CEBPA, HSF2, ESRRG, AK1, CSF3, KRAS |
| 9 | T- versus B-cell specification |
IL2, TCR, PAX5, SMARCC1, TP53, IFNG, IL7, TCF3, EBF1, TNF |
| 10 | Myelo- and lymphopoietic factors |
CEBPA, GATA1, SRF, TGFB1, RNF20, ETS1, GATA2, PPARA, NR5A1, IL4 |
Ly-6D− and Ly-6D+ CLPs differ in inflammatory and B-cell fate transcription factor signatures
We next performed Gene Set Enrinchment Analysis (GSEA) on the effects of CpG induced inflammation on Ly-6D− CLPs and Ly-6D+ CLPs to look for specific differences between these two populations. First we performed GSEA anaylsis using gene sets derived from the MSigDB v4.0 database to query multiple inflammatory signaling pathways (gene set lists and rankings available as supplemental data). Accepting only gene sets as significant with a false discovery rate of 10% and a p-value of less than 0.05, we found that both TLR and IFN-γ signaling pathways were activated in both Ly-6D− CLPs and Ly-6D+ CLPs. However, similar to results obtained in the clustering analysis, IL-12 signaling was only activated in inflammation exposed Ly-6D+ CLPs. Furthermore, interferon-alpha and IL-7 pathways were suppressed only in Ly-6D− CLPs, suggesting unique signaling changes in this population as well.
We then generated our own gene sets for the B-cell specification factors EBF1, Pax5, and E2A, as well as the T-cell specification factors Notch1, Ikaros, and Gata-3 as these are not found in MSigDB. These sets were derived by obtaining the lists of genes that were noted to be directly transcriptionally regulated by each of these factors by the Ingenuity software. Performing GSEA using these gene sets using the same cut-offs (rankings available as supplemental data) demonstrated that in both Ly-6D− CLPs and Ly-6D+ CLPs, all B-cell specification programs were inhibited. However, none of the T-cell specification programs were inhibited or activated in Ly-6D− CLPs, suggesting that the T-cell skewing may be due to a loss of B-cell specification in CLPs, rather than a gain of T-cell specification. This is consistent with previous reports of Pax5 acting to inhibit T-cell fates, but having no effect on myeloid fates, and explains why a loss of B-cell specification alone would not alter DC potential but increase T-cell potential.
HOXa9 is downregulated in Ly-6D− CLPs during CpG induced inflammation
In order to better understand the most upstream effects of CpG-induced inflammation on B-cell lymphopoiesis, we next focused on the Ly-6D− CLPs, which have in vivo T-cell and DC potential and appear to be the earliest susceptible precursor to in vivo CpG treatment as the defect appears at the transition to the Ly-6D+ CLP stage. We explored possible factors that could account for the pattern of differentially expressed genes between PBS and CpG exposures again using the upstream analysis module of the Ingenuity Pathway Analysis program. Interestingly, the transcription factor HOXa9 was predicted to have the most down-regulated activity (Table 2), as it is an important B-cell lineage coordination factor.[23–25, 35] Intriguingly, HOXa9 was suggested as a regulator of the Cluster 6 genes, and appears itself as a member of Cluster 10 (those genes that are differentially regulated between Ly-6D− CLPs and Ly-6D+ CLPs). Additionally, in concert with what would be expected, many of the predicted up-regulated factors were related to inflammatory cytokines produced in our model. As in the GSEA analyses, T-cell promoting genes were not predicted to be upregulated, perhaps because many require stronger Notch signaling to be induced than is present in the bone marrow.
