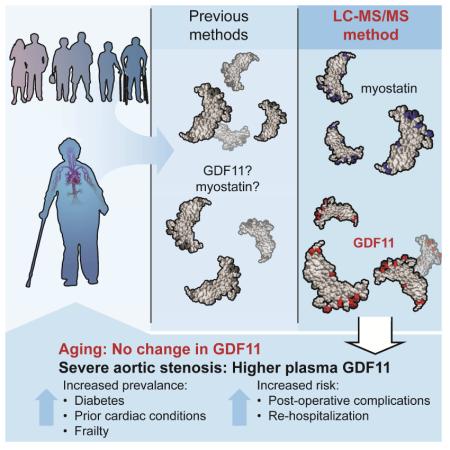
Abstract
Growth and differentiation factor 11 (GDF11) is a transforming growth factor β superfamily member with a controversial role in aging processes. We have developed a highly specific LC-MS/MS assay to quantify GDF11, resolved from its homologue, myostatin (MSTN), based on unique amino acid sequence features. Here, we demonstrate that MSTN, but not GDF11, declines in healthy men throughout aging. Neither GDF11 nor MSTN levels differ as a function of age in healthy women. In an independent cohort of older adults with severe aortic stenosis, we show that individuals with higher GDF11 were more likely to be frail and have diabetes or prior cardiac conditions. Following valve replacement surgery, higher GDF11 at surgical baseline was associated with rehospitalization and multiple adverse events. Cumulatively, our results show that GDF11 levels do not decline throughout aging but are associated with comorbidity, frailty, and greater operative risk in older adults with cardiovascular disease.
Introduction
Circulating proteins that change in abundance across the lifespan have the potential to serve as instructive biomarkers and, potentially, targetable modifiers of aging conditions. Members of the transforming growth factor β (TGF-β) superfamily have emerged as candidates that may link seemingly autonomous age-related diseases, including cardiovascular disease (CVD), diabetes, and frailty (Burks and Cohn, 2011; McPherron, 2010; Pardali and Ten Dijke, 2012). GDF11 and MSTN are highly-related TGF-β superfamily members and putative regulators of aging processes. However, recent studies have yielded contradictory findings with respect to age-associated changes in GDF11 and MSTN and their distinct roles in the genesis of aging-related conditions.
GDF11 and MSTN have striking homology, differing in amino acid sequence by only 11 residues (Nakashima et al., 1999). While sequence similarity has challenged partitioned detection of GDF11 and MSTN, structural insights suggest that amino acid sequence variation may confer important differences in inhibitory protein and receptor binding affinities (Cash et al., 2012; Cash et al., 2009; Padyana et al., 2016). Furthermore, expression pattern analyses and loss of function studies support non-redundant biological functions (McPherron et al., 1997, 1999; Nakashima et al., 1999). MSTN is expressed primarily in skeletal muscle and is a robust negative growth regulator (McPherron et al., 1997). Accordingly, therapeutic strategies to block myostatin signaling are aggressively being pursued as a means to improve muscle mass and function in the context of aging and disease (White and Lebrasseur, 2014).
GDF11 is more widely expressed and is requisite for axial skeleton patterning throughout embryogenesis (McPherron et al., 1999; Nakashima et al., 1999). In contrast to MSTN, the role of GDF11 in growth and regenerative processes is controversial. Initial reports exploiting heterochronic parabiosis identified GDF11 as a protein capable of reversing cardiac hypertrophy (Loffredo et al., 2013), skeletal muscle dysfunction (Sinha et al., 2014), and neurogenic potential (Katsimpardi et al., 2014). Declining GDF11 in circulation throughout normal aging was implicated as a causal factor in diminished systemic regenerative capacity in mice. Similarly, a recent human study that utilized a proteomic quantification method unable to distinguish GDF11 from MSTN demonstrated that combined GDF11+MSTN levels were higher in men, younger individuals, and people with lower risk of CVD events and death. The rejuvenative influence of GDF11 was called into question by Egerman et al., who showed that circulating GDF11 levels increase in rats and humans as a function of age and in fact, impede skeletal muscle regeneration (Egerman et al., 2015). Correspondingly, recent studies showed no effect of GDF11 on skeletal muscle satellite cell expansion in vitro (Hinken et al., 2016) and no cardiac improvements following daily delivery of recombinant GDF11 to 24-month old mice (Smith et al., 2015). The investigators who reported age-related decreases in GDF11 have also published corroborative results derived from antibody-based methods (Poggioli et al., 2016). Opposing conclusions underscore the requirement of methods sufficiently precise to quantify GDF11 and MSTN independently within explicit physiological contexts.
