Abstract
Giang, Michael, Demosthenes G. Papamatheakis, Dan Nguyen, Ricardo Paez, Carla Blum Johnston, Joon Kim, Alexander Brunnell, Quintin Blood, Ravi Goyal, Lawrence D. Longo, and Sean M. Wilson. Muscarinic receptor activation affects pulmonary artery contractility in sheep: the impact of maturation and chronic hypoxia on endothelium-dependent and endothelium-independent function. High Alt Med Biol. 17:122–132, 2015.—Muscarinic receptor activation in the pulmonary vasculature can cause endothelium-dependent vasodilation and smooth muscle-dependent vasoconstriction. Chronic hypoxia (CH) can modify both of these responses. This study aimed to assess the combined influence of CH and maturation on endothelium-dependent and endothelium-independent muscarinic-induced vasoreactivity. This was accomplished by performing wire myography on endothelium-intact or endothelium-disrupted pulmonary arterial rings isolated from normoxic or CH fetal and adult sheep. In endothelium-intact arteries, vasodilation was evaluated using cumulative bradykinin doses in phenylephrine and carbachol precontracted pulmonary arterial segments; and vasoconstriction was examined using cumulative doses of carbachol following bradykinin predilation. Effects of nonselective (atropine) and selective M1 (pirenzepine), M2 (AFDX116), and M3 (4-DAMP and Dau5884) muscarinic receptor antagonists were assessed in disrupted arteries. In normoxic arteries, bradykinin relaxation was twofold greater in the adult compared to fetus, while carbachol contraction was fourfold greater. In adult arteries, CH increased bradykinin relaxation and carbachol contraction. In vessels with intact endothelium, maturation and CH augmented maximal response and efficacy for carbachol constriction and bradykinin relaxation. Approximately 50%–80% of adult normoxic and CH endothelium-disrupted arteries contracted to acetylcholine, while ∼50% of fetal normoxic and ∼10% of CH arteries responded. Atropine reduced carbachol-induced contraction in all vessels. Adult normoxic vessels were most responsive to M3 antagonism, fetal to M2 antagonism, while M1 inhibition had no effect. Overall, muscarinic-induced pulmonary arterial contraction is partially endothelium dependent and appears to develop after birth. Fetuses are more reliant on M3 receptors while M2 receptors predominate in adults, whereas CH augments muscarinic-dependent pulmonary vasoconstriction in both.
Key Words: : acetylcholine, contractility, hypoxia, maturation, muscarinic receptors
Introduction
The endothelium is critically important to the regulation of pulmonary arterial blood flow, as it participates actively in vasoreactivity. Endothelial activation is dependent upon the actions of multiple factors, including those from the autonomic nervous system, endocrine system, as well as paracrine factors derived locally (Haworth, 2006; Goldenberg and Kuebler, 2015). Neural control of the pulmonary vasculature is based on release of select neurotransmitters from either sympathetic or parasympathetic neurons. The former result in vasoconstriction, while the latter cause vasodilation (Haworth, 2006). Classically, and in most vessels, parasympathetic-induced vasodilation is thought to be mediated through the release of acetylcholine. In the lung, however, acetylcholine release from parasympathetic nerves is best known for causing bronchoconstriction (Semenov et al., 2012).
Muscarinic receptor (mAChR) activation in the pulmonary vasculature is widely accepted to cause dilation of vessels by stimulating the functional endothelium (Greenberg et al., 1987). mAChR activation of the endothelium reduces pulmonary arterial tension or vascular pressures in multiple animals, including mice, rats, and sheep in addition to humans (Storme et al., 1999; Peyter et al., 2008; Rowell et al., 2009; Pankey et al., 2014). Endothelial cell mAChR stimulation induces intracellular Ca2+ responses that activate multiple signaling pathways, including endothelial nitric oxide synthase (eNOS), phospholipase A2 (PLA2), and myoendothelial junctions (Norel et al., 1996; Walch et al., 2001; Papamatheakis et al., 2013).
The various signaling systems work in unison to cause endothelium-dependent relaxation. This relaxation is mediated through the generation of NO, prostacyclins, and direct modulation of the smooth muscle cell membrane potential (Norel et al., 1996; Walch et al., 2001). In humans and other species, however, mAChR activation can also elicit pulmonary artery constriction through activation of receptors found on vascular smooth muscle (Altiere et al., 1994; Norel et al., 1996; Walch et al., 1999). In this regard, a recent study from our group showed that muscarinic stimulation does not relax ovine fetal pulmonary arteries with functional endothelium (Xue et al., 2008).
The constrictor influences of mAChR activation and the neural innervation patterns are well described for bronchial smooth muscle. Interestingly, the mechanisms associated with neural growth are similar to those that govern vascular growth (Carmeliet and Tessier-Lavigne, 2005) and suggest parallelisms in their development. Recent evidence indicates that congenital diaphragmatic hernia leads to a decrease in parasympathetic neuronal innervation in the developing lung and airway branching abnormalities (Lath et al., 2012; Rhodes et al., 2015). Such reports suggest that maternal environmental stress may have similar impacts on fetal vascular and neuronal development.
Studies also show that disruption of endothelial and smooth muscle cell function by chronic hypoxia (CH) may cause loss of endothelium-dependent pulmonary vasorelaxation (Altiere et al., 1994; Shimoda et al., 2000b). Specifically, CH can suppress normal endothelium-dependent vasodilatory responses. This can be through an increase in endothelial production of endothelin-1 and conversion of angiotensin I to angiotensin II, as well as by an increase in smooth muscle reactivity to these vasoconstrictors (Shimoda et al., 2000a, 2000b).
Our previously published Ca2+ signaling and vasoreactivity findings led us to postulate that CH and maturation each would differentially modify mAChR-dependent pulmonary arterial vasoreactivity (Xue et al., 2008; Goyal et al., 2011; Papamatheakis et al., 2011, 2012; Hadley et al., 2012; Blood et al., 2013). The potential loss of endothelium-dependent pulmonary arterial vasodilation and acetylcholine-dependent vasoconstriction led us to test the hypothesis that mAChR vasoconstrictor responses predominate in the pulmonary vasculature of high-altitude sheep compared to normoxic controls. To address this hypothesis, we used wire myography and pharmacological profiling approaches to evaluate the role of various mAChR isoforms potentially involved in muscarinic-dependent pulmonary vasoreactivity.
Methods
Experimental animals
All experimental procedures were performed within the regulations of the Animal Welfare Act, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, “The Guiding Principles in the Care and Use of Animals” approved by the Council of the American Physiological Society, and the Animal Care and Use Committees of Loma Linda University (LLU) and the University of Mississippi. Many of the experimental procedures and protocols are based on our previously published methodology (Goyal et al., 2011; Papamatheakis et al., 2011, 2012, 2013; Blood et al., 2013). All animals were purchased from Nebeker Ranch (Lancaster, CA; 720 m elevation). The normoxic control animals were brought to LLU (353 m elevation; ewe arterial PaO2 = 95 ± 5 Torr) for experimental study. The chronic hypoxic experimental animals were acclimatized to high altitude (3801 m elevation, ewe PaO2 = 60 ± 5 Torr) at the Barcroft Laboratory, White Mountain Research Station (Bishop, CA), for ∼110 days (Longo et al., 1996; Goyal et al., 2011; Papamatheakis et al., 2012).
The hypoxic animals were then transported to LLU for experimental study. To maintain hypoxic conditions, a tracheal catheter was placed in the ewe shortly after arrival. The tracheal catheter allowed N2 to be delivered to the animal at a rate adjusted to maintain PaO2 at ∼60 Torr, which is equivalent to the PaO2 at the White Mountain Research Station (Kamitomo et al., 1993). This PaO2 was maintained until the time of the experimental study. Within 1–5 days of arriving at LLU, sheep were sacrificed with Euthasol (pentobarbital sodium 100 mg/kg and phenytoin sodium 10 mg/kg; Virbac, Ft. Worth, TX), a proprietary euthanasia solution.
Lungs were removed and used immediately for contractility experiments at LLU. In some of the early studies, tissues were shipped through overnight courier on ice to the University of Mississippi for experimental study. In vasodilation studies, special care was taken during arterial isolation and wire mounting to avoid endothelial disruption. In experiments in which pulmonary arterial contraction to muscarinic stimulation was studied, the endothelium was intentionally disrupted by inserting a wire through the arterial lumen and gently rotating, as previously described (Goyal et al., 2011; Papamatheakis et al., 2012; Blood et al., 2013).
Tissue preparation
Approximately fourth to fifth branch order pulmonary arteries with internal diameters of about 500–700 μm were isolated from normoxic or chronic hypoxic adult ewes or full-term fetuses of either sex that were 138–141 days of gestation. Typically, the pulmonary arterial rings were taken from a single lobe to reduce potential issues of regional heterogeneity. The lung parenchyma was removed from the pulmonary arteries for contractility studies. Arteries were then cut into 5 mm long rings in ice-cold phosphate-free balanced salt solution of the following composition (mM): 126 NaCl; 5 KCl; 10 HEPES; 1 MgCl2; 2 CaCl2; 10 glucose; pH 7.4 (adjusted with NaOH). All contraction studies were performed with a modified Krebs–Henseleit (K-H) solution containing the following in mM: 120 NaCl; 4.8 KCl; 1.2 K2HPO4; 25 NaHCO3; 1.2 MgCl2; 2.5 CaCl2; 10 glucose.