Table 2. Ingenuity Pathway Analysis of Ly-6D− CLPs from CpG and PBS treated mice.
| Upstream Regulator | Activation Z-score |
p-value of overlap |
|---|---|---|
| HOXA9 | −3.08 | 5.45e-06 |
| POR | −2.813 | 1.65e-04 |
| HOXA7 | −2.789 | 1.49e-07 |
| RB1 | −2.262 | 3.17e-04 |
| CD3 | −2.076 | 8.76e-06 |
| IFN-γ | 3.857 | 3.71e-07 |
| CD38 | 3.536 | 3.67e-07 |
| IL5 | 3.019 | 5.71e-07 |
| STAT3 | 2.954 | 2.74e-05 |
| IL6 | 2.691 | 1.82e-08 |
| IL1B | 2.673 | 1.56e-04 |
| CSF2 | 2.670 | 4.23e-05 |
| IL4 | 2.638 | 1.84e-08 |
| IFN-α/β | 2.191 | 3.84e-05 |
| IfnαR | 2.135 | 3.79e-04 |
| IKBKB | 2.122 | 1.74e-05 |
| APP | 2.087 | 1.72e-05 |
HOXa9 and EBF-1 are downregulated in CLPs exposed to CpG-induced inflammation
We then interrogated transcriptional levels of HOXa9 and some of its targets by qRTPCR. We found that Ly-6D− CLPs from CpG-treated mice had lower levels of HOXa9 than their PBS treated counterparts. Known HOXa9 targets important for B-cell specification, Flt3, IL-7Rα and RAG1,[23–25, 35] were also decreased (Fig. 5D). Such loss of IL-7Rα in the Ly-6D− CLPs is consistent with the loss of IL-7 signaling seen by GSEA. As many of these targets are also known to be mediated by the transcription factor E2A, we looked at E2A and its unique target FOXO1 but found that neither were significantly decreased, suggesting that alterations in HOXa9 may preferentially account for the decreases in Flt3, RAG1, and IL-7Rα in CpG treated mice (Fig. 5E). While E2A activation is generally controlled at the protein level, unchanged levels of its target FOXO1 suggest it is not inhibited enough to substantially decrease transcriptional profiles.[36, 37] EBF-1, a target of IL-7Rα and an important factor for initiating the B-cell program,[29] was also decreased in Ly-6D− CLPs, (Fig. 5D). Together these data suggest that important factors for the B-cell program are decreased with CpG treatment.
Discussion
Hyperinflammatory syndrome, the rapid elevation of many inflammatory cytokines leading to immune dysfunction, is seen in a variety of auto-inflammatory and infectious diseases including. This inflammation leads to widespread organ pathology including hepatitis, fever, cytokinemia, and pan-cytopenias, including B- and T-cell lymphopenias. This report characterizes the effects of this inflammation on B-cell lymphopoiesis using a well-characterized hyperinflammatory model in which mice are administered repeated doses of the TLR9 agonist CpG.
Our in vitro and in vivo data suggest that TLR9 and IFN-γ are acting independently to inhibit B-cell development at multiple stages. Differential cell yields from CLPs exposed to CpG in vitro suggest that TLR9 skews CLPs away from the B-cell lineage and specifically towards the T-cell but not DC lineages. In contrast, IFN-γ has similar and stronger inhibitory effects on B- and T-cell yields in vitro, suggesting that it is acting via a different pathway, perhaps by inducing death or inhibiting proliferation in a lineage non-specific manner, such as by decreased sensitivity to IL-7. CpG and IFN-γ also decrease B-cell yields from Pre B cells. Together this suggests that CpG and IFN-γ differentially act on CLPs, but also that they both inhibit Pre B-cell growth. Consistent with the in vitro data, we show that CpG and IFN-γ have independent and additive affects suppressing B-cell lymphopoiesis in vivo. Not surprisingly, the intrinsic effects of TLR9 signaling seem to be masked by the IFN-γ produced in vivo demonstrating the importance of both systems for dissecting these events. The combination of these multiple inflammatory signals in vivo lead to a profound loss of B-cell lymphopoeisis with a skewed potential for T-cell lymphopoesis. While stromal cell factors may contribute to the effects we saw in vitro, however, we show that both TLR9 and IFN-γR signaling are CLP intrinsic in mixed cultures. Accordingly, when we attempted stromal cell free cultures under B-cell directing conditions, B-cell yields were significantly decreased, albeit with much lower overall yields across all of the cultures (data not shown).