We recently described a novel immunoplexed liquid chromatography with tandem mass spectrometry (LC-MS/MS) assay applicable for accurate quantification of MSTN and two of its key inhibitors, follistatin-related gene (FLRG) protein and growth and serum protein-1 (GASP-1) (Bergen et al., 2015). We have expanded this technology to accurately measure levels of circulating GDF11. Here, we leverage immunoplexed LC-MS/MS to assess and compare age-associated changes in GDF11 and MSTN in healthy women and men from 20 to 80+ years of age. Moreover, we apply this method to define associations between clinical phenotypes and circulating concentrations of GDF11 and MSTN in a well-characterized cohort of older adults undergoing valve replacement for severe aortic stenosis, an age-associated condition and the most common form of valvular CVD in developed countries (Osnabrugge et al., 2013)
Results
Resolution of circulating GDF11 and MSTN abundance by LC-MS/MS
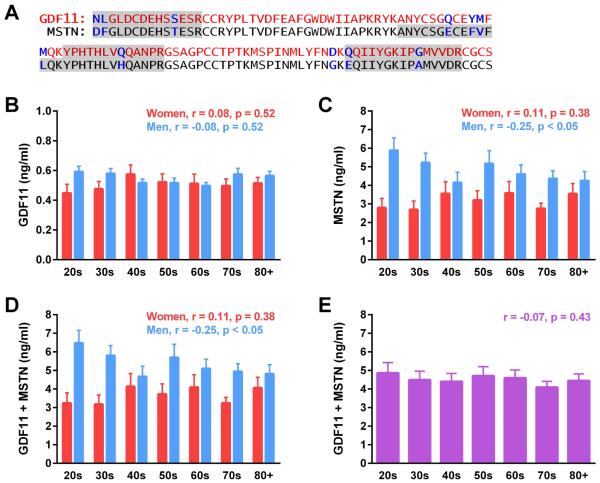
High amino acid sequence homology (Fig.1A) and low circulating concentrations have challenged previous attempts at measuring GDF11 and MSTN in vivo. LC-MS/MS overcomes this barrier by monitoring multiple, distinct residues within GDF11 and MSTN (Fig.1A), allowing for accurate resolution of homologue quantity within biological samples. Monitored transitions and assay parameters employed for assessment of GDF11 and MSTN are detailed in Table S1, and comparative GDF11 and MSTN peptide spectra are depicted in Figure S1. The lower limits of detection and quantification (LOD, LOQ) for GDF11 were both 194 pg/ml, determined by spiking authentic protein into BSA. A four hour digestion utilizing “winged” internal standard peptides to account for digestion variability was found to provide consistent results with no evidence of residual intact internal standard peptide (Hoofnagle et al., 2016; Shuford et al., 2012). Inter- and intra-assay precision for GDF11 ranged from 9.6-18.4% and 1.5-10.6%, respectively, for recombinant concentrations and pooled human serum (Tables S2 and S3). Recovery rates were ascertained relative to the calculated levels of endogenous GDF11 plus spiked in concentrations and ranged from 64-91% (Table S4.) Assay parameters for measurement of MSTN, including LOD, LOQ, assay precision, and recovery rates, have been recently described (Bergen et al., 2015).
Figure 1. Quantitative analysis of GDF11 and MSTN by LC-MS/MS throughout human aging.
(A) Amino acid sequence of active human GDF11 (red) and MSTN (black), indicating differing residues (blue). Shading demarcates MS signal peptides monitored for quantification. (B) GDF11 and (C) MSTN levels were quantified in healthy women and men corresponding to the indicated ages in years. Concentrations of GDF11 and MSTN were combined and compared in a (D) sex-dependent and (E) sex-independent manner throughout aging (n=10 per sex and decade; linear regression assessment.)
Aging-related changes in GDF11 and MSTN
Previous studies exploring the relationship of GDF11 to aging have been contradictory. Thus, we utilized our quantitative LC-MS/MS assay to measure serum protein levels in 70 women and 70 men, age 21-93. GDF11 levels did not statistically differ as a function of age or sex (Fig. 1B). MSTN levels were highest in men in their 20s and statistically declined throughout subsequent decades (Fig. 1C). Female MSTN levels did not change as a function of age (Fig. 1C). Consistent with our previous study (Bergen et al., 2015), young women had lower circulating MSTN levels than young men Fig. 1C). As methods used in several previous studies were unable to distinguish GDF11 from MSTN, we also report combined GDF11 and MSTN levels. GDF11+MSTN levels declined in men but not women throughout aging, in register with MSTN levels (Fig. 1D). When data from both sexes were combined, we did not observe statistically significant differences in circulating GDF11+MSTN levels throughout aging (Fig. 1E). Of note, GDF11 represented, on average, 15.5% and 11.2% of composite GDF11+MSTN values for women and men, respectively. Thus, GDF11 levels are not altered in women or men as a function of age, and previous observations of age-dependent decreases in GDF11 levels likely reflect changes in MSTN levels.
GDF11 and MSTN in older adults with CVD
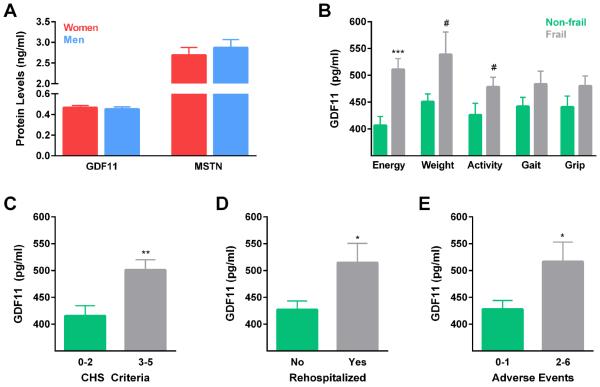
Given previous reports that GDF11 may influence cardiovascular health, we next tested whether plasma protein levels are associated with health parameters and post-operative outcomes in an extensively characterized cohort of older adults undergoing surgical valve replacement for treatment of severe aortic stenosis. Our study sample consisted of 41 women and 55 men, age 65-94. Plasma GDF11 levels ranged from 220 to 841 pg/ml and plasma myostatin concentrations ranged from 0.64 to 6.27 ng/ml. In contrast to our healthy aging subjects, neither GDF11 nor MSTN levels statistically differed as a function of sex (Fig. 2A). For phenotypic comparisons, we stratified participants into low, middle, and high GDF11 or MSTN tertiles (Table 1, Table 2). GDF11 groups did not vary by sex or age (Table 1), but consistent with our data from healthy subjects, the tertile with the lowest myostatin trended to be of older age (p=0.066) (Table 2). We observed a trend indicative of higher body weight in individuals with low GDF11 levels (Table 1). There were no differences in the prevalence of hypertension or hyperlipidemia, recognized risk factors for adverse health outcomes, as a function of GDF11 or MSTN levels (Table 1, Table 2).