Contraction studies
Pulmonary arterial rings were suspended in organ baths (Radnoti Glass Instruments, Inc., Monrovia, CA) that contained 5 or 10 mL of modified K-H solution maintained at 37°C. Arteries in modified K-H were aerated with a 95% O2/5% CO2 gas mixture (pH = 7.4). Each arterial ring was suspended between two tungsten wires passed through the lumen. One wire was anchored to a glass hook at the bottom of the organ chamber while the other was connected to a tissue hook attached to a low compliance force transducer (Radnoti Glass Instruments, Inc.), which measured isometric force (Goyal et al., 2011; Papamatheakis et al., 2011, 2012; Hadley et al., 2012; Blood et al., 2013). The transducers were connected to an analogue to digital data interface (Digidata 1200/pCLAMP 8.1; Molecular Devices, Sunnyvale, CA; PowerLab 16/30 AD Instruments; Colorado Springs, CO; or MP100; BIOPAC Systems, Inc., Goleta, CA) attached to a computer. The changes in tension were recorded using pCLAMP 8.1 software (Molecular Devices) at the University of Mississippi, Chart 5.5 (AD Instruments) or AcqKnowledge 3.9 (BIOPAC Systems, Inc.) at LLU, and the obtained data were stored on magnetic media for later analysis.
At the beginning of each experiment, vessels were equilibrated without tension for a minimum of 30 minutes. Tension in the vessel rings was then increased to ∼0.75 g and allowed to equilibrate, as previously described (Goyal et al., 2011; Papamatheakis et al., 2011, 2012; Hadley et al., 2012; Blood et al., 2013). To allow for comparative evaluation of smooth muscle contraction and relaxation, isolated pulmonary arterial rings bathed in modified K-H were stimulated with 125 mM KCl (high-K solution) to cause membrane depolarization and subsequent L-type Ca2+ channel activation with contraction (Papamatheakis et al., 2012). In experiments of Figures 1C, 1D, 3C, 4, 5A, 5B, and 6–8, subsequent stimulations were normalized to a control response obtained with the high-K solution (%TKmax). To evaluate vessel reactivity responses of Figure 2A–D, the relaxation of pulmonary arteries to cumulative concentrations of bradykinin was examined in arterial segments precontracted with 10 μM phenylephrine (PE). In these experiments, the tension was normalized to the maximal PE-induced tension (%TPE), as denoted in each figure panel.
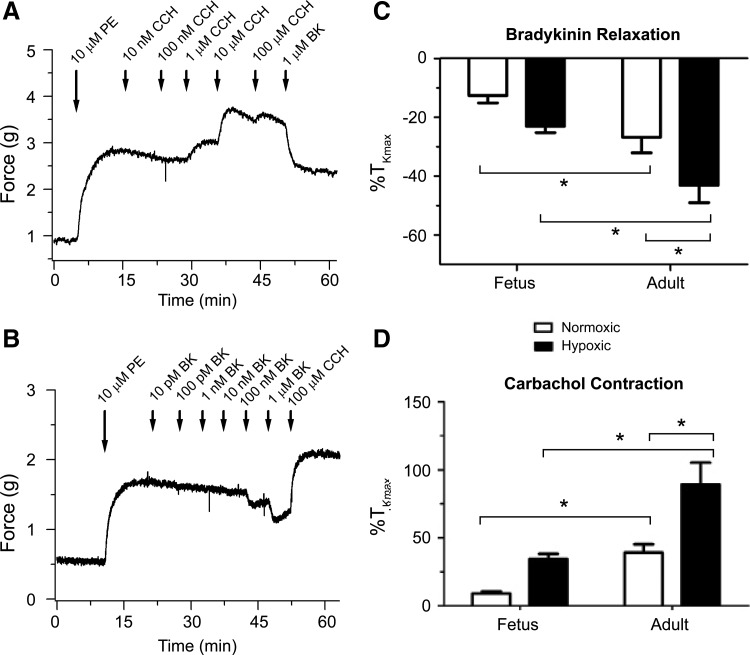
FIG. 1.
Bradykinin relaxes while carbachol contracts pulmonary arteries with an intact endothelium. Isometric tension recordings of adult normoxic pulmonary arterial rings stimulated with 10 μM phenylephrine (PE) and then contracted with cumulative concentrations of 10 nM–100 μM carbachol (CCh) followed by addition of 1 μM bradykinin (A) and relaxed with cumulative concentrations of 10 pM–1 μM bradykinin (BK) followed by addition of 100 μM CCH (B). Mean ± SEM of maximal contraction to 10 μM phenylephrine is shown for 1 μM BK addition after cumulative carbachol additions (C) and 100 μM CCh addition (D) after cumulative bradykinin additions. Statistical significance is noted between normoxic fetus and adult and hypoxic fetus and adult based on a two-way ANOVA with a Bonferroni post-test analysis (*p < 0.05). (C) Based on 12 adult normoxic arteries (4 animals), 14 adult hypoxic arteries (5 animals), 13 fetal normoxic arteries (4 animals), and 22 fetal hypoxic arteries (6 animals). (D) Based on 12 adult normoxic arteries (4 animals), 13 adult hypoxic arteries (5 animals), 13 fetal normoxic arteries (4 animals), and 14 fetal hypoxic arteries (5 animals). ANOVA, analysis of variance; SEM, standard error of the mean.
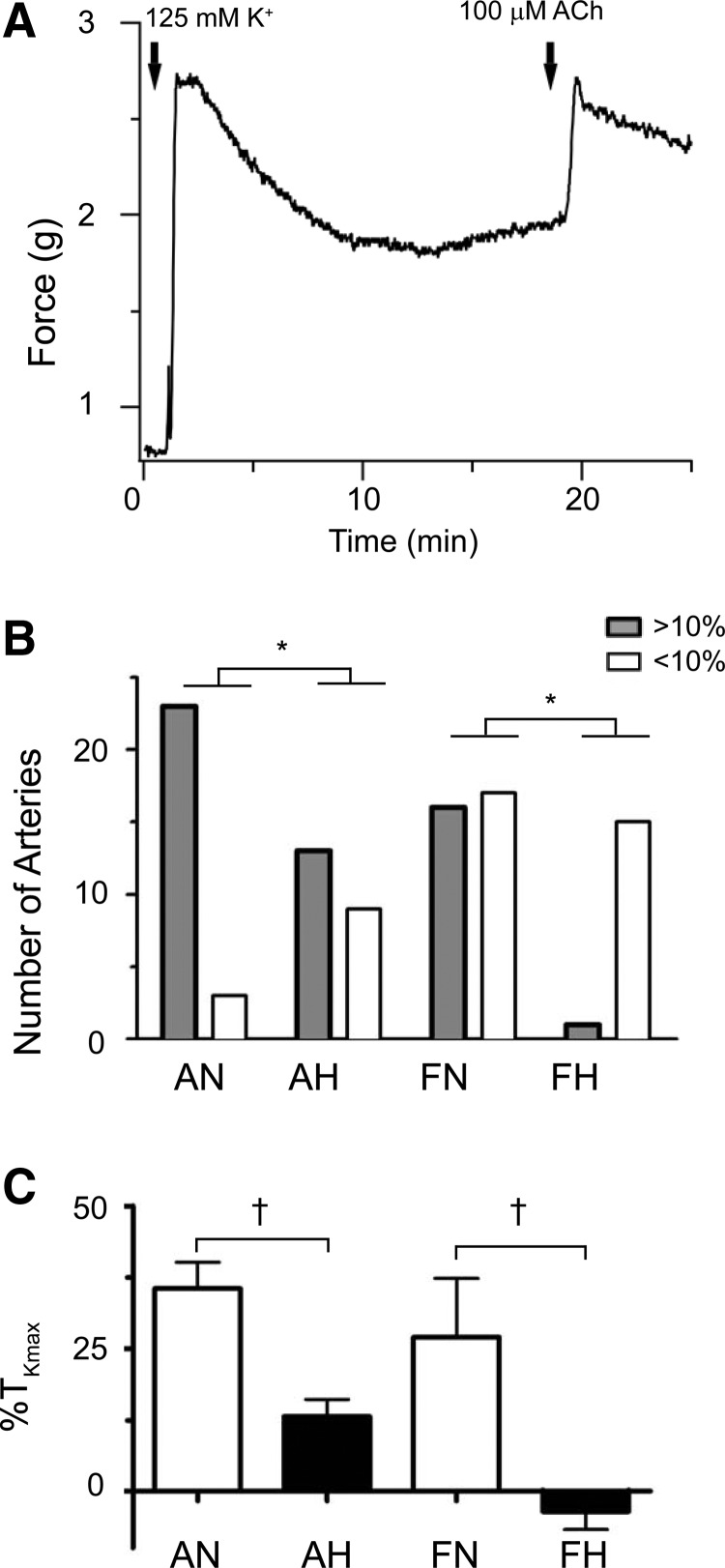
FIG. 3.