Previous studies have suggested that TLR9 signaling can drive CLPs towards the DC fate.[20] However, these studies examined bulk CLPs, and thus the loss of Ly-6D+ CLPs during inflammation would bias the bulk CLP population towards the Ly-6D− CLPs that have broader differentiation capabilities. This would make it appear as if bulk CLPs have greater DC potential. In our report we separate the Ly-6D− and Ly-6D+ populations revealing their true B-cell and DC potentials.
The complex inflammatory environment induced by sustained TLR9 activation leads to distinct transcriptional programs in CLP populations. While both Ly-6D− and Ly-6D+ CLPs seem to be affected similarly by TLR9 and IFN-γ signals as evidenced by GSEA, there were some significant qualitative differences in their responses as identified by clustering analysis and GSEA. GSEA also revealed a loss of the B-cell specification program in both CLP populations without a change in the T-cell specification program. Based on the actions of Pax5 and other B-cell specification factors on T-cell fate but not myeloid fates, the net result of a loss of the B-cell program should cause an increase in T-cell potential with unaltered DC potential, just as we see in our results. Since we examined only CLPs in the bone marrow, it is possible that an enhanced T-cell specification program can only be seen when CLPs receive a strong Notch signal such as in the thymus. Interestingly, based on our results from the mixed TLR9 sufficient/deficient bone marrow chimeras in TLR9 deficient hosts, it appears that CLP extrinsic TLR9 signals prevent thymopoiesis perhaps by reducing available Notch signals.
Via both a bioinformatics approach and by examining transcript levels, we identified loss of HOXa9 and its indirect target EBF-1 as a potential pathway by which inflammation suppresses B-cell lymphopoiesis. It is interesting to note that NF-κB activation suppresses HOXa9 transcription in a number of different cell types,[38] consistent with a role for inflammation in this process. IFN-γ inhibits Il-7 responsiveness,[39] which in turn could lower EBF-1 levels.[29, 37] It is possible that these alterations in transcription represent either a greater shift in the CpG exposed Ly-6D− CLP population away from the B-cell lineage or the direct results of inflammation on individual cells.
Sustained TLR9 mediated inflammation can be seen in a number of clinical scenarios. It is intriguing to speculate that immuno-paralysis in sepsis, and B-cell autoimmunity in lupus might in part arise from dysfunctional B-cell lymphopoiesis due to TLR stimulation and cytokine release. Other hyperinflammatory scenarios where IFN-γ is a dominant inflammatory cytokine, such as HLH, have been associated with B-cell lymphopenias and suppression of genes important for B-cell lymphopoiesis.[4, 40] Indeed, when we enumerated bone marrow B-cell precursors in both the perforin and SAP deficient murine models of HLH infected with lymphocytic choriomenigitis virus, we saw precursors losses similar to our CpG model. These losses were not seen in wild type mice infected with lymphocytic choriomenigitis virus, suggesting that the hyperinflammation and not the infection itself, was important for the effect (unpublished observations). This work provides a possible explanation for some of the mechanisms that underlie dysfunctional B-cell lymphopoiesis in these hyperinflammatory syndrome settings.
In conclusion, we have shown that TLR9 induced hyperinflammation results in decreased B-cell lymphopoiesis in part via distinct IFN-γR and TLR9 pathways. We have also shown that CLPs exposed to the in vivo inflammatory environment demonstrate diminished capability to differentiate into B cells even when removed from the inflammation, possibly due to reduced levels of transcription factors important for B-cell development. Together these findings provide insights into the mechanisms that cause inflammation and hypercytokinemia to inhibit B-cell development. As specific cytokine blocking therapeutics continue to proliferate in clinical application, enhanced understanding of how cytokines regulate B-cell development in both homeostasis and inflammation will be important to predict both efficacy and toxicity of these agents during their use.