Figure 2. Frailty, rehospitalization, and multiple adverse postoperative events are associated with elevated GDF11 levels in older adults with severe aortic stenosis.
(A) Quantified levels of circulating GDF11 and MSTN do not differ among older women (n=41) and men (n=55) with CVD. CHS criteria were applied for frailty assessment and were compared with plasma GDF11 concentrations. (B) Participants with normal test performance are indicated by green bars, and those with impairments are indicated by gray bars. Energy and endurance were determined through Center of Epidemiological Studies Depression Scale self-report questions. (Normal energy, n=48, low energy, n=48; t-test, ***p<0.001.) Unintentional weight loss of 10 pounds or greater within the past year was determined by verbal report or medical record examination. (No loss, n=87, unintentional loss, n=9; t-test, #p=0.064.) The Physical Activity Scale for the Elderly was used to estimate activity; a score of less than 100 equated to low activity. (Normal activity, n=36, low activity, n=60; t-test, #p=0.066.) Average time required to walk 5 meters over three trials was used to probe gait speed. Less than 0.83 meters per second was considered slow gait. (Normal gait, n=62, slow gait, n=33; t-test, p=0.150.) Dominant hand grip strength was measured by serial tests with an electronic dynamometer, with normal strength defined as above 17-21 kg for women and 29-32 kg for men, normalized to BMI. (Normal grip, n=52, weak grip, n=44; t-test, p=0.163). (C) Individuals displaying impairments in 2 or fewer CHS criteria were considered non-frail, and individuals with 3 or more criteria were considered frail (Non-frail, n=45, frail, n=50; t-test, **p=0.002.) (D) Of the 73 participants in which follow-up information was ascertained, 22 were rehospitalized at least once (No n=51, Yes n=22, t-test, *p=0.011.) (E) Participants who experienced multiple adverse events post-surgery had higher GDF11 levels at baseline than those that experienced one or no negative events (0-1 AE n=52, 3-6 AE n=21, t-test, *p=0.011.)
Table 1.
Study sample demographic characteristics, comorbid conditions or events, and estimated CVD risk, stratified by circulating GDF11 levels.
| GDF11 Level | ||||
|---|---|---|---|---|
| 220-400 pg/ml (n=35) |
401-500 pg/ml (n=33) |
501-841 pg/ml (n=28) |
||
| Characteristic | Mean (±SD) or Number (%) | p-value | ||
| Sex | 0.3571 | |||
| Female | 12 (34.3%) | 17 (51.5%) | 12 (42.9%) | |
| Male | 23 (65.7%) | 16 (48.5%) | 16 (57.1%) | |
| Age in years | 79.1 (8.1) | 80.8 (7.5) | 82.2 (6.2) | 0.2682 |
| Weight in kilograms | 91.3 (24.5) | 80.6 (18.3) | 82.8 (12.9) | 0.0662 |
| Height in centimeters | 169.3 (12.2) | 163.8 (10.1) | 166.6 (10.6) | 0.1272 |
| BMI | 31.7 (7.4) | 29.9 (6.0) | 30.0 (5.3) | 0.4702 |
| Comorbid condition or event | ||||
| Diabetes | 5 (14.3%) | 9 (27.3%) | 13 (46.4%) | 0.0191 |
| Hypertension | 31 (88.6%) | 30 (90.9%) | 25 (89.3%) | 0.9501 |
| Hyperlipidemia | 24 (68.6%) | 22 (66.7%) | 20 (71.4%) | 0.9231 |
| Peripheral vascular disease | 12 (34.3%) | 16 (48.5%) | 11 (39.3%) | 0.4851 |
| Previous stroke | 3 (8.6%) | 1 (3.0%) | 5 (17.9%) | 0.1381 |
| Previous percutaneous coronary angioplasty | 7 (20.0%) | 13 (39.4%) | 13 (46.4%) | 0.0681 |
| Previous coronary artery bypass | 5 (14.3%) | 10 (30.3%) | 12 (42.9%) | 0.0411 |
| Atrial fibrillation or flutter | 9 (25.7%) | 9 (27.3%) | 10 (35.7%) | 0.6571 |
| Previous pacemaker | 2 (5.7%) | 1 (3.0%) | 4 (14.3%) | 0.2191 |
| Any prior cardiac condition | 14 (40.0%) | 22 (66.7%) | 20 (71.4%) | 0.0211 |
| CVD classification or risk | ||||
| NYHA classification | 2.5 (0.7) | 2.7 (0.6) | 3.0 (0.7) | 0.0422 |
| STS predicted risk of mortality (%) | 5.7 (3.1) | 7.1 (4.2) | 8.3 (3.8) | 0.0442 |
Chi-Square
ANOVA F-test
Table 2.
