Abstract
Miele, Catherine H., Alan R. Schwartz, Robert H. Gilman, Luu Pham, Robert A. Wise, Victor G. Davila-Roman, Jonathan C. Jun, Vsevolod Y. Polotsky, J. Jaime Miranda, Fabiola Leon-Velarde, and William Checkley. Increased cardiometabolic risk and worsening hypoxemia at high altitude. High Alt Med Biol. 17:93–100, 2016.—Metabolic syndrome, insulin resistance, diabetes, and dyslipidemia are associated with an increased risk of cardiovascular disease. While excessive erythrocytosis is associated with cardiovascular complications, it is unclear how worsening hypoxemia of any degree affects cardiometabolic risk factors in high-altitude populations. We studied the relationship between daytime resting oxyhemoglobin saturation and cardiometabolic risk factors in adult participants living in Puno, Peru (3825 m above sea level). We used multivariable logistic regression models to study the relationship between having a lower oxyhemoglobin saturation and markers of cardiometabolic risk. Nine hundred and fifty-four participants (mean age 55 years, 52% male) had information available on pulse oximetry and markers of cardiometabolic risk. Average oxyhemoglobin saturation was 90% (interquartile range 88%–92%) and 43 (4.5%) had excessive erythrocytosis. Older age, decreased height-adjusted lung function, and higher body mass index (BMI) were associated with having an oxyhemoglobin saturation ≤85%. When adjusting for age, sex, socioeconomic status, having excessive erythrocytosis, and site, we found that each 5% decrease in oxyhemoglobin saturation was associated with a higher adjusted odds of metabolic syndrome (OR = 1.35, 95% CI: 1.07–1.72, p < 0.04), insulin resistance as defined by homeostasis model assessment-insulin resistance (HOMA-IR) >2 mass units (OR = 1.29, 95% CI: 1.00–1.67, p < 0.05), hemoglobin A1c ≥6.5% (OR = 1.66, 95% CI: 1.09–2.51, p < 0.04), and high sensitivity C-reactive protein (hs-CRP) ≥3 mg/L (OR = 1.46, 95% CI: 1.09–1.96, p < 0.01). In high-altitude populations in Puno, Peru, a higher BMI and lower pulmonary function were associated with lower resting daytime oxyhemoglobin saturation. Lower resting oxyhemoglobin saturation, in turn, was associated with higher odds of having multiple unfavorable cardiometabolic factors. Worsening hypoxia of any degree in high-altitude dwellers may be an independent risk factor for cardiovascular disease.
Key Words: : altitude, diabetes, hypoxemia, insulin resistance, metabolic syndrome
Introduction
Cardiovascular disease is the leading cause of death worldwide (Despres et al., 2008; Mottillo et al., 2010) and is responsible for 18 million deaths each year (World Health Organization, 2015). Metabolic syndrome represents a cluster of cardiovascular and metabolic risk factors, including abdominal obesity (as a proxy for body mass index [BMI]), atherogenic dyslipidemia, hypertension, and insulin resistance, all of which have been associated with increased risk of cardiovascular disease and death (Grundy et al., 2004; Wilson et al., 2005). Population-based studies have found that severe pulmonary disease and obstructive sleep apnea are also associated with increased cardiovascular disease morbidity and mortality (Kannel et al., 1983; Schroeder et al., 2003). Since both pulmonary disease and sleep apnea lead to hypoxemia, it has been hypothesized that hypoxemia is responsible for detrimental cardiovascular outcomes.
High altitude provides a natural laboratory to study the association between worsening hypoxemia and cardiometabolic risk. The partial pressure of oxygen at high altitudes (>2500 m above sea level) is reduced when compared to that at sea level, resulting in chronic hypoxemia of varying degrees in highlanders. In some, hypoxemia may be exaggerated and lead to the development of chronic mountain sickness, a prevalent condition in the Peruvian Andes that leads to cardiovascular decompensation, stroke, and heart failure (Monge, 1942; Whayne, 2004; León-Velarde et al., 2005; Vargas and Spielvogel, 2006).
There is conflicting evidence that high-altitude dwellers may have either increased (Baracco et al., 2007; Malaga et al., 2010) or decreased odds of having metabolic syndrome (Woolcott et al., 2014) when compared with sea-level counterparts. Laboratory studies have demonstrated that intermittent hypoxemia leads to insulin resistance (Polotsky et al., 2004) and low-grade systemic inflammation (Ryan et al., 2005), likely contributing to the development of cardiovascular disease; however, a better understanding of underlying mechanisms of hypoxemia-associated cardiometabolic disease risk is lacking. Current evidence has focused on the role of intermittent hypoxemia on cardiometabolic disease and not on the role of chronic hypoxemia. Therefore, high-altitude research could provide relevant epidemiological data to more clearly elicit the link between chronic hypoxemia and cardiometabolic risk.
In a prior study, we found that excessive erythrocytosis, the hallmark of Chronic Mountain Sickness, was associated with higher odds of having metabolic syndrome (De Ferrari et al., 2014). The overall prevalence of excessive erythrocytosis was 4.5%, but our earlier analyses did not explore the relationship between oxyhemoglobin saturation of varying degrees and cardiometabolic risk profiles. We hypothesize that worsening hypoxemia of any degree, even before the development of excessive erythrocytosis or Chronic Mountain Sickness, contributes to increased cardiometabolic risk and thus it may be a marker of increased cardiometabolic disease risk.
Methods
Setting and design
We leveraged data from an existing cohort of adult participants living at high altitude to evaluate the relationship between degree of hypoxemia and cardiometabolic risk. The CRONICAS study is a longitudinal, population-based study aimed to determine the prevalence and risk factors for chronic pulmonary and cardiovascular disease across four disparate regions in Peru. The study protocol has been described in detail elsewhere (Miranda et al., 2012). Data for our study were taken from the high-altitude population in Puno, Peru. Puno is a southwestern city in the Andes, located on the shores of Lake Titicaca at 3825 m above sea level. A large proportion of participants were of Aymara and Quechua ethnicity, the second and third largest ethnic group in Peru after mestizos (i.e., mixed Amerindian and European ancestry). Within Puno, there are two separate sites: an urban setting located at the city center (population 120,000) and a rural setting made up of inhabitants from the surrounding farming communities.