Materials and Methods
Mice
6 week to 12 week old mice were used throughout these studies. No differences in sex or age were noted across experiments. C57BL6, C57BL/6.SJL IFN-γKO and IFN-γRKO mice were obtained form Jackson Laboratories. TLR9KO and IFN-αRKO mice were used as previously described,[41, 42] and bred in our colony. Bone marrow chimeras were administered 950 rads of gamma irradiation followed by intravenous injection of 4–7 million bone marrow cells. Chimeric mice were allowed 10 weeks to rest before use in experiments. Mice were maintained at the University of Pennsylvania and Children’s Hospital of Philadelphia animal facilities. All procedures were performed with consent from institutional ethics boards.
Antibodies and flow cytometry
The lineage panel included antibodies against TCRβ, CD3, CD19, B220, NK1.1, CD11c, CD11b, GR-1, Ter119, FcεR, and CD8a. Aqua live-dead was used to visualize dead cells. All populations were first gated on forward and side scatter to limit dead cells and doublets. Samples were then gated as Aqua low for live cells. Antibodies were obtained from Biolegend, eBioscience, and BD Biosciences. Cells were run on LSRII/II or FACS Aria sorters at the University of Pennsylvania flow cytometry core. FACS data were analyzed using FlowJo software. Gating strategies for all of the flow cytometry panels can be found in Supporting Information Figures 1–6.
CpG model and isolation of samples
Mice were injected intraperitoneally on days 0, 2, 4, 7, and 9 with phosphate buffered saline (PBS), 50 mcg CpG 1826 DNA (synthesized by IDT), or 10 ng IFN-γ (peprotech) in 200 or 100 µl of volume. On day 10 mice were euthanized and organs were taken for analysis. Two tibias and femurs per mouse were flushed using a 27.5 gauge needle with cold PBS. To ensure full and equal removal of cells, bones were dissected open and any remaining cells were flushed out. Red blood cells were lysed using ammonium chloride-potassium bicarbonate lysis buffer.
OP9 in vitro culture assays
OP9 and OP9-DL1 stromal cells were used essentially as described [30, 31]. Both B- and T-cell cultures were supplemented with 10 ng/ml of IL-7, Flt3-ligand, and stem cell factor (peprotech). DC cultures were supplemented with 1 ng/ml of IL-7, 100 ng/ml Flt3-ligand, and 10 ng/ml stem cell factor. OP9 and OP9-dl1 cells were plated at 20,00 cells/ml on day -2 and irradiated with 25 grey gamma irradiation on day 0. Stromal cells rested for 2–6 hours before media was changed and 500 CLPs or 1000 Pro or Pre B cells were plated per well. Ly-6D+, Pro, and Pre B-cell co-cultures were allowed to grow untouched for 6–9 days and Ly-6D− co-cultures for 13–15 days. CpG was administered at 0.6 µg/ml and IFN-γ at 100 pg/ml to individual cultures. Cell numbers from OP9 experiments represent the number of events collected per volume per time for a given well to allow for comparison.
Statistical Analyses
Student’s t-tests were performed for all single comparisons. For data with multiple single comparisons, 1-way ANOVAs were performed with post-hoc Tukey’s test for pair-wise comparisons. For data comparing two independent variables, 2-way ANOVAs were performed using Prism software. The p-value for significance of the interaction coefficient was used to determine if one variable (e.g. treatment) acts differentially when in the presence of the second variable (e.g. genotype) and is therefore used to test whether a treatment acts in a cell intrinsic or extrinsic manner.