Study sample demographic characteristics, comorbid conditions or events, and estimated CVD risk, stratified by circulating MSTN levels.
| MSTN Level | ||||
|---|---|---|---|---|
| ≤2 ng/ml (n=32) |
2-3.1 ng/ml (n=32) |
≥3.1 ng/ml (n=32) |
||
| Characteristic | Mean (±SD) or Number (%) | p-value | ||
| Sex | 0.1241 | |||
| Female | 13 (40.6%) | 18 (56.3%) | 10 (31.3%) | |
| Male | 19 (59.4%) | 14 (43.8%) | 22 (68.8%) | |
| Age in years | 83.0 (7.0) | 79.9 (6.5) | 78.9 (8.2) | 0.0662 |
| Weight in kilograms | 81.8 (17.0) | 89.6 (25.4) | 84.1 (15.9) | 0.2792 |
| Height in centimeters | 167.8 (12.0) | 165.4 (10.6) | 166.6 (11.0) | 0.6872 |
| BMI | 28.9 (4.8) | 32.5 (7.9) | 30.4 (5.7) | 0.0732 |
| Comorbid condition or event | ||||
| CHS frailty score | 2.7 (1.4) | 2.4 (1.4) | 2.2 (1.5) | 0.3862 |
| Diabetes | 8 (25.0%) | 8 (25.0%) | 11 (34.4%) | 0.6291 |
| Hypertension | 28 (87.5%) | 30 (93.8%) | 28 (87.5%) | 0.6401 |
| Hyperlipidemia | 22 (68.8%) | 19 (59.4%) | 25 (78.1%) | 0.2701 |
| Peripheral vascular disease | 12 (37.5%) | 15 (46.9%) | 12 (37.5%) | 0.6781 |
| Previous stroke | 4 (12.5%) | 2 (6.3%) | 3 (9.4%) | 0.6921 |
| Previous percutaneous coronary angioplasty | 12 (37.5%) | 10 (31.3%) | 11 (34.4%) | 0.8711 |
| Previous coronary artery bypass | 7 (21.9%) | 7 (21.9%) | 13 (40.6%) | 0.1561 |
| Atrial fibrillation or flutter | 14 (43.8%) | 6 (18.8%) | 8 (25.0%) | 0.0731 |
| Previous pacemaker | 1 (3.1%) | 5 (15.6%) | 1 (3.1%) | 0.0851 |
| Any prior cardiac condition | 18 (56.2%) | 18 (56.2%) | 20 (62.5%) | 0.8421 |
| CVD classification or risk | ||||
| NYHA classification | 2.8 (0.7) | 2.8 (0.8) | 2.6 (0.6) | 0.3692 |
| STS predicted risk of mortality (%) | 7.8 (4.2) | 6.3 (3.3) | 6.7 (3.9) | 0.3452 |
Chi-Square
ANOVA F-test
GDF11, comorbid conditions, functional status, and mortality risk
We next interrogated potential associations between GDF11 and MSTN levels and baseline comorbid conditions. Increased circulating GDF11 was identified in a greater proportion of study participants with diabetes (p=0.019). Forty-six percent of individuals with high GDF11 had diabetes, in contrast to only 14.3% with low levels. Elevated GDF11 was also associated with a history of previous cardiac conditions (p=0.021), with a significant relationship drawn with previous coronary artery bypass (p=0.041). Participants with the highest GDF11 levels at surgery had significantly higher NYHA class ranking than those with lower GDF11 levels (p=0.042), and were uniquely categorized as high risk based on a mean STS predicted risk of mortality score greater than 8% (Thourani et al., 2015) (p=0.044) (Table 1). Critically, we did not identify differences in aortic stenosis severity or related cardiac dysfunction, as measured by ejection fraction, mean gradient, aortic valve velocity, valve area, valve area index, or left ventricular mass index, as a function of circulating GDF11 concentration (Table S5). In contrast to GDF11, we were unable to draw any statistical associations between MSTN and comorbid conditions, NYHA classification, or mortality risk (Table 2).
GDF11 as a biomarker of frailty
Frailty is a strong predictor of poor post-operative outcomes, yet biomarkers of frailty have not been forthcoming. Accordingly, we tested whether circulating GDF11 and MSTN protein levels are associated with frailty status using Cardiovascular Health Study (CHS) criteria, specified by unintentional weight loss, grip strength, endurance, gait speed, and physical activity (Fried et al., 2001). Participants who reported low energy and endurance had significantly higher levels of circulating GDF11 than those who reported normal energy (p<0.001). A trend towards increased plasma GDF11 concentrations was observed in individuals demonstrating unintentional weight loss (p=0.064) and low activity (p=0.066), relative to peers reporting no weight loss and normal activity (Fig. 2B). Similarly, individuals with slow gait (p=0.150) and weak grip strength (p=0.163) appeared to possess higher GDF11 levels than counterparts with normal gait and appropriate grip strength, although these associations did not reach statistical significance. Using the presence of 3 or more CHS criteria as an operational frailty definition, we compared GDF11 concentrations between frail and non-frail study participants. Mean GDF11 levels increased by 21% as a function of frailty (p=0.002) (Fig.2C). Unlike GDF11, MSTN levels were not associated with frailty status (Table 2). To evaluate whether the relationship between GDF11 and frailty remained after adjusting for potential confounding demographic and comorbidity characteristics, we applied a multivariable model using a penalized stepwise logistic regression approach for variable selection. After variable adjustment, GDF11 was still significantly related to frailty (p=0.003) (Table S6).
GDF11 and adverse health outcomes
Multimorbidity and frailty are independent predictors of poor post-operative outcomes (Makary et al., 2010; Rowe et al., 2014). Thus, we speculated that elevated GDF11 levels at the time of surgery, which were associated with both comorbid conditions and frailty, might also predict adverse outcomes. Of the 96 individuals who underwent surgical or transcatheter aortic stenosis treatment, we received post-operative information in the form of follow-up visits at 1, 3, and/or 6 months post-surgery, self-reported surveys, and/or communication with a primary care provider for 73 study participants. One or more adverse outcomes were observed in 33 individuals, which included myocardial infarction, new arrhythmia, new conduction abnormality, stroke, deep vein thrombosis, pulmonary emboli, pneumonia, pleural effusion, renal insufficiency, seizure disorder, hypotension, tachycardia, bradycardia, urinary tract infection, other infection, acute dementia, rehospitalization, or death. The most common adverse event was rehospitalization, an outcome experienced by 22 participants (30% of the study sample that provided follow-up information) at least once within one year post-operation. Individuals who were rehospitalized had significantly higher GDF11 levels at baseline than non-rehospitalized counterparts (Fig.2D). Moreover, participants that experienced multiple adverse health outcomes had higher baseline GDF11 levels than those with only one or no post-operative health complications (Fig. 2E). The associations between GDF11 and rehospitalization or multiple adverse post-operative events remained statistically significant after adjusting for potential confounding demographic and comorbidity variables (Table S6).
