Abstract
Background: Although most studies of levothyroxine–liothyronine combination therapy employ once-daily hormone administration, the kinetics of once-daily liothyronine have been studied infrequently. The aim of this study was to document both the peak and trough serum triiodothyronine (T3) levels that occur with once-daily liothyronine administration, along with changes in thyroid-responsive parameters.
Methods: Participants with hypothyroidism were studied prospectively at an academic institution. Patients were switched from levothyroxine monotherapy to liothyronine monotherapy with 15 μg liothyronine for two weeks, and then continued liothyronine at doses of 30–45 μg for a further four weeks in an open-label, single-arm study. Weekly trough levels of T3 were documented. In addition, hourly T3 concentrations immediately following liothyronine tablet administration were documented for eight hours during the sixth week of therapy. Serum thyrotropin (TSH) and free thyroxine (fT4) concentrations were documented. Biochemical markers, markers of energy metabolism, anthropometric parameters, well-being, and hyperthyroid symptoms were also assessed.
Results: Mean serum TSH levels increased from 1.56 ± 0.81 mIU/L at baseline to 5.90 ± 5.74 mIU/L at two weeks and 3.84 ± 3.66 mIU/L at six weeks. Trough T3 levels decreased from 99.5 ± 22.9 to 91.9 ± 40.2 at two weeks and recovered to 96.1 ± 32.2 at six weeks. The peak T3 concentration after dosing of liothyronine during week 6 was 292.8 ± 152.3 ng/dL. fT4 levels fell once levothyroxine was discontinued and plateaued at 0.44 ng/dL at week 4. The sex hormone binding globulin (SHBG) concentration decreased at week 2 (p = 0.002). Hyperthyroid symptoms and SF36-PCS scores increased significantly at weeks 4–5 of liothyronine therapy (p = 0.04–0.005). Preference for liothyronine therapy increased from 6% to 39% over the study period.
Conclusions: Once-daily dosing of liothyronine at doses of 30–45 μg did not return serum TSH to the values seen during levothyroxine therapy. There were significant excursions in serum total and free T3 concentrations with once-daily therapy. Trials of combination therapy are likely to be associated with similar excursions, albeit of a lesser magnitude. Only the physical component score of the SF36 questionnaire and hyperthyroid symptoms changed significantly with conversion to liothyronine monotherapy. Sustained release preparations with stable serum T3 profiles may have entirely different outcomes.
Introduction
One of the more complex issues to be considered when treating hypothyroidism is how to ensure that patients undergoing treatment are restored to optimal health (1). Areas of uncertainty are whether quality of life is decreased in patients with hypothyroidism who are taking levothyroxine (LT4) monotherapy (1–5), whether the inherent problem with LT4 monotherapy is the failure of deiodinases to compensate adequately for the high thyroxine (T4)/triiodothyronine (T3) ratio in athyreotic patients (6), and whether combination therapy with LT4 and liothyronine (LT3) is able to reverse the quality-of-life impairments (1,7–9).
Several studies have shown decreased psychological well-being, more thyroid-related symptoms, more dissatisfaction, increased fatigue, more cognitive impairment, and decreased well-being in patients being treated for hypothyroidism compared with various control groups (1–4). A recent study has also shown that patients with hypothyroidism are at more risk of being diagnosed with a psychiatric disorder and being treated with anti-depressive or anti-anxiety medications (10).
The studies reported thus far have not clearly shown benefits of combination therapy on health-related quality of life, mood, or neurocognitive functioning (1). When combining the patients participating in crossover studies, similar numbers of patients had a preference for combination therapy (n = 128) as had no preference (n = 101). Combining the numbers from the parallel design studies, more patients had no preference (n = 573) than had a preference for combination therapy (n = 130) (1). Drawing conclusions from current studies is confounded by their heterogeneity. This heterogeneity involves study design, outcome measures, and results, and includes use of different T3 doses and frequency of dosing.
The failure of combination therapy studies to show benefits for patients could be associated with failure to achieve steadily sustained T3 concentrations, which could potentially have either direct or genomic effects. Of 13 combination therapy studies, nine used once-daily LT3 therapy, whereas four used twice daily therapy (1). The fluctuation in free T3 (fT3) levels during LT4/LT3 combination with daily administration of 10 μg LT3 has previously been shown in a study of 10 patients (11). The hypothesis that steady T3 levels are necessary for improved outcomes would need to be tested with either a sustained release preparation or, at a minimum, three times daily administration of current T3 preparation (12). When a clinical trial of a sustained release T3 preparation, given in a dose that is physiologic for humans, has been conducted, this will greatly advance the field. A metabolite of T3, T3 sulfate, has been shown to provide steady T3 levels for at least 48 hours, but this property has only been demonstrated in profoundly hypothyroid patients undergoing a thyroid hormone withdrawal protocol for treatment of differentiated thyroid cancer (13). The present study allowed documentation of the fluctuation of T3 levels, along with documentation of multiple other parameters during once-daily administration of T3 monotherapy.
Methods
Overview
This was a phase 2 study of doses of 15, 30, and 45 μg of Thyromax® (BCT303). This preparation is a LT3 tablet made with microcrystalline cellulose and magnesium stearate. It was hoped to have a sustained profile of T3 release, but as shown in a phase 1 study (NCT01581463), the preparation had a similar serum T3 profile to Cytomel (14). The present trial was registered at ClinicalTrials.gov as clinical trial NCT01800617. In this study, hypothyroid patients were initially treated with 15 μg LT3, and then increased to either 30 or 45 μg LT3 instead of their regular LT4. As designed, the aim of the study was to show for regulatory purposes that daily T3 administration provided comparable treatment of hypothyroidism to LT4. The present analysis does not concern any of the regulatory implications of the trial, but simply comments on the biochemical and clinical changes that were noted during the course of the trial.