Study participants
We identified a sex- and age-stratified random sample of adults aged ≥35 years. We aimed to enroll 1000 subjects in Puno and stratified recruitment to include ∼500 participants each from the urban and rural settings (Miranda et al., 2012). We recorded oxyhemoglobin saturations during baseline evaluation in 95% of participants (489 urban and 465 rural participants). Exclusion criteria were pregnancy, inability to participate in questionnaires or spirometer due to physical disability, and active pulmonary tuberculosis. Fieldworkers obtained verbal consent from study participants before enrollment. The study was approved by the Institutional Review Boards at Universidad Peruana Cayetano Heredia and A.B. PRISMA in Lima, Peru, and the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Data collection
Trained technicians measured weight, height, and blood pressure. Data collected include several factors potentially associated with chronic disease, such as age, sex, years of education, occupation, demographic information, other socioeconomic variables, smoking habits, biomass fuel use, and self-reported medical conditions and self-reported family medical conditions (Miranda et al., 2012). All interviews were conducted in Spanish, Aymara, or Quechua according to the participant's primary language of preference. We used a modified version of WHO STEPS approach questionnaire for surveillance of noncommunicable disease (World Health Organization, 2014). Pulse oximetry was measured using a handheld device (Rad-5v, Masimo, Irvine, CA) while participants were awake, upright, and resting comfortably (Luks and Swenson, 2011). Oxyhemoglobin saturation was recorded after the reading stabilized for more than 10 seconds. The Rad-5v has an oxyhemoglobin saturation accuracy range of 70%–100% with ±3%. Fasting blood samples were obtained and analyzed in a single facility using a standardized approach. Hemoglobin was determined by the automated sodium lauryl sulfate method for the detection of methemoglobin. All tests were processed in a centralized testing facility (Miranda et al., 2012). Plasma glucose was measured using an enzymatic colorimetric method (GOD-PAP; Modular P-E/Roche-Cobas). Trained technicians measured pre- and post-bronchodilator spirometry with the Easy-On-PC spirometer (ndd Medizintechnik AG) following standard guidelines (Miller et al., 2005).
Definitions
We defined oxyhemoglobin saturation as low if pulse oximetry reading was ≤85% and acceptable if >85%; excessive erythrocytosis as having a hemoglobin ≥21 g/dL in men and ≥19 g/dL in women (León-Velarde et al., 2005); and metabolic syndrome following the 2009 harmonized definition that incorporated the following region-specific cutoffs (Alberti et al., 2009): elevated waist circumference (≥90 cm in men and ≥80 in cm women); elevated triglycerides (≥150 mg/dL) or drug treatment; reduced high-density lipoprotein (HDL)-cholesterol (<40 mg/dL in males and <50 mg/dL in females) or drug treatment; elevated blood pressure (systolic ≥130 mmHg or diastolic ≥85 mmHg) or antihypertensive drug treatment; and elevated fasting glucose (≥100 mg/dL) or drug treatment. Insulin resistance was defined using homeostasis model assessment (HOMA-IR) and was calculated from fasting glucose and insulin values using the following equation: 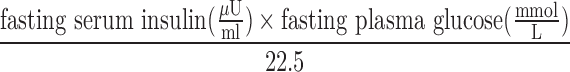 (Matthews et al., 1985). Diabetes was defined as having fasting glucose ≥126 mg/dL or currently taking diabetes medications (Miranda et al., 2012). Since there are no established reference equations for lung function in Peruvians, we used the Global Lung Function Initiative mixed ethnic population reference equations (Quanjer et al., 2012). We created a wealth index according to assets, household facilities, household income, and occupation (Howe et al., 2012) and used this as a marker of socioeconomic status. We included site (urban or rural settings) in our analysis to account for other living related differences that are not included in the wealth index.
(Matthews et al., 1985). Diabetes was defined as having fasting glucose ≥126 mg/dL or currently taking diabetes medications (Miranda et al., 2012). Since there are no established reference equations for lung function in Peruvians, we used the Global Lung Function Initiative mixed ethnic population reference equations (Quanjer et al., 2012). We created a wealth index according to assets, household facilities, household income, and occupation (Howe et al., 2012) and used this as a marker of socioeconomic status. We included site (urban or rural settings) in our analysis to account for other living related differences that are not included in the wealth index.
Biostatistical methods
The primary aim of this analysis was to evaluate the relationship between resting oxyhemoglobin saturation and cardiometabolic risk factors at high altitude. We first evaluated participant characteristics related to low oxyhemoglobin saturation in single and multivariable regression, and identified that BMI was likely in the causal pathway of the relationship between exaggerated hypoxemia and cardiometabolic risk factors. We based our further analyses on this assumption (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ham).
We performed multivariate logistic regressions for metabolic syndrome, for each of its components, and for other select cardiometabolic, lipid, and inflammatory markers, including systolic and diastolic blood pressure, low-density lipoprotein (LDL)-cholesterol, high sensitivity C-reactive protein (hs-CRP), fasting insulin, fasting glucose, HOMA-IR, elevated HbA1c, and diabetes to estimate the increase odds for each 5% decrease in oxyhemoglobin saturation adjusted for age, sex, excessive erythrocytosis, wealth index, and site. Given that metabolic syndrome and its components are strongly related to BMI, and that BMI is likely in the causal pathway linking hypoxemia to other cardiometabolic risk factors (Supplementary Fig. S1), we conducted a second multivariable regression in which we included the component of BMI that was orthogonal to oxyhemoglobin saturation in a sensitivity analysis. We first calculated residuals of a linear model in which we regressed oxyhemoglobin saturation on BMI. We then added the calculated residuals of BMI to the above multivariable logistic regression. We describe these methods in further detail in the Supplementary Data. We performed analyses in STATA 12 (StataCorp LP, College Station, Texas, USA) and R (www.r-project.org).