Chronic hypoxia decreases endothelium-disrupted pulmonary arterial responsiveness to acetylcholine in fetal sheep. Isometric tension recording of an adult normoxic pulmonary arterial ring (A) stimulated with 125 mM KCl (first solid arrow) and then followed by addition of 100 μM acetylcholine (ACh) (second solid arrow). (B) The number of arterial rings that contracted in response to ACh using 10% of maximal contraction attained from 125 mM KCl (%TKmax) as the cutoff point in differentiating between responders (>10%) and nonresponders (<10%) is shown. Responders are depicted as solid gray bars, while nonresponders are depicted by clear bars. A chi-square test was performed to evaluate for changes in the frequency of response with statistical significance noted (*p < 0.05). No difference was noted between adult normoxic and adult hypoxic groups. Bars in (C) depict mean ± SEM contraction of pulmonary arteries at 100 μM of ACh compared to %TKmax. Statistical significance is noted between normoxic and hypoxic group based on a Mann–Whitney U test (†p < 0.05). No differences were noted between the fetal and adult normoxic groups. Based on 26 adult normoxic arteries (5 animals), 33 adult hypoxic arteries (9 animals), 22 fetal normoxic arteries (6 animals), and 16 fetal hypoxic arteries (7 animals).
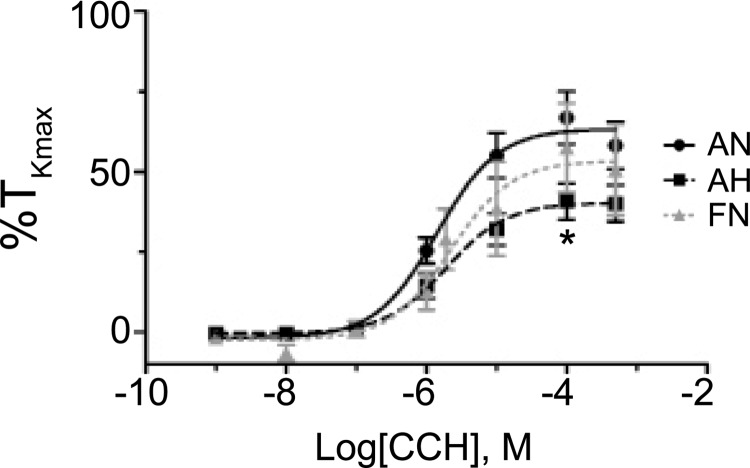
FIG. 4.
Chronic hypoxia decreases pulmonary arterial responsiveness to carbachol in adult sheep. Dose–response contraction curves of pulmonary arterial rings to 1 nm–100 μM carbachol (CCh) added in a cumulative manner normalized to %TKmax for adult normoxic (filled black circles, solid line), adult hypoxic (filled black squares, dashed line), and fetal normoxic (filled gray triangles, dashed line). Lines show resultant fits with a Hill equation to the dose–response relationships, and markers show mean ± SEM. The data were analyzed by two-way ANOVA with a Bonferroni post-test analysis for each dose compared to control. Statistical significance is noted between adult normoxic and adult hypoxic groups (*p < 0.05). No differences were noted between the fetal and adult normoxic groups. Data were not generated for the fetal chronic hypoxic group because of the low percentage of responsive vessels. This figure and control traces for subsequent figures are based on 49 adult normoxic arteries (16 animals), 23 adult hypoxic arteries (7 animals), and 15 fetal normoxic arteries (11 animals).
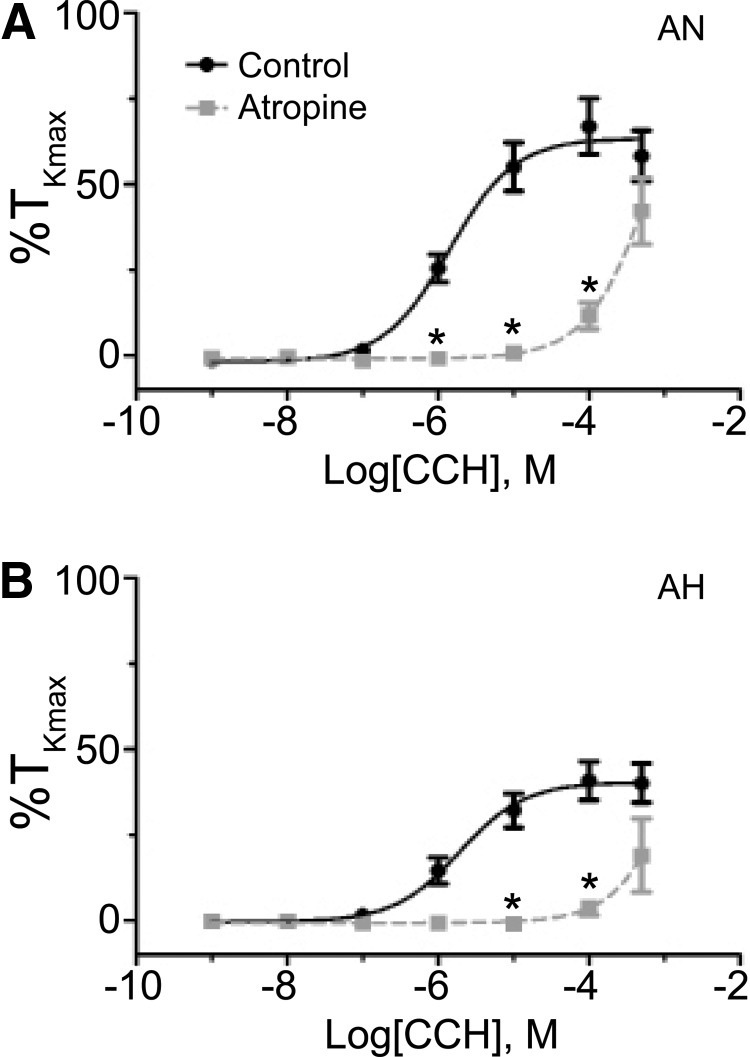
FIG. 5.
Muscarinic receptors are important to carbachol-induced pulmonary arterial contraction. Dose–response contraction curves of pulmonary arterial rings to 1 nm–100 μM carbachol (CCh) added in a cumulative manner normalized to %TKmax following addition of 1 μM atropine for adult normoxic (A) and adult hypoxic (B) pulmonary arteries. The control response is depicted with filled black circles and a solid line, while the atropine response is depicted with filled gray squares and a dashed line. Data are not shown for fetal normoxic arteries due to insufficient response. Lines show resultant fits with a Hill equation to the dose–response relationships, and markers show mean ± SEM. The data were analyzed by two-way ANOVA with a Bonferroni post-test analysis for each dose compared to control. Statistical significance is noted between control and atropine groups (*p < 0.05). Atropine data are based on 20 adult normoxic arteries (7 animals) and 3 adult hypoxic arteries (3 animals).
FIG. 6.
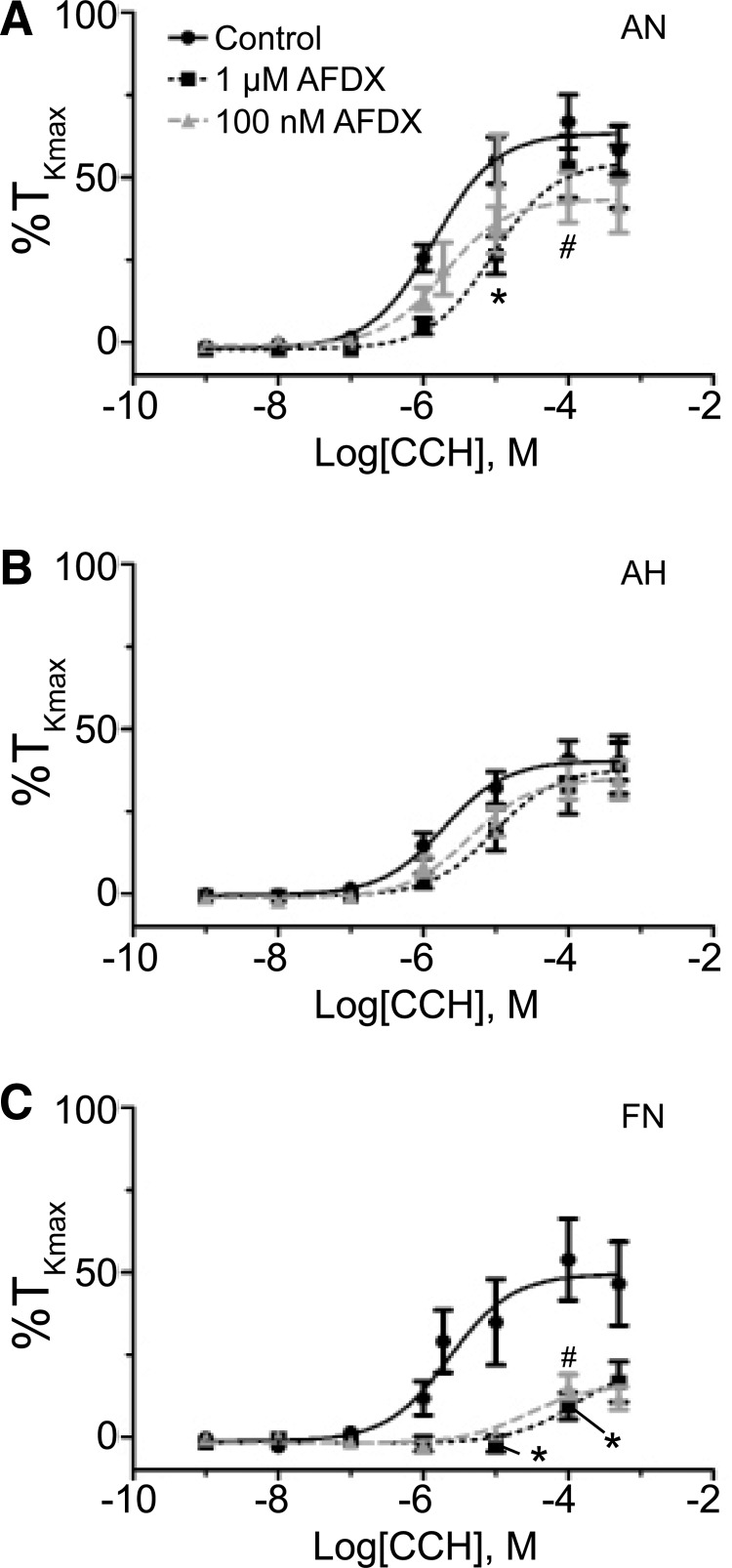
The M2 receptor is important to muscarinic-mediated contraction in pulmonary arteries from fetal and adult normoxic sheep. Dose–response curves of pulmonary arterial rings to 1 nm–100 μM carbachol (CCh) added in a cumulative manner normalized to %TKmax following addition of 100 nM or 1 μM of AFDX for adult normoxic (A), adult hypoxic (B), and fetal normoxic (C) animals. The control response is depicted with filled black circles with solid line. The 1 μM AFDX response is represented with filled black squares and a dashed line, while the 100 nM AFDX response is shown with filled gray triangles and a dashed line. Lines show resultant fits with a Hill equation to the dose–response relationships, and markers show mean ± SEM. The data were analyzed by two-way ANOVA with a Bonferroni post-test analysis for each dose compared to control. Statistical significance is noted between control and 1 μM AFDX group (*p < 0.05) and control and 100 nM AFDX group (#p < 0.05). AFDX adult normoxic data are based on 21 (100 nM) and 25 (1 μM) arteries (6 and 9 animals, respectively), adult hypoxic data are based on 12 (100 nM) and 17 (1 μM) arteries (3 and 6 animals, respectively), and fetal normoxic data are based on 8 (100 nM) and 12 (1 μM) arteries (4 and 5 sheep, respectively).