Participation recruitment and study procedures
The study was approved by the Georgetown University Institutional Review Board. Participants aged 18–65 years with hypothyroidism of any etiology were recruited. They were required to have no significant medical problems, other than their thyroid disease, in order to be eligible for the study. Examples of medical conditions that resulted in exclusion included cardiac disease, pulmonary conditions, diabetes, and malignancy. Initial screening involved a questionnaire that was administered over the telephone. Patients were required to be taking a LT4 dose of ≥75 μg in order to select participants without significant endogenous thyroid function. Patients taking steroids or any medications known to affect thyroid hormone metabolism, thyroid hormone absorption, or thyroxine-binding globulin were also excluded. Women who were pregnant, lactating, or taking oral contraceptives were ineligible. Patients who appeared to be eligible for the study were scheduled for a visit to the Georgetown Clinical Research Unit (GCRU). They were then consented for the study and signed a written informed consent form. They were determined to be in general good health based on the results of medical history, physical examination, 12-lead electrocardiogram (ECG), and a screening thyrotropin (TSH). Women underwent a screening pregnancy test. Participants were eligible if clinical laboratory testing showed that they had a normal serum TSH (0.4–4.5 mIU/L) while taking LT4.
Eligible participants were studied for six weeks. On the first day of the study (baseline), the participants refrained from taking their usual LT4 tablet and reported to the GCRU in a fasting state, after not having eaten since 10:00 pm the previous day. Vital signs, including heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), respiratory rate (RR), temperature, and weight (BW), were obtained after a minimum of five minutes of rest in a seated position. Metabolic parameters were also documented: respiratory quotient and resting energy expenditure were measured using a Vmax™ Encore Metabolic Cart. An ECG was recorded. Baseline thyroid function tests (TSH, total T4, fT4, total T3, and fT3) were obtained, as well as measurement of thyroid hormone-responsive biochemical markers (lipid profile, sex hormone binding globulin [SHBG], and ferritin). Ten milliliters of blood were drawn for this and all subsequent weekly blood tests. Questionnaires documenting hyperthyroid symptoms, hypothyroid symptoms, preference for LT3, SF36 physical health score (PCS), and mental health score (MCS) were obtained. The participant was then given a week's supply of 15 μg LT3 tablets and asked to take this instead of their LT4. They returned to the GCRU one week later. At the second visit to the GCRU (wk 1–15), all assessments were repeated, and each participant was given a further one week's supply of 15 μg LT3 tablets.
One week later (wk 2–15), all assessments were repeated, and each participant was provided with a week's supply of 30 or 45 μg doses of LT3. If the patient's pre-study dose of LT4 was <100 μg, the dose provided was 30 μg. However, if the patient's pre-study dose of LT4 was ≥100 μg, the dose provided was 45 μg. The 45 μg dose of LT3 was given as both a 15 μg and a 30 μg tablet. At each of the four subsequent weekly visits (wk 3–30/45 to wk 5–30/45), all assessments were repeated, and a week's supply of LT3 was provided. If any patient had a TSH >5 mIU/L one week after starting their 30 μg or 45 μg dose, their daily dose was increased by 15 μg at their next visit, up to a maximum dose of 60 μg.
At the final study visit (wk 6–30/45), patients again presented to the GCRU prior to taking their LT3 dose. Baseline thyroid function tests (TSH, total T4, fT4, total T3, and fT3) and biochemical markers were obtained at 8:00 a.m., with 8:00 a.m. being designated as time 0. The patient's current dose of LT3 was administered orally at approximately 8:05 a.m. with a glass of water. Thyroid function tests (T3, fT3, fT4, T4, and TSH) were obtained 30 minutes after taking the T3, then at hourly intervals for eight additional samples. A set of vital signs and an electrocardiogram were obtained at four-hour intervals. Light meals were provided at approximately 9:30 a.m. and 12:00 noon.
Laboratory testing
Blood samples were collected in a plain red-top tube and allowed to clot. The resulting serum was sent to the main Georgetown laboratory for thyroid function tests, lipid profile, SHBG, and ferritin to be measured. For the week 6 pharmacokinetic study, the samples collected after LT3 administration were assayed for thyroid function tests only. For safety purposes, and so appropriate adjustments could be made in the LT3 dose, all samples were assayed on the day they were drawn. The reference range for the thyroid assays were TSH 0.4–4.5 mIU/L (immunometric assay), fT4 0.8–1.8 ng/dL, T4 4.7–13.3 μg/dL, fT3 2.18–3.98 pg/mL, and total T3 76–181 ng/dL (all immunoassays).
Statistical analysis
A change in each parameter (vital signs, metabolic parameters, thyroid function tests, biochemical markers, and questionnaire data) from the baseline to each following week was compared using repeated measures analysis of variance. Similar analysis was also performed to compare all weeks to the second week (when T3 values were lowest and TSH values were highest). Some parameters were log transformed to reduce skewness.