Results
Participant characteristics
A total of 954 participants had information available on pulse oximetry and markers of cardiometabolic risk. There were no significant differences in age (p = 0.86), sex (p = 0.89), daily smoking (p = 0.82), or prevalence of metabolic syndrome (p = 0.17) among participants included in this analysis and those who were not. Daily smoking (≥1 cigarette per day) was low at both sites (1.3%), whereas daily biomass fuel use was pervasive in the rural setting when compared to the urban setting (p < 0.001). A total of 43 participants (4.5%) had excessive erythrocytosis. We found that urban participants had a higher BMI, increased prevalence of metabolic syndrome and its components (increased waist circumference, blood pressure, triglycerides, fasting glucose, and reduced HDL-cholesterol), elevated LDL-cholesterol, hs-CRP, fasting insulin, fasting glucose, HbA1c, and increased prevalence of insulin resistance (HOMA-IR) than did participants in rural Puno (Table 1).
Table 1.
Prevalence of Cardiovascular Risk Factors by Site
| High altitude | ||||
|---|---|---|---|---|
| Characteristics, n (%) or mean ± SD | Urban Puno (507) | Rural Puno (506) | Total (1013) | p |
| Age in years | 55.2 ± 12.1 | 55.5 ± 12.5 | 55.3 ± 12.3 | 0.82 |
| Male | 250 (49.3) | 240 (47.4) | 490 (48.3) | 0.02 |
| Height (cm) | 156.9 ± 9.0 | 155.5 ± 8.0 | 156.2 ± 8.5 | <0.01 |
| BMI (kg/m2) | 27.9 ± 4.4 | 25.1 ± 3.73 | 26.4 ± 4.3 | <0.001 |
| Daily smoker | 11 (2.2) | 1 (0.2) | 12 (1.2) | <0.01 |
| Daily biomass fuel use | 25 (5.0) | 484 (96.6) | 508 (50.7) | <0.001 |
| Metabolic syndrome | 232 (45.8) | 138 (27.3) | 370 (36.5) | <0.001 |
| Waist circumference ≥90 cm in men and ≥80 in women | 385 (75.9) | 245 (48.3) | 630 (62.2) | <0.001 |
| Systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg | 92 (18.3) | 134 (26.8) | 226 (22.5) | 0.001 |
| HDL <40 mg/dL in males and <50 mg/dL in females | 329 (67.3) | 274 (57.0) | 603 (62.2) | <0.001 |
| Triglycerides ≥150 mg/dL | 115 (23.6) | 66 (13.7) | 181 (18.7) | 0.001 |
| Fasting glucose ≥100 mg/dL | 107 (21.9) | 54 (11.2) | 161 (16.6) | <0.001 |
| Other cardiovascular risk factors | ||||
| Systolic blood pressure ≥140 mmHg | 56 (11.1) | 43 (8.6) | 99 (9.9) | 0.18 |
| Diastolic blood pressure ≥90 mmHg | 53 (10.5) | 48 (9.6) | 101 (10.1) | 0.63 |
| LDL ≥160 mg/dL | 90 (18.4) | 49 (10.2) | 139 (14.3) | <0.001 |
| hs-CRP ≥3 mg/L | 103 (21.1) | 32 (12.9) | 165 (17.0) | <0.01 |
| Fasting insulin >25 mIU/L | 16 (3.3) | 6 (1.25) | 22 (2.3) | 0.04 |
| HOMA-IR >2 mass units | 190 (38.9) | 100 (20.8) | 290 (29.9) | <0.001 |
| Fasting glucose ≥126 mg/dL | 28 (5.7) | 15 (3.1) | 43 (4.4) | 0.05 |
| Hemoglobin A1c ≥6.5% | 47 (9.6) | 25 (5.2) | 72 (7.4) | <0.01 |
| Diabetes | 33 (6.8) | 16 (3.3) | 49 (5.1) | 0.02 |
BMI, body mass index; HOMA-IR, homeostasis model assessment-insulin resistance; HDL, high-density lipoprotein; hs-CRP, high sensitivity C-reactive protein; LDL, low-density lipoprotein.
Risk factors for low oxyhemoglobin saturation
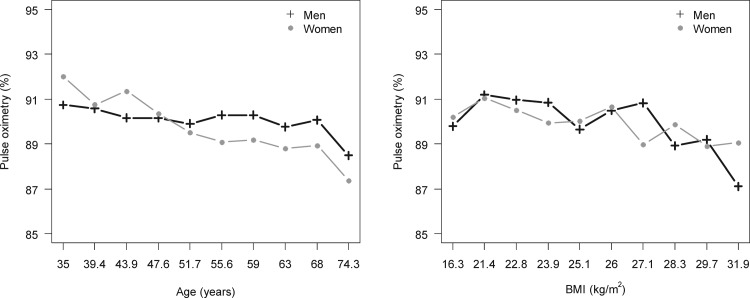
Higher BMI, lower lung function (either height-corrected as FEV1/height2 and FVC/height2, or as percent predicted FEV1 and FVC), and a lower post-bronchodilator FEV1/FVC ratio were all associated with having a lower oxyhemoglobin saturation (Table 2). Elevated hemoglobin, resting heart rate, and hs-CRP were also seen in individuals with low oxyhemoglobin saturation. Oxyhemoglobin saturation was lower with older age and higher BMI in both men and women (Fig. 1) independent of percent predicted FVC (Supplementary Table S2). In multivariable regression, factors associated with a low oxyhemoglobin saturation were age (OR = 1.37 per 5 years of age, 95% CI: 1.20–1.56, p < 0.001), BMI (OR = 2.08 per 5 kg/m2 increase, 95% CI: 1.47–2.94, p < 0.001), and having a percent predicted post-bronchodilator FVC <80% (OR = 3.62, 95% CI: 1.35–12.76, p = 0.01) (Supplementary Table S2).
Table 2.