FIG. 7.
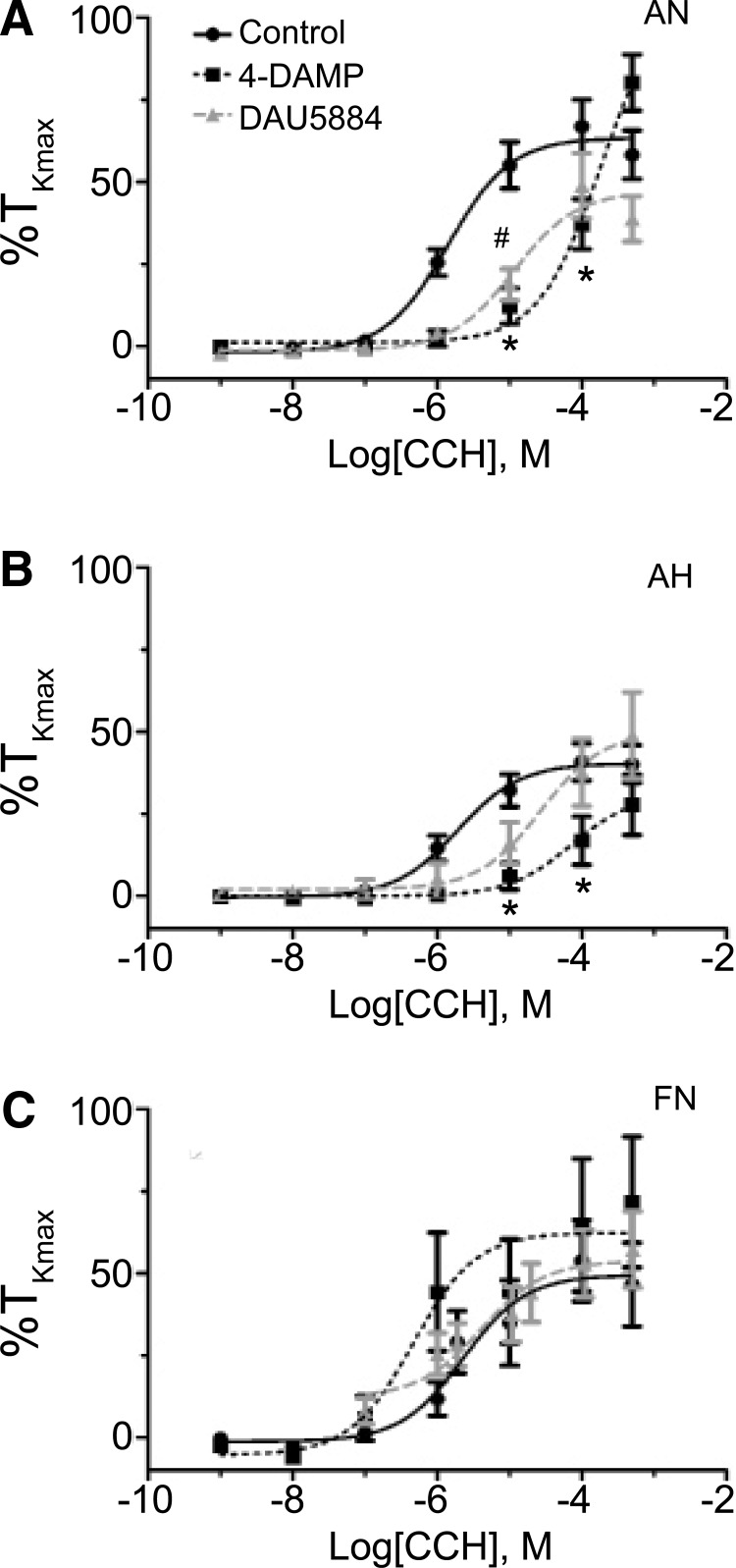
The M3 receptor is important to carbachol-mediated contraction in pulmonary arteries of normoxic and chronic hypoxic adult sheep, but not those from fetuses. Dose–response curves of pulmonary arterial rings to 1 nm–100 μM carbachol (CCh) added in a cumulative manner normalized to %TKmax following addition of 100 nM 4-DAMP or 10 μM DAU 5884 for adult normoxic (A), adult hypoxic (B), and fetal normoxic (C) animals. The control response is shown with filled black circles and a solid line. The 4-DAMP response is depicted with filled black squares and a dashed line, while the DAU 5884 response is illustrated with filled gray triangles and a dashed line. Lines show resultant fits with a Hill equation to the dose–response relationships, and markers show mean ± SEM. The data were analyzed by two-way ANOVA with a Bonferroni post-test analysis for each dose compared to control. Statistical significance is noted between control and 4-DAMP groups (*p < 0.05) or DAU 5884 (#p < 0.05). 4-DAMP data are based on 16 adult normoxic arteries (5 animals), 13 adult hypoxic arteries (5 animals), and 6 fetal normoxic arteries (3 animals), whereas Dau 5884 data are based on 21 adult normoxic arteries (6 animals), 8 adult hypoxic arteries (3 animals), and 13 fetal normoxic arteries (6 animals).
FIG. 8.
