Abstract
White matter microstructure forms a complex and dynamical system that is critical for efficient and synchronized brain function. Neuroimaging findings in children with autism spectrum disorder (ASD) suggest this condition is associated with altered white matter microstructure, which may lead to atypical macroscale brain connectivity. In this study, we used diffusion tensor imaging measures to examine the extent that white matter tracts are interrelated within ASD and typical development. We assessed the strength of inter-regional white matter correlations between typically developing and ASD diagnosed individuals. Using hierarchical clustering analysis, clustering patterns of the pairwise white matter correlations were constructed and revealed to be different between the two groups. Additionally, we explored the use of graph theory analysis to examine the characteristics of the patterns formed by inter-regional white matter correlations and compared these properties between ASD and typical development. We demonstrate that the ASD sample has significantly less coherence in white matter microstructure across the brain compared to that in the typical development sample. The ASD group also presented altered topological characteristics, which may implicate less efficient brain networking in ASD. These findings highlight the potential of graph theory based network characteristics to describe the underlying networks as measured by diffusion magnetic resonance imaging and furthermore indicates that ASD may be associated with altered brain network characteristics. Our findings are consistent with those of a growing number of studies and hypotheses that have suggested disrupted brain connectivity in ASD.
Key words: : autism spectrum disorder, brain network, diffusion tensor imaging, graph theory, network analysis, white matter microstructure
Introduction
Autism spectrum disorder (ASD) is a complex, heterogeneous neurodevelopmental condition diagnostically defined by a triad of core features that cluster together within individuals: qualitative differences in social interaction and social communication, and repetitive and stereotyped behaviors and interests (American Psychiatric Association, 2013). Though the exact etiology of ASD is unknown, increasing evidence from neuroimaging research suggests that aberrant brain connectivity may contribute to these cognitive and behavioral deficits (Belmonte et al., 2004; Lainhart, 2006; Minshew and Williams, 2007; Müller et al., 2011; Vissers et al., 2012), impairing the ability to integrate and communicate information across the brain (Just et al., 2004). Essential to the normative functioning of the brain is the white matter microstructure, which links discrete gray matter regions together and coordinates highly efficient, temporally precise communication between them (Pajevic et al., 2014), thus providing the structural foundation of brain connectivity. Due to the inherent role that the brain's white matter has in facilitating brain messaging and communication, such deviations in neural connectivity may implicate atypical white matter microstructure in the functional and cognitive deficits observed within the ASD phenotype (Ameis and Catani, 2015; Travers et al., 2012).
A number of anatomical and microstructural neuroimaging findings have shown ASD to be associated with altered cerebral white matter, with the majority of such results coming from magnetic resonance imaging (MRI) volumetry and diffusion tensor imaging (DTI) (Ameis and Catani, 2015; Lange et al., 2010, 2015; Prigge et al., 2013a; Travers et al., 2012). For example, the largest of the commissural white matter pathways, the corpus callosum, has been found to be reduced in size (Egaas et al., 1995; Prigge et al., 2013b) and have reduced white matter integrity in ASD (Alexander et al., 2007a; Lange et al., 2010). Such atypicalities in the corpus callosum have more recently been shown to occur longitudinally throughout development (Lange et al., 2015; Travers et al., 2015) and begin as early as 6 months (Wolff et al., 2015). Other imaging studies have found reduced white matter integrity throughout numerous tracts in the brain, including the cingulum bundle (Barnea-Goraly et al., 2004), arcuate fasciculus (Barnea-Goraly et al., 2010; Fletcher et al., 2010; Noriuchi et al., 2010), internal capsules (Brito et al., 2009; Shukla et al., 2011), frontal white matter pathways (Courchesne and Pierce, 2005a), temporal-parietal junctions (Barnea-Goraly et al., 2010; Brieber et al., 2007), and others. For a more complete review, see (Just et al., 2012; Schipul et al., 2011; Travers et al., 2012). Moreover, the recurrent finding of abnormal brain maturation in individuals with autism has been revealed as a hallmark characteristic of ASD and thought to be a result of atypical white matter development during early infancy (Belmonte et al., 2004; Courchesne and Pierce, 2005b; Courchesne et al., 2007, 2004; Lainhart et al., 1997; Lange et al., 2015, 2010; Piven et al., 1995; Stevenson et al., 1997; Travers et al., 2015; Wolff et al., 2015). Together, the observations of atypical integrity and development suggests that the white matter microstructure has a significant role in the biological basis and pathogenesis of ASD.
Additionally, there is substantial heterogeneity in the phenotype of ASD, and it is unclear whether ASD is associated with atypical white matter microstructure across multiple tracts of the brain or if these atypicalities are more localized. In addition to understanding how brain features may differ between ASD and typical development (TD), it is equally important to understand how the microstructure of the brain covaries with other brain regions in TD and ASD and examine how such features of neural microstructure may differ between these two groups (Zielinski et al., 2012). In particular, significant microstructural correlations of DTI parameters have been shown to exist in normal adult populations and are hypothesized to reflect anatomic and/or functional similarities between white matter tracts (Li et al., 2012; Wahl et al., 2010). Moreover, changes of microstructural correlations from birth to puberty suggest that underlying developmental processes are important for the strengthening of inter-tract correlations, which may allow the brain to meet the demands of more advanced cognitive processes as one transitions from adolescence to early adulthood (Mishra et al., 2013). Understanding such “signatures” regarding the white matter microstructure within ASD and how these characteristics may differ between that of typically developing individuals are therefore critical. If white matter microstructural abnormalities are localized to specific tracts in ASD, then the microstructural white matter tracts may be less coherent in ASD than in TD. Also, variations in white matter tract properties across the brain over a broad age range may provide information regarding abnormal brain development. Such information may provide valuable insight to identify meaningful subgroups within the autism spectrum.
The development of complex network analysis (Boccaletti et al., 2006; Strogatz, 2001) has begun to allow neuroimaging research to abstractly view the brain as a set of elements (nodes) that are interrelated to one another via functional and/or structural associations (edges) (Rubinov and Sporns, 2010; Zalesky et al., 2010). Such graph models have been utilized to investigate relationships between brain structure and function and used as a tool to characterize the network topology and organization of the brain (Bullmore and Sporns, 2009; Sporns and Kötter, 2004; Zalesky et al., 2010). Graph metrics such as modularity, small-worldness, clustering, local and global efficiency, and others (Rubinov and Sporns, 2010) have been used to quantify the complex topology of these brain networks into meaningful features (Bullmore and Sporns, 2009). Furthermore, these characteristic properties have been observed to differ in Alzheimer's disease (Sanz-Arigita et al., 2010; Zhao et al., 2012), multiple sclerosis (He et al., 2009), schizophrenia (Alexander-Bloch et al., 2010; Bassett et al., 2008; Liu et al., 2008), attention deficit and hyperactivity disorder (Liu et al., 2015; Wang et al., 2009), epilepsy (Bernhardt et al., 2011) and in infants with intrauterine growth restriction (Batalle et al., 2012). These network analyses have been used to examine correlation-based networks derived from measures of cortical thickness, structural MRI, and other neuroimaging techniques (Bassett and Bullmore, 2009), and have been used to identify topological alterations in clinical diseases and disorders including, epilepsy (Bernhardt et al., 2011), Alzheimer's disease (Phillips et al., 2015), and others (Guye et al., 2010). These results provide converging evidence to support the potential of these MRI-based network measures as possible means that can be utilized to glean information about the structure of correlation-based networks and monitor neurodevelopmental and neurological disorders.
Given the involvement and diversity of white matter microstructure abnormalities that are reported in ASD (Just et al., 2012; Lange et al., 2015, 2010; Schipul et al., 2011; Travers et al., 2015, 2012), investigating the characteristics of the inter-regional correlations of white matter integrity may provide insight into the structural coherence of underlying white matter tracts in ASD. Specifically, it would be important to understand whether or not observed white matter atypicalities are consistently widespread in ASD, consistently specific in ASD, or a mix of consistent and inconsistent. Characterization of the intricate patterns of white matter between ASD and TD would therefore be particularly informative about the intrinsic organization of underlying white matter microstructure and potentially enhance our understanding of how deviant white matter may lead to cognitive and behavioral impairments in ASD.
In this study, DTI was used to investigate whether patterns of inter-regional correlation differed in a large, cross-sectional sample of individuals with ASD and typical development. We constructed correlation matrices from measures of fractional anisotropy and assessed the strength of the inter-correlations between these two groups. Using hierarchical clustering, white matter regions and tracts were grouped to reveal patterns of microstructural relatedness and to compare the observed patterns between those of typical development and ASD. Finally, in an exploratory analysis, metrics from complex network analysis were used to examine the network characteristics of white matter inter-regional correlation networks. We evaluated the ability of diffusion MRI measures to demonstrate group differences in global brain network features from inter-regional correlations. Additional analyses examined whether ASD and TD groups differed in global and local complex network features of the brain, such as mean degree, characteristic path length, global and local efficiency, mean clustering coefficient, modularity, and small-worldness.
Materials and Methods
Participants
The study protocol was approved by both the University of Utah and University of Wisconsin-Madison Institutional Review Boards. Participants included 92 males with ASD and 43 TD nonpsychiatric control males between the ages of 3.3 and 36.8 years. Consent was obtained for all participants, and both parental consent and participant assent were obtained for participants under the age of 18 years. This cross-sectional cohort of individuals was selected from a broader sample of ASD and TD individuals that have been imaged and assessed as part of on-going longitudinal investigation of brain and behavioral development in ASD from childhood into adulthood (Lange et al., 2015; Travers et al., 2015, 2014; Zielinski et al., 2012, 2014). Exclusion criteria included history of severe head injury, seizure disorder, hypoxia-ischemia, genetic disorder associated with ASD (identified with fragile-X testing or karyotype), known medical cause of ASD diagnosis (e.g., known patient history, and physical exam), and/or other neurological disorders. All 135 participants were recruited, assessed, and underwent MRI scanning at the University of Utah. See Table 1 for additional participant information.
Table 1.