Characteristics of Participants by Oxyhemoglobin Saturation Categories
| n (%) or mean ± SD | SpO2 ≤ 85% | SpO2 86%–90% | SpO2 ≥91% | Total | p |
|---|---|---|---|---|---|
| Demographics | |||||
| Sample size | 103 (10.8) | 428 (44.9) | 423 (44.3) | 954 | |
| Age (years) | 63.5 ± 12.7 | 57.1 ± 12.1 | 52.9 ± 12.0 | 55.9 ± 12.5 | <0.001 |
| Male | 39 (44.8) | 185 (46.3) | 202 (51.7) | 426 (48.5) | 0.24 |
| Daily smoker | 2 (2.3) | 4 (1.0) | 5 (1.3) | 11 (1.3) | 0.62 |
| Daily biomass fuel use | 35 (40.2) | 161 (40.5) | 230 (59.9) | 426 (49.0) | <0.001 |
| Clinical parameters | |||||
| Height (cm) | 154.7 ± 9.2 | 155.4 ± 8.4 | 157.1 ± 8.4 | 156.1 ± 8.5 | <0.01 |
| Weight (kg) | 65.4 ± 15.7 | 65.7 ± 11.9 | 62.9 ± 10.9 | 64.4 ± 12.0 | <0.01 |
| BMI (kg/m2) | 27.2 ± 5.3 | 27.2 ± 4.3 | 25.5 ± 3.7 | 26.4 ± 4.2 | <0.001 |
| Systolic blood pressure (mmHg) | 115.4 ± 17.4 | 113.0 ± 17.2 | 114.2 ± 16.9 | 113.1 ± 16.5 | 0.27 |
| Diastolic blood pressure (mmHg) | 72.5 ± 10.2 | 72.8 ± 10.7 | 74.2 ± 10.2 | 73.4 ± 10.5 | 0.15 |
| Hypertension | 11 (10.7) | 44 (10.3) | 39 (9.2) | 94 (9.9) | 0.84 |
| Heart rate (bpm) | 72.2 ± 9.8 | 69.1 ± 10.3 | 66.4 ± 10.3 | 68.3 ± 10.4 | <0.001 |
| Oxygen saturation (%) | 83.1 ± 2.5 | 88.6 ± 1.3 | 92.7 ± 1.7 | 89.8 ± 3.4 | <0.001 |
| Hemoglobin (g/dL) | 17.8 ± 2.6 | 17.0 ± 1.9 | 16.4 ± 1.6 | 16.8 ± 1.9 | <0.001 |
| LDL-cholesterol (mg/dL) | 119.9 ± 37.9 | 125.2 ± 33.9 | 119.2 ± 36.4 | 122.1 ± 35.6 | <0.04 |
| HDL-cholesterol (mg/dL) | 41.7 ± 12.0 | 42.7 ± 11.5 | 43.2 ± 10.9 | 42.8 ± 11.3 | 0.26 |
| hs-CRP (log(mg/dL)) | 4.0 ± 6.0 | 3.4 ± 10.9 | 2.1 ± 5.1 | 2.9 ± 8.3 | <0.001 |
| Fasting insulin (mIU/L) | 8.9 ± 12.5 | 8.9 ± 9.8 | 6.7 ± 5.5 | 7.9 ± 8.6 | <0.001 |
| Fasting glucose (mg/dL) | 96.5 ± 30.3 | 93.5 ± 26.4 | 92.2 ± 26.8 | 93.3 ± 27.0 | <0.04 |
| HbA1c (%) | 6.3 ± 1.4 | 6.0 ± 0.7 | 5.8 ± 0.9 | 5.9 ± 0.9 | <0.001 |
| HOMA-IR (mass units) | 2.3 ± 3.5 | 2.2 ± 3.2 | 1.6 ± 1.5 | 1.9 ± 2.6 | <0.001 |
| Diabetic | 10 (9.7) | 20 (4.7) | 19 (4.5) | 49 (5.1) | 0.08 |
| Pulmonary function | |||||
| FEV1/height2 (L/m2) | 0.98 ± 0.29 | 1.13 ± 0.25 | 1.24 ± 0.25 | 1.17 ± 0.17 | <0.001 |
| FVC/height2 (L/m2) | 1.28 ± 0.37 | 1.45 ± 0.30 | 1.57 ± 0.30 | 1.49 ± 0.32 | <0.001 |
| Post FEV1/FVC ratio | 76.8 ± 7.1 | 78.3 ± 6.1 | 78.8 ± 6.1 | 78.4 ± 6.2 | <0.04 |
| Percent predicted FEV1 (%) NHANES III Mexican Americans | 103.1 ± 21.7 | 110.8 ± 17.0 | 115.2 ± 17.4 | 112.0 ± 18.0 | <0.001 |
| Percent predicted FVC (%) NHANES III, Mexican Americans | 103.1 ± 21.1 | 110.1 ± 15.2 | 115.0 ± 16.4 | 111.6 ± 16.8 | <0.001 |
Spo2, oxygen saturation as measured by pulse oximetry.
FIG. 1.
Relationship between oxyhemoglobin saturation and either age or BMI. We plotted the mean oxyhemoglobin saturation by deciles of age and BMI, respectively, and stratified by sex. Numbers in the x-axis represent the starting value of each decile. The graphs show a dose-response relationship with age or BMI corresponding to an incrementally lower oxyhemoglobin saturation, in both men and women. BMI, body mass index.