A relationship between the T4/T3 ratio and fT4/fT3 ratio and other parameters was tested using a mixed-effect model adjusting for time in weeks as a continuous fixed effect and random effect among patients. Some parameters were again log transformed to reduce skewness. A mixed linear model with time-varying covariates was also used to assess the association of T3 and fT3 concentrations with TSH concentrations, fT4 concentrations, T4 concentrations, vital signs, metabolic parameters, biochemical markers, and questionnaire data. Two-tailed p-values of <0.05 were considered statistically significant. Adjustment for multiplicity was performed with a Bonferroni correction.
The maximum observed concentration (Cmax) and the time at which this occurred (Tmax) were obtained from graphic representation of the data. The area under the curve (AUC) from 0.5 hours to 8 hours was calculated using the trapezoidal rule using the actual times of measurements. Baseline correction was performed using the concentration at 0.5 hours. Mean with standard deviation or median with interquartile range were calculated according to sex and race/ethnicity. The Wilcoxon rank sum test was used to compare the differences by sex and race/ethnicity. Pearson and Spearman correlation coefficients were calculated to examine the correlation of physiologic parameters (height, weight, body mass index, and age) with Cmax, Tmax, and AUC. The correlation between parameters was tested using a mixed-effect model adjusting for age, sex, and time.
Data were analyzed using SAS v9.3 (SAS Institute, Cary, NC).
Results
Thirty-one participants were screened for the study. Eighteen individuals were eligible and wished to participate, and all completed the entire study. The characteristics of these participants are shown in Table 1. Their mean age was 38.1 ± 10.1 years (range 24–56 years). Of the 18 subjects, 16 (89%) were women, and 72% were Caucasian, 17% African American, and 11% Asian or Hispanic. The average pre-study LT4 dose was 102 ± 30 μg. None of the participants were taking any confounding medications at the time of the study. As per the study protocol, all participants initially took 15 μg of LT3 daily. The final LT3 dose was 30 μg in nine participants, 45 μg in eight participants, and 60 μg in one participant (see Table 1). Participants completed the study between April 2013 and July 2014.
Table 1.
Patient Baseline Characteristics and Thyroid Hormone Doses
| Participant number | Sex | Age (years) | Race/ethnicity | Etiology of hypothyroidism | Patient weight (kg) | Serum TSH while taking LT4 (mIU/L) | LT4 dose (μg) | Weight-based LT4 dose (μg/kg) | Final LT3 dose (μg) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 25 | Hisp | Hashimoto's | 81.9 | 0.8 | 137 | 1.67 | 30 |
| 2 | F | 24 | Caus | Hashimoto's | 65 | 0.9 | 88 | 1.35 | 30 |
| 3 | F | 35 | Asian | Hashimoto's | 55.5 | 2.4 | 75 | 1.35 | 30 |
| 4 | M | 43 | Caus | Hashimoto's | 94.7 | 1.7 | 75 | 0.79 | 30 |
| 5 | F | 24 | Caus | Hashimoto's | 68.3 | 2.67 | 75 | 1.09 | 30 |
| 6 | F | 45 | Caus | Hashimoto's | 81 | 2.7 | 100 | 1.23 | 45 |
| 7 | F | 56 | AA | Hashimoto's | 66.5 | 1.8 | 112 | 1.68 | 45 |
| 8 | F | 31 | Caus | Hashimoto's | 79.6 | 1.2 | 100 | 1.25 | 45 |
| 9 | F | 40 | Caus | Hashimoto's | 78.9 | 0.8 | 137 | 1.73 | 45 |
| 10 | F | 41 | Caus | Hashimoto's | 69.2 | 0.98 | 75 | 1.08 | 30 |
| 11 | F | 32 | AA | Hashimoto's | 131.2 | 1.56 | 75 | 0.57 | 30 |
| 12 | F | 24 | Caus | Hashimoto's | 100.2 | 1.49 | 100 | 0.99 | 45 |
| 13 | F | 35 | Caus | Hashimoto's | 88.3 | 3.28 | 75 | 0.85 | 30 |
| 14 | F | 47 | Caus | Hashimoto's | 119 | 1.29 | 175 | 1.47 | 45 |
| 15 | F | 47 | Caus | Hashimoto's | 52.8 | 0.4 | 100 | 1.89 | 45 |
| 16 | M | 50 | AA | Thyroidectomy | 96.9 | 2.19 | 150 | 1.55 | 60 |
| 17 | F | 50 | Caus | Hashimoto's | 68.3 | 1.11 | 75 | 1.09 | 30 |
| 18 | F | 36 | Caus | Hashimoto's | 74.5 | 0.97 | 112 | 1.50 | 45 |
TSH, thyrotropin; LT4, levothyroxine; LT3, liothyronine; F, female; M, male; Hisp, Hispanic; Caus, Caucasian; AA, African American.