Summary of Subject Characteristic Including Age, Total Motion Index, Height, Head Circumference, Verbal IQ, Performance IQ, and Full-Scale IQ for the Complete Investigated Cohort
| ASD group | TD group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N = 92 | N = 43 | ||||||||
| Range | Median | Mean | SD | Range | Median | Mean | SD | p | |
| Age (years) | 3.33–36.83 | 12.38 | 13.99 | 7.94 | 4.0–29.5 | 16.17 | 16.24 | 6.39 | 0.1078 |
| TMI | 0.69–6.10 | 1.88 | 2.00 | 0.94 | 0.45–6.70 | 2.01 | 2.32 | 1.16 | 0.1164 |
| Height (cm) | 76.45–196.5 | 158.50 | 149.59 | 27.47 | 112–194.2 | 171.75 | 163.84 | 22.54 | 0.008 |
| HC (cm) | 49.0–60.7 | 54.40 | 54.77 | 2.86 | 51.8–59.3 | 56.35 | 55.86 | 2.13 | 0.0441 |
| VIQ | 51–145 | 98.0 | 97.39 | 22.73 | 94–151 | 116.0 | 116.58 | 13.64 | <0.001 |
| PIQ | 64–135 | 100.0 | 100.63 | 17.84 | 90–155 | 114.0 | 117.97 | 16.44 | <0.001 |
| FSIQ | 61–137 | 97.0 | 98.15 | 20.03 | 95–153 | 117.0 | 119.16 | 15.36 | <0.001 |
Ranges, medians, means, and standard deviations (SD) are provided. All subjects were males. Between group differences were calculated using independent sample t-tests.
Bold values denote significant (p < 0.05) difference between ASD and TD groups.
ASD, autism spectrum disorder; FSIQ, full-scale IQ; HC, head circumference; PIQ, performance IQ; TD, typical development; TMI, total motion index; VIQ, verbal IQ.
Participants with ASD were diagnosed according to the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994), Autism Diagnostic Observation Schedule-Generic (ADOS-G) (Lord et al., 2000), Diagnostic Statistical Manual-IV (American Psychiatric Association, 1994), and the International Statistical Classification of Diseases and Related Health Problems-10th revision (ICD-10) criteria (World Health Organization, 2007). Standardized psychiatric assessment, neuropsychological assessment, IQ testing, and assessment with the ADOS-G (Lord et al., 2000) were performed on TD participants to confirm typical development. Typically developing and ASD participants did not significantly differ on age t(133) = 1.62, p = 0.11, however, participants diagnosed with ASD had a significantly decreased full-scale IQ (FSIQ), t(133) = 5.96, p < 0.001.
Imaging protocol
Magnetic resonance images were collected on a Siemens Tim Trio 3.0 T scanner equipped with an eight-channel receive-only head radio-frequency coil. Diffusion-weighted imaging (DWI) data were obtained using a single shot spin-echo echo-planar imaging pulse sequence. Bipolar gradients with dual-echo refocusing was used to reduce eddy currents (Reese et al., 2003). Parallel acquisition, with a geometric reduction factor of two, was used to reduce image distortions from magnetic field inhomogeneity and reduce acquisition time. Imaging parameters consisted of repetition time (TR) = 7000 ms, echo time (TE) = 84 ms, averages = 4, and bandwidth = 1346 Hz/pixel. Imaging field of view was 25 × 25 cm with an acquisition matrix of 128 × 128, providing a 2 × 2 mm in-plane resolution. Coverage across the cerebrum and cerebellum was achieved by acquiring 60 axial-oriented contiguous slices with a slice thickness of 2.5 mm. Diffusion data were acquired with diffusion encoded along 12 noncollinear directions with b = 1000 sec/mm2 and a single nondiffusion weighted (b = 0 sec/mm2) image. The total time of the DTI acquisition was 6.5 min.
In addition to DWI data, a wide range of pulse sequences, including a 3D MP-RAGE, 2D proton-density, T2-weighted, and 2D FLAIR, were collected for clinical review but were not used in this study.
Image analysis
All image processing and analyses were conducted at the University of Wisconsin-Madison. Individual diffusion weighted images were co-registered to account for any subtle distortion, translation, and rotation from bulk head motion and eddy currents using an affine registration tool (Jenkinson et al., 2002) from the FMRIB software library (FSL; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) software suite. Gradient directions were additionally corrected for rotations (Leemans and Jones, 2009). Nonparenchyma signals were removed using FSL's brain extraction tool (BET) (Smith, 2002) and diffusion tensors were fit at each voxel using the robust estimation of tensors by outlier rejection (RESTORE) (Chang et al., 2005) algorithm as part of the Camino software package (Cook et al., 2006). Eigenvalues (λ1, λ2, λ3) were calculated from these voxel-wise estimates of the diffusion tensor and quantitative maps of fractional anisotropy (FA) were then derived (Basser and Pierpaoli, 1996).
DTI-TK was used to generate a population-specific template using affine and diffeomorphic diffusion tensor registration (Zhang et al., 2006) using the full diffusion tensors. White matter tracts from the JHU ICBM-DTI-81 template (Mori et al., 2005; Oishi et al., 2008) were spatially aligned to this population template using the Advanced Normalization Tools (ANTs) diffeomorphic spatial registration algorithms (Avants et al., 2008) and nearest neighbor interpolation. The normalized JHU ICBM-DTI-81 template was then warped into each subject's native space by applying the inverse of the spatial transformations estimated in the population-specific template generation step. Native-space FA maps were subsequently parcellated into the 48 white matter tracts (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/brain) contained within this template and the median FA value for each tract was computed for each participant. The median FA was selected as the measure of interest for a given region rather than the mean, as the median is less sensitive to voxels with extreme values (Travers et al., 2015).
Comparison of ASD and TD white matter correlation
To evaluate white matter structural relatedness, a matrix of the correlation between FA of each pair of white matter tracts was generated for the ASD and TD groups. Each element of these symmetric, 48 × 48 correlation matrices was defined as the nonparametric Spearman's rank correlation coefficient between the medians FA of two white matter tracts. It is well known that FA changes with age (Lange et al., 2010; Lebel and Beaulieu, 2011; Travers et al., 2015; Walker et al., 2012) and therefore before calculating correlation coefficients between white matter tract pairs, generalized additive models were used to regress the effects of age out of the pairwise FA correlations. These semi-parametric methods were used because age-related changes may not be fully captured by specific parametric models (Travers et al., 2015). A total motion index (TMI) was additionally calculated for each participant to account for the effects of potential group differences in head motion during scanning, as described (Benner et al., 2011; Yendiki et al., 2014). Measures of TMI suggested that head motion did not differ between groups (p = 0.12), however, this measure was included as a covariate in the regression models to ascertain that group differences were not a result of differences in head motion. The residuals from this regression were determined and replaced the raw FA values in computing the correlation coefficients between two pairs of white matter tracts. Box's M-test (Box, 1954) was used to examine significant (p < 0.001) statistical differences between the correlation matrices of the ASD and TD groups (i.e., whether the TD correlation matrix was more consistent across the cells than the ASD correlation matrix).
Once the DTI-derived correlation matrices were found to be significantly different between typically developing and ASD individuals, the discrete entries of the correlation matrices were compared between both groups. Spearman's rank correlation coefficients were transformed to z scores using Fisher's z-transformation (Fisher, 1915) and the strength of the pairwise inter-regional correlations were compared between the autism and TD groups.
Hierarchical clustering of inter-regional correlation matrices
Hierarchical clustering was employed to identify patterns of microstructural relatedness within the typical development and ASD groups, as previously described (Mishra et al., 2013; Wahl et al., 2010). For clustering purposes, the quantity 1-ρ, where ρ corresponds to the Spearman's rank correlation coefficient, was calculated for all entries of the group-specific correlation matrices and used as a measure of dissimilarity between white matter regions (Mishra et al., 2013; Wahl et al., 2010). The hierarchical clustering analysis was performed using the “hclust” function within R version 3.2.1, while the pvclust” function in R (Suzuki and Shimodaira, 2006) was used to generate p-values of the formed clusters by performing bootstrap resampling with 10,000 repetitions. An unbiased p-value of 0.05, corresponding to a 95% confidence level, was selected as the threshold for determining whether formed clusters were significant.
Effects of FSIQ and age on microstructural relatedness
To ensure that observed differences from the previous analysis did not result from the significant differences in FSIQ scores between the ASD and TD groups (Table 1), we performed a secondary analysis by repeating the above procedure on a subset of ASD and TD individuals, matched for age and FSIQ (Supplementary Table S2). Correlation matrices for these subgroups were calculated in the same way, first regressing age from the median FA measures using the generalized additive models, followed by computing the Spearman's rank correlation coefficient between the regression residuals for each pair of white matter tracts. Box's M-test (Box, 1954) was again used to examine significant (p < 0.001) statistical differences between the age and IQ matched ASD and TD matrices. Fisher's z-transformation (Fisher, 1915) was once more used to assess the strength of the pairwise correlations between the ASD and TD groups and hierarchical clustering with bootstrap resampling was used to examine the patterns of microstructural relatedness.
Next, we sought to examine whether the observed patterns of microstructural relatedness change with age. While we have previously tried to account for such age-related effects by using the residuals from model regressions, patterns of microstructural relatedness have been shown to strengthen from birth to late childhood (Mishra et al., 2013). Thus, we questioned whether such changes in the microstructural correlations would also be observed for ASD and TD individuals from childhood into adulthood. To perform this analysis, ASD and TD participants younger and older than 15 years of age were first separated into two age groups. ASD and TD microstructural correlation matrices were next constructed for each age group as previously described. Fisher's z-transformation (Fisher, 1915) was again used to assess the strength of the pairwise correlations between age groups and between the ASD and TD groups. Hierarchical clustering with bootstrap resampling was used to examine the age group patterns of microstructural relatedness.
Post hoc white matter network analysis
While comparison of the correlation matrices between ASD and TD subjects allows identification of an overall difference between the patterns of white matter inter-relatedness between these two groups, the information discerned from this global comparison is limited. Therefore, these results do not enable us to distinguish how such differences between ASD and TD may be related to the network structure formed by the white matter microstructure. The application of graph theory techniques to neuroimaging data has been increasingly used to study relationships of structural covariance and connectivity (Bassett and Bullmore, 2009; Guye et al., 2010; Rubinov and Sporns, 2010; Sporns, 2013); thus, we hypothesized that such measures may also be applicable to examine the properties and group differences of the microstructure correlation matrices in this study. Hence, in a post hoc analysis, we investigated the global and local network features of the white matter correlation matrices from the overall cohort of ASD and TD participants using graph theoretic analyses.