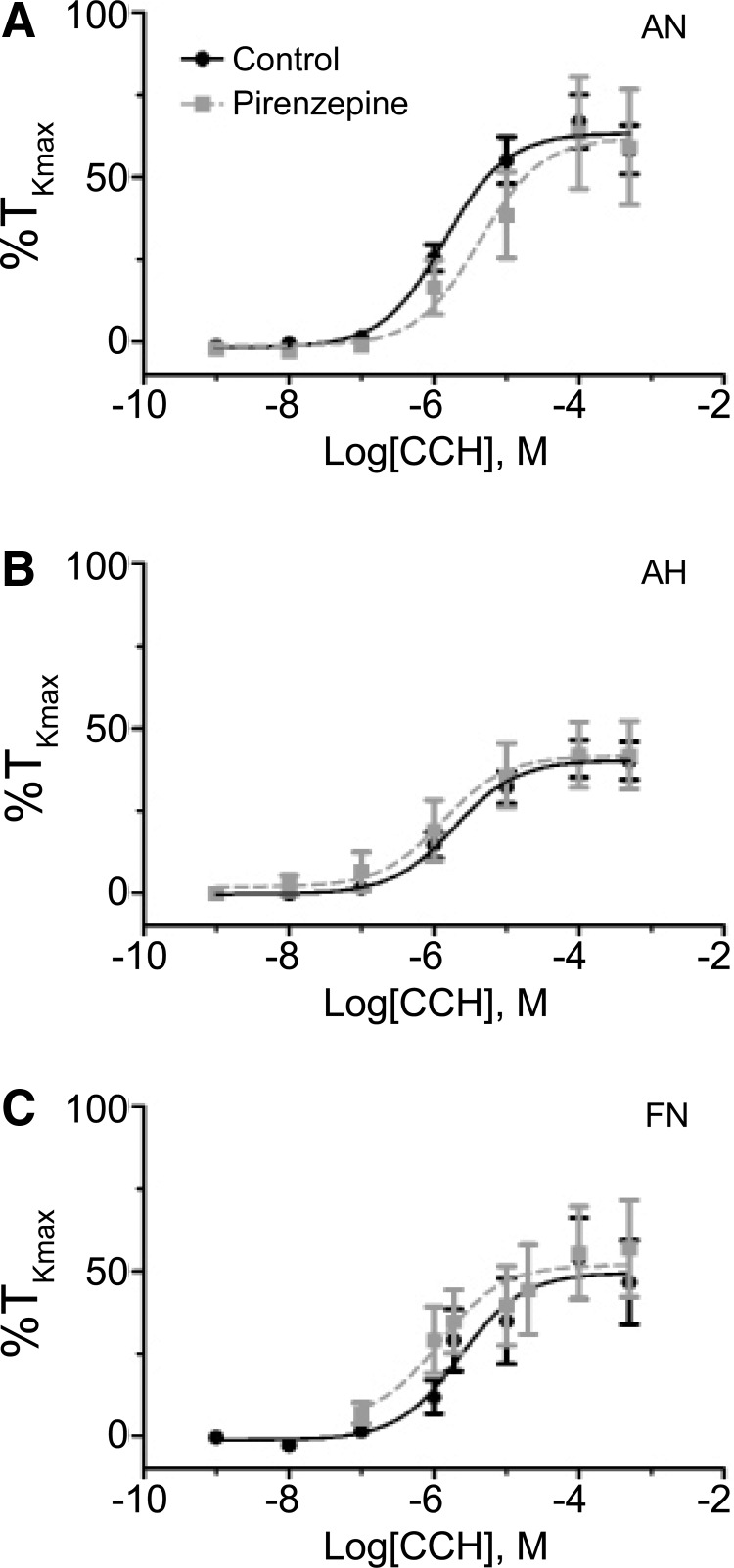
The M1 receptor is not important to carbachol-induced pulmonary arterial contraction. Dose–response curves of pulmonary arterial rings to 1 nm–100 μM carbachol (CCh) added in a cumulative manner normalized to %TKmax following addition of 100 nM pirenzepine for adult normoxic (A), adult hypoxic (B), and fetal normoxic (C) animals. The control response is depicted with filled black circles and a solid line, while the pirenzepine response is depicted with filled gray squares and a dashed line. Lines show resultant fits with a Hill equation to the dose–response relationships, and markers show mean ± SEM. The data were analyzed by two-way ANOVA with a Bonferroni post-test analysis for each dose compared to control. No significant differences were observed. PIR data are based on 16 adult normoxic arteries (5 animals), 14 adult hypoxic arteries (5 animals), and 14 fetal normoxic arteries (5 animals).
FIG. 2.
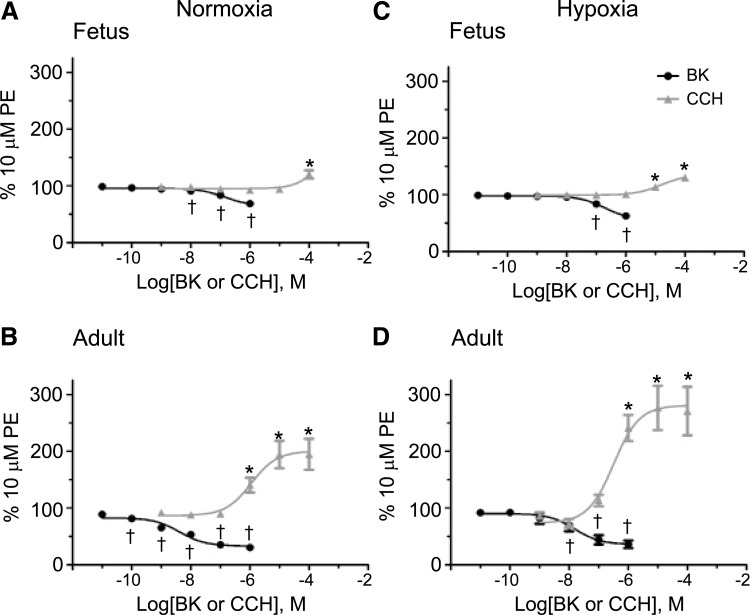
Maturation and chronic hypoxia modify bradykinin- and carbachol-induced pulmonary arterial reactivity. Dose–response curves of pulmonary arterial rings exposed to cumulative concentrations of 10 pM–1 μM bradykinin (BK) and 1 nM–100 μM carbachol (CCh) from normoxic fetuses (A) and adults (B) as well as chronic hypoxic fetuses (C) and adults (D). BK addition is documented by solid black circles and CCh addition by solid gray triangles. Lines show resultant fits with a Hill equation to the dose–response relationships and markers show mean ± SEM. The data were analyzed by two-way ANOVA with a Bonferroni post-test analysis for each dose compared to control. Statistical significance is noted between bradykinin baseline (10 pM) and other concentrations (†p < 0.05) and between carbachol baseline (1 nM) and other concentrations (*p < 0.05).
Bradykinin dose-dependent relaxation was evaluated using cumulative concentrations of drug from 10 pM to 1 μM. Alternatively, dose–response contraction characteristics were examined in phenylephrine precontracted arteries that were stimulated with cumulative concentrations of carbachol from 1 nM to 100 or 500 μM in log dose increments, without washing between each concentration increase (Blood et al., 2013).
A total of 536 arteries were used for these experiments. The number of animals and arteries used for these studies is provided in the figure legends and tables.
Chemicals and drugs
Unless otherwise noted, reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Specific muscarinic isoform receptor antagonists were purchased from Tocris Bioscience (Minneapolis, MN) (Eglen et al., 1996). This includes pirenzepine (PIR), a selective M1 muscarinic receptor antagonist (Norel et al., 1996); AFDX 116 (11-[[2-[(Diethylamino)methyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one), a selective M2 muscarinic receptor antagonist (Altiere et al., 1994); and 4-DAMP (1,1-Dimethyl-4-diphenylacetoxypiperidinium iodide) as well as Dau 5884 (8-Methyl-8-azabicyclo-3-endo[3.2.1] oct-3-yl-1,4-dihydro-2-oxo-3(2H)-quinazolinecarboxylic acid ester hydrochloride) that are selective M3 muscarinic receptor antagonists (Gosens et al., 2003).
Statistical methods
All time series recordings were graphed with IGOR pro 6.0 (Wavemetrics, Lake Oswego, OR), and the data presented as mean ± standard error of the mean. Statistical analyses were made using GraphPad Prism 5.0 (La Jolla, CA). Data were evaluated for normality before any comparative statistical analysis. Statistical difference between Gaussian distributed groups was determined with a two-tailed unpaired Student's t-test. A Mann–Whitney U test was used for comparisons of non-Gaussian data. For contractility studies, comparisons were made within and among groups using a two-way analysis of variance (ANOVA) with a Dunnett's post-hoc analysis, one-way ANOVA with a Bonferroni post-hoc analysis, and with chi-square analysis to examine for changes in the frequency of response, as appropriate, and denoted in the figure legends. Dose–response curves were fitted in Prism 5.0 using a Hill equation (Goyal et al., 2011; Papamatheakis et al., 2011, 2012; Hadley et al., 2012; Blood et al., 2013). The N values reported reflect the total number of arterial segments tested. A p-value of <0.05 was accepted as statistically significant.
Results
The first series of studies were designed to determine the extent to which there were fundamental effects of maturation or CH on carbachol-induced pulmonary arterial contraction when the endothelium was intact. We also made comparisons to bradykinin-induced relaxation to demonstrate the functionality of the endothelium under conditions in which carbachol elicited vasoconstriction. Specifically, we examined whether bradykinin would induce relaxation after carbachol-induced stimulation, and conversely, whether carbachol would induce contraction after bradykinin-induced relaxation. Results of these studies, which also confirm endothelial integrity, are shown in Figures 1 and 2.
Representative myography traces from pulmonary arteries of adult normoxic sheep contracted with 10 μM PE are shown in Figure 1A and B. These traces document bradykinin-induced relaxation in the setting of carbachol prestimulation of pulmonary arteries (Fig. 1A), as well as carbachol-mediated contraction when arteries were pretreated with bradykinin (Fig. 1B). Figure 1C shows that the maximum response of bradykinin-induced relaxation in the setting of carbachol prestimulation increases with maturation in normoxic animals, and with CH in adult sheep. More specifically, in adult vessels, bradykinin-induced relaxation of vessels prestimulated with carbachol was approximately twofold greater compared to the fetus in normoxic (−13% ± 3% TKmax, n = 13 in fetus vs. −27% ± 5% TKmax, n = 12 in adult) and hypoxic groups (−23% ± 2% TKmax, n = 22 in fetus vs. −43% ± 6% TKmax, n = 14 in adult). When considering CH, bradykinin-induced relaxation was statistically different only in the adult, with ∼1.5-fold greater relaxation in hypoxic animals compared to their normoxic counterparts.