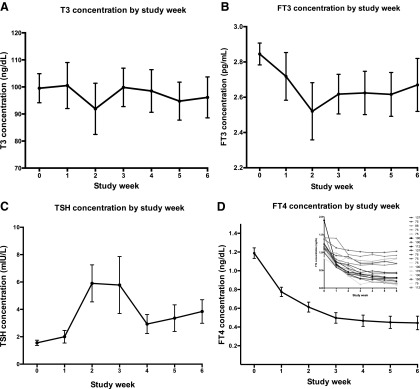
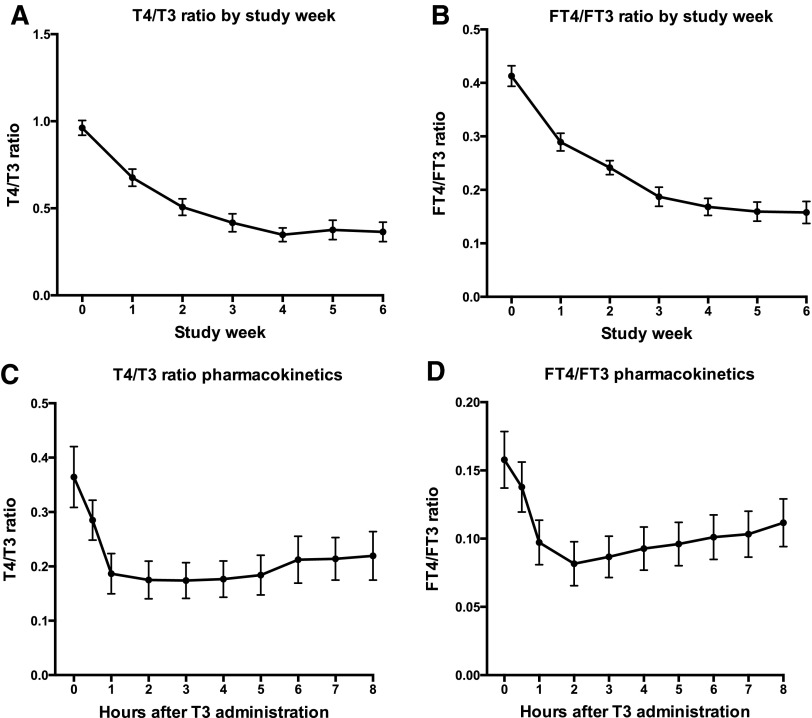
The changes in total T3, TSH, and fT4 over the successive weeks of the study are shown in Table 2. These analytes and changes in fT3 are depicted graphically in Figure 1. Trough T3 levels decreased from 99.5 ± 22.9 ng/dL to 91.9 ± 40.2 ng/dL at two weeks and had recovered to 96.1 ± 32.2 ng/dL at six weeks, although none of these changes were significant (Fig. 1A). Mean serum TSH levels increased significantly from 1.56 ± 0.81 mIU/L at baseline to 5.90 ± 5.74 mIU/L at two weeks (p = 0.0002). TSH values trended to normalize at week 4, but were again significantly elevated above baseline at 3.84 ± 3.66 mIU/L at six weeks (p = 0.003; Fig. 1C). fT4 concentration reached a nadir of 0.47 ng/dL by week 4 and remained steady and significantly decreased (p < 0.0001) at 0.44–0.45 ng/dL thereafter (Fig. 1D). The small graph shown as an insert shows how participants fell into two groups: those who maintained some residual T4 production, and those who had minimal T4 production. The LT4 dose (μg) of the participants is shown in the right-sided legend. Both T4/T3 and fT4/fFT3 ratios decreased as the study progressed, reaching a minimum value at four weeks and maintaining approximately the same value at weeks 5 and 6 (Fig. 2A and B). There was a negative correlation between TSH and T3; as T3 increased by one unit, log10 TSH decreased by 0.00751 (or TSH decreased by 0.751%; p < 0.0001). One unit in fT3 decreased log10 TSH by 0.4775 (TSH decreased by 47.75%; p < 0.0001).
Table 2.
Thyroid Analytes Over Study Week
| Week-T3 dose | N | M | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| A. T3 (ng/dL) concentrations over time | |||||
| Baseline | 18 | 99.54 | 22.90 | 77.20 | 174.60 |
| Wk 1–15 | 18 | 100.53 | 36.12 | 60.40 | 194.20 |
| Wk 2–15 | 18 | 91.91 | 40.16 | 38.00 | 183.00 |
| Wk 3–30/45 | 18 | 101.02 | 30.62 | 50.50 | 173.90 |
| Wk 4–30/45 | 18 | 98.51 | 33.47 | 69.90 | 189.50 |
| Wk 5–30/45 | 18 | 94.44 | 30.70 | 62.60 | 183.80 |
| Wk 6–30/45 | 18 | 96.14 | 32.16 | 59.60 | 184.60 |
| B. TSH (mIU/L) concentrations over time | |||||
| Baseline | 18 | 1.56 | 0.81 | 0.19 | 3.28 |
| Wk 1–15 | 18 | 2.00 | 1.95 | 0.22 | 7.80 |
| Wk 2–15 | 18 | 5.90 | 5.74 | 0.20 | 19.00 |
| Wk 3–30/45 | 18 | 5.87 | 9.10 | 0.08 | 31.40 |
| Wk 4–30/45 | 18 | 2.93 | 2.96 | 0.07 | 10.70 |
| Wk 5–30/45 | 18 | 3.36 | 4.12 | 0.07 | 16.50 |
| Wk 6–30/45 | 18 | 3.84 | 3.66 | 0.04 | 12.00 |
| C. fT4 concentrations (ng/dL) over time | |||||
| Baseline | 18 | 1.19 | 0.24 | 0.84 | 1.90 |
| Wk 1–15 | 18 | 0.78 | 0.21 | 0.58 | 1.40 |
| Wk 2–15 | 18 | 0.61 | 0.22 | 0.34 | 1.06 |
| Wk 3–30/45 | 18 | 0.51 | 0.24 | 0.10 | 1.02 |
| Wk 4–30/45 | 18 | 0.47 | 0.26 | 0.17 | 0.99 |
| Wk 5–30/45 | 18 | 0.45 | 0.28 | 0.15 | 1.00 |
| Wk 6–30/45 | 18 | 0.44 | 0.31 | 0.10 | 1.03 |
SD, standard deviation; T3, triiodothyronine; fT4, free thyroxine.