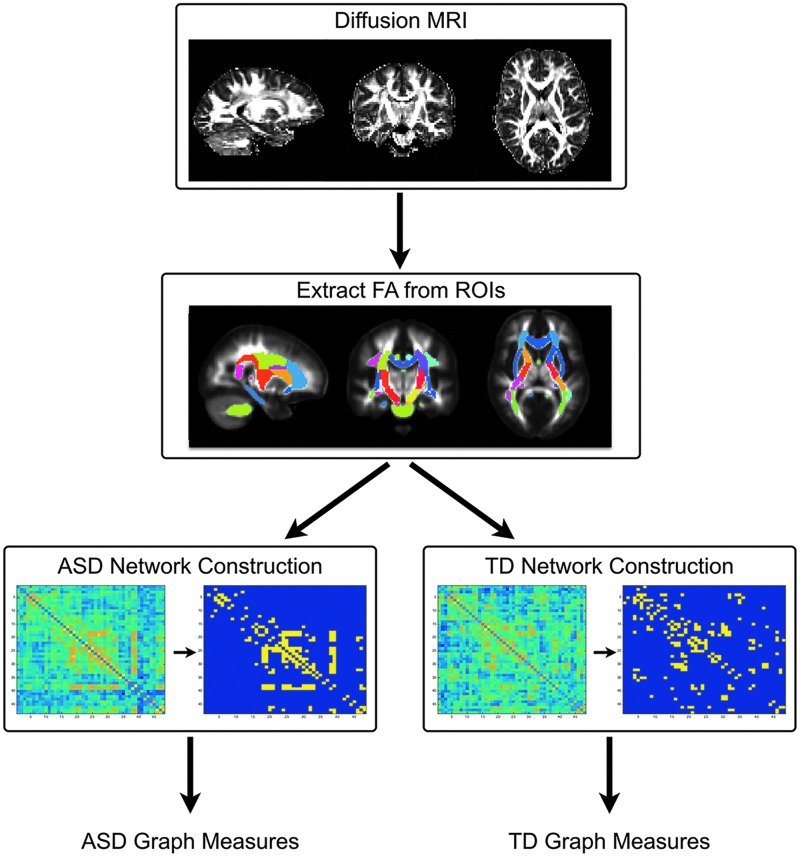
From the group-specific correlation matrices, we constructed a binarized adjacency matrix (Bullmore and Sporns, 2009; Ginestet et al., 2011) of inter-regional correlations. Each element of this matrix contained either a 1, representing a correlation between two white matter tracts existed, or a 0, representing no correlation, for both the ASD and TD groups. While such an approach has been used to examine the structural relatedness formed by measures of cortical thickness, structural MRI, and other neuroimaging strategies (Bernhardt et al., 2011; Bassett and Bullmore, 2009; Guye et al., 2010; He et al., 2007; Phillips et al., 2015), this representation is useful in the current context, as it allows identification and visualization of patterns between regional DTI relatedness measures within the ASD and TD groups. A global threshold was applied to the group-specific correlation matrices to create the adjacency matrices. Since topological features of a network may change with different threshold levels, with the resulting network becoming increasingly sparse as the threshold increases (He et al., 2007), we first explored the influence of the threshold on the mean degree (described below) of the network and selected the most appropriate global threshold from this analysis. Figure 1 provides an overall schematic of how brain networks were constructed from the group FA maps.
FIG. 1.
Overall schematic depicting the construction of the structural brain networks and graph theory measures. ASD, autism spectrum disorder; TD, typical development. Color images available online at www.liebertpub.com/brain
These group-specific adjacency matrices constitute as a description of the underlying white matter network (He et al., 2007; He and Evans, 2010) and thus allow a graph theory approach to compare and evaluate the similarities and differences in these networks between the ASD and TD groups. To perform this analysis, the binarized adjacency matrix for each group was described as an undirected graph, in which graph nodes correspond to white matter tracts, and the edges represent significant correlations between the white matter tracts. Network measures could be subsequently calculated from the undirected graphs of the ASD and TD groups. The network measures were examined and their interpretations are described below.
Mean degree
One of the more fundamental and important measures of a network is a node's degree (Bullmore and Sporns, 2009) and corresponds to the number of links (i.e., nonzero edges) connected to the individual node (Rubinov and Sporns, 2010). The mean degree of a network is therefore the average degree over all nodes in the network and provides a measure of the overall network density (Rubinov and Sporns, 2010).
Characteristic path length
The characteristic path length, L, of a graph is defined as the smallest number of connections required to connect one node to another, averaged over all node pairs within the graph (Watts and Strogatz, 1998). The characteristic path length corresponds to a property that reflects the network's capacity to transfer information globally (Ginestet et al., 2011).
Clustering coefficient
The clustering coefficient, C, is defined as the average of clustering coefficients over all nodes in the graph, where Ci represents the clustering coefficient of node i and corresponds to the fraction of existing edges to all possible edges. This measure is interpreted to represent an average measure of the local connectedness of the network (Ginestet et al., 2011).
Global and local efficiency
Similar to characteristic path length and the clustering coefficient, global and local efficiency describe a network's ability to communicate information. Global efficiency is inversely related to the characteristic path length and is typically considered to be a measure of the network's overall ability for information transfer and integrated processing (Bullmore and Sporns, 2012). Local efficiency, on the other hand, is related to the clustering coefficient and is interpreted as the average global efficiency of specific network modules (Bullmore and Sporns, 2012; Rubinov and Sporns, 2010). A network with high global efficiency can be viewed as one that efficiently transfers information across the network (a global feature), whereas having a high local efficiency implies efficient local transfer of information, on average (Ginestet et al., 2011).
Modularity
An important measure in the study of complex networks is modularity. Complex networks may be organized by a number of modules, which are defined as densely connected nodes that have relatively few connections between other modules (Bullmore and Sporns, 2009). Modularity, therefore, is a measure that estimates a network's modular structure (Bullmore and Sporns, 2009; Rubinov and Sporns, 2010), where networks with high modularity have dense connections between nodes within modules, but sparse connections between nodes of different modules. Identifying whether a network is made up of groups of modules could provide information about the dynamics and organization of the network, with different modules having a specific role or function within the network (Newman, 2006).
Small-world properties
In addition to these measures, we examined the small-worldness of the ASD and TD networks. Small-worldness is defined as a type of network that exhibits groups of highly clustered vertices (high clustering coefficient), with a limited number edges connecting the vertex assemblies (low path length) (Watts and Strogatz, 1998). Clustering coefficients (Creal) and characteristic path length (Lreal) of the FA-based anatomical networks were compared with those of 1000 random graphs (Crand and Lrand) with the same number of nodes, mean degree, and degree distribution. We calculated the small-world parameters: 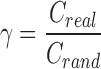 ,
, 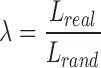 , and
, and 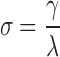 , in which a small-world network should fulfill the following conditions: γ > 1, λ ∼ 1, and therefore σ > 1 (Achard et al., 2006; Watts and Strogatz, 1998). Small-worldness is an important characteristic of a network as it represents an optimal balance between global integration and local processing (Batalle et al., 2012; Sporns et al., 2004).
, in which a small-world network should fulfill the following conditions: γ > 1, λ ∼ 1, and therefore σ > 1 (Achard et al., 2006; Watts and Strogatz, 1998). Small-worldness is an important characteristic of a network as it represents an optimal balance between global integration and local processing (Batalle et al., 2012; Sporns et al., 2004).
As described previously, the correlation threshold level has significant impact on the topological characteristics. When this threshold level reaches a value such that the mean degree of the resulting network is less than the natural logarithm of the number of nodes contained in the network [i.e., for the current study: mean degree < ln(# nodes) = 3.87], small-world characteristics are not estimable (Achard et al., 2006; Watts and Strogatz, 1998). Thus, the global threshold used to construct the network adjacency matrices was selected through a sensitivity analysis that estimated the influence of the threshold level on the mean degree of the network. The threshold that was the strongest while preserving estimable small-world properties for both the ASD and TD networks (i.e., mean degee >3.87) was selected as the optimal threshold and used for further analyses.
Graph theory measures were calculated within MATLAB (MathWorks, Natick, MA) using the freely available Brain Connectivity Toolbox (BCT, www.brain-connectivity-toolbox.net/). Group comparisons between graph topological features were tested using permutation tests based on 5000 permutations.
Results
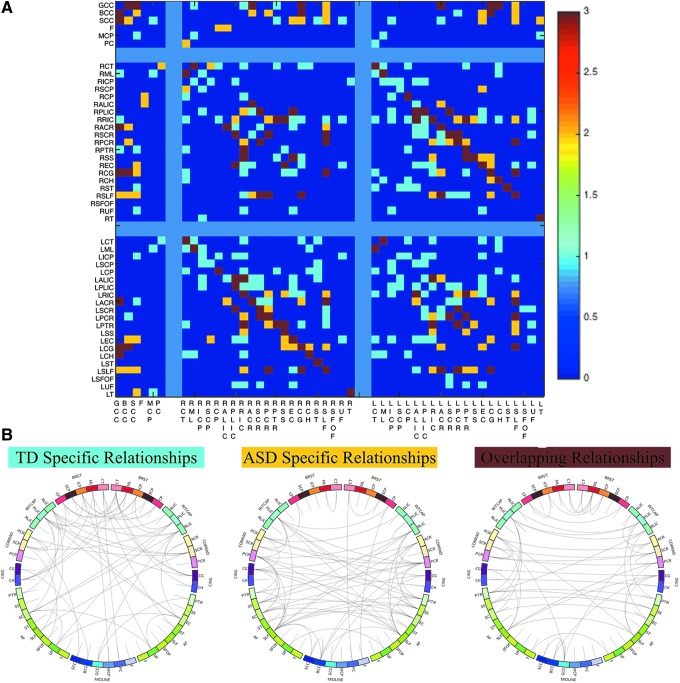
The inter-regional correlation matrices of the TD and ASD groups and the difference between these matrices are depicted in the top panel of Figure 2, while the bottom panel provides a gravitational plot representation created within Gephi (Bastian et al., 2009) of the inter-regional correlations for both groups. Within the gravitational plot representation, each tract is displayed as a circular node while each node is linked together by an edge that is representative of the correlation of FA between the two regions. Shorter edges and more closely spaced nodes signify a stronger correlation. Qualitatively, it can be appreciated that the ASD graph contains more nodes that are further apart, signifying a less inter-related white matter microstructure than the typically developing group. This observation can also be appreciated from the cooler colors in the correlation matrix of the ASD group. Quantitatively, Box's M-test comparing the ASD and TD FA correlation matrices showed an overall significant difference in the inter-relatedness of white matter metrics between the groups (p < 0.001).