Cardiometabolic risk factors associated with lower resting daytime oxygen saturation
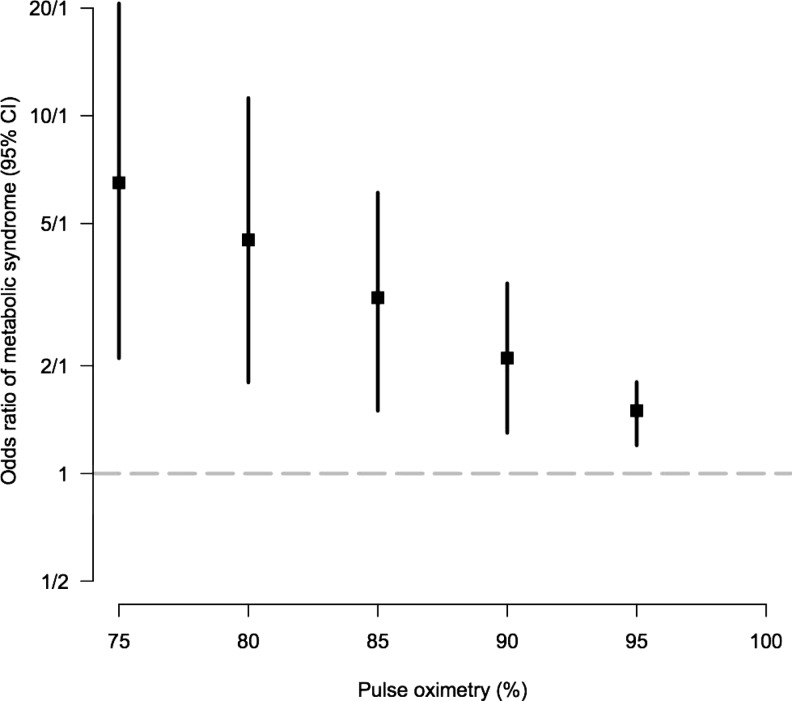
We found higher odds of having metabolic syndrome for each 5% decrease in oxyhemoglobin saturation (Fig. 2 and Supplementary Table S1). We performed adjusted analysis (Table 3) to further evaluate the relationship of a 5% change in oxyhemoglobin saturation and various cardiometabolic factors. In analyses adjusted for age, sex, excessive erythrocytosis, wealth index, and setting, a 5% decrease in oxyhemoglobin saturation was strongly related to higher odds of having a metabolic syndrome, a higher waist circumference, HbA1c ≥6.5%, HOMA-IR >2 mass units, and hs-CRP ≥3 mg/L.
FIG. 2.
Odds ratio of metabolic syndrome based on 5% decrease in oxyhemoglobin saturation. We plotted the odds ratios and 95% confidence intervals for every 5% increase in oxyhemoglobin saturation. Relationship between oxyhemoglobin saturation and age and BMI in men and women. We plotted the mean oxyhemoglobin saturation for each 1 year or 1 kg/m2 increased in age and BMI, respectively. This was done for men and woman independently. The graphs show a dose–response with increased age and BMI corresponding to incremental decreased oxyhemoglobin saturation. BMI, body mass index.
Table 3.
Odds Ratio of Prevalence of Individual Components of Metabolic Syndrome Per 5% Decrease in Resting Daytime SpO2 Adjusted for Multiple Variables
| Model adjusted for age, sex, excessive erythrocytosis, wealth index, and site | Model adjusted for age, sex, excessive erythrocytosis, wealth index, site, and residuals of BMI | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Metabolic syndrome | 1.35 | 1.07–1.72 | <0.01 | 1.45 | 1.10–1.90 | <0.01 |
| Waist circumference ≥90 cm in men and ≥80 in women | 1.29 | 1.01–1.66 | <0.05 | |||
| Systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg | 0.77 | 0.59–1.03 | 0.08 | 0.76 | 0.57–1.02 | 0.07 |
| HDL <40 mg/dL in males and <50 mg/dL in females | 1.21 | 0.95–1.54 | 0.12 | 1.27 | 0.98–1.63 | 0.07 |
| Triglycerides ≥150 mg/dL | 1.04 | 0.77–1.40 | 0.79 | 1.04 | 0.77–1.40 | 0.82 |
| Fasting glucose ≥100 mg/dL | 1.07 | 0.79–1.45 | 0.65 | 1.07 | 0.79–1.45 | 0.68 |
| Other cardiovascular risk factors | ||||||
| Systolic blood pressure ≥140 mmHg | 0.98 | 0.66–1.45 | 0.91 | 0.97 | 0.64–1.46 | 0.87 |
| Diastolic blood pressure ≥90 mmHg | 0.82 | 0.56–1.21 | 0.32 | 0.79 | 0.53–1.18 | 0.25 |
| LDL ≥160 mg/dL | 1.09 | 0.79–1.52 | 0.59 | 1.09 | 0.79–1.52 | 0.60 |
| hs-CRP ≥3 mg/L | 1.46 | 1.09–1.96 | 0.01 | 1.59 | 1.17–2.15 | <0.01 |
| Fasting insulin >25 mIU/L | 1.55 | 0.77–3.14 | 0.22 | 1.31 | 0.62–2.77 | 0.48 |
| Fasting glucose ≥126 mg/dL | 0.98 | 0.56–1.70 | 0.94 | 0.96 | 0.55–1.68 | 0.88 |
| Hemoglobin A1c ≥6.5% | 1.66 | 1.09–2.51 | <0.04 | 1.66 | 1.09–2.54 | 0.02 |
| HOMA-IR >2 mass units | 1.29 | 1.00–1.67 | <0.05 | 1.31 | 0.98–1.74 | 0.07 |
| Diabetes | 1.12 | 0.67–1.87 | 0.67 | 1.08 | 0.64–1.83 | 0.77 |
Sensitivity analyses
As previously mentioned, given that BMI is likely in the assumed causal pathway linking hypoxemia to other cardiometabolic risk factors (Supplementary Fig. S1), we used residuals of BMI as a sensitivity analysis. In a model adjusted for age, sex, excessive erythrocytosis, wealth index, and residuals of BMI, lower oxyhemoglobin saturation maintained associations with HbA1c ≥6.5% and hs-CRP ≥3 mg/L, while the association between oxyhemoglobin saturation and HOMA-IR >2 mass units was no longer significant (Table 3). When stratified by sex, although not having sufficient power for adequate stratification, we found that the relationship between oxyhemoglobin saturation and HbA1c showed similar trends in men (OR = 1.50, 95% CI: 0.76–3.12, p = 0.23) and in women (OR = 1.65, 95% CI: 0.95–2.87, p = 0.08). Effect sizes were in a similar direction and magnitude for regressions stratified by site (Supplementary Tables S3 and S4).