Bradykinin-induced relaxation was not significantly enhanced in fetal hypoxic compared to fetal normoxic arteries. Figure 1D shows the maximum response of carbachol-induced pulmonary arterial contraction following bradykinin treatment and highlights similar findings as above. Maturation increased carbachol contraction approximately fourfold in normoxic (9% ± 1% TKmax, n = 13 in fetus vs. 39% ± 6% TKmax, n = 12 in adult) as well as hypoxic arteries (34% ± 3% TKmax, n = 14 in fetus vs. 89% ± 16% TKmax, n = 13 in adult). Moreover, CH more than doubled carbachol-induced contractility in adult arteries. CH did not increase carbachol-mediated contraction in the fetus. Overall, these data confirm that even in the presence of an intact endothelium, mAChR activation will contract pulmonary arteries and that the magnitude of this contraction is increased by maturation as well as CH in the adult.
Figure 2 shows the dose–response relationship and associated potency and maximum response of carbachol-induced contraction and bradykinin-mediated relaxation in fetal and adult pulmonary arteries with an intact endothelium. As shown, maturation impacts both constriction with carbachol and relaxation with bradykinin in phenylephrine precontracted pulmonary arteries regardless of oxygenation status. Adult normoxic arteries constricted significantly at 1 μM carbachol, while fetal normoxic arteries did not show a response until 100 μM. In addition, chronically hypoxic fetal arteries exhibited significant constriction at 10 μM carbachol. Interestingly, both normoxic and hypoxic adult animals contracted in response to carbachol, with potency shifted to the left compared to the fetuses, responding substantially at 1 μM carbachol. The vasodilatory responses to bradykinin follow a similar pattern, as described for constriction due to carbachol. Adult pulmonary arteries typically responded at lower doses compared to those from the fetus. This difference was more pronounced in normoxic conditions, with adult pulmonary arteries responding at 100 pM bradykinin and fetal pulmonary arteries responding at 10 nM bradykinin. A similar but less pronounced difference was noted in CH, where significant bradykinin-induced relaxation in fetuses was observed at 100 nM compared to 10 nM in adults. Table 1 summarizes the maximum response and potency values for the dose–response curves depicted in Figure 2.
Table 1.
Potency and Maximum Response (EMax) of Carbachol-Induced Pulmonary Arterial Contraction and Bradykinin-Induced Pulmonary Arterial Relaxation in Vessels with Intact Endothelium from Adult and Fetuses in Both Normoxic and Hypoxic Conditions
| Carbachol | Bradykinin | |||||
|---|---|---|---|---|---|---|
| Intact endothelium | Potency | EMax | N | Potency | EMax | N |
| AN | −5.95 ± 0.29 M | 200 ± 13% | 14 | −8.38 ± 0.20 Ma | 33% ± 3%a | 16 |
| AH | −6.49 ± 0.26 M | 281 ± 18%b | 9 | −7.80 ± 0.30 Ma | 36% ± 7%a | 17 |
| FN | ND | ND | 13 | −6.86 ± 0.25 M | 65% ± 5% | 14 |
| FH | −4.80 ± 0.18 Mc | 135 ± 4%c | 22 | −6.71 ± 0.19 M | 56% ± 5% | 27 |
Potency values are in Log Molar concentration values, while EMax is in %TKMax. ND as the nonlinear curve fit was unable to fit the data properly. N is the number of arteries examined for each group.
p < 0.05, Significantly different from fetal counterpart by a two-way analysis of variance with a Bonferroni's Multiple Comparison Test.
p < 0.05, Different from AN by a one-way analysis of variance with a Bonferroni's Multiple Comparison Test.
p < 0.05 Significantly different from AN and AH groups.
AH, adult hypoxic; AN, adult normoxic; FH, fetal hypoxic; FN, fetal normoxic; ND, not determined.
After determining that carbachol contracts arteries with a functional endothelium, we then examined the direct action of muscarinic stimulation on smooth muscle per se. This was investigated by measuring the contractile response to 100 μM acetylcholine in arteries with disrupted endothelium that were depolarized with 125 mm KCl. In these studies, the percentage of arteries responding more than 10% of the maximal contraction due to 125 mM KCl was taken as being reactive with representative traces and summary data presented in Figure 3.
Figure 3A shows a representative trace of ACh-induced contraction for an adult normoxic vessel. Figure 3B shows that overall in the adult normoxic group, vasoconstrictive responses were noted in 88% (23 out of a total of 26 arteries) and in 48% (16 of 33) of the adult hypoxic group. In the fetal normoxic group, 59% (13 of 22) responded, and 6% (1 of 16) responded in the fetal hypoxic group. Figure 3C shows the average response to 100 μM acetylcholine in reference to the average response due to 125 mM KCl in each of the four groups. This was 36% ± 5% TKmax in adult normoxic (n = 26), 13% ± 3% TKmax in adult hypoxic (n = 33), 27% ± 10% TKmax in fetal normoxic (n = 22), and −4% ± 3% TKmax in fetal hypoxic (n = 16) pulmonary arteries. Since both the number of disrupted fetal hypoxic arteries responding to carbachol and the magnitude of the response were extremely low, the fetal hypoxic group was excluded from subsequent experiments.
The next series of experiments were designed to determine the maximum response and potency of the contraction response to carbachol in endothelium-disrupted vessels. With regard to potency, Figure 4 and Table 2 show that carbachol-induced contraction was similar among the fetal normoxic, adult normoxic, and adult hypoxic groups. The maximum response in comparison, as shown in Figure 4 and Table 2, illustrates that carbachol-induced contraction in the adult normoxic group was not significantly different than the fetal normoxic group, but was significantly greater than that of the adult hypoxic group.
Table 2.
Potency and Maximum Response (EMax) of Carbachol-Induced Pulmonary Arterial Contraction in the Presence and Absence of Atropine (Nonselective), or AFDX 116 (M2 at 100 nM or M1 and M3 at 1 μM Selective), 4-DAMP or DAU 5884 (M3 Selective), or Pirenzepine (M1 Selective) Muscarinic Receptor Antagonists
| AN | AH | FN | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Denuded vessels Animal group | Potency | EMax | N | Potency | EMax | N | Potency | EMax | N |
| CCh | −5.85 ± 0.16 M | 63% ± 4% | 49 | −5.72 ± 0.2 M | 40% ± 3% | 23 | −5.64 ± 0.35 M | 49% ± 7% | 17 |
| CCh + 1 μM Atropine | −3.10 ± 0.56 M | ND | 19 | −5.72 ± 0.2 M | ND | 19 | NA | NA | |
| CCh + 100 nM AFDX 116 | −5.70 ± 0.23 M | 43% ± 3%a | 21 | −5.36 ± 0.22 M | 34% ± 3% | 12 | −4.56 ± 0.31 Ma | 16% ± 3%a | 8 |
| CCh + 1 μM AFDX 116 | −5.04 ± 0.19 Ma | 55% ± 5% | 25 | −5.02 ± 0.27 M | 42% ± 5% | 17 | −3.92 ± 0.42 Ma | 36% ± 8%a | 12 |
| CCh + 10 μM 4-DAMP | −3.73 ± 0.16 Ma | 109% ± 15% | 16 | −4.14 ± 0.42 M | 31% ± 8% | 13 | −6.33 ± 0.54 M | 62% ± 10% | 6 |
| CCh + 100 nM DAU5884 | −4.94 ± 0.22 Ma | 47% ± 5% | 21 | −4.56 ± 0.35 M | 50% ± 8% | 8 | −5.39 ± 0.42 M | 54% ± 6% | 12 |
| CCh + 100 nM Pirenzepine | −5.38 ± 0.36 M | 62% ± 9% | 16 | −5.88 ± 0.38 M | 42% ± 5% | 14 | −5.92 ± 0.52 M | 52% ± 7% | 14 |
Indicates statistical significance, p < 0.05 relative to control by a one-way analysis of variance with a Bonferroni's Multiple Comparison Test. Maximum response is relative to the %TKmax. N is the number of arteries used for the studies.
NA, not applicable.
Subsequently, we evaluated suppression of carbachol-mediated contraction when all three mAChR subtypes were inhibited. This was performed by examining the ability of atropine to block carbachol contraction, with comparisons made to the control data shown in Figure 5. Atropine was chosen because it is a well-documented muscarinic antagonist that blocks all mAChR isoforms (Eglen et al., 1996; Caulfield and Birdsall, 1998). Figure 5 shows that 1 μM atropine significantly disrupted carbachol-induced contractility in adult normoxic as well as hypoxic pulmonary arteries by decreasing potency, with data summarized in Table 2. Unfortunately, too few fetal normoxic arteries responded to carbachol in the presence of atropine to allow for proper analysis, and thus, these data are not shown. Moreover, the magnitude in the shift in potency made it such that the changes in maximum response could not be adequately evaluated because the resultant curve fits failed to plateau. Nevertheless, these data illustrate that atropine greatly reduced carbachol-induced contractility in disrupted pulmonary arteries.
Based on the results of nonselective mAChR blockade with atropine, the next series of studies focused on delineating the roles of specific mAChR isoforms (M1, M2, and M3) in the contractile response. The M2 receptor subtype was blocked with 100 nM AFDX 116 and this was compared to 1 μM AFDX 116 that purportedly can also block M1 as well as M3 receptors (Hammer et al., 1986; Brown et al., 2013). Figure 6A and B and Table 2 show that the potency of carbachol-induced arterial contraction was not significantly affected by the presence of 100 nM AFDX 116, in both normoxic and hypoxic adult sheep. In adult normoxic (Fig. 6A) but not in adult hypoxic arteries (Fig. 6B), the potency to carbachol was shifted to the right by 1 μM AFDX 116. Table 2 shows that the maximum response for contraction was also not significantly affected, regardless of the AFDX 116 concentration, in both adult normoxic and hypoxic arteries.