FIG. 1.
Thyroid analyte concentrations according to study week (mean ± standard error).
FIG. 2.
Total and free thyroid hormone ratios by study week and during the pharmacokinetic portion of the study (mean ± standard error).
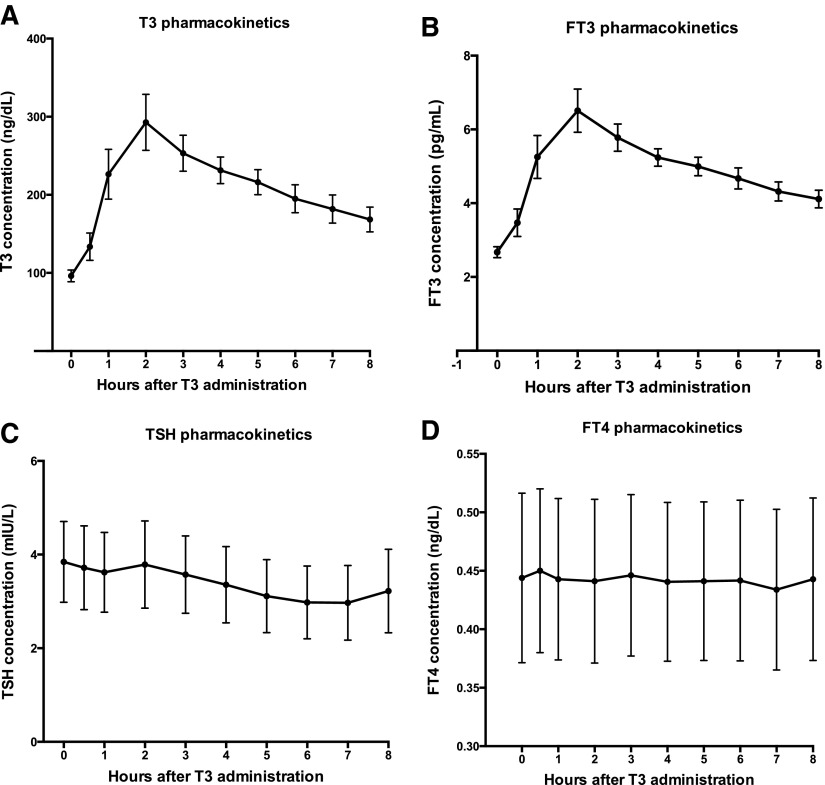
The pharmacokinetics after dosing of the LT3 are shown in Figure 3. The peak T3 concentration after LT3 administration during week 6 was 292.8 ± 152.3 ng/dL, rising from a baseline value of 96.1 ± 7.6 ng/dL (Fig. 3A). Similar patterns were seen in the trough and peak fT3 concentrations, with the trough being 2.67 ± 0.15 pg/mL and the peak 6.51 ± 2.48 pg/mL (Fig. 3B). The Tmax was 2.4 ± 1.5 h and 2.22 ± 1.26 h for T3 and fT3, respectively. The mean AUC 0–8 h for T3, corrected for baseline, was 655.6 ± 404.6 ng/h/dL. The mean AUC 0–8 h for fT3, corrected for baseline, was 12.4 ± 7.2 pg/h/dL. There were no differences in AUC, Cmax, or Tmax according to sex or age. Serum TSH and fT4 did not change acutely following the administration of the daily LT3 dose (Fig. 3C and D).
FIG. 3.
Thyroid analytes during the pharmacokinetic portion of the study during week 6 (mean ± standard error).