FIG. 2.
(A) White matter inter-regional correlation matrices for the typically developing (left) and ASD (middle) participants. Correlations are separated by midline, right, and left hemispheres to easily appreciate within- and inter-hemisphere microstructural correlations. White matter tracts from the JHU ICBM-DTI-81 template were used. See Supplementary Table S1 for reference of the tract abbreviations. Comparison using Fisher Z-transform (right) shows, in general, stronger inter-regional correlations compared to the ASD group. (B) Gravitational plots of the correlations between the FA measurements of 48 white matter tracts between the TD (left in red) and ASD (right, in green) groups. Nodes represent regions of interest, while edge lengths represent the magnitudes of correlation coefficients between pairs of regions of interest. Shorter edges depict larger correlation coefficients, which signify more closely spaced nodes in the correlation space. As can be seen, the white matter microstructure of different tracts in the ASD group was significantly less interrelated (less correlated) than that of the typically developing group (p < 0.001, Box M-test). TD, typical development. Color images available online at www.liebertpub.com/brain
In general, the TD group had stronger inter-regional correlations compared to the ASD group, with 55% (620/1128) of the independent entries in the TD correlation matrix having higher values than the ASD correlation matrix. The strongest of correlations within the TD group existed between the homologous pairs of the left and right posterior limb of the internal capsule (ρ = 0.87), while the left and right posterior corona radiata exhibited the strongest correlation in the ASD group (ρ = 0.79). Using the Fisher z-transformation to compare these correlation matrices (top right Fig. 2) shows that 48 of these pairwise differences are significantly higher in the TD group, while 18 pairwise differences were higher in the ASD group (p < 0.05).
Patterns of microstructural relatedness within and between the TD and ASD groups were further examined using hierarchical clustering applied to the group correlation matrices, while confidence levels for the resulting clusters were calculated from a bootstrapping approach using 10,000 repetitions (Suzuki and Shimodaira, 2006). The results from this clustering are displayed as a dendrogram and are depicted in Figure 3 for the TD (left panel) and ASD (right panel) groups. Values at branches correspond to the approximately unbiased (AU) p-values, bootstrap probability values, and cluster ranks, as computed with “pvclust,” while significant clusters with AU >95 are outlined by red rectangles (Suzuki and Shimodaira, 2006). Dendrograms from both groups show that, in general, the strongest within-group correlations are among homologous tracts, though not all pairs of tracts exhibit this feature. Within the TD group, two statistically significant clusters were formed and incorporated all examined white matter regions. These two clusters appear to create a partition between projection tracts (e.g., corticospinal tracts, medial leminiscus, cerebellar peduncle) and the association (e.g., cingulum, superior/inferior occipito-frontal fasicului, superior longitudinal fasciculi) and commissure tracts (e.g., corpus callosum). Conversely, in the ASD group, 12 distinct clusters were formed, however, these were partitioned into smaller groups and mostly between homologous white matter regions.
FIG. 3.
Hierarchical clustering of inter-regional correlations for typical development and ASD. Values at branches correspond to the approximately unbiased (AU) p-values, bootstrap probability values, and cluster ranks, as computed with “pvclust,” while significant clusters with AU >95 are outlined by red rectangles. Within-group correlations among homologous tracts displayed the strongest of relationships. Color images available online at www.liebertpub.com/brain
Effects of FSIQ and age on microstructural relatedness
Follow-up analysis examined these same measures with an age and FSIQ matched subset to ensure the observed differences did not result from the difference in IQ scores between the ASD and TD groups. Correlation matrices between ASD and TD in this age and IQ matched subset (Fig. 4) were also found to be significantly different (p < 0.001) consistent with the group differences observed with the full sample. Within the TD group, the average correlations were larger in the left hemisphere compared to the right hemisphere (ρLEFT = 0.2180; ρRIGHT = 0.2136), while in the ASD group, the right hemispheric correlations were larger (ρLEFT = 0.16; ρRIGHT = 0.22). As before, the TD group had a higher mean correlation among homologous white matter tracts (ρ = 0.61) compared to the ASD group (ρ = 0.58). The strongest correlations in the TD group were again found between the homologous pairs of the left and right posterior limb of the internal capsule (ρ = 0.89), while the left and right superior longitudinal fasciculus exhibited the strongest correlation in the ASD group (ρ = 0.78). While homologous pairs were again observed to exhibit stronger correlations than nonhomologous pairs within both groups. The resulting dendrograms (Supplementary Fig. S1) additionally show distinct clustering patterns between the groups. In particular, the genu, body, and splenium of the corpus callosum of the TD group together formed a significant grouping, while in the ASD group these three regions can be seen to be separately partitioned.
FIG. 4.
Inter-regional correlation matrices for age and FSIQ-matched subjects. Correlations are divided as in Figure 2. Consistent with the differences observed with the full sample, these matrices were found to be significantly different (p < 0.001, Box M-test), with the typical development group having, in general, stronger and more widespread inter-regional correlations. FSIQ, full-scale IQ. Color images available online at www.liebertpub.com/brain
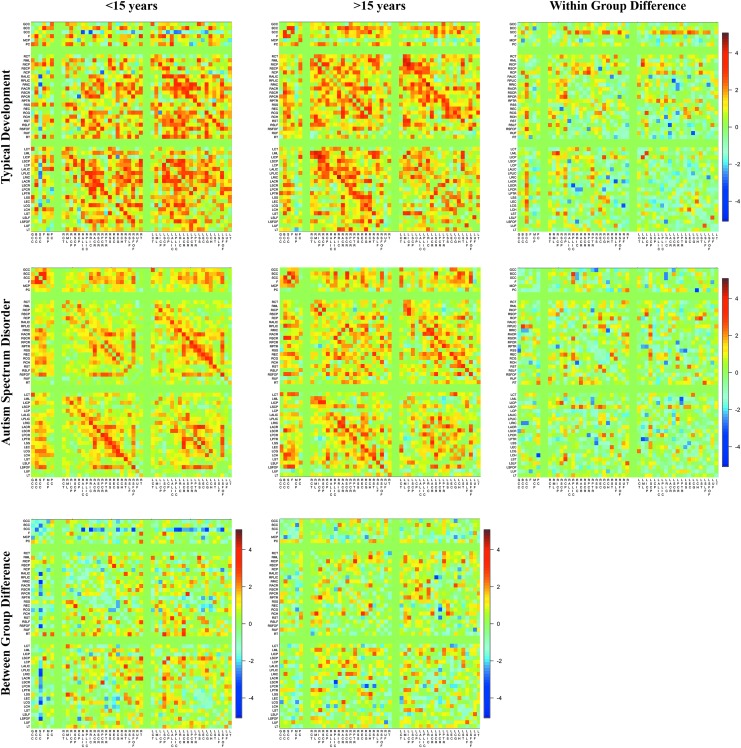
The developmental effects of white matter inter-relatedness were broadly assessed by separating the ASD and TD participants into two age groups of younger and older than 15 years of age. Correlation matrices for the younger and older age groups are shown in Figure 5, while the hierarchical clustering dendrogram is shown in Supplementary Figure S2 for both TD and ASD participants. Fisher z-transformations of the correlation matrices from both the TD and ASD groups indicate that compared to younger participants, older participants have stronger inter-regional correlations. While similarities to those of the full cohort of participants exist, some important differences can be noticed. Within the younger TD group, the strongest of correlations exist between the right and left extreme capsule (ρ = 0.81), while in the older sample we observe the right and left posterior limb of the internal capsules to have the strongest relationships (ρ = 0.91). However, in the ASD group, the strongest correlation exists between the left and right superior corona radiata (ρ = 0.81) in the younger age group and between the right and left medial lemenicus (ρ = 0.79) in the older participants. Additionally, the pairing of homologous white matter regions appears to be more prominent in the older ages for the TD group, while pairing of homologous regions appears more prevalent in the younger ASD participants.
FIG. 5.
Inter-regional correlation matrices from typically developing and ASD participants younger and older than 15 years of age. Correlations are divided as in Figure 2. Correlation differences between age groups (bottom panel) and within-group (right panel) are additionally shown. In general, older participants have stronger inter-regional correlations, suggesting the inter-regional correlations change with advancing age. Correlations between homologous white matter regions appear stronger in the older ages for the TD group, while these correlations appear stronger in the younger ASD participants. Color images available online at www.liebertpub.com/brain
Post hoc white matter network analysis
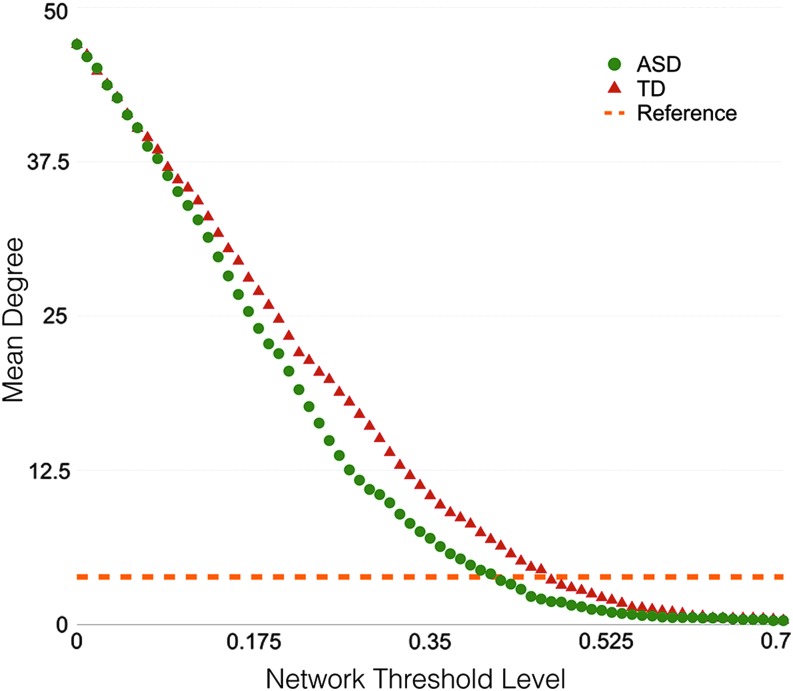
Figure 6 displays the mean degree of the resulting inter-regional FA-based network with respect to the correlation threshold for both the ASD and TD groups. Mean network degree appears to decrease rapidly as the correlation threshold increases. A line denoting the natural logarithm of the number of nodes of the network is also displayed and serves as a reference to which the small-world properties are no longer estimable (Achard et al., 2006; Watts and Strogatz, 1998). Thus, for the FA-based network, a correlation threshold of r > 0.41 was selected as the global threshold to apply to the adjacency matrices, as this threshold was the maximum value such that the mean degree of the ASD and TD networks was greater than 3.87 (i.e., ln(48)).