Affymetrix Microarray
Mice were treated with PBS (n=3) or CpG (n=3) as described above. On day 10 Ly-6D− and Ly-6D+ CLPs from each mouse were sorted directly into lysis buffer from the Qiagen RNAeasy Micro kit Plus using a FACS Ariaand purification was performed as per Qiagen protocol. Further analysis and processing of the RNA for quality, amplification and hybridization to the mouse Affymetrix ST 2.0 Chip were performed by the NAPcore at the Children’s Hospital of Philadelphia as previously described.[22] Microarray data have been uploaded to the Gene Expression Omnibus repository (GEO accession number GSE59762). The array dataset was modeled using a 2-way comparison of the Ly-6D− vs Ly-6D+ and PBS vs CpG. Principle component analysis was performed using Partek Genomics Suite. Genome-wide significant genes from the Ly-6D− PBS vs. CpG comparison were analyzed with the Ingenuity Pathway Analysis program using a cutoff of a p value less than 0.00001 and an absolute value Z score greater than or equal to 2. Gene Set Enrichment Analysis (GSEA) was performed using the publicly available software from the Broad Institute. Preranked unfiltered lists sorted by fold-change were generated comparing gene expression differences between PBS and CpG treatments for Ly-6D− and Ly-6D+ populations. 1000 Permutations over gene sets were performed for statistical testing. K-means clustering with k=10 was performed using the Genepattern platform (Broad Institute) with probes showing genome wide significant changes between any two groups with a false discovery rate of 20%. Intensity values were row normalized prior to clustering.
qRT-PCR
Mice were treated with PBS (n=3) or CpG (n=3) and RNA was isolated from sorted CLPs as described above. cDNA was made according to manufacturers instructions (Superscript III First-Strand Synthesis System, Invitrogen). qPCR was performed using the LifeTechnologies protocol. Primers were obtained from Applied Biosciences. Real time PCR reactions were done using an ABI700 sequence detection system and the 2XSYBR green master mix according to the manufacturer’s specifications. The delta-delta CT method was used to quantify results. Values for each gene were normalized to β-actin and one of the PBS treated samples for fold change comparison.
Supplementary Material
Acknowledgments
The authors would like to thank Michael P. Cancro and David M. Allman from the Department of Pathology at the University of Pennsylvania for their critical review of the manuscript and Martha Jordan, Taku Kambayashi, Avinash Bhandoola, Kim Nichols and Gary Koretzky for their support and helpful discussions. The authors also thank the NapCore at the Children’s Hospital of Philadelphia for their help running the microarray and technical support and the flow cytometry core at the University of Pennsylvania for technical help. EMB was funded by National Institute of NHLBI grant number RO1HL112836 and a Howard Hughes Medical Institute early career investigator award.
Abbreviations
- CLP
common lymphoid progenitor
- IFN-γ
Interferon gamma
- DC
dendritic cell
- pDC
plasmacytoid DC
- cDC
conventional DC
- IFN-γKO
Inteferon-gamma null
- IFN-γRKO
Interferon-gamma receptor null
- TLR9KO
TLR null
- GEO
Gene expression Omnibus
- IPA
Ingenuity Pathway Analysis.
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest
References
- 1.Behrens EM, Canna SW, Slade K, Rao S, Kreiger PA, Paessler M, Kambayashi T, et al. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest. 2011;121:2264–2277. doi: 10.1172/JCI43157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canna SW, Wrobel J, Chu N, Kreiger PA, Paessler M, Behrens EM. Interferon-gamma mediates anemia but is dispensable for fulminant toll-like receptor 9-induced macrophage activation syndrome and hemophagocytosis in mice. Arthritis Rheum. 2013;65:1764–1775. doi: 10.1002/art.37958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang M, Behrens EM, Atkinson TP, Shakoory B, Grom AA, Cron RQ. Genetic defects in cytolysis in macrophage activation syndrome. Curr Rheumatol Rep. 2014;16:439. doi: 10.1007/s11926-014-0439-2. [DOI] [PubMed] [Google Scholar]