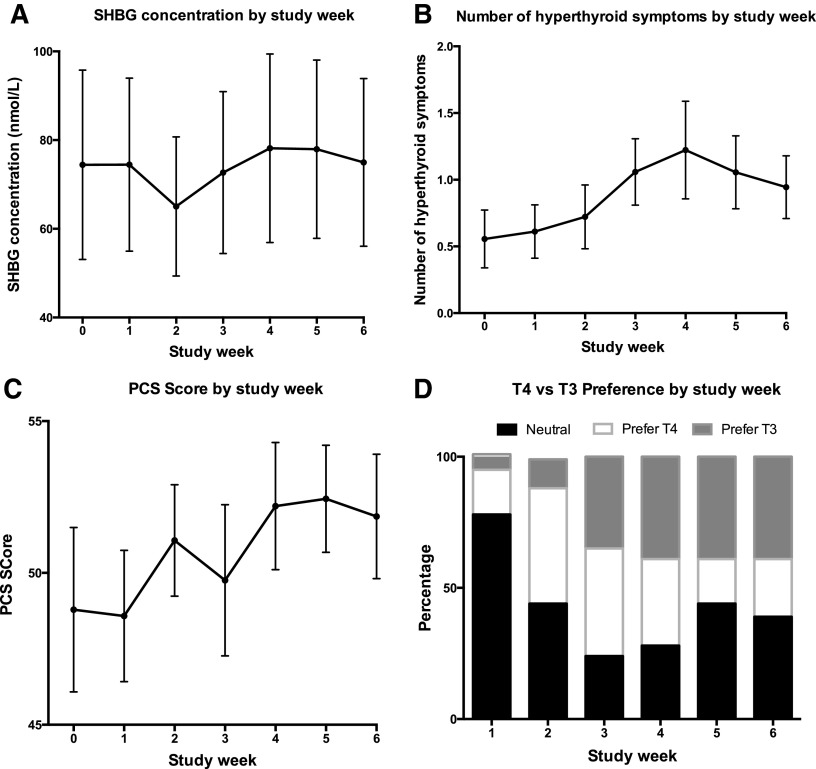
Vital signs (HR, temperature, SBP, DBP, RR) did not change during the study. The lowest values for weight and body mass index were at week 5, but these changes were not significant. Lipid profile and ferritin did not change with the conversion to T3 therapy. SHBG decreased significantly at week 2 when participants were taking 15 μg LT3 (p = 0.002), but was unchanged at weeks 0–1 compared with weeks 3–6 (Fig. 4A). Hyperthyroid symptoms were increased at weeks 4 and 5 (p = 0.005 and 0.03, respectively; Fig. 4B). SF36-PCS scores also increased significantly at weeks 4 and 5 (p = 0.04 and 0.03, respectively; Fig. 4C). Respiratory quotient, resting energy expenditure, hypothyroid symptoms, and SF36-MCS scores did not change during the study. Over the course of the study, there was no correlation between HR, weight, SF36-PCS, SF36-MCS, and hypothyroid symptoms and the serum T3 concentrations. However, there was a significant correlation between hyperthyroid symptoms and serum T3 levels (p = 0.04). For the six weeks that patients were taking T3, participant preference for T3 therapy increased, such that 39% of participants preferred T3 during weeks 4–6 (see Fig. 4D). The percentage preferring T4 plateaued at 17–22%; the percentage with no preference plateaued at 39–44%. None of these parameters had different trends in the six patients whose fT4 concentrations remained at >0.5 ng/dL by the end of the study compared with the 12 patients whose fT4 concentrations fell to <0.5 ng/dL (see insert in Fig. 1D).
FIG. 4.
(A)–(C) Changes in markers of thyroid status over the course of the study (mean ± standard error). (D) Changes in treatment preference.
Discussion
There are several potential reasons that trials of combination therapy versus monotherapy have not clearly demonstrated positive effects of the combination therapy. It could be that LT3 does not in fact have salutary effects. It could also be that positive effects will only be seen in therapy that produces relatively stable levels of T3, as are seen in normal physiology (15–17). Alternatively, it could be that the correct population, which may actually benefit from such therapy, such as those who are dissatisfied at baseline or affected by specific polymorphisms, has not yet been targeted. It is also possible that the initial study that suggested benefit (18), and also the patient preference seen in some studies (1), could be due to a placebo effect, a pharmacologic “drug effect,” or a need for T3 in specific tissues only that may not have been detected using the particular instruments used in some studies. If euthyroidism in specific tissues is particularly important, instruments would need to be included that assess the impact of T3 on that relevant tissue or organ.
A much-cited retrospective study raised the possibility that a positive response to use of combination therapy may be associated with the presence of a type 2 deiodinase polymorphism (19). Although prospective clinical trials that examine the outcomes of combination therapy based on deiodinase polymorphism status or activity of deiodinases have not yet been published, several preclinical investigations have focused on the issue of deiodinase activity. Mathematical modeling suggests decreased deiodinase activity, as well as decreased fT3/fT4 ratios, in LT4-treated patients, with decreases being more notable in athyreotic patients (20). As a complement to prior animal studies by Escobar-Morreale et al. (21,22), a recent study conducted in rats suggested impairment of type 2 deiodinase activity in the whole body during LT4 monotherapy due to deiodinase inactivation compared with maintenance of deiodinase activity in the hypothalamus (6). The lesser degree of inactivation in the hypothalamus leads to efficient T3 production in the hypothalamus and normalization of TSH before T3 normalizes in the rest of the body. Accompanying the inactivation of type 2 deiodinase in other tissues, lower serum T3 and higher T4/T3 ratios were seen in rats during monotherapy and, also importantly, with intermittent T3 administration compared with combination therapy employing a subcutaneous slow release T3 pellet.
The present study shows that daily administration of T3 was associated with peaks and troughs in T3 concentration. However, TSH and fT4 remained steady on both a weekly and hourly basis. Vital signs, anthropometric measures, metabolic parameters, and thyroid hormone-responsive biochemical markers did not change with T3 administration. Given the complete overlap of the Tmax of BCT303 with that of Cytomel, results of this present study should be generalizable to Cytomel (14). On the other hand, a study using three times daily administration of T3 in athyreotic patients that achieved steady T3 levels suggested that there may be hepatic effects of T3, as well as effects on body weight, without effects on heart rate or resting energy expenditure (23).