FIG. 6.
Mean degree as a function of the correlation threshold for the TD (red) and ASD (green) groups. The mean degree of the brain white matter networks decreases as the correlation threshold increases. A reference line (in orange) denotes the minimum mean degree such that small-world properties are estimable. From this analysis, a correlation threshold of 0.45 was selected as the optimal global threshold, as this value was the largest threshold such that small-world properties could still be calculated. Color images available online at www.liebertpub.com/brain
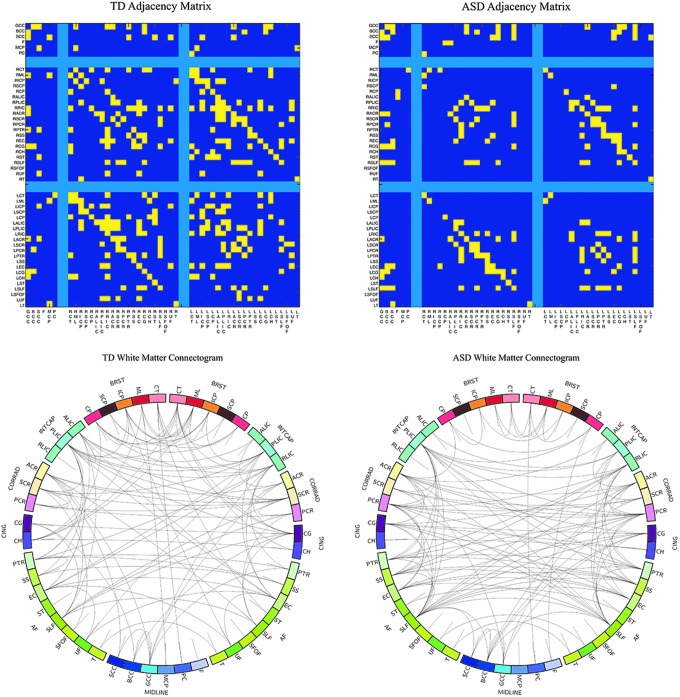
Using the above results, the FA correlation matrices were binarized to construct adjacency matrices for each group by thresholding the FA correlation matrix at the r > 0.41 level. The adjacency matrices constructed from the FA correlations and a graphical visualization [constructed using Circos (Krzywinski et al., 2009)] of the similarities and differences of the ASD and TD networks are shown in Figure 7. The ASD FA network consisted of 99 links, involving 83.33% (40 of 48) of the white matter regions, while the TD FA network contained 166 significant links across 95.83% (46 of 48) of the white matter regions. Fifty-three of the corresponding links overlapped both the ASD and TD networks (Fig. 8). The total number of links within the left and right hemispheres were greater in the TD group (left = 35; right = 32) compared to the ASD group (left = 17; right = 15), while the TD group additionally had a larger number of links between the left and right hemispheres (76 compared to 41). Within the TD brain network, the right hemispheric retrolenticular limb of the internal capsule contained the largest number of links; while the left hemispheric superior longitudinal fasciculus contained the most links for the ASD FA network.
FIG. 7.
Adjacency matrices and graphical visualization (“connectogram”) of brain white matter networks for the TD and ASD groups. Individual white matter tracts were broadly grouped by region (outside labels), while the individual tract is indicated by the inside of the circle. Adjacency matrices are separated by right and left hemisphere as in Figure 2. Connectograms are symmetric about the center, with left hemisphere tracts on the left, and right hemisphere tracts on the right. Connectograms were constructed using the Circos software package (Krzywinski et al., 2009). Color images available online at www.liebertpub.com/brain
FIG. 8.
(A) Matrix visualization of the distinct and overlapping white matter relationships between the TD and ASD groups. TD-specific inter-tract relationships are displayed in light blue, ASD-specific white matter tract relationships are shown in orange, and overlapping relationships are displayed in red. (B) Graphical visualization of group-specific and overlapping white matter relationships. Color images available online at www.liebertpub.com/brain
We calculated the network topological properties from the FA-derived structural networks and for the network of white matter tracts within the right and left hemispheres, separately, and tested for group differences using permutation testing. Results from this analysis are provided in Table 2. The mean clustering coefficient, global efficiency, mean local efficiency, and mean degree of the FA network were found to differ significantly between the ASD and TD groups, with all four of these metrics being reduced in the ASD group. Similarly, these were found to be different between TD and ASD right hemisphere white matter tracts, while TD and ASD measures of global efficiency, mean local efficiency, and mean degree were found to differ across the left hemisphere. The characteristic path length and network modularity were not found to be significantly different between ASD and TD FA-based networks. Interestingly, both FA-based networks displayed small-world characteristics, however, none of these network parameters were found to be significantly different between the TD and ASD networks.
Table 2.
Graph Theory Measures Calculated for Autism Spectrum Disorder and Typical Development Groups
| ASD | TD | p | |
|---|---|---|---|
| Full network | |||
| Mean degree | 4.13 (0.83) | 6.92 (0.68) | <0.001 |
| Clustering coefficient | 0.26 (0.02) | 0.39 (0.03) | 0.0014 |
| Characteristic path length | 3.19 (0.56) | 2.31 (0.55) | 0.2350 |
| Global efficiency | 0.26 (0.02) | 0.46 (0.03) | <0.001 |
| Mean local efficiency | 0.33 (0.03) | 0.55 (0.02) | <0.001 |
| Modularity | 0.40 (0.08) | 0.37 (0.08) | 0.8354 |
| Small-worldness properties | |||
| Γ | 1.62 (0.46) | 2.18 (0.46) | 0.5820 |
| Λ | 1.33 (0.32) | 1.09 (0.14) | 0.2080 |
| σ | 1.22 (0.46) | 2.00 (0.46) | 0.5260 |
| Right hemisphere | |||
| Mean degree | 1.43 (0.43) | 3.05 (0.51) | <0.001 |
| Clustering coefficient | 0.04 (0.06) | 0.33 (0.06) | <0.001 |
| Characteristic path length | 2.04 (0.49) | 2.33 (0.49) | 0.6778 |
| Global efficiency | 0.15 (0.03) | 0.41 (0.03) | <0.001 |
| Mean local efficiency | 0.04 (0.06) | 0.39 (0.06) | <0.001 |
| Modularity | 0.39 (0.08) | 0.37 (0.08) | 0.8802 |
| Small-worldness properties | |||
| γ | 0.38 (0.45) | 2.39 (0.47) | 0.3260 |
| λ | 0.99 (0.03) | 0.97 (0.12) | 0.9520 |
| σ | 0.39 (0.45) | 2.46 (0.47) | 0.3200 |
| Left hemisphere | |||
| Mean degree | 1.62 (0.54) | 3.33 (0.65) | <0.001 |
| Clustering coefficient | 0.19 (0.06) | 0.30 (0.06) | 0.1452 |
| Characteristic path length | 1.52 (0.50) | 2.23 (0.49) | 0.2920 |
| Global efficiency | 0.14 (0.03) | 0.39 (0.02) | <0.001 |
| Mean local efficiency | 0.19 (0.06) | 0.36 (0.06) | 0.05 |
| Modularity | 0.29 (0.08) | 0.40 (0.08) | 0.3158 |
| Small-worldness properties | |||
| γ | 2.09 (0.44) | 1.75 (0.44) | 0.2280 |
| λ | 0.82 (0.12) | 1.14 (0.09) | 0.6820 |
| σ | 2.54 (0.44) | 1.52 (0.44) | 0.4740 |
Between group comparisons of graph theory measures were conducted using permutation testing based upon 5000 permutations.
Bold values denote significant (p < 0.05) difference between ASD and TD groups.
Discussion
This study sought to compare the regional inter-relatedness of white matter microstructure in ASD versus typical development at the group level. We report organizational alterations in white matter microstructure and group differences in topological properties of the structural architecture constructed from DTI parameters. Significant inter-regional correlations were found to exist within the white matter of TD and ASD groups, while the strengths and clustering configurations of these microstructural correlations were found to differ. These microstructural patterns were found to display small-world structure in both groups, which is consistent with previous studies employing complex network analysis and graph theory (Sporns et al., 2004). The group with ASD, however, exhibited a reduced mean clustering coefficient, global and local efficiency, and mean degree, compared to those of typical development. These results indicate a complex imbalance between structural organization and connectivity in ASD (Batalle et al., 2012), suggesting that white matter microstructure in the disorder may be less coherently organized and may be suboptimal for the communication of information across the brain in the ASD group. These findings are in agreement with and add to a growing body of evidence that suggest white matter microstructure plays an important role in autism.
Significant group differences in the overall microstructural relatedness of circuit integrity (FA) between individuals with ASD and those with typical development are demonstrated. These differences appear to be characterized by the ASD group having a less inter-related white matter microstructure. Follow-up analyses provided additional evidence that this overall group difference was not related to age or IQ differences between TD and ASD groups. Our results indicate that, at the group level, individuals with ASD have significantly less uniformity in white matter microstructure across multiple tracts of the brain compared to individuals with typical development. While this is consistent with previous studies that have shown ASD to be associated with reduced white matter integrity across many white matter pathways (Just et al., 2012; Schipul et al., 2011; Travers et al., 2012), it is important to note that these results do not implicate specific white matter regional differences between typically developing and ASD individuals. Our results indicate, however, that some of the relationships between white matter regions are reduced within the white matter microstructure of individuals with ASD across the brain. One can thus hypothesize that factors such as aberrant early brain development and/or atypical myelination, occurring prenatally and during the first few years of life (Deoni et al., 2012), could lead to such overall microstructural differences. Future research is needed to investigate these and other hypotheses to better understand the biologically based mechanisms underlying ASD and typical development and thus improve the lives of individuals with autism.