Discussion
Aging is the primary risk factor for the majority of chronic diseases. Recent efforts to understand this fundamental link have included the search for circulating factors that may be biomarkers or modifiers of biological age. GDF11 has been proposed as one such factor, but has been surrounded by controversy attributed, in part, to challenges associated with the accurate measurement of circulating levels and their dissociation from circulating MSTN levels. Here, using a novel, highly precise immunoplexed LC-MS/MS assay, we show that, cross-sectionally, circulating concentrations of GDF11 do not decline as a function of advancing age in humans. Furthermore, in older adults with CVD, we show that increased circulating GDF11 levels are associated with a higher prevalence of comorbid conditions, frailty, and a larger number of adverse health outcomes following aortic valve replacement surgery.
Initially, GDF11 was identified, using an aptamer-based proteomic profiling platform, as a circulating rejuvenating factor in young mice that declines with age (Loffredo et al., 2013). It was later learned that this methodology is unable to distinguish GDF11 from MSTN, thwarting interpretation. A recent study in humans utilizing a similar aptameric strategy identified an association between higher GDF11+MSTN levels with younger age and lower risk of CVD events and death (Olson et al., 2015). This study also demonstrated higher GDF11+MSTN levels in men. Our results, derived from a novel mass spectrometric method capable of accurately resolving GDF11 from MSTN, show that GDF11 levels do not decline throughout aging and do not statistically differ between sexes in healthy adults. MSTN, in contrast, is highest in men in their 20s and declines throughout aging. This observation is substantiated by our results demonstrating reduced MSTN levels in aged and sarcopenic males compared to young men (Bergen et al., 2015), as well as recent results demonstrating cross-reactivity of an assay historically used for MSTN detection and declining MSTN levels in murine aging (Rodgers and Eldridge, 2015). Trends toward age-dependent differences in GDF11+MSTN observed by Olson et al., therefore, are likely attributable to differences in MSTN levels, the far more abundant homologue, rather than changes in GDF11 levels.
Early exploration of putative rejuvenative properties suggested that GDF11 mediates cardioprotective effects, including reversal of age-related hypertrophy (Loffredo et al., 2013). Recent studies have contradicted these claims, showing trends for age-related increases in circulating GDF11 concentrations in rodents and humans (Egerman et al., 2015), and no beneficial effects of GDF11 treatment on cardiac myocyte size, total cardiac volume, or cardiac performance. Rather, GDF11 treatment dose-dependently increases ventricular myocyte hypertrophy in culture (Smith et al., 2015). Similarly, the beneficial influence of GDF11 on skeletal muscle regeneration following injury (Sinha et al., 2014) has been challenged (Egerman et al., 2015; Hinken et al., 2016). To establish relevance in humans and disentangle controversy, we measured GDF11 levels in a well-characterized patient population of older adults undergoing surgery for severe aortic stenosis. We observed a higher prevalence of cardiac conditions and prior cardiac procedures, worse NYHA classification scores, and STS scores greater than 8% (indicative of high risk for morbidity and mortality) in older adults with higher compared to lower plasma GDF11 concentrations. Importantly, these associations were independent of obesity, hypertension, and hyperlipidemia, which are well-established risk factors for CVD and its complications (Grundy et al., 1999). No similar associations were drawn for MSTN. While these findings do not clarify whether increases in GDF11 are adaptive or maladaptive in patients with end-stage valvular heart disease, our data support the conclusion that GDF11 may be a unique biomarker to discern between biological and chronological aging.
We have also identified striking associations between high circulating GDF11 levels and increased disease burden, reduced resiliency, and elevated post-operative risk. In particular, the prevalence of diabetes was 3-fold higher in individuals with the highest compared to the lowest plasma concentrations of GDF11. Moreover, despite equivalent age and severity of aortic stenosis, participants categorized as frail by CHS criteria, a combination of performance-based (i.e., grip strength and gait speed) and self-reported (i.e., questions of activity and endurance) measures, had significantly higher GDF11 levels compared to peers categorized as non-frail. We believe this is of great importance as chronological age and disease severity are inadequate determinants of surgical risk. Frailty, on the other hand, is a strong predictor of post-operative vulnerability in older adults (Makary et al., 2010), and a biomarker of frailty could be of significant utility in the clinical setting. In accordance, we observed that participants who had multiple postoperative complications had 21% higher plasma concentrations of GDF11, and those who were rehospitalized after surgery had 20% higher GDF11 levels. These data suggest that GDF11 may indeed be a modifier of aging, as circulating levels are associated with deficits in multiple physiological systems. Additional research is needed to determine whether GDF11 is a predictor of compromised physiological reserves and subsequent risk, or “biological age”, in other clinical populations.
We believe the current controversy surrounding GDF11 as either a rejuvenating factor or a pro-aging protein is related to the difficulties inherent to its measurement. In the present study, we have overcome methodological challenges by developing an immunoplexed LC/MS-MS assay with excellent specificity for GDF11 and MSTN. However, we are not yet able to determine the relative proportions of either latent versus mature GDF11 or MSTN, or unbound versus bound (to propeptide, GASP1, FLRG, or other binding proteins) mature GDF11 or MSTN. Indeed, additional methodological advances that resolve these limitations will yield important insights into the abundance and regulation of these fascinating proteins. An additional contributor to the inconsistent conclusions about GDF11’s role in aging may be the experimental contexts (e.g., species, models, doses of recombinant protein) in which it has been studied. These issues have been discussed exhaustively elsewhere (Egerman et al., 2015; Harper et al., 2016; McNally, 2016; Walker et al., 2016).