This study has multiple limitations. The pharmacokinetic portion of the study was eight hours in duration, rather than 24 hours. A small number of participants were studied in a non-controlled manner in a short duration study examining surrogate outcomes. The limitation of being single-arm and unblinded is somewhat less concerning from the standpoint of a placebo effect, as there was no significant effect on parameters. The small group of participants studied was also heterogeneous with respect to sex and race/ethnicity, but consisted mostly of women, thus comprising the group most affected by hypothyroidism. Another limitation of the study is that it is clear from the mean fT4 value achieved after six weeks off LT4 therapy that many of these individuals were not athyreotic but were able to maintain endogenous T4 production. The requirement for patients to be taking ≥75 μg of LT4 in order to be eligible for the study was intended to exclude patients with residual thyroid function. However, clearly, a higher dose requirement such as 88 or 100 μg of LT4 should have been chosen. An additional limitation is that only LT3 tablets of 15 μg and 30 μg were available, and so alterations in T3 dose could only be made in 15 μg increments. The T3 doses employed did not normalize TSH back to baseline, possibly accounting for failure to observe changes with LT3 therapy. This was due to a shortcoming in the protocol, which did not allow for further adjustment of the LT3 dose unless the serum TSH was >5 mIU/L.
However, the strength of this study is the documentation of multiple parameters after conversion to LT3 therapy. It should be noted that SHBG values did not change during the study, with the exception of decreasing on the smaller 15 μg dose of LT3. Although the participants were not devoid of circulating T4, the T3/T4 ratio did change substantially during the study. Thus, this study has the potential to be able to show alterations in surrogate outcomes due to switching patients from once-daily LT4 monotherapy to once-daily LT3 monotherapy.
Despite the study limitations, the data generated serve a useful purpose. They show that repeated once-daily administration of T3 is associated with substantial peaks and troughs, which would have been occurring, although to a lesser magnitude, in the 9 of 13 combination therapy trials that employed once-daily administration (1). The significance of these peaks and troughs may be that they do not have the same downstream effects as steadily sustained T3 concentrations. The difference between intermittent versus continuous delivery of T3 was demonstrated in a rat study, where only the continuous delivery replicated the higher T3 levels seen in intact animals, accompanied by closer replication of the measures of skeletal muscle functioning, liver functioning, and mRNA levels of T3-responsive genes seen in controls (6). There has been considerable discussion about whether serum T3 levels should be a therapeutic target during thyroid hormone treatment and whether LT3 therapy should be added to LT4 (1). Traditionally, T3 levels have not been thought to be a target, but this issue has been described as an area needing further research (1). Serum T3 levels are proposed as a potential target by others (24). In the latter study, low FT3 levels were used to identify those who were less efficient in converting T4 to T3 and who might potentially benefit from combination therapy. Unfortunately, due to fluctuating T3 levels with the daily therapy, short study duration, and absence of a control group, this study does not establish physiologic or psychologic consequences of raising T3 levels that would inform this debate.
It could be hypothesized that the lack of convincing superiority of combination therapy in terms of health outcomes in the prior multiple trials could be linked to failure to maintain constant T3 levels, as are seen in normal physiology. Certainly, the present study failed to show any change in vital signs, anthropometric measures, metabolic parameters, and thyroid hormone-responsive biochemical markers. The sole changes observed were slight increases in SF36 PCS scores and hyperthyroid symptoms at weeks 4 and 5 and associated preference for T3. It is unclear whether this preference would have been sustained; certainly, the preference expressed cannot be relied on, as the study was uncontrolled.
In conclusion, it is possible that based on the lack of steadily maintained T3 levels in trials conducted thus far, and the different results seen in animal studies with provision of steady T3 levels compared with those seen with non-physiologic fluctuations in T3 levels, combination therapy has not been adequately tested. Combination therapy cannot be considered to have no advantages over monotherapy until a well-designed long-term trial of combination therapy with a sustained release or, at a minimum, three times daily administration has been conducted. Three times daily therapy is challenging with respect to patient adherence (12). The potential for a sustained release T3 is clearly limited by the maximum time over which absorption takes place in the gastrointestinal tract. Once any T3 preparation has been absorbed, its half-life would be similar to conventional T3. Efforts to create an extended release product are thus likely to be focused on a T3 product that has a prolonged residence or delayed breakdown in the gastrointestinal tract.
Acknowledgments
The authors could not have performed this study without the invaluable expert assistance of Amber Surian who served as study coordinator. The authors also wish to thank sincerely the members of the data safety and monitoring board: Dr. Meeta Sharma, Dr. Andrea Singer, Dr. Jane Otado, and Dr. Alice Aukaegbu for their dedicated service and generous donation of their time.
J.J. is supported by Federal funds from Grant # R01AG033867 and also Grant # UL1TR001409 (previously UL1TR000101) from the National Center for Advancement of Translational Science, NIH, through the Clinical and Translational Science Awards Program (CTSA), a trademark of DHHS, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise. Statistical analyses were provided by the Design, Biostatistics, and Population Studies component of the Georgetown-Howard Universities Center for Clinical and Translational Science.
K. R. Latham of ITL Pharma, Kingsport, TN, provided drug and participant compensation for this study. KRL holds the IND for the T3 preparation used in this study (BCT303). KRL is the inventor and ITL Pharma is the manufacturer of BCT303.
Author Disclosure Statement
K.D.B. serves on the Food and Drug Administration Endocrine Advisory Committee as an ad hoc member. J.J. has no other conflicts of interest relevant to this article.