Discussion
In this high-altitude population-based study of 954 residents of Puno, Peru, we found that older age, lower lung function, and higher BMI were associated with a lower resting daytime oxyhemoglobin saturation. In turn, a lower oxyhemoglobin saturation was associated with higher odds of having cardiometabolic risk factors such as metabolic syndrome, HbA1c (≥6.5%), and hs-CRP (≥3 mg/L) even when accounting for age, sex, excessive erythrocytosis, wealth index, site, and BMI. A higher BMI did not appear to fully account for the cardiometabolic risk suggesting that low oxyhemoglobin saturation itself plays an independent role in augmenting cardiometabolic risk factors at high altitude.
Several findings reported in our study are largely consistent with the literature and confirm established physiological principles. First, in this high-altitude cohort, the prevalence of metabolic syndrome and elevated fasting glucose (>100 mg/dL) was 36.5% and 16.6%, respectively, which is similar to previous findings among Andean populations (Chirinos et al., 2013). Second, we found obesity to be linked with worsening hypoxemia, likely, in part, due to its effects on lung volume (Jones, 2006). Finally, it is well established that obesity is a major causal factor in the development of metabolic disorders. In particular, central adiposity, more common among urban residents of Puno (Medina-Lezama et al., 2010), was accompanied by dyslipidemia, fasting hyperglycemia, and insulin resistance. In light of the effects of obesity, it was not surprising that adjusting for residuals of BMI in our sensitivity analysis attenuated some of the associations between oxyhemoglobin saturation and metabolism. At face value, our results differ from some studies that have shown decreased prevalence of cardiovascular risk factors in high-altitude dwellers (Sherpa et al., 2010, 2013; Woolcott et al., 2014). However, these studies did not examine oxyhemoglobin saturation, which can vary greatly between subjects at the same altitude (Luks and Swenson, 2011) and which reflects true hypoxemia at high altitudes.
An important and novel finding of our study is that exaggerated hypoxemia in its own right was associated with cardiometabolic risk in our study setting. We found that participants in urban Puno had a higher mean BMI compared to those in rural Puno, but both cohorts had a similar prevalence of elevated fasting glucose, HbA1c, and diabetes and implicating a factor other than BMI may explain observed differences in metabolic risk factors. In addition, our multivariable regression model adjusted for BMI also showed a strong inverse relationship between oxyhemoglobin saturation and prevalence of metabolic syndrome, HbA1c ≥6.5%, and hs-CRP ≥3 mg/L. Elevated hs-CRP in individuals with lower resting daytime oxyhemoglobin saturation may serve as a marker of increased cardiovascular risk at high altitude (Grundy et al., 2004).
While not the primary focus of this study, there are several plausible mechanisms by which hypoxemia may affect glucose metabolism and inflammation. First, hypoxemia activates the sympathetic nervous system, chiefly through arterial chemoreceptors. In humans and animals, various forms of hypoxia-induced glucose intolerance may be significantly attenuated by sympathetic blockade or by chemoreceptor denervation (Peltonen et al., 2012; Jun et al., 2014; Shin et al., 2014). Chronic activation of the sympathetic nervous system may be a pathway toward the development of metabolic syndrome (Brotman et al., 2002). Alternatively, new findings suggest that hypoglycemia stimulates and hyperglycemia inhibits peripheral chemoreceptor responsiveness, and may imply that serum glucose is a driver for sympathetic tone thereby affecting oxygenation through respiratory drive (Ortega-Saenz et al., 2013).
Second, hypoxemia in tissues may cause systemic inflammation by activation of hypoxia-inducible factor 1-alpha in hypertrophied obese adipose tissue (He et al., 2011) and through the inhibition of adipocytokines (Hosogai et al., 2007). Interestingly, many adaptations to tissue hypoxia in muscle theoretically facilitate glucose disposal, an effect witnessed both in vivo (Mackenzie et al., 2011, 2012) and in vitro (Azevedo et al., 1995). Hypoxia facilitates angiogenesis (Iyer et al., 1998), which increases tissue glucose delivery; facilitates glucose transport through upregulation of GLUT1 and GLUT4 (Chou et al., 2004; Sakagami et al., 2014); upregulates AMP-activated protein kinase, which increases insulin sensitivity (Kahn et al., 2005); and shunts glucose toward glycolysis and lactate pathways rather than mitochondrial oxidation (Wheaton et al., 2011), resulting in higher total body glucose flux (Roberts et al., 1985; Palmer et al., 2014). Many of these changes are facilitated, in whole or in part, by HIF-1α (Semenza, 1999).
Our finding that, at altitude, hypoxemia was associated with insulin resistance suggests that sympathetic activation and adipose tissue hypoxia may prevail over metabolic adaptations occurring in muscle. This physiology may explain how the synergy of obesity and hypoxemia may lead to metabolic complications, as demonstrated in a mouse model of sleep apnea (Polotsky et al., 2004). In terms of lipid metabolism, hypoxemia may increase adipose tissue lipolysis, upregulate hepatic lipoprotein secretion, and/or suppress lipoprotein clearance (Drager et al., 2010). It is also possible that the presence of hypoxemia is a marker of lung disease, which in turn may increase the risk of cardiovascular disease (Schroeder et al., 2003; Lee et al., 2011). In our study, we found that participants with lower oxyhemoglobin saturations also had lower height-corrected forced expiratory volumes independent of BMI.
Our study has several strengths. First, our study is a population-based sample derived in a high-altitude setting in two disparate socioeconomic settings, allowing for increased environmental, dietary, and lifestyle variability. Second, we have a large sample size and collected extensive anthropometric, clinical, social, and laboratory data to best characterize cardiovascular risk factors. Limitations to our study include the cross-sectional nature and inability to determine if these cardiometabolic risk factors correlate to increased mortality in this population. We also relied on a single pulse oximetry spot-check for oxyhemoglobin saturation values, which may affect the accuracy of an individual's true value. However, we believe that a large sample size may have helped to minimize the effects of a spot-check accuracy on our inferences. Finally, the validity of our results depends on the assumptions made in our directed acyclic graph (Supplementary Data) demonstrating the multiple collinear risk factors that influence both oxygen saturation and increased cardiometabolic risk.