In summary, our data suggest that GDF11 is a possible biomarker of advanced biological aging and impaired organismal resiliency to surgical stress, but is not a reliable indicator of chronological aging. GDF11 levels do not decline in men or women as a function of age. In older adults with aortic stenosis who underwent surgery, however, we consistently observed significant associations between baseline circulating concentrations of GDF11 and comorbidity, frailty, multiple postoperative complications, and rehospitalization. These observations were unique to GDF11, and not observed with MSTN. Additional research is needed to understand the generalizability of these findings, the utility of GDF11 as predictor of health outcomes, and ultimately, the potential contexts in which GDF11 signaling may be harnessed for therapeutic benefit.
Experimental Procedures
Study Participants and Protocol
These studies were approved by the Mayo Clinic Institutional Review Board. For the aging study, samples were derived from 140 healthy individuals (70 women and 70 men), collected at Mayo Clinic in Rochester, MN between November 2000 and May 2006 who were randomly selected from the local population. Participants providing samples ranged in age from 21 to 93 years old. Additional patient cohort characteristics have been previously described (Lebrasseur et al., 2012; Riggs et al., 2004). For the CVD study, a convenience sample of 96 individuals (41 women and 55 men) age 65 years and older diagnosed with severe aortic stenosis and scheduled for surgical or transcatheter aortic valve replacement were recruited between July 2013 and May 2015 at Mayo Clinic in Rochester, MN. Demographic characteristics and medical history, including previous surgical events and diagnoses, were ascertained by interview, physical exam, and electronic medical record review at baseline. The New York Heart Association functional classification (NYHA) system was used to define CVD severity (New York Heart Association. and Ferrer, 1994). The Society of Thoracic Surgeons (STS) scoring system was used to predict the risk of mortality (Puskas et al., 2012). Clinical parameters of frailty were based upon the Cardiovascular Health Study (CHS) criteria, as outlined by the following metrics: weak grip strength by electronic dynamometer (less than 17-21 kg for women and 29-32 kg for men, normalized to BMI), slow walk speed by a handheld ultrasonic monitor (less than 0.83 meters per second), self-report of low endurance and energy on the Center for Epidemiological Studies Depression Scale (self-report of exhaustion), unintended weight loss (greater than or equal to 10 pounds in the prior year), and low physical activity by the Physical Activity Scale for the Elderly (men, less than 383 kcal expended per week; women, less than 270 kcal expended per week) (Fried et al., 2001). Adverse peri- and post-operative events were recorded prior to discharge from hospital and at 1, 3, and 6 months post-dismissal.
Sample Preparation
For the aging study, fasted serum samples were collected and stored at −80°C until proteomic assessment. The following sample preparation methods were applied to a plate-based format utilizing Eppendorf deepwell 96/2000μl Lo Bind® plates. For the CVD study, fasted blood samples collected in EDTA at the time of the surgical procedure were centrifuged and stored at −80°C until proteomic assessment. Participant plasma samples, standards, and controls were analyzed as recently described using a tube-based format (Bergen et al., 2015). Briefly, 400 μL of sample was diluted with 600 μL Phosphate buffered saline (PBS) (Bio-Rad, Hercules, CA, USA) containing 0.03 % 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) (Thermo Fisher Scientific, Waltham, MA, USA). Biotinylated anti GDF11 (cat# MAB19581, R&D systems, Minneapolis, MN, USA), biotinylated anti-MSTN (cat# BAF788), biotinylated anti-GASP-1 (cat# BAF2070), and biotinylated anti-FLRG (cat# BAF1288) (0.25μg/μL in PBS with 0.1% BSA) were immobilized to Dynabeads® M-280 Streptavidin (Invitrogen, Carlsbad, CA,USA) (10mg/mL in PBS) at a ratio of 40 μL antibody to 125 μL of magnetic bead suspension. Individual suspensions were combined, rinsed with serial PBS washes, and diluted to a final volume of 625 μL with PBS. Fifteen microliters of immobilized antibody mixture was added to each sample, which were incubated at 4°C with rotation overnight. Following consecutive PBS washes, 15 μL of 8 M urea, 15 mM tris(2-carboxyethyl)phosphine hydrochloride (TCEP) (Sigma, St. Louis, MO, USA) in 50 mM tris buffer was added to each sample with an internal standard peptide mixture (24nM KRSRRDFGLDCDEHSTESRCCRYP, 60nM VFLQKYPHTHLVHQANPRGSAGP, 48nM PKRYKANYCSGECEFVFLQKYPHTH, 6nM YFNGKEQIIYGKIPAMVVDRCGCS, 12nM YFNDKQQIIYGKIPGMVVDRCGCS, 60nM KRSRRNLGLDCDEHSSESRCCRYP, 60nM MFMQKYPHTHLVQQANPRGSAGP) and incubated for 30 minutes at room temperature. Alkylation was achieved by application of 20 μL of 60 mM iodoacetamide (Sigma) (30 mM final) and 30 min room temperature incubation in darkness. Urea was diluted to 1.1M with the addition of 70 μL of 50 mM tris buffer pH 8.2. Five microliters of Trypsin/Lys-C Mix (Promega, Madison, WI, USA) (0.2 μg/μL) was added to each sample and was incubated at 37°C for 4 hours. Trypsin digestion was terminated through the addition of 5 μL of 4.7% trifluoroacetic acid (TFA) (Thermo Fisher Scientific) with 0.02 %
Zwittergent® 3-16 (CalBiochem, EMD Millipore, Billerica, MA, USA). Digested samples were transferred to auto-sampler vials and analyzed by LC-MS/MS.