References
- 1.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM. 2014. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid 24:1670–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louwerens M, Appelhof BC, Verloop H, Medici M, Peeters RP, Visser TJ, Boelen A, Fliers E, Smit JW, Dekkers OM. 2012. Fatigue and fatigue-related symptoms in patients treated for different causes of hypothyroidism. Eur J Endocrinol 167:809–815 [DOI] [PubMed] [Google Scholar]
- 3.Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM. 2002. Psychological well-being in patients on “adequate” doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol 57:577–585 [DOI] [PubMed] [Google Scholar]
- 4.Wekking EM, Appelhof BC, Fliers E, Schene AH, Huyser J, Tijssen JG, Wiersinga WM. 2005. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur J Endocrinol 153:747–753 [DOI] [PubMed] [Google Scholar]
- 5.Eskelinen SI, Vahlberg TJ, Isoaho RE, Lopponen MK, Kivela SL, Irjala KM. 2007. Associations of thyroid stimulating hormone and free thyroxine concentrations with health and life satisfaction in elderly adults. Endocr Pract 13:451–457 [DOI] [PubMed] [Google Scholar]
- 6.Werneck de Castro JP, Fonseca TL, Ueta CB, McAninch EA, Abdalla S, Wittmann G, Lechan RM, Gereben B, Bianco AC. 2015. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Invest 125:769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grozinsky-Glasberg S, Fraser A, Nahshoni E, Weizman A, Leibovici L. 2006. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 91:2592–2599 [DOI] [PubMed] [Google Scholar]
- 8.Ma C, Xie J, Huang X, Wang G, Wang Y, Wang X, Zuo S. 2009. Thyroxine alone or thyroxine plus triiodothyronine replacement therapy for hypothyroidism. Nucl Med Commun 30:586–593 [DOI] [PubMed] [Google Scholar]
- 9.Joffe RT, Brimacombe M, Levitt AJ, Stagnaro-Green A. 2007. Treatment of clinical hypothyroidism with thyroxine and triiodothyronine: a literature review and metaanalysis. Psychosomatics 48:379–384 [DOI] [PubMed] [Google Scholar]
- 10.Thvilum M, Brandt F, Almind D, Christensen K, Brix TH, Hegedus L. 2014. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: a nationwide register study. Thyroid 24:802–808 [DOI] [PubMed] [Google Scholar]
- 11.Saravanan P, Siddique H, Simmons DJ, Greenwood R, Dayan CM. 2007. Twenty-four hour hormone profiles of TSH, free T3 and free T4 in hypothyroid patients on combined T3/T4 therapy. Exp Clin Endocrinol Diabetes 115:261–267 [DOI] [PubMed] [Google Scholar]
- 12.Celi FS, Zemskova M, Linderman JD, Babar NI, Skarulis MC, Csako G, Wesley R, Costello R, Penzak SR, Pucino F. 2010. The pharmacodynamic equivalence of levothyroxine and liothyronine: a randomized, double blind, cross-over study in thyroidectomized patients. Clin Endocrinol 72:709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santini F, Giannetti M, Ricco I, Querci G, Saponati G, Bokor D, Rivolta G, Bussi S, Braverman LE, Vitti P, Pinchera A. 2014. Steady-state serum T3 concentrations for 48 hours following the oral administration of a single dose of 3,5,3′-triiodothyronine sulfate (T3S). Endocr Pract 20:680–689 [DOI] [PubMed] [Google Scholar]
- 14.Jonklaas J, Burman KD, Wang H, Latham KR. 2015. Single-dose T3 administration: kinetics and effects on biochemical and physiological parameters. Ther Drug Monit 37:110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen S, Bruun NH, Pedersen KM, Laurberg P. 2003. Biologic variation is important for interpretation of thyroid function tests. Thyroid 13:1069–1078 [DOI] [PubMed] [Google Scholar]
- 16.Brabant G, Brabant A, Ranft U, Ocran K, Köhrle J, Hesch RD, von zur Mühlen A. 1987. Circadian and pulsatile thyrotropin secretion in euthyroid man under the influence of thyroid hormone and glucocorticoid administration. J Clin Endocrinol Metab 65:83–88 [DOI] [PubMed] [Google Scholar]
- 17.Azukizawa M, Pekary AE, Hershman JM, Parker DC. 1976. Plasma thyrotropin, thyroxine, and triiodothyronine relationships in man. J Clin Endocrinol Metab 43:533–542 [DOI] [PubMed] [Google Scholar]
- 18.Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ., Jr 1999. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med 340:424–429 [DOI] [PubMed] [Google Scholar]
- 19.Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, Dayan CM. 2009. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab 94:1623–1629 [DOI] [PubMed] [Google Scholar]
- 20.Hoermann R, Midgley JE, Larisch R, Dietrich JW. 2015. Integration of peripheral and glandular regulation of triiodothyronine production by thyrotropin in untreated and thyroxine-treated subjects. Horm Metab Res 47:674–680 [DOI] [PubMed] [Google Scholar]
- 21.Escobar-Morreale HF, del Rey FE, Obregon MJ, de Escobar GM. 1996. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology 137:2490–2502 [DOI] [PubMed] [Google Scholar]
- 22.Escobar-Morreale HF, Obregon MJ, Escobar del Rey F, Morreale de Escobar G. 1995. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest 96:2828–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celi FS, Zemskova M, Linderman JD, Smith S, Drinkard B, Sachdev V, Skarulis MC, Kozlosky M, Csako G, Costello R, Pucino F. 2011. Metabolic effects of liothyronine therapy in hypothyroidism: a randomized, double-blind, crossover trial of liothyronine versus levothyroxine. J Clin Endocrinol Metab 96:3466–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Midgley JEM, Larisch R, Dietrich JW, Hoermann R. 2015. Variation in the biochemical response to L-thyroxine therapy and relationship with peripheral thyroid hormone conversion efficiency. Endocr Connect 4:196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]