Conclusion
Among high-altitude dwellers in Peru, a lower oxyhemoglobin saturation was associated with higher odds of having metabolic syndrome, elevated HbA1c, and elevated hs-CRP even after accounting for those who had excessive erythrocytosis. Therefore, our findings suggest that any degree of hypoxemia may play a role in the development of diabetes, dyslipidemia, and systemic inflammation in high-altitude populations. It is likely that although obesity may be less prevalent at high altitude, individuals with a lower oxyhemoglobin saturation have a higher risk for cardiometabolic disease. High-altitude settings provide a natural laboratory to better understand the effects of worsening hypoxemia on cardiometabolic health.
Supplementary Material
Acknowledgments
The authors are indebted to all participants who kindly agreed to participate in the study. Special thanks to all field teams for their commitment and hard work, especially to Lilia Cabrera, Rosa Salirrosas, Viterbo Aybar, Sergio Mimbela, and David Danz for their leadership in each of the study sites, as well as Marco Varela for data coordination. This project was funded in whole with Federal funds from the United States National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268200900033C. C.H.M. was further supported by the National institute of Health Fogarty International Center (5R25TW009340) and the University North Carolina Center for AIDS Research. W.C. was further supported by a Pathway to Independence Award (R00HL096955) from the National Heart, Lung, and Blood Institute.
Author Disclosure Statement
No competing financial interests exist.
References
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, and Smith SC, Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. (2009). Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645 [DOI] [PubMed] [Google Scholar]
- Azevedo JL, Jr., Carey JO, Pories WJ, Morris PG, and Dohm GL. (1995). Hypoxia stimulates glucose transport in insulin-resistant human skeletal muscle. Diabetes 44:695–698 [DOI] [PubMed] [Google Scholar]
- Baracco R, Mohanna S, and Seclén S. (2007). A comparison of the prevalence of metabolic syndrome and its components in high and low altitude populations in peru. Metab Syndr Relat Disord 5:55–62 [DOI] [PubMed] [Google Scholar]
- Brotman DJ, and Girod JP. (2002). The metabolic syndrome: a tug-of-war with no winner. Cleve Clin J Med 69:990–994 [DOI] [PubMed] [Google Scholar]
- Chirinos DA, Morey-Vargas OL, Goldberg RB, Chirinos JA, and Medina-Lezama J. (2013). Metabolic syndrome in Andean populations. Global Heart 8:349–354.e1 [DOI] [PubMed] [Google Scholar]
- Chou SW, Chiu LL, Cho YM, Ho HY, Ivy JL, Ho CF, and Kuo CH. (2004). Effect of systemic hypoxia on GLUT4 protein expression in exercised rat heart. Jpn J Physiol 54:357–363 [DOI] [PubMed] [Google Scholar]
- De Ferrari A, Miranda JJ, Gilman RH, Dávila-Román VG, León-Velarde F, Rivera-Ch M, Huicho L, Bernabé-Ortiz A, Wise RA, Checkley W; CRONICAS Cohort Study Group (2014). Prevalence, clinical profile, iron status and subject-specific traits for excessive erythrocytosis in Andean adults living permanently at 3825 meters above sea level. Chest 145:1327–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodes-Cabau J, Bertrand OF, and Poirier P. (2008). Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 28:1039–1049 [DOI] [PubMed] [Google Scholar]
- Drager LF, Jun JC, and Polotsky VY. (2010). Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab 24:843–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Hansen B, Smith SC, Jr., Cleeman JI, and Kahn RA; American Heart Association; National Heart, Lung, and Blood Institute; American Diabetes Association. (2004). Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation 109:433–438 [DOI] [PubMed] [Google Scholar]
- He Q, Gao Z, Yin J, Zhang J, Yun Z, and Ye J. (2011). Regulation of HIF-1alpha activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab 300:E877–E885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, and Shimomura I. (2007). Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56:901–911 [DOI] [PubMed] [Google Scholar]
- Howe LD, Galobardes B, Matijasevich A, Gordon D, Johnston D, Onwujekwe O, Patel R, Webb EA, Lawlor DA, and Hargreaves JR. (2012). Measuring socio-economic position for epidemiological studies in low- and middle-income countries: a methods of measurement in epidemiology paper. Int J Epidemiol 41:871–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, et al. (1998). Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12:149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL. (2006). The effects of body mass index on lung volumes. Chest 130:827–833 [DOI] [PubMed] [Google Scholar]
- Jun JC, Shin MK, Devera R, Yao Q, Mesarwi O, Bevans-Fonti S, and Polotsky VY. (2014). Intermittent hypoxia-induced glucose intolerance is abolished by alpha-adrenergic blockade or adrenal medullectomy. Am J Physiol Endocrinol Metab 307:E1073–E1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, and Hardie DG. (2005). AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25 [DOI] [PubMed] [Google Scholar]
- Kannel WB, Hubert H, and Lew EA. (1983). Vital capacity as a predictor of cardiovascular disease: the Framingham study. Am Heart J 105:311–315 [DOI] [PubMed] [Google Scholar]
- Lee HM, Truong ST, and Wong ND. (2011). Evidence of lung function for stratification of cardiovascular disease risk. Korean Circ J 41:171–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Velarde F, Maggiorini M, Reeves JT, et al. (2005). Consensus statement on chronic and subacute high altitude disease. High Alt Med Biol 6:147–157 [DOI] [PubMed] [Google Scholar]
- Luks AM, and Swenson ER. (2011). Pulse oximetry at high altitude. High Alt Med Biol 12:109–119 [DOI] [PubMed] [Google Scholar]
- Mackenzie R, Maxwell N, Castle P, Brickley G, and Watt P. (2011). Acute hypoxia and exercise improve insulin sensitivity (S(I) (2*)) in individuals with type 2 diabetes. Diabetes Metab Res Rev 27:94–101 [DOI] [PubMed] [Google Scholar]