Hierarchical clustering of the correlational distances to form a dendrogram of the microstructural relationships provides a unique, data-driven method to assess underlying patterns of brain microstructure. Such methods have been previously used in DTI studies across adults (Wahl et al., 2010) and during early child development (Mishra et al., 2013), however, to the best of our knowledge, this is the first investigation to use this approach to examine microstructural differences within ASD. Our findings are consistent with previous analyses that show homologous regions and tracts to exhibit, in general, stronger inter-regional correlations (Mishra et al., 2013; Wahl et al., 2010), however, we find the clustering patterns to differ between individuals with ASD and those that are typically developing. In particular, the right and left posterior limb of the internal capsule had the strongest correlation (ρ = 0.87) within the TD group, while these homologous pairs were ranked sixth among the correlations in the ASD group. In the ASD group, the weakest of FA correlations between homologous white matter pathways were found between the left and right superior cerebellar peduncle (ρ = 0.35), possibly reflecting a localized alteration of the microstructural organization of the superior cerebellar peduncles (Sivaswamy et al., 2010). Such atypicalities may lead to dysfunction of these cerebellar efferent pathways, impairing the connectivity of other brain networks, such as the cerebellar-thalamo-cortical network, and altering sensorimotor, adaptive social behaviors, and other cognitive processes (Catani et al., 2008; Nair et al., 2013; Sivaswamy et al., 2010).
The differences in the patterns of significant clusters between the TD and ASD groups are additionally interesting. Within the TD group, two significant clusters emerged from the hierarchical clustering, incorporating all the examined white matter regions and appearing to partition projection tracts from the association and commissure tracts. Conversely, within the ASD group, 12 smaller and distinct clusters were formed. While these patterns may reflect structural and/or functional similarities between the regional white matter measures (Wahl et al., 2010), such patterns may also be indicative of disrupted microstructural organization and connectivity of white matter. The clustering of more localized brain regions, as seen in the correlation/association matrices and dendrograms of the ASD group, may reflect the hypothesized local overconnectivity in ASD, while the lack of larger groups of white matter regions may reflect a disruption of connectivity between larger brain networks (Wass, 2011). This hypothesis would also suggest that the larger clusters formed within the TD group are indicative of a more tightly coupled white matter microstructure.
We found in addition that the global and mean local efficiencies of the FA-based structural networks to be significantly different between the ASD and TD groups. Both global and mean local efficiency, network-based metrics related to information transmission characteristics of that network (Bullmore and Sporns, 2012, 2009), were found to be reduced in the ASD network. The ASD FA-based network is also observed to have a decreased mean network degree and mean clustering coefficient, compared to the TD network, suggesting that the ASD network consisted of, on average, of a sparser set of associations between white matter tracts. Taken together, these results may allude to a structural framework in which altered brain connectivity exists (Zielinski et al., 2012). Such a model of local overconnectivity would predict white matter tracts to exhibit increased fiber volume and higher fiber density (Wass, 2011). Furthermore, such increased local fiber density could impair the conveyance of neural signals to broader brain regions, causing individual brain regions to become independent and isolated from the rest of the brain, and therefore having a detrimental effect on global connectivity (Just et al., 2012; Kana et al., 2011). As brain structure and its organization influence brain function (Johnson and Munakata, 2005), such alterations of structural connectivity will undoubtedly impact brain function. Alterations of the network of the white matter microstructure could, therefore, be responsible for widely reported deficits of functional connectivity in ASD (Anderson et al., 2011; Cherkassky et al., 2006; Kana et al., 2006; Kleinhans et al., 2008; Müller et al., 2011). Studies examining the relationships between structural and functional connectivity have been limited (Uddin et al., 2013), and therefore future studies that explore relationships between structural and functional connectivity are critical to gain further understanding of how white matter microstructure alterations impact the ASD phenotype.
Differences in small-worldness between the ASD brain network model and typically developing controls were not found to be statistically significant. Complex networks that possess small-world properties are thought to be ideal, as these types of networks provide an optimal balance of local processing and global information propagation (Sporns et al., 2004). While such types of networks have been reported to exist in a wide range of natural and technological systems (Boccaletti et al., 2006; Strogatz, 2001; Watts and Strogatz, 1998), graph analyses have revealed small-world characteristics in structural brain networks (Bullmore and Bassett, 2011; Bullmore and Sporns, 2009). Though this study did not find such differences in small-world features in structural white matter networks, such properties may be altered within autism (Wang et al., 2009). Speculatively, differences in “small-worldness” of ASD brain networks could reflect a less optimal topological network organization. Disruption of such structural small-world characteristics could result in alterations of the interactions between brain regions that are needed to support cognitive and behavioral functioning (Damoiseaux and Greicius, 2009), providing further support for the hypothesis that ASD is a disorder of widespread alterations of the white matter microstructure.
While there is converging evidence that suggest brain connectivity is altered in ASD, even in children as young as 24 months of age (Lewis et al., 2014), a critical question remains as yet unanswered, namely of when in brain development do these atypicalities first present themselves? The human brain undergoes many changes throughout much of the lifespan, while most rapidly developing during the first years of life (Casey et al., 2005; Dean et al., 2014; Deoni et al., 2012; Evans and Brain Development Cooperative Group, 2006; Giedd et al., 1999; Giedd and Rapoport, 2010; Lange et al., 1997). Developmental processes, such as synapse formation, dendritic sprouting, gyrification, and myelination, establish and refine the structural and functional brain networks that ultimately enable local and global processing of complex information (Durston and Casey, 2006). Indeed, we found the patterns of microstructural correlations to change between participants younger and older than 15 years of age, suggesting developmental and other biological processes occurring during this time may contribute and alter such microstructural relationships. Such age-related changes have been examined and noted to affect microstructural relationships from infancy to young adolescence, with correlations becoming stronger as one ages (Mishra et al., 2013). Morever, age-related changes of topological properties of structural brain networks have additionaly been observed throughout typical development (Liu et al., 2014; Montembeault et al., 2012; Wu et al., 2012; Yap et al., 2011; Zielinski et al., 2010). For example, Wu et al. (2012) observed robust decreases in local efficiency and increases in global efficiency from young to middle age, which they attributed these findings to maturational changes that enable more efficient information processing (Wu et al., 2012) in healthy individuals. Small-world characteristics appear to be present by 2 years of age (Yap et al., 2011). Other demographic and environmental factors may additionally influence the underlying patterns of these structural networks, however, these await future research. Longitudinal studies of infants and children at risk for developing ASD may provide the opportunity to discern when differences in the patterns of microstructural relatedness arise while studies that investigate the relationships of genetic and environmental factors on brain network properties will help determine the role that these elements have on the formation of such differences. Equally important are longitudinal studies that examine the development and maturation of network-based brain structure, function, and connectivity from early childhood into adulthood in autism as such studies will provide invaluable insight into how different patterns of change in networks are related to clinical course and outcome.
Although the results reported in this study suggest alterations of white matter microstructure in ASD, the cause of such differences remains unclear. DTI-derived coefficients (i.e., FA) describe local measures of water diffusion and anisotropy. While these are sensitive to the underlying white matter microstructure, a broad range of microstructural changes can influence these parameters (Alexander et al., 2007b; Beaulieu, 2002; Jones et al., 2013). Thus, it is challenging to attribute such findings or differences to specific white matter mechanisms that may be responsible. As an alternative, quantitative imaging techniques, such as neurite orientation dispersion and density imaging (Zhang et al., 2012), quantitative magnetization transfer imaging (Alexander et al., 2011), and relaxometry (Deoni, 2010), while being more complex to implement than DTI, may allow a more specific interpretation of findings between individuals with ASD and typically developing individuals. Moreover, the development of novel quantitative biomarkers that target the direct measurement of specific white matter characteristics, such as the myelin water fraction (Deoni et al., 2008; MacKay et al., 1994) or the myelin g-ratio (Stikov et al., 2015; Dean et al., 2016), may be increasingly useful for understanding specific microstructural differences that arise between individuals with ASD and typical development.
This study has several possible limitations. First, the decision to examine atlas-based regions instead of taking a whole-brain approach may limit the resolution of network-level effects. While the use of such voxel-wise methods are desirable for future research, the use of atlas-based regions allows us characterize the microstructural correlations between two pairs of white matter regions more directly. A second possible limitation is that our DTI-based brain networks were obtained from the FA correlation matrices using global, conservative threshold criteria, allowing us to only examine relationships having strong correlations. Determination of a definitive threshold for the construction of complex brain networks is a methodological problem that has been described in previous studies utilizing network analyses (He et al., 2007; Serrano et al., 2009), while others have proposed alternative strategies for constructing brain-based networks (Alexander-Bloch et al., 2010; Zalesky et al., 2010). Nonetheless, previous studies have suggested that global and alternative thresholding techniques provide broadly consistent network-level results (Alexander-Bloch et al., 2010). Finally, this study is cross-sectional and there is an uneven balance among the number of typically developing controls and individuals with ASD. Although cross-sectional studies are informative, longitudinal studies of brain network measures computed from individuals would enable individual network topological differences to be studied, an important aspect of understanding the heterogeneity associated with ASD (Lange et al., 2015; Travers et al., 2015), and normative brain development (Dean et al., 2014; Giedd et al., 1999; Lebel and Beaulieu, 2011). Investigating individual variability of topological characteristics would likely be informative for future research.
Conclusion
In conclusion, this study presents a complex network analysis of the inter-related characteristics of white matter microstructure in a large sample of individual with ASD and typical development. Its results demonstrate that networks formed within the brains of individuals with ASD are less interrelated and may be suboptimal regarding efficient integration and processing of complex information. Further, its results highlight the impact that white matter microstructure has on the ASD phenotype, and adds to a growing body of literature, suggesting that ASD is associated with widespread alterations of underlying white matter microstructure. Given the role white matter plays in the coordination of rapid and synchronized brain communication, the network deficiencies observed here are likely to have a lasting influence on many behavioral and cognitive characteristics of ASD.