- 4.Hinze CH, Fall N, Thornton S, Mo JQ, Aronow BJ, Layh-Schmitt G, Griffin TA, et al. Immature cell populations and an erythropoiesis gene-expression signature in systemic juvenile idiopathic arthritis: implications for pathogenesis. Arthritis Res Ther. 2010;12:R123. doi: 10.1186/ar3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biermann MH, Veissi S, Maueroder C, Chaurio R, Berens C, Herrmann M, Munoz LE. The role of dead cell clearance in the etiology and pathogenesis of systemic lupus erythematosus: dendritic cells as potential targets. Expert Rev Clin Immunol. 2014;10:1151–1164. doi: 10.1586/1744666X.2014.944162. [DOI] [PubMed] [Google Scholar]
- 6.Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 7.Simmons P, Kaushansky K, Torok-Storb B. Mechanisms of cytomegalovirus-mediated myelosuppression: perturbation of stromal cell function versus direct infection of myeloid cells. Proc Natl Acad Sci U S A. 1990;87:1386–1390. doi: 10.1073/pnas.87.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young HA, Klinman DM, Reynolds DA, Grzegorzewski KJ, Nii A, Ward JM, Winkler-Pickett RT, et al. Bone marrow and thymus expression of interferon-gamma results in severe B-cell lineage reduction, T-cell lineage alterations, and hematopoietic progenitor deficiencies. Blood. 1997;89:583–595. [PubMed] [Google Scholar]
- 9.Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock. 2010;34:55–59. doi: 10.1097/SHK.0b013e3181cd8c08. [DOI] [PubMed] [Google Scholar]
- 10.Allman D, Li J, Hardy RR. Commitment to the B lymphoid lineage occurs before DH-JH recombination. J Exp Med. 1999;189:735–740. doi: 10.1084/jem.189.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inlay MA, Bhattacharya D, Sahoo D, Serwold T, Seita J, Karsunky H, Plevritis SK, et al. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23:2376–2381. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansson R, Zandi S, Welinder E, Tsapogas P, Sakaguchi N, Bryder D, Sigvardsson M. Single-cell analysis of the common lymphoid progenitor compartment reveals functional and molecular heterogeneity. Blood. 2010;115:2601–2609. doi: 10.1182/blood-2009-08-236398. [DOI] [PubMed] [Google Scholar]
- 13.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borrow P, Hou S, Gloster S, Ashton M, Hyland L. Virus infection-associated bone marrow B cell depletion and impairment of humoral immunity to heterologous infection mediated by TNF-alpha/LTalpha. Eur J Immunol. 2005;35:524–532. doi: 10.1002/eji.200425597. [DOI] [PubMed] [Google Scholar]
- 16.Cain DW, Snowden PB, Sempowski GD, Kelsoe G. Inflammation triggers emergency granulopoiesis through a density-dependent feedback mechanism. PLoS One. 2011;6:e19957. doi: 10.1371/journal.pone.0019957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Q, Dong C, Cooper MD. Impairment of T and B cell development by treatment with a type I interferon. J Exp Med. 1998;187:79–87. doi: 10.1084/jem.187.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binder D, Fehr J, Hengartner H, Zinkernagel RM. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med. 1997;185:517–530. doi: 10.1084/jem.185.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YL, Chen TT, Pai LM, Wesoly J, Bluyssen HA, Lee CK. A type I IFN-Flt3 ligand axis augments plasmacytoid dendritic cell development from common lymphoid progenitors. J Exp Med. 2013;210:2515–2522. doi: 10.1084/jem.20130536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welner RS, Pelayo R, Nagai Y, Garrett KP, Wuest TR, Carr DJ, Borghesi LA, et al. Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection. Blood. 2008;112:3753–3761. doi: 10.1182/blood-2008-04-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canna SW, Costa-Reis P, Bernal WE, Chu N, Sullivan KE, Paessler ME, Behrens EM. Brief report: alternative activation of laser-captured murine hemophagocytes. Arthritis Rheumatol. 2014;66:1666–1671. doi: 10.1002/art.38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwin K, Dolence JJ, Shapiro MB, Medina KL. Differential requirement for Hoxa9 in the development and differentiation of B, NK, and DC-lineage cells from Flt3+ multipotential progenitors. BMC Immunol. 2013;14:5. doi: 10.1186/1471-2172-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gwin K, Frank E, Bossou A, Medina KL. Hoxa9 regulates Flt3 in lymphohematopoietic progenitors. J Immunol. 2010;185:6572–6583. doi: 10.4049/jimmunol.0904203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gwin KA, Shapiro MB, Dolence JJ, Huang ZL, Medina KL. Hoxa9 and Flt3 signaling synergistically regulate an early checkpoint in lymphopoiesis. J Immunol. 2013;191:745–754. doi: 10.4049/jimmunol.1203294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263–267. doi: 10.1038/376263a0. [DOI] [PubMed] [Google Scholar]