- Mackenzie R, Maxwell N, Castle P, Elliott B, Brickley G, and Watt P. (2012). Intermittent exercise with and without hypoxia improves insulin sensitivity in individuals with type 2 diabetes. J Clin Endocrinol Metab 97:E546–E555 [DOI] [PubMed] [Google Scholar]
- Malaga G, Zevallos-Palacios C, Lazo M de LA, and Huayanay C. (2010). [High frequency of dyslipidemia and impaired fasting glycemia in a high altitude Peruvian population]. Rev Peru Med Exp Salud Publica 27:557–561 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, and Turner RC. (1985). Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Medina-Lezama J, Pastorius CA, Zea-Diaz H, Bernabe-Ortiz A, Corrales-Medina F, Morey-Vargas OL, Chirinos DA, Munoz-Atahualpa E, Chirinos-Pacheco J, and Chirinos JA; On behalf of the PREVENCION Investigators. (2010). Optimal Definitions for Abdominal Obesity and the Metabolic Syndrome in Andean Hispanics: The PREVENCION Study. Diabetes Care 33:1385–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, and Wanger J; ATS/ERS Task Force. (2005). Standardisation of spirometry. Eur Respir J 26:319–338 [DOI] [PubMed] [Google Scholar]
- Miranda JJ, Bernabe-Ortiz A, Smeeth L, Gilman RH, and Checkley W; CRONICAS Cohort Study Group. (2012). Addressing geographical variation in the progression of non-communicable diseases in Peru: the CRONICAS cohort study protocol. BMJ Open 2:e000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge C. (1942). Life in the andes and chronic mountain sickness. Science 95:79–84 [DOI] [PubMed] [Google Scholar]
- Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, and Eisenberg MJ. (2010). The metabolic syndrome and cardiovascular risk. J Am Coll Cardiol 56:1113–1132 [DOI] [PubMed] [Google Scholar]
- Ortega-Saenz P, Pardal R, Levitsky K, Villadiego J, Munoz-Manchado AB, Duran R, Bonilla-Henao V, Arias-Mayenco I, Sobrino V, Ordóñez A, Oliver M, Toledo-Aral JJ, and López-Barneo J. (2013). Cellular properties and chemosensory responses of the human carotid body. J Physiol 591:6157–6173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BF, and Clegg DJ. (2014). Ascent to altitude as a weight loss method: the good and bad of hypoxia inducible factor activation. Obesity 22:311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen GL, Scalzo RL, Schweder MM, Larson DG, Luckasen GJ, Irwin D, Hamilton KL, Schroeder T, and Bell C. (2012). Sympathetic inhibition attenuates hypoxia induced insulin resistance in healthy adult humans. J Physiol 590:2801–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, and O'Donnell CP. (2004). Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 552:253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MSM, Zheng J, and Stocks J. (2012). Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 40:1324–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Butterfield GE, Cymerman A, Reeves JT, Wolfel EE, and Brooks GA. (1985). Acclimatization to 4,300-m altitude decreases reliance on fat as a substrate. J Appl Physiol 81:1762–1771 [DOI] [PubMed] [Google Scholar]
- Ryan S, Taylor CT, and McNicholas WT. (2005). Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112:2660–2667 [DOI] [PubMed] [Google Scholar]
- Sakagami H, Makino Y, Mizumoto K, Isoe T, Takeda Y, Watanabe J, Fujita Y, Takiyama Y, Abiko A, and Haneda M. (2014). Loss of HIF-1alpha impairs GLUT4 translocation and glucose uptake by the skeletal muscle cells. Am J Physiol Endocrinol Metab 306:E1065–E1076 [DOI] [PubMed] [Google Scholar]
- Schroeder EB, Welch VL, Couper D, Nieto FJ, Liao D, Rosamond WD, and Heiss G. (2003). Lung function and incident coronary heart disease: the atherosclerosis risk in communities study. Am J Epidemiol 158:1171–1181 [DOI] [PubMed] [Google Scholar]
- Semenza GL. (1999). Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 15:551–578 [DOI] [PubMed] [Google Scholar]
- Sherpa LY, Deji , Stigum H, Chongsuvivatwong V, Nafstad P, and Bjertness E. (2013). Prevalence of metabolic syndrome and common metabolic components in high altitude farmers and herdsmen at 3700 m in Tibet. High Alt Med Biol 14:37–44 [DOI] [PubMed] [Google Scholar]
- Sherpa LY, Deji , Stigum H, Chongsuvivatwong V, Thelle DS, and Bjertness E. (2010). Obesity in Tibetans aged 30–70 living at different altitudes under the north and south faces of Mt. Everest. Int J Environ Res Public Health 7:1670–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MK, Yao Q, Jun JC, Bevans-Fonti S, Yoo D-Y, Han W, Mesarwi O, Richardson R, Fu YY, Pasricha PJ, Schwartz AR, Shirahata M, and Polotsky VY. (2014). Carotid body denervation prevents fasting hyperglycemia during chronic intermittent hypoxia. J Appl Physiol 117:765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas E, and Spielvogel H. (2006). Chronic mountain sickness, optimal hemoglobin, and heart disease. High Alt Med Biol 7:138–149 [DOI] [PubMed] [Google Scholar]
- Whayne TF., Jr. (2014). Cardiovascular medicine at high altitude. Angiology 65:459–472 [DOI] [PubMed] [Google Scholar]
- Wheaton WW, and Chandel NS. (2011). Hypoxia. 2. Hypoxia regulates cellular metabolism. Am J Physiol Cell Physiol 300:C385–C393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO STEPwise Approach to Survalance (STEPS). Available at www.whoint/chp/steps/manual/en/indexhtml (last accessed May31, 2014)
- Wilson PW, D'Agostino RB, Parise H, Sullivan L, and Meigs JB. (2005). Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 112:3066–3072 [DOI] [PubMed] [Google Scholar]
- Woolcott OO, Castillo OA, Gutierrez C, Elashoff RM, Stefanovski D, and Bergman RN. (2014). Inverse association between diabetes and altitude: a cross-sectional study in the adult population of the United States. Obesity 22:2080–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Cardiovascular diseases (CVDs). Available at www.who.int/mediacentre/factsheets/fs317/en/ (last accessed January2015)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.