Liquid chromatography-tandem mass spectrometry and protein analysis
5 μL of the acidified samples were loaded onto a 0.25μL bed OptiPak trap (Optimize Technologies, Oregon) custom-packed with 5um, 200A Magic C8 (Bruker-Michrom, Auburn, CA) stationary phase. An aqueous loading buffer composed of 0.2% FA and 0.05% TFA was used to wash the loaded trap at a flow rate of 10μL/min. Following the wash, a 10-port valve was used to place the trap in-line with a 35cmx100μm PicoFrit column, self-packed with Michrom Magic 3μm C18 AQ stationary phase, using a Dionex UltiMate® 3000 RSLC liquid chromatography (LC) system (Thermo-Fisher Scientific, Sunnyvale, CA). The LC flow rate was set at 400nL/min and the gradient was 2%-50% B in 20 min, 50%-95%B from 20-22 min, held at 95%B from 20-24 min and re-equilibrated to 2%B from 26-39 minutes. Mobile phase A was 2% acetonitrile (ACN) in water with 0.2% FA and mobile phase B was ACN/isopropanol/water (80/10/10 by volume) with 0.2% FA. The target peptides were analyzed using a QExactive mass spectrometer (Thermo-Fisher Scientific, Bremen, Germany). The instrument was configured to acquire 1 full MS scan followed by 10 consecutive targeted MS2 scans directed by a scheduled inclusion list including m/z values for the native and labeled forms of the target peptides. The MS1 full scans were collected at 70,000 resolving power (measured at m/z 200) with an AGC value of 1E6 over a m/z range of 250-2500. The targeted MS2 scans were acquired at 17,500 resolving power, an AGC target of 3E6 and a maximum ionization time of 50ms. Target transition chromatograms were extracted from the full scan MS2 data using a 10ppm window and data was analyzed as recently described (Bergen et al., 2015).
Statistical Analyses
The Pearson correlation coefficient was used to summarize the relationship between the biomarkers and age. We summarized continuous variables as mean (SD) and reported categorical variables as percentages. Continuous and categorical variables were compared by GDF11 levels using the ANOVA F-test and the chi-square test, respectively. Binary variables were compared using t-tests. Penalized logistic regression with stepwise variable selection (stepPlr package in R) was used to identify comorbidity and demographic variables associated with measures of frailty or adverse post-operative outcomes. These variables were then added to a multivariable model with GDF11; a spline was used to account for the non-linear relationship between GDF11 and the endpoint. Analyses were performed using JMP 10.0, R 3.2.0, SAS 9.4, and GraphPad Prism 6.05.
Supplementary Material
In Brief.
XXX et al develop a LC-MS/MS assay for precise quantification of GDF11 and its closely related homologue, myostatin, and show that circulating concentrations of GDF11 do not decline in humans during aging. Furthermore, in older adults with cardiovascular disease, increased circulating GDF11 levels are associated with negative health outcomes.
Highlights.
- Immunoplexed liquid chromatography mass spectrometric assessment of GDF11 and MSTN
- Circulating GDF11 levels do not change in healthy adults throughout the lifespan
- Previous reports of declining GDF11 levels likely correspond to changes in MSTN
- In CVD, GDF11 is associated with comorbidity, frailty, and post-operative outcomes
Acknowledgments
We are grateful to the women and men who participated in this study. This work was supported by Robert and Arlene Kogod, the Hoeft family, the Pritzker Foundation, the Glenn Foundation for Medical Research, and the National Institutes of Health (NIH) grants AR027065 and AG004875 to SK. This study was also supported by the Mayo Clinical Center for Clinical and Translational Science, Grant Number UL1 TR000135 from the National Center for Advancing Translational Science, a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
This study was designed by MJS, CJB, KLG, RMS, SK, JDM, HRB, and NKL. HRB, PMV, BK, and TAW collected data, which was analyzed and interpreted by MJS, EJA, SK, JDM, HRB, and NKL. The manuscript was drafted by MJS, EJA, PMV, and NKL and revised by MJS, MMM, KLG, RMS, SK, JDM, HRB, and NKL. The authors have no conflicts to disclose.