In comparison to the lack of effect in adult vessels, Figure 6C shows that both concentrations of AFDX 116 decreased the potency for carbachol-induced contractility in fetal normoxic vessels. Table 2 shows that although 100 nM AFDX 116 shifted the potency by ∼1-log unit to the right, this did not reach statistical significance. However, 1 μM AFDX 116 shifted the potency of carbachol by nearly 2-log units. Maximum response on the contrary was lowered by addition of either 100 nM AFDX 116 or 1 μM AFDX 116. Overall, these data suggest a substantial role for M2 receptors in fetal normoxic vessels, but a far lesser role in vessels from adults.
We then focused on examining the specific role of M3 receptors to carbachol-mediated contraction. M3 receptors were blocked with two structurally distinct antagonists, 10 μM DAU 5884 and 100 nM 4-DAMP. We chose to use two antagonists, so as to mitigate any potential nonspecific effects of each antagonist on other mAChR (Gosens et al., 2003).
Figure 7 indicates that both DAU 5884 and 4-DAMP decreased the potency for carbachol-induced contractility in adult normoxic vessels (Fig. 7A) shifting the curve to the right by ∼1- and 2-log units, respectively. Figure 7B and Table 2 show that M3 receptor inhibition with 4-DAMP caused modest, but statistically significant, potency shifts in adult hypoxic sheep, although this was not observed with DAU 5884. Moreover, M3 muscarinic receptor inhibition failed to impact reactivity of pulmonary arteries from fetal normoxic sheep (Fig. 7C and Table 2). In general, the maximum response for carbachol-induced contraction was not affected in any experimental group (Fig. 7 and Table 2).
For the last series of studies, we examined the role of M1 receptors by treating pulmonary arteries with 100 nM pirenzepine, a selective M1 muscarinic receptor antagonist (Altiere et al., 1994; Norel et al., 1996). Interestingly, pirenzepine failed to affect carbachol-induced contractility when compared to the control data, as shown in Figure 8 and Table 2, in any of the experimental groups.
Discussion
These experiments are the first to examine the combined influence of maturation and CH on mAChR-induced vasoreactivity in pulmonary arteries of fetal and adult sheep. The primary findings of this study indicate that mAChR activation causes pulmonary arterial contraction (Figs. 1 and 2), and that a portion of this contraction is dependent on a functional pulmonary endothelium.
Our experiments support the concept that mAChR-mediated contractility develops after birth, with somewhat larger responses in the adult compared to the fetus (Fig. 3). Our data also illustrate a mAChR isoform switch from the M2 isoform dominating in the fetus to the M3 isoform dominating in the adult, with the M1 receptor not appearing to play a significant role (Figs. 4–8). Finally, we show that CH dramatically augments mAChR—contractility in vessels with functional endothelium of both the fetus and adult (Fig. 2), an effect that appears to offset CH-induced losses in direct smooth muscle contraction (Fig. 3).
The matching of ventilation and perfusion is an integral aspect of respiratory physiology, with both airway and vascular reactivity playing active roles. Airway caliber is largely regulated through parasympathetic innervation, and muscarinic-dependent responses are central to this process (Gosens et al., 2003). Based on the structural and functional relationships between the airways and the pulmonary vasculature, the natural response would be that an increase in airway contraction and subsequent decrease in alveolar ventilation would cause a coordinated reduction in perfusion to these regions (Sylvester et al., 2012; Swenson, 2013).
Many studies have focused on the intrinsic ability of pulmonary arteries to constrict in response to hypoxia and this is regarded as central in the matching of ventilation to perfusion (Sylvester et al., 2012; Swenson, 2013). Potentially, muscarinic-dependent pulmonary arterial contractility could provide a secondary mechanism to regulate vascular perfusion and match alveolar ventilation to perfusion. In this regard, parasympathetic nerves that stimulate airway contraction could simultaneously contract the arteries, coordinating a reduction in vascular perfusion. One functional implication of parasympathetic-induced contraction of the pulmonary vasculature is that inhibiting muscarinic receptor activity may provide a novel therapeutic avenue for enhancing both ventilation and perfusion by simultaneously relaxing pulmonary arteries and airways.
Our data indicate that the role of muscarinic receptors in the regulation of pulmonary vascular contractility begins to develop prenatally, but this process continues after birth, with adults having greater responses than fetuses. There are two components to this postnatal increase in responsiveness: one, a larger percentage of adult arteries respond to muscarinic receptor stimulation, and two, adult arteries have an increased maximal contraction when compared to arteries from the fetus. Although there is rich neuronal innervation in fetal lungs of a number of species, the primary focus of previous studies has been on airway innervation (Sparrow et al., 2003; Buels and Fryer, 2012; Aven and Ai, 2013). In addition, despite evidence for cholinergic innervation of the vasculature (Haberberger et al., 1997), much less is known about its role in pulmonary vascular function.
There is the possibility that there may be postnatal maturation of parasympathetic nerve function, which then leads to enhanced pulmonary vascular reactivity. From a functional perspective, delaying maturation of muscarinic pulmonary artery contraction until after birth may help the newborn negotiate the transition period of delivery, where maximal oxygen uptake is needed to meet metabolic demands of the brain and other tissues. In the adult, the need for muscarinic innervation of the pulmonary vasculature may simply be another mechanism to facilitate the matching of ventilation to perfusion.
CH-related suppression of the development of muscarinic contractile responses in endothelium-disrupted arteries of the fetus and enhanced reactivity in endothelium-intact arteries are novel findings. The latter suggests that muscarinic receptor stimulation of the endothelium may induce vasoconstrictor pathways, although a portion of the increased reactivity to carbachol in these vessels may be due to phenylephrine-induced sensitization and pretone (Sylvester et al., 2012). Nevertheless, the endothelium-dependent pulmonary arterial contraction may involve release of relevant vasoconstrictors from the endothelium. The CH-related diminished reactivity of disrupted fetal arteries parallels the lessening of contraction in hypoxic arteries of the adult. Discovering that the responses of fetuses and adults to CH are similar suggests there may be common adaptation mechanisms to long-term low arterial oxygen tensions.
The fact that the chronically hypoxic fetus is subjected to lower oxygen tensions through the maternal circulation, and is not breathing, leads to the premise that these adjustments may represent preparation for birth in a rarified environment. Alternatively, these changes may be compensatory processes to offset enhanced endothelium-dependent contraction. Although the CH-induced loss in direct smooth muscle contraction in response to carbachol did not fully counterbalance the augmentation in endothelium-dependent contraction, potentially blunted muscarinic receptor-induced contractility in the newborn could help maintain low vascular tone in the early postnatal period. This would promote pulmonary blood flow and improve oxygen uptake.
The abbreviated muscarinic-dependent pulmonary arterial contraction in the adult may serve a similar purpose, helping to restrain CH-induced development of pulmonary hypertension that occurs in many species, including humans. Weakened muscarinic-mediated contraction could help prevent exacerbated hypoxia-induced vasoconstrictor responses, which would maintain adequate pulmonary blood flow in the chronically hypoxic adult. Conceivably, this would restrict right ventricular afterload and lessen right ventricular dysfunction. Reduced muscarinic receptor contraction could therefore help protect the individual from right ventricular hypertrophy and progressive right heart failure.
The finding that contraction of fetal smooth muscle cells is largely mediated by M2 receptors while M2 and M3 receptors are important in the adult is novel. Previous studies have shown that isolated adult human pulmonary arteries have only M3 receptors on smooth muscle cells and that M2 receptors are not important (Norel et al., 1996). Similarly, adult rabbit pulmonary arteries primarily contract in response to M3 stimulation (Altiere et al., 1994). The similarities in sheep and human lung development suggest that contraction of pulmonary arteries in prenatal infants may also depend on M2 receptors. If this is true, antagonism of M2 receptors could present a new therapeutic target to provide relief from various forms of pulmonary hypertension in the newborn. One limitation to this pharmacological analysis is that there is the potential for off-target effects. As noted, AFDX can block multiple muscarinic receptor isoforms in a dose-dependent manner (Hammer et al., 1986; Brown et al., 2013). Similarly, the other antagonists used in the present studies may also block more than one muscarinic receptor isoform, with various degrees of efficacy and potency (Gosens et al., 2003).
This study provides in vitro evidence that muscarinic receptor activation on the endothelium as well as smooth muscle may be important to ventilation–perfusion matching in the lung, building from previous studies in animals and humans (Sylvester et al., 2012; Swenson, 2013). Our studies on chronically hypoxic animals suggest that muscarinic receptor antagonists could be therapeutically important, allowing for improved pulmonary perfusion in neonatal and adult patients with pulmonary hypertension. Moreover, our in vitro observations indicate that M2 antagonists would be beneficial in neonates, while M3 antagonists would appear to be more important in the adult.