Supplementary Material
Acknowledgments
We sincerely thank the children, adolescents, and adults with autism, the individuals with typical development, and all the families who participated in this study. This work was supported by the National Institute of Mental Health (RO1 MH080826 to J.E.L., A.L.A., N.L., E.D.B.; RO1 MH084795 to J.E.L., P.T.F., N.L.; RO1 MH097464 to J.E.L., M.L., N.L., A.L.A.; K08 MH100609 to B.A.Z., and KO8 MH092697 to J.S.A.), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (T32 HD007489 to D.C.D., B.G.T., and P30 HD003352 to the Waisman Center), the Poelman Foundation (to E.D.B.), the Primary Children's Foundation (Early Career Development Award to B.A.Z.), and the Hartwell Foundation (Postdoctoral Fellowship Award to B.G.T.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institute of Child Health and Development, or the National Institutes of Health. We appreciate and thank Dr. Vikas Singh, Won Hwa Kim, Zhan Xu, Anne M. Bartosic, Annahir Cariello, Celeste Knoles, Sam Doran, Kristine McLaughlin, and Abigail Freeman for their useful discussions and contributions to this project.
Author Disclosure Statement
No competing financial interests exist.
References
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore ET. 2006. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci 26:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, Tromp DPM, Zakszewski E, Field AS. 2011. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect 1:423–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, Miller JN, Lu J, Jeong E-K, McMahon WM, Bigler ED, Lainhart JE. 2007a. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage 34: 61–73 [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. 2007b. Diffusion tensor imaging of the brain. Neurotherapeutics 4:316–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch AF, Gogtay N, Meunier D, Birn R, Clasen L, Lalonde F, Lenroot R, Giedd J, Bullmore ET. 2010. Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Front Syst Neurosci 4:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameis SH, Catani M. 2015. Altered white matter connectivity as a neural substrate for social impairment in autism spectrum disorder. Cortex 62C:158–181 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. 1994. Diagnostic and statistical manual of mental health disorders (DSM-IV). American Psychiatric Association, Washington, DC [Google Scholar]
- American Psychiatric Association. 2013. Diagnostic and statistical manual of mental health disorders (DSM-V). American Psychiatric Association, Washington, DC [Google Scholar]
- Anderson JS, Druzgal TJ, Froehlich A, DuBray MB, Lange N, Alexander AL, Abildskov T, Nielsen JA, Cariello AN, Cooperrider JR, Bigler ED, Lainhart JE. 2011. Decreased interhemispheric functional connectivity in autism. Cereb Cortex 21:1134–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, Epstein C, Grossman M, Gee J. 2008. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12:26–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. 2004. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry 55:323–326 [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Lotspeich LJ, Reiss AL. 2010. Similar white matter aberrations in children with autism and their unaffected siblings: a diffusion tensor imaging study using tract-based spatial statistics. Arch Gen Psychiatry 67:1052–1060 [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. 1996. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111:209–219 [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. 2008. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci 28:9239–9248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET. 2009. Human brain networks in health and disease. Curr Opin Neurol 22:340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian M, Heymann S, Jacomy M. 2009. Gephi: an open source software for exploring and manipulating networks. Proc Third International ICWSM 17:951–961 [Google Scholar]
- Batalle D, Eixarch E, Figueras F, Muñoz-Moreno E, Bargallo N, Illa M, Acosta-Rojas R, Amat-Roldan I, Gratacos E. 2012. Altered small-world topology of structural brain networks in infants with intrauterine growth restriction and its association with later neurodevelopmental outcome. Neuroimage 60:1352–1366 [DOI] [PubMed] [Google Scholar]
- Beaulieu C. 2002. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 15:435–455 [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. 2004. Autism and abnormal development of brain connectivity. J Neurosci 24:9228–9231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner T, van der Kouwe AJW, Sorensen AG. 2011. Diffusion imaging with prospective motion correction and reacquisition. Magn Reson Med 66:154–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N. 2011. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex 21:2147–2157 [DOI] [PubMed] [Google Scholar]
- Boccaletti S, Latora V, Moreno Y, Chavez M, Hwang D. 2006. Complex networks: structure and dynamics. Phys Rep 424:175–308 [Google Scholar]
- Box GEP. 1954. Some theorems on quadratic forms applied in the study of analysis of variance problems, II. Effects of inequality of variance and of correlation between errors in the two-way classification. Ann Math Stat 25:484–498 [Google Scholar]
- Brieber S, Neufang S, Bruning N, Kamp Becker I, Remschmidt H, Herpertz Dahlmann B, Fink GR, Konrad K. 2007. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J Child Psychol Psychiatry 48:1251–1258 [DOI] [PubMed] [Google Scholar]
- Brito AR, Vasconcelos MM, Domingues RC, Hygino da Cruz LC, Rodrigues L, de S, Gasparetto EL, Calçada CABP. 2009. Diffusion tensor imaging findings in school-aged autistic children. J Neuroimaging 19:337–343 [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Bassett DS. 2011. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol 7:113–140 [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Sporns O. 2009. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198 [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Sporns O. 2012. The economy of brain network organization. Nat Rev Neurosci 13:336–349 [DOI] [PubMed] [Google Scholar]
- Casey B, Tottenham N, Liston C, Durston S. 2005. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci 9:104–110 [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Daly E, Embiricos N, Deeley Q, Pugliese L, Curran S, Robertson D, Murphy DGM. 2008. Altered cerebellar feedback projections in Asperger syndrome. Neuroimage 41:1184–1191 [DOI] [PubMed] [Google Scholar]
- Chang LC, Jones DK, Pierpaoli C. 2005. RESTORE: robust estimation of tensors by outlier rejection. Magn Reson Med 53:1088–1095 [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. 2006. Functional connectivity in a baseline resting-state network in autism. Neuroreport 17:1687–1690 [DOI] [PubMed] [Google Scholar]
- Cook PA, Bai Y, Nedjati-Gilani S. 2006. Camino: open-source diffusion-MRI reconstruction and processing. In: Presented at the Proceedings of the International Society of Magnetic Resonace Imaging in Medicine, Seattle, Washington, p. 2759 [Google Scholar]
- Courchesne E, Pierce K. 2005a. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol 15:225–230 [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. 2005b. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci 23:153–170 [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. 2007. Mapping early brain development in autism. Neuron 56:399–413 [DOI] [PubMed] [Google Scholar]
- Courchesne E, Redcay E, Kennedy DP. 2004. The autistic brain: birth through adulthood. Curr Opin Neurol 17:489–496 [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. 2009. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Funct 213:525–533 [DOI] [PubMed] [Google Scholar]
- Dean DC, III, O'Muircheartaigh J, Dirks H, Travers BG, Adluru N, Alexander AL, Deoni SCL. 2016. Mapping an index of the myelin g-ratio in infants using magnetic resonance imaging. Neuroimage 132:225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, III, O'Muircheartaigh J, Dirks H, Waskiewicz N, Walker L, Doernberg E, Piryatinsky I, Deoni SCL. 2014. Characterizing longitudinal white matter development during early childhood. Brain Struct Funct 220:1921–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL. 2010. Quantitative relaxometry of the brain. Top Magn Reson Imaging 21:101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL, Dean DC, III, O'Muircheartaigh J, Dirks H, Jerskey BA. 2012. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage 63:1038–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL, Rutt BK, Arun T, Pierpaoli C, Jones DK. 2008. Gleaning multicomponent T1 and T2 information from steady‐state imaging data. Magn Reson Med 60:1372–1387 [DOI] [PubMed] [Google Scholar]
- Durston S, Casey BJ. 2006. What have we learned about cognitive development from neuroimaging? Neuropsychologia 44:2149–2157 [DOI] [PubMed] [Google Scholar]
- Egaas B, Courchesne E, Saitoh O. 1995. Reduced size of corpus callosum in autism. Arch Neurol 52:794–801 [DOI] [PubMed] [Google Scholar]
- Evans AC; Brain Development Cooperative Group. 2006. The NIH MRI study of normal brain development. Neuroimage 30:184–202 [DOI] [PubMed] [Google Scholar]
- Fisher RA. 1915. Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika 10:507 [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C, Alexander AL, Bigler ED, Lange N, Lainhart JE. 2010. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage 51:1117–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2:861–863 [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. 2010. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron 67:728–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestet CE, Nichols TE, Bullmore ET, Simmons A. 2011. Brain network analysis: separating cost from topology using cost-integration. PLoS One 6:e21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guye M, Bettus G, Bartolomei F, Cozzone PJ. 2010. Graph theoretical analysis of structural and functional connectivity MRI in normal and pathological brain networks. MAGMA 23:409–421 [DOI] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC. 2007. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex 17:2419. [DOI] [PubMed] [Google Scholar]
- He Y, Dagher A, Chen Z, Charil A, Zijdenbos A, Worsley K, Evans A. 2009. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain 132:3366–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Evans A. 2010. Graph theoretical modeling of brain connectivity. Curr Opin Neurol 23:341. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841 [DOI] [PubMed] [Google Scholar]
- Johnson M, Munakata Y. 2005. Processes of change in brain and cognitive development. Trends Cogn Sci 9:152–158 [DOI] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R. 2013. White matter integrity, fiber count, and other fallacies: the do“s and don”ts of diffusion MRI. Neuroimage 73:239–254 [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. 2004. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain 127:1811–1821 [DOI] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, Varma S. 2012. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev 36:1292–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. 2006. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain 129:2484–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Libero LE, Moore MS. 2011. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys Life Rev 8:410–437 [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. 2008. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain 131:1000–1012 [DOI] [PubMed] [Google Scholar]
- Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. 2009. Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainhart JE. 2006. Advances in autism neuroimaging research for the clinician and geneticist. Am J Med Genet 142C:33–39 [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Piven J, Wzorek M, Landa R, Santangelo SL, Coon H, Folstein SE. 1997. Macrocephaly in children and adults with autism. J Am Acad Child Adolesc Psychiatry 36:282–290 [DOI] [PubMed] [Google Scholar]