References
- Bergen HR, 3rd, Farr JN, Vanderboom PM, Atkinson EJ, White TA, Singh RJ, Khosla S, LeBrasseur NK. Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: insights using a new mass spectrometry-based assay. Skelet Muscle. 2015;5:21. doi: 10.1186/s13395-015-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks TN, Cohn RD. Role of TGF-beta signaling in inherited and acquired myopathies. Skelet Muscle. 2011;1:19. doi: 10.1186/2044-5040-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash JN, Angerman EB, Kattamuri C, Nolan K, Zhao H, Sidis Y, Keutmann HT, Thompson TB. Structure of myostatin.follistatin-like 3: N-terminal domains of follistatin-type molecules exhibit alternate modes of binding. J Biol Chem. 2012;287:1043–1053. doi: 10.1074/jbc.M111.270801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash JN, Rejon CA, McPherron AC, Bernard DJ, Thompson TB. The structure of myostatin:follistatin 288: insights into receptor utilization and heparin binding. Embo J. 2009;28:2662–2676. doi: 10.1038/emboj.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, et al. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab. 2015 doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr., Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- Harper SC, Brack A, MacDonnell S, Franti M, Olwin BB, Bailey BA, Rudnicki MA, Houser SR. Is Growth Differentiation Factor 11 a Realistic Therapeutic for Aging-Dependent Muscle Defects? Circ Res. 2016;118:1143–1150. doi: 10.1161/CIRCRESAHA.116.307962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinken AC, Powers JM, Luo G, Holt JA, Billin AN, Russell AJ. Lack of evidence for GDF11 as a rejuvenator of aged skeletal muscle satellite cells. Aging Cell. 2016 doi: 10.1111/acel.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofnagle AN, Whiteaker JR, Carr SA, Kuhn E, Liu T, Massoni SA, Thomas SN, Townsend RR, Zimmerman LJ, Boja E, et al. Recommendations for the Generation, Quantification, Storage, and Handling of Peptides Used for Mass Spectrometry– Based Assays. Clinical Chemistry. 2016;62:48–69. doi: 10.1373/clinchem.2015.250563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrasseur NK, Achenbach SJ, Melton LJ, 3rd, Amin S, Khosla S. Skeletal muscle mass is associated with bone geometry and microstructure and serum insulin-like growth factor binding protein-2 levels in adult women and men. J Bone Miner Res. 2012;27:2159–2169. doi: 10.1002/jbmr.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall'Osso C, Khong D, Shadrach JL, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- McNally EM. Questions and Answers About Myostatin, GDF11, and the Aging Heart. Circ Res. 2016;118:6–8. doi: 10.1161/CIRCRESAHA.115.307861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron AC. Metabolic Functions of Myostatin and Gdf11. Immunol Endocr Metab Agents Med Chem. 2010;10:217–231. doi: 10.2174/187152210793663810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet. 1999;22:260–264. doi: 10.1038/10320. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Toyono T, Akamine A, Joyner A. Expression of growth/differentiation factor 11, a new member of the BMP/TGFbeta superfamily during mouse embryogenesis. Mech Dev. 1999;80:185–189. doi: 10.1016/s0925-4773(98)00205-6. [DOI] [PubMed] [Google Scholar]
- New York Heart Association. Ferrer MI. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th Boston: Little, Brown: 1994. [Google Scholar]
- Olson KA, Beatty AL, Heidecker B, Regan MC, Brody EN, Foreman T, Kato S, Mehler RE, Singer BS, Hveem K, et al. Association of growth differentiation factor 11/8, putative anti-ageing factor, with cardiovascular outcomes and overall mortality in humans: analysis of the Heart and Soul and HUNT3 cohorts. Eur Heart J. 2015;36:3426–3434. doi: 10.1093/eurheartj/ehv385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, Bogers AJ, Piazza N, Kappetein AP. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Padyana AK, Vaidialingam B, Hayes DB, Gupta P, Franti M, Farrow NA. Crystal structure of human GDF11. Acta Crystallogr F Struct Biol Commun. 2016;72:160–164. doi: 10.1107/S2053230X16001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardali E, Ten Dijke P. TGFbeta signaling and cardiovascular diseases. Int J Biol Sci. 2012;8:195–213. doi: 10.7150/ijbs.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggioli T, Vujic A, Yang P, Macias-Trevino C, Uygur A, Loffredo FS, Pancoast JR, Cho M, Goldstein J, Tandias RM, et al. Circulating Growth Differentiation Factor 11/8 Levels Decline With Age. Circ Res. 2016;118:29–37. doi: 10.1161/CIRCRESAHA.115.307521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puskas JD, Kilgo PD, Thourani VH, Lattouf OM, Chen E, Vega JD, Cooper W, Guyton RA, Halkos M. The society of thoracic surgeons 30-day predicted risk of mortality score also predicts long-term survival. Ann Thorac Surg. 2012;93:26–33. doi: 10.1016/j.athoracsur.2011.07.086. discussion 33-25. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ, III, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- Rodgers BD, Eldridge JA. Reduced Circulating GDF11 Is Unlikely Responsible for Age-Dependent Changes in Mouse Heart, Muscle, and Brain. Endocrinology. 2015;156:3885–3888. doi: 10.1210/en.2015-1628. [DOI] [PubMed] [Google Scholar]
- Rowe R, Iqbal J, Murali-Krishnan R, Sultan A, Orme R, Briffa N, Denvir M, Gunn J. Role of frailty assessment in patients undergoing cardiac interventions. Open Heart. 2014;1:e000033. doi: 10.1136/openhrt-2013-000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuford CM, Sederoff RR, Chiang VL, Muddiman DC. Peptide Production and Decay Rates Affect the Quantitative Accuracy of Protein Cleavage Isotope Dilution Mass Spectrometry (PC-IDMS) Molecular & cellular proteomics. 2012;11:814–823. doi: 10.1074/mcp.O112.017145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SC, Zhang X, Gross P, Starosta T, Mohsin S, Franti M, Gupta P, Hayes D, Myzithras M, Kahn J, et al. GDF11 Does Not Rescue Aging-Related Pathological Hypertrophy. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.115.307527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thourani VH, Suri RM, Gunter RL, Sheng S, O'Brien SM, Ailawadi G, Szeto WY, Dewey TM, Guyton RA, Bavaria JE, et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann Thorac Surg. 2015;99:55–61. doi: 10.1016/j.athoracsur.2014.06.050. [DOI] [PubMed] [Google Scholar]
- Walker RG, Poggioli T, Katsimpardi L, Buchanan SM, Oh J, Wattrus S, Heidecker B, Fong YW, Rubin LL, Ganz P, et al. Biochemistry and Biology of GDF11 and Myostatin: Similarities, Differences, and Questions for Future Investigation. Circ Res. 2016;118:1125–1142. doi: 10.1161/CIRCRESAHA.116.308391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TA, Lebrasseur NK. Myostatin and Sarcopenia: Opportunities and Challenges - A Mini-Review. Gerontology. 2014 doi: 10.1159/000356740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.