Acknowledgments
We would like to thank Rachael Wilson for technical assistance with the contraction studies and Craig Wolfe for assistance in figure preparation. This material is based on the work supported by NIH HD-069746 (S.M.W.), P01HD031226, and R01HD003807 (L.D.L.). Additional funding for research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number 2 R25 GM060507. M.G. was a Walter E. Macpherson Medical Student Summer Research Fellow. R.P. and C.B.J. were summer research fellows in the Apprenticeship Bridge to College and Undergraduate Training Programs through the Initiative for Maximizing Student Development Program in the Center for Health Disparities and Molecular Medicine at Loma Linda University School of Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- Altiere RJ, Travis DC, Roberts J, and Thompson DC. (1994). Pharmacological characterization of muscarinic receptors mediating acetylcholine-induced contraction and relaxation in rabbit intrapulmonary arteries. J Pharmacol Exp Ther 270:269–276 [PubMed] [Google Scholar]
- Aven L, and Ai X. (2013). Mechanisms of respiratory innervation during embryonic development. Organogenesis 9:194–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AB, Terry MH, Merritt TA, Papamatheakis DG, Blood Q, Ross JM, Power GG, Longo LD, and Wilson SM. (2013). Effect of chronic perinatal hypoxia on the role of rho-kinase in pulmonary artery contraction in newborn lambs. Am J Physiol Regul Integr Comp Physiol 304:R136–R146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Koarai A, Sturton RG, Nicholson AG, Barnes PJ, and Donnelly LE. (2013). A role for M(2) and M(3) muscarinic receptors in the contraction of rat and human small airways. Eur J Pharmacol 702:109–115 [DOI] [PubMed] [Google Scholar]
- Buels KS, and Fryer AD. (2012). Muscarinic receptor antagonists: Effects on pulmonary function. Handb Exp Pharmacol 317–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, and Tessier-Lavigne M. (2005). Common mechanisms of nerve and blood vessel wiring. Nature 436:193–200 [DOI] [PubMed] [Google Scholar]
- Caulfield MP, and Birdsall NJ. (1998). International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50:279–290 [PubMed] [Google Scholar]
- Eglen RM, Hegde SS, and Watson N. (1996). Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev 48:531–565 [PubMed] [Google Scholar]
- Goldenberg NM, and Kuebler WM. (2015). Endothelial cell regulation of pulmonary vascular tone, inflammation, and coagulation. Compr Physiol 5:531–559 [DOI] [PubMed] [Google Scholar]
- Gosens R, Nelemans SA, Grootte Bromhaar MM, McKay S, Zaagsma J, and Meurs H. (2003). Muscarinic M3-receptors mediate cholinergic synergism of mitogenesis in airway smooth muscle. Am J Respir Cell Mol Biol 28:257–262 [DOI] [PubMed] [Google Scholar]
- Goyal R, Papamatheakis DG, Loftin M, Vrancken K, Dawson AS, Osman NJ, Blood AB, Pearce WJ, Longo LD, and Wilson SM. (2011). Long-term maternal hypoxia: the role of extracellular Ca2+ entry during serotonin-mediated contractility in fetal ovine pulmonary arteries. Reprod Sci 18:948–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B, Rhoden K, and Barnes PJ. (1987). Endothelium-dependent relaxation of human pulmonary arteries. Am J Physiol 252:H434–H438 [DOI] [PubMed] [Google Scholar]
- Haberberger R, Schemann M, Sann H, and Kummer W. (1997). Innervation pattern of guinea pig pulmonary vasculature depends on vascular diameter. J Appl Physiol (1985) 82:426–434 [DOI] [PubMed] [Google Scholar]
- Hadley SR, Blood Q, Rubalcava M, Waskel E, Lumbard B, Le P, Longo LD, Buchholz JN, and Wilson SM. (2012). Maternal high-altitude hypoxia and suppression of ryanodine receptor-mediated Ca2+ sparks in fetal sheep pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 303:L799–L813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer R, Giraldo E, Schiavi GB, Monferini E, and Ladinsky H. (1986). Binding profile of a novel cardioselective muscarine receptor antagonist, AF-DX 116, to membranes of peripheral tissues and brain in the rat. Life Sci 38:1653–1662 [DOI] [PubMed] [Google Scholar]
- Haworth SG. (2006). Pulmonary endothelium in the perinatal period. Pharmacol Rep 58 Suppl:153–164 [PubMed] [Google Scholar]
- Kamitomo M, Alonso JG, Okai T, Longo LD, and Gilbert RD. (1993). Effects of long-term, high-altitude hypoxemia on ovine fetal cardiac output and blood flow distribution. Am J Obstet Gynecol 169:701–707 [DOI] [PubMed] [Google Scholar]
- Lath NR, Galambos C, Rocha AB, Malek M, Gittes GK, and Potoka DA. (2012). Defective pulmonary innervation and autonomic imbalance in congenital diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol 302:L390–L398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo LD, Ueno N, Zhao Y, Pearce WJ, and Zhang L. (1996). Developmental changes in alpha 1-adrenergic receptors, IP3 responses, and NE-induced contraction in cerebral arteries. Am J Physiol 271:H2313–H2319 [DOI] [PubMed] [Google Scholar]
- Norel X, Walch L, Costantino M, Labat C, Gorenne I, Dulmet E, Rossi F, and Brink C. (1996). M1 and M3 muscarinic receptors in human pulmonary arteries. Br J Pharmacol 119:149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankey EA, Kassan M, Choi SK, Matrougui K, Nossaman BD, Hyman AL, and Kadowitz PJ. (2014). Vasodilator responses to acetylcholine are not mediated by the activation of soluble guanylate cyclase or TRPV4 channels in the rat. Am J Physiol Heart Circ Physiol 306:H1495–H506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamatheakis DG, Blood AB, Kim JH, and Wilson SM. (2013). Antenatal hypoxia and pulmonary vascular function and remodeling. Curr Vasc Pharmacol 11:616–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamatheakis DG, Patel JJ, Blood Q, Merritt TT, Longo LD, and Wilson SM. (2012). Depolarization-dependent contraction increase after birth and preservation following long-term hypoxia in sheep pulmonary arteries. Pulm Circ 2:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamatheakis DG, Vemulakonda S, Blood Q, Goyal R, Rubalcava M, Vrancken K, Bennett A, Dawson A, Osman NJ, Blood AB, Pearce WJ, Longo LD, and Wilson SM. (2011). Preservation of serotonin-mediated contractility in adult sheep pulmonary arteries following long-term high-altitude hypoxia. High Alt Med Biol 12:253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyter AC, Muehlethaler V, Liaudet L, Marino M, Di Bernardo S, Diaceri G, and Tolsa JF. (2008). Muscarinic receptor M1 and phosphodiesterase 1 are key determinants in pulmonary vascular dysfunction following perinatal hypoxia in mice. Am J Physiol Lung Cell Mol Physiol 295:L201–L213 [DOI] [PubMed] [Google Scholar]
- Rhodes J, Saxena D, Zhang G, Gittes GK, and Potoka DA. (2015). Defective parasympathetic innervation is associated with airway branching abnormalities in experimental CDH. Am J Physiol Lung Cell Mol Physiol 309:L168–L174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell KO, Hall J, Pugh PJ, Jones TH, Channer KS, and Jones RD. (2009). Testosterone acts as an efficacious vasodilator in isolated human pulmonary arteries and veins: Evidence for a biphasic effect at physiological and supra-physiological concentrations. J Endocrinol Invest 32:718–723 [DOI] [PubMed] [Google Scholar]
- Semenov I, Herlihy JT, and Brenner R. (2012). In vitro measurements of tracheal constriction using mice. J Vis Exp pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda LA, Sham JS, Shimoda TH, and Sylvester JT. (2000a). L-type Ca(2+) channels, resting [Ca(2+)](i), and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol 279:L884–L894 [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Sham JS, and Sylvester JT. (2000b). Altered pulmonary vasoreactivity in the chronically hypoxic lung. Physiol Res 49:549–560 [PubMed] [Google Scholar]
- Sparrow MP, Weichselbaum M, Tollet J, McFawn PK, and Fisher JT. (2003). The Lung: Development, Aging, and Environment. Academic Press, San Diego, CA [Google Scholar]
- Storme L, Rairigh RL, Parker TA, Kinsella JP, and Abman SH. (1999). Acute intrauterine pulmonary hypertension impairs endothelium-dependent vasodilation in the ovine fetus. Pediatr Res 45:575–581 [DOI] [PubMed] [Google Scholar]
- Swenson ER. (2013). Hypoxic pulmonary vasoconstriction. High Alt Med Biol 14:101–110 [DOI] [PubMed] [Google Scholar]
- Sylvester JT, Shimoda LA, Aaronson PI, and Ward JP. (2012). Hypoxic pulmonary vasoconstriction. Physiol Rev 92:367–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch L, Brink C, and Norel X. (2001). The muscarinic receptor subtypes in human blood vessels. Therapie 56:223–226 [PubMed] [Google Scholar]
- Walch L, Norel X, Leconte B, Gascard JP, and Brink C. (1999). Cholinergic control of human and animal pulmonary vascular tone. Therapie 54:99–102 [PubMed] [Google Scholar]
- Xue Q, Ducsay CA, Longo LD, and Zhang L. (2008). Effect of long-term high-altitude hypoxia on fetal pulmonary vascular contractility. J Appl Physiol (1985) 104:1786–1792 [DOI] [PubMed] [Google Scholar]