- Lange N, DuBray MB, Lee JE, Froimowitz MP, Froehlich A, Adluru N, Wright B, Ravichandran C, Fletcher PT, Bigler ED, Alexander AL, Lainhart JE. 2010. Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res 3:350–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange N, Giedd JN, Xavier Castellanos F, Vaituzis AC, Rapoport JL. 1997. Variability of human brain structure size: ages 4–20 years. Psychiatry Res Neuroimaging 74:1–12 [DOI] [PubMed] [Google Scholar]
- Lange N, Travers BG, Bigler ED, Prigge MB, Froehlich AL, Nielsen JA, Cariello AN, Zielinski BA, Anderson JS, Fletcher PT, Alexander AA, Lainhart JE. 2015. Longitudinal volumetric brain changes in autism spectrum disorder ages 6–35 years. Autism Res 8:82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. 2011. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 31:10937–10947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A, Jones DK. 2009. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61:1336–1349 [DOI] [PubMed] [Google Scholar]
- Lewis JD, Evans AC, Pruett JR, Botteron K, Zwaigenbaum L, Estes A, Gerig G, Collins L, Kostopoulos P, McKinstry R, Dager S, Paterson S, Schultz RT, Styner M, Hazlett H, Piven J. 2014. Network inefficiencies in autism spectrum disorder at 24 months. Transl Psychiatry 4:e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-O, Yang FG, Nguyen CT, Cooper SR, LaHue SC, Venugopal S, Mukherjee P. 2012. Independent component analysis of DTI reveals multivariate microstructural correlations of white matter in the human brain. Hum Brain Mapp 33:1431–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Chen Y, Lin P, Wang J. 2015. Small-world brain functional networks in children with attention-deficit/hyperactivity disorder revealed by EEG synchrony. Clin EEG Neurosci 46:183–191 [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T. 2008. Disrupted small-world networks in schizophrenia. Brain 131:945–961 [DOI] [PubMed] [Google Scholar]
- Liu Z, Ke L, Liu H, Huang W, Hu Z. 2014. Changes in topological organization of functional PET brain network with normal aging. PLoS One 9:e88690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. 2000. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30:205–223 [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. 1994. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24:659–685 [DOI] [PubMed] [Google Scholar]
- MacKay A, Whittall K, Adler J, Li D, Paty D, Graeb D. 1994. In vivo visualization of myelin water in brain by magnetic resonance. Magn Reson Med 31:673–677 [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. 2007. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol 64:945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V, Cheng H, Gong G, He Y, Dong Q, Huang H. 2013. Differences of inter-tract correlations between neonates and children around puberty: a study based on microstructural measurements with DTI. Front Hum Neurosci 7:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montembeault M, Joubert S, Doyon J, Carrier J, Gagnon J-F, Monchi O, Lungu O, Belleville S, Brambati SM. 2012. The impact of aging on gray matter structural covariance networks. Neuroimage 63:754–759 [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM. 2005. MRI Atlas of Human White Matter. Amsterdam: Elsevier Science [Google Scholar]
- Müller R-A, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. 2011. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex 21:2233–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Müller R-A. 2013. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain 136:1942–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MEJ. 2006. Modularity and community structure in networks. Proc Natl Acad Sci U S A 103:8577–8582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Yoshiura T, Kira R, Shigeto H, Hara T, Tobimatsu S, Kamio Y. 2010. Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Res 1362:141–149 [DOI] [PubMed] [Google Scholar]
- Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, Akhter K, Hua K, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Zhang J, Huang H, Miller MI, van Zijl PCM, Mazziotta J, Mori S. 2008. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage 43:447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajevic S, Basser PJ, Fields RD. 2014. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DJ, McGlaughlin A, Ruth D, Jager LR, Soldan A. 2015. Graph theoretic analysis of structural connectivity across the spectrum of Alzheimer's disease: the importance of graph creation methods. Neuroimage Clin 7:377–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. 1995. An MRI study of brain size in autism. Am J Psychiatry 152:1145–1149 [DOI] [PubMed] [Google Scholar]
- Prigge MB, Bigler ED, Fletcher PT, Zielinski BA, Ravichandran C, Anderson J, Froehlich A, Abildskov T, Papadopolous E, Maasberg K, Nielsen JA, Alexander AL, Lange N, Lainhart J. 2013a. Longitudinal Heschl's gyrus growth during childhood and adolescence in typical development and autism. Autism Res 6:78–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MB, Lange N, Bigler ED, Merkley TL, Neeley ES, Abildskov TJ, Froehlich AL, Nielsen JA, Cooperrider JR, Cariello AN, Ravichandran C, Alexander AL, Lainhart JE. 2013b. Corpus callosum area in children and adults with autism. Res Autism Spectr Disord 7:221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. 2003. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med 49:177–182 [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. 2010. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069 [DOI] [PubMed] [Google Scholar]
- Sanz-Arigita EJ, Schoonheim MM, Damoiseaux JS, Rombouts SARB, Maris E, Barkhof F, Scheltens P, Stam CJ. 2010. Loss of “small-world” networks in Alzheimer's disease: graph analysis of fMRI resting-state functional connectivity. PLoS One 5:e13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipul SE, Keller TA, Just MA. 2011. Inter-regional brain communication and its disturbance in autism. Front Syst Neurosci 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano MA, Boguñá M, Vespignani A. 2009. Extracting the multiscale backbone of complex weighted networks. Proc Natl Acad Sci U S A 106:6483–6488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Müller R-A. 2011. Tract‐specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. J Child Psychol Psychiatry 52:286–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaswamy L, Kumar A, Rajan D, Behen M, Muzik O, Chugani D, Chugani H. 2010. A diffusion tensor imaging study of the cerebellar pathways in children with autism spectrum disorder. J Child Neurol 25:1223–1231 [DOI] [PubMed] [Google Scholar]
- Smith SM. 2002. Fast robust automated brain extraction. Hum Brain Mapp 17:143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. 2013. The human connectome: origins and challenges. Neuroimage 80:53–61 [DOI] [PubMed] [Google Scholar]
- Sporns O, Chialvo D, Kaiser M, Hilgetag C. 2004. Organization, development and function of complex brain networks. Trends Cogn Sci 8:418–425 [DOI] [PubMed] [Google Scholar]
- Sporns O, Kötter R. 2004. Motifs in brain networks. PLoS Biol 2:e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RE, Schroer RJ, Skinner C, Fender D, Simensen RJ. 1997. Autism and macrocephaly. Lancet 349:1744–1745 [DOI] [PubMed] [Google Scholar]
- Stikov N, Campbell JSW, Stroh T, Lavelée M, Frey S, Novek J, Nuara S, Ho M-K, Bedell BJ, Dougherty RF, Leppert IR, Boudreau M, Narayanan S, Duval T, Cohen-Adad J, Picard P-A, Gasecka A, Côté D, Pike GB. 2015. In vivo histology of the myelin g-ratio with magnetic resonance imaging. Neuroimage 118:397–405 [DOI] [PubMed] [Google Scholar]
- Strogatz SH. 2001. Exploring complex networks. Nature 410:268–276 [DOI] [PubMed] [Google Scholar]
- Suzuki R, Shimodaira H. 2006. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 22:1540–1542 [DOI] [PubMed] [Google Scholar]
- Travers BG, Adluru N, Ennis C, Tromp D. 2012. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res 5:289–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Bigler ED, Tromp DPM, Adluru N, Froehlich AL, Ennis C, Lange N, Nielsen JA, Prigge MB, Alexander AL, Lainhart JE. 2014. Longitudinal processing speed impairments in males with autism and the effects of white matter microstructure. Neuropsychologia 53:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Tromp DPM, Adluru N, Lange N, Destiche D, Ennis C, Nielsen JA, Froehlich AL, Prigge MB, Fletcher PT, Anderson JS, Zielinski BA, Bigler ED, Lainhart JE, Alexander AL. 2015. Atypical development of white matter microstructure of the corpus callosum in males with autism: a longitudinal investigation. Mol Autism 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. 2013. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci 7:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, Geurts HM. 2012. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev 36:604–625 [DOI] [PubMed] [Google Scholar]
- Wahl M, Li Y-O, Ng J, LaHue SC, Cooper SR, Sherr EH, Mukherjee P. 2010. Microstructural correlations of white matter tracts in the human brain. Neuroimage 51:531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L, Gozzi M, Lenroot R, Thurm A, Behseta B, Swedo S, Pierpaoli C. 2012. Diffusion tensor imaging in young children with autism: biological effects and potential confounds. Biol Psychiatry 72:1043–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhu C, He Y, Zang Y, Cao Q, Zhang H, Zhong Q, Wang Y. 2009. Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Hum Brain Mapp 30:638–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass S. 2011. Distortions and disconnections: disrupted brain connectivity in autism. Brain Cogn 75:18–28 [DOI] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. 1998. Collective dynamics of “small-world” networks. Nature 393:440–442 [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Gerig G, Lewis JD, Soda T, Styner MA, Vachet C, Botteron KN, Elison JT, Dager SR, Estes AM, Hazlett HC, Schultz RT, Zwaigenbaum L, Piven J, Network FTI. 2015. Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain 138:2046–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2007. International Statistical Classification of Diseases and Related Health Problems. Tenth Revision. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- Wu K, Taki Y, Sato K, Kinomura S, Goto R, Okada K, Kawashima R, He Y, Evans AC, Fukuda H. 2012. Age-related changes in topological organization of structural brain networks in healthy individuals. Hum Brain Mapp 33:552–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap P-T, Fan Y, Chen Y, Gilmore JH, Lin W, Shen D. 2011. Development trends of white matter connectivity in the first years of life. PLoS One 6:e24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B. 2014. Spurious group differences due to head motion in a diffusion MRI study. Neuroimage 88:79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET. 2010. Network-based statistic: identifying differences in brain networks. Neuroimage 53:1197–1207 [DOI] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. 2012. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61:1000–1016 [DOI] [PubMed] [Google Scholar]
- Zhang H, Yushkevich PA, Alexander DC, Gee JC. 2006. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med Image Anal 10:764–785 [DOI] [PubMed] [Google Scholar]
- Zhao X, Liu Y, Wang X, Liu B, Xi Q, Guo Q, Jiang H, Jiang T, Wang P. 2012. Disrupted small-world brain networks in moderate Alzheimer's disease: a resting-state fMRI study. PLoS One 7:e33540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Anderson JS, Froehlich AL, Prigge MB, Nielsen JA, Cooperrider JR, Cariello AN, Fletcher PT, Alexander AL, Lange N, Bigler ED, Lainhart JE. 2012. scMRI reveals large-scale brain network abnormalities in autism. PLoS One 7:e49172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou J, Seeley WW. 2010. Network-level structural covariance in the developing brain. Proc Natl Acad Sci U S A 107:18191–18196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Prigge MB, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS, Fletcher PT, Zygmunt KM, Travers BG, Lange N, Alexander AL, Bigler ED, Lainhart JE. 2014. Longitudinal changes in cortical thickness in autism and typical development. Brain 137:1799–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.