- 27.Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556–562. doi: 10.1038/44076. [DOI] [PubMed] [Google Scholar]
- 28.Pongubala JM, Northrup DL, Lancki DW, Medina KL, Treiber T, Bertolino E, Thomas M, et al. Transcription factor EBF restricts alternative lineage options and promotes B cell fate commitment independently of Pax5. Nat Immunol. 2008;9:203–215. doi: 10.1038/ni1555. [DOI] [PubMed] [Google Scholar]
- 29.Tsapogas P, Zandi S, Ahsberg J, Zetterblad J, Welinder E, Jonsson JI, Mansson R, et al. IL-7 mediates Ebf-1-dependent lineage restriction in early lymphoid progenitors. Blood. 2011;118:1283–1290. doi: 10.1182/blood-2011-01-332189. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt TM, Zuniga-Pflucker JC. T-cell development, doing it in a dish. Immunol Rev. 2006;209:95–102. doi: 10.1111/j.0105-2896.2006.00353.x. [DOI] [PubMed] [Google Scholar]
- 31.Cho SK, Webber TD, Carlyle JR, Nakano T, Lewis SM, Zuniga-Pflucker JC. Functional characterization of B lymphocytes generated in vitro from embryonic stem cells. Proc Natl Acad Sci U S A. 1999;96:9797–9802. doi: 10.1073/pnas.96.17.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu X, Chakravarty SD, Ivashkiv LB. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol Rev. 2008;226:41–56. doi: 10.1111/j.1600-065X.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiao Y, Giannopoulou EG, Chan CH, Park SH, Gong S, Chen J, Hu X, et al. Synergistic activation of inflammatory cytokine genes by interferon-gamma-induced chromatin remodeling and toll-like receptor signaling. Immunity. 2013;39:454–469. doi: 10.1016/j.immuni.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Ivashkiv LB. IFN-gamma abrogates endotoxin tolerance by facilitating Toll-like receptor-induced chromatin remodeling. Proc Natl Acad Sci U S A. 2010;107:19438–19443. doi: 10.1073/pnas.1007816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izon DJ, Rozenfeld S, Fong ST, Komuves L, Largman C, Lawrence HJ. Loss of function of the homeobox gene Hoxa-9 perturbs early T-cell development and induces apoptosis in primitive thymocytes. Blood. 1998;92:383–393. [PubMed] [Google Scholar]
- 36.Bain G, Maandag EC, Izon DJ, Amsen D, Kruisbeek AM, Weintraub BC, Krop I, et al. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994;79:885–892. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 37.Seet CS, Brumbaugh RL, Kee BL. Early B cell factor promotes B lymphopoiesis with reduced interleukin 7 responsiveness in the absence of E2A. J Exp Med. 2004;199:1689–1700. doi: 10.1084/jem.20032202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trivedi CM, Patel RC, Patel CV. Differential regulation of HOXA9 expression by nuclear factor kappa B (NF-kappaB) and HOXA9. Gene. 2008;408:187–195. doi: 10.1016/j.gene.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Garvy BA, Riley RL. IFN-gamma abrogates IL-7-dependent proliferation in pre-B cells, coinciding with onset of apoptosis. Immunology. 1994;81:381–388. [PMC free article] [PubMed] [Google Scholar]
- 40.Sumegi J, Barnes MG, Nestheide SV, Molleran-Lee S, Villanueva J, Zhang K, Risma KA, et al. Gene expression profiling of peripheral blood mononuclear cells from children with active hemophagocytic lymphohistiocytosis. Blood. 2011;117:e151–e160. doi: 10.1182/blood-2010-08-300046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leitner WW, Bergmann-Leitner ES, Hwang LN, Restifo NP. Type I Interferons are essential for the efficacy of replicase-based DNA vaccines. Vaccine. 2006;24:5110–5118. doi: 10.1016/j.vaccine.